Abstract
A hallmark of smooth muscle cells is their ability to adapt their functions to meet temporal and chronic fluctuations in their demands. These functions include force development and growth. Understanding the mechanisms underlying the functional plasticity of smooth muscles, the major constituent of organ walls, is fundamental to elucidating pathophysiological rationales of failures of organ functions. Also, the knowledge is expected to facilitate devising innovative strategies that more precisely monitor and normalize organ functions by targeting individual smooth muscles. Evidence has established a current paradigm that the myosin light chain phosphatase (MLCP) is a master regulator of smooth muscle responsiveness to stimuli. Cellular MLCP activity is negatively and positively regulated in response to G-protein activation and cAMP/cGMP production, respectively, through the MYPT1 regulatory subunit and an endogenous inhibitor protein named CPI-17. In this article we review the outcomes from two decade of research on the CPI-17 signaling and discuss emerging paradoxes in the view of signaling pathways regulating smooth muscle functions through MLCP.
Keywords: Artery, sympathetic regulation, G-protein, PKC, ROCK
Introduction
Almost every hollow organ wall is lined with smooth muscle cells that regulate organ functions by governing the motility and altering the thickness and elasticity. Unlike skeletal or cardiac muscle, smooth muscle possesses extensive functional diversity depending on the specific demands of different organs. The diversity of smooth muscle responsiveness is attributed to differences in electro-mechanical couplings (graded depolarization vs. action potentials) and pharmaco-mechanical couplings (variation in receptors and G-protein coupling) (1). In addition, accumulating lines of evidence suggest that every smooth muscle cell is capable of adapting the extent of the force development and the proliferation to meet functional demands. Plus, pathological cues, such as physical and chemical stresses, can trigger reprogramming of gene regulation in smooth muscle cells and lead to functional changes.
A better understanding of the mechanisms underlying the diversity and on-demand plasticity in smooth muscle functions is expected to facilitate the development of strategies for precision medicine, including more accurate diagnosis and effective treatment of diseases. Differences in the expression and functions of cytoskeletal proteins and many regulatory proteins, including ion channels, receptors, kinases, and phosphatases, help to further define smooth muscle characteristics. Accumulating lines of evidence strongly suggest that the myosin light chain phosphatase (MLCP) signaling contributes to regulating smooth muscle responsiveness. In this review paper, we summarize evidence that led to the current paradigm and discuss pathophysiological roles of the MLCP signaling based on our two-decade study of CPI-17, the master regulator of MLCP in smooth muscles.
Paradigms and Paradoxes in Ca2+-Sensitization Research
Discovery of Ca2+ sensitization/desensitization of smooth muscle contraction
Early studies on myosin regulatory light chain (MLC20) phosphorylation and smooth muscle contraction revealed a principal pathway for smooth muscle contraction–when Ca2+ binds to calmodulin, the MLC20 kinase (MLCK) phosphorylates MLC20 and induces smooth muscle contraction (1,2,3,4,5). Recent studies using MLCK knockout mouse support this MLCK paradigm, even though many kinases are reported to phosphorylate MLC20 (6, 7). These non-MLCK-type kinases possibly contribute to pathological dual phosphorylation of MLC20 (8,9,10).
Additional studies revealed that this excitation-contraction coupling is modified through the MLCP (11,12,13,14,15). In the earlier study, an augmentation in the Ca2+-induced force was detected in intact smooth muscle strips, in which aequorin was injected as an ectopic Ca2+ indicator (16). Studies using the membrane permeabilization technique with staphylococcus aureus α-toxin had broken through a barrier preventing access to the excitation-contraction coupling in smooth muscle and contributed to the precise determination of the Ca2+-force relationship. Since small molecules less than 1,000 Da, such as ions and nucleotides, can permeate though pores formed by the α-toxins, the intracellular [Ca2+] can be controlled without losing receptor-G-protein coupling and the downstream signaling proteins (17, 18). The outcomes revealed fluctuations in the Ca2+-force relationship in response to G-protein activation.
When G-protein-coupled receptors (GPCRs) or G-proteins in α-toxin-permeabilized smooth muscle strips were stimulated with agonist or GTP, the muscle strips contracted at a given submaximum [Ca2+] (17, 18). This G-protein-mediated force development causes an increase in MLC20 phosphorylation without changing the relationships between MLC20 phosphorylation and force in both phasic and tonic smooth muscles (19). The phenomenon, called Ca2+ sensitization, was also confirmed in intact smooth muscle strips from the transgenic mouse expressing a MLCK biosensor (20, 21). Ca2+ sensitization is a common feature of multiple types of smooth muscles, including artery, vein, urinary bladder and ileum. Notably, the extent of unhydrolyzable GTP (GTPγS)-induced Ca2+ sensitization is greater in tonic muscles compared to phasic muscles, whereas maximum contraction with high [Ca2+] is further enhanced by GTPγS in phasic but not in tonic muscle. Thus, the GTP signaling may be a factor that defines smooth muscle characteristics (19). The GPCR-induced Ca2+ sensitization occurs through MLCP inhibition but not MLCK activation (22). Mediators between GPCR activation and MLCP inhibition are thought to be PKC and RhoA/ROCK (1, 11,12,13), however the contribution of PKC to the Ca2+ sensitization was controversial due to mixed results from multiple smooth muscle cell types treated by different permeabilization techniques (23,24,25,26,27). For example, PKC more strongly contributes to G-protein-mediated Ca2+ sensitization in the order of femoral artery, portal vein, urinary bladder, and ileum (26, 28). Thus, the balance between PKC and ROCK can be a determinant of smooth muscle responsiveness.
In addition to Ca2+ sensitization, cGMP induces a decrease in the Ca2+ sensitivity of MLC20 phosphorylation in parallel with the relaxation (29, 30). Importantly, the Ca2+ desensitization occurs without changes in the relationship between MLC20 phosphorylation and force (30). This occurs when MLCP activity further activated and/or dis-inhibited (30). Thus, evidence clearly suggests that MLCP mediates multiple pathways into MLC20 phosphorylation and modulates the responsiveness of smooth muscle contraction.
It is also noteworthy that phasic, but not tonic, smooth muscle tissues have a unique function, Ca2+-induced Ca2+ desensitization–a stepwise increase in [Ca2+] triggers a burst of MLC phosphorylation and contraction, which is followed by spontaneous declines (31, 32). The data suggest that the Ca2+-induced Ca2+ desensitization occurs beyond a threshold of [Ca2+], although underlying mechanisms have yet to be understood.
The myosin light chain phosphatase (MLCP) in Ca2+ sensitization/desensitization
Multiple types of Ser/Thr phosphatases are expressed in smooth muscle. Pato et al. classified the Ser/Thr phosphatases in smooth muscle homogenates into four types, SMP-I to IV (33), with the dominant MLCP as SMP-IV. SMP-IV was later characterized as a heterotrimeric holoenzyme, consisting of a protein phosphatase-1 (PP1) catalytic subunit δ / β isoform associated with the regulatory subunit complex of MYPT1 (MYosin Phosphatase Target subunit 1), and M20 (an accessory subunit bound to MYPT1) (34) (Fig. 1, top left). Roles of other SMPs in smooth muscle functions have yet to be fully understood; these SMPs likely consist of PP2A/4/6 and PP2C (33).
Fig. 1.
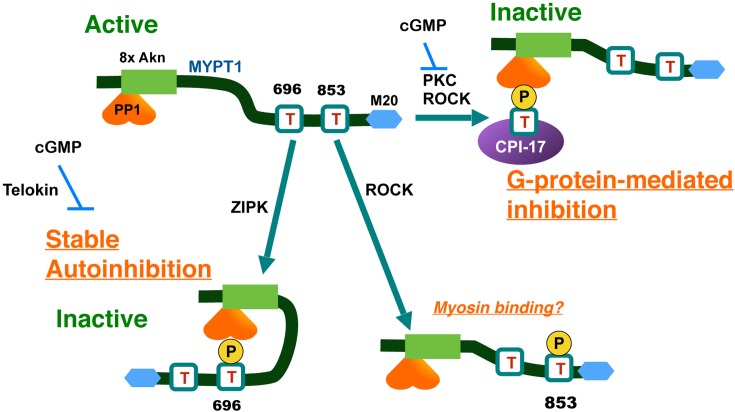
The molecular basis of the regulation of MLCP through phosphorylation of MYPT1 and CPI-17. Phosphorylation of MYPT1 at Thr696 spontaneously occurs and causes autoinhibition of MLCP by direct contact to the active site. Thr853 phosphorylation is elevated in response to ROCK activation, but it has no little effects on MLCP activity. In response to GPCR stimulation, PKC and ROCK phosphorylate CPI-17 at Thr38, and the phospho-Thr38 directly docks at the active site of MLCP and inhibits the activity. MYPT1, myosin phosphatase target subunit-1; M20, an accessory subunit of MLCP; CPI-17, PKC-activated PP1 inhibitor protein with Mr of 17 kDa; 8xAkn, 8 ankyrin repeats; PP1, type-1 Ser/Thr phosphatase catalytic subunit.
Because MYPT1 confers substrate specificity to PP1 through the allosteric interaction and compartmentalization to myosin filaments (35,36,37,38), it has drawn a major attention among researchers (13, 14). In 1995, Trinkle-Mulcahy et al. showed that stimulating permeabilized smooth muscle strips with GTPγS induces phosphorylation of MYPT1 in parallel with the force development and MLCP inhibition (39). Ichikawa et al. showed that the activity of MLCP purified from smooth muscle is reduced when MYPT1 at Thr696 is phosphorylated by a protein kinase copurified with MLCP (40). This MLCP associated kinase was later identified as a variant of ZIPK (Fig. 1) (41).
Independently, Kimura et al. reported that RhoA/ROCK induces MYPT1 phosphorylation and MLCP inhibition (42). Indeed, the purified ROCK phosphorylates MYPT1 at Thr696 and Thr853 (Fig. 1) (43). Unlike Thr696, ROCK is the only kinase that is reported to phosphorylate Thr853, and the Thr853 phosphorylation now serves as an indicator of cellular ROCK activity (14). These lines of evidence are the foundations for the current paradigm that the ROCK-MYPT1 signaling axis plays a critical role in the Ca2+ sensitization of smooth muscle and regulation of cell motility in other cell types.
An update on the MYPT1 phosphorylation paradigm
A large volume of studies have been conducted to improve understanding of MYPT1 phosphorylation at Thr696 and Thr853 in smooth muscles. The majority of reports agree that Thr696 phosphorylation is spontaneously high in resting smooth muscle tissues and is barely elevated in response to ROCK activation by any agonist stimuli (44,45,46). Therefore, it is unlikely that phosphorylation of MYPT1 at Thr696 is responsible for G-protein-mediated Ca2+ sensitization force of smooth muscle.
By contrast, Thr853 phosphorylation is significantly increased in response to agonist stimuli and decreased in response to treatment with ROCK antagonists (44,45,46). Thr853 phosphorylation of purified MLCP reduces affinity to myosin filaments (43), supporting the paradigm of the ROCK-MYPT1-mediated MLCP inhibition.
Further study using the reconstituted recombinant MLCP complex (47, 48) defined the roles of each phosphorylation of MYPT1 at Thr696 and Thr853 on MLCP activity. The recombinant MLCP activity is reduced to approximately 20% when it is phosphorylated with purified ROCK. When MYPT1 at Thr696 is substituted with Ala (T696A version), the ROCK phosphorylation no longer reduced the MLCP activity, indicating that Thr696 phosphorylation is necessary for the inhibition (Fig. 1, bottom, left). The phospho-Thr696 residue directly docks at the active site of PP1, causing autoinhibition (Fig. 1, bottom left) (48, 49). When the peptide mimicking MYPT1 Thr696 site is added to permeabilized smooth muscle strips, it is spontaneously phosphorylated in the tissues and inhibits the endogenous MLCP (49). In ileum, the phospho-Thr696-mediated MLCP autoinhibition is eliminated in response to cGMP treatment (49), possibly due to inference by telokin (Fig. 1, bottom left) (50). On the other hand, ROCK phosphorylation inhibits the activity of the recombinant MLCP with the MYPT1 T853A version and with the wild type MYPT1 (Fig. 1, bottom right) (47, 48). These data clearly show that, when MYPT1 is phosphorylated at Thr853, the effects on the MLCP activity are negligible (47, 48).
The phenotype of the transgenic mice agreed with the biochemical data of the recombinant MLCP (48, 51). When the MYPT1 T696A protein (lacking Thr696) in smooth muscle was replaced with the endogenous protein, the tonic component of force development and MLC phosphorylation was reduced, suggesting that loss of the spontaneous autoinhibition elevates MLCP activity. The expression of the MYPT1 T853A version does not cause noticeable changes of agonist-induced MLC phosphorylation and force development in bladder and ileum smooth muscles (51, 52). Therefore, MYPT1 Thr853 phosphorylation seems to be dispensable for the agonist-induced Ca2+ sensitization of smooth muscle, although it is elevated in response to ROCK activation.
Other pathways may regulate MLCP through MYPT1. Multiple MYPT1 residues, including the adjacent Ser695 and Ser852 residues, are phosphorylated in smooth muscle (53), some of which potentially play a role in MLCP regulation. PKG, MRIP and Par4 are also reported to bind to MYPT1 and regulate MLCP (54,55,56,57,58). Additionally, alternative splicing of MYPT1 has been linked to Ca2+ sensitization and PKG pathways and likely plays a role in the regulatory circuit (59, 60).
Importantly, conditional smooth muscle-specific ablation of MYPT1 gene augmented the sustained force of mesenteric artery strips, which is associated with higher blood pressure reading (61). MYPT1-knockout also caused the sustained force development of bladder and ileum strips (52, 62). Remarkably, sensitivity to PKC and ROCK inhibitors varies among smooth muscles. For example, both ROCK and PKC inhibitors can inhibit agonist-induced contraction and MLC20 phosphorylation in MYPT1-null artery strips, suggesting that both kinases can inhibit the endogenous phosphatase(s) for MLC20 even though MYPT1 is not expressed (61). Furthermore, NO-induced relaxation of the mesenteric artery strips from MYPT1-knockout mouse was not significantly different from control (61), suggesting alternative pathways that mediate cGMP elevation and smooth muscle relaxation. It appears that MYPT1 is dispensable in terms of both Ca2+-sensitization and desensitization of arterial smooth muscle. On the other hand, when MYPT1 gene was deleted, ileum strips became insensitive to PKC or ROCK inhibitor (52). A ROCK inhibitor, and to a lesser extent a PKC inhibitor, suppressed the contraction of MYPT1-null bladder strips (62). These data suggest that physiological contributions of MYPT1 to the regulation of contraction depend on the type of tissues, likely due to their demands. The whole picture of the ROCK-MYPT1 signaling pathways that regulate smooth muscle contraction remains unfinished and need to be re-evaluated.
Discovery and characterization of an endogenous inhibitor protein for MLCP
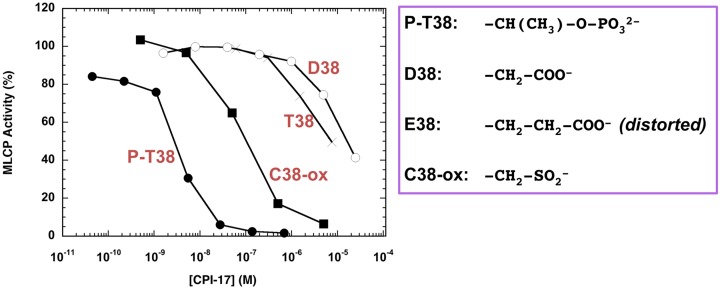
Another player that mediates G-protein activation into MLCP inhibition is CPI-17, a PKC-activated Phosphatase Inhibitor protein with a molecular weight of 17 kDa. CPI-17 was first identified in 1995 in pig aorta smooth muscle homogenates as an endogenous MLCP inhibitor protein that exhibits elevated potency upon phosphorylation, along with the endogenous kinase (63, 64). Later, PKC α and PKC δ were identified as the dominant kinases for CPI-17 in pig aorta homogenates (65). Phosphorylation and thio-phosphorylation of CPI-17 at Thr38 is sufficient and necessary to convert it into a potent inhibitor (P-T38 (phosphorylated) vs. T38 (unphosphorylated) in Fig. 2). By contrast, Asp substitution (D38 in Fig. 2) is insufficient to mimic the phosphorylation-dependent activation of CPI-17 (66). Meanwhile, Glu-substitution causes distortions in the structure due to instability of recombinant protein (66). Substitution with oxidized Cys (sulfinyl- or sulfonyl-Cys) also fails to fully potentiate CPI-17 (C38-ox in Fig. 2). The conformational change and the binding of CPI-17 to MLCP seem to rely on phospho-ester at Thr residue, in addition to negative charges.
Fig. 2.
Inhibition of MLCP by CPI-17 with oxidized Cys. MLCP assay was conducted in the presence of recombinant CPI-17 proteins as listed. The activity without CPI-17 was set as 100%, and the relative activity is shown. T38, unphosphorylated wild type; P-T38, phosphorylated at Thr38; D38, Asp substitution at the 38 position; C38-ox, oxidized Cys at the 38th position.
Although MYPT1 is ubiquitously expressed (67), the expression of CPI-17 is highly restricted in smooth muscles and neurons (28, 64, 68). The CPI-17 gene (PPP1R14A) is detected in most vertebrates (69), although it remains unknown whether the physiological roles are conserved among species. It has three homologues: PHI-1, KEPI, and GBPI (70,71,72). The primary structure around the inhibitory phosphorylation site of CPI-17 at Thr38 is highly conserved among the CPI-17 family, although CPI-17 and GBPI are more potent to MLCP than PHI-1 and KEPI (73). Compared with CPI-17, our knowledge of other family members is limited. The CPI-17 structure, which is likely conserved among the CPI-17 family based on the sequence similarity, consists of a four-helix bundle with intrinsically disordered N-terminal and C-terminal tail domains (66, 74, 75). Curiously, other PP1 inhibitors, such as inhibitor-1, inhibitor-2, DARPP32, and NIPP1, plus p21, p27, and other endogenous kinase inhibitors, are generally classified into intrinsically disordered proteins with molecular weight of around 20 kDa (76).
Selective regulation of cellular MLCP by CPI-17
Lines of evidence clearly show that CPI-17 is a master regulator of MLCP in smooth muscles (72, 76). When CPI-17 is phosphorylated, the phospho-Thr38 selectively docks at the active site of PP1 associated with MYPT1, with a Kd value at sub nM range, causing a potent inhibition (63, 77). The data of the in silico simulation and the in vitro binding assay suggest that phospho-CPI-17 directly contact a part of MYPT1 (75). Once phospho-CPI-17 binds to MLCP, it is slowly dephosphorylated and recycled (78, 79). Williams et al. found this type of inhibitory mechanism in PP2A-P-endosulphine system and named it “unfair competition inhibition” (80). The unfair competition model may also explain the autoinhibition of MLCP by Thr696 phosphorylation, because it is slowly auto-dephosphorylated (48).
In addition to MLCP, more than 100 other PP1 holoenzymes are predicted to exist in mammalian cells, and they efficiently dephosphorylate and inactivate CPI-17 (79). Recombinant CPI-17 effectively induces Ca2+ sensitization of MLC20 phosphorylation and force in permeabilized smooth muscle strips and cell culture, indicating that MLCP is inhibited in smooth muscle tissues (81). Thus, upon CPI-17 phosphorylation, MLCP is selectively regulated in smooth muscle cells (79).
The extent of Ca2+ sensitization force, defined as a force development at a constant [Ca2+], depends on the extent of CPI-17 in tissues. When the endogenous CPI-17 is washed away by the permeabilization with Triton-X100, phorbol ester-induced Ca2+ sensitization force is eliminated (82). Addition of recombinant CPI-17 to the CPI-17-null skinned tissues restores the Ca2+-sensitization force (82, 83). Ectopic expression of CPI-17 in intact cells or smooth muscles augments MLC phosphorylation and contraction and elevates blood pressure readings (84). Clearly, CPI-17 targets cellular MLCP and regulates smooth muscle contraction in smooth muscles.
Signaling pathways regulating CPI-17 phosphorylation and smooth muscle contraction/relaxation
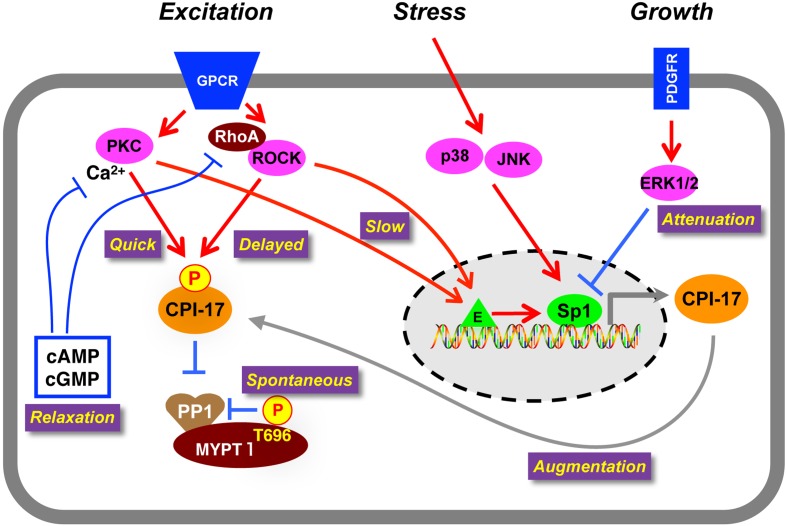
A large number of agonists induce CPI-17 phosphorylation in various smooth muscle tissues, according to published reports. Histamine-induced CPI-17 phosphorylation in rabbit femoral artery is partially reduced by treatment with GF109203x or Y27632, suggesting that both PKC and ROCK contribute to CPI-17 phosphorylation (85). Multi-parametric time-resolving analysis revealed that PKC and ROCK play distinguishable roles in Ca2+ sensitization signaling (Fig. 3). In artery, α1-adrenergic receptor (AR) activation triggers quick and sustained MLC phosphorylation and force development. When PKC is inhibited, phosphorylation of CPI-17 and MLC20 at initial phase is eliminated, resulting in a delayed force development (86). On the other hand, when ROCK is inhibited, phosphorylation of CPI-17 and MLC20 at the sustained phase is effectively reduced, causing phasic response (86). These results suggest that GPCR stimulus triggers a sequential activation of PKC and ROCK, each of which is responsible for the quick and sustained elevation of CPI-17 phosphorylation and Ca2+ sensitization.
Fig. 3.
On-demand regulation of smooth muscle responsiveness through the CPI-17-MLCP signaling. Spontaneous phosphorylation of MYPT1 at Thr696 sets the basal MLCP activity. GPCR stimulation induces a sequential activation of PKC followed by ROCK, resulting in a bi-phasic phosphorylation of CPI-17. Production of cAMP and cGMP causes inactivation of PKC and ROCK and consequent CPI-17 dephosphorylation. Stress-induced activation of JNK and p38MAPK augments CPI-17 expression through Sp1 at the gene promoter. The CPI-17 promoter is suppressed in response to growth factor stimulus, attenuating the Ca2+ sensitization signaling. PKC and ROCK are capable of activating CPI-17 expression, suggesting that chronic excitation of smooth muscle augments the responsiveness through the CPI-17 upregulation. Sp1: transcription factor specific protein 1, E: transcription enhancer(s).
Dephosphorylation of CPI-17 occurs with nitric oxide (NO) release and an increase in cAMP/cGMP (87,88,89). NO production triggers a rapid decline of CPI-17 phosphorylation in parallel with MLC dephosphorylation, preceding the relaxation. PKC activation with phorbol ester sustains CPI-17 phosphorylation and attenuates NO-induced relaxation of smooth muscle (89). Thus, NO-induced PKC inactivation followed by CPI-17 dephosphorylation is necessary for the quick relaxation of smooth muscle. In a possible cause for the PKC inactivation, NO production quickly reduces cytoplasmic Ca2+. This reduction in Ca2+ is followed by a slow ROCK inactivation, suggesting that PKC is a quick regulator of CPI-17 phosphorylation and dephosphorylation, and ROCK is a sustained regulator of CPI-17 phosphorylation and dephosphorylation (86, 89). The ratio of PKC and ROCK may partly define the responsiveness of each smooth muscle, and the contribution depends on the size of arteries (see below) (90). Notably, the bi-phasic action of PKC and ROCK potentially biases the way pharmacological data are interpreted, since inhibition of PKC is effective to initial phases upon stimulus while ROCK is effective to sustained phases upon stimulus.
Importantly, CPI-17 phosphorylation occurs prior to or at least in parallel with MLC20 phosphorylation (86). Also, CPI-17 dephosphorylation precedes a decrease in MLC20 phosphorylation upon NO production (89). Therefore, the CPI-17 phosphorylation signaling can satisfy a requirement being an upstream regulator of MLC20 phosphorylation (86, 89). In contrast, any changes in ROCK-induced MYPT1 phosphorylation are much slower than that of MLC phosphorylation in either case of α1-AR agonist or NO stimulation, suggesting that MYPT1 phosphorylation may contribute to the chronic regulation of MLC20 phosphorylation (86, 89).
In addition to PKC and ROCK, multiple kinases, such as ZIPK, ILK, and PAK, are capable of phosphorylating recombinant CPI-17 at Thr38 (91,92,93). Roles of these potential kinases in phosphorylation of CPI-17 in smooth muscles remains to be fully understood, although the contributions of ZIPK to CPI-17 phosphorylation may be limited (94, 95).
CPI-17 is a determinant of the smooth muscle responsiveness to agonist stimulation
The expression level of CPI-17 in smooth muscles is linked to individual contractile characteristics. It is higher in tonic muscles, such as artery and trachea, and lower in phasic muscles, such as urinary bladder and vas deferens (28).
CPI-17 expression also positively correlates with the extent of phorbol ester-induced Ca2+ sensitization force (28). We also learned about this correlation when we found that CPI-17 is not expressed in smooth muscles in farm chickens (83). In chicken mesenteric artery strips that do not express CPI-17, but the levels of RhoA, ROCK, PKC and MYPT1 are comparable with those in rabbit artery (83). Stimulation with agonists, such as endothelin-1, phenylephrine, and phorbol ester, induces a subtle Ca2+ sensitization force, but in rabbit and pigeon arteries that do express CPI-17, these agonists induce a robust contraction (83). The higher Ca2+ sensitivity of chicken smooth muscle compared to rabbit and pigeon smooth muscle suggests that the loss of CPI-17 is compensated through unidentified mechanisms (83). Notably, GTPγS induces a partial Ca2+ sensitization of chicken smooth muscle, suggesting minor pathways independent from CPI-17; it is unclear whether MYPT1 Thr696 phosphorylation is involved in the GTPγS-induced Ca2+ sensitization of chicken smooth muscle (83). Interestingly, a recent database search revealed that a CPI-17 gene is not detected in fowls, a phylogenetic group of birds, that includes chicken, turkey, and duck, and it remains unclear how this loss of CPI-17 impacts on the biological characteristics of these birds.
In each smooth muscle cell, CPI-17 expression fluctuates depending on physiological and pathological conditions (reviewed in (76)). In artery, CPI-17 expression is upregulated during embryonic development and downregulated in response to pathological dedifferentiation due to vascular injury (96). Up- and down-regulations of CPI-17 levels have been linked to hyper- and hypo-responsiveness of smooth muscle contraction, such as pulmonary hypertension, asthma, inflammatory bowel disease, urinary incontinence, and sepsis (76, 97). Clearly CPI-17 is a functional marker of smooth muscle contraction/relaxation.
Myocardin, a transcriptional co-factor activating the serum response factor, has been identified as a driver of smooth muscle-specific gene expression (98, 99), although it plays negligible roles in CPI-17 gene expression (100). Instead, the CPI-17 gene promoter is governed by Sp1 binding to multiple GC boxes at the 5′-flanking region; these boxes are assisted by adjacent GATA binding (Fig. 3) (100). The Sp1 binding is regulated through multiple kinase pathways. Augmentation and attenuation of the Sp1 binding occurs in response to the activation of PKC/ROCK/p38MAPK/JNK, leading to up-regulation of CPI-17, and to the activation of ERK, leading to down-regulation of CPI-17 (Fig. 3) (100). Of particular note, the PKC/ROCK-induced upregulation of CPI-17 expression may contribute to augmentation of the Ca2+ sensitization force under high demand for smooth muscles (Fig. 3) (100).
The view of regulatory circuits for CPI-17 expression is not yet complete, because mechanisms selectively activating CPI-17 promoter in smooth muscle by a ubiquitous Sp1 transcription factor remains to be established. Genetic analyses predict additional regulatory elements at distal regions and in introns (69, 101), which may contribute to the pathophysiological regulation of CPI-17 gene.
Roles of the CPI-17-MLCP signaling axis in the sympathetic regulation of vascular tones
CPI-17 expression depends on the location of arteries: expression is higher in smaller resistance vessels compared with larger ones, such as aorta (90, 102). On the other hand, there is no significant difference in PKCα expression between mesenteric artery and aorta (90). Importantly, sensitivity of CPI-17 phosphorylation to α1-AR agonist stimulus depends on the size of arteries; phenylephrine stimulation induces greater CPI-17 phosphorylation in mesenteric artery compared to aorta smooth muscle (90). CPI-17 phosphorylation responds to α1A-AR stimulation that is dominant in mesentery artery (90), which is highly innervated with sympathetic nerves. PKC inhibitors potently suppress α1A-AR-induced contraction of mesenteric artery and phosphorylation of MLC20 and CPI-17, whereas ROCK plays a lesser role (90). On the other hand, α1D-AR stimulation is dominant in aorta smooth muscle, where α1-AR agonist stimulates an increase in [Ca2+] through Ca2+ release and Ca2+ influx but not the PKC-CPI-17 signaling pathway (90). Although almost all regulatory proteins are expressed in any sizes of arteries, the responsiveness of these smooth muscle are clearly distinguishable. The heterogeneity in the CPI-17 signaling pathways warrants further investigation.
Another note: there is a bias in Ca2+ signaling that induces CPI-17 phosphorylation (86, 90). In arteries, the Ca2+ release from SR plays a major role in the rapid increase in CPI-17 phosphorylation in response to α1-AR stimulation (86, 90). The depletion of the SR Ca2+ store using ryanodine, to a lesser extent the inhibition of Ca2+ influx, suppressed phenylephrine-induced CPI-17 phosphorylation (86, 90). These results revealed a sequence of the signaling pathways in small arteries; the α1-AR stimulation induces 1) Ca2+ release from the SR through IP3 production, 2) a rapid activation of Ca2+-dependent PKC, 3) CPI-17 phosphorylation and 4) MLCP inhibition, which occurs in parallel with Ca2+-induced MLCK activation. We propose this Ca2+ sensitization pathway; “Ca2+-dependent Ca2+ sensitization” of MLC phosphorylation and contraction. The Ca2+-induced Ca2+ sensitization pathway augments the initial force development in small arteries. Additional pathways, such as ROCK-mediated Ca2+-independent Ca2+ sensitization, maintain the tonic force (86). Strangely, Ca2+-induced Ca2+ sensitization is not operated in large aorta smooth muscle, although agonist-induced SR Ca2+ release exists (90). We must continue to characterize each pathway for fully elucidating the mechanisms that regulate organ motility and functions, such as the regulatory systems for circulation.
The α1A-AR-induced Ca2+-induced Ca2+ sensitization pathway is likely responsible for the sympathetic regulation of the blood pressure. The concept is supported by findings that blood pressure in the transgenic mouse elevates in response to the ectopic expression of CPI-17 via the smooth-muscle actin promoter (84). Recent data suggest that pathological orthostatic hypotension, in which α1A-AR-induced CPI-17 phosphorylation is diminished, occurs in response to a deficiency in the CPI-17-MLCP signaling pathway (Kitazawa & Kitazawa, manuscript in preparation).
New dimensions in the CPI-17-MLCP signaling axis regulating smooth muscle functions
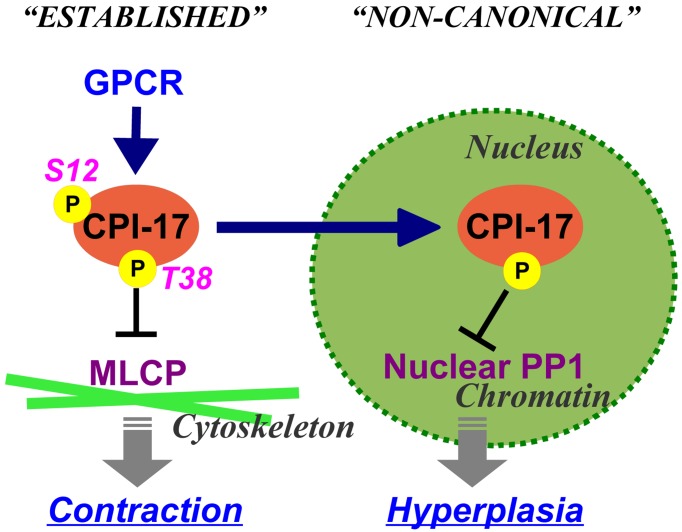
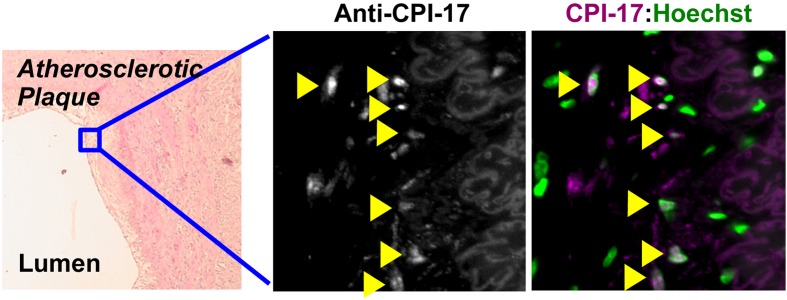
CPI-17 functions are governed not only by phosphorylation and expression, but also by cellular distribution (Fig. 4). CPI-17 is a 17-kDa soluble protein and is distributed in the cytoplasm of mature smooth muscle. In a rat vascular injury model and in human atherosclerotic plaques, on the other hand, it accumulates in nuclei of neointimal cells (Fig. 5) (103). CPI-17 consistently concentrates in nuclei of the smooth muscle cell culture under growth conditions (103). In addition, CPI-17 is expressed in a subset of cancer cells, and accumulates into the nucleus (103).
Fig. 4.
Functional pleiotropy of CPI-17 due to subcellular distribution. CPI-17 is accumulated in the nucleus of proliferating smooth muscle and cancer cells. The nuclear CPI-17 regulates phosphorylation of histone(s) and MEF2C, but not canonical MLCP substrates, such as myosin light chain and ezrin/moecin/radixin. A nuclear localization signal exists at the N-terminal tail of CPI-17. Phosphorylation of CPI-17 at Ser12 interferes the nuclear accumulation.
Fig. 5.
CPI-17 is accumulated in nuclei of neointimal cells at atherosclerotic plaques. Paraffin-embedded human atherosclerotic arterial sections (OriGene #CS808380) were subjected to indirect fluorescence immunostaining using anti-CPI-17 antibody, followed by confocal microscopy. Left: haematoxylin and eosin stain; middle: anti-CPI-17 stain; right: overlay of anti-CPI-17 stain and Hoechst stain. Arrowheads indicate nuclear accumulation of CPI-17.
An unconventional nuclear localization signal exists at the N-terminal tail of CPI-17 (103). Ser12 within the domain can be phosphorylated (63). Phospho-mimetic Asp-substitution at Ser12 interferes the nuclear accumulation of CPI-17 (103). Consistently, immunostaining revealed a mild exclusion of phospho-Ser12-CPI-17 out of the nucleus, suggesting that Ser12 phosphorylation plays a role in the subcellular distribution of CPI-17 (103).
What is the function(s) of the nuclear CPI-17? An active nuclear localization signal also exists in MYPT1, although PP1 binding inactivates it and interferes with MLCP nuclear accumulation (104, 105). CPI-17 gene silencing resulted in elevations in phosphorylation of histone(s) and MEF2C, but not the substrates of MLCP (103, 106). Clearly, nuclear CPI-17 inhibits a group of PP1 responsible for histone phosphorylation, but not MLCP, and likely regulates chromatin functions (Fig. 4). CPI-17 knockdown suppressed the proliferation of cancer cells (103). Based on these results, it appears that pathological cues redistribute CPI-17 into nuclei and positively regulates cell proliferation, independently from the MLCP signaling (Fig. 4). On the other hand, there are lines of evidence that CPI-17 activates Ras GTPase through MLCP inhibition and consequent merlin phosphorylation in cancer cells (107, 108). A remnant fraction of the cytoplasmic CPI-17 may suffice to augment Ras signaling. CPI-17 pathways in cancer cells need further investigation.
The functional pleiotropy of CPI-17 may explain why CPI-17 signaling is silenced in the larger arteries, smooth muscle from genetically hypertensive rats and from other organs and models (90, 109,110,111,112). The multi-functionality may be a common feature of the endogenous regulator proteins for kinases/phosphatases, such as DARPP32, phosphatase inhibitor-1, inhibitor-2, a Raf kinase inhibitor protein (RKIP), and cyclin-dependent kinase inhibitors (p21CIP/WAF, p27KIP1, and p57) (113,114,115,116,117,118,119,120,121,122,123). For example, a CDK inhibitor p27KIP1 is capable of inhibiting RhoA and p21CIP/WAF is capable of inhibiting ROCK; both functions link the cell cycle signaling and the cytoskeletal reorganization (123,124,125,126,127). DARPP32 is a cytoplasmic phosphatase inhibitor enriched in neurons, and it also inhibits PKA depending on the phosphorylation state (113). Further study is needed for establish the pathological pleiotropy of CPI-17 in smooth muscles and beyond.
Conclusion
“If you cannot explain it simply, you do not understand it well enough.”–Albert Einstein
Our accumulated knowledge about the molecular basis of Ca2+ sensitization signaling has helped resolve mysteries in the excitation-contraction coupling of smooth muscle. It becomes evident that wide diversity exists in the signaling pathways regulating Ca2+ sensitivity of MLC20 phosphorylation. The discovery of CPI-17 has provided new insights into the mechanisms underlying the pharmaco-mechanical coupling in smooth muscle contraction. In particular, we now know that differences in CPI-17 expression depend on the location and types of smooth muscle and on species. These differences in expression likely contribute to different contributions of PKC to the excitation-contraction coupling, helping settled controversy over the role of PKC in smooth muscle.
As we gain deeper insights into the Ca2+ sensitization signaling, new mysteries emerge. For example; what is the mechanisms underlying ROCK-induced MLCP inhibition? Also, how and why does CPI-17 signaling appear to be disconnected from G-protein activation in some types of smooth muscles or under specific conditions? Before closing the discussion of the smooth muscle signaling pathways, we have to highlight a potential caveat–that our current knowledge fully relies on our trust in the specificity of antibodies. To better understand smooth muscle regulation, we must devise new technology and methodology that quantitatively determine real-time phosphorylation and localization of proteins in cells. By understanding the diversity and plasticity of the signaling pathways regulating smooth muscle functions, we will be able to reach to a true form of precision medicine, in which each smooth muscle disorder will be treated effectively and specifically.
Conflict of Interest
Research material license agreement of products in EMD Millipore (M.E.)
Acknowledgements
Special thanks for the support and encouragement from Professor Fumi Morita, Hokkaido University, who passed.
References
- 1.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994; 372(6503): 231–6. doi: 10.1038/372231a0 [DOI] [PubMed] [Google Scholar]
- 2.Dabrowska R, Aromatorio D, Sherry JM, Hartshorne DJ. Composition of the myosin light chain kinase from chicken gizzard. Biochem Biophys Res Commun. 1977; 78(4): 1263–72. doi: 10.1016/0006-291X(77)91429-2 [DOI] [PubMed] [Google Scholar]
- 3.Yazawa M, Kuwayama H, Yagi K. Modulator protein as a Ca2+-dependent activator of rabbit skeletal myosin light-chain kinase. Purification and characterization. J Biochem. 1978; 84(5): 1253–8. [DOI] [PubMed] [Google Scholar]
- 4.Dillon PF, Aksoy MO, Driska SP, Murphy RA. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science. 1981; 211(4481): 495–7. doi: 10.1126/science.6893872 [DOI] [PubMed] [Google Scholar]
- 5.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001; 276(7): 4527–30. doi: 10.1074/jbc.R000028200 [DOI] [PubMed] [Google Scholar]
- 6.He WQ, Peng YJ, Zhang WC, Lv N, Tang J, Chen C, Zhang CH, Gao S, Chen HQ, Zhi G, Feil R, Kamm KE, Stull JT, Gao X, Zhu MS. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology. 2008; 135(2): 610–20. doi: 10.1053/j.gastro.2008.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He WQ, Qiao YN, Zhang CH, Peng YJ, Chen C, Wang P, Gao YQ, Chen C, Chen X, Tao T, Su XH, Li CJ, Kamm KE, Stull JT, Zhu MS. Role of myosin light chain kinase in regulation of basal blood pressure and maintenance of salt-induced hypertension. Am J Physiol Heart Circ Physiol. 2011; 301(2): H584–91. doi: 10.1152/ajpheart.01212.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh MP. Vascular smooth muscle myosin light chain diphosphorylation: mechanism, function, and pathological implications. IUBMB Life. 2011; 63(11): 987–1000. doi: 10.1002/iub.527 [DOI] [PubMed] [Google Scholar]
- 9.Takeya K. Highly sensitive myosin phosphorylation analysis in the renal afferent arteriole. J Smooth Muscle Res. 2016; 52: 45–55. doi: 10.1540/jsmr.52.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeya K, Wang X, Sutherland C, Kathol I, Loutzenhiser K, Loutzenhiser RD, Walsh MP. Involvement of myosin regulatory light chain diphosphorylation in sustained vasoconstriction under pathophysiological conditions. J Smooth Muscle Res. 2014; 50: 18–28. doi: 10.1540/jsmr.50.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfitzer G. Invited review: regulation of myosin phosphorylation in smooth muscle. J Appl Physiol 1985. 2001; 91(1): 497–503. [DOI] [PubMed] [Google Scholar]
- 12.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003; 83(4): 1325–58. doi: 10.1152/physrev.00023.2003 [DOI] [PubMed] [Google Scholar]
- 13.Hartshorne DJ, Ito M, Erdödi F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J Biol Chem. 2004; 279(36): 37211–4. doi: 10.1074/jbc.R400018200 [DOI] [PubMed] [Google Scholar]
- 14.Grassie ME, Moffat LD, Walsh MP, MacDonald JA. The myosin phosphatase targeting protein (MYPT) family: a regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1δ. Arch Biochem Biophys. 2011; 510(2): 147–59. doi: 10.1016/j.abb.2011.01.018 [DOI] [PubMed] [Google Scholar]
- 15.Perrino BA. Calcium Sensitization Mechanisms in Gastrointestinal Smooth Muscles. J Neurogastroenterol Motil. 2016; 22(2): 213–25. doi: 10.5056/jnm15186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan JP, Morgan KG. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984; 351(1): 155–67. doi: 10.1113/jphysiol.1984.sp015239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura J, Kolber M, van Breemen C. Norepinephrine and GTP-γ-S increase myofilament Ca2+ sensitivity in α-toxin permeabilized arterial smooth muscle. Biochem Biophys Res Commun. 1988; 157(2): 677–83. doi: 10.1016/S0006-291X(88)80303-6 [DOI] [PubMed] [Google Scholar]
- 18.Kitazawa T, Kobayashi S, Horiuti K, Somlyo AV, Somlyo AP. Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem. 1989; 264(10): 5339–42. [PubMed] [Google Scholar]
- 19.Kitazawa T, Gaylinn BD, Denney GH, Somlyo AP. G-protein-mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1991; 266(3): 1708–15. [PubMed] [Google Scholar]
- 20.Isotani E, Zhi G, Lau KS, Huang J, Mizuno Y, Persechini A, Geguchadze R, Kamm KE, Stull JT. Real-time evaluation of myosin light chain kinase activation in smooth muscle tissues from a transgenic calmodulin-biosensor mouse. Proc Natl Acad Sci USA. 2004; 101(16): 6279–84. doi: 10.1073/pnas.0308742101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizuno Y, Isotani E, Huang J, Ding H, Stull JT, Kamm KE. Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. Am J Physiol Cell Physiol. 2008; 295(2): C358–64. doi: 10.1152/ajpcell.90645.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitazawa T, Masuo M, Somlyo AP. G protein-mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proc Natl Acad Sci USA. 1991; 88(20): 9307–10. doi: 10.1073/pnas.88.20.9307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masuo M, Reardon S, Ikebe M, Kitazawa T. A novel mechanism for the Ca(2+)-sensitizing effect of protein kinase C on vascular smooth muscle: inhibition of myosin light chain phosphatase. J Gen Physiol. 1994; 104(2): 265–86. doi: 10.1085/jgp.104.2.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida M, Suzuki A, Itoh T. Mechanisms of vasoconstriction induced by endothelin-1 in smooth muscle of rabbit mesenteric artery. J Physiol. 1994; 477(Pt 2): 253–65. doi: 10.1113/jphysiol.1994.sp020188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horowitz A, Clément-Chomienne O, Walsh MP, Morgan KG. Epsilon-isoenzyme of protein kinase C induces a Ca(2+)-independent contraction in vascular smooth muscle. Am J Physiol. 1996; 271(2 Pt 1): C589–94. [DOI] [PubMed] [Google Scholar]
- 26.Jensen PE, Gong MC, Somlyo AV, Somlyo AP. Separate upstream and convergent downstream pathways of G-protein- and phorbol ester-mediated Ca2+ sensitization of myosin light chain phosphorylation in smooth muscle. Biochem J. 1996; 318(Pt 2): 469–75. doi: 10.1042/bj3180469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker LA, Gailly P, Jensen PE, Somlyo AV, Somlyo AP. The unimportance of being (protein kinase C) epsilon. FASEB J. 1998; 12(10): 813–21. [DOI] [PubMed] [Google Scholar]
- 28.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca(2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol. 2001; 535(Pt 2): 553–64. doi: 10.1111/j.1469-7793.2001.t01-1-00553.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu X, Somlyo AV, Somlyo AP. Cyclic GMP-dependent stimulation reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphate. Biochem Biophys Res Commun. 1996; 220(3): 658–63. doi: 10.1006/bbrc.1996.0460 [DOI] [PubMed] [Google Scholar]
- 30.Lee MR, Li L, Kitazawa T. Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J Biol Chem. 1997; 272(8): 5063–8. doi: 10.1074/jbc.272.8.5063 [DOI] [PubMed] [Google Scholar]
- 31.Kitazawa T, Somlyo AP. Modulation of Ca2+ sensitivity by agonists in smooth muscle. Adv Exp Med Biol. 1991; 304: 97–109. doi: 10.1007/978-1-4684-6003-2_10 [DOI] [PubMed] [Google Scholar]
- 32.Murahashi T, Fujita A, Kitazawa T. Ca2+ -induced Ca2+ desensitization of myosin light chain phosphorylation and contraction in phasic smooth muscle. Mol Cell Biochem. 1999; 190(1–2): 91–8. doi: 10.1023/A:1006981302514 [DOI] [PubMed] [Google Scholar]
- 33.Pato MD, Adelstein RS. Purification and characterization of a multisubunit phosphatase from turkey gizzard smooth muscle. The effect of calmodulin binding to myosin light chain kinase on dephosphorylation. J Biol Chem. 1983; 258(11): 7047–54. [PubMed] [Google Scholar]
- 34.Alessi D, MacDougall LK, Sola MM, Ikebe M, Cohen P. The control of protein phosphatase-1 by targetting subunits. The major myosin phosphatase in avian smooth muscle is a novel form of protein phosphatase-1. Eur J Biochem. 1992; 210(3): 1023–35. doi: 10.1111/j.1432-1033.1992.tb17508.x [DOI] [PubMed] [Google Scholar]
- 35.Shimizu H, Ito M, Miyahara M, Ichikawa K, Okubo S, Konishi T, Naka M, Tanaka T, Hirano K, Hartshorne DJ, Nakano T. Characterization of the myosin-binding subunit of smooth muscle myosin phosphatase. J Biol Chem. 1994; 269(48): 30407–11. [PubMed] [Google Scholar]
- 36.Chen YH, Chen MX, Alessi DR, Campbell DG, Shanahan C, Cohen P, Cohen PT. Molecular cloning of cDNA encoding the 110 kDa and 21 kDa regulatory subunits of smooth muscle protein phosphatase 1M. FEBS Lett. 1994; 356(1): 51–5. doi: 10.1016/0014-5793(94)01231-8 [DOI] [PubMed] [Google Scholar]
- 37.Shirazi A, Iizuka K, Fadden P, Mosse C, Somlyo AP, Somlyo AV, Haystead TA. Purification and characterization of the mammalian myosin light chain phosphatase holoenzyme. The differential effects of the holoenzyme and its subunits on smooth muscle. J Biol Chem. 1994; 269(50): 31598–606. [PubMed] [Google Scholar]
- 38.Hirano K, Phan BC, Hartshorne DJ. Interactions of the subunits of smooth muscle myosin phosphatase. J Biol Chem. 1997; 272(6): 3683–8. doi: 10.1074/jbc.272.6.3683 [DOI] [PubMed] [Google Scholar]
- 39.Trinkle-Mulcahy L, Ichikawa K, Hartshorne DJ, Siegman MJ, Butler TM. Thiophosphorylation of the 130-kDa subunit is associated with a decreased activity of myosin light chain phosphatase in α-toxin-permeabilized smooth muscle. J Biol Chem. 1995; 270(31): 18191–4. doi: 10.1074/jbc.270.31.18191 [DOI] [PubMed] [Google Scholar]
- 40.Ichikawa K, Ito M, Hartshorne DJ. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity. J Biol Chem. 1996; 271(9): 4733–40. doi: 10.1074/jbc.271.9.4733 [DOI] [PubMed] [Google Scholar]
- 41.MacDonald JA, Borman MA, Murányi A, Somlyo AV, Hartshorne DJ, Haystead TA. Identification of the endogenous smooth muscle myosin phosphatase-associated kinase. Proc Natl Acad Sci USA. 2001; 98(5): 2419–24. doi: 10.1073/pnas.041331498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 1996; 273(5272): 245–8. doi: 10.1126/science.273.5272.245 [DOI] [PubMed] [Google Scholar]
- 43.Velasco G, Armstrong C, Morrice N, Frame S, Cohen P. Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1M at Thr850 induces its dissociation from myosin. FEBS Lett. 2002; 527(1-3): 101–4. doi: 10.1016/S0014-5793(02)03175-7 [DOI] [PubMed] [Google Scholar]
- 44.Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol. 2003; 546(Pt 3): 879–89. doi: 10.1113/jphysiol.2002.029306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niiro N, Koga Y, Ikebe M. Agonist-induced changes in the phosphorylation of the myosin- binding subunit of myosin light chain phosphatase and CPI17, two regulatory factors of myosin light chain phosphatase, in smooth muscle. Biochem J. 2003; 369(Pt 1): 117–28. doi: 10.1042/bj20021040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson DP, Susnjar M, Kiss E, Sutherland C, Walsh MP. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochem J. 2005; 389(Pt 3): 763–74. doi: 10.1042/BJ20050237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999; 274(52): 37385–90. doi: 10.1074/jbc.274.52.37385 [DOI] [PubMed] [Google Scholar]
- 48.Khasnis M, Nakatomi A, Gumpper K, Eto M. Reconstituted human myosin light chain phosphatase reveals distinct roles of two inhibitory phosphorylation sites of the regulatory subunit, MYPT1. Biochemistry. 2014; 53(16): 2701–9. doi: 10.1021/bi5001728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khromov A, Choudhury N, Stevenson AS, Somlyo AV, Eto M. Phosphorylation-dependent autoinhibition of myosin light chain phosphatase accounts for Ca2+ sensitization force of smooth muscle contraction. J Biol Chem. 2009; 284(32): 21569–79. doi: 10.1074/jbc.M109.019729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khromov AS, Momotani K, Jin L, Artamonov MV, Shannon J, Eto M, Somlyo AV. Molecular mechanism of telokin-mediated disinhibition of myosin light chain phosphatase and cAMP/cGMP-induced relaxation of gastrointestinal smooth muscle. J Biol Chem. 2012; 287(25): 20975–85. doi: 10.1074/jbc.M112.341479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen CP, Chen X, Qiao YN, Wang P, He WQ, Zhang CH, Zhao W, Gao YQ, Chen C, Tao T, Sun J, Wang Y, Gao N, Kamm KE, Stull JT, Zhu MS. In vivo roles for myosin phosphatase targeting subunit-1 phosphorylation sites T694 and T852 in bladder smooth muscle contraction. J Physiol. 2015; 593(3): 681–700. doi: 10.1113/jphysiol.2014.283853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao N, Chang AN, He W, Chen CP, Qiao YN, Zhu M, Kamm KE, Stull JT. Physiological signalling to myosin phosphatase targeting subunit-1 phosphorylation in ileal smooth muscle. J Physiol. 2016; 594(12): 3209–25. doi: 10.1113/JP271703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grassie ME, Sutherland C, Ulke-Lemée A, Chappellaz M, Kiss E, Walsh MP, MacDonald JA. Cross-talk between Rho-associated kinase and cyclic nucleotide-dependent kinase signaling pathways in the regulation of smooth muscle myosin light chain phosphatase. J Biol Chem. 2012; 287(43): 36356–69. doi: 10.1074/jbc.M112.398479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha. Science. 1999; 286(5444): 1583–7. doi: 10.1126/science.286.5444.1583 [DOI] [PubMed] [Google Scholar]
- 55.Michael SK, Surks HK, Wang Y, Zhu Y, Blanton R, Jamnongjit M, Aronovitz M, Baur W, Ohtani K, Wilkerson MK, Bonev AD, Nelson MT, Karas RH, Mendelsohn ME. High blood pressure arising from a defect in vascular function. Proc Natl Acad Sci USA. 2008; 105(18): 6702–7. doi: 10.1073/pnas.0802128105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Surks HK, Richards CT, Mendelsohn ME. Myosin phosphatase-Rho interacting protein. A new member of the myosin phosphatase complex that directly binds RhoA. J Biol Chem. 2003; 278(51): 51484–93. doi: 10.1074/jbc.M305622200 [DOI] [PubMed] [Google Scholar]
- 57.Koga Y, Ikebe M. p116Rip decreases myosin II phosphorylation by activating myosin light chain phosphatase and by inactivating RhoA. J Biol Chem. 2005; 280(6): 4983–91. doi: 10.1074/jbc.M410909200 [DOI] [PubMed] [Google Scholar]
- 58.Vetterkind S, Lee E, Sundberg E, Poythress RH, Tao TC, Preuss U, Morgan KG. Par-4: a new activator of myosin phosphatase. Mol Biol Cell. 2010; 21(7): 1214–24. doi: 10.1091/mbc.E09-08-0711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richards CT, Ogut O, Brozovich FV. Agonist-induced force enhancement: the role of isoforms and phosphorylation of the myosin-targeting subunit of myosin light chain phosphatase. J Biol Chem. 2002; 277(6): 4422–7. doi: 10.1074/jbc.M111047200 [DOI] [PubMed] [Google Scholar]
- 60.Khatri JJ, Joyce KM, Brozovich FV, Fisher SA. Role of myosin phosphatase isoforms in cGMP-mediated smooth muscle relaxation. J Biol Chem. 2001; 276(40): 37250–7. doi: 10.1074/jbc.M105275200 [DOI] [PubMed] [Google Scholar]
- 61.Qiao YN, He WQ, Chen CP, Zhang CH, Zhao W, Wang P, Zhang L, Wu YZ, Yang X, Peng YJ, Gao JM, Kamm KE, Stull JT, Zhu MS. Myosin phosphatase target subunit 1 (MYPT1) regulates the contraction and relaxation of vascular smooth muscle and maintains blood pressure. J Biol Chem. 2014; 289(32): 22512–23. doi: 10.1074/jbc.M113.525444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai MH, Chang AN, Huang J, He W, Sweeney HL, Zhu M, Kamm KE, Stull JT. Constitutive phosphorylation of myosin phosphatase targeting subunit-1 in smooth muscle. J Physiol. 2014; 592(14): 3031–51. doi: 10.1113/jphysiol.2014.273011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. J Biochem. 1995; 118(6): 1104–7. [DOI] [PubMed] [Google Scholar]
- 64.Eto M, Senba S, Morita F, Yazawa M. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI17) in smooth muscle: its specific localization in smooth muscle. FEBS Lett. 1997; 410(2-3): 356–60. doi: 10.1016/S0014-5793(97)00657-1 [DOI] [PubMed] [Google Scholar]
- 65.Eto M, Kitazawa T, Yazawa M, Mukai H, Ono Y, Brautigan DL. Histamine-induced vasoconstriction involves phosphorylation of a specific inhibitor protein for myosin phosphatase by protein kinase C alpha and delta isoforms. J Biol Chem. 2001; 276(31): 29072–8. doi: 10.1074/jbc.M103206200 [DOI] [PubMed] [Google Scholar]
- 66.Ohki S, Eto M, Shimizu M, Takada R, Brautigan DL, Kainosho M. Distinctive solution conformation of phosphatase inhibitor CPI-17 substituted with aspartate at the phosphorylation-site threonine residue. J Mol Biol. 2003; 326(5): 1539–47. doi: 10.1016/S0022-2836(03)00048-2 [DOI] [PubMed] [Google Scholar]
- 67.Okubo S, Ito M, Takashiba Y, Ichikawa K, Miyahara M, Shimizu H, Konishi T, Shima H, Nagao M, Hartshorne DJ, Nakano T. A regulatory subunit of smooth muscle myosin bound phosphatase. Biochem Biophys Res Commun. 1994; 200(1): 429–34. doi: 10.1006/bbrc.1994.1467 [DOI] [PubMed] [Google Scholar]
- 68.Eto M, Bock R, Brautigan DL, Linden DJ. Cerebellar long-term synaptic depression requires PKC-mediated activation of CPI-17, a myosin/moesin phosphatase inhibitor. Neuron. 2002; 36(6): 1145–58. doi: 10.1016/S0896-6273(02)01107-8 [DOI] [PubMed] [Google Scholar]
- 69.Dippold RP, Fisher SA. A bioinformatic and computational study of myosin phosphatase subunit diversity. Am J Physiol Regul Integr Comp Physiol. 2014; 307(3): R256–70. doi: 10.1152/ajpregu.00145.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eto M, Karginov A, Brautigan DL. A novel phosphoprotein inhibitor of protein type-1 phosphatase holoenzymes. Biochemistry. 1999; 38(51): 16952–7. doi: 10.1021/bi992030o [DOI] [PubMed] [Google Scholar]
- 71.Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE. Molecular genetics of nicotine dependence and abstinence: whole genome association using 520,000 SNPs. BMC Genet. 2007; 8: 10. doi: 10.1186/1471-2156-8-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eto M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J Biol Chem. 2009; 284(51): 35273–7. doi: 10.1074/jbc.R109.059972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Erdodi F, Kiss E, Walsh MP, Stefansson B, Deng JT, Eto M, Brautigan DL, Hartshorne DJ. Phosphorylation of protein phosphatase type-1 inhibitory proteins by integrin-linked kinase and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 2003; 306(2): 382–7. doi: 10.1016/S0006-291X(03)00976-8 [DOI] [PubMed] [Google Scholar]
- 74.Ohki S, Eto M, Kariya E, Hayano T, Hayashi Y, Yazawa M, Brautigan D, Kainosho M. Solution NMR structure of the myosin phosphatase inhibitor protein CPI-17 shows phosphorylation-induced conformational changes responsible for activation. J Mol Biol. 2001; 314(4): 839–49. doi: 10.1006/jmbi.2001.5200 [DOI] [PubMed] [Google Scholar]
- 75.Eto M, Kitazawa T, Matsuzawa F, Aikawa S, Kirkbride JA, Isozumi N, Nishimura Y, Brautigan DL, Ohki SY. Phosphorylation-induced conformational switching of CPI-17 produces a potent myosin phosphatase inhibitor. Structure. 2007; 15(12): 1591–602. doi: 10.1016/j.str.2007.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eto M, Brautigan DL. Endogenous inhibitor proteins that connect Ser/Thr kinases and phosphatases in cell signaling. IUBMB Life. 2012; 64(9): 732–9. doi: 10.1002/iub.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Senba S, Eto M, Yazawa M. Identification of trimeric myosin phosphatase (PP1M) as a target for a novel PKC-potentiated protein phosphatase-1 inhibitory protein (CPI17) in porcine aorta smooth muscle. J Biochem. 1999; 125(2): 354–62. doi: 10.1093/oxfordjournals.jbchem.a022294 [DOI] [PubMed] [Google Scholar]
- 78.Hayashi Y, Senba S, Yazawa M, Brautigan DL, Eto M. Defining the structural determinants and a potential mechanism for inhibition of myosin phosphatase by the protein kinase C-potentiated inhibitor protein of 17 kDa. J Biol Chem. 2001; 276(43): 39858–63. doi: 10.1074/jbc.M107302200 [DOI] [PubMed] [Google Scholar]
- 79.Eto M, Kitazawa T, Brautigan DL. Phosphoprotein inhibitor CPI-17 specificity depends on allosteric regulation of protein phosphatase-1 by regulatory subunits. Proc Natl Acad Sci USA. 2004; 101(24): 8888–93. doi: 10.1073/pnas.0307812101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams BC, Filter JJ, Blake-Hodek KA, Wadzinski BE, Fuda NJ, Shalloway D, Goldberg ML. Greatwall-phosphorylated Endosulfine is both an inhibitor and a substrate of PP2A-B55 heterotrimers. eLife. 2014; 3: e01695. doi: 10.7554/eLife.01695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li L, Eto M, Lee MR, Morita F, Yazawa M, Kitazawa T. Possible involvement of the novel CPI-17 protein in protein kinase C signal transduction of rabbit arterial smooth muscle. J Physiol. 1998; 508(Pt 3): 871–81. doi: 10.1111/j.1469-7793.1998.871bp.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kitazawa T, Takizawa N, Ikebe M, Eto M. Reconstitution of protein kinase C-induced contractile Ca2+ sensitization in triton X-100-demembranated rabbit arterial smooth muscle. J Physiol. 1999; 520(Pt 1): 139–52. doi: 10.1111/j.1469-7793.1999.00139.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kitazawa T, Polzin AN, Eto M. CPI-17-deficient smooth muscle of chicken. J Physiol. 2004; 557(Pt 2): 515–28. doi: 10.1113/jphysiol.2004.064543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Su W, Xie Z, Liu S, Calderon LE, Guo Z, Gong MC. Smooth muscle-selective CPI-17 expression increases vascular smooth muscle contraction and blood pressure. Am J Physiol Heart Circ Physiol. 2013; 305(1): H104–13. doi: 10.1152/ajpheart.00597.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kitazawa T, Eto M, Woodsome TP, Brautigan DL. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem. 2000; 275(14): 9897–900. doi: 10.1074/jbc.275.14.9897 [DOI] [PubMed] [Google Scholar]
- 86.Dimopoulos GJ, Semba S, Kitazawa K, Eto M, Kitazawa T. Ca2+-dependent rapid Ca2+ sensitization of contraction in arterial smooth muscle. Circ Res. 2007; 100(1): 121–9. doi: 10.1161/01.RES.0000253902.90489.df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Etter EF, Eto M, Wardle RL, Brautigan DL, Murphy RA. Activation of myosin light chain phosphatase in intact arterial smooth muscle during nitric oxide-induced relaxation. J Biol Chem. 2001; 276(37): 34681–5. doi: 10.1074/jbc.M104737200 [DOI] [PubMed] [Google Scholar]
- 88.Bonnevier J, Arner A. Actions downstream of cyclic GMP/protein kinase G can reverse protein kinase C-mediated phosphorylation of CPI-17 and Ca(2+) sensitization in smooth muscle. J Biol Chem. 2004; 279(28): 28998–9003. doi: 10.1074/jbc.M404259200 [DOI] [PubMed] [Google Scholar]
- 89.Kitazawa T, Semba S, Huh YH, Kitazawa K, Eto M. Nitric oxide-induced biphasic mechanism of vascular relaxation via dephosphorylation of CPI-17 and MYPT1. J Physiol. 2009; 587(Pt 14): 3587–603. doi: 10.1113/jphysiol.2009.172189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kitazawa T, Kitazawa K. Size-dependent heterogeneity of contractile Ca2+ sensitization in rat arterial smooth muscle. J Physiol. 2012; 590(21): 5401–23. doi: 10.1113/jphysiol.2012.241315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.MacDonald JA, Eto M, Borman MA, Brautigan DL, Haystead TAJ. Dual Ser and Thr phosphorylation of CPI-17, an inhibitor of myosin phosphatase, by MYPT-associated kinase. FEBS Lett. 2001; 493(2-3): 91–4. doi: 10.1016/S0014-5793(01)02277-3 [DOI] [PubMed] [Google Scholar]
- 92.Deng JT, Sutherland C, Brautigan DL, Eto M, Walsh MP. Phosphorylation of the myosin phosphatase inhibitors, CPI-17 and PHI-1, by integrin-linked kinase. Biochem J. 2002; 367(Pt 2): 517–24. doi: 10.1042/bj20020522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takizawa N, Koga Y, Ikebe M. Phosphorylation of CPI17 and myosin binding subunit of type 1 protein phosphatase by p21-activated kinase. Biochem Biophys Res Commun. 2002; 297(4): 773–8. doi: 10.1016/S0006-291X(02)02302-1 [DOI] [PubMed] [Google Scholar]
- 94.Moffat LD, Brown SB, Grassie ME, Ulke-Lemée A, Williamson LM, Walsh MP, MacDonald JA. Chemical genetics of zipper-interacting protein kinase reveal myosin light chain as a bona fide substrate in permeabilized arterial smooth muscle. J Biol Chem. 2011; 286(42): 36978–91. doi: 10.1074/jbc.M111.257949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.MacDonald JA, Sutherland C, Carlson DA, Bhaidani S, Al-Ghabkari A, Swärd K, Haystead TA, Walsh MP. A Small Molecule Pyrazolo[3,4-d]Pyrimidinone Inhibitor of Zipper-Interacting Protein Kinase Suppresses Calcium Sensitization of Vascular Smooth Muscle. Mol Pharmacol. 2016; 89(1): 105–17. doi: 10.1124/mol.115.100529 [DOI] [PubMed] [Google Scholar]
- 96.Kim JI, Young GD, Jin L, Somlyo AV, Eto M. Expression of CPI-17 in smooth muscle during embryonic development and in neointimal lesion formation. Histochem Cell Biol. 2009; 132(2): 191–8. doi: 10.1007/s00418-009-0604-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reho JJ, Zheng X, Benjamin JE, Fisher SA. Neural programming of mesenteric and renal arteries. Am J Physiol Heart Circ Physiol. 2014; 307(4): H563–73. doi: 10.1152/ajpheart.00250.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004; 84(3): 767–801. doi: 10.1152/physrev.00041.2003 [DOI] [PubMed] [Google Scholar]
- 99.Zheng XL. Myocardin and smooth muscle differentiation. Arch Biochem Biophys. 2014; 543: 48–56. doi: 10.1016/j.abb.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 100.Kim JI, Urban M, Young GD, Eto M. Reciprocal regulation controlling the expression of CPI-17, a specific inhibitor protein for the myosin light chain phosphatase in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2012; 303(1): C58–68. doi: 10.1152/ajpcell.00118.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boopathi E, Hypolite JA, Zderic SA, Gomes CM, Malkowicz B, Liou HC, Wein AJ, Chacko S. GATA-6 and NF-κB activate CPI-17 gene transcription and regulate Ca2+ sensitization of smooth muscle contraction. Mol Cell Biol. 2013; 33(5): 1085–102. doi: 10.1128/MCB.00626-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Woodsome TP, Eto M, Kitazawa T. Characterization and localization of CPI-17 in arterial smooth muscle cells. Biophys J. 2002; 82(1): 419c. [Google Scholar]
- 103.Eto M, Kirkbride JA, Chugh R, Karikari NK, Kim JI. Nuclear localization of CPI-17, a protein phosphatase-1 inhibitor protein, affects histone H3 phosphorylation and corresponds to proliferation of cancer and smooth muscle cells. Biochem Biophys Res Commun. 2013; 434(1): 137–42. doi: 10.1016/j.bbrc.2013.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eto M, Kirkbride JA, Brautigan DL. Assembly of MYPT1 with protein phosphatase-1 in fibroblasts redirects localization and reorganizes the actin cytoskeleton. Cell Motil Cytoskeleton. 2005; 62(2): 100–9. doi: 10.1002/cm.20088 [DOI] [PubMed] [Google Scholar]
- 105.Wu Y, Murányi A, Erdodi F, Hartshorne DJ. Localization of myosin phosphatase target subunit and its mutants. J Muscle Res Cell Motil. 2005; 26(2-3): 123–34. doi: 10.1007/s10974-005-2579-5 [DOI] [PubMed] [Google Scholar]
- 106.Pagiatakis C, Gordon JW, Ehyai S, McDermott JC. A novel RhoA/ROCK-CPI-17-MEF2C signaling pathway regulates vascular smooth muscle cell gene expression. J Biol Chem. 2012; 287(11): 8361–70. doi: 10.1074/jbc.M111.286203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jin H, Sperka T, Herrlich P, Morrison H. Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature. 2006; 442(7102): 576–9. doi: 10.1038/nature04856 [DOI] [PubMed] [Google Scholar]
- 108.Riecken LB, Zoch A, Wiehl U, Reichert S, Scholl I, Cui Y, Ziemer M, Anderegg U, Hagel C, Morrison H. CPI-17 drives oncogenic Ras signaling in human melanomas via Ezrin-Radixin-Moesin family proteins. Oncotarget. 2016; 7(48): 78242–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Freitas MR, Eto M, Kirkbride JA, Schott C, Sassard J, Stoclet JC. Y27632, a Rho-activated kinase inhibitor, normalizes dysregulation in alpha1-adrenergic receptor-induced contraction of Lyon hypertensive rat artery smooth muscle. Fundam Clin Pharmacol. 2009; 23(2): 169–78. doi: 10.1111/j.1472-8206.2008.00658.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhetwal BP, An C, Baker SA, Lyon KL, Perrino BA. Impaired contractile responses and altered expression and phosphorylation of Ca(2+) sensitization proteins in gastric antrum smooth muscles from ob/ob mice. J Muscle Res Cell Motil. 2013; 34(2): 137–49. doi: 10.1007/s10974-013-9341-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang Y, Hermanson ME, Eddinger TJ. Tonic and phasic smooth muscle contraction is not regulated by the PKCα - CPI-17 pathway in swine stomach antrum and fundus. PLoS ONE. 2013; 8(9): e74608. doi: 10.1371/journal.pone.0074608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moreno-Domínguez A, Colinas O, El-Yazbi A, Walsh EJ, Hill MA, Walsh MP, Cole WC. Ca2+ sensitization due to myosin light chain phosphatase inhibition and cytoskeletal reorganization in the myogenic response of skeletal muscle resistance arteries. J Physiol. 2013; 591(5): 1235–50. doi: 10.1113/jphysiol.2012.243576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999; 402(6762): 669–71. doi: 10.1038/45251 [DOI] [PubMed] [Google Scholar]
- 114.Bibb JA, Nishi A, O’Callaghan JP, Ule J, Lan M, Snyder GL, Horiuchi A, Saito T, Hisanaga S, Czernik AJ, Nairn AC, Greengard P. Phosphorylation of protein phosphatase inhibitor-1 by Cdk5. J Biol Chem. 2001; 276(17): 14490–7. [DOI] [PubMed] [Google Scholar]
- 115.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004; 10(3): 248–54. doi: 10.1038/nm1000 [DOI] [PubMed] [Google Scholar]
- 116.Stipanovich A, Valjent E, Matamales M, Nishi A, Ahn JH, Maroteaux M, Bertran-Gonzalez J, Brami-Cherrier K, Enslen H, Corbillé AG, Filhol O, Nairn AC, Greengard P, Hervé D, Girault JA. A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature. 2008; 453(7197): 879–84. doi: 10.1038/nature06994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Satinover DL, Leach CA, Stukenberg PT, Brautigan DL. Activation of Aurora-A kinase by protein phosphatase inhibitor-2, a bifunctional signaling protein. Proc Natl Acad Sci USA. 2004; 101(23): 8625–30. doi: 10.1073/pnas.0402966101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sami F, Smet-Nocca C, Khan M, Landrieu I, Lippens G, Brautigan DL. Molecular basis for an ancient partnership between prolyl isomerase Pin1 and phosphatase inhibitor-2. Biochemistry. 2011; 50(30): 6567–78. doi: 10.1021/bi200553e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li M, Stukenberg PT, Brautigan DL. Binding of phosphatase inhibitor-2 to prolyl isomerase Pin1 modifies specificity for mitotic phosphoproteins. Biochemistry. 2008; 47(1): 292–300. doi: 10.1021/bi701819k [DOI] [PubMed] [Google Scholar]
- 120.Lorenz K, Lohse MJ, Quitterer U. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature. 2003; 426(6966): 574–9. doi: 10.1038/nature02158 [DOI] [PubMed] [Google Scholar]
- 121.Galea CA, Wang Y, Sivakolundu SG, Kriwacki RW. Regulation of cell division by intrinsically unstructured proteins: intrinsic flexibility, modularity, and signaling conduits. Biochemistry. 2008; 47(29): 7598–609. doi: 10.1021/bi8006803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Denicourt C, Dowdy SF. Cip/Kip proteins: more than just CDKs inhibitors. Genes Dev. 2004; 18(8): 851–5. doi: 10.1101/gad.1205304 [DOI] [PubMed] [Google Scholar]
- 123.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008; 14(2): 159–69. doi: 10.1016/j.devcel.2008.01.013 [DOI] [PubMed] [Google Scholar]
- 124.Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004; 18(8): 862–76. doi: 10.1101/gad.1185504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tanaka H, Yamashita T, Asada M, Mizutani S, Yoshikawa H, Tohyama M. Cytoplasmic p21(Cip1/WAF1) regulates neurite remodeling by inhibiting Rho-kinase activity. J Cell Biol. 2002; 158(2): 321–9. doi: 10.1083/jcb.200202071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sahai E, Olson MF, Marshall CJ. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 2001; 20(4): 755–66. doi: 10.1093/emboj/20.4.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee S, Helfman DM. Cytoplasmic p21Cip1 is involved in Ras-induced inhibition of the ROCK/LIMK/cofilin pathway. J Biol Chem. 2004; 279(3): 1885–91. doi: 10.1074/jbc.M306968200 [DOI] [PubMed] [Google Scholar]