Summary
The rise of a pest species represents a unique opportunity to address how species evolve new behaviors and adapt to novel ecological niches [1]. We address this question by studying the egg-laying behavior of Drosophila suzukii, an invasive agricultural pest species that has spread from Southeast Asia to Europe and North America in the last decade [2]. While most closely related Drosophila species lay their eggs on decaying plant substrates, D. suzukii oviposits on ripening fruit, thereby causing substantial economic losses to the fruit industry [3, 4, 5, 6, 7, 8]. D. suzukii has evolved an enlarged, serrated ovipositor that presumably plays a key role by enabling females to pierce the skin of ripe fruit [9]. Here, we explore how D. suzukii selects oviposition sites, and how this behavior differs from that of closely related species. We have combined behavioral experiments in multiple species with neurogenetics and mutant analysis in D. suzukii to show that this species has evolved a specific preference for oviposition on ripe fruit. Our results also establish that changes in mechanosensation, olfaction, and presumably gustation have contributed to this ecological shift. Our observations support a model in which the emergence of D. suzukii as an agricultural pest is the consequence of the progressive modification of several sensory systems, which collectively underlie a radical change in oviposition behavior.
Keywords: Drosophila suzukii, oviposition, olfaction, egg-laying evolution, pest species, chemosensation, evolution, behavior, strawberry
Highlights
-
•
The pest Drosophila suzukii prefers to lay eggs on ripening fruit
-
•
Closely related Drosophila species prefer to lay eggs on rotten fruit
-
•
Female flies use chemosensation and mechanosensation to choose an oviposition site
-
•
Orco-dependent detection of ripe fruit odors elicits oviposition in D. suzukii
Karageorgi et al. show that the invasive pest Drosophila suzukii has evolved a preference to lay its eggs on ripening fruit. The authors dissect the sensory bases of this preference, pointing to a multi-step evolutionary scenario involving the tuning of different sensory modalities.
Results and Discussion
D. suzukii Females Have Evolved a Preference to Lay Eggs in Ripe Rather Than Rotten Strawberries
We analyzed the oviposition behavior of D. suzukii and some close relatives using strawberries (genus Fragaria), a main target of D. suzukii, at different stages of maturation [10]. We first compared ripe, pristine strawberries (hereafter referred to as “ripe fruit”), purchased from a local grocery store, to strawberries from a similar batch left to decay for 4 days (hereafter referred to as “rotten fruit”; see Supplemental Experimental Procedures). We then assessed the egg-laying behavior of D. suzukii and five closely related species [11] on ripe and rotten fruit using a two-choice oviposition assay (Figure 1A). We counted the number of eggs laid in each fruit after 19 hr and calculated an oviposition substrate preference index (PI). Using this two-choice assay, we observed a robust oviposition preference for most species and striking differences between D. suzukii and the other species (Figures 1B and S1A). D. suzukii females laid almost all of their eggs on ripe fruit, whereas D. ananassae, D. melanogaster, D. eugracilis, and D. takahashii demonstrated the opposite behavior, targeting rotten fruit almost exclusively. Remarkably, D. biarmipes showed an intermediate behavior with no marked preference for either substrate. To test whether the fruit skin is a deterrent barrier resulting in these different behaviors, we repeated the choice assay with ripe fruits sliced in half, to expose their flesh, and rotten fruits. We found that D. biarmipes laid approximately equal numbers of eggs on both substrates, while D. melanogaster maintained a strong preference for the rotten fruit (Figure S1B). These results establish that the preference for laying eggs on rotten fruit is ancestral to this group of species, and that a preference for oviposition on ripe fruit has evolved in the lineage leading to D. suzukii.
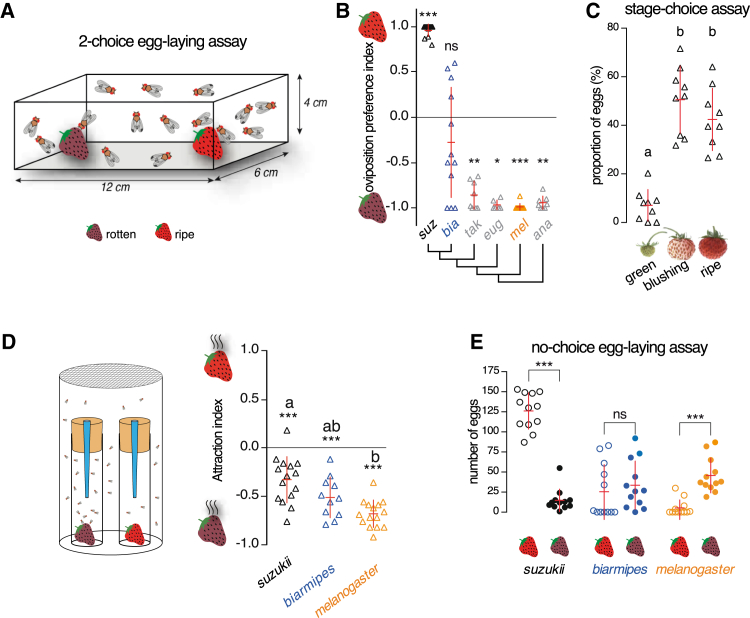
Figure 1.
D. suzukii Females Have an Evolutionarily Novel Preference for Laying Eggs in the Early Stages of Strawberry Maturation
(A) Two-choice oviposition assay on whole fruits, where ten mated Drosophila females (mixed with five males) lay their eggs on two different substrates (ripe versus rotten strawberries). Eggs were counted at the end of each assay.
(B) Oviposition preference (two-choice assay as depicted in A) of six closely related species of the D. melanogaster species group. Whereas most species show a strong preference to lay their eggs on rotten fruit, D. suzukii females have radically shifted their preference to lay eggs on ripe fruit. Interestingly, D. biarmipes, a close relative of D. suzukii, displays a mild but not statistically significant preference for rotten fruit. All p values were calculated via Wilcoxon matched-pairs signed-rank test. p values in this and all subsequent figures: ∗∗∗p ≤ 0.001; ∗∗p ≤ 0.01; ∗p ≤ 0.05; ns, p > 0.05, i.e., non-significant difference. In this and all subsequent figures, the red bars indicate the mean ± SD unless otherwise noted. Replicates in preference experiments are represented by triangles (n = 6 to 12 replicates per species).
(C) When given a choice between three stages of the same strawberry cultivar (Fontaine strawberries; see Supplemental Experimental Procedures), D. suzukii selects predominantly the ripening (blushing) or mature (ripe) fruits. Significant differences are denoted by letters (Friedman test; X2(2) = 14, p < 0.001, followed by Conover’s test; p ≤ 0.01). n = 9 replicates per species.
(D) Olfactory attraction assay (trap; design of the assay is shown on the left). All three species (D. suzukii, D. biarmipes, and D. melanogaster) are more attracted to rotten strawberry odors, but D. suzukii relatively less than D. melanogaster. p values were calculated via one-sample t test for each species; significant differences between species are denoted by letters (ANOVA followed by Tukey’s test for multiple comparison; p < 0.001). n = 11 or 15 replicates per species.
(E) A no-choice oviposition assay, similar to the two-choice assay in (A), but in which ten females and five males were presented with one type of fruit (ripe or rotten), reveals oviposition on ripe or rotten strawberries for D. suzukii, D. biarmipes, and D. melanogaster. This shows that D. suzukii can lay eggs on rotten fruits, although not much, and conversely that D. melanogaster can lay eggs on ripe fruits, although not much. D. biarmipes appears to lay eggs indifferently on both substrates. All p values were calculated via Mann-Whitney test. In this and all subsequent figures, the number of eggs laid per replicate is presented with open (ripe fruit) or filled (rotten fruit or other) circles. n = 12 replicates per species.
See also Figure S1.
To further determine the preferred range of fruit ripening stages [12] targeted by D. suzukii for oviposition, we offered D. suzukii females the choice between “green,” “early/late blushing,” and “ripe” fruit (Figures S1C and S1D). Although flies managed to lay a few eggs in the green fruit, the vast majority of eggs were laid in fruit of later maturation stages (Figure 1C). We concluded that D. suzukii has access to strawberries at the onset of their maturation but strongly prefers blushing and ripe fruit stages. These results are consistent with previous observations that used different strawberry cultivars and other species of berry [10, 13, 14]. Together, they establish that D. suzukii, compared to other closely related species, has shifted its oviposition target from rotten to earlier stages of fruit maturation. We explored this behavioral shift further by focusing on three species: the genetic model D. melanogaster; D. biarmipes, a close relative of D. suzukii not known as a ripe fruit pest; and D. suzukii itself.
The Preference of D. suzukii for Ripe Fruit Is Specific to Oviposition
We sought to determine whether the preference of D. suzukii for ripe fruit is specific to oviposition or a facet of a general ecological shift, as has been observed for other drosophilids [15]. We first found, using an olfactory trap assay, that all three species are more attracted to the odor of rotten fruit, although D. suzukii shows a weaker preference (Figure 1D). Hence, the preference for ripe fruit that D. suzukii displays in the context of oviposition seems specific to this behavior. Its attraction to rotten fruit may instead relate to feeding. To examine this possibility, we compared feeding behaviors of all three species with an assay similar to that depicted in Figure 1A, but using either ripe or rotten fruit alone (no-choice assay). While D. suzukii fed more on ripe strawberries than D. melanogaster (as indicated by the red color of the abdomen), both species show a similarly strong appetite for rotten fruit (Figure S1E). We conclude that D. suzukii is attracted to rotten fruit mostly for feeding and targets ripe fruit mostly for oviposition, in agreement with published data [16].
We next evaluated the intrinsic capacity of each fruit stage to elicit oviposition using a no-choice oviposition assay. We observed that D. suzukii laid more eggs on ripe strawberries alone than on rotten strawberries alone; by contrast, D. melanogaster laid more eggs when exposed to rotten fruit, while D. biarmipes laid similar numbers of eggs on both fruit stages (Figure 1E). These results suggest that the relative preferences observed in the two-choice assay result directly from the absolute capacity of each substrate to elicit oviposition for each species. We went on to dissect the properties of the fruit that females select as their preferred egg-laying substrates.
D. suzukii Tolerates Stiffer Substrates for Oviposition
The enlarged ovipositor of D. suzukii presumably endows the females with the capacity to more easily pierce the stiff skin of a ripe fruit [9]. We wondered whether flies exploit the stiffness of the fruit skin, which decreases with maturation [17], to assess oviposition substrate quality. We exposed females to substrates that differ only in stiffness, in the form of Petri dish halves filled with agarose at different concentrations and equally sweetened with glucose to stimulate ovipositioning (Figure 2A, top). We then measured and correlated the stiffness of agarose at different concentrations to that of strawberries at different stages (Figure S2A). Exposed to a choice between agarose at 0.25% (stiffness of a rotten fruit) and any stiffer substrate, up to 2.25% agarose (earlier fruit maturation stages), D. melanogaster and D. biarmipes always strongly preferred softer substrates. By contrast, D. suzukii displayed hardly any preference for softer substrates until the stiffer substrate reached 1.75% agarose (Figure 2A). We concluded that all three species exploit substrate stiffness for oviposition site selection, but in different ranges. The relaxation of the stiffness threshold observed in D. suzukii implies functional changes in the mechanosensory system of this species.
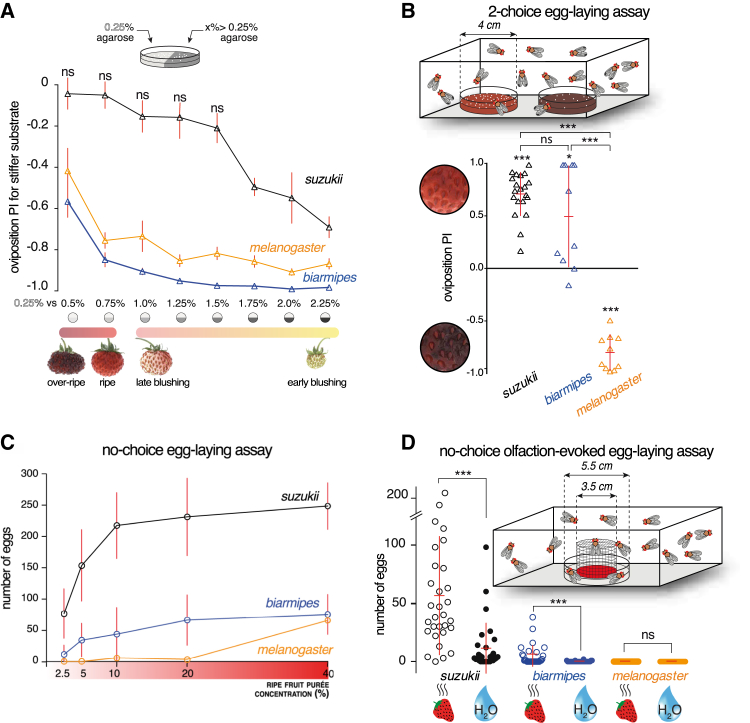
Figure 2.
Changes in Mechanosensory and Chemosensory Systems Underlie the Evolution of Oviposition Site Preference
(A) Two-choice oviposition assay for substrate stiffness (schematic depicted above the graph). For each point (n = 8 replicates), a group of females had the choice to lay eggs on 0.25% agarose or an increasingly stiffer substrate from 0.5% to 2.25% agarose in steps of 0.25 percentage point. All substrates were equally supplemented with glucose. The result is expressed as a preference index for the stiffer substrate (red bars indicates here mean ± SEM rather than mean ± SD, for the sake of figure clarity). Whereas D. melanogaster and D. biarmipes showed a consistent strong preference for the softer substrate —even when the alternative was only marginally stiffer (0.25% versus 0.5%)—D. suzukii was relatively indifferent to the substrate stiffness until the concentration difference was higher than 1.5 percentage points. The turning point in the preference of each species matched the stiffness of the fruit stage in which they preferred to lay eggs in choice assays (Figures 1B, 1C, and S1A). All p values were calculated via Wilcoxon tests for each point with comparison to a theoretical value of 0 (no preference). p ≤ 0.01 for all samples, except where “ns” is indicated.
(B) Two-choice oviposition assay for substrates of different chemical composition. Ten Drosophila females (and five males) had the choice between two substrates of equal stiffness (1% agar) but different chemical composition. One egg-laying plate contained 35% w/v ripe strawberry purée, while the other plate contained 35% w/v rotten strawberry purée. The results recapitulate the preference of D. melanogaster and D. suzukii observed in a similar two-choice assay using whole fruits (Figure 1A), while D. biarmipes shows in this assay a mild and variable preference for ripe fruit purée. p values were calculated via Wilcoxon tests with comparison to a theoretical value of 0 (no preference) for each species, and via Kruskal-Wallis test followed by Dunn’s test (p < 0.001) for interspecies comparisons. n = 10–19 replicates per species.
(C) Oviposition in D. suzukii increases in response to increasing concentrations of ripe strawberry purée. D. melanogaster hardly responds to this substrate, while D. biarmipes shows a moderate response. logEC50 of D. suzukii (5.7%) and D. biarmipes (8.6%) are significantly different (measured via a non-linear regression analysis of dose response, p < 0.001); the logEC50 of D. melanogaster (23.9%) is not statistically comparable to the other species due to a poor fit of the data. n = 16 replicates per species. Each replicate includes ten females.
(D) Oviposition response elicited by the odor of ripe fruit or water in D. suzukii, D. biarmipes, and D. melanogaster. In this no-choice egg-laying assay, females were placed in a chamber similar to the one depicted in Figure 1A and were offered an agar plate for egg laying. A source of odor placed at the center of the plate was covered by a mesh and therefore could not be directly contacted by the flies. D. suzukii laid abundantly on the agar when exposed to the odor of ripe strawberry purée but laid significantly less when exposed to water. By contrast, strawberry odors hardly elicited egg laying in D. biarmipes and did not elicit egg laying at all in D. melanogaster. p values were calculated via Mann-Whitney test. n = 28–30 replicates per condition (15 females and 0 males per replicate).
See also Figures S1 and S2.
Chemical Cues Drive Species-Specific Oviposition Substrate Preferences
Chemical composition also changes with fruit maturation [12, 18]. To test its influence on oviposition site selection, we used substrates of fixed stiffness (1% agarose) containing either ripe or rotten strawberry purée (see Supplemental Experimental Procedures). We then tested oviposition preference in a two-choice assay (Figure 2B, top) in darkness (to circumvent slight color differences). The chemical stimuli were sufficient to recapitulate the egg-laying preference of D. melanogaster and D. suzukii on whole fruits (compare Figures 1B and 2B), while D. biarmipes showed an intermediate behavior (Figure 2B). We concluded that the chemical composition of the substrate is a primary determinant guiding oviposition site selection for D. suzukii and D. melanogaster. For D. biarmipes, the contrasting results obtained with whole fruits (Figure 1B) or agar-based substrates (Figure 2B) suggest that this species has evolved a mild preference for the chemicals of ripe fruit that is balanced with a strong preference for soft substrates. The previous results suggest that chemical cues from ripe fruit elicit variable oviposition responses in different species. To compare the quantitative response to these cues, we exposed D. suzukii, D. biarmipes, and D. melanogaster females to a dilution series of ripe strawberry purée plates. We observed that the egg laying increased with the concentration of ripe fruit purée (Figure 2C). Although the dose response was shared by all species, D. suzukii responded much more, and at lower concentrations of ripe fruit purée, than D. melanogaster or, to some degree, D. biarmipes. These results show that the oviposition site preference of D. suzukii for ripe fruit is mediated at least in part by chemical cues. They also suggest that the chemosensory system involved in oviposition has changed in D. suzukii compared to D. biarmipes and D. melanogaster.
Strawberry Odors Are Sufficient to Evoke Oviposition in D. suzukii
We then set out to determine how D. suzukii females perceive the chemical cues that elicit their oviposition on ripening fruit. We first tested the sufficiency of olfaction to respond to these cues and elicit oviposition. Specifically, we asked whether the odor of ripe strawberries alone could evoke egg laying in D. suzukii, D. biarmipes, and D. melanogaster. We placed flies in a chamber containing agar plates with, at the center, a cup filled with ripe strawberry purée or water; the cup was covered with a metallic mesh allowing the flies to smell but not to contact its content (Figure 2D). The odor of ripe strawberries alone elicited oviposition by D. suzukii on plain agar, and to a lesser extent by D. biarmipes as well (Figure 2D). By contrast, it did not elicit D. melanogaster to lay eggs at all (Figure 2D). To eliminate the possibility that D. melanogaster did not lay eggs simply because females disliked plain agar, we created conditions for an oviposition baseline by supplementing the agar with 5% fructose. We then surveyed oviposition enhancement from this baseline upon exposure to fruit odor and found that the odor of ripe fruit enhanced oviposition in D. suzukii, but not in D. melanogaster (Figure S2B). Finally, we demonstrated that the oviposition enhancement in D. suzukii is not the indirect result of a stronger attraction to the odor source. First, replacing strawberry odors by acetoin, a potent attractant of D. melanogaster [19] and D. suzukii (Figure S2C), did not enhance D. suzukii oviposition (Figure S2B). Second, D. suzukii and D. melanogaster were equally attracted to ripe strawberry volatiles (Figure S2D). Together, these results reveal that ripe strawberry odors are sufficient to elicit oviposition in D. suzukii.
OR-Mediated Olfaction Elicits Oviposition in D. suzukii
To measure the contribution of olfaction to the selection of an oviposition site, we first ablated the antennae (the main olfactory organs) of female D. suzukii. In a two-choice assay with fruit purée plates, the ablated flies displayed a reduced preference for ripe fruit compared to the non-ablated control flies (Figure S2E), mostly due to a reduction of egg laying on ripe fruit substrate (Figure S2F). We concluded that olfaction from antennae is partially necessary for selecting between ripe and rotten fruits in D. suzukii. This also indicates that the maxillary palps (the other olfactory organs) or the perception of chemosensory cues by direct contact can partly compensate for the absence of antennae. We further analyzed the role of olfaction in egg laying in D. suzukii by impairing olfaction genetically. We focused on the odorant receptor (OR) system, one of the two olfactory receptor families expressed in antennae chemosensory neurons [20, 21]. We targeted the obligate co-receptor Orco, or the neurons that express it, to interfere with OR-mediated olfaction [19, 22].
We first generated a D. suzukii Orco-Gal4 line [19] to target Orco-positive sensory neurons (Figure S3A) and a UAS-CD4-tdTomato line [23] to visualize the projections of Orco-Gal4-positive cells. In D. suzukii, the Orco-Gal4 and UAS-CD4-tdTomato combination labels neurons projecting to the antennal lobe (Figures S3B4 and B5), consistent with what has been described in D. melanogaster (Figures S3C4 and C5). These neurons also express endogenous Orco, detected by an antibody in the antenna (Figures S3B1–B3 and C1–C3), showing that the Orco-Gal4 construct targets Orco-positive cells.
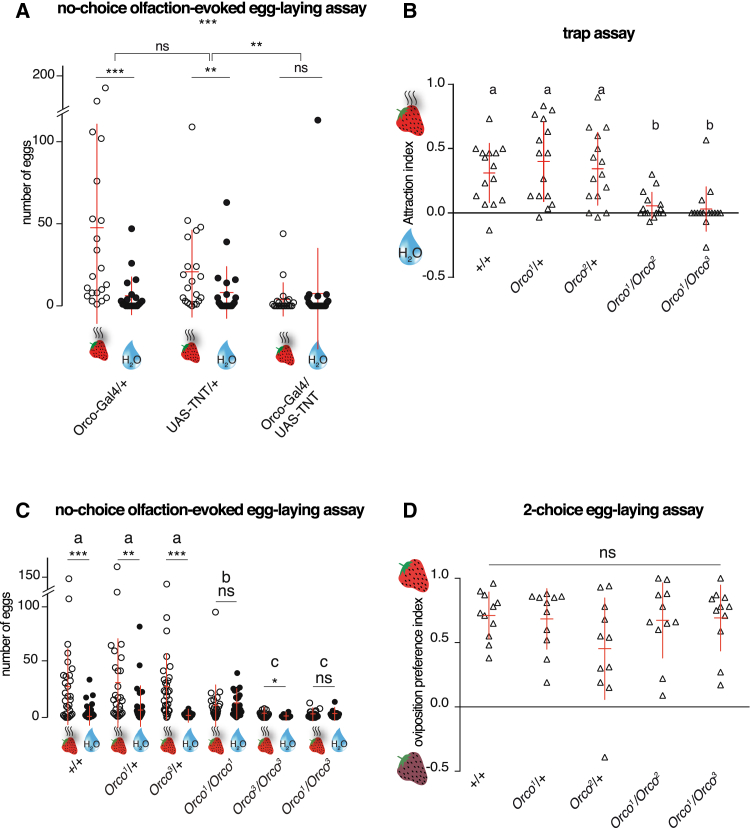
Figure 3.
Orco and Orco-Expressing Neurons Mediate Oviposition Enhancement in Response to the Odor of Ripe Strawberries in D. suzukii
(A) In a no-choice olfaction-evoked egg-laying assay (as depicted in Figure 2D), the enhanced egg laying elicited by the odor of ripe fruits in D. suzukii was abolished when neurotransmission was specifically blocked in Orco-positive neurons. n = 21 replicates per condition (15 females and 0 males per replicate). p values were calculated via Mann-Whitney test for each genotype, using a negative binomial generalized linear model followed by a general linear hypothesis test for multiple comparisons with a false discovery rate (FDR) correction method. n = 21 replicates per condition (15 females and 0 males per replicate).
(B) The loss of Orco function in D. suzukii prevents attraction to the odors of ripe strawberries in a trap assay against water. Significant differences are denoted by letters (ANOVA followed by Tukey’s test for multiple comparison; p < 0.001). n = 10–13 replicates per condition (15 males and 15 females per replicate).
(C) The enhanced egg laying elicited by the odor of ripe fruits in a no-choice olfaction-evoked egg-laying assay was also significantly diminished or abolished in D. suzukii when the function of Orco is lost. p values were calculated via Mann-Whitney test for each genotype, using a negative binomial generalized linear model followed by a general linear hypothesis test for multiple comparisons with a FDR correction method to compare the genotypes. n = 26–30 replicates per condition (10 females and 0 males per replicate).
(D) In a two-choice assay with whole strawberries, in which the flies could contact and presumably taste the fruits, D. suzukii Orco mutants preferred to lay eggs on ripe fruits as much as wild-type females did. n = 11 replicates per condition (15 females and 0 males per replicate) (ANOVA followed by Tukey’s test for multiple comparison; p < 0.001).
See also Figures S3 and S4.
To block synaptic transmission and impair Orco neuron-mediated olfaction, we created a D. suzukii UAS-TNT transgenic line [24] and crossed it to our Orco-Gal4 line. In a trap assay with acetoin, an odor perceived by Orco-expressing neurons [19], we observed a severe reduction of attraction to acetoin (Figure S3D) in Orco-Gal4, UAS-TNT flies but normal locomotion (Figure S3E), confirming that these constructs impair OR-mediated olfaction. We then subjected Orco-Gal4, UAS-TNT D. suzukii females to the olfaction-evoked oviposition assay (Figure 2D) with ripe strawberry odors. We found that olfaction-evoked oviposition was almost abolished compared to the control genotypes (Figure 3A), showing that Orco-positive neurons are involved in the oviposition elicited by ripe fruit odors in D. suzukii.
We also generated D. suzukii Orco mutants using the CRISPR-Cas9 system [25]. We obtained three alleles, named Dsuz\Orco1, Dsuz\Orco2, and Dsuz\Orco3 (Figure S4A). These mutants are protein null (Figure S4B). While wild-type D. suzukii antennae respond to ripe strawberry odor and to the control odor acetoin in electroantennograms (EAGs), mutant antennae did not respond to either smell (Figure S4E). Consistent with this data, the attraction of Dsuz\Orco mutants to acetoin (Figure S4C) or ripe strawberry odors (Figure 3B) was severely impaired, although their locomotion was unaffected (Figure S4D), revealing that they are loss-of-function alleles.
We then exposed Dsuz\Orco mutants to the olfaction-evoked oviposition assay (as in Figure 2D). The mutant females were not stimulated, or were significantly less stimulated, to lay eggs in response to ripe strawberry odors compared with the control genotypes (Figure 3C), similar to what we observed upon silencing the Orco-expressing neurons (Figure 3A). Altogether, these results reveal that the OR subsystem is essential in D. suzukii for the perception of ripe fruit volatiles and the oviposition elicited by these odors.
Finally, we tested the oviposition preference of the Dsuz\Orco mutants in a two-choice assay with whole ripe and rotten strawberries (Figure 1A). We found no difference in oviposition preference between the Dsuz\Orco mutants and the wild-type controls (Figure 3D), revealing that OR-mediated olfaction becomes redundant for egg-laying site selection when other sensory stimuli are available. Consistent with this, when females in which Orco neuron output is silenced could contact strawberry purée, and presumably taste it, they laid eggs at levels comparable to wild-type flies (Figure S3F). These results suggest that contact chemosensation, in conjunction with olfaction, also contributes to the oviposition behavior of D. suzukii on ripe fruit.
A Multi-step Evolutionary Scenario for the Making of a Pest Species
The evolution of D. suzukii as a pest species could be regarded as the result of a single key innovation: its enlarged, serrated ovipositor that enables females to pierce the skin of many ripe fruits. We have found that the egg-laying substrate preference of D. suzukii has evolved in concert with its morphology and was instrumental in the shift to a new reproductive niche. Our work shows that the divergence in oviposition behavior is associated with the modification of multiple sensory modalities, namely mechanosensation and chemosensation, that determine differences in the egg-laying site choice between D. melanogaster, D. biarmipes, and D. suzukii.
The comparison of D. suzukii with multiple closely related species, in particular D. biarmipes, suggests a possible scenario for the evolution of D. suzukii as a pest species (Figure 4). In this scenario, oviposition in species like D. melanogaster is strongly elicited by rotten fruit and is inhibited by stiff substrates. In the lineage leading to D. suzukii, the oviposition response to ripe fruit has progressively increased, as in D. biarmipes, whose behavior is intermediate between that of D. melanogaster and D. suzukii. Such species, however, can only exploit ripe or slightly damaged fruit of sufficient softness. Presumably, the small ovipositor in these species prevented the full exploitation of the ripe fruit niche. Only in D. suzukii, and its close relative D. subpulchrella [9], did chemosensory specialization for ripe fruit cues, broadening of substrate stiffness preference, and evolution of an enlarged ovipositor come together and endow the flies with the capacity to fully use ripe fruit as oviposition substrates. Finally, additional physiological adaptations (e.g., metabolic changes [27]) may have provided D. suzukii with the potential to adapt to different environments and invade new geographical areas. In this stepwise scenario, the evolution of the ovipositor of D. suzukii was certainly a key acquisition, but it was secondary to the behavioral changes that endowed some ancestors with an opportunistic egg-laying behavior toward ripe fruits.
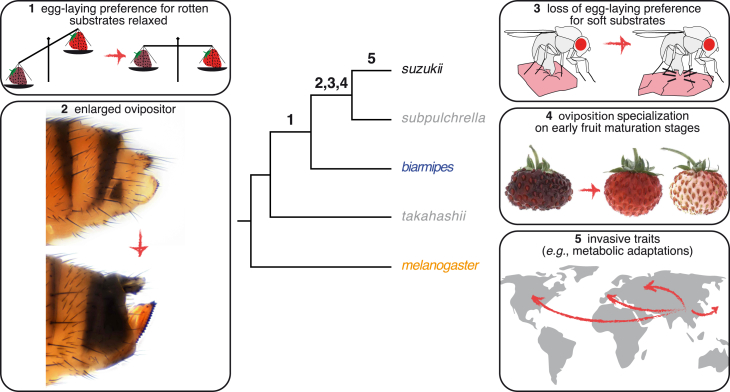
Figure 4.
A Possible Evolutionary Scenario for the Shift in D. suzukii Oviposition Behavior
The emergence of D. suzukii as a pest species is associated with multiple evolutionary changes, either identified or suspected. These comprise (1) a broadening of the range of possible oviposition sites to include earlier fruit maturation stages, (2) the morphological expansion and strengthening of the ovipositor, (3) the tuning of mechanosensory preferences to accept stiffer substrates, (4) the tuning of chemosensory modalities to earlier stages of fruit maturation in the context of egg laying, and (5) physiological and metabolic changes enabling the fly to spread across a broad geographic range. The order of these changes is speculative, but the intermediate state of D. biarmipes for some of these traits suggests the genetic potentiation of the clade containing these species to gain such traits [26].
We propose that the evolutionary origin of D. suzukii as a pest species was therefore made possible by the progressive tuning of multiple sensory systems, which might be mirrored in changes in its sensory receptor genes [28, 29] or the determinants of neuronal connectivity. Our results suggest that these traits may have emerged in a clade predisposed [26] for this behavioral shift.
Author Contributions
B.P. and N.G. conceived the project. B.P., N.G., M.K., L.B.B., and S.L. designed the experiments. M.K., L.B.B., and S.L. carried out the behavioral experiments and the statistical analyses. C.M. and M.K. built the constructs used in Figure 3 and S3 (Orco-Gal4 [C.M.]; UAS-CD4-tdTomato and UAS-TNT [M.K.]). M.C. characterized the expression of Orco and reporter genes (Figures S3 and 4). B.P., C.M., and M.K. generated all transgenic and CRISPR mutant lines. N.G. documented the strawberry stages. I.C.G.K. and K.P.S. designed and analyzed the electrophysiology experiments (Figure S4E), which K.P.S. carried out. B.P. and N.G. wrote the manuscript with the help of all authors.
Acknowledgments
We are grateful to B. Detailleur for the design and construction of behavioral chambers, to K. Olbricht and A. Schneider for the gift and maintenance (respectively) of Fontaine cultures, to S. Travaillard for the feeding experiment, to R. Benton for the anti-Orco antibody and the D. melanogaster Orco-Gal4 stock, to the SICOLY cooperative for providing frozen strawberry purée, and to J. Ewbank and J. Green for comments on the manuscript. We acknowledge the University of California, San Diego Drosophila Species Stock Center for fly stocks. M.K. acknowledges funding from the Marie Curie FP7 Programme through FLiACT (ITN) and the Fondation pour la Recherche Médicale (FRM-FDT20150532044). This project was supported by funding from Ludwig-Maximilians Universität München (N.G.), the European Research Council under the European Union Seventh Framework Programme (FP/2007-2013)/ERC grant agreement 615789 (B.P.), the A∗MIDEX project (ANR-11-IDEX-0001-02) funded by the “Investissements d’Avenir” French Government program managed by the French National Research Agency (ANR) (B.P.), and the CRC870 (Project A04)/German Research Foundation (I.C.G.K.).
Published: March 9, 2017
Footnotes
Supplemental Information includes four figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2017.01.055.
Contributor Information
Nicolas Gompel, Email: gompel@biologie.uni-muenchen.de.
Benjamin Prud’homme, Email: benjamin.prudhomme@univ-amu.fr.
Supplemental Information
References
- 1.Gould F. The evolutionary potential of crop pests. Am. Sci. 1991;79:496–507. [Google Scholar]
- 2.Fraimout A., Debat V., Fellous S., Hufbauer R.A., Foucaud J., Pudlo P., Marin J.-M., Price D.K., Cattel J., Chen X. Deciphering the routes of invasion of Drosophila suzukii by means of ABC random forest. Mol. Biol. Evol. 2017 doi: 10.1093/molbev/msx050. Published online January 24, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolda M.P., Goodhue R.E., Zalom F.G. Spotted wing Drosophila: potential economic impact of a newly established pest. Agric. Resource Econ. Update, Univ. Calif. Giannini Foundation Agric. Econ. 2010;13:5–8. [Google Scholar]
- 4.Burrack H.J., Fernandez G.E., Spivey T., Kraus D.A. Variation in selection and utilization of host crops in the field and laboratory by Drosophila suzukii Matsumara (Diptera: Drosophilidae), an invasive frugivore. Pest Manag. Sci. 2013;69:1173–1180. doi: 10.1002/ps.3489. [DOI] [PubMed] [Google Scholar]
- 5.Cini A., Anfora G., Escudero-Colomar L.A., Grassi A., Santosuosso U., Seljak G., Papini A. Tracking the invasion of the alien fruit pest Drosophila suzukii in Europe. J. Pest Sci. 2014;87:559–566. [Google Scholar]
- 6.Ioriatti C., Walton V., Dalton D., Anfora G., Grassi A., Maistri S., Mazzoni V. Drosophila suzukii (Diptera: Drosophilidae) and its potential impact to wine grapes during harvest in two cool climate wine grape production regions. J. Econ. Entomol. 2015;108:1148–1155. doi: 10.1093/jee/tov042. [DOI] [PubMed] [Google Scholar]
- 7.Rota-Stabelli O., Blaxter M., Anfora G. Drosophila suzukii. Curr. Biol. 2013;23:R8–R9. doi: 10.1016/j.cub.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Keesey I.W., Knaden M., Hansson B.S. Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J. Chem. Ecol. 2015;41:121–128. doi: 10.1007/s10886-015-0544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atallah J., Teixeira L., Salazar R., Zaragoza G., Kopp A. The making of a pest: the evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc. Biol. Sci. 2014;281:20132840. doi: 10.1098/rspb.2013.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong X., Bräcker L., Bölke N., Plata C., Zeitlmayr S., Metzler D., Olbricht K., Gompel N., Parniske M. Strawberry accessions with reduced Drosophila suzukii emergence from fruits. Front. Plant Sci. 2016;7:1880. doi: 10.3389/fpls.2016.01880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prud’homme B., Gompel N., Rokas A., Kassner V.A., Williams T.M., Yeh S.D., True J.R., Carroll S.B. Repeated morphological evolution through cis-regulatory changes in a pleiotropic gene. Nature. 2006;440:1050–1053. doi: 10.1038/nature04597. [DOI] [PubMed] [Google Scholar]
- 12.Fait A., Hanhineva K., Beleggia R., Dai N., Rogachev I., Nikiforova V.J., Fernie A.R., Aharoni A. Reconfiguration of the achene and receptacle metabolic networks during strawberry fruit development. Plant Physiol. 2008;148:730–750. doi: 10.1104/pp.108.120691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J.C., Bruck D.J., Curry H., Edwards D., Haviland D.R., Van Steenwyk R.A., Yorgey B.M. The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag. Sci. 2011;67:1358–1367. doi: 10.1002/ps.2225. [DOI] [PubMed] [Google Scholar]
- 14.Bernardi D., Andreazza F., Botton M., Baronio C.A., Nava D.E. Susceptibility and Interactions of Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae) in Damaging Strawberry. Neotrop. Entomol. 2017;46:1–7. doi: 10.1007/s13744-016-0423-9. [DOI] [PubMed] [Google Scholar]
- 15.Goldman-Huertas B., Mitchell R.F., Lapoint R.T., Faucher C.P., Hildebrand J.G., Whiteman N.K. Evolution of herbivory in Drosophilidae linked to loss of behaviors, antennal responses, odorant receptors, and ancestral diet. Proc. Natl. Acad. Sci. USA. 2015;112:3026–3031. doi: 10.1073/pnas.1424656112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori B.A., Whitener A.B., Leinweber Y., Revadi S., Beers E.H., Witzgall P., Becher P.G. Enhanced yeast feeding following mating facilitates control of the invasive fruit pest Drosophila suzukii. J. Appl. Ecol. 2016;54:170–177. [Google Scholar]
- 17.Posé S., García-Gago J.A., Santiago-Domenech N., Pliego-Alfaro F., Quesada M.A., Mercado J.A. Strawberry fruit softening: role of cell wall disassembly and its manipulation in transgenic plants. Genes Genomics. 2011;5:40–48. [Google Scholar]
- 18.Kim Y.H., Kim K.H., Szulejko J.E., Parker D. Quantitative analysis of fragrance and odorants released from fresh and decaying strawberries. Sensors (Basel) 2013;13:7939–7978. doi: 10.3390/s130607939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsson M.C., Domingos A.I., Jones W.D., Chiappe M.E., Amrein H., Vosshall L.B. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Benton R., Vannice K.S., Gomez-Diaz C., Vosshall L.B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vosshall L.B., Wong A.M., Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- 22.Benton R., Sachse S., Michnick S.W., Vosshall L.B. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han C., Jan L.Y., Jan Y.N. Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proc. Natl. Acad. Sci. USA. 2011;108:9673–9678. doi: 10.1073/pnas.1106386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umezaki Y., Yasuyama K., Nakagoshi H., Tomioka K. Blocking synaptic transmission with tetanus toxin light chain reveals modes of neurotransmission in the PDF-positive circadian clock neurons of Drosophila melanogaster. J. Insect Physiol. 2011;57:1290–1299. doi: 10.1016/j.jinsphys.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Gratz S.J., Rubinstein C.D., Harrison M.M., Wildonger J., O’Connor-Giles K.M. CRISPR-Cas9 Genome Editing in Drosophila. Curr. Protoc. Mol. Biol. 2015;111:1–20. doi: 10.1002/0471142727.mb3102s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blount Z.D., Barrick J.E., Davidson C.J., Lenski R.E. Genomic analysis of a key innovation in an experimental Escherichia coli population. Nature. 2012;489:513–518. doi: 10.1038/nature11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen P., Kim A.Y., Jung J.K., Donahue K.M., Jung C., Choi M.Y., Koh Y.H. The Biochemical Adaptations of Spotted Wing Drosophila (Diptera: Drosophilidae) to Fresh Fruits Reduced Fructose Concentrations and Glutathione-S Transferase Activities. J. Econ. Entomol. 2016;109:973–981. doi: 10.1093/jee/tow019. [DOI] [PubMed] [Google Scholar]
- 28.Hickner P.V., Rivaldi C.L., Johnson C.M., Siddappaji M., Raster G.J., Syed Z. The making of a pest: Insights from the evolution of chemosensory receptor families in a pestiferous and invasive fly, Drosophila suzukii. BMC Genomics. 2016;17:648. doi: 10.1186/s12864-016-2983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramasamy S., Ometto L., Crava C.M., Revadi S., Kaur R., Horner D.S., Pisani D., Dekker T., Anfora G., Rota-Stabelli O. The Evolution of Olfactory Gene Families in Drosophila and the Genomic Basis of chemical-Ecological Adaptation in Drosophila suzukii. Genome Biol. Evol. 2016;8:2297–2311. doi: 10.1093/gbe/evw160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.