Abstract
The incidence of prostate cancer (PC) is growing rapidly throughout the world, in probable association with the adoption of western style diets. Thus, understanding the molecular pathways triggering the development of PC is crucial for both its prevention and treatment. Here, we investigated the role of the metabolism-associated protein, CREB3L4, in the proliferation of PC cells. CREB3L4 was upregulated by the synthetic androgen, R1881, in LNCaP PC cells (an androgen-dependent cell line). Knockdown of CREB3L4 resulted in decreased androgen-dependent PC cell growth. LNCaP cells transfected with siCREB3L4 underwent G2/M arrest, with upregulation of the proteins cyclin B1, phospho-CDK1, p21Waf1/Cip1, and INCA1, and downregulation of cyclin D1. Moreover, depletion of CREB3L4 resulted in significantly decreased expression of a subset of androgen-receptor (AR) target genes, including PSA, FKBP5, HPGD, KLK2, and KLK4. We also demonstrated that CREB3L4 directly interacts with the AR, and increases the binding of AR to androgen response elements (AREs). We also identified a role for the unfolded protein response (and its surrogate, IRE1α), in activating CREB3L4. Cumulatively, we postulate that CREB3L4 expression is mediated by an AR-IRE1α axis, but is also directly regulated by AR-to-ARE binding. Thus, our study demonstrates that CREB3L4 plays a key role in PC cell proliferation, which is promoted by both AR and IRE1α.
Prostate cancer is one of the most commonly diagnosed cancers among men in industrialized nations, and is a leading cause of cancer-related deaths1. Proliferation of prostate cancer cells is known to depend on androgen receptor (AR) signaling2. The AR promotes the growth and regulates the activity of the normal prostate, and remains expressed in nearly all prostate tumors, even in recurrent androgen-independent tumors3,4. Since AR affects prostate cancer development through the regulation of not only transcription networks, but also genomic stability and DNA repair5, prostate cancer treatment relies on strategies targeting AR activity. Although androgen deprivation therapy (ADT) is initially effective in most cases, acquired resistance (termed castrate-resistant prostate cancer (CRPC)) typically develops, which is often characterized by acquired mutations in AR signal pathway genes2,6,7.
The prostate-specific antigen (PSA) gene is the best-characterized androgen-responsive gene in the prostate. Biochemical and genetic studies revealed that both the enhancer and the promoter of the PSA gene are androgen-responsive (independent of each other), but for the maximum expression of PSA, both its promoter and enhancer activities are necessary8.
It has been well shown that the endoplasmic reticulum (ER) plays a key role in normal prostate function, and various stimuli that cause ER stress could result in prostate pathologies, including benign prostate hyperplasia (BPH) and prostate cancer9. Indeed, there is a strong correlation between the occurrence of prostate cancer and the expression of ER stress protein markers, mainly via AR signal transduction10. In addition, activated AR directly binds the inositol-requiring enzyme 1 (IRE1α), as well as x-box binding protein 1 (XBP1). The latter two proteins target the ribosome-associated membrane protein 4 (RAMP4) and the ER degradation-enhancing alpha-mannosidase-like 1 (EDEM1) genes, in prostate cancer cells11. Indeed, AR and UPR gene expression are positively correlated in human prostate cancer patient samples11.
CREB3L4 (cyclic AMP-responsive element-binding protein 3-like 4, also called AIbZIP, Tisp40, or ATCE1) is an ER membrane-bound bZIP domain-containing transcription factor12. It was reported that Creb3l4 is expressed in preadipocytes and functions as a gatekeeper, inhibiting adipogenesis13. Creb3l4 disruption causes ER stress, formation of abnormal epididymal sperm nuclei, and caspase activation12, leading to apoptosis of meiotic and postmeiotic germ cells14. CREB3L4 is abundantly expressed in the prostate, and is much more highly expressed in cancerous than non-cancerous prostate cells15,16. Specifically, CREB3L4 is abundantly expressed in high-grade prostatic intraepithelial neoplasia (PIN), and all grades of adenocarcinomas, as compared to the normal prostate15. Thus, CREB3L4 could represent a potential biomarker for distinguishing benign from malignant prostatic cancer17. Although CREB3L4 expression is known to be regulated by androgen15, its role in prostate cancer progression and development is not well understood.
To this end, we explored a role for CREB3L4 in the androgen-dependent prostate cancer cell line, LNCaP. Our overall results demonstrate a role for CREB3L4 in modulating AR action, suggesting an interrelationship, and an AR-ER stress-CREB3L4 signaling axis, in prostate cancer cell proliferation.
Results
CREB3L4 is overexpressed in androgen-dependent LNCaP cells, and is further induced by androgen
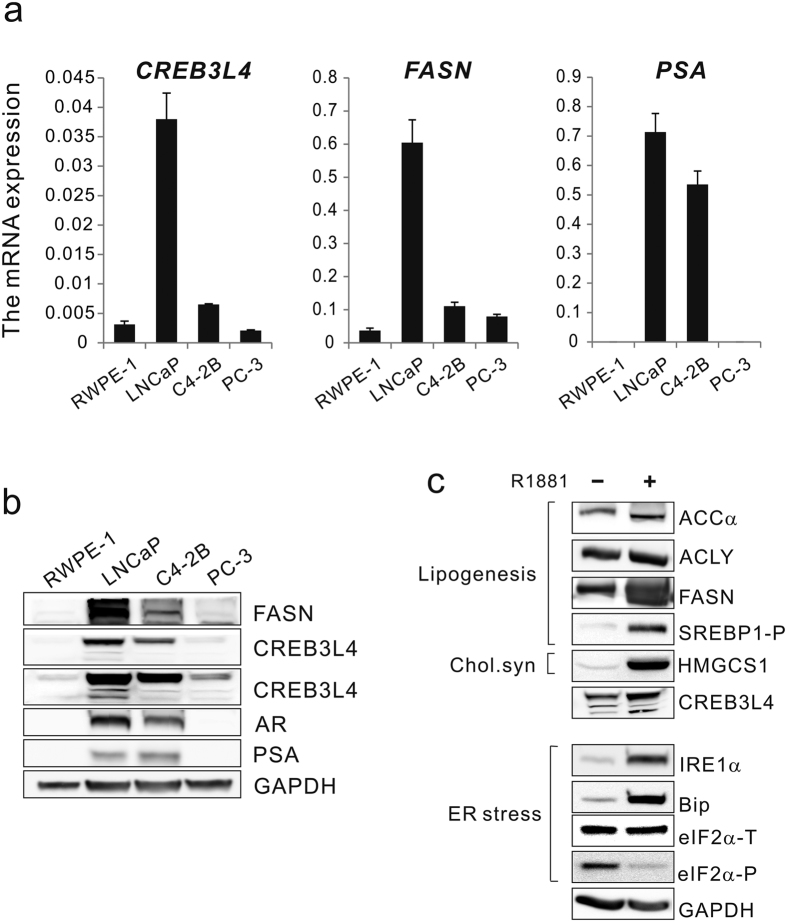
To explore the biological role of CREB3L4 in prostate cancer, we first observed the expression level of CREB3L4 in prostate cancer cell lines, including the androgen-dependent prostate cancer (ADPC) cell line LNCaP, the androgen-insensitive metastatic subline C4-2B (derived from parental ADPC LNCaP cells), the androgen-independent prostate cancer (AIPC) cell line PC-3, and the nontumorigenic prostatic epithelial cell line RWPE-1. As shown in Fig. 1a, LNCaP cells expressed the highest levels of CREB3L4, fatty acid synthase (FASN), and PSA, as compared to the other cell lines. Along with its mRNA, CREB3L4 protein expression was higher in LNCaP than other cell lines (Fig. 1b). Western blot revealed that CREB3L4 expression was significantly increased by the synthetic androgen R1881, compared to untreated LNCaP cells (Fig.1c). These results support previous data showing the expression of genes related to fatty acid and cholesterol biosynthesis, and IRE1α, all significantly increased by R1881, whereas the protein kinase RNA-like endoplasmic reticulum kinase (PERK)-eukaryotic initiation factor 2 (eIF2α)-phosphorylation ER stress pathway, was suppressed by androgen11,18. From this background, we used LNCaP cells as an experimental model for studying androgen-dependent regulation of CREB3L4, with specific regard to prostate cancer cell proliferation.
Figure 1. CREB3L4 is overexpressed in androgen-dependent prostate cancer cell lines, and is induced by androgen.
(a) Total RNA was extracted from cultured prostate cancer cell lines and real time qPCR performed. Levels of all mRNAs were normalized to those of RPL19 mRNA. (b) Whole cell lysate protein (40 μg) of various prostate cancer cell lines was resolved by SDS/PAGE, and immunoblotted for the respective proteins indicated, with GAPDH intensity used as a loading control. (c) LNCaP cells were incubated for 1 day in the absence or presence of 10 nM R1881, in 1% CT-FBS medium, to optimize androgen effect(s). FASN, fatty acid synthase; PSA, prostate-specific antigen; ACCα, acetyl CoA carboxylase alpha; ACLY, acetyl-CoA lyase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; RPL19, ribosomal protein L19.
CREB3L4 is required for LNCaP cell proliferation
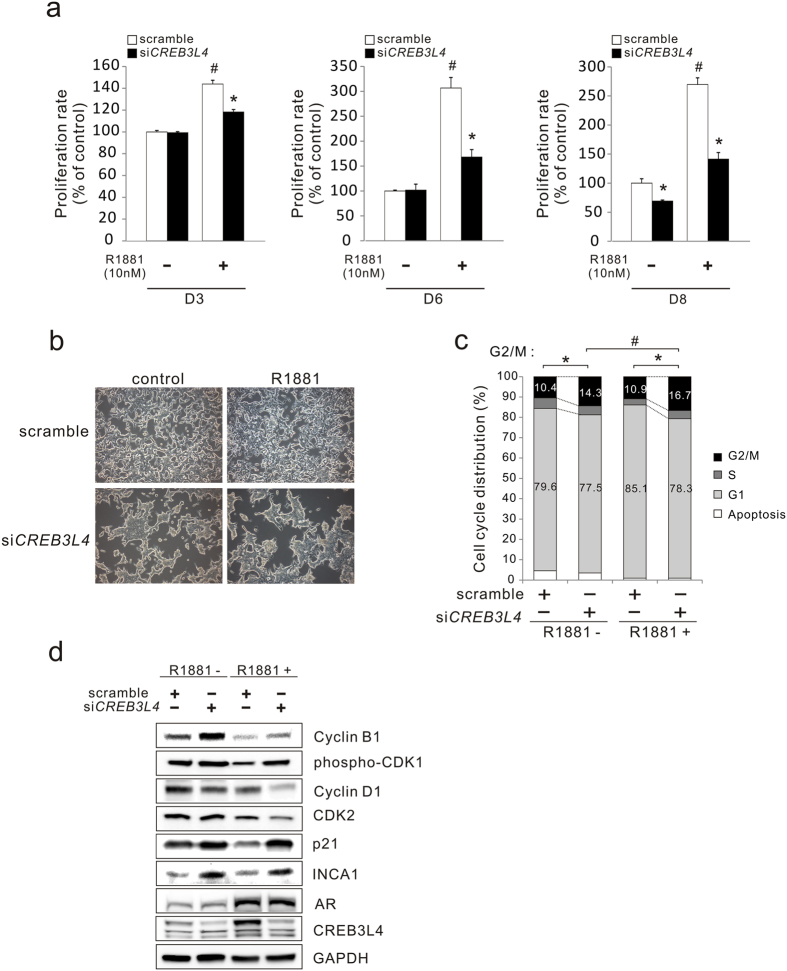
We next investigated whether CREB3L4 affects the growth of LNCaP cells. For this, we transfected siCREB3L4, and observed proliferation of cells for a period of 8 days (D3, D6, D8) in the presence or absence of R1881 (Fig. 2a). LNCaP transfection with siCREB3L4 with or without R1881 inhibited proliferation at D3 by 20% (p < 0.05), D6 by 50% (p < 0.05), and D8 by 50% (p < 0.05), respectively. Specifically, from D3 to D8 after transfection, androgen-dependent proliferation of LNCaP cells was suppressed by siCREB3L4 transfection (Fig. 2a). siCREB3L4 also inhibited cell proliferation, even in the absence of androgen, at D8 (Fig. 2a). Microscopic observation also indicated that CREB3L4 knockdown resulted in LNCaP growth inhibition (Fig. 2b). We observed decreased numbers of G1 phase cells, with increased numbers of G2/M-arrested cells, regardless of the presence of androgen (Fig. 2c), although androgen even further increased G2/M arrest, by suppressing CREB3L4. These findings further support a role for CREB3L4 in LNCaP cell proliferation. To confirm whether CREB3L4 affects cell cycle regulation in cell growth, we assessed expression of proteins involved in the cell cycle, especially G2/M arrest-related proteins (Fig. 2d). We found that suppression of CREB3L4 resulted in the induction of cyclin B1, with repression of cyclin D1, causing mitotic clonal delay. Furthermore, expression of p21Waf1/Cip1 and INCA1 (inhibitor of cyclin-dependent kinase (CDK) interacting with cyclin A1), in complex with CDK2, respectively19, was increased by siCREB3L4 (Fig. 2d). In addition, siCREB3L4 increased phospho-CDK1 with decreased cyclin D1 and CDK2 in the presence of androgen, making it likely that CREB3L4 affects G2/M arrest. These data suggest that CREB3L4 affects the proliferation of LNCaP cells by inhibiting cell cycle transitions.
Figure 2. CREB3L4 regulates prostate cancer cell growth.
(a) LNCaP cells were transfected with scrambled or siCREB3L4 siRNAs. After 24 hr, the cells were treated with 10-nM R1881 or DMSO/EtOH control in 5% CT-FBS-containing medium, which was refreshed every 2 days. At 3/5/8 days after transfection, proliferation rates of the cells were measured by CCK-8 assay. (b) LNCaP cells were transfected with scrambled or siCREB3L4, in the presence or absence of R1881 (10-nM). At 48-hr after transfection, microscopic analysis of the cells was performed (x20). (c) Cell cycle profiles, of LNCaP cells transfected with CREB3L4-specific siRNA (siCREB3L4), in the presence or absence of 10-nM R1881 in 5% CT-FBS medium, were analyzed by propodium iodide (PI) staining and flow cytometry. As shown, there were significant differences in the G2/M population between scrambled versus siCREB3L4, in the absence/presence of R1881 (*P < 0.05), and siCREB3L4 versus siCREB3L4, in the presence of R1881 (#P < 0.05). (d) LNCaP cells were transfected with scrambled or siCREB3L4. After 24 hr, the cells were treated with R1881 (10 nM) or DMSO/EtOH (control) in 5% CT-FBS-containing medium, for 24-hr. Whole cell lysate protein (40-μg) was resolved by SDS/PAGE, and immunoblotted for the respective proteins indicated, with GAPDH used as a loading control. Bars represent means ± S.E. (error bars), n = 3, *P < 0.05, scrambled versus siCREB3L4, in the absence/presence of R1881; #P < 0.05, scrambled versus scrambled, in the absence of R1881. DMSO, dimethyl sulfoxide; EtOH, ethyl alcohol; CT-FBS, charcoal treated-fetal bovine serum.
CREB3L4 regulates AR-mediated transcription
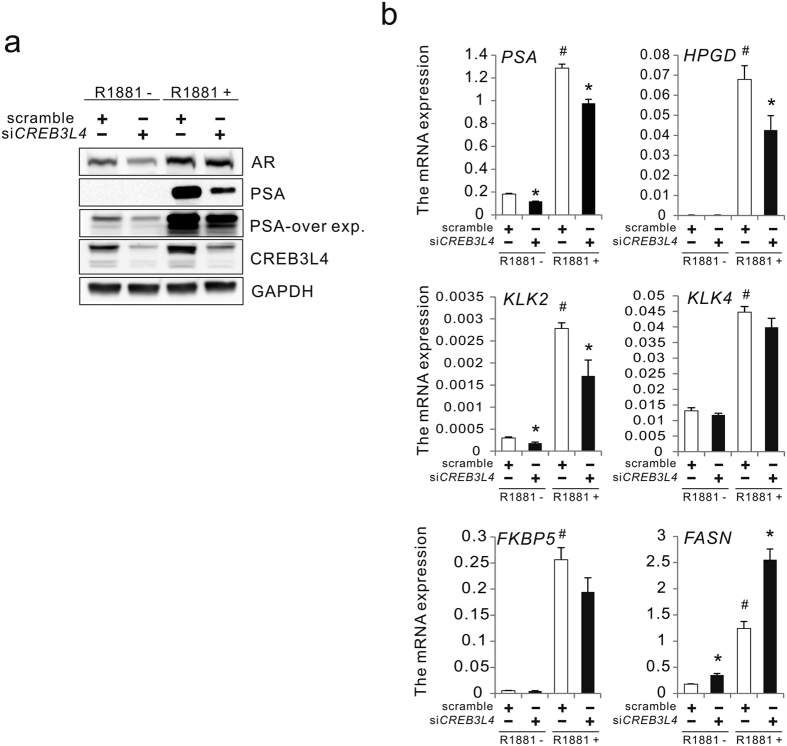
Since AR plays a crucial role in prostate cancer cell proliferation, and is a common therapeutic target, we assessed possible roles for CREB3L4 in the AR signal cascade. Treatment of siCREB3L4-LNCaP cells with R1881 resulted in a significant reduction in protein levels of PSA, a well-known target of AR, even while AR expression was not changed (Fig. 3a). siCREB3L4 also decreased PSA expression levels in the absence of androgen (Fig. 3a). To confirm the effect(s) of CREB3L4 on the expression of various AR-downstream genes, their expression was observed in androgen-treated cells expressing siCREB3L4. Knockdown of CREB3L4 significantly decreased the expression of AR target genes, such as PSA, HPGD, FKBP5, KLK2, and KLK4, all of whose expression is tightly associated with prostate cell proliferation20 (Fig. 3b). These data suggest that CREB3L4 may act as a positive regulator of AR.
Figure 3. CREB3L4 regulates AR-mediated transcription.
(a) LNCaP cells were transfected with scrambled or siCREB3L4. After 24 hr, the cells were treated with 10-nM R1881 or DMSO/EtOH control in 5% CT-FBS-containing medium, for 24 hr. Whole cell lysate protein (40 μg) was resolved by SDS/PAGE and immunoblotted for the respective proteins indicated, with GAPDH as a loading control. (b) Real-time PCR analysis showing the effect of siCREB3L4 on the activation of AR target genes. LNCaP cells treated with siCREB3L4 were harvested after treatment with 10-nM R1881. Total RNA was analyzed by real-time quantitative PCR, with the levels of all mRNAs normalized to those of RPL19 mRNA, and expressed as folds-change. Statistical significance of differences between experimental groups was assessed by nonparametric Mann-Whitney test. Values were expressed as means ± S.E. (error bars), n = 3, *P < 0.05, scrambled versus siCREB3L4, in absence/presence of R1881; #P < 0.05, scrambled versus scrambled, in the absence of R1881. DMSO, dimethyl sulfoxide; EtOH, ethyl alcohol; CT-FBS, charcoal treated-fetal bovine serum.
CREB3L4 enhances AR activity through direct interaction with AR
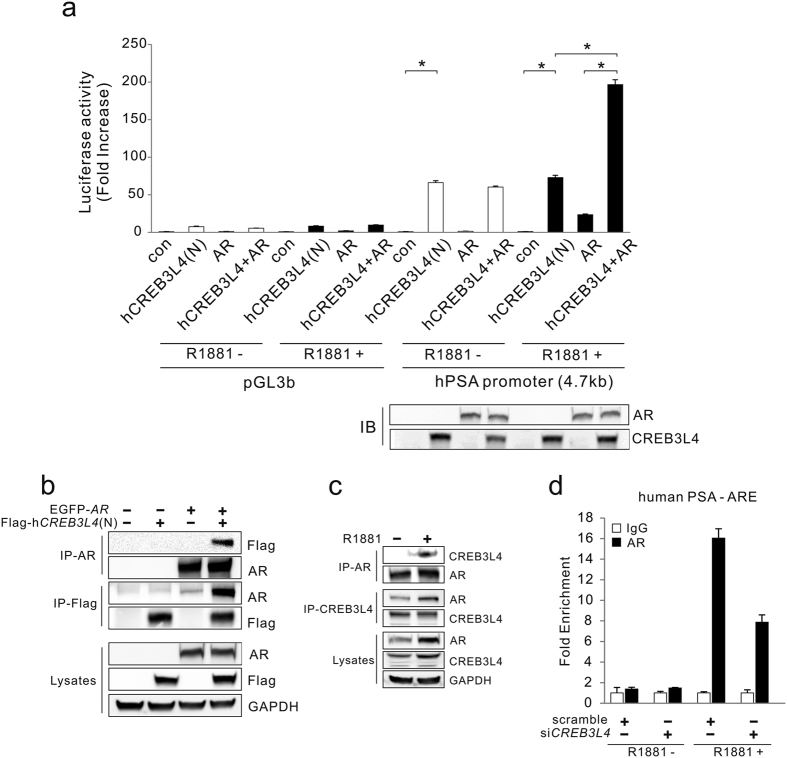
Then, a question arises. What is the role of CREB3L4 in the AR-dependent signaling pathway? To examine the relationship between CREB3L4 and AR, with regard to prostate cancer cell proliferation, we performed reporter assays using a PSA gene promoter construct. Those reporter assays showed that human nuclear form of CREB3L4 (hCREB3L4N) activated the PSA promoter activity by 50-fold, in the absence of androgen and AR (Fig. 4a). This data is consistent with our other results showing that PSA expression is suppressed in siCREB3L4-LNCaP cells in the absence of androgen (Fig. 3a). These data indicate that PSA gene expression could be directly regulated by CREB3L4.
Figure 4. CREB3L4 enhances AR transactivation through interacting with AR.
(a) Plasmids expressing hCREB3L4N (nuclear protein) and/or AR, under the pGL3b or human PSA gene promoters, were transfected into HEK293 cells, followed by treatment with or without 10-nM R1881. After 24 hr, cell lysates were subjected to luciferase assay. Luciferase activities were normalized to Renilla luciferase activities to adjust for transfection efficiency. Normalized activities are shown as means ± S.E. (error bars), n = 3, and expressed as folds-increase, relative to basal activity. (b) HEK293 cells were transfected with AR, and CREB3L4 expression vectors, and proteins immunoprecipitated (IP’ed) using an anti-AR and anti-Flag for CREB3L4 antibodies. IP protein was then resolved by SDS/PAGE and immunoblotted for the respective proteins indicated. (c) Interaction of endogenous AR and CREB3L4 in LNCaP cells. Cells were treated with 10-nM R1881 for 24 hr. Endogenous AR and CREB3L4 protein from LNCaP cells were precipitated with anti-AR or anti-CREB3L4 antibodies, and the interaction between these proteins determined by co-IP. (d) ChIP assay performed in LNCaP cells transfected with siCREB3L4, with R1881 treatment. Normal IgG was used as a negative control for IP. AR response element (ARE) of PSA gene promoter was amplified to determine ChIP’ed DNA, which was normalized to total input DNA.
In the presence of androgen, however, AR activated the PSA gene promoter reporter by 25-fold (Fig. 4a). However, AR-mediated transactivation of PSA promoter activity in the presence of androgen was significantly increased, by 200-fold, when the hCREB3L4N was cotransfected (Fig. 4a). This result suggests that hCREB3L4N upregulates AR-mediated transactivation, in a ligand-dependent manner. Consequently, we next explored the mechanism of how CREB3L4 activates AR signaling. We first examined the interaction between CREB3L4 and AR by co-immunoprecipitation (co-IP), showing that the two proteins interact with each other (Fig. 4b). Furthermore, the interaction between endogenous CREB3L4 and AR in LNCaP cells was also increased in the presence of androgen (Fig. 4c). To observe a possible role for CREB3L4 in AR-to-DNA recruitment, we performed chromatin immunoprecipitation (ChIP) assays. Androgen-induced AR recruitment to an androgen-response element (ARE), within the PSA gene promoter, was abolished in siCREB3L4-transfected cells (Fig. 4d). This result suggests that CREB3L4 interacts with AR, and assists AR recruitment to ARE. Taken together, these data suggest that not only can CREB3L4 upregulate the PSA gene AR-independently, it also enhances AR-mediated transactivation of its target genes by acting as an activator.
Androgen-induced CREB3L4 expression is regulated both directly, by AR, and indirectly, via IRE1α pathway signaling
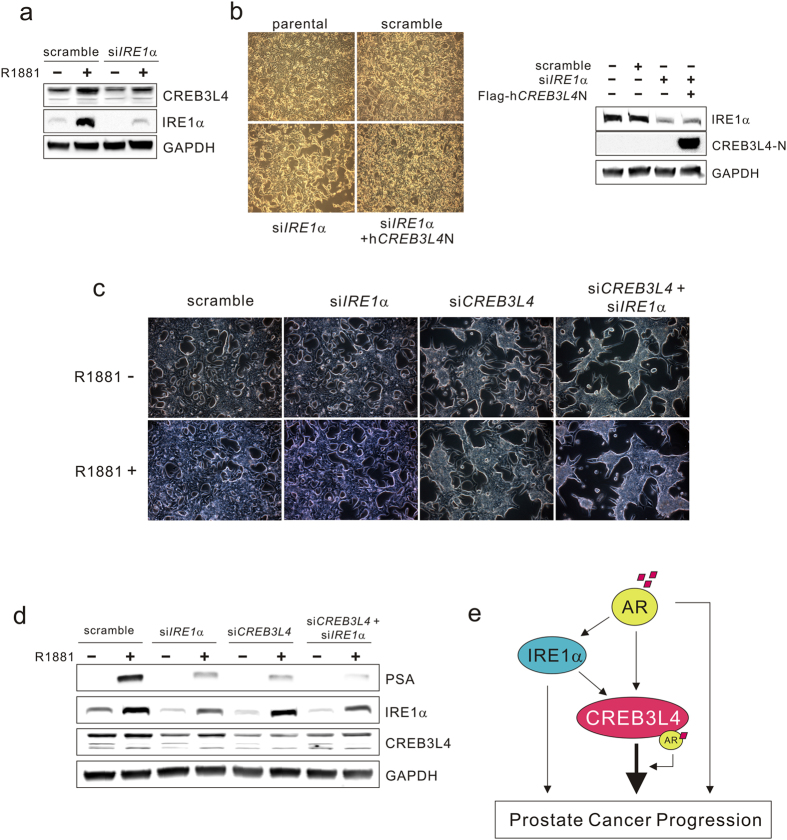
We next observed that androgen promotes LNCaP cell proliferation by inducing CREB3L4 expression, which cooperatively upregulates AR target genes. Thus, we speculated whether CREB3L4 may also play a critical role in regulating prostate cancer cell proliferation, through interacting with AR. We first questioned the molecular mechanism of how CREB3L4 gene expression is regulated in prostate cancer cells. It was reported that CREB3L4 expression is increased by androgen15,16, which we confirmed in Fig. 1c. Recently, canonical unfolded protein response (UPR) pathways were also shown to be directly and divergently regulated by androgens in prostate cancer cells, through modulation of AR transactivation11, including that of IRE1α, with inhibition of the PERK signaling pathway11. In addition, full-length CREB3L4 is cleaved to its nuclear form, upon ER stress, in LNCaP cells21. From this basis, we assumed that androgen-induced CREB3L4 expression could also be mediated through an AR-IRE1α signaling branch of the ER stress response. To test this hypothesis, we examined the relationship between IRE1α pathway signaling and CREB3L4 in AR-induced prostate cancer cell proliferation. These studies revealed that androgen-induced expression of CREB3L4 was inhibited by depletion of IRE1α (Fig. 5a), which also significantly decreased LNCaP cell proliferation. Ectopic expression of CREB3L4 in IRE1α-knockdown cells restored proliferation back to the level of control (i.e., non-transfected) LNCaP cells (Fig. 5b). Analogously, siCREB3L4-mediated inhibition of cell proliferation was further inhibited cell growth by siIRE1α (Fig. 5c). To examine the relationship between IRE1α and CREB3L4 in AR activity, we observed PSA expression levels following knockdown of IRE1α and/or CREB3L4. PSA levels were reduced by siCREB3L4, with even further reduction in cells cotransfected with siIREα (Fig. 5d). We next determined whether AR directly regulates CREB3L4 gene expression, via CHIP assays. Using Vector NTI Suite, we found four putative androgen response elements (AREs) (inverted repeated (IR) sequence-IR3; AGAACANNNTGTTCT; ARE1~ARE4) within the 3 kb region of the human CREB3L4 promoter (Supplementary Fig. 1a). Moreover, as might be expected, we observed that R1881 increases endogenous AR recruitment to the putative ARE4 region of the CREB3L4 promoter (Supplementary Fig. 1b). These results suggest that androgen-induced CREB3L4 gene expression is in part regulated by AR directly. Furthermore, activation of ER stress response signals by AR contributes to CREB3L4 upregulation, resulting in activation of the androgen-dependent transcriptional cascades.
Figure 5. Androgen-induced CREB3L4 expression is regulated by IRE1α pathway.
(a) LNCaP cells were transfected with scrambled or siIRE1α. After 24 hr, the cells were treated with 10-nM R1881 or DMSO/EtOH control in 5% CT-FBS-containing medium, for 24 hr. Whole cell lysates (40 μg) was resolved by SDS/PAGE and immunoblotted for the respective proteins indicated, with GAPDH used as a loading control. (b) LNCaP cells were transfected with scrambled or siIRE1α with hCREBL4N overexpression vector. 48 hr after transfection, microscopic analysis of the cells was performed (x10, upper panel). Western blot was performed to confirm the expression of IRE1α and CREB3L4 expression (lower panel). (c) LNCaP cells were transfected with siIRE1α and/or siCREB3L4 in the presence or absence of R1881 (10-nM). At 48 hr after transfection, microscopic analysis of the cells was performed (x20) (d), and analyzed by western blot, to confirm the expression of PSA, IRE1α, and CREB3L4, with GAPDH as a loading control. (e) Proposed mechanism of action of CREB3L4 in prostate cancer progression.
Discussion
CREB3L4 is highly expressed in the prostate gland, and upregulated even further in prostate cancer15,22. Indeed, CREB3L4 is now being evaluated as a potential marker for prostatic diseases, based especially on its ability to discriminate between benign and malignant prostate tumors17. However, the biological role of CREB3L4 in prostate cancer cell proliferation previously remained unknown. In this study, we found that CREB3L4 is essential for prostate cancer cell proliferation, through its modulation of AR activity, which reciprocally, is upstream of CREB3L4 expression (perhaps suggesting some type of positive feedback). We also showed that CREB3L4 directly associates with AR, to enhance AR-induced transactivation of its downstream genes, in prostate cancer cells. Furthermore, we observed that AR-induced CREB3L4 expression is also mediated by IRE1α, ER stress response signaling. Our data thus suggest that the novel link between AR-CREB3L4 and the IRE1α pathway is an axis critical for driving prostate cancer progression (Fig. 5e).
The unfolded protein response (UPR) has critical roles in development and normal physiology, as well as in pathological states such as cancer23. The IRE1α-XBP1 pathway is known to be essential for tumor survival24, and loss of XBP1 sensitized cancer cells to death from oxidative stress25. Specifically, androgens induce a UPR response in prostate cancer cells, by activating the IRE1α-XBP1 signaling branch, to regulate growth and survival of prostate cancer cells11. XBP1 expression is also increased in clinical prostate cancer specimens11. Since CREB3L4 is upregulated by ER stress14, we assumed that androgen-induced CREB3L4 expression is mediated through the IRE1α pathway. We confirmed that AR-induced CREB3L4 expression is abolished through knockdown of IRE1α (Fig. 5a). In addition, we observed that CREB3L4 promoter activity is significantly increased by overexpressing hXBP1, a transcription factor gene downstream of the IRE1α pathway (Supplementary Fig. 1c). These results suggest that androgen-induced CREB3L4 gene expression is in part regulated by AR directly (due to physical interaction with the CREB3L4 promoter), and in part mediated indirectly, via an IRE1α pathway. Thus, we posit that CREB3L4 is an essential mediator of AR-IRE1α-induced prostate cancer progression.
CREB3L4 knockdown also increased the number of cells in G2/M, and upregulated p21Waf1/Cip1 and INCA1, both inhibitors CDK, regardless of the presence of androgen (Fig. 2c,d). However, phosphorylation of CDK1, after siCREB3L4 transfection, was further increased in the presence, but not absence, of androgen (Fig. 2d). Moreover, CDK1 phosphorylation coincided with a small, but statistically significant, G2/M arrest in siCREB3L4-transfected and R1881-treated cells, vs. androgen-untreated siCREB3L4 cells. This result shows that androgen could play an additive role in siCREB3L4-mediated arrest of G2/M phase prostate cancer cells. Indeed, we observed that siCREB3L4 transfection resulted in significant growth inhibition of LNCaP cells, in the presence of R1881, at D6 and D8 (Fig. 2a). This result suggests that androgen-induced CREB3L4 significantly increases cell proliferation.
There is a question whether CREB3L4 can even function in the absence of androgen. To address this, we showed that cell proliferation by D8, even in the absence of R1881, was decreased by siCREB3L4 (Fig. 2a,b). CREB3L4 also increased PSA expression independently of AR (Fig. 4a), and had a greater activating effect on the PSA promoter, compared to that of AR alone (Fig. 4a). Furthermore, transfection of siCREB3L4 decreased PSA protein levels in the absence of androgen (Fig. 3a). We also observed IRE1α downregulation, by CREB3L4 knockdown, in the absence of androgen (Fig. 5d). Thus, it is possible that IRE1α is also regulated by CREB3L4, similar to PSA. Therefore, we could not exclude androgen-independent effects of CREB3L4 on LNCaP cell proliferation. However, CREB3L4 had a more robust effect in the androgen-dependent state, compared to the androgen-independent state (Fig. 2a). We speculate that regulation of the AR-IRE1α-CREB3L4 pathway in the presence of androgen, may maximize the effect of androgen on proliferation of prostate cancer cells.
How does CREB3L4 regulate androgen-dependent cancer cell proliferation? We showed here that CREB3L4 knockdown resulted in decreased androgen-induced PSA expression, without affecting AR expression (Fig. 3a). Besides, CREB3L4 depletion decreased AR binding to an ARE within the PSA gene promoter (Fig. 4d). This indicates that CREB3L4 promotes AR binding to AREs, and transactivation of the associated target genes, by directly interacting with AR. However, we could not confirm recruitment of CREB3L4 to the ARE within the PSA gene promoter, nor binding to an unfolded protein response element (data not shown), in contrast to another study26. Thus, it yet remains necessary to identify CREB3L4-binding sites, within gene promoters, to better understand its regulation of PSA and other AR-target genes. We assume that independently bound CREB3L4 may interact with AR (bound to its ARE) on the PSA promoter, presumably by bending, “looping,” or some other unknown mechanism. Hence, more study is required to understand CREB3L4 activation of PSA, or other downstream genes, in a manner that is synergistic with AR.
Previous reports have demonstrated that the enzymes involved in fatty acid synthesis and cholesterol synthesis are upregulated in prostate cancer cells by androgens27,28. Thus, it was surprising to note that the expression of FASN was increased in siCREB3L4 knockdown cells. The mechanism of downregulation of FASN by the AR-CREB3L4 axis may be different from that of other AR target genes. Distinct cofactor complexes with CREB3L4/AR may occur in its regulation of lipogenesis-related genes. Thus, the molecular mechanism of this distinct regulation needs further study.
Collectively, we demonstrated that CREB3L4 is required for proliferation of prostate cancer cells, and that CREB3L4 is a crucial activator of AR function. Indeed, CREB3L4 directly interacts with, and facilitates, AR recruitment to the AREs of AR target genes, maximizing their expression, (e.g., PSA) (Fig. 3b). Additionally, we demonstrated that androgen-induced CREB3L4 expression is in part regulated by AR directly, and in part indirectly, by IRE1α signaling, suggesting that a distinct AR-ER stress-CREB3L4 regulatory axis also plays a role in prostate cancer proliferation. A schematic of the mechanism of action of CREB3L4, in facilitating prostate cancer proliferation, is shown in Fig. 5e. In summary, our findings demonstrate that CREB3L4 plays a key role in prostate cancer cell proliferation, justifying its further study as a possible prostate cancer biomarker and therapeutic target.
Methods
Cell culture and Transient Transfection Assay
HEK293 (embryonic kidney) and PC3 (androgen-independent prostate cancer cells) were obtained from ATCC (Manassas, VA, USA) and grown in Dulbecco’s modified Eagle’s medium (Hyclone, Logan, UT, USA), supplemented with 10% (v/v) fetal bovine serum, 100 units/ml penicillin, and 100-μg/ml streptomycin. LNCaP (an androgen-dependent human prostate cancer cell line; ATCC number CRL1740) and RWPE-1 (nontumorigenic, prostatic epithelial cell line; ATCC number CRL11609) cells were grown at the Roswell Park Memorial Institute (RPMI, Buffalo, NY, USA) in medium 1640, supplemented with 10% FBS, and penicillin/streptomycin. C4-2B cell lines were supplied as a kind gift from Dr. H.G Yoon29. LNCaP cells were maintained in 10% FBS-containing media, and for all experiments, this media was replaced with 5% charcoal treated (CT)-FBS media to selectively remove hormones, growth factors, and steroids the day before observing the effects of R1881. Transient transfection and luciferase assays were performed using Fugene HD reagent (Promega, Madison, WI, USA) and a Dual luciferase assay kit (Promega). Luciferase activities were normalized to Renilla firefly activities, to adjust for transfection efficiency. Normalized luciferase activities are shown as means ± S.E. Data are expressed as fold-increases, relative to the basal activity of the reporters, in the absence of overexpression vectors.
Flow cytometry analysis
siRNA-transfected LNCaP cells were harvested by trypsinization, and fixed in ice-cold 70% ethanol overnight. The cells were then washed twice with cold PBS, and incubated for 30 min at room temperature in 1-mL PBS containing 50 μg/ml propidium iodide (Invitrogen) and 50 μg/ml RNaseA (Sigma, St. Louis, MO, USA). The stained samples were then analyzed with a LSR-II flow cytometer (Becton Dickinson, Franklin Lakes, NY, USA).
Cell viability assay
siRNA-transfected LNCaP cells were trypsinized and seeded in 5% CT-FBS-containing media, in the presence/absence of 10 nM R1881, in 96-well plates (7.5 × 103 cells/well). Media containing 5% CT-FBS, with 10 nM R1881 or DMSO/EtOH (control), was changed every 2 days. At D3/D6/D8 after transfection, cell viability was measured, using the Cell Counting Kit-8 (Sigma) according to the manufacturer’s instructions. Briefly, 10-μl of CCK-8 solution was added to each well, and plates incubated for 4 hr, followed by measurement of absorbance at 450 nm, using a microplate reader.
Total RNA isolation and quantitative real-time PCR
Total RNA was isolated from LNCaP/PC3/RWPE1/C4-2B cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. This RNA was used to generate cDNA using the GoScript Reverse Transcription System (Promega). Quantitative real-time PCR (qPCR) was performed using the Step One Real-Time PCR Systems instrumentation and software (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s protocol. The relative amount of mRNA in each sample was normalized to that of the gene RPL19. Primers used in PCR were as follows: CREB3L4-f, 5′-GGCCTTCAAGAGAGTGAGCCTG-3′; CREB3L4-r, f, 5′-AACTGGGCAGGATGATGAGAGC-3′; RPL19-f, 5′-CACATCCACAAGCTGAAGGCAGAC-3′; RPL19-r, 5′-CGTGCTTCCTTGGTCTTAGACCTG-3′. The primers, FASN, PSA, HPGD, KLK2, KLK4, and FKBP5, were used for the real time PCR18,20.
Western blotting
Proteins from LNCaP/PC3/RWPE1/C42B cells were isolated using RIPA solution (Thermo Scientific, Rockford, IL, USA) containing appropriate protease inhibitors. Protein concentration was determined using the BCA protein assay (Thermo Scientific), and 40 μg were subjected to sodium dodecyl sulfate - polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Whatman, Dassel, Germany). Membranes were blocked with 5% non-fat milk and incubated with the following primary antibodies: anti-CREB3L4 (AT1618a, Abgent Inc., San Diego, CA, USA), anti-INCA1 (sc-243077) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), anti-AR (#5153s), anti-PSA (#2475s), anti-GAPDH (#2118), anti-IRE1α (#3294s), anti-Bip (#3711p), anti-eIF2α-total (#2103s), anti-eIF2α-phospho (#3597s), anti-cyclin B1 (#12231), anti-phospho CDK1 (#4539), anti-cyclin D1 (#2922), anti-CDK2 (#2546), anti-p21Waf1/Cip1 (#2947) (all from Cell Signaling, St. Louis, MO, USA), anti-HMGCS1 (ab194971, Abcam, Cambridge, MA, USA), and anti-Flag (F3165, Sigma). Antibodies for FAS, ACCα, ACLY, and SREBP1 were kindly provided by Dr. K.S. Kim18. Membranes were then incubated with horseradish peroxidase-conjugated-anti-mouse or -anti-rabbit goat secondary antibodies (Thermo Scientific), at a 1:4000 dilution, in 5% non-fat milk in PBST buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, 0.1% Tween-20), for 1 h at room temperature. Antibody-bound proteins were visualized using an enhanced chemiluminescence detection system (West Pico & West Dura, Thermo Scientific). The protein bands were also detected using a Fujifilm LAS-3000 Imager (FUJIFILM Corporation, Tokyo, Japan).
Chromatin immunoprecipitation (ChIP) assay
ChIP experiments were carried out according to the standard protocol (Merck KGaA, Darmstadt, Germany). LNCaP cells were plated in 15-cm tissue culture plates and transfected with siCREB3L4 siRNA, combined with 10 nM R1881 treatment. Cell lysates were then incubated for 15 hr at 4 °C with 5-μg anti-AR (#5153, Cell Signaling) or normal rabbit IgG (sc-2027, SantaCruz Biotechnology) antibodies. After reversal of crosslinking, immunoprecipitated DNA was quantified by qPCR. All reactions were normalized relative to total input to account for chromatin sample preparation differences (ΔCt), determined as the difference between the α-AR IP sample (ΔCt [AR]) and α-IgG IP sample (ΔCt [IgG]) for fold enrichment. Relative expression was determined by the method ΔΔCt [AR - IgG] = ΔCt [AR] - ΔCt [IgG], and fold-change in occupancy = 2(−ΔΔCt[AR-IgG])30. The primer used for PCR of the AR promoter binding region (distal ARE) of the PSA gene is described in ref. 31. Primers sequence for PCR of four putative AREs were as follows: ARE1-f, 5′-AACCTGGATTCTGGTCCAAGTTCTA-3′; ARE1-r, 5′-AACTCCTGACCTCGTCATCTGC-3′; ARE2-f, 5′-TCAAATCATCTCTGCACATACA-3′; ARE2-r, AACCAAGATTTGGATGCTTCAG-3′; ARE3-f, 5′-GGAGCTTGCAGTGAGCCGAGATC-3′; ARE3-r, 5′-ACTGCTACTAATTATCCTTATGAAG-3′; ARE4-f, 5′-TGCATGGAACCGTGATCGCACCAC-3′; ARE4-r, 5′-AAGCTGAAGACTTAGGTTTCGGAG-3′.
Immunoprecipitation assay
Constructs expressing Flag-tagged hCREB3L4N and GFP-tagged AR were cotransfected into HEK293 cells using FuGENE HP transfection reagent (Promega). To confirm the endogenous interaction between CREB3L4 and AR, LNCaP cells were treated with 10-nM R1881 for 48 hr, harvested, and lysed in cold cell lysis buffer (50-mM Tris [pH7.4], 150-mM NaCl, 0.2% Triton X-100, 0.3% NP-40), containing appropriate protease inhibitors. Whole cell lysates (1000 μg) were precleared, with protein G-agarose (#05015952001, Roche, Mannheim, Germany), followed by incubation with anti-AR, anti-CREB3L4, or anti-Flag antibodies, for 16 h with protein G-agarose, for 2 h at 4 °C. After centrifugation, the protein G-agarose pellets were washed several times with washing buffer (1/3 dilution of cell lysis buffer in PBS) and resuspended in sample buffer, before being subjected to SDS-PAGE.
Small interfering RNA (siRNA)
RNA oligonucleotides for human CREB3L4 (forward, 5′-GCUAGAUCAGUGGAGCCCAGCAUUU-3′) (Invitrogen) were synthesized. For silencing IRE1α, siRNA against IRE1α (Santa Cruz, sc-40705) was used. Medium GC Duplex was used as a negative control (Thermo Scientific, # 12935300). Each siRNA (40 nM) was transfected into appropriate experimental sets of LNCaP cells, using Lipofectamine RNA iMAX (Invitrogen) for at least 48 h, followed by cell lysis for RNA and protein preparation.
Construction of plasmids
Expression constructs encoding the nuclear form of hCREB3L4 and hXBPs were PCR-amplified and cloned directly into the pcDNA3.1-Flag2 vector. The construct pEGFP-C1-AR was purchased from Addgene (Cambridge, MA, USA). The human PSA promoter, covering about 4.7 kb, was a generous gift from Dr. K.S. Kim (Yonsei Univ., South Korea). The mouse Creb3l4 gene promoter covering a −2500/+209 region was PCR-amplified and cloned directly into the pGL4b luciferase reporter vector.
Additional Information
How to cite this article: Kim, T.-H. et al. The role of CREB3L4 in the proliferation of prostate cancer cells. Sci. Rep. 7, 45300; doi: 10.1038/srep45300 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We would like to thank Dr. KS Kim and Dr. HG Yoon for providing plasmids, antibodies and cell lines. This work was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) and Ministry of Education, Science and Technology (MEST), Republic or Korea (NRF-2014R1A2A2A01004396 to YHA, NRF- 2013R1A1A2060537 to THK) and Korea Mouse Phenotyping project(2013M3A9D5072550) of Ministry of Science, ICT and Future Planning through the National Research Foundation.
Footnotes
The authors declare no competing financial interests.
Author Contributions T.H.K. substantially contributed to the study’s conception and design, the acquisition, analysis, and interpretation of data, and drafting of the article. J.M.P. substantially contributed to the analysis and interpretation of data, and drafting of the article. M.Y.K contributed substantially to data acquisition and interpretation. Y.H.A. contributed to the study’s conception, drafting of the article, and data interpretation. All authors read and gave their final approval of the version to be published.
References
- Watson P. A., Arora V. K. & Sawyers C. L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer 15, 701–711 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. L. et al. Androgen deprivation leads to increased carbohydrate metabolism and hexokinase 2-mediated survival in Pten/Tp53-deficient prostate cancer. Oncogene, doi: 10.1038/onc.2016.223. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadi M. V., Walsh P. C. & Barrack E. R. Immunohistochemical study of androgen receptors in metastatic prostate cancer. Comparison of receptor content and response to hormonal therapy. Cancer 67, 3057–3064 (1991). [DOI] [PubMed] [Google Scholar]
- van der Kwast T. H. et al. Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int J Cancer 48, 189–193 (1991). [DOI] [PubMed] [Google Scholar]
- Mills I. G. Maintaining and reprogramming genomic androgen receptor activity in prostate cancer. Nat Rev Cancer 14, 187–198 (2014). [DOI] [PubMed] [Google Scholar]
- Grasso C. S. et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 487, 239–243 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y., Myers M. & Brown M. Formation of the androgen receptor transcription complex. Mol Cell 9, 601–610 (2002). [DOI] [PubMed] [Google Scholar]
- Storm M., Sheng X., Arnoldussen Y. J. & Saatcioglu F. Prostate cancer and the unfolded protein response. Oncotarget, doi: 10.18632/oncotarget.9912. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa T. et al. Androgen-induced expression of endoplasmic reticulum (ER) stress response genes in prostate cancer cells. Oncogene 21, 8749–8758 (2002). [DOI] [PubMed] [Google Scholar]
- Sheng X. et al. Divergent androgen regulation of unfolded protein response pathways drives prostate cancer. EMBO Mol Med 7, 788–801 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada R., Kanemoto S., Kondo S., Saito A. & Imaizumi K. The signalling from endoplasmic reticulum-resident bZIP transcription factors involved in diverse cellular physiology. J Biochem 149, 507–518 (2011). [DOI] [PubMed] [Google Scholar]
- Kim T. H. et al. Identification of Creb3l4 as an essential negative regulator of adipogenesis. Cell Death Dis 5, e1527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamori I. et al. The testes-specific bZip type transcription factor Tisp40 plays a role in ER stress responses and chromatin packaging during spermiogenesis. Genes Cells 11, 1161–1171 (2006). [DOI] [PubMed] [Google Scholar]
- Labrie C. et al. Androgen-regulated transcription factor AIbZIP in prostate cancer. J Steroid Biochem Mol Biol 108, 237–244 (2008). [DOI] [PubMed] [Google Scholar]
- Qi H. et al. AIbZIP, a novel bZIP gene located on chromosome 1q21.3 that is highly expressed in prostate tumors and of which the expression is up-regulated by androgens in LNCaP human prostate cancer cells. Cancer Res 62, 721–733 (2002). [PubMed] [Google Scholar]
- Levesque M. H., El-Alfy M., Berger L., Labrie F. & Labrie C. Evaluation of AIbZIP and Cdc47 as markers for human prostatic diseases. Urology 69, 196–201 (2007). [DOI] [PubMed] [Google Scholar]
- Lee M. Y. et al. KLF5 enhances SREBP-1 action in androgen-dependent induction of fatty acid synthase in prostate cancer cells. Biochem J 417, 313–322 (2009). [DOI] [PubMed] [Google Scholar]
- Baumer N. et al. Inhibitor of cyclin-dependent kinase (CDK) interacting with cyclin A1 (INCA1) regulates proliferation and is repressed by oncogenic signaling. J Biol Chem 286, 28210–28222 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. et al. BAP18 coactivates androgen receptor action and promotes prostate cancer progression. Nucleic Acids Res, doi: 10.1093/nar/gkw472. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Aicha S. et al. Transcriptional profiling of genes that are regulated by the endoplasmic reticulum-bound transcription factor AIbZIP/CREB3L4 in prostate cells. Physiol Genomics 31, 295–305 (2007). [DOI] [PubMed] [Google Scholar]
- Cunha A. C., Weigle B., Kiessling A., Bachmann M. & Rieber E. P. Tissue-specificity of prostate specific antigens: comparative analysis of transcript levels in prostate and non-prostatic tissues. Cancer Lett 236, 229–238 (2006). [DOI] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13, 89–102 (2012). [DOI] [PubMed] [Google Scholar]
- Romero-Ramirez L. et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res 64, 5943–5947 (2004). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Preventing oxidative stress: a new role for XBP1. Cell Death Differ 16, 847–857 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamori I. et al. Tisp40, a spermatid specific bZip transcription factor, functions by binding to the unfolded protein response element via the Rip pathway. Genes Cells 10, 575–594 (2005). [DOI] [PubMed] [Google Scholar]
- Swinnen J. V. et al. Androgens, lipogenesis and prostate cancer. J Steroid Biochem Mol Biol 92, 273–279 (2004). [DOI] [PubMed] [Google Scholar]
- Swinnen J. V., Brusselmans K. & Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care 9, 358–365 (2006). [DOI] [PubMed] [Google Scholar]
- Soo-Yeon P. et al. SUMOylation of TBL1 and TBLR1 promotes androgen-independent prostate cancer cell growth. Oncotarget, doi: 10.18632/oncotarget.9002. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A., Deplancke B., Walhout A. J. & Tissenbaum H. A. Chromatin immunoprecipitation (ChIP) coupled to detection by quantitative real-time PCR to study transcription factor binding to DNA in Caenorhabditis elegans. Nat Protoc 3, 698–709 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie-Inoue K., Bono H., Okazaki Y. & Inoue S. Identification and functional analysis of consensus androgen response elements in human prostate cancer cells. Biochem Biophys Res Commun 325, 1312–1317 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.