Abstract
Glioblastoma multiforme (GBM) is the most common and aggressive primary neoplasm of the brain. Poor prognosis is mainly attributed to tumor heterogeneity, invasiveness, and drug resistance. microRNA-based therapeutics represent a promising approach due to their ability to inhibit multiple targets. In this work, we aim to restore the oncosuppressor activity of microRNA-34a (miR-34a) in GBM. We developed a cationic carrier system, dendritic polyglycerolamine (dPG-NH2), which remarkably improves miRNA stability, intracellular trafficking, and activity. dPG-NH2 carrying mature miR-34a targets C-MET, CDK6, Notch1 and BCL-2, consequently inhibiting cell cycle progression, proliferation and migration of GBM cells. Following complexation with dPG-NH2, miRNA is stable in plasma and able to cross the blood–brain barrier. We further show inhibition of tumor growth following treatment with dPG-NH2–miR-34a in a human glioblastoma mouse model. We hereby present a promising technology using dPG-NH2–miR-34a polyplex for brain-tumor treatment, with enhanced efficacy and no apparent signs of toxicity.
Key words: MicroRNA-34a, Glioblastoma, Polyglycerol-based polyplex
Graphical Abstract
Schematic representation of aminated polyglycerol-based dPG-NH2–miR-34a polyplex taken-up by glioblastoma cells, inhibiting their proliferation and migration and accumulating selectively at the tumor site in the brain following extravasation via the tumor leaky vessels.

Glioblastoma multiforme (GBM) is a Grade IV primary brain tumor. Its aggressive nature and evasiveness to treatments make it one of the most lethal cancers.1 Current treatment involving maximal surgical resection followed by chemotherapy, radiotherapy and anti-angiogenic agents usually remains palliative.2, 3 Due to GBM's diffusive and invasive nature, complete removal of the tumor is impossible by conventional surgery and hence results in high recurrence rate.4 In almost all cases, recurrent tumors are resistant to all available treatments.5, 6, 7, 8 Hence, novel agents targeting relevant pathways are desperately needed. The heterogeneous nature of GBM and anatomic complexities crossing the blood–brain barrier9, 10 make its treatment a difficult goal. Moreover, the molecular mechanisms of drug resistance displayed by GBM are only partially understood. Cancer cell proliferation, growth and death are regulated by intricate networks of signaling pathways. Inhibiting any one specific pathway mostly results in the activation of compensating mechanisms. Hence, we propose to target multiple biological pathways in order to effectively impede cancer progression and recurrence, and overcome drug resistance.
MicroRNAs (miRNAs, miRs) are master regulators of the genome that play a key role in the regulation of cell cycle, proliferation and cancer progression. Both downregulation and upregulation of miRNAs have been recently implicated in the development of GBM.11, 12
The therapeutic use of miRNAs has gained much attention because their mechanisms of action are in line with our current perception of cancer as a multiple pathways-associated disease. miR-34a was originally uncovered as a potential tumor suppressor that is downregulated and induces apoptosis in neuroblastoma cells.13 Shortly afterwards, a few studies almost simultaneously reported that miR-34a functions as a transcriptional target of p53.14, 15, 16, 17 Since then, a number of studies have addressed the deregulation of miR-34a in various cancers including colon, leukemia, lymphoma, brain, bone, skin, prostate, ovary, pancreas, liver and lung.18, 19, 20, 21, 22, 23, 24, 25 miR-34a was found to affect tumor cell apoptosis, senescence, proliferation and invasion, via regulation of hundreds of predicted mRNA targets including CDK6, C-MET, Notch1 and Bcl-2.13, 16, 18, 20, 26, 27 Li et al28 showed that miR-34a is downregulated in GBM and inhibits cell proliferation, survival, migration and invasion. Following transfection with miR-34a prior to inoculation into mice, GBM cells formed smaller xenografts.28 miR-34a overexpression also inhibits various malignancy endpoints in glioma stem cells, inducing glioma stem cell differentiation.29 This important work suggested that miR-34a plays a key tumor suppressive role in GBM.28, 29 We therefore aim to evaluate the therapeutic potential of miR-34a in GBM. Mirna Therapeutics' lead product candidate, MRX34, designed to deliver miR-34a mimic, is currently in Phase 1 clinical trial for unresectable liver cancer and other hematological malignancies (http://www.mirnarx.com/pipeline/mirna-MRX34.htmL).
We developed dendritic polyglycerolamine (dPG-NH2), a polyglycerol-based nanocarrier to deliver small interfering RNA (siRNA) and miRNA to tumors in vivo.30, 31, 32, 33 dPG-NH2 is a cationic hyperbranched polymer, which improves siRNA stability, intracellular trafficking, silencing efficacy, and accumulation in the tumor environment due to the enhanced permeability and retention effect. dPG-NH2 exhibited low cytotoxicity and high efficacy in delivering active siRNA into cells and successfully silenced a luciferase gene, ectopically overexpressed in a human GBM cell line.33
In the current work, we show that dPG-NH2–miR-34a polyplex restores the tumor suppressing function of miR-34a in GBM. Treatment of several GBM cells with dPG-NH2–miR-34a polyplex inhibits cells' proliferation and migration, potentially via the direct modulation of miR-34a-downstream targets CDK6, C-MET, Bcl-2 and Notch1. More significantly, miR-34a restoration led to remarkable reduction in tumor growth in a mouse model of human GBM. Restoration of miR-34a tumor suppressor activity may hold significant promise as a novel molecular therapy for human GBM. The ability of dPG-NH2–miR-34a to impact multiple cellular pathways also suggests that miR-34a can act synergistically with conventional, cytotoxic therapies.
Methods
Ethics statement
All animal experiments were approved by Tel-Aviv University animal care and use committee (IACUC) and conducted in accordance with NIH guidelines for humane care. Experiments involving human tissues were performed following informed consent of patients and approved by our institutional review board for research involving human subjects (IRB).
dPG-NH2 synthesis
Synthesis of dendritic polyglycerol (dPG-NH2) analogues was executed according to a three-step protocol as previously reported34, 35, 36 (brief description in Supplementary Material).
Electrophoresis mobility shift assay (EMSA)
The optimal ratio for the polyplex formation was studied as described previously.33
psiCHECK luciferase reporter assay
The activity of a dPG-NH2–miR-34a polyplex toward a consensus target sequence cloned into the 3′-untranslated region (3′-UTR) of Renilla luciferase was monitored by a dual luciferase assay in U-87 MG, U251 and U373 GBM cells. More detailed description of the assay can be found at the Supplementary Material section.
Physico-chemical characterization of the polyplex
Dynamic light scattering (DLS) and zeta potential determination were performed using a ZetaSizer Nano ZS instrument (λ = 633 nm; Malvern Instruments Ltd., UK). Scanning electron microscope (SEM) images were taken by Quanta 200 FEG Environmental SEM (FEI, Oregon, USA) at high vacuum and 10.0 kV. Samples preparation is described in the Supplementary Material section.
Patient-derived human primary glioblastoma cells
Fresh GBM specimens were obtained from Tel Aviv Medical Center (Tel-Aviv, Israel). Each tumor specimen was cut to 2 mm diameter pieces and cultured in DMEM medium containing 20% fetal bovine serum (FBS), 1% l-glutamine, 1% penicillin and streptomycin. Following continuous media replacement, viable cancer cells remained attached to culture plates and kept growing in culture, while stroma and cell debris were washed away.
Confocal microscopy
microRNA-34a (miR-34a) (50 pmol) (Biospring, Germany) was mixed with FITC-labeled dPG-NH2 (100 pmol) in serum-free medium, incubated for 20 min at RT, and then added to U-87 MG cells previously seeded on cover glasses. Twenty four hours later, cells were fixed with 4% paraformaldehyde and immunostained with anti-EEA1 (BD Biosciences) and anti-LAMP1 (Cell Signaling, USA) antibodies, followed by rhodamine-labeled secondary antibodies (Jackson, USA). Cover glasses were mounted by Vectashild (Vector Laboratories, USA) and analyzed by Leica TCS SP5 confocal imaging (Leica Microsystems, Germany).
Real time PCR
RNA samples from A172, U-87 MG and T98 human GBM cell lines transfected with 200 nM miR-34a or negative control microRNA (NC-miR) complexed with 500 nM dPG-NH2 and harvested 24 h later were reverse transcribed (miSCript II RT, Qiagen) and miR-34a expression levels were evaluated by miSCript miRNA PCR assay (Qiagen) according to the manufacturer's protocol. Target genes expression levels were assessed by SYBR green real time PCR (SensiFAST™ SYBR, Bioline; StepOne plus, Life Technologies). Sequences of primers used are detailed in the Supplementary Material section.
Cell proliferation assay
Human GBM cell lines and patient-derived primary glioblastoma cells were treated with dPG-NH2–miR-34a or dPG-NH2-NC miR (200 nM dPG-NH2, 100 nM miRNA), or left untreated. Four days later, cells proliferation was assessed by Coulter Counter (Beckman Coulter).
Cell cycle analysis
U-87 MG cells were transfected with dPG-NH2–miR-34a, dPG-NH2–NC-miR (100 nM miRNA, 200 nM dPG-NH2) or left untreated. Following 72 h cells were labeled with propidium iodide (25 μg/mL), 0.1% Triton X-100 and DNase-free RNase A (5 μg/mL) and incubated in the dark on ice for 30 min. The stained cells (1 × 106) were then analyzed for DNA content with a flow cytometer (FACScalibur, Becton–Dickinson).
Migration assay
Cells migration was evaluated using transwells chambers with pore size of 8 mm (Costar Corp., Corning, NY, USA). U-87 MG and A172 cells were treated with dPG-NH2–miR-34a, dPG-NH2–NC-miR (200 nM miR) or left untreated. GBM cells (48 h after treatment) or untreated HUVEC were seeded at the upper chamber of transwells. Following 2 h, 10% FBS-containing media (for GBM cells) or GBM conditioned media (for HUVEC) were added to the lower chamber. Cells were allowed to migrate to the lower chamber for 4 h, followed by fixation and staining (Hema 3, Fisher HealthCare). More detailed protocol can be found in the Supplementary Material section.
Stability of microRNA in plasma
50 pmol of miR-34a, either naked or complexed with dPG-NH2 (N/P 9) was incubated with mouse plasma (Sigma), at 37 °C for the indicated times, followed by 15 min at room temperature with or without sodium heparin (0.25 IU final conc.). Samples were analyzed by electrophoresis.
Cytokines induction assay
Peripheral blood mononuclear cells (PBMCs) were incubated with dPG-NH2, miR-34a and dPG-NH2–miR-34a for 4 h at 37 °C. Lipopolysacharides (Sigma) were used as positive control (1 μg/mL), PBS as negative control. Upon incubation, RNA was isolated from cell pellets and reverse transcribed using EZ-RNA II isolation kit and EZ-first strand cDNA synthesis kit (Biological industries). TNF-α and IL-6 levels were assessed by SYBR green based real-time PCR and normalized to GAPDH (primers in Supplementary Material).
Animal studies
U-87 MG human cells (1 × 106) were injected subcutaneously to 6- to 8-week-old male SCID mice (Harlan Laboratories, Israel). Tumor size was measured using caliper and volume was calculated using standard formula (length × width2 × 0.52). Once tumors reached the size of 50 mm3, mice were injected intratumorally with PBS, dPG-NH2–miR-34a and dPG-NH2–NC-miR (4 mg/kg miRNA, 10 mg/kg dPG-NH2) on days 0, 3 and 6. Animals were monitored twice a week for general health, body weight and tumor volume.
Intracranial GBM cells inoculation
SCID mice anesthetized with ketamine–xylazine were secured to a stereotaxic device and a 1-cm incision was made on the skull midline between the ears. A small hole was drilled 1.5 mm left, 0.5 mm anterior and 2.5 mm ventral to the bregma. mCherry-labeled U-87 MG cells (2 × 105) were slowly inoculated and incision was closed by wound clips. Animals were monitored twice a week for general health and body weight. Tumor growth was followed by Maestro CRI™ intravital non-invasive imaging.
Polyplex accumulation follow-up and half-life in plasma
Cy5-labeled dPG-NH2–miR-34a (2 mg/kg miRNA, 5 mg/kg dPG-NH2) was injected intravenously to 6- to 8-week-old male SCID mice bearing subcutaneous and intracranial U-87 MG, and to non-tumor bearing mice. Polyplex accumulation in tumor and organs was followed with Maestro CRI™. One hour and five hours after administration, mice were euthanized and intracardially perfused with saline, and tumor and organs were resected and analyzed for Cy5-dPG-NH2 accumulation. Blood samples were taken from the mice orbital sinus on different time points following polyplex injection. Plasma was isolated and fluorescence intensity (λEx = 630 nm, λEm = 670 nm) was measured using SpectraMax M5 plate reader.
Results
Synthesis and physicochemical characterization of dPG-NH2–miR-34a polyplex
Hyperbranched polyglycerol with high amine loading (Figures 1, A, S1) was synthesized, as previously reported.33 To assess the capability of dPG-NH2 to complex miRNA, we incubated several amounts of the polymer with a constant amount of miR-34a and analyzed the efficacy of the polyplex formation by electrophoresis mobility shift assay (Figure 1, B). Similar results were obtained with dPG-NH2–NC-miR polyplex (data not shown). While a neutral polyplex formed at N/P 2.5, maximal miR-34 activity and minimal cytotoxicity with dPG-NH2–miR-34a polyplex were achieved at N/P 9 (Figure 1, C and D), therefore this ratio was selected for all consecutive experiments.
Figure 1.
Physicochemical characterization of dPG-NH2-miR-34a polyplex. A. Schematic structure of dPG-NH2-miRNA polyplex. B. Electrophoresis Mobility Shift Assay with dPG-NH2-miR-34a at N/P ratios of 0, 0.4, 0.9, 2.5, 4.5 and 9. C-D. U-87 MG glioblastoma cells were treated with dPG-NH2-miR-34a or dPGNH2-NC-miR at N/P ratios of 2.2, 4.5 and 9 at dPG-NH2 concentrations of 100, 200 and 400 nM, respectively. C. Activity of dPG-NH2-miR polyplexes at different N/P ratios was monitored by a dual luciferase assay. D. Cells viability was assessed by XTT proliferation assay. E. Zeta potential. F. Size distribution. G. SEM image. H. Table summarizing zeta potential, hydrodynamic diameter and average diameter of dPG-NH2-miR-34a polyplex (N/P 9).
dPG-NH2–miR-34a polyplexes were analyzed for their hydrodynamic diameter, zeta potential and morphology using DLS analyzer and SEM respectively. Neutralization of the negatively charged surface of miRNA following complexation with dPG-NH2 was confirmed by zeta potential measurements. Mixture of miRNA with dPG-NH2 at pH 7.4, yielded polyplexes with positive charges. Surface charge values, hydrodynamic volume and diameter of dPG-NH2–miRNA polyplex at N/P ratio of 9 were determined (Figure 1, E-H). The measured particle's hydrodynamic diameter was 113.9 ± 35.73 nm with a polydispersity index of 0.063. Such low size dispersity of the polyplexes evidences the strong interaction and stability of the complex between dPG-NH2 and miR-34a. The size distribution showed a high correlation with the diameter obtained in the SEM analysis (100 ± 17 nm). This diameter reflects assemblies of several dPG-NH2–miRNAs and is in accordance with previously reported data.31 The measured zeta potential was slightly positive (16.4 ± 3.60 mV) as expected.33
dPG-NH2–miR-34a polyplex internalizes into GBM cells via endocytosis
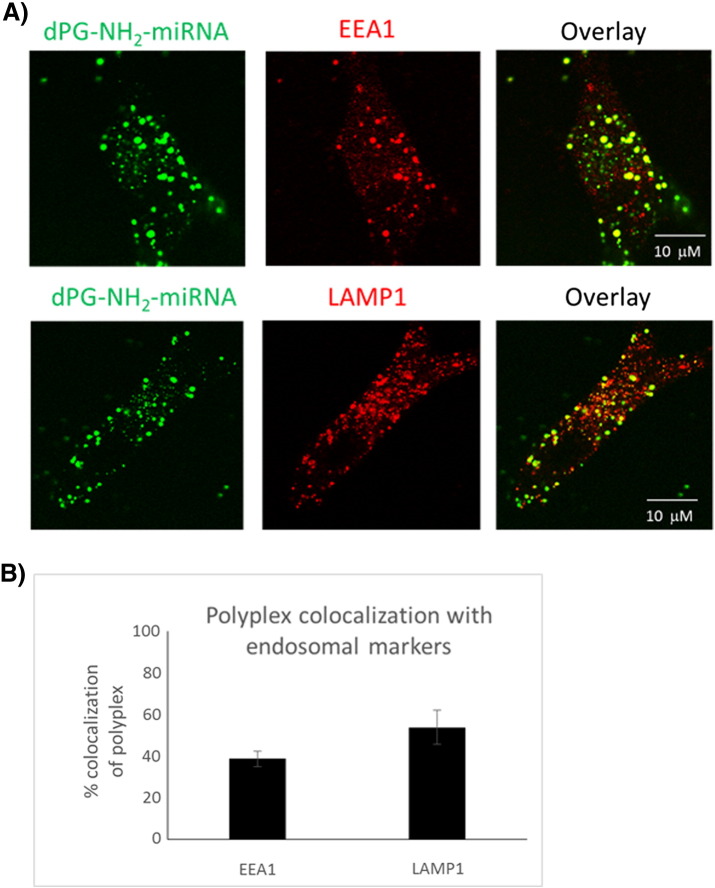
Using confocal microscopy, we followed miRNA internalization to human GBM cells, and consequently confirmed the high efficacy of FITC-labeled dPG-NH2 as a nanocarrier for small RNA molecules. In order to determine dPG-NH2 polyplex internalization mechanism, we performed colocalization studies with subcellular markers: early endosome antigen 1 (EEA1) and lysosomal-associated membrane protein 1 (LAMP-1). High levels of colocalization of dPG-NH2 polyplexes with EEA1 and LAMP1 markers (Figure 2), confirmed that their cellular uptake is mediated by endocytosis via the endosome–lysosome path.
Figure 2.
Intracellular trafficking of dPG-NH2–miRNA polyplex in U-87 MG cells. (A) Co-localization of FITC-labeled dPG-NH2 miRNA polyplex with EEA1 and LAMP1. Confocal images following 24 h incubation of cells with FITC-dPG-NH2 miRNA polyplex. (B) Quantitative analysis using Imaris software (Bitplane AC, Switzerland). Data represent mean ± stdev. from five different fields.
dPG-NH2–miR-34a polyplex-treated human GBM cells express higher levels of miR-34a and lower levels of its target genes
miR-34 regulates several processes that promote cancer development through downregulation of their target mRNAs. As of today, more than 77 miR-34 targets have been validated, including MET,17, 20 CDK6,26, 37 Notch138, 39 and Bcl-2.17, 40, 41 Following treatment with dPG-NH2–miR-34a, we observed remarkably higher levels of miR-34a and lower expression levels of its target genes: C-MET, CDK6, Notch1 and Bcl-2 in several human GBM cell lines (Figure 3). This means not only that miR-34a is effectively internalized into the cells cytoplasm, but it is also released from the dPG-NH2 carrier, escaping from the endosome. Then, it binds to its target sequences in the cytoplasm and successfully inhibits gene expression.
Figure 3.
dPG-NH2–miR-34a polyplex-treated human GBM cells express higher levels of miR-34a and lower levels of its target genes. (A-E) A172, U-87 MG and T98 human GBM cells were treated with dPG-NH2–miR-34a, dPG-NH2–NC-miR or left untreated. Twenty-four hours later, RNA was isolated and qPCR was performed to evaluate miR-34a, C-MET, CDK6, Notch1 and Bcl-2 expression levels. ***P value ≤ 0.01, **P value ≤ 0.05 related to untreated control and to NC-miR.
dPG-NH2–miR-34a polyplex inhibits GBM miR-34a activity, cell cycle progression and cell survival
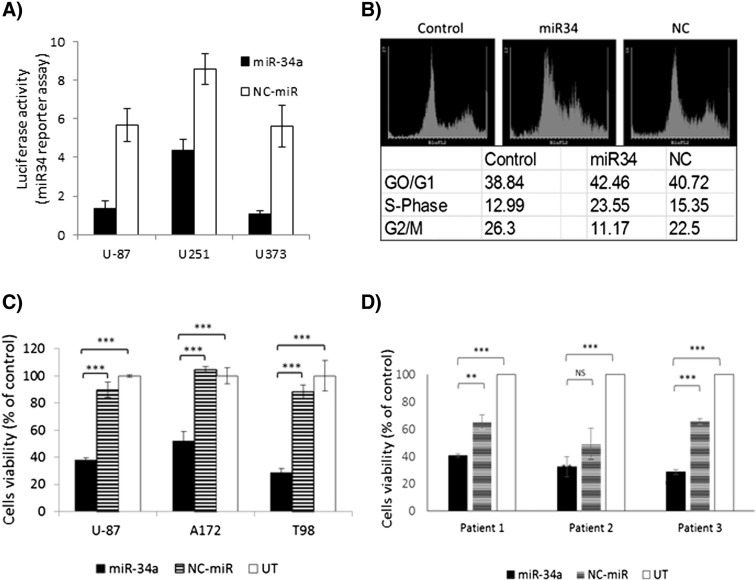
We further evaluated the activity of miR-34a mimic, delivered to the cell cytoplasm by dPG-NH2, using psiCHECK (Promega™) plasmid constructs. psiCHECK™-2-based construct was prepared, containing one copy of the full target (nucleotide sequence fully complementary to the miR-34a guide strand). Results showed a remarkable downregulation of the miR-34a target sequences following treatment of several GBM cells with dPG-NH2–miR-34a polyplex (Figure 4, A). Moreover, miR-34a overexpression induced cell cycle arrest in S-phase (Figure 4, B) and inhibited viability and proliferation of human GBM cell lines (Figure 4, C). Further support to the remarkable anti-tumorigenic effect of dPG-NH2–miR-34a on GBM can be found in Figure 4, D that shows reduced viability following treatment with miR-34a polyplex in fresh GBM cells derived from three different human patients. These findings demonstrate that miR-34a mimic delivered to the cell cytoplasm by dPG-NH2 is highly active and able to restore the tumor suppressor function of miR-34a in GBM.
Figure 4.
dPG-NH2–miR-34a polyplex inhibits GBM miR-34a activity, cell cycle progression and cell survival. (A) Activity of a dPG-NH2–miR-34a polyplex monitored by a dual luciferase assay in U-87 MG, U251 and U373 GBM cells. (B-D) GBM cells were treated with dPG-NH2–miR-34a or dPG-NH2–NC-miR (100 nM miR). (B) U-87 MG cells were analyzed by flow cytometry 72 h following treatment with polyplex. (C-D) Four days later, cell proliferation was assessed by Coulter Counter. ***P value ≤ 0.01, **P value ≤ 0.05 related to untreated control and/or NC-miR. (C) U-87 MG, A172 and T98G human GBM cell lines. (D) Human patient-derived GBM cells.
dPG-NH2–miR-34a polyplex inhibits migration of GBM cells toward serum and migration of endothelial cells toward conditioned media (CM) from GBM cells
Some of the known targets of miR-34a, like C-MET, are directly involved in the regulation of cell migration, a highly integrated, multi-step process that plays an important role in cancer progression. Therefore, we evaluated the effect of dPG-NH2–miR-34a polyplex on the invasion ability and angiogenic potential of GBM cells. We found a remarkable inhibition in the ability of GBM cells to migrate toward serum, as well as the ability of endothelial cells to migrate toward CM from GBM cells (Figure 5). Images of experiments represented in B and C are shown in the supplementary information (Figure S2).
Figure 5.
dPG-NH2–miR-34a polyplex inhibits migration of GBM cells toward serum and migration of endothelial cells toward GBM cells. Representative images and quantification of migration experiments. (A) U-87 MG. ***P value ≤ 0.01, **P value ≤ 0.05 related to control and to NC-miR. (B) A172 cells. (C) Human umbilical vein endothelial cells (HUVEC) toward conditioned media (C.M.) from GBM cells treated with dPG-NH2–miR-34/NC-miR polyplex.
dPG-NH2–miR-34a polyplex is stable in plasma and do not induce an immune response ex vivo
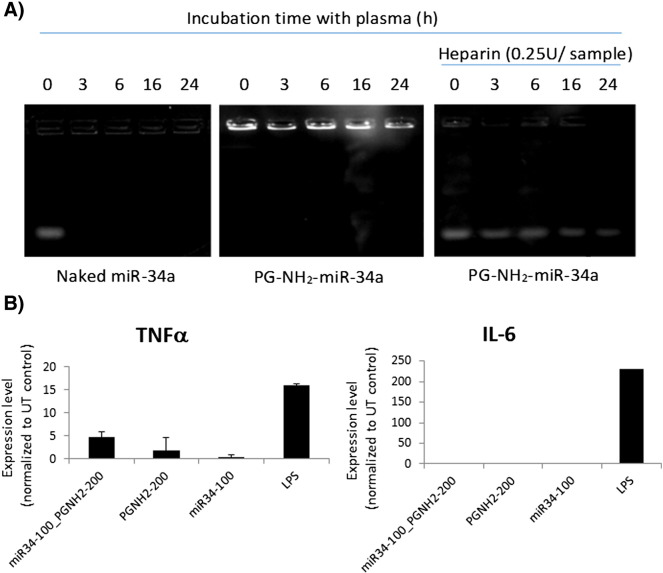
The relative stability of dPG-NH2–miR-34a polyplex was evaluated by measuring miRNA release in the presence of the competing polyanion heparin. Heparin, capable of displacing RNA from polycation/RNA complexes, was chosen as a model substance for this assay. The lowest concentration of heparin required for miRNA displacement to occur provides an estimation of the complex stability against polyanions.42 Following incubation of dPG-NH2–miR-34a polyplex with increasing levels of heparin, we found that the level required for miRNA release from the polyplex is 0.15 IU (International Units)/20 μl reaction volume (Figure S3). This equals to 750 IU/100 ml, while the average heparin levels in human plasma are 15 IU/100 ml.43 We next evaluated the integrity of the polyplex versus that of the naked miRNA following incubation with murine plasma. Summarizing the results presented in Figure 6, A, we may conclude that dPG-NH2 confers good protection to miRNA against plasma-induced degradation.
Figure 6.
miR-34a and dPG-NH2–miR-34a polyplex physiological characterization. (A) Naked miR-34a and dPG-NH2–miR-34a were incubated at 37 °C in mouse plasma, with or without heparin, and analyzed by gel electrophoresis. (B) Tumor necrosis factor-alpha (TNF-α) and interleukin 6 (IL-6) cytokines secreted from human PBMCs following treatment with dPG-NH2, miR-34a or dPG-NH2–miR-34a (concentration in nM). Lipopolysaccharide (LPS) was used as positive control, PBS as negative control.
One additional important aspect for successful miRNA delivery is preventing a robust adverse effect such as a cytokines induction, lymphocyte activation or interferon response. Unmodified duplex siRNA/miRNA may be recognized by the immune system as an unwanted invader and trigger an immune response. The miR-34a mimic used in the described work was chemically modified at the 2′ position of ribose (2'-O-Methyl), to block recognition by Toll-like receptors (TLRs) and thwart the immune response. However, recent reports indicate that cationic polymers like dPG-NH2 can potentially activate significant immunological activity mediated by TLRs.44 In order to address this issue, we evaluated the effect of dPG-NH2, miR-34a, and dPG-NH2–miR-34a polyplex on TNF-α and IL-6 cytokines secretion from human peripheral blood mononuclear cells (PBMCs). The results show no IL-6 and a moderate TNF-α stimulation (Figure 6, B), suggesting no major activation of immune response by dPG-NH2 or miR-34a.
dPG-NH2–miR-34 polyplex reaches both subcutaneous and orthotopically inoculated glioblastoma tumors following systemic administration
In order to evaluate the feasibility of systemic administration of polyplex to treat GBM tumors, we followed the accumulation of fluorescently labeled dPG-NH2–miR-34 in both subcutaneous and intracranially inoculated GBM tumors (Figure 7, A and B, respectively). Cy5-labeled dPG-NH2 complexed with miR-34a was injected intravenously (i.v.) to mice bearing U-87 MG human glioblastoma. The polyplex reached the tumor site within 5 min. dPG-NH2–miR-34a was capable of crossing the compromised blood–brain barrier at the tumor site and reach the GBM, although it was not accumulated there for long (Figure 7, B). Thirty minutes following intravenous administration, the level of Cy5-labeled polyplex localized in the intracranial tumor was considerably reduced, suggesting its fast clearance. In the subcutaneous tumor the Cy5-labeled polyplex was retained for a slightly longer time. Probably due to its larger size, the subcutaneous tumor displayed a more robust EPR (enhanced permeability and retention) effect, as opposed to the intracranial tumor. However, as clearly seen from the PK experiment, shown in Figure 7, C, the polyplex has a very short t1/2 in the plasma (4 min). It seems enough for some extravasation into the tumor, however, the polyplex also accumulated in reticuloendothelial system (RES) organs (lung and liver). Subsequently, we are now working on the development of a second generation of dPG-NH2 that will hopefully be able to increase the accumulation and retention time of the polyplex at the tumor site and minimize its presence in normal tissues.
Figure 7.
Systemically-administered dPG-NH2–miR-34a polyplex accumulates in glioblastoma tumors. (A-B) Cy5-dPG-NH2 (blue) complexed with miR-34a was injected intravenously (i.v.) to mice bearing subcutaneous (A) and intracranial mCherry-labeled (B) U-87 MG tumors. Accumulation of Cy5-dPG-NH2–miR-34a in the tumor was followed via intravital non-invasive imaging (Maestro CRI™), quantified and plotted in a graph (in vivo). Mice were euthanized, and tumors and organs were resected and imaged. Cy5-polyplex distribution in tumor and organs are plotted in a graph (ex vivo). Percent colocalization represents the % of Cy5 fluorescence in mCherry-labeled (U-87 MG tumor) area. (C) Half-life of polyplex in plasma. Cy5-dPG-NH2–miR-34a was i.v. injected to non-tumor-bearing mice. Polyplex levels were monitored in blood samples taken from mice at several time points (n = 3). Fluorescence intensity (λEx = 630 nm, λEm = 670 nm) was measured using SpectraMax M5 plate reader and is plotted in a graph ± stdev.
dPG-NH2–miR-34a polyplex inhibits GBM tumor growth in vivo
Following our findings that after systemic administration, the polyplex reaches the tumor site but is rapidly cleared, we decided to evaluate dPG-NH2 as a prototype miRNA nanocarrier for intratumoral administration. We performed an in vivo experiment to evaluate its potential to restore the tumor suppressing function of miR-34a in GBM, following intratumoral administration of dPG-NH2–miR-34a. U-87 MG human GBM cells were subcutaneously inoculated in SCID mice. Once palpable tumors developed, 10 mg/kg dPG-NH2 complexed with 4 mg/kg miR-34a, NC-miR, or PBS was injected intratumorally, in line with our previous study,33 with a slight adjustment to the optimal N/P ratio of the new dPG-NH2–miRNA polyplexes (Figure 1). According to the same study, the silencing effect of luciferase siRNA delivered intratumorally with the same vehicle lasted roughly for 3 days.33 Therefore, dPG-NH2–miRNA polyplex was administered every 3 days.
As shown in Figure 8, A, a substantial tumor growth inhibition was achieved, following 3 consecutive treatments with dPG-NH2–miR-34a polyplex. While tumors in mice treated with PBS reached the size of 1.2 cm3 around day 15, tumors treated with dPG-NH2–miR-34a polyplex grew to that size almost 35 days later. No weight loss or deterioration in general health and behavior was observed.
Figure 8.
dPG-NH2–miR-34a polyplex inhibits U-87 MG tumor growth. (A) Tumor growth. (B) Kaplan–Meier analysis following 3 consecutive treatments (marked by arrows in A) with dPG-NH2–miR-34a (miR-34a), dPG-NH2–NC-miR (NC-miR), or PBS. Data in tumor volume graph represent mean ± s.e.m. For miR-34a treated toward NC-miR treated mice, P < 0.05 on days 20 to 30, P < 0.01 on days 32 to 62.
Survival was significantly prolonged in the miR-34a-treated mice. While the median survival for the PBS-treated group and the NC miR-treated group was 20 and 40 days, respectively, for the dPG-NH2–miR-34a treated group it was 55 days (Figure 8, B).
Some non-specific anti-tumor activity was observed following treatment with dPG-NH2–NC-miR polyplex. Unfortunately, although several sequences were evaluated, we were not able to generate a completely inert negative control. We expect to reduce the non-specific effects of the NC-miR polyplex after optimization of the formulation that will hopefully enable to deliver lower doses of miR and achieve a maximal therapeutic effect.
Discussion
Poor prognosis for GBM is mainly attributed to undefined tumor margins, critical location of the tumor mass and presence of chemo- and radio-resistant tumor cells.45, 46 Tumor heterogeneity is one of the main causes for drug resistance. Therefore, miRNA-based therapeutics represent a promising approach that can potentially overcome resistance mechanisms by inhibiting multiple targets. miRNAs are dysregulated in almost all solid and hematological malignancies. Several miRNAs that play important roles in glioma has been discovered to date.47 Increased understanding of miRNA role in glioma biology has led to the approach of restoring normal miRNA expression and function and improving the prognosis of glioma patients.
Altered modulation of drug response mediated by miR-34a has been reported in a number of cellular models, including GBM. miR-34a is a key regulator of tumor suppression, downregulated in human glioma tumors as compared to normal brain, and in mutant-p53 gliomas as compared to wild type-p53 tumors.48 Therefore, restoring the oncosuppressor miR-34a provides a promising strategy against GBM. However, despite the interest in the use of miR-34a as new anticancer agent, the delivery issue represents a major obstacle. Reaching the right target in the cancer cell cytoplasm still remains the Achilles' heel of miRNA-based treatments. miRNA molecules are rapidly degraded in biological fluids, poorly taken-up into cells and homogeneously distributed throughout the body.49 Here we show that we were able to stabilize the miRNA when complexing it with our nanocarrier as compared to the free miRNA incubated in plasma (Figure 6, A). We hereby show, for the first time, successful delivery of miR-34a to human GBM cells both in vitro and in vivo using dPG-NH2. dPG-NH2 formed a stable complex with miRNA (Figure 1), of circa 100 nm size and zeta potential of 16.4 mV, at N/P 9, that showed optimal activity.
dPG-NH2–miR-34a polyplex was successfully internalized into the cytoplasm of human GBM cells via the endosome-lysosome system (Figure 2), leading to increased expression levels of miR-34a followed by downregulation of a set of essential target genes: C-MET, CDK6, Notch1 and BCL-2 (Figure 3). C-MET is a receptor tyrosine kinase that activates a wide range of cellular signaling pathways, including those involved in proliferation, motility, migration and invasion. It has been found to be aberrantly activated in human cancers via mutation, amplification or protein overexpression.50 CDK6, a cell cycle regulator, is significantly upregulated in glioma cells, and its elevated expression correlates well with the grade of glioma malignancy.51 CDK6 knockdown dramatically inhibits proliferation and survival of tumor cells and reduces the expression level of drug resistance genes such as Multidrug Resistance (MDR) and Multidrug Resistance associated Protein (MRP).51 The Notch signaling pathway is involved in cell fate decisions during normal development and in the generation of several cancers.52 Notch signaling is activated in primary GBM53 and it plays an important role in the cellular response to hypoxia and angiogenesis,54 two key processes in the development of human gliomas.55 BCL-2 has been widely characterized ant it is known to block programmed cell death rather than promote proliferation.56 BCL-2 was further shown to play an additional role in the malignant phenotype of glioma cells, that is, to enhance migration and invasion by altering the expression of a set of metalloproteinases and their inhibitors.57 The four target genes evaluated here were previously established as transcriptional targets of miR-34a that play key roles in glioma cells.28 In our study, we demonstrate that they are all tightly regulated by dPG-NH2–miR-34a polyplex.
dPG-NH2–miR-34a polyplex treatment further induced a substantial inhibition of miR-34a-driven luciferase activity. Not surprisingly, G1/S cell cycle arrest and decreased cell survival in several human glioblastoma cell lines were observed. Remarkable inhibition in cells survival and proliferation following treatment with dPG-NH2–miR-34a was also measured in human patient-derived GBM cells (Figure 4). This last finding may lead to a higher correlation with clinical outcomes compared to conventional cell line-derived models. Determining the function or phenotype of patient-derived GBM cells following treatment will hopefully lead to more comprehensive knowledge of tumor development as well as mechanisms of resistance that are often found in the clinic.
Moreover, dPG-NH2–miR-34a polyplex led to reduced migration of GBM cells toward serum and migration of endothelial cells toward glioblastoma cells (Figure 5). These findings imply not only anti-tumorigenic but also anti-metastatic activity of the polyplex. The above data shows that dPG-NH2 is a promising nanocarrier, which not only neutralizes the negative charge of miRNA, crosses the cell membrane and releases the active miRNA into the cell cytoplasm; but also shields the miRNA molecule against degradation by proteases.
Our polyplex successfully reached both subcutaneous and intracranial GBM tumors following intravenous administration (Figure 7). As previously demonstrated,58 the blood–brain barrier in GBM tumors is hyperpermeable, enabling extravasation of polyplexes. Nevertheless, dPG-NH2–miR-34a was also detected in healthy organs and most of it was found in the kidneys within a few hours on its way for rapid clearance. We therefore present dPG-NH2 as a prototype miRNA nanocarrier for local delivery in its current structure and formulation. Substantial tumor growth inhibition was achieved and mouse survival was remarkably prolonged following local treatment of GBM with dPG-NH2–miR-34a polyplex (Figure 8), while no weight loss occurred. We further observed some anti-tumor activity of dPG-NH2–NC-miR polyplex. Adding exogenous miRNA to the system can result in repression of non-physiological target mRNAs since miRNA–target interaction is strongly concentration-dependent.59 Optimizing the effective dose that will not induce off-target effects is in our future plans.
dPG-NH2 can be used as an effective delivery for miR-34a and other miRNAs for the local treatment of GBM. When injected intravenously, this polyplex is rapidly cleared from the circulation and also reaches the reticuloendothelial system (RES) including organs such as the liver and the lung. In order to exploit this nanocarrier further for systemic administration, we are currently working on increasing its size and its ability to overcome the immune system by addition of poly(ethylene glycol) (PEG). We expect that a new generation of dPG-NH2 with improved pharmacokinetics will lead to improved selective accumulation in the tumor site. Here, we demonstrated that miRNA replacement provides a new therapeutic concept for the treatment of GBM. The broad anti-oncogenic activity of miR-34a holds the prospect of creating a new therapeutic modality that is effective against heterogeneous tumors such as GBM.
Footnotes
R.S.-F. and R.H. would like to thank the German–Israel Foundation (GIF) for financial support. The Satchi-Fainaro laboratory's research leading to these results has received partial funding from the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ERC Consolidator Grant Agreement No. 617445, from the MAGNETON Program of the Office of the Chief Scientist of the Israel Ministry of Industry, Trade & Labor, and from the Israel Science Foundation (918/14). R.H. acknowledges financial support from the Bundesmiminsterium für Bildung und Forschung (BMBF) within the Biotransporter project (project number: 13N11536) and the SFB 765.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nano.2016.05.016.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Supplementary materials.
References
- 1.Nicholas MK, Lukas RV, Chmura S, Yamini B, Lesniak M, Pytel P. Molecular heterogeneity in glioblastoma: therapeutic opportunities and challenges. Semin Oncol. 2011;38:243–253. doi: 10.1053/j.seminoncol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Weller M. Novel diagnostic and therapeutic approaches to malignant glioma. Swiss Med Wkly. 2011;141:w13210. doi: 10.4414/smw.2011.13210. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Ong BY, Ranganath SH, Lee LY, Lu F, Lee HS, Sahinidis NV. Paclitaxel delivery from PLGA foams for controlled release in post-surgical chemotherapy against glioblastoma multiforme. Biomaterials. 2009;30:3189–3196. doi: 10.1016/j.biomaterials.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 6.Friedman GK, Spiller SE, Harrison DK, Fiveash JB, Reddy AT. Treatment of children with glioblastoma with conformal radiation, temozolomide, and bevacizumab as adjuncts to surgical resection. J Pediatr Hematol Oncol. 2013;35:e123–e126. doi: 10.1097/MPH.0b013e318282cd7f. [DOI] [PubMed] [Google Scholar]
- 7.Vredenburgh JJ, Desjardins A, Reardon DA, Peters KB, Herndon JE, 2nd, Marcello J. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res. 2011;17:4119–4124. doi: 10.1158/1078-0432.CCR-11-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai A, Tran A, Nghiemphu PL, Pope WB, Solis OE, Selch M. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29:142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holland EC. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci U S A. 2000;97:6242–6244. doi: 10.1073/pnas.97.12.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleeker FE, Molenaar RJ, Leenstra S. Recent advances in the molecular understanding of glioblastoma. J Neuro-Oncol. 2012;108:11–27. doi: 10.1007/s11060-011-0793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi L, Chen J, Yang J, Pan T, Zhang S, Wang Z. MiR-21 protected human glioblastoma U87MG cells from chemotherapeutic drug temozolomide induced apoptosis by decreasing Bax/Bcl-2 ratio and caspase-3 activity. Brain Res. 2010;1352:255–264. doi: 10.1016/j.brainres.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Li W, Yang Y, Lu Y, He C, Hu G. MicroRNA-21 targets LRRFIP1 and contributes to VM-26 resistance in glioblastoma multiforme. Brain Res. 2009;1286:13–18. doi: 10.1016/j.brainres.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 13.Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26:5017–5022. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- 14.Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26:731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Tarasov V, Jung P, Verdoodt B, Lodygin D, Epanchintsev A, Menssen A. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6:1586–1593. doi: 10.4161/cc.6.13.4436. [DOI] [PubMed] [Google Scholar]
- 17.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci U S A. 2007;104:15472–15477. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dijkstra MK, van Lom K, Tielemans D, Elstrodt F, Langerak AW, van't Veer MB. 17p13/TP53 deletion in B-CLL patients is associated with microRNA-34a downregulation. Leukemia. 2009;23:625–627. doi: 10.1038/leu.2008.264. [DOI] [PubMed] [Google Scholar]
- 20.Li N, Fu H, Tie Y, Hu Z, Kong W, Wu Y. miR-34a inhibits migration and invasion by down-regulation of c-Met expression in human hepatocellular carcinoma cells. Cancer Lett. 2009;275:44–53. doi: 10.1016/j.canlet.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 21.Gallardo E, Navarro A, Vinolas N, Marrades RM, Diaz T, Gel B. miR-34a as a prognostic marker of relapse in surgically resected non-small-cell lung cancer. Carcinogenesis. 2009;30:1903–1909. doi: 10.1093/carcin/bgp219. [DOI] [PubMed] [Google Scholar]
- 22.Li WB, Ma MW, Dong LJ, Wang F, Chen LX, Li XR. MicroRNA-34a targets notch1 and inhibits cell proliferation in glioblastoma multiforme. Cancer Biol Ther. 2011;12:477–483. doi: 10.4161/cbt.12.6.16300. [DOI] [PubMed] [Google Scholar]
- 23.Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg E, Hershkovitz L, Itzhaki O, Hajdu S, Nemlich Y, Ortenberg R. Regulation of cancer aggressive features in melanoma cells by microRNAs. PLoS One. 2011;6:e18936. doi: 10.1371/journal.pone.0018936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun F, Fu H, Liu Q, Tie Y, Zhu J, Xing R. Downregulation of CCND1 and CDK6 by miR-34a induces cell cycle arrest. FEBS Lett. 2008;582:1564–1568. doi: 10.1016/j.febslet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 27.Wei JS, Song YK, Durinck S, Chen QR, Cheuk AT, Tsang P. The MYCN oncogene is a direct target of miR-34a. Oncogene. 2008;27:5204–5213. doi: 10.1038/onc.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Guessous F, Zhang Y, Dipierro C, Kefas B, Johnson E. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009;69:7569–7576. doi: 10.1158/0008-5472.CAN-09-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guessous F, Zhang Y, Kofman A, Catania A, Li Y, Schiff D. microRNA-34a is tumor suppressive in brain tumors and glioma stem cells. Cell Cycle. 2010;9:1031–1036. doi: 10.4161/cc.9.6.10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan K, Ebert B, Voigt J, Wang Q, Dai Y, Haag R. In vivo tumor imaging using a novel RNAi-based detection mechanism. Nanomedicine. 2012;8:393–398. doi: 10.1016/j.nano.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Fischer W, Calderon M, Schulz A, Andreou I, Weber M, Haag R. Dendritic polyglycerols with oligoamine shells show low toxicity and high siRNA transfection efficiency in vitro. Bioconjug Chem. 2010;21:1744–1752. doi: 10.1021/bc900459n. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed F, Pakunlu RI, Brannan A, Bates F, Minko T, Discher DE. Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J Control Release. 2006;116:150–158. doi: 10.1016/j.jconrel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Ofek P, Fischer W, Calderon M, Haag R, Satchi-Fainaro R. In vivo delivery of small interfering RNA to tumors and their vasculature by novel dendritic nanocarriers. FASEB J. 2010;24:3122–3134. doi: 10.1096/fj.09-149641. [DOI] [PubMed] [Google Scholar]
- 34.Sunder A, Kramer M, Hanselmann R, Mulhaupt R, Frey H. Molecular Nanocapsules Based on Amphiphilic Hyperbranched Polyglycerols. Angew Chem. 1999;38:3552–3555. doi: 10.1002/(sici)1521-3773(19991203)38:23<3552::aid-anie3552>3.3.co;2-7. [DOI] [PubMed] [Google Scholar]
- 35.Sunder A, Heinemann J, Frey H. Controlling the growth of polymer trees: concepts and perspectives for hyperbranched polymers. Chemistry. 2000;6:2499–2506. doi: 10.1002/1521-3765(20000717)6:14<2499::aid-chem2499>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 36.Kramer M, Stumbe JF, Grimm G, Kaufmann B, Kruger U, Weber M. Dendritic polyamines: simple access to new materials with defined treelike structures for application in nonviral gene delivery. Chembiochem. 2004;5:1081–1087. doi: 10.1002/cbic.200300905. [DOI] [PubMed] [Google Scholar]
- 37.Toyota M, Suzuki H, Sasaki Y, Maruyama R, Imai K, Shinomura Y. Epigenetic silencing of microRNA-34b/c and B-cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer Res. 2008;68:4123–4132. doi: 10.1158/0008-5472.CAN-08-0325. [DOI] [PubMed] [Google Scholar]
- 38.Bae Y, Yang T, Zeng HC, Campeau PM, Chen Y, Bertin T. miRNA-34c regulates Notch signaling during bone development. Hum Mol Genet. 2012;21:2991–3000. doi: 10.1093/hmg/dds129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du R, Sun W, Xia L, Zhao A, Yu Y, Zhao L. Hypoxia-induced down-regulation of microRNA-34a promotes EMT by targeting the Notch signaling pathway in tubular epithelial cells. PLoS One. 2012;7:e30771. doi: 10.1371/journal.pone.0030771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 41.Cole KA, Attiyeh EF, Mosse YP, Laquaglia MJ, Diskin SJ, Brodeur GM. A functional screen identifies miR-34a as a candidate neuroblastoma tumor suppressor gene. Mol Cancer Res. 2008;6:735–742. doi: 10.1158/1541-7786.MCR-07-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merdan T, Callahan J, Petersen H, Kunath K, Bakowsky U, Kopeckova P. Pegylated polyethylenimine-Fab' antibody fragment conjugates for targeted gene delivery to human ovarian carcinoma cells. Bioconjug Chem. 2003;14:989–996. doi: 10.1021/bc0340767. [DOI] [PubMed] [Google Scholar]
- 43.Engelberg H. Plasma heparin levels. Correlation with serum cholesterol and low-density lipoproteins. Circulation. 1961;23:573–577. doi: 10.1161/01.cir.23.4.573. [DOI] [PubMed] [Google Scholar]
- 44.Huang Z, Yang Y, Jiang Y, Shao J, Sun X, Chen J. Anti-tumor immune responses of tumor-associated macrophages via toll-like receptor 4 triggered by cationic polymers. Biomaterials. 2013;34:746–755. doi: 10.1016/j.biomaterials.2012.09.062. [DOI] [PubMed] [Google Scholar]
- 45.Alifieris C, Trafalis DT. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol Ther. 2015 doi: 10.1016/j.pharmthera.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Bradley D, Rees J. Updates in the management of high-grade glioma. J Neurol. 2014;261:651–654. doi: 10.1007/s00415-013-7032-x. [DOI] [PubMed] [Google Scholar]
- 47.Tumilson CA, Lea RW, Alder JE, Shaw L. Circulating microRNA biomarkers for glioma and predicting response to therapy. Mol Neurobiol. 2014;50:545–558. doi: 10.1007/s12035-014-8679-8. [DOI] [PubMed] [Google Scholar]
- 48.Misso G, Di Martino MT, De Rosa G, Farooqi AA, Lombardi A, Campani V. Mir-34: a new weapon against cancer? Mol Ther Nucleic acids. 2014;3:e194. doi: 10.1038/mtna.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabernero J, Shapiro GI, LoRusso PM, Cervantes A, Schwartz GK, Weiss GJ. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–417. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]
- 50.Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. 2011;3:S7–S19. doi: 10.1177/1758834011422556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li B, He H, Tao BB, Zhao ZY, Hu GH, Luo C. Knockdown of CDK6 enhances glioma sensitivity to chemotherapy. Oncol Rep. 2012;28:909–914. doi: 10.3892/or.2012.1884. [DOI] [PubMed] [Google Scholar]
- 52.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 53.Stockhausen MT, Kristoffersen K, Poulsen HS. The functional role of Notch signaling in human gliomas. Neuro-Oncology. 2010;12:199–211. doi: 10.1093/neuonc/nop022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rehman AO, Wang CY. Notch signaling in the regulation of tumor angiogenesis. Trends Cell Biol. 2006;16:293–300. doi: 10.1016/j.tcb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Fischer I, Gagner JP, Law M, Newcomb EW, Zagzag D. Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol. 2005;15:297–310. doi: 10.1111/j.1750-3639.2005.tb00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693s–1700s. [PubMed] [Google Scholar]
- 57.Wick W, Wagner S, Kerkau S, Dichgans J, Tonn JC, Weller M. BCL-2 promotes migration and invasiveness of human glioma cells. FEBS Lett. 1998;440:419–424. doi: 10.1016/s0014-5793(98)01494-x. [DOI] [PubMed] [Google Scholar]
- 58.Satchi-Fainaro R, Mamluk R, Wang L, Short SM, Nagy JA, Feng D. Inhibition of vessel permeability by TNP-470 and its polymer conjugate, caplostatin. Cancer Cell. 2005;7:251–261. doi: 10.1016/j.ccr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Ebert MS, Sharp PA. MicroRNA sponges: progress and possibilities. RNA. 2010;16:2043–2050. doi: 10.1261/rna.2414110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.