Abstract
Soil nitrification potential (NP) activities of ammonia-oxidizing archaea and bacteria (AOA and AOB, respectively) were evaluated across a temperature gradient (4–42 °C) imposed upon eight soils from four different sites in Oregon and modeled with both the macromolecular rate theory and the square root growth models to quantify the thermodynamic responses. There were significant differences in response by the dominant AOA and AOB contributing to the NPs. The optimal temperatures (Topt) for AOA- and AOB-supported NPs were significantly different (P<0.001), with AOA having Topt>12 °C greater than AOB. The change in heat capacity associated with the temperature dependence of nitrification (ΔCP‡) was correlated with Topt across the eight soils, and the ΔCP‡ of AOB activity was significantly more negative than that of AOA activity (P<0.01). Model results predicted, and confirmatory experiments showed, a significantly lower minimum temperature (Tmin) and different, albeit very similar, maximum temperature (Tmax) values for AOB than for AOA activity. The results also suggested that there may be different forms of AOA AMO that are active over different temperature ranges with different Tmin, but no evidence of multiple Tmin values within the AOB. Fundamental differences in temperature-influenced properties of nitrification driven by AOA and AOB provides support for the idea that the biochemical processes associated with NH3 oxidation in AOA and AOB differ thermodynamically from each other, and that also might account for the difficulties encountered in attempting to model the response of nitrification to temperature change in soil environments.
Introduction
Both ammonia-oxidizing archaea (AOA) and bacteria (AOB) co-habit diverse soils. In some situations, AOA outnumber AOB by two to three orders of magnitude, and in other cases, their abundances are more similar (Leininger et al., 2006, Adair and Schwartz, 2008, Schauss et al., 2009, Taylor et al., 2012). Considerable debate has occurred about the relative contributions of AOA and AOB to soil nitrification, and the factors that may influence those contributions (Schleper, 2010, Hatzenpichler, 2012, Prosser and Nicol, 2012). There is direct and indirect evidence that factors such as increased CO2, N concentration and form, pH, and temperature differentially influence AOA and AOB contributions to nitrification (Hu et al., 2015, Liu et al., 2015, Sun et al., 2015). For example, growth of pure cultures of AOB isolates is usually optimal ⩽30 °C (Jiang and Bakken, 1999), and one AOB, Nitrosomonas cryotolerans, can grow at 4–5 °C (Jones et al., 1988). These observations corroborate several studies that showed the temperature optimum of soil nitrification is often ⩽30 °C (Malhi and McGill, 1982; Dalias et al., 2002; Avrahami et al., 2003; Fierer et al., 2003), and soil nitrification has been measured at temperatures as low as 2 °C (Cookson et al., 2002).
However, a few reports have described nitrification in soils from western United States and Australia with temperature optima of 35–40 °C (Myers, 1975; Stark and Firestone, 1996). The isolation of the AOA Nitrososphaera gargensis and Nitrosocaldus yellowstonii from geothermal sources with temperature optima of 46 and 65–72 °C, respectively (de la Torre et al., 2008; Hatzenpichler et al., 2008), and the discovery of AOA soil isolates, Nitrosotalea devanaterra Nd1, ‘Candidatus Nitrosocosmicus franklandus' and Nitrososphaera viennensis, growing optimally at 35–40 °C (Tourna et al., 2011; Lehtovirta-Morley et al., 2014; Lehtovirta-Morley et al., 2016), led us to speculate that AOA might be primarily responsible for soil nitrification ⩾30 °C. In a field study, we previously observed increases of AOA numbers, and evidence of their activity, in cropped soils under late summer/early fall conditions when soil temperatures reached 30–35 °C (Taylor et al., 2012), and studies from a UK agricultural soil incubated at temperatures between 10 and 30 °C showed that the thaumarchaeal composition (determined by analysis of the relative abundance of 16S ribosomal RNA) shifted during incubation at 25 and 30 °C, whereas there was no change in the AOB community composition (Tourna et al., 2008; Offre et al., 2009). In contrast, AOA were enriched from Arctic soils incubated at 20 °C, and rates of nitrification were measured in three Arctic soils incubated at 15 °C, where AOB amoA numbers were below detection (Alves et al., 2013).
In previous studies, we showed that the relative contributions of AOB and AOA to nitrifying activity varied among four pairs of noncropped and cropped soils sampled throughout the state of Oregon (Taylor et al., 2013, 2015; Giguere et al., 2015). In this study, the AOA and AOB contributions to the nitrification potential (NP) of the above-mentioned soils were evaluated over a temperature range that they annually experience (4–42 °C). We hypothesized that AOA and AOB would exhibit different characteristic responses to temperature, and to quantify these differences we used two models: the square root growth model (SQRT) that has been used to relate soil respiratory and growth processes to temperature (Birgander et al., 2013; van Gestel et al., 2013), and the macromolecular rate theory model (MMRT) utilized by Schipper et al. (2014) to model the temperature response of a variety of microbial activities, including nitrification.
Materials and methods
Chemicals
Vanadium chloride, 1-octyne (C8) and NH4Cl were obtained from Sigma-Aldrich (St Louis, MO, USA). Acetylene was obtained from Airgas (Radnor, PA, USA).
Collection of soils
In the spring of 2014, soil samples, representing different soil types from different climate regions of Oregon, were collected from cropped fields and noncropped locations at four locations (three replicates of each) at Oregon State University Agricultural Experimental Stations located in Corvallis, Pendleton, Madras and Klamath Falls, Oregon (Taylor et al., 2013; Giguere et al., 2015). Four to five soil samples were recovered to a depth of 10 cm from each replicate site via a random walk process. A composited sample was prepared for each replicate site and brought to the laboratory where they were sieved <4.75 mm. The soils were stored at 4 °C before experimentation.
Response of soil NPs to temperature
NPs were determined on soil samples as described previously (Taylor et al., 2010b), except slurries were shaken at a range of temperatures (4–42 °C) in deionized water containing 1 mM NH4+. Some NP treatments included octyne (4 μM) to distinguish between AOA and AOB activities, using a procedure described by Taylor et al. (2013). AOA are octyne resistant, and AOB are octyne sensitive (total nitrification−nitrification by octyne-resistant AOA). NP controls were comprised of soil suspensions to which acetylene was added (10 μM) to evaluate the possibility of acetylene-resistant heterotrophic nitrification, and also to assess the significance of NO3− consumption due to assimilation or denitrification. Each treatment had three replicates. Accumulation of NO2−+NO3− was monitored over a 3-day interval in incubations performed at 4, 10 and 16 °C, whereas 1 day was sufficient for incubations at higher temperatures. The accumulation of NO2−+NO3− in the NPs was considered to be the rate of nitrification. NO2− accumulated to various fractions of the total NO2−+NO3− in actively nitrifying soil slurries over the temperature range, and generally increased as temperature increased but did not change the overall rate of NO2−+NO3− accumulation. The accumulation of NO2−+NO3− in the 1- or 3-day NPs was linear at all temperatures, indicating that there was no change in the nitrifier abundance or adaptation/recovery of NP activity over the course of the NP assays. There was no significant NO3− consumption or production in plus acetylene controls (data not shown), indicating all NO2−+NO3− accumulation was likely due to NH4+-dependent nitrification, and that alternate sinks for NO2− or NO3− were not significant under NP conditions.
Modeling of the nitrification response to temperature
Each replicate of the NP temperature response data was modeled using two methods. The first was the SQRT developed by Ratkowsky et al. (1983) to describe bacterial growth response to temperature, but has been used more recently to describe soil respiratory and growth processes to temperature (Birgander et al., 2013; van Gestel et al., 2013). Here it was used to model the NP response to temperature:
Four unknown parameters were fit to this model in MATLAB (The MathWorks Inc., Natwick, MA, USA): (i) ‘a' is a parameter associated with the temperature response of activity at temperatures below the temperature at which the highest nitrification rates were obtained (Topt); (ii) Tmin represents a theoretical constant describing the x-intercept, and is referred to as the apparent minimum temperature at which activity is measured; (iii) ‘b' describes the decrease in nitrifying activity above Topt; and (iv) Tmax represents the maximum temperature where activity may be detected. Topt is described by the Equation
The second model used was MMRT utilized by Schipper et al. (2014) to model the temperature response of a variety of microbial activities, including nitrification, and is described by the Equation
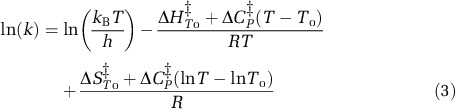 |
where k is the rate constant, Tnaught (To) is the reference temperature at which the fitting process is initiated, kB is Boltzmann's constant, h is Planck's constant and R is the universal gas constant (8.314 J K−1 mol−1). Three unknown parameters were fit to the model using SigmaPlot (Systat Software, San Jose, CA, USA): (i) the change of enthalpy of AOA or AOB supported nitrification (ΔHTo‡), (ii) the change of entropy of the AOA or AOB supported nitrification (ΔSTo‡), and (iii) the change in heat capacity associated with temperature dependence of NO2−+NO3− accumulation attributed to AOA or AOB (ΔCP‡). The temperature for optimum activity Topt is described by:
 |
The temperature where the AOA- or AOB-supported NO2−+NO3− accumulation rate shows maximum sensitivity to changes in temperature (Ts_max) is described by:
 |
From the modeling activities, we obtained information that predicted there were different temperature responses of AOA- and AOB-driven activities. As a consequence, a series of experiments were conducted to (i) gain further insights into how well the recovery of acetylene-inactivated NO2−+NO3− production activity in both AOA- and AOB (biosynthetic competence) matched NO2−+NO3− production activity across the temperature range, (ii) confirm the predicted Tmin and Tmax of AOA and AOB activity, and (iii) determine whether Ts_max values reflected simply a thermodynamic response of preexisting activity potential, or whether they were influenced by the recruitment/activation of nitrifying activity of subpopulations with different temperature profiles. This series of experiments was conducted in Pendleton noncropped soils where both AOA and AOB consistently demonstrated their highest rates of NO2−+NO3− production activity.
The effect of temperature on acetylene inactivation of soil slurries
(i) Does biosynthetic competence match NO2−+NO3− production activity in AOA and AOB? The ability of acetylene to irreversibly denature NH3 monooxygenase (AMO) depends upon the enzyme being potentially active and capable of using acetylene as a substrate under the experimental conditions; recovery of AMO activity is dependent upon the ability to carry out de novo protein synthesis under the same conditions. Therefore, we used the recovery of nitrification potential (RNP) assay as a surrogate to assess the protein synthesis (biosynthetic) potential of the AOB and AOA at a range of temperatures (10, 16, 23, 30 or 37 °C) in Pendleton noncropped soil. Details of the RNP assay have been described previously (Taylor et al., 2010b). Soils were preincubated at field capacity for 2–3 days at specific temperatures of 10, 16, 23 or 30 °C, or for a shorter duration (18 h) at 37 °C. Then, the soils were slurried in deionized water containing 1 mM NH4+, exposed to acetylene (10 μM Caq) for 6 h at each of the specific temperatures, after which acetylene was removed by evacuation under vacuum, and RNP determined plus and minus bacterial protein synthesis inhibitors (RNPab, 800 μg ml−1 kanamycin plus 400 μg ml−1 spectinomycin) at the specific inactivation temperatures. At 10 °C, soil was allowed to recover activity for 90 h, whereas the magnitude of recovery at other temperatures was determined after 48–72 h. Controls included (a) no alkyne amendment, (b) plus acetylene (10 μM Caq) and (c) plus octyne (4 μM Caq) treatments to establish the AOA- and AOB-dependent activities at each temperature.
(ii) Effect of temperature on the magnitude of acetylene inactivation of optimal nitrification activity to experimentally determine Tmin and Tmax. Noncropped Pendleton soil was preincubated at temperatures ranging from 4 to 42 °C as described above. Soils were slurried in distilled H2O containing 1 mM NH4+ and exposed to acetylene (10 μM Caq) for 6 h at each specific preincubation temperature. Then, acetylene was removed by vacuum, and soil slurries were incubated plus and minus 4 μM (Caq) octyne with shaking at 16 or 37 °C (the optimal temperatures for AOB or AOA activity in this soil, respectively, see Figure 1). Controls included no alkyne amendment, plus acetylene (10 μM Caq) and plus octyne (4 μM Caq) treatments to evaluate the effect of the temperature inactivation step on activity at the optimal temperatures. Maximum RNPAOA at 37 °C in this soil normally occurred between 24 and 48 h after acetylene removal, and maximum RNPAOB at 16 °C occurred between 48 and 72 h after acetylene removal. Accumulation of NO2−+NO3− after ⩽12 h of incubation was considered to be residual NH3-oxidizing activity that had escaped acetylene inactivation. The octyne-resistant residual activity was considered to be residual AOA activity, whereas residual AOB activity was calculated as the difference between the plus and minus octyne treatments. To confirm that 6 h of incubation in the presence of acetylene was sufficient to completely inhibit all NH3-oxidizing activity occurring at temperatures of 10, 23 and 30 °C, a longer exposure time (10 h) was evaluated. There was no significant difference (P>0.1) between the residual activity after the two periods of acetylene inactivation.
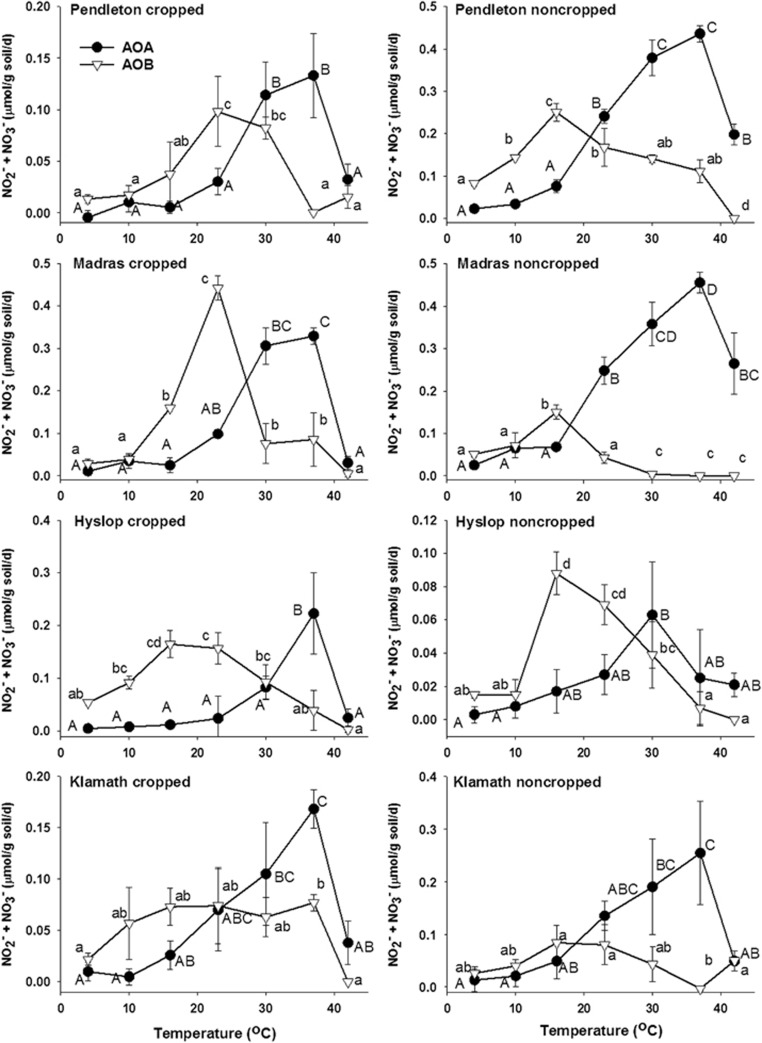
Figure 1.
Nitrification potential response of AOA and AOB in cropped and noncropped soils to a range of temperatures (4–42 °C). Symbols with error bars represent the s.d. of the average of three replicate measurements. Upper case letters indicate significant differences (P<0.05) in rates of nitrification by AOA at each temperature. Lower case letters indicate significant differences (P<0.05) between rates of nitrification by AOB at each temperature. Temperatures that have letters in common were not significantly different.
Statistical analysis
To determine the significant differences (P<0.05) in AOA or AOB NP activities over the 4–42 °C range, analysis of variance (ANOVA) tests with the Tukey–Kramer adjustment were performed using the three replicates in Statgraphics Version 17.1.06 (Statpoint Technologies, Warrenton, VA, USA) for all pair-wise comparisons among temperatures within each soil. With the sites as replicates, ANOVA using the default Type III Sum of Squares was performed in Statgraphics on the average of the mean of each of the model coefficients to determine the statistical significance of each factor. ANOVA using the Holm–Sidak method was used for pair-wise comparisons in SigmaPlot (Systat Software, San Jose, CA, USA) to determine whether there were significant differences in AOA or AOB activity after acetylene treatment.
Results
AOA and AOB contributions to NO2−+NO3− production over a temperature profile
The soil AOA and AOB contributions to the NPs of four pairs of adjacent cropped and noncropped soils were evaluated over a temperature range of 4–42 °C using the octyne method to discriminate between AOA and AOB activities (Figure 1). Regardless of soil origin or cropping history, AOA consistently expressed their maximum NP rates between 30 and 37 °C where they contributed virtually all of the NP. In contrast, AOB expressed their maximum NP rates at either 16 or 23 °C, contributing most of the total NP in cropped and noncropped soils. The experiments were repeated with soils collected from the same sites in the summer and fall of 2014 and the same trends were observed (data not shown).
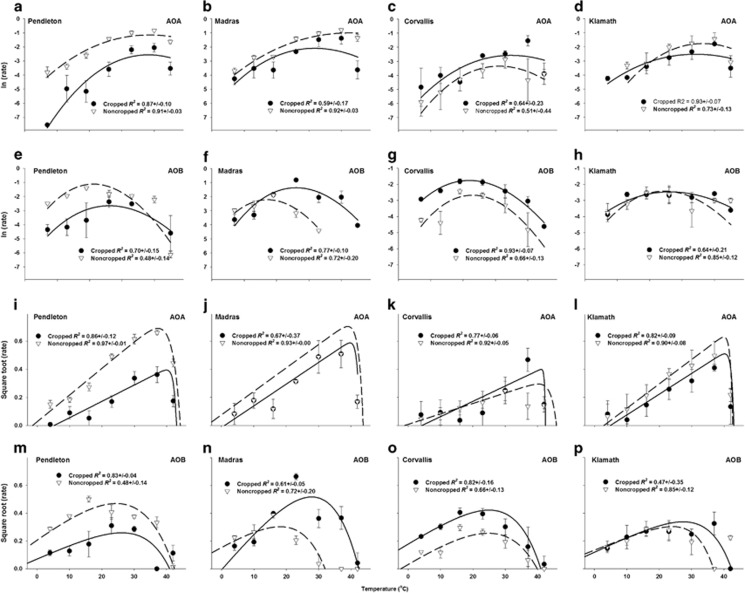
To further quantify the differences in temperature response of AOA and AOB driven activities, two complementary models that have been previously used to describe the response of soil processes to temperature, MMRT and SQRT, were applied to the data (Figure 2; Supplementary Tables 1 and 2). Based on estimates of least squared error, the SQRT model fit the temperature response better than the MMRT model (0.03±0.02 and 1.77±1.40 for SQRT and MMRT); however, there was not a significant difference in how well each model fit either the AOA or AOB temperature response (P>0.2). Conversely, there was no difference between the SQRT and MMRT models on how well a regression of the model outcome approximated the experimental data; the models yielded coefficients of determination (R2) with an average of 0.75±0.13 for MMRT and 0.77±0.15 for SQRT models. In addition, there was no significant difference of any of the MMRT or SQRT model parameters between cropped and noncropped soils (P>0.05).
Figure 2.
The fit of the MMRT (a–h) and SQRT (i–p) models to rates of NO2−+NO3− production by AOA and AOB in cropped (solid lines) and noncropped (broken lines) using the average of the model fit from each replicate. Symbols with error bars represent the s.d. of the average of the experimental data.
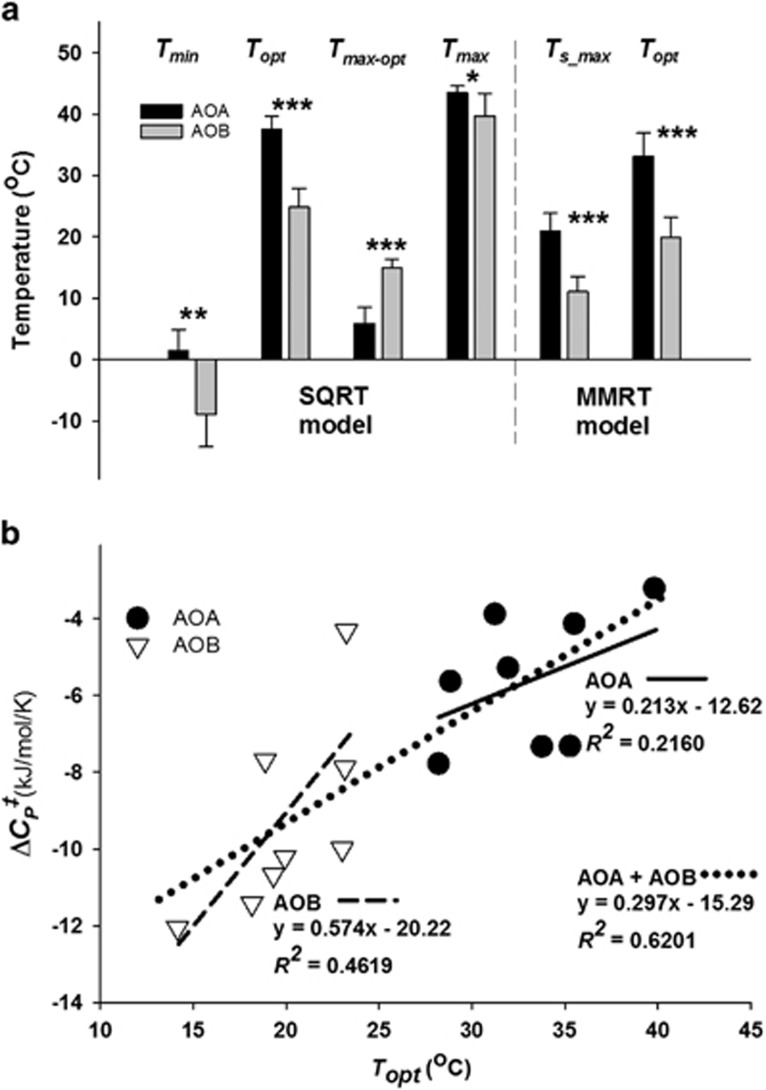
When the AOA and AOB activity responses to temperature were compared, several model parameters were highly significantly different (Figure 3a). Both models identified Topt values (the optimal temperature at which activity is achieved) for AOA that were significantly greater than estimates of Topt for AOB (P<0.000, Figure 3a), with the Topt derived from the SQRT model being significantly greater (4.7±2.6 °C) than that derived from the MMRT model (P<0.000, Supplementary Tables 1 and 2). The SQRT model estimated a Topt for AOA activity that was 12.8 ± 4.4 °C higher than that of AOB activity, and the Topt for AOA activity estimated by the MMRT model was 13.1±6.3 °C higher than that of AOB.
Figure 3.
Significant outcomes from modeling the NP response to temperature in eight Oregon soils. (a) SQRT and MMRT model parameters that had significantly different values for NO2−+NO3− production by soil AOA and AOB. The pairs of bars represent the average of the mean AOA or AOB value of a given parameter, and error bars represent the s.d. The asterisks assign the level of significance to each pair of bars (*P<0.01, **P<0.001, ***P<0.0001). (b) Comparison of the ΔCP‡–Topt–relationships of AOA and AOB in soil. Symbols represent the values for individual soils. The dotted regression line demonstrates the ΔCP‡–Topt–relationship for all AOA and AOB values across the entire temperature range. The solid and dashed regression lines are for the AOA and AOB ΔCP‡–Topt relationship, respectively.
The SQRT model generated a theoretical apparent minimum temperature of activity (Tmin) for AOB that was significantly lower than that of AOA (−8.8±5.3 and 1.4±3.5 °C for AOB and AOA, respectively, P<0.001, Figure 3a). In addition, SQRT yielded a maximum temperature of activity, Tmax, for AOA that was significantly greater than that of AOB (43.5±1.1 and 39.7±3.7 °C, P<0.01). The temperature interval between Topt and Tmax (Tmax-opt) was significantly greater for AOB than AOA (14.9±1.5 and 5.9±2.6 °C, P<0.000), which was in agreement with the significant difference in the b parameter (Supplementary Tables 1 and 2, P<0.01) that describes the much steeper decline in AOA activity above Topt compared with AOB. The MMRT model yielded a significant difference in the temperature at which the rate of nitrification showed maximum sensitivity to changes in temperature (Ts_max, P<0.000) between AOA and AOB activity (20.9±3.0 and 11.1±2.4 °C for AOA and AOB), and also in the heat capacity term ΔCP‡ (Supplementary Tables 1 and 2, P<0.01).
The curvature of the ln(rate) vs temperature plots of both AOA and AOB activities (Figures 2a–h) indicated that enthalpy and entropy (ΔHTo‡ and ΔSTo‡, respectively) of the overall nitrification processes were temperature-dependent (Equation (3)), and the significant difference in ΔCP‡ pointed to potential differences in thermodynamic properties of AOA and AOB. A large negative ΔCP‡ of an enzyme reaction is associated with a lower Topt (Hobbs et al., 2013; Schipper et al., 2014). When the relationship between the response of Topt and ΔCP‡ of soil AOA and AOB activities was compared (Figure 3b), larger negative ΔCP‡ values correlated with lower Topt across the spectrum of soils (R2=0.6201); and there was a clear trend for the ΔCP‡ of soil AOB-driven activity to be more negative than of soil AOA-driven activity, implying differences in the thermodynamic properties of NO2−+NO3− production supported by the two groups of nitrifiers.
Experimental validation of insights into AOA and AOB activity differences gained from modeling of temperature-influenced parameters
A series of experiments were conducted to gain further insights into (i) protein biosynthetic competence of AOA and AOB across a temperature profile, (ii) how the predicted cardinal values of Tmin and Tmax reflect AOA and AOB activity, and (iii) how properties of Ts_max of AOA and AOB activity might be influenced by recruitment of AMO activity exhibiting different temperature profiles. These experiments were carried out in Pendleton noncropped soils because the high rates of both AOA and AOB activities expressed in this soil allowed for the execution of these detailed experiments.
Evaluation of protein biosynthetic potential in soil across a temperature profile
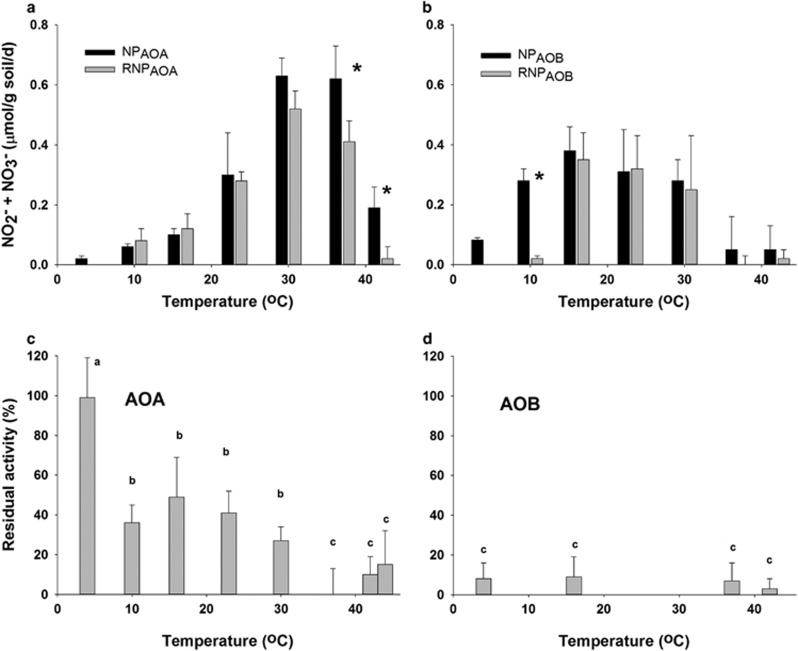
Using the RNP assay as a surrogate to assess protein synthesizing potential of AOA and AOB, the NP and RNP were compared across the temperature range in Pendleton noncropped soil (Figure 4). AOA demonstrated RNP (RNPAOA) at 10–37 °C, illustrating their effective protein synthesis potential at temperatures where significant nitrifying activity occurred (Figure 4a). However, at 37 °C, RNPAOA was significantly less than the NPAOA and RNPAOA did not occur at all at 42 °C, suggesting that high temperatures that support short-term rates of NO2−+NO3− producing activity might not necessarily support biosynthetic potential and growth of all AOA population members. In contrast, AOB demonstrated RNPAOB only at 16, 23 and 30 °C (Figure 4b) despite expressing substantial NO2−+NO3− producing activity over a wider temperature range (4–37 °C), suggesting a much narrower temperature range of protein synthesis and growth potential than for NO2−+NO3− producing activity per se.
Figure 4.
The effect of acetylene exposure on AOA and AOB in Pendleton noncropped soil. Evaluation of biosynthesis potential (RNP) of AOA (a) or AOB (b) in comparison with potential nitrification activity (NP) over a range of temperatures in Pendleton noncropped soil. Soils were inactivated and activity recovered at the same temperature. Error bars represent the s.d. of three replicates. The asterisks (*) represent the NP and RNP that were significantly different at the same temperature (P>0.05). The effect of acetylene on the residual Topt activity of AOA at 37 °C (c) or AOB at 16 °C (d). Soil was acetylene-inactivated at 4 through 46 °C for 6h, degassed, and transferred to 37 °C for measurement of AOA residual activity, or 16 °C for measurement of AOB residual activity. Residual activity measured between 0 and 12 h was considered to be due to AMO that was not acetylene-inactivated. Error bars represent the s.d. of three replicates. Lower case letters indicate significant differences between residual activities (P<0.01). Bars with the same letter are not significantly different.
Experimental validation of Tmin and Tmax, and evidence for different and overlapping contributions to soil nitrification by different groups of ammonia oxidizers
We considered the possibility that subgroups of AOA and AOB might occupy different temperature niches within the overall range of temperatures and would possess different Tmin and Tmax. It is known that acetylene is an irreversible inactivator of AMO of both AOA and AOB during active substrate turnover (Hyman and Wood, 1985; Taylor et al., 2013; Vajrala et al., 2014), and that de novo protein synthesis is required for activity to resume. Our hypothesis was that if the AMO of AOA or AOB are not actively turning over substrate at specific temperatures, they should not be inactivated by acetylene. We also hypothesized that we would observe heat inactivation of NO2−+NO3− accumulating activity above their modeled Tmax. To evaluate these hypotheses, Pendleton noncropped soil was inactivated with acetylene at a range of temperatures from 4 to 46 °C, and the residual activities after acetylene exposure measured at 16 °C (temperature of optimal AOB activity) and 37 °C (temperature of optimal AOA activity).
The SQRT model predicted Tmax of 44.2 and 42.4 °C for AOA and AOB, respectively, in Pendleton noncropped soil. To determine whether both AOA and AOB were capable of oxidizing NH3 or enzyme turnover at temperatures >40 °C, Pendleton noncropped soil was exposed to acetylene at 42, 44 or 46 °C, and then transferred to 16 or 37 °C. After acetylene exposure of soil at 42 °C, there was no residual AOA- or AOB-driven activity significantly greater than zero over 12 h of incubation (Figures 4c and d). These data indicate that both AOA and AOB were capable of expressing sufficient AMO activity for acetylene to inactivate all NO2−+NO3− production activity, even though AOB-driven NO2−+NO3− accumulation could not be measured at 42 °C over 24 h. Both AOA and AOB retained all their NO2−+NO3− production activities when returned to 16 or 37 °C, respectively, in non-acetylene exposed controls after incubation at 42 °C, demonstrating there was no irreversible heat inactivation of cellular functions at this temperature. After acetylene exposure at 44 °C, there was no significant residual post-acetylene exposure AOA activity, again indicating that AOA expressed sufficient AMO turnover at 44 °C for acetylene to inactivate all activity, but in the non-acetylene controls all activity was retained. In contrast, there was no residual AOB activity in either the non-acetylene or acetylene treatments exposed to 44 °C, suggesting that the upper limit of AOB thermal stability had been exceeded. Incubations at 46 °C heat-inactivated both AOA and AOB activity regardless of acetylene treatment. Despite the close similarity of Tmax values for AOA and AOB activities, the outcome of the experiment confirmed the model prediction.
The SQRT model also predicted significant differences in Tmin of AOA and AOB, and when acetylene inactivation was carried out at 4 °C and the soil moved to 37 °C, all of the octyne-resistant AOA activity was immediately expressed, indicating that none of the AOA AMO activity was actively turning over substrate during acetylene exposure at 4 °C, and that Tmin of the active soil AOA must be >4 °C. In contrast, there was no residual AOB activity significantly greater than zero at 16 °C after acetylene exposure at 4 °C (Figure 4d) verifying the model prediction that the soil AOB were active at 4 °C and possess a lower Tmin than the soil AOA.
When acetylene inactivation of Pendleton noncropped soil was performed at 10 °C, however, and the slurries subsequently transferred to 37 °C, only a fraction of the non-acetylene exposed octyne-resistant activity (27–49%) was immediately expressed, indicating that a fraction (51–63%) of the AOA potential activity had become active at the lower incubation temperatures between 10 and 30 °C and inactivated by acetylene. Longer exposures to acetylene at ⩽30 °C did not increase the fraction of activity that was inactivated. The response to acetylene inactivation across the temperature range suggested that there may be different types of AMO within the AOA population with one response type having a Tmin between 4 and 10 °C, and another AMO response type with a Tmin that must be higher than 30 °C. Evidence of different temperature responsive AOA AMO activities could indicate that Ts_max of AOA might be explained by different AOA AMO types with different Tmin values active across different ranges of temperature.
In the case of AOB, there was no significant residual nitrifying activity at 16 °C after acetylene exposure at any temperature, indicating that the soil AOB expressed sufficient AMO activity across the whole temperature range to be inactivated by acetylene. This indicates that the AOB actively contributing to the measured NO2−+NO3− production in the Pendleton noncropped soil may have AMO possessing the same temperature response profile, and that Ts_max describes the thermodynamic response of AOB AMO to temperature increase.
Discussion
We have measured the temperature response of the AOA and AOB actively contributing to NO2−+NO3− production in eight Oregon soils, and collected data demonstrating that soil AOA and AOB possess different temperature-dependent characteristics and contribute differentially to soil nitrification over a range of temperature. The temperature responses were evaluated in well-aerated soil slurry NPs containing saturating NH4+. Although we readily concede that our results might not necessarily extrapolate to all soils, and in situ whole soil nitrification, nonetheless, several interesting points have arisen from this work that are worthy of comment and further study. For example, because it appears that AOA and AOB AMO possess different responses to temperature, it is perhaps not surprising that it has been challenging to model the response of soil nitrification to temperature (Stark, 1996), and for Schipper et al. (2014) to determine the parameters Topt and ΔCP‡ when the temperature range of their data set only extended from 5 to 20 °C—a range below the Topt of AOA in the soils used in our study. Similarly, a trait-based modeling approach to nitrification was limited by both the lack of data obtained with AOA and AOB laboratory isolates, and a comprehensive data set with which to test the model (Bouskill et al., 2012). By distinguishing between AOA and AOB contributions to soil NPs with the octyne method over a range of temperatures, and then evaluating those data using models with either empirical or thermodynamic underpinnings, we were able to parameterize some of the characteristics of nitrification driven by AOA and AOB, respectively. Our results agree with, and in part explain what has previously been observed, but also contribute new insights into how AOA and AOB may contribute differentially to nitrification in soils. The discovery of Nitrospira capable of complete nitrification (comammox) potentially adds further complexity to the interpretation of the AOA and AOB contributions in soil (Daims et al., 2015; van Kessel et al., 2015). Nonetheless, limited data imply that comammox is highly sensitive to octyne (Daims, personal communication) and, therefore, should not interfere with any conclusions drawn about octyne-resistant AOA activity. How important the contributions of comammox to nitrification are in soils will remain unknown until tools to discriminate between comammox activity and that of AOA and AOB have been developed.
Significance of the model results
The SQRT and MMRT models were in agreement that Topt for AOA activity was significantly greater than that of AOB. This observation was true for soils that had never been cropped or N fertilized, as well as for adjacent cropped soils of the same soil series that are regularly cultivated and N fertilized. Although it is unknown if the mechanism of octyne inhibition may itself be temperature-dependent, the temperature niche separation determined with the octyne method agrees with previous studies that found differences in AOA and AOB amoA gene abundances or differential labeling with 13CO2 in response to different incubation temperatures (Avrahami et al., 2011; Wu et al., 2013; Zeng et al., 2014), and with the isolation of soil AOA that grow optimally at 35–40 °C (Tourna et al., 2011; Kim et al., 2012; Lehtovirta-Morley et al., 2014, 2016). Some of these AOA isolates were obtained at an initial cultivation of 37 °C (Lehtovirta-Morley et al., 2011, 2016; Tourna et al., 2011); however, another isolate was initially enriched at 25 °C (Kim et al., 2012). Virtually, all AOB isolates grow best at ⩽30 °C (Jones and Morita, 1985; Stein and Arp, 1998; Jiang and Bakken, 1999; Norton et al., 2008). Examples of the response of specific rates of activity by AOB isolates to comprehensive temperature ranges are rare in the literature (Jiang and Bakken, 1999), and the influence of initial cultivation or growth temperatures on the cultures' specific rates of activity in response to temperature are uncertain. Four AOB strains that were grown at 22 °C had their highest rates of activity at 25–30 °C (Jiang and Bakken, 1999), and the AOB Nitrosomonas sp. 4W30 (initially isolated at 10 °C) had highest specific rates of activity at 20 and 32 °C when cultivated at 5 and 25 °C, respectively (Jones and Morita, 1985). The temperature response of only a few AOA isolates has been examined, and in these cases, the response of specific growth rates, not activities, to different temperatures were measured (Jung et al., 2011; Kim et al., 2012; Lehtovirta-Morley et al., 2014, 2016). Comparing coefficients generated using growth data of isolates may not be a fair comparison with the specific rates of soil-nitrifying activity that we measured in this study. For example, when the Topt values for respiratory (glucose mineralization) and growth (leucine incorporation) processes were compared in a soil, the Topt for respiration was >20 °C higher than the Topt of growth (Birgander et al., 2013), suggesting that the thermodynamic properties of growth and respiration can differ among mixed soil populations. In this context, we showed that, although the temperature range of AOB activity was wide (4–42 °C), the ability of AOB to conduct de novo protein synthesis to replace acetylene-inactivated AMO covered a much narrower temperature range. Clearly, more comparative studies are needed with AOA and AOB isolates, taking both growth and NH3-oxidizing activity into consideration.
The SQRT model predicted significantly higher Tmax values for AOA- and AOB-driven nitrification (P<0.01). We experimentally demonstrated in Pendleton noncropped soil that both AOA and AOB (Tmax estimates of 44.2±0.9 °C and 42.4±0.1 °C) had sufficient rates of NH4+ turnover at 42 °C for their respective AMO to be irreversibly inactivated by acetylene, resulting in no residual activity upon subsequent incubation at their respective Topt values. We also found that their heat tolerance was very similar, with AOB withstanding temperatures ⩽44 °C, and AOA ⩽46 °C. Perhaps it makes sense that AOA and AOB in the same soil should have similar thermal stability. Previous studies have shown that changes in the temperature response of soil microbes, using 14C leucine incorporation occurred as a result of 1–2-month incubations of soil at elevated temperatures (Barcenas-Moreno et al., 2009; Birgander et al., 2013). As a result, the highest temperature in an environment, even transiently over seasons, can select for organisms that have similar heat tolerance (van Gestel et al., 2013; Schipper et al., 2014). With the acetylene inactivation approach, we confirmed the model predictions of a significantly lower Tmin value for AOB than AOA. Through this approach, we also obtained evidence that raising the soil temperature to 10 °C resulted in appearance of some activity of AOA AMO, but not all of it, suggesting the existence in the AOA population of different temperature responsive forms of AMO. This result contrasts with the lack of evidence for different temperature responsive forms of AMO among the AOB. With regard to model parameters, these results could suggest different interpretations of the Ts_max parameter for AOA and AOB. Because the AOB demonstrated a single temperature response, Ts_max could indicate a purely thermodynamic response of NH3-oxidizing activity to temperature increase, whereas the higher Ts_max for AOA could be due to recruitment of additional AMO types within the AOA population with different thermodynamic response profiles.
Although the ΔCP‡ parameter modeled from the MMRT model should be considered as an integration of multiple biochemical reactions that define nitrification, it is intriguing to consider ΔCP‡ in context with specific properties of AMO. For example, Hobbs et al. (2013) demonstrated through site-directed mutagenesis studies with the enzyme α-glucosidase (MaIL) that enzymes with larger negative ΔCP‡ values have more conformational states than enzymes possessing less negative ΔCP‡ values. The soil AOB in our study expressed larger negative ΔCP‡ values than AOA (P<0.01), suggesting a conformationally more flexible AOB AMO, which according to the model should be less restrained at its active site. This ‘flexibility' might correspond with the broad substrate range of AMO of AOB (Hyman et al., 1988), and correlate with Topt and Ts_max values that were consistently lower than the corresponding values ascribed to their soil AOA counterparts. By comparison, a less negative ΔCP‡ for AOA might extrapolate to a more rigid and constrained version of AMO that may correspond with a more limited substrate range as shown by Taylor et al. (2015). This property might also explain the inability of acetylene to inactivate a fraction of AOA AMO at lower temperatures. It is well documented, albeit for unknown reasons, that the efficacy of acetylene as a substrate for both alkene and aromatic monooxygenases varies considerably (Hyman et al., 1988; Ensign et al., 1992; Yeager et al., 1999; Taylor et al., 2010a). With a less negative ΔCP‡, optimal AOA activity could be constrained to higher soil temperatures, closer to Tmax, resulting in a much narrower Tmax-opt than the AOB (6±3 and 15±2 for AOA and AOB, respectively), and a significantly steeper decline (‘b' parameter) in AOA-dependent activity above Topt (P<0.01). The significant difference in ΔCP‡ for AOA and AOB might also provide support for the idea that the two groups of NH3 oxidizers utilize different biochemical mechanisms for NH3 oxidation to NO2− (Walker et al., 2010; Martens-Habbena et al., 2015; Kozlowski et al., 2016).
It is of some importance to consider how the ΔCP‡ values we have determined in this study fit into context with other data on temperature dependence of soil processes. The ΔCP‡ values of soil AOB in this study (−9.3±2.5 kJ mol−1 K−1) fell within the same range as ΔCP‡ values determined from a study of soil nitrification evaluated between 5 and 20 °C (−10.1±2.9 kJ mol−1 K−1; Russell et al., 2002;Schipper et al., 2014). In our study, NO2−+NO3− production activity over this temperature range was dominated by AOB. ΔCP‡ values were also modeled from a study on temperature dependency of soil methane oxidation and values obtained were also very similar to that of AOB-driven soil nitrification (−7.3±4.7 kJ mol−1 K−1; Schipper et al., 2014). It is well known that AMO of AOB is genetically similar to particulate methane monooxygenase, and that both enzymes are capable of oxidizing both substrates (Holmes et al., 1995; Semrau et al., 1995; Arp and Stein, 2003), whereas we do not know if CH4 is a substrate for AOA AMO. It remains to be seen if ΔCP‡ values of soil nitrification from other soil environments will yield confirmatory results, and how ΔCP‡ values based on rates of activity of NH3-oxidizing isolates will add to our understanding of the biochemical mechanisms behind this process and how they respond to temperature change.
Value of application of SQRT and MMRT models to environmental data
By modeling the rates of NO2−+NO3− production activity by AOA and AOB in soil, we obtained parameters that reflect the response of the AOA and AOB actively contributing to nitrification, and not just the response of the few that can be isolated into culture. This approach allowed us to determine that key characteristics (Tmin, Tmax, Topt, Ts_max and ΔCP‡) within each of the two groups of NH3 oxidizers were consistent across the study sites, and are perhaps intrinsic attributes of these groups. The modeled and experimental results yielded a clear trend for differences in Tmin, Topt, Ts_max and ΔCP‡ of soil AOA and AOB activities, implying fundamental differences in temperature-influenced properties of nitrification driven by the two groups of NH3 oxidizers. These thermodynamic parameters could be utilized in trait-based modeling systems to gain understanding into how past climates have shaped the current communities of NH3 oxidizers, and predict future response to climate change.
Acknowledgments
We thank J Rouske, L Schipper, V Arcus and M Dolan for assistance with modeling, and faculty and staff at the Agricultural Experiment Stations with soil collection. We also thank the anonymous reviewers for critical comments which lead to a stronger paper. This research was supported by USDA NIFA award 2012-67019-3028, and an Oregon Agricultural Research Foundation competitive grant.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
The authors declare no conflict of interest.
Supplementary Material
References
- Adair KL, Schwartz E. (2008). Evidence that ammonia-oxidizing archaea are more abundant than ammonia-oxidizing bacteria in semiarid soils of northern Arizona, USA. Microbial Ecol 56: 420–426. [DOI] [PubMed] [Google Scholar]
- Alves RJ, Wanek W, Zappe A, Richter A, Svenning MM, Schleper C et al. (2013). Nitrification rates in Arctic soils are associated with functionally distinct populations of ammonia-oxidizing archaea. ISME J 7: 1620–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arp DJ, Stein LY. (2003). Metabolism of inorganic N compounds by ammonia-oxidizing bacteria. Crit Rev Biochem Mol Biol 38: 471–495. [DOI] [PubMed] [Google Scholar]
- Avrahami S, Liesack W, Conrad R. (2003). Effects of temperature and fertilizer on activity and community structure of soil ammonia oxidizers. Environ Microbiol 5: 691–705. [DOI] [PubMed] [Google Scholar]
- Avrahami S, Jia ZJ, Neufeld JD, Murrell JC, Conrad R, Kusel K. (2011). Active autotrophic ammonia-oxidizing bacteria in biofilm enrichments from simulated creek ecosystems at two ammonium concentrations respond to temperature manipulation. Appl Environ Microbiol 77: 7329–7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcenas-Moreno G, Gomez-Brandon M, Rousk J, Baath E. (2009). Adaptation of soil microbial communities to temperature: comparison of fungi and bacteria in a laboratory experiment. Global Change Biol 15: 2950–2957. [Google Scholar]
- Birgander J, Reischke S, Jones DL, Rousk J. (2013). Temperature adaptation of bacterial growth and 14C-glucose mineralisation in a laboratory study. Soil Biol Biochem 65: 294–303. [Google Scholar]
- Bouskill NJ, Tang J, Riley WJ, Brodie EL. (2012). Trait-based representation of biological nitrfication: model development testing, and predicted community composition. Front Microbiol 3: 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson WR, Cornforth IS, Rowarth JS. (2002). Winter soil temperature (2-15 degrees C) effects on nitrogen transformations in clover green manure amended or unamended soils; a laboratory and field study. Soil Biol Biochem 34: 1401–1415. [Google Scholar]
- Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M et al. (2015). Complete nitrification by Nitrospira bacteria. Nature 528: 504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalias P, Anderson JM, Bottner P, Couteaux MM. (2002). Temperature responses of net nitrogen mineralization and nitrification in conifer forest soils incubated under standard laboratory conditions. Soil Biol Biochem 34: 691–701. [Google Scholar]
- de la Torre JR, Walker CB, Ingalls AE, Konneke M, Stahl DA. (2008). Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol. Environ Microbiol 10: 810–818. [DOI] [PubMed] [Google Scholar]
- Ensign SA, Hyman MR, Arp DJ. (1992). Cometabolic degradation of chlorinated alkenes by alkene monooxygenase in a propylene-grown Xanthobacter strain. Appl Environ Microbiol 59: 3038–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierer N, Schimel JP, Holden PA. (2003). Influence of drying-rewetting frequency on soil bacterial community structure. Microb Ecol 45: 63–71. [DOI] [PubMed] [Google Scholar]
- Giguere AT, Taylor AE, Myrold DD, Bottomley PJ. (2015). Nitrification responses of soil ammonia-oxidizing archaea and bacteria to ammonium concentrations. Soil Sci Soc Am J 79: 1366–1374. [Google Scholar]
- Hatzenpichler R, Lebedeva EV, Spieck E, Stoecker K, Richter A, Daims H et al. (2008). A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proc Natl Acad Sci USA 105: 2134–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenpichler R. (2012). Diversity, physiology, and niche differentiation of ammonia-oxidizing archaea. Appl Environ Microbiol 78: 7501–7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs JK, Jiao WT, Easter AD, Parker EJ, Schipper LA, Arcus VL. (2013). Change in heat capacity for enzyme catalysis determines temperature dependence of enzyme catalyzed rates. ACS Chem Biol 8: 2388–2393. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Costello A, Lidstrom ME, Murrell JC. (1995). Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol Lett 132: 203–208. [DOI] [PubMed] [Google Scholar]
- Hu HW, Zhang LM, Yuan CL, Zheng Y, Wang JT, Chen DL et al. (2015). The large-scale distribution of ammonia oxidizers in paddy soils is driven by soil pH, geographic distance, and climatic factors. Front Microbiol 4: 938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman MR, Wood PM. (1985). Suicidal inactivation and labeling of ammonia monooxygenase by acetylene. Biochem J 227: 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman MR, Murton IB, Arp DJ. (1988). Interaction of ammonia monooxygenase from Nitrosomonas europaea with alkanes, alkenes, and alkynes. Appl Environ Microbiol 54: 3187–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang QQ, Bakken LR. (1999). Comparison of Nitrosospira strains isolated from terrestrial environments. FEMS Microbiol Ecol 30: 171–186. [DOI] [PubMed] [Google Scholar]
- Jones RD, Morita RY. (1985). Low-temperature growth and whole-cell kinetics of a marine ammonium oxidizer. Mar Ecol Prog Ser 21: 239–243. [Google Scholar]
- Jones RD, Morita RY, Koops HP, Watson SW. (1988). A new marine ammonium-oxidizing bacterium, Nitrosomonas cryotolerans Sp-Nov. Canadian J Microbiol 34: 1122–1128. [Google Scholar]
- Jung MY, Park SJ, Min D, Kim JS, Rijpstra WI, Sinninghe Damste JS et al. (2011). Enrichment and characterization of an autotrophic ammonia-oxidizing archaeon of mesophilic crenarchaeal group I.1a from an agricultural soil. Appl Environ Microbiol 77: 8635–8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JG, Jung MY, Park SJ, Rijpstra WIC, Damste JSS, Madsen EL et al. (2012). Cultivation of a highly enriched ammonia-oxidizing archaeon of thaumarchaeotal group I.1b from an agricultural soil. Environ Microbiol 14: 1528–1543. [DOI] [PubMed] [Google Scholar]
- Kozlowski JA, Stieglmeier M, Schleper C, Klotz MG, Stein LY. (2016). Pathways and key intermediates required for obligate aerobic ammonia-dependent chemolithotrophy in bacteria and Thaumarchaeota. ISME J 10: 1836–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtovirta-Morley LE, Stoecker K, Vilcinskas A, Prosser JI, Nicol GW. (2011). Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc Natl Acad Sci USA 108: 15892–15897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtovirta-Morley LE, Ge CR, Ross J, Yao HY, Nicol GW, Prosser JI. (2014). Characterisation of terrestrial acidophilic archaeal ammonia oxidisers and their inhibition and stimulation by organic compounds. FEMS Microbiol Ecol 89: 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtovirta-Morley LE, Ross J, Hink L, Weber EB, Gubry-Rangin C, Thion C et al. (2016). Isolation of 'Candidatus Nitrosocosmicus franklandus', a novel ureolytic soil archaeal ammonia oxidiser with tolerance to high ammonia concentration. FEMS Microbiol Ecol. 92: fiw057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW et al. (2006). Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442: 806–809. [DOI] [PubMed] [Google Scholar]
- Liu SS, Wang F, Xue K, Sun B, Zhang YG, He ZL et al. (2015). The interactive effects of soil transplant into colder regions and cropping on soil microbiology and biogeochemistry. Environ Microbiol 17: 566–576. [DOI] [PubMed] [Google Scholar]
- Malhi SS, McGill WB. (1982). Nitrification in three Alberta soils - Effect of temperature, moisture and substrate concentration. Soil Biol Biochem 14: 393–399. [Google Scholar]
- Martens-Habbena W, Qin W, Horak REA, Urakawa H, Schauer AJ, Moffett JW et al. (2015). The production of nitric oxide by marine ammonia-oxidizing archaea and inhibition of archaeal ammonia oxidation by a nitric oxide scavenger. Environ Microbiol 17: 2261–2274. [DOI] [PubMed] [Google Scholar]
- Myers RJK. (1975). Temperature effects on ammonification and nitrification in a tropical soil. Soil Biol Biochem 7: 83–86. [Google Scholar]
- Norton JM, Klotz MG, Stein LY, Arp DJ, Bottomley PJ, Chain PS et al. (2008). Complete genome sequence of Nitrosospira multiformis, an ammonia-oxidizing bacterium from the soil environment. Appl Environ Microbiol 74: 3559–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offre P, Prosser JI, Nicol GW. (2009). Growth of ammonia-oxidizing archaea in soil microcosms is inhibited by acetylene. FEMS Microbiol Ecol 70: 99–108. [DOI] [PubMed] [Google Scholar]
- Prosser JI, Nicol GW. (2012). Archaeal and bacterial ammonia-oxidisers in soil: the quest for niche specialisation and differentiation. Trends Microbiol 20: 523–531. [DOI] [PubMed] [Google Scholar]
- Ratkowsky DA, Lowry RK, McMeekin TA, Stokes AN, Chandler RE. (1983). Model for bacterial culture growth rate throughout the entire biokinetic temperature range. J Bacteriol 154: 1222–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell CA, Fillery IRP, Bootsma N, McInnes KJ. (2002). Effect of temperature and nitrogen source on nitrification in a sandy soil. Commun Soil Sci Plant Anal 33: 1975–1989. [Google Scholar]
- Schauss K, Focks A, Leininger S, Kotzerke A, Heuer H, Thiele-Bruhn S et al. (2009). Dynamics and functional relevance of ammonia-oxidizing archaea in two agricultural soils. Environ Microbiol 11: 446–456. [DOI] [PubMed] [Google Scholar]
- Schipper LA, Hobbs JK, Rutledge S, Arcus VL. (2014). Thermodynamic theory explains the temperature optima of soil microbial processes and high Q(10) values at low temperatures. Global Change Biol 20: 3578–3586. [DOI] [PubMed] [Google Scholar]
- Schleper C. (2010). Ammonia oxidation: different niches for bacteria and archaea? ISME J 4: 1092–1094. [DOI] [PubMed] [Google Scholar]
- Semrau JD, Chistoserdov A, Lebron J, Costello A, Davagnino J, Kenna E et al. (1995). Particulate methane monooxygenase genes in methanotrophs. J Bacteriol 177: 3071–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JM. (1996). Modeling the temperature response of nitrification. Biogeochem 35: 433–445. [Google Scholar]
- Stark JM, Firestone MK. (1996). Kinetic characteristics of ammonium-oxidizer communities in a California oak woodland-annual grassland. Soil Biol Biochem 28: 1307–1317. [Google Scholar]
- Stein LY, Arp DJ. (1998). Ammonium limitation results in the loss of ammonia-oxidizing activity in Nitrosomonas europaea. Appl Environ Microbiol 64: 1514–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun RB, Guo XS, Wang DZ, Chu HY. (2015). Effects of long-term application of chemical and organic fertilizers on the abundance of microbial communities involved in the nitrogen cycle. Appl Soil Ecol 95: 171–178. [Google Scholar]
- Taylor A, Arp D, Bottomley P, Semprini L. (2010a). Extending the alkene substrate range of vinyl chloride utilizing Nocardioides sp. strain JS614 with ethene oxide. Appl Microbiol Biotechnol 87: 2293–2302. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Zeglin LH, Dooley S, Myrold DD, Bottomley PJ. (2010b). Evidence for different contributions of archaea and bacteria to the ammonia-oxidizing potential of diverse Oregon soils. Appl Environ Microbiol 76: 7691–7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AE, Zeglin L, Wanzek TA, Myrold DD, Bottomley PJ. (2012). Dynamics of ammonia oxidizing archaea and bacteria populations and contributions to soil nitrification potentials. ISME J 6: 2024–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AE, Vajrala N, Giguere AT, Gitelman AI, Arp DJ, Myrold DD et al. (2013). Use of aliphatic n-alkynes to discriminate soil nitrification activities of ammonia-oxidizing Thaumarchaea and Bacteria. Appl Environ Microb 79: 6544–6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AE, Taylor K, Tennigkeit B, Palatinszky M, Stieglmeier M, Myrold DD et al. (2015). Inhibitory effects of C2 to C10 1-alkynes on ammonia oxidation in two Nitrososphaera species. Appl Environ Microbiol 81: 1942–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourna M, Freitag TE, Nicol GW, Prosser JI. (2008). Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ Microbiol 10: 1357–1364. [DOI] [PubMed] [Google Scholar]
- Tourna M, Stieglmeier M, Spang A, Konneke M, Schintlmeister A, Urich T et al. (2011). Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci USA 108: 8420–8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajrala N, Bottomley PJ, Stahl DA, Arp DJ, Sayavedra-Soto LA. (2014). Cycloheximide prevents the de novo polypeptide synthesis required to recover from acetylene inhibition in Nitrosopumilus maritimus. FEMS Microbiol Ecol 88: 495–502. [DOI] [PubMed] [Google Scholar]
- van Gestel NC, Reischke S, Bååth E. (2013). Temperature sensitivity of bacterial growth in a hot desert soil with large temperature fluctuations. Soil Biol Biochem 65: 180–185. [Google Scholar]
- van Kessel MAHJ, Speth DR, Albertsen M, Nielsen PH, Op den Camp HJM, Kartal B et al. (2015). Complete nitrification by a single microorganism. Nature 528: 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CB, de la Torre JR, Klotz MG, Urakawa H, Pinel N, Arp DJ et al. (2010). Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA 107: 8818–8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Ke XB, Hernandez M, Wang BZ, Dumont MG, Jia ZJ et al. (2013). Autotrophic growth of bacterial and archaeal ammonia oxidizers in freshwater sediment microcosms incubated at different temperatures. Appl Environ Microbiol 79: 3076–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager CM, Bottomley PJ, Arp DJ, Hyman MR. (1999). Inactivation of toluene 2-monooxygenase in Burkholderia cepacia G4 by alkynes. Appl Environ Microbiol 65: 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Zhao DY, Yu ZB, Huang R, Wu QLL. (2014). Temperature responses of ammonia-oxidizing prokaryotes in freshwater sediment microcosms. PloS One 9: e100653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.