Abstract
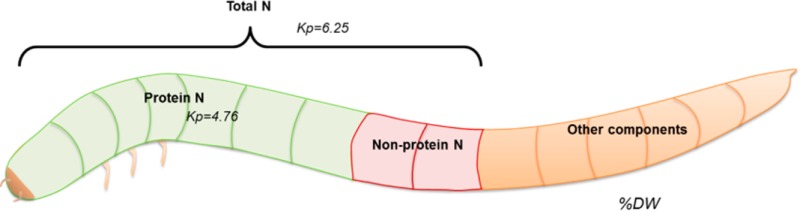
Insects are considered a nutritionally valuable source of alternative proteins, and their efficient protein extraction is a prerequisite for large-scale use. The protein content is usually calculated from total nitrogen using the nitrogen-to-protein conversion factor (Kp) of 6.25. This factor overestimates the protein content, due to the presence of nonprotein nitrogen in insects. In this paper, a specific Kp of 4.76 ± 0.09 was calculated for larvae from Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens, using amino acid analysis. After protein extraction and purification, a Kp factor of 5.60 ± 0.39 was found for the larvae of three insect species studied. We propose to adopt these Kp values for determining protein content of insects to avoid overestimation of the protein content.
Keywords: protein extraction, Tenebrio molitor, Alphitobius diaperinus, Hermetia illucens, black soldier fly, yellow mealworm, lesser mealworm, edible insects, amino acids, nitrogen-to-protein conversion factor (Kp)
Introduction
There is increasing interest in alternative protein sources to feed the increasing world population.1 Insects represent one of the potential sources to exploit. The high protein content, 40–75% on dry matter basis, makes insects a promising protein alternative for both food and feed.2 Their nutritional composition and ease of rearing makes insects especially interesting for food and feed production when they are in the larval stage.3 To use insects as an alternative food protein source, efficient protein extraction is a prerequisite, as potential consumers do not like to recognize the insects as such.
The protein content of different insect species in the literature is mainly based on nitrogen content using the nitrogen-to-protein conversion factor (Kp) of 6.25 generally used for proteins.2,4−8 The presence of nonprotein nitrogen (NPN) in insects, for example, chitin, nucleic acids, phospholipids, and excretion products (e.g., ammonia) in the intestinal tract, could lead to an overestimation of the protein content.9,10 Finke estimated that the amount of nitrogen present from chitin would not significantly increase the total amount of nitrogen.11
The aim of this research was to determine the specific nitrogen-to-protein conversion factor (Kp) for larvae of three insect species and their protein extracts using amino acid composition data. In this way an accurate protein content can be determined from the analysis of the nitrogen content. Larvae of Tenebrio molitor (yellow mealworm), Alphitobius diaperinus (lesser mealworm), and Hermetia illucens (black soldier fly) were used.
Materials and Methods
T. molitor
and A. diaperinus larvae were purchased from Kreca Ento-Feed BV (Ermelo, The Netherlands). H. illucens larvae were kindly provided by the Laboratory of Entomology (Wageningen University, The Netherlands). Larvae were frozen with liquid nitrogen and stored at −22 °C. The larvae from the three species were freeze-dried before chitin, nitrogen, and amino acid analysis.
The dry matter content and ash content were determined gravimetrically by drying and incinerating the samples at, respectively, 105 and 525 °C overnight in triplicate.
For carbohydrate analysis, larvae were frozen and ground in liquid nitrogen. The ground larvae were freeze-dried and subsequently hydrolyzed and analyzed for carbohydrates according to the method of Gilbert-López et al.12 with some modifications. An ICS-3000 Ion Chromatography HPLC system equipped with a Dionex CarboPac PA-1 column (2 × 250 mm) in combination with a Dionex CarboPac PA guard column (2 × 25 mm) and a pulsed electrochemical detector in pulsed amperometric detection mode was used (ThermoFisher Scientific, Breda, The Netherlands). A flow rate of 0.25 mL min–1 was used, and the column was equilibrated with H2O. Elution was performed as follows: 0–35 min, H2O; 35–50 min, 0–40% 1 M sodium acetate in 100 mM NaOH; 50–55 min, 1 M sodium acetate in 100 mM NaOH; 55–60 min, 150 mM NaOH; 70–85 min, H2O. Detection of the monosaccharides was possible after post column addition of 0.5 M sodium hydroxide (0.15 mL min–1). Elution was performed at 20 °C, and to discriminate between glucose and glucosamine an additional run was performed at 28 °C using the same settings.
Fat content was determined gravimetrically after petroleum ether extraction using Soxhlet in duplicate.13
For protein extraction, frozen larvae were blended at 4 °C in 0.1 M citric acid–0.2 M disodium phosphate buffer at pH 6 in a ratio of 1:4 (w/v) using a kitchen blender (Philips, Eindhoven, The Netherlands). The obtained solutions were centrifuged for 20 min at 25800g and 15 °C using a high-speed centrifuge (Beckman Coulter, Woerden, The Netherlands). The supernatant was filtered twice through cellulose filter paper (grade: 424, VWR, USA) and dialyzed at 4 °C at a cutoff of 12–14 kDa (Medicell Membranes Ltd., London, UK). Dialyzed protein extracts were considered as soluble protein extract and stored at −20 °C after freeze-drying. Extraction was performed in duplicate.
Amino acid composition was determined in duplicate by using the ISO13903:2005 method,14 adjusted for microscale. The amide nitrogen from Asn/Gln was measured together with Asp/Glu. The amount of tryptophan was determined on the basis of AOAC 988.15. Total protein content was calculated from the total amino acid content.
Nitrogen content (Nt) was determined in triplicate according to the Dumas method using a Flash EA 1112 NC analyzer (Thermo Fisher Scientific Inc., Waltham, MA, USA) following the manufacturer’s protocol. Average Kp values were calculated from the ratio of the sum of amino acid residue weights to Nt. Kp values were statistically evaluated by analysis of variance (ANOVA) with the SPSS 23 program. The percentage protein nitrogen from total nitrogen was determined by total amino acid nitrogen (Naa)/Nt. The lower limit of this percentage was calculated on the basis of the theoretical value with 100% Asp/Glu and the upper level with 100% Asn/Gln.15
Results and Discussion
Nutritional Composition of Whole Insects
The amino acid profile from both whole larvae and their protein extract contains high amounts of all essential amino acids (Table 1). Overall, amino acid profiles were comparable as observed before for T. molitor, A. diaperinus,4 and H. illucens.8,16 From the amino acid profiles, the total nitrogen from amino acids and the accurate protein content were determined (Table 2).
Table 1. Amino Acid Composition (g/100 g Protein) and Total Amino Acid (AA) Content (w/w % dw) for Whole Larvae from Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens and Their Protein Extractsa.
| T. molitor | A. diaperinus | H. illucens | T. molitor extract | A. diaperinus extract | H. illucens extract | |
|---|---|---|---|---|---|---|
| His | 3.56 (±0.05) | 3.97 (±0.01) | 3.85 (±0.02) | 2.61 (±0.00) | 2.98 (±0.00) | 3.64 (±0.01) |
| Ile | 4.99 (±0.02) | 4.61 (±0.00) | 4.59 (±0.01) | 5.54 (±0.01) | 5.04 (±0.01) | 5.18 (±0.00) |
| Leu | 8.33 (±0.02) | 7.32 (±0.01) | 7.45 (±0.07) | 9.36 (±0.02) | 8.03 (±0.00) | 7.99 (±0.00) |
| Lys | 6.14 (±0.08) | 7.05 (±0.01) | 6.91 (±0.02) | 6.12 (±0.05) | 8.42 (±0.02) | 9.16 (±0.00) |
| Met | 1.52 (±0.04) | 1.59 (±0.01) | 2.00 (±0.01) | 1.44 (±0.01) | 1.68 (±0.02) | 2.53 (±0.02) |
| Cys | 1.13 (±0.01) | 0.96 (±0.00) | 0.97 (±0.02) | 1.92 (±0.01) | 1.29 (±0.00) | 1.32 (±0.01) |
| Tyr | 5.80 (±0.01) | 8.49 (±0.01) | 6.54 (±0.02) | 5.01 (±0.03) | 6.91 (±0.02) | 6.28 (±0.00) |
| Phe | 3.64 (±0.00) | 5.17 (±0.05) | 4.49 (±0.05) | 5.10 (±0.00) | 5.76 (±0.01) | 7.18 (±0.01) |
| Val | 6.42 (±0.04) | 5.76 (±0.01) | 6.10 (±0.06) | 6.16 (±0.00) | 5.50 (±0.03) | 5.61 (±0.00) |
| Trp | 1.50 (±0.01) | 1.47 (±0.04) | 1.87 (±0.01) | nd | nd | nd |
| Thr | 4.52 (±0.03) | 4.31 (±0.00) | 4.34 (±0.01) | 5.85 (±0.02) | 5.09 (±0.00) | 4.95 (±0.00) |
| Ser | 5.03 (±0.01) | 4.41 (±0.00) | 4.54 (±0.01) | 5.06 (±0.02) | 4.54 (±0.00) | 3.99 (±0.01) |
| Asx | 9.21 (±0.09) | 9.38 (±0.05) | 10.62 (±0.18) | 14.29 (±0.13) | 12.78 (±0.01) | 12.56 (±0.06) |
| Glx | 12.30 (±0.18) | 13.01 (±0.04) | 13.68 (±0.01) | 13.53 (±0.14) | 14.85 (±0.06) | 12.13 (±0.03) |
| Gly | 4.98 (±0.03) | 4.20 (±0.00) | 4.92 (±0.05) | 4.25 (±0.00) | 3.79 (±0.00) | 3.88 (±0.01) |
| Ala | 7.40 (±0.16) | 6.58 (±0.03) | 6.23 (±0.08) | 4.89 (±0.01) | 4.43 (±0.00) | 4.66 (±0.02) |
| Pro | 7.96 (±0.18) | 6.36 (±0.08) | 5.85 (±0.12) | 4.80 (±0.06) | 4.58 (±0.11) | 4.38 (±0.01) |
| Arg | 5.57 (±0.02) | 5.35 (±0.00) | 5.06 (±0.05) | 4.07 (±0.03) | 4.25 (±0.01) | 4.57 (±0.01) |
| sum AA | 44.74 (±0.11) | 49.58 (±0.52) | 36.00 (±0.31) | 67.91 (±1.31) | 72.74 (±0.82) | 67.77 (±0.60) |
Asx, no separate analysis of Asp/Asn; Glx, no separate analysis of Glu/Gln (mean ± SD, n = 2). nd, not determined.
Table 2. Total Nitrogen (Nt), Protein Nitrogen (Naa), Chitin Nitrogen (NGlcN), Naa/Nt Ratio, and Nitrogen-to-Protein Conversion Factors (Kp) of Different Whole Larvae and Protein Extracts from Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens (% dw)a.
| Naa |
protein content |
protein yield
(%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nt | Asp-Glu | Asn-Gln | NGlcN | Naa/Nt (%) | Kp | Kp new | Kp 6.25 | Kp new | Kp 6.25 | |
| T. molitor | 9.41 (±0.03) | 7.20 (±0.02) | 8.31 (±0.04) | 0.624 (±0.041) | 77 < x < 88 | 4.75 | 44.8 (±0.1) | 58.8 (±0.2) | ||
| A. diaperinus | 10.21 (±0.05) | 7.84 (±0.11) | 9.13 (±0.12) | 0.304 (±0.018) | 77 < x < 89 | 4.86 | 48.6 (±0.2) | 63.8 (±0.3) | ||
| H. illucens | 7.70 (±0.06) | 5.71 (±0.05) | 6.72 (±0.07) | 0.529 (±0.000) | 74 < x < 87 | 4.67 | 36.7 (±0.3) | 48.1 (±0.4) | ||
| T. molitor extract | 12.15 (±0.53) | 10.31 (±0.26) | 12.52 (±0.29) | nd | 85 < x < 103 | 5.59 | 68.1 (±3.0) | 76.0 (±3.3) | 23.5 (±0.4) | 17.6 (±1.3) |
| A. diaperinus extract | 13.00 (±1.09) | 11.09 (±0.15) | 13.43 (±0.18) | nd | 85 < x < 103 | 5.59 | 72.8 (±6.1) | 81.3 (±7.0) | 19.1 (±0.7) | 16.9 (±2.1) |
| H. illucens extract | 12.06 (±0.13) | 10.52 (±0.11) | 12.48 (±0.13) | nd | 87 < x < 103 | 5.62 | 67.6 (±0.7) | 75.4 (±0.8) | 17.1 (±1.9) | 14.4 (±1.4) |
| av larvae | 76 < x < 88 | 4.76 (±0.09) | ||||||||
| av insect protein extract | 86 < x < 103 | 5.60 (±0.02) | ||||||||
Protein extraction yield and content based on estimated Kp and Kp 6.25 (mean ± S.D, n = 2). nd, not determined.
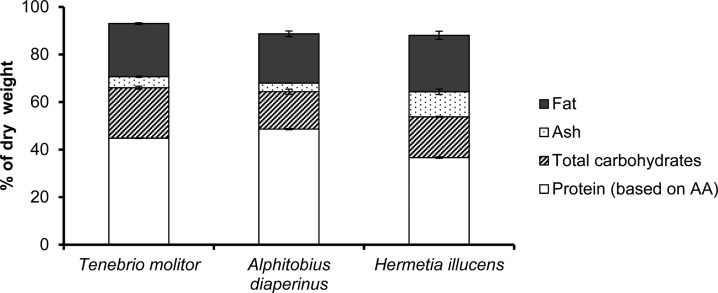
General composition data are summarized in Figure 1. The protein values based on amino acid content for T. molitor and A. diaperinus were lower compared to those of Yi et al.4A. diaperinus showed the highest protein content based on total amino acid content within the tested species. The total carbohydrate content within the three species ranged from 15 to 21%. The fat content for the three species ranged from 21 to 24% based on dry matter. In the literature, fat contents between 27 and 49% for T. molitor,4,6,13 between 20 and 22% for A. diaperinus(4,16) and between 13 and 36% for H. illucens(8,16) have been reported. Differences in chemical composition were probably caused by different diets.6,17 Our results show that proteins, fats, and carbohydrates accounted for around 90% of the total dry matter; the remainder might come from other organic components, that is, phenols and nucleic acids.
Figure 1.
General composition (% dw) of whole insect larvae from Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. The protein content was based on amino acid (AA) composition.
Nitrogen-to-Protein Conversion Factors
To determine the protein content from total nitrogen content, the Kp and ratio Naa/Nt were calculated (Table 2). Interestingly, comparable Kp values were found among larvae of the three species with an average Kp value of 4.76 ± 0.09, despite the fact that H. illucens belongs to a different order (Diptera) from T. molitor and A. diaperinus (which are Tenebriodinae family members within the Coleoptera order). This Kp value was significantly lower (P < 0.001) than the general nitrogen factor of 6.25, which has been used up to now to calculate the protein content of insects.4−6,8,16 The Kp values found for insects are similar to those calculated for different tropical plants (Kp range 3.7–5.0)18 and microalgae (Kp range 2.53–5.77),12,15,19 as well as different grains and legumes (Kp range 5.09–5.38).20 Higher values between 5.14 and 6.26 were found for meat, fish, and egg.21
This new Kp value gives a more accurate estimation of protein content by taking the presence of NPN into account. This leads to >20% lower values for protein content compared to literature values, which are based on Kp of 6.25. Therefore, the protein content of T. molitor calculated in this study was 45%, which falls in the low range (45–65%) found in the literature based on Kp of 6.25.6,22 The protein content falls out of the range for the larvae of A. diaperinus, for which a value of 49% was found compared to the literature values of 58–65% protein.4,16,22 Also for H. illucens, a lower value of 36% was found compared to the range of 37–56% from the literature.16,23 When protein content is calculated from our data using a Kp of 6.25, the results do fall again into the ranges reported in the literature.
The average Kp value of 5.60 ± 0.24 obtained for soluble protein extracted from insects was significantly (P < 0.001) higher compared to that for whole larvae, due to the removal of NPN. Again, comparable Kp values among the three species were found.
Nonprotein Nitrogen
The calculated Naa/Nt ratio showed the presence of 11–26% NPN in whole larvae of all three insect species (Table 2). T. molitor contained 12–23% NPN, which is in line with Finke.7 The NPN of 16–26% present in H. illucens is higher compared to the 2% found in the literature, whereas the amino acid composition and content were similar.8 Besides the analytical procedures, differences in composition and recovery might be also caused by different diets fed to the insects.17
Carbohydrates, such as chitin and chitosan, have glucosamine or N-acetylglucosamine with nitrogen as building blocks. During the hydrolysis conditions used, N-acetylglucosamine was converted into N-glucosamine. The total amount of (N-acetyl)glucosamine within polymers for the three insect species was 4.4–9.1% (w/w), corresponding to approximately one-third of the carbohydrates present, similar to results based on acid detergent fiber fraction for T. molitor.24
The chitin content comprised 3.0–6.8% nitrogen of the total nitrogen. Apart from chitin, NPN might originate from nucleic acids.9 Part of the NPN can also come from inorganic nitrogen. Examples of inorganic nitrogen are excretion products in the intestinal tract of the larvae, such as uric acid, urea, and ammonia.10 This is in agreement with the removal of most NPN during dialysis of the protein extracts.
Protein Extraction Yields
The average Kp values for the whole larvae and extracts were used to determine the protein content and extraction yield based on nitrogen (Table 2). Protein extraction yields between 17.1 and 23.5% were calculated using the insect-specific Kp factors, and these were higher compared to those obtained with the general Kp of 6.25 (14.4–17.6%). This is due to a larger overestimation of the protein content within the whole larvae when the factor of 6.25 was used caused by NPN.
When insect larvae are considered as an alternative protein source, overestimation of the protein content, due to the presence of NPN, should be avoided. To avoid overestimation of protein content in insects, we propose the use of a Kp value of 4.76 for the quantification of protein content in whole larvae and a Kp of 5.60 for the protein extracts derived from insects.
Acknowledgments
We kindly acknowledge Dennis Oonincx for providing the Hermetia illucens larvae. We are grateful to the Animal Nutrition group from Wageningen University & Research for performing the amino acid analysis.
Glossary
Abbreviations Used
- Kp
ratio of the sum of nitrogen from amino acid residue weights to total nitrogen from Dumas measurement
- Nt
total nitrogen content based on Dumas measurement
- Naa
total nitrogen from amino acid analysis
- NGlcN
total nitrogen from glucosamine
This research was funded by the Protein Innovation Program STW together with the Dutch Ministry of Economic Affairs within the project In2Food.
The authors declare no competing financial interest.
References
- van Huis A.; van Itterbeeck J.; Klunder H.; Mertens E.; Halloran A.; Muir G.; Vantomme P.. Edible Insects. Future Prospects for Food and Feed Security; FAO: Rome, Italy, 2013; Vol. 171. [Google Scholar]
- Bukkens S. G. F. The nutritional value of edible insects. Ecol. Food Nutr. 1997, 36 (2–4), 287–319. 10.1080/03670244.1997.9991521. [DOI] [Google Scholar]
- Ghaly A. E.; Alkoaik F. N. The yellow mealworm as a novel source of protein. Am. J. Agric. Biol. Sci. 2009, 4 (4), 319–331. 10.3844/ajabssp.2009.319.331. [DOI] [Google Scholar]
- Yi L.; Lakemond C. M. M.; Sagis L. M. C.; Eisner-Schadler V.; Van Huis A.; Van Boekel M. A. J. S. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141 (4), 3341–3348. 10.1016/j.foodchem.2013.05.115. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Vázquez-Gutiérrez J. L.; Johansson D. P.; Landberg R.; Langton M. Yellow mealworm protein for food purposes – extraction and functional properties. PLoS One 2016, 11 (2), 1–17. 10.1371/journal.pone.0147791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpold B. A.; Schlüter O. K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57 (5), 802–823. 10.1002/mnfr.201200735. [DOI] [PubMed] [Google Scholar]
- Finke M. D. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 2002, 21 (3), 269–285. 10.1002/zoo.10031. [DOI] [Google Scholar]
- Finke M. D. Complete nutrient content of four species of feeder insects. Zoo Biol. 2013, 32 (1), 27–36. 10.1002/zoo.21012. [DOI] [PubMed] [Google Scholar]
- Mariotti F.; Tomé D.; Mirand P. P. Converting nitrogen into protein—beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48 (2), 177–184. 10.1080/10408390701279749. [DOI] [PubMed] [Google Scholar]
- Weihrauch D.; Donini A.; O’Donnell M. J. Ammonia transport by terrestrial and aquatic insects. J. Insect Physiol. 2012, 58 (4), 473–487. 10.1016/j.jinsphys.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Finke M. D. Estimate of chitin in raw whole insects. Zoo Biol. 2007, 26, 105–115. 10.1002/zoo.20123. [DOI] [PubMed] [Google Scholar]
- Gilbert-López B.; Mendiola J. A.; Fontecha J.; van den Broek L. A. M.; Sijtsma L.; Cifuentes A.; Herrero M.; Ibáñez E. Downstream processing of Isochrysis galbana: a step towards microalgal biorefinery. Green Chem. 2015, 17 (9), 4599–4609. 10.1039/C5GC01256B. [DOI] [Google Scholar]
- Tzompa-Sosa D. A.; Yi L.; van Valenberg H. J. F.; van Boekel M. A. J. S.; Lakemond C. M. M. Insect lipid profile: aqueous versus organic solvent-based extraction methods. Food Res. Int. 2014, 62, 1087–1094. 10.1016/j.foodres.2014.05.052. [DOI] [Google Scholar]
- ISO 13903:2005. Animal feeding stuff – determination of amino acids content; international organization for standardization; Geneva, Switzerland, 2005.
- Schwenzfeier A.; Wierenga P. A.; Gruppen H. Isolation and characterization of soluble protein from the green microalgae Tetraselmis sp. Bioresour. Technol. 2011, 102 (19), 9121–9127. 10.1016/j.biortech.2011.07.046. [DOI] [PubMed] [Google Scholar]
- Bosch G.; Zhang S.; Oonincx D. G. A. B.; Hendriks W. H. Protein quality of insects as potential ingredients for dog and cat foods. J. Nutr. Sci. 2014, 3 (29), 1–4. 10.1017/jns.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oonincx D. G. A. B.; Dierenfeld E. S. An investigation into the chemical composition of alternative invertebrate prey. Zoo Biol. 2012, 31, 40–54. 10.1002/zoo.20382. [DOI] [PubMed] [Google Scholar]
- Milton K.; Dintzis F. R. Nitrogen-to-protein conversion factors for tropical plant samples. Biotropica 1981, 13 (3), 177–181. 10.2307/2388122. [DOI] [Google Scholar]
- Lourenço S. O.; Barbarino E.; Lavín P. L.; Lanfer Marquez U. M.; Aidar E. Distribution of intracellular nitrogen in marine microalgae: calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39 (1), 17–32. 10.1080/0967026032000157156. [DOI] [Google Scholar]
- Mosse J. Nitrogen-to-protein conversion factor for ten cereals and six legumes or oilseeds. A reappraisal of its definition and determination. Variation according to species and. J. Agric. Food Chem. 1990, 38, 18–24. 10.1021/jf00091a004. [DOI] [Google Scholar]
- Sosulski F. W.; Imafidon G. I. Amino acid composition and nitrogen-to-protein conversion factors for animal and plant foods. J. Agric. Food Chem. 1990, 38 (6), 1351–1356. 10.1021/jf00096a011. [DOI] [Google Scholar]
- van Broekhoven S.; Oonincx D. G. A. B.; van Huis A.; van Loon J. J. A. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. 10.1016/j.jinsphys.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Sánchez-Muros M. J.; Barroso F. G.; Manzano-Agugliaro F. Insect meal as renewable source of food for animal feeding: a review. J. Cleaner Prod. 2014, 65, 16–27. 10.1016/j.jclepro.2013.11.068. [DOI] [Google Scholar]
- Barker D.; Fitzpatrick M. P.; Dierenfeld E. S. Nutrient composition of selected whole invertebrates. Zoo Biol. 1998, 17, 123–134. . [DOI] [Google Scholar]