Abstract
We take an in vivo fluorescence approach to investigate photosynthetic adaptation by ecologically divergent members of the A/B clade of the hot spring cyanobacterium Synechococcus, the most thermotolerant of which defines the upper thermal limit for photosynthesis. During Synechococcus diversification, both photosystem II and the light-harvesting phycobilisome have evolved greater thermostability as the group has invaded higher temperature habitats, particularly for the most thermotolerant lineage. This enhanced function at higher temperatures has come at the cost of reduced performance at lower temperatures, and these trade-offs contribute to niche specialization in the clade. Molecular evolutionary analyses revealed specific adaptive protein changes in the most thermotolerant lineage. Our study advances our understanding of the origins of Synechococcus diversity.
Introduction
Cyanobacteria are the dominant primary producers in alkaline hot springs at temperatures below ca. 73 °C, the upper limit for photosynthetic life (Ward et al., 2012). In these environments, members of the Synechococcus A/B clade exhibit markedly divergent thermal ecologies (Miller and Castenholz, 2000; Allewalt et al., 2006), with more thermotolerant lineages in the group descended from a moderately thermophilic ancestor (Miller and Castenholz, 2000; Miller et al., 2013). The Synechococcus radiation therefore makes an excellent system for investigating how microorganisms have diverged during adaptation to novel environments. For example, although thermostability of the carbon-fixing enzyme RuBisCO was not under selection during much of the history of Synechococcus diversification, an evolved increase in stability was essential for the invasion of the highest temperature habitats that support photoautotrophy (Miller et al., 2013).
Temperature has a major effect on photosynthesis, and photosystem II (PSII), the multisubunit complex that extracts electrons from water at its oxygen-evolving complex (OEC) by light-driven redox chemistry, is the most temperature sensitive component of the photosynthetic apparatus (Berry and Björkman, 1980). Temperature also impacts the ability to efficiently harvest solar energy. In most cyanobacteria, light is primarily harvested for photosynthesis by phycobilisomes (PBS), supramolecular, phycobiliprotein-containing antennae that funnel excitation energy to the photosystem reaction centers (Glazer, 1985). High temperature stress can result in the reversible dissociation and energetic uncoupling of PBS from a photosystem or, under severe stress, irreversible PBS denaturation (Inoue et al., 2000). To better understand how Synechococcus has diverged during adaptation to different thermal environments, we have investigated the temperature dependence of PSII and PBS stability and function with an in vivo fluorescence approach.
Materials and methods
Temperature dependence of fluorescence
Strains were grown under a 12/12 h light–dark cycle at 55 °C as in Miller and Castenholz (2000). We monitored fluorescence emission at 680 nm of cell suspensions containing 1 μg±0.04 (s.e.) Chla per ml with a Photon Technology International model QM-7/2005 (Lawrenceville, NJ, USA) rapid temperature change spectrofluorometer equipped with a Quantum Northwest TLC 50 thermoelectric temperature-controlled cuvette holder. For each sample, cells were excited with either 437 or 605 nm light to excite either PSII or PBS, respectively. Fluorescence was measured beginning at 40 °C and then over 2.5 °C increments as the sample was heated until the temperature at which either complete inhibition of PSII (437 nm) or PBS denaturing (605 nm), respectively, was reached. Cells were stirred with a magnetic stir bar, and the temperature in the cuvette was independently monitored with an Omega temperature probe for all measurements. Experiments were conducted in triplicate for independent cultures of each strain.
Correlation analyses of thermotolerance traits
To estimate Pearson's correlation coefficients between thermotolerance measures, we used phylogenetic generalized least-squares models (Martins and Hansen, 1997) implemented in Compare version 4.6 (http://compare.bio.indiana.edu/), which account for the statistical dependence of phenotypic data collected from related organisms by converting phylogeny branch lengths into units of expected phenotypic divergence. Branch length data were from the phylogeny in Ward et al. (2012; Figure 1a).
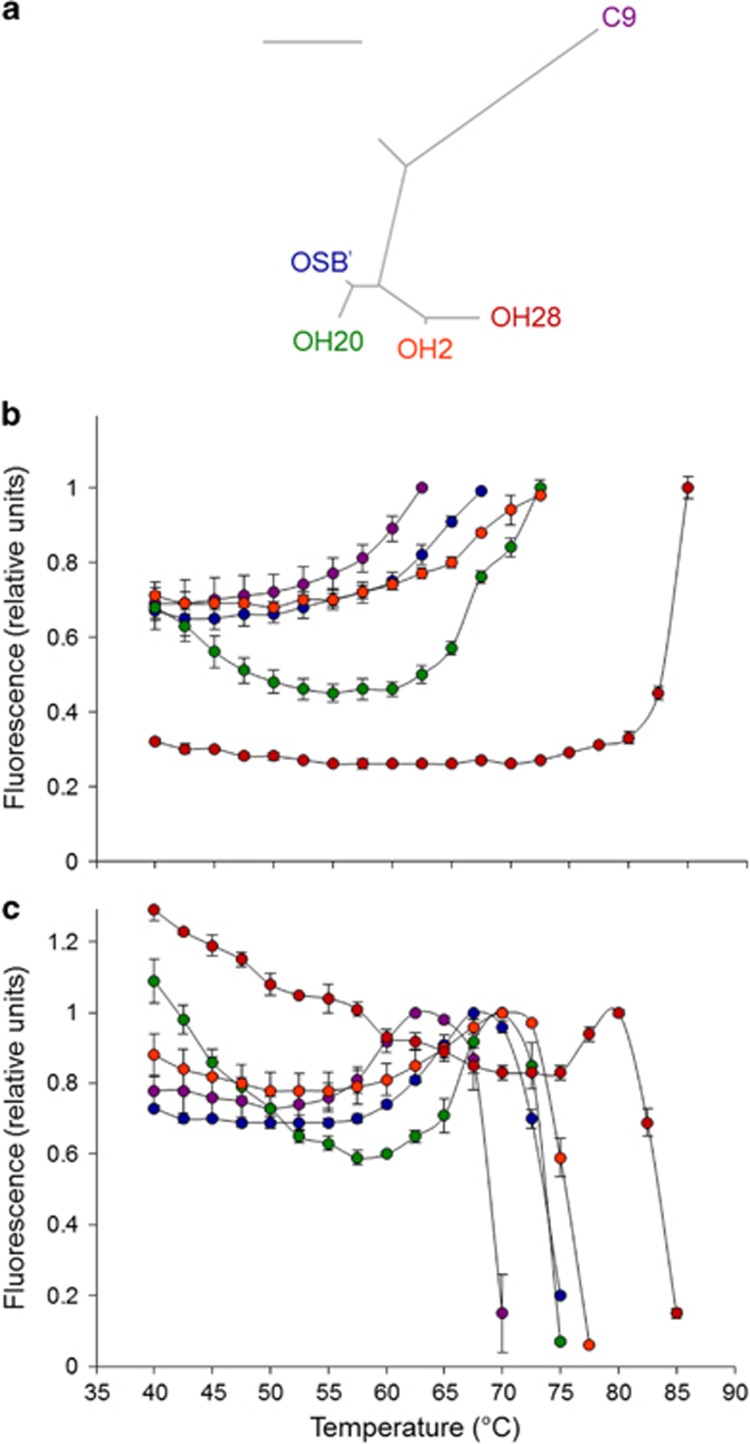
Figure 1.
(a) Bayesian phylogeny of the 16S rRNA gene for strains in the Synechococcus A/B clade and outgroup taxon Synechococcus strain C9. Topology and branch lengths are from Ward et al. (2012). The scale bar is 0.05 nucleotide substitutions per site. All nodes in the tree have posterior probability support of 100%. (b) Temperature dependence of relative in vivo fluorescence monitored at 680 nm for Synechococcus strains excited at 437 nm. Strain colors are as in (a). Error bars are s.e. A relative fluorescence value of 1.0 corresponds to the following mean absolute fluorescence values (photons per s)±s.e.: 96 871±14 070.0 (C9); 23 575±1892.1 (OSB′); 42 808±3975.8 (OH20); 43 869±3975.8 (OH2); 21 692±690.3 (OH28). (c) Temperature dependence of relative in vivo fluorescence monitored at 680 nm for Synechococcus strains excited at 605 nm. Strain colors are as in (a). Error bars are s.e. A relative fluorescence value of 1.0 corresponds to the following mean absolute fluorescence values (photons per s)±s.e.: 315 815±19 215.7 (C9); 108 273±6220.4 (OSB′); 226 643±16 087.7 (OH20); 338 726±16 340.4 (OH2); 28 335±432.1 (OH28).
Comparative sequence analysis
Genomic DNA was isolated as in Miller and Castenholz (2000), and fragments of genes encoding components of PSII and the PBS were amplified using primers (Supplementary Table S1) designed from the Synechococcus strains A and OSB′ genomes (GenBank accession nos CP000239 and CP000240; Bhaya et al., 2007). These were directly sequenced on an ABI 3130 at the University of Montana DNA Sequencing Facility (Missoula, MT, USA). Sequence data were deposited in GenBank (accession numbers KX758263–KX758291). For each gene, we implemented two maximum-likelihood models of codon evolution with the program codeml in PAML version 3.14: a branch-site model (Yang and Nielsen, 2002) that tests for adaptive evolution at the subset of codon sites along a specified lineage by allowing ω (that is, dN/dS) to vary both among codon sites and among branches; and a null model that constrains ω between 0 and 1 (that is, neutral evolution). Models were compared with likelihood ratio tests. For analyses for which the null model could be rejected, positively selected codons were identified by Empirical Bayes analysis (probability cutoff of 90%).
Results and discussion
Strains from the Synechococcus A/B clade (Figure 1a) and the thermophilic outgroup strain Synechococcus C9, which had been isolated from different thermal niches along the environmental gradients of North American alkaline hot springs, have diverged in their temperature dependence of growth (Miller and Castenholz, 2000; Allewalt et al., 2006; Table 1). These differences have persisted during laboratory maintenance: less thermotolerant strains C9 and OSB′ did not grow at or above 61 °C, strain OH20 grew at 61 °C but not at 64 °C, strain OH2 grew at 64 °C and only OH28 was capable of growth at or above 65 °C. We used fluorescence signals of divergent Synechococcus strains grown at a common garden temperature of 55 °C (Table 1) to test whether there have been evolutionary changes in the temperature dependence of PSII and PBS function during the diversification of this group.
Table 1. Synechococcus strain growth at 55 °C and temperature dependence.
| Strain | 55 °C growth rate (doublings day)±s.e. | Topt (°C) | Tmax (°C) |
|---|---|---|---|
| C9 | 0.79±0.008 | 45 | 55 |
| OSB′ | 0.56±0.059 | 50 | 60 |
| OH20 | 0.76±0.039 | 57 | 62 |
| OH2 | 0.57±0.017 | 57 | 63 |
| OH28 | 0.37±0.020 | 65 | 70 |
Abbreviations: Topt, observed temperature of maximum growth rate values from Miller and Castenholz (2000) and Allewalt et al. (2006); Tmax, observed maximum temperature supporting growth from Miller and Castenholz (2000) and Allewalt et al. (2006).
Because fluorescence and photochemical energy capture are competing de-excitation processes following light absorption, heat-induced impairment of PSII function results in an increased fluorescence yield (Berry and Björkman, 1980) as electron transport is inhibited between primary and secondary electron acceptors QA and QB (Cao and Govindjee, 1990). The temperature dependence of fluorescence emission during the excitation of PSII with blue light varied among divergent Synechococcus A/B strains and Synechococcus strain C9 (Figure 1b). Differences among strains explain 98% of the variation in the temperature at which the fluorescence maximum is reached (that is, the temperature of PSII inactivation; P<0.0001), which indicates that the onset of heat-induced damage has evolved during Synechococcus diversification. Based on t-tests with a Bonferroni correction to account for multiple pairwise comparisons, the temperature of PSII inactivation increased in the order: C9<OSB′<OH20=OH2<OH28 (P⩽0.005 for all significant comparisons). This is most striking for Synechococcus strain OH28, for which PSII function was not compromised at temperatures sufficient to inactivate all PSII centers of less thermotolerant strains. This strain was also characterized by lower baseline levels of relative chlorophyll fluorescence compared with the other strains (Figure 1b). In addition to photochemical quenching, other relaxation processes that compete with fluorescence following chlorophyll excitation that may potentially differ between OH28 and other strains include energy transfer to other pigments (including PSI), heat dissipation and the distribution of chlorophyll between PSII and PSI. Whether any of these fluorescence-quenching processes contribute to these differences, as well as whether there is a mechanistic connection to the extreme temperature tolerance of PSII in strain OH28, is a subject for future investigations. After accounting for the phylogenetic dependence of the data (see Materials and methods), the temperature of PSII inactivation was strongly positively correlated with both strain optimal growth temperature (R=0.98) and maximum growth temperature (R=0.98) measured in previous studies (Table 1; Miller and Castenholz, 2000; Allewalt et al., 2006). The effect of short-term heat stress on PSII function is therefore associated with the long-term temperature dependence of strain fitness.
We next assayed the thermostability of Synechococcus PBS. When PBS are excited at 605 nm, fluorescence emission monitored at 680 nm has two major sources: PSII and the PBS itself (Campbell et al., 1998). Fluorescence is at a minimum when both photochemical efficiency and energetic coupling between PBS and PSII are high (Pospíšil et al., 1998; Tamary et al., 2012). With further heating, fluorescence initially increases to a local maximum due to both decreased photochemical efficiency and heat-induced energetic uncoupling of membrane-diffusible PBS from the photosystems, but then sharply declines as PBS irreversibly denature (Inoue et al., 2000). As we observed above for PSII, there has also been substantial evolution of PBS thermostability during Synechococcus diversification, most notably for strain OH28 (Figure 1c). Differences among strains in the measured temperature of the fluorescence maximum under heating were very highly significant overall (P<0.0001), ranged from 62.5 °C (strain C9) to 80 °C (strain OH28), and increased in the order: C9<OSB′=OH20=OH2<OH28 (P⩽0.005 for all significant t-test comparisons with a Bonferroni correction). The temperature of this fluorescence maximum is strongly positively correlated with both optimal (R=0.96) and maximum (R=0.99) growth temperatures of Synechococcus (Table 1). Although the fluorescence maximum was not significantly different for strains OSB′, OH20 and OH2, these strains were distinguished by subtle but statistically significant differences in PBS stability based on the dynamics of the steep fluorescence decrease that is indicative of phycobiliprotein denaturation (Figure 1c; P<0.02 for the comparison between OH20 and OSB′ at 72.5 °C and P<0.001 for comparisons between OH2 and both OH20 and OSB′ at 75 °C). Strains with higher temperature maxima for growth (Table 1), therefore, have more stable PBS. In addition, the position of the fluorescence minimum, where photochemical quenching is greatest, is observed at higher temperatures in strain OH28 compared with other strains (Figure 1c), indicating that enhanced function at higher temperatures comes at the cost of reduced photochemical efficiency at lower temperatures. The inability of the photosynthetic apparatus to function well at both high and low temperatures thus contributes to the niche specialization of Synechococcus.
Taken together, the above results emphasize that enhanced thermostability of both PSII and PBS has contributed to the adaptation to higher temperatures during diversification of the Synechococcus A/B clade, particularly in the most thermotolerant lineage represented by strain OH28. To gain insights on the molecular basis of these changes, we implemented maximum-likelihood models of codon evolution (Yang and Bielawski, 2000) for several PSII and PBS genes from these strains. These included genes from the PSII core (psbA, psbB, psbD) and psbO, which encodes an extrinsic protein that forms a cap over the oxygen-evolving complex, as well as genes contributing to the membrane-associated core (apcA, apcB, apcD, apcE) and distal rods (cpcA, cpcB) of the PBS (Supplementary Table S1). Owing to the degeneracy of the genetic code, a nucleotide substitution in a protein-coding gene may or may not alter the amino-acid sequence. These models estimate the ratio of nonsynonymous (that is, amino-acid changing; dN) to synonymous (dS) substitution rates (ω=dN/dS). Nonsynonymous mutations are typically expected to be deleterious and therefore selected against (ω<1), whereas an excess of nonsynonymous substitutions (ω>1) is indicative of adaptive evolution (Yang and Bielawski, 2000). For each locus, we implemented a branch-site model (Yang and Nielsen, 2002) that tested for adaptive evolution at a few key sites along each branch of the Synechococcus phylogeny by allowing ω to vary both among codon sites and among branches (see Materials and methods). These models can be statistically compared by a likelihood ratio test with a null model for which ω is constrained between 0 and 1.
Amino-acid changes were very rare overall for all genes (ω<0.1). Despite these functional constraints on protein evolution, two genes exhibited evidence for a class of codon sites that was under positive selection along the strain OH28 terminal branch of the Synechococcus phylogeny presented in Figure 1a. The first was psbA, which encodes the D1 protein. D1 contributes most of the residues that bind and stabilize the oxygen-evolving complex, the reaction center and other electron transport components on both the donor and acceptor sides of PSII (Ferreira et al., 2004). One site (codon 222) was assigned to the positively selected class (ω=4.52; 2ΔL=16.76, P=0.0002) with high probability (97%). This site, which is threonine in strain OH28 but a highly conserved serine in other publicly available psbA sequences, is near the binding pocket of the secondary electron acceptor QB. This is of potential interest, given that inhibition of electron transport between QA and QB is likely responsible for heat-induced PSII inactivation (Cao and Govindjee, 1990). The other positively selected gene in our analyses was cpcA (ω=3.17; 2ΔL=7.56, P=0.025), which encodes the α-subunit of the PBS rod phycobiliprotein phycocyanin. One site (codon 111), at which glutamine has been replaced by methionine in strain OH28, was inferred to belong to the positively selected site class (90%). This residue makes contacts with the α84 phycocyanobilin chromophore and may contribute to changes in the protein environment surrounding the chromophores that are responsible for the observed blue shift in the absorption maximum of phycocyanin from ~620 to 608 nm in strain OH28 (unpublished data) and a related strain (Edwards et al., 1996).
These results also inform our understanding of the impacts of acute thermal stress at the physiological limits of photosynthetic metabolism. Whereas, in most strains, PSII is inactivated at approximately the same temperature as that of PBS dissociation from the photosystems (Figure 1), PSII of strain OH28 is more thermostable than the PBS. That is, the phenotypic effects of adaptive amino-acid substitutions that have occurred along the OH28 lineage (either positively selected substitutions detected by our models of codon evolution and/or unobserved changes at other genetic loci) have had a larger impact on the temperature dependence of PSII function, resulting in an asymmetric response exclusive to this lineage during Synechococcus diversification. Although the temperatures required to inactivate PSII and PBS exceed the thermal limit for sustained growth of each strain (Table 1; Miller and Castenholz, 2000; Allewalt et al., 2006), this shift is nonetheless significant, because it changes the nature of the physiological response of the photosynthetic apparatus during short-term exposure to extreme but non-lethal thermal stress that may occur in geothermal environments.
Acknowledgments
We thank two anonymous reviewers for their comments and suggestions on an earlier version of the paper. We also thank Reid Longley for his technical assistance with the fluorescence experiments and Big Sky High School (Missoula, MT, USA) interns Amanda Tripp and Amy Smith, who learned the polymerase chain reaction as a part of this project. This work was supported by US National Science Foundation award MCB-0347627 and NASA Astrobiology Institute award NNA15BB04A.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
The authors declare no conflict of interest.
Supplementary Material
References
- Allewalt JP, Bateson MM, Revsbech NP, Stack K, Ward DM. (2006). Effect of temperature and light on growth of and photosynthesis by Synechococcus isolates typical of those predominating in the Octopus Spring microbial mat community of Yellowstone National Park. Appl Environ Microbiol 72: 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J, Björkman O. (1980). Photosynthetic response and adaptation to temperature in higher plants. Ann Rev Plant Physiol 31: 491–543. [Google Scholar]
- Bhaya D, Grossman AR, Steunou AS, Khuri N, Cohan FM, Hamamura N et al. (2007). Population level functional diversity in a microbial community revealed by comparative genomic and metagenomic analyses. ISME J 1: 703–713. [DOI] [PubMed] [Google Scholar]
- Campbell D, Hurry V, Clarke AK, Gustafsson P, Oquist G. (1998). Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol Mol Biol Rev 62: 667–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Govindjee. (1990). Chlorophyll a fluorescence transient as an indicator of active and inactive photosystem II in thylakoid membranes. Biochim Biophys Acta 105: 180–188. [DOI] [PubMed] [Google Scholar]
- Edwards MR, MacColl R, Eisele LE. (1996). Some physical properties of an unusual C-phycocyanin isolated from a photosynthetic thermophile. Biochim Biophys Acta 1276: 64–70. [Google Scholar]
- Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. (2004). Architecture of the photosynthetic oxygen-evolving center. Science 303: 1831–1838. [DOI] [PubMed] [Google Scholar]
- Glazer AN. (1985). Light-harvesting by phycobilisomes. Annu Rev Biophys Biophys Chem 14: 47–77. [DOI] [PubMed] [Google Scholar]
- Inoue N, Emi T, Yamane Y, Kashino Y, Koike H, Satoh K. (2000). Effects of high-temperature treatments on a thermophilic cyanobacterium Synechococcus vulcanus. Plant Cell Physiol 41: 515–522. [DOI] [PubMed] [Google Scholar]
- Martins EP, Hansen TF. (1997). Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am Nat 149: 646–667. [Google Scholar]
- Miller SR, Castenholz RW. (2000). Evolution of thermotolerance in hot spring cyanobacteria of the genus Synechococcus. Appl Environ Microbiol 66: 4222–4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SR, McGuirl MA, Carvey D. (2013). The evolution of RuBisCO stability at the thermal limit of photoautotrophy. Mol Biol Evol 30: 752–760. [DOI] [PubMed] [Google Scholar]
- Pospíšil P, Skotnica J, Nauš J. (1998). Low and high temperature dependence of minimum F0 and maximum FM chlorophyll fluorescence in vivo. Biochim Biophys Acta 163: 95–99. [DOI] [PubMed] [Google Scholar]
- Tamary E, Kiss V, Nevo R, Adam Z, Bernát G, Rexroth S et al. (2012). Structural and functional alterations of cyanobacterial phycobilisomes induced by high-light stress. Biochim Biophys Acta 1817: 319–327. [DOI] [PubMed] [Google Scholar]
- Ward DM, Castenholz RW, Miller SR. (2012) Cyanobacteria in geothermal habitats. In: Whitton BE (ed). Ecology of Cyanobacteria II. Springer: Dordrecht, The Netherlands. [Google Scholar]
- Yang Z, Bielawski J. (2000). Statistical methods for detecting molecular adaptation. Trends Ecol Evol 15: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Nielsen R. (2002). Codon-substitution models for detecting molecular adaptation at individual sites along specific lineages. Mol Biol Evol 19: 908–917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.