Abstract
The chronic nature and associated complications of nonhealing wounds have led to the emergence of nanotechnology-based therapies that aim at facilitating the healing process and ultimately repairing the injured tissue. A number of engineered nanotechnologies have been proposed demonstrating unique properties and multiple functions that address specific problems associated with wound repair mechanisms. In this outlook, we highlight the most recently developed nanotechnology-based therapeutic agents and assess the viability and efficacy of each treatment, with emphasis on chronic cutaneous wounds. Herein we explore the unmet needs and future directions of current technologies, while discussing promising strategies that can advance the wound-healing field.
Short abstract
A myriad of advanced nanotechnology-driven therapies were designed for targeting specific problems of chronic wound healing. The clinical application of these therapies still requires intensive research for the standardization of nanotechnologies.
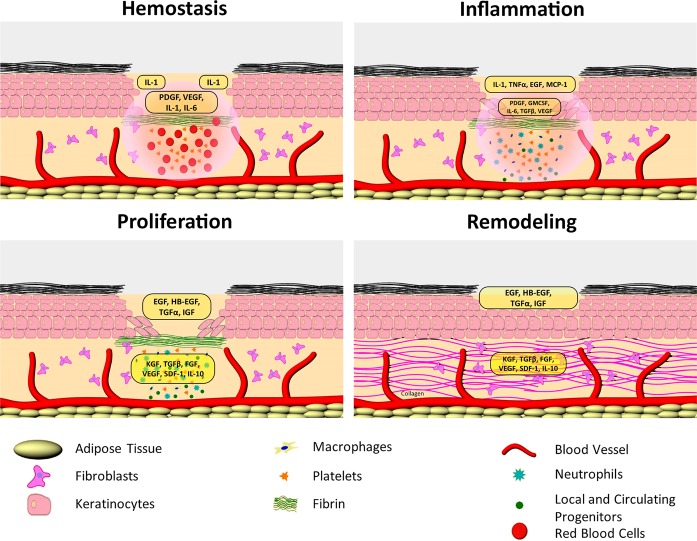
Wound healing in skin is an evolutionarily conserved, highly coordinated, spatiotemporally regulated process. It occurs over the sequential yet overlapping phases of hemostasis, inflammation, proliferation, and remodeling.1,2 The phases of the cutaneous wound healing are executed by coordinated action of multiple cell types including keratinocytes, fibroblasts, and immune, endothelial, and progenitor cells (Figure 1).1,3 These steps involve multiple cellular and molecular events tightly controlled by numerous growth factors, chemokines, and cytokines. In addition, the dynamic relationship between skin and microbiome is another major contributor to the outcome of this process. Signaling networks involving the interleukin (IL) family and growth factors are required to coordinate cell–cell and cell–extracellular matrix (ECM) interactions essential to fully heal a wound, with imbalances leading to a nonhealing, chronic wound.1,4,5 Chronic wounds are characterized by unresolved inflammation, nonmigratory epidermis, impaired fibroblast function and ECM deposition, decreased angiogenesis, increased levels of proteases, and bacterial colonization and/or infection.4,6−10 The incidence of chronic wounds (venous, diabetic foot, or pressure ulcers) is on the rise, reaching epidemic proportions, which justifies the increased interest in finding more efficient therapies.1 Nanotechnology-based diagnostics and treatment approaches offer an excellent opportunity to target the complexity of the normal wound-healing process, cell type specificity, and plethora of regulating molecules as well as pathophysiology of chronic wounds. Conceptually, cutaneous wound healing has major advantages for use of nanotherapeutics. It requires topical delivery, can be multifactorial and cell-type specific, and the therapeutic agent is used for a limited time or until the wound has healed.
Figure 1.
Phases of cutaneous wound healing depicting the cells and molecules responsible for the regaining of a healthy barrier.
To date, there are only four FDA-approved therapies for chronic cutaneous wounds, including a bioengineered human skin equivalent, two dermal substitutes, and recombinant human platelet derived growth factor (rhPDGF).11−15 Human skin equivalent and dermal substitutes have been suggested to act as a “smart” biomaterial by interacting with the wound environment to promote a healthy healing process. PDGF exhibits beneficial effects during wound healing by stimulating chemotaxis of neutrophils, macrophages, fibroblasts, and smooth muscle cells.16,17 However, 44 to 70% of treated patients affected with chronic ulcers remain unhealed, even in the clinical trials that lead to FDA approval.12,18,19 Better understanding of the wound-healing process has also contributed to the emergence of contemporary dressing-based therapies (Table 1).20,21 Thus, modern dressings were custom designed to provide the beneficial microenvironment for successful healing by controlling wound moisture and absorbing excess exudate.22 Active dressings may locally alter the wound’s biochemical environment by targeting bacterial load and excessive protease levels, or providing exogenous collagen matrices which serve as a scaffold for tissue ingrowth when endogenous collagen is disrupted by the proteolytic wound environment (Table 1).23,24 Despite trials demonstrating the benefits of improved dressings incorporating recombinant growth factors and cells, many of the modalities used in clinical practice are based on safety data and clinical experience rather than evidence of efficacy.25
Table 1. Current Traditional and Modern Approaches Used for Wound Healinga.
| classification |
||||
|---|---|---|---|---|
| type of therapy | benefits | limitations | traditional | modern |
| biomaterial-based dressings (grafts and bioengineered skin substitutes) | restoration of functional components of the tissue; severe burns or chronic wounds with loss of important portion of the skin | reduced vascularization, poor mechanical integrity, and immune rejection | ×28,29 | |
| cell/growth factor therapy | regenerative strategies for targeting chronic wounds | rapid breakdown of growth factors/impairing stem cells proliferation by chronic wound fluid | ×30,31 | |
| artificial dressings (e.g., polymers) | mimic some physical and biological properties pertinent to native tissues including high water content, biocompatibility, and biodegradability | lack of bioactive component | ×23 | |
| silver dressings | good clinical efficacy, simplicity, and affordability | toxic at specific concentration | ×21 | |
| natural substances (e.g., herbs, honey, maggots) | simplicity, and affordability | unexpected allergic reaction, variable clinical results | ×21 | |
The failure of many novel approaches in delivering specific outcomes such as wound closure, control of fluid loss, and exhibiting properties such as durability, elasticity, and histocompatibility has led to the introduction of numerous nanotechnological advances.32 Furthermore, the field of chronic wounds is in dire need of robust, more efficacious treatments that are able to address a dysfunctional healing process at multiple cellular levels. These findings underscore the need for development and implementation of novel nanotechnology-driven therapies. Nanotherapeutic approaches that employ materials engineered with at least one dimension within the nanoscale (1–100 nm) were pioneered to efficiently control wound healing and minimize any possible complication that might surface during this process.32,33 The major advantage of nanomaterials over their bulk counterparts is the versatility and tunability of the nanomaterial’s physicochemical properties (e.g., hydrophobicity, charge, size).20 Furthermore, the high surface area to volume ratio endows nanostructures with unique features.32 For example, nanoscale particles provide for a high probability of interaction with the biological target and an enhanced penetration into the wound site.33 As a result, nanoparticles have an ability to deliver a sustained and controlled release of therapeutics that results in an accelerated healing process.23
In this outlook, we highlight the most recent advances in nanotechnology that contributed to a paradigm shift in wound-healing therapeutics. We will briefly introduce the newly developed nanomaterials, nanoengineering processes, and gene, growth factor, and stem cell therapies for wound healing. New insights in the field of nanotechnology-based targeted delivery of therapeutic agents for wound healing are also featured.
Nanotechnology in Wound Healing
An increasing number of innovative nanotherapies have emerged in the field of wound healing and are currently under clinical investigation (Table 2). Various nanoscale strategies were explored for targeting different phases of wound repair (Figure 2), including nanomaterials. There are two main categories of nanomaterials used in wound healing: (1) nanomaterials that exhibit intrinsic properties beneficial for wound treatment and (2) nanomaterials employed as delivery vehicles for therapeutic agents.20,34
Table 2. Select Examples of Nanotechnologies Recently Reported for Wound-Healing Applications.
| type of nanotechnology | specific characteristics | application | references |
|---|---|---|---|
| Nanomaterials | |||
| silver nanomaterials | antibacterial and anti-inflammatory properties, and better appearance of healed wounds | burn wounds and diabetic ulcers | (35,36) |
| copper nanoparticles | antimicrobial activity and enhanced pace of wound healing (biosynthesized copper nanoparticles) | excisional wound in rat model | (37) |
| cerium nanoparticles (nanoceria) | antioxidant properties and faster wound closure | full-thickness murine wounds | (38) |
| nanosized bioactive glass particles | inducing cell proliferation, angiogenesis, and wound closure | in vitro wound-healing assay using cultured human umbilical vein endothelial cells (HUVECs) | (39) |
| zinc oxide nanomaterials | infection control in wound healing, promoting angiogenesis, cell proliferation, and chemotaxis | wound-healing assay using endothelial cells | (40) |
| carbon-based nanomaterials | antioxidant and anti-inflammatory properties; affect cell proliferation | in vitro assay using primary human keratinocytes | (41) |
| Nanomaterials for Delivery of Therapeutic Agents | |||
| chitosan–pectin–titanium dioxide nanodressing | antimicrobial, biocompatibility, and inherent bioactivity of chitosan; mechanical and antibacterial properties of TiO2; gelling properties of pectin | excisional wounds in rats | (42) |
| gold nanoparticles | functionalized with antibiotics, antioxidants and reactive oxygen species scavengers; used for gene delivery | diabetic murine wounds | (43) |
| nanoparticles bearing nitric oxide | acceleration of wound closure, reducing inflammation, and increasing fibroblast cells, collagen deposition, and neovascularization; antibiofilm activity | wounds in nonobese, diabetic, immunodefficient NOD-SCID mice; Pseudomonas aeruginosa-infected murine excisional wounds | (44,45) |
| lipids containing nanomaterials (e.g., liposomes) | delivery of phytodrugs with antioxidant and anti-inflammatory properties | in vitro and in vivo models of full-thickness skin defects | (46) |
| metal (silver) incorporated electrospun mats | reduction of silver ions by the polymer-based fibers (polyvinyl alcohol); targeting chronic wound biofilms | antibacterial activity against Staphylococcus aureus and Escherichia coli | (47) |
| Scaffolds | |||
| poly(lactide-co-glycolic acid) (PLGA)/silk fibroin (SF) hybrid nanofibrous scaffold | L929 cells attachment and proliferation; optimization of the ratio PLGA/SF is required | excisional wound model in diabetic rats | (48) |
| gelatin and poly-ε-caprolactone (PCL) nanofibers | fabrication of the nanofibers using needleless electrospinning technology; cell adhesion and proliferation | full thickness wounds in rats | (49) |
| fibrin–collagen–fibrin porous scaffold | matrices for the motility of fibroblasts, keratinocytes, and epidermal cells | skin regeneration | (50) |
| anodic aluminum oxide (AAO) | highly ordered porous structure; efficient synthesis; biocompatible, naturally inert, and nonreactive | in vitro migration of keratinocytes | (51,52) |
| Gene Therapy | |||
| dendrimers | gene therapy; delivery of minicircle plasmid DNA encoding vascular endothelial growth factor (VEGF); enhancement of angiogenesis | diabetic murine wounds | (53) |
| electrospun poly(l-lactide) PLA and PCL nanofibers | loaded with DNA plasmids encoding keratinocyte growth factor; improvement in the rate of wound reepithelialization, keratinocyte proliferation, and granulation response | murine wounds | (54) |
| spherical nucleic acid (SNA) gold nanoparticles | use of siRNA-based ganglioside-monosialic acid 3 synthase (GM3S) SNA to knock down the expression of GM3S mRNA | diabetic murine wounds | (55) |
| Growth Factor Therapy | |||
| PLGA nanoparticles loaded with vascular endothelial growth factor (VEGF) | combined effects of PLGA and VEGF; enhanced bioactivity of VEGF | nondiabetic and diabetic murine wounds | (56) |
| electrospun core/shell basic fibroblast growth factor (bFGF)/PCL–PEG block copolymer fibers | immobilization of EGF growth factor on the nanofibers; dual release of bFGF and EGF for a higher keratinocyte and fibroblast cellular proliferation | diabetic murine wounds | (57) |
| Stem Cell Therapy | |||
| nanofiber scaffolds functionalized with bone-marrow-derived mesenchymal stem cells (BM-MSCs) | complete and earlier wound closure than control group; involvement of BM-MSCs in epidermal differentiation | acute full-thickness burn wounds | (58) |
| aloe vera–PCL (AV/PCL) nanoscaffold with human umbilical cord Wharton’s jelly stem cells (hWJSCs) | synergistic effect of stem cells and nanoscaffold combined with the antibacterial effect of aloe vera | excisional and diabetic murine wounds | (59) |
Figure 2.
Schematic representation of the nanotechnology-based therapies employed in wound healing.
Nanomaterials as Intrinsic Therapeutic Agents
Metallic and Metal Oxide Nanomaterials
Silver is best known for its antibacterial effect mediated through the blockage of respiratory enzyme pathways and alteration of microbial DNA and the cell wall.60 The advantage of using silver for wound healing is that it is effective against multiresistant and biofilm-forming bacteria.47,61,62 Chronic wounds are characterized by bacterial biofilms, bacterial communities enclosed in a protective self-produced extracellular polymeric substance.63 Wound-associated biofilms have been shown to induce apoptosis, release of reactive oxygen species and inflammatory cytokines, contributing to chronic inflammation and inhibition of re-epithelialization.2,64,65 Moreover, microorganisms within biofilms represent a therapeutic challenge due to their persistent nature and resistance to conventional antimicrobial therapy.66,67
Silver compounds such as silver nitrate and silver sulfadiazine are extensively employed to treat chronic wound and burn infections.68,69 However, these compounds, especially silver sulfadiazine, might result in tissue toxicity. To circumvent this drawback, silver nanoparticles with high surface-to-volume ratio were synthesized which rendered these materials more efficient at low concentrations, thus resulting in reduced toxicity in comparison to traditional silver compounds.69 It was demonstrated that pure silver nanoparticles were able to treat inflammation through cytokine modulation and induce wound healing with decreased scar formation.35 Different shapes and sizes of silver nanostructures were also investigated and shown to achieve different degrees of antibacterial activity.70 Kelestemur et al. demonstrated that surface modification of silver nanoparticles with the thiolated oligonucleotide (5′-HS-(CH2)6- TAATGCTGAAGG-3) reduced the cytotoxic effect by prolonging the release of silver ions.71 Another form of silver, nanocrystalline silver, is also considered beneficial due to its efficiency in releasing aggregates of silver nanoparticles that can cover large wound surface area.36 Nanocrystalline silver dressings like Acticoat provided sustained release of Ag+, thereby overcoming the potential consumption of silver by interaction with target cells or inactivation by protein and anion complexes in the wound fluid.36 Nevertheless, the use of silver-based nanomaterials for wound healing still suffers from drawbacks such as blue-gray coloration of the skin upon prolonged use, and reoccurrence of silver-resistant bacteria.72−74
The intrinsic antibacterial properties of zinc oxide nanoparticles (nZnO) prompt the use of these nanomaterials in several hydrogel-based wound dressings.75,76 nZnO are less toxic to mammalian cells than silver nanoparticles, which makes these nanoparticles a suitable choice as an inorganic antibacterial agent.77,78 In a recent study, nZnO were introduced into a microporous chitosan hydrogel bandage.75 The prepared composite bandage had a high swelling ability that allowed the absorption of wound exudates, resulting in activation of platelets and blood clotting. In vivo studies demonstrated that the bandage possessed essential antibacterial properties and cytocompatibility as it promoted re-epithelialization and collagen deposition. In another study, ZnO nanoflowers (flower-like morphology of ZnO nanostructures) exhibited a proangiogenic activity, which was confirmed by in vitro and in vivo assays.40 However, despite the high potential of metallic nanoparticles in treating drug-resistant bacteria, these materials possess high toxicity, thus limiting their use in wound healing.79
Nonmetallic Nanomaterials
Carbon-based nanomaterials have been proposed for wound-healing applications. Carbon fullerenes exhibit interesting properties that offset several pathological mechanisms responsible for obstructing the wound-healing process.80 Some fullerene derivatives have demonstrated antioxidant and anti-inflammatory properties, which designates these materials as therapeutic agents for wound healing.80 A recent study reported the use of graphene oxide (GO) nanosheets for photothermal treatment of bacterial and fungal wound infection.81 A synergistic effect against different pathogens was elicited by action of GO and the heat generated from the irradiation of these nanomaterials with a near-infrared Nd:YAG laser. The application of the laser and GO resulted in a faster healing of infected wounds, yielding a noninvasive alternative to antibiotic treatment.
Polymeric nanoparticles, specifically naturally occurring polymers like chitosan nanoparticles, have been studied for their antibacterial activity and pro-wound-healing properties.32 Polymeric nanomaterial therapy involves the use of polymeric materials as dressings or as delivery vehicles.20 The natural polymer chitosan was selected as a dressing material because of its biocompatibility, biodegradability, hemostatic activity, and antibacterial properties.42 Chitosan composites usually exhibit unique properties that are not individually displayed by chitosan or the incorporated materials.82
Nanostructures as Carriers of Therapeutic Agents
Nitric Oxide Containing Nanocarriers
The nitric oxide free radical is one of the several intrinsic pro-wound-healing agents.83 The highest NO synthase (NOS) activity is observed during the early phases of wound healing when NO is synthesized by inflammatory cells.84,85 NO plays an important role in inflammation, cellular proliferation, ECM matrix deposition, angiogenesis, and matrix remodeling.23,86−88 Moreover, NO is an antibacterial agent effective against a broad range of bacteria, including biofilm forming microorganisms, through an oxidation process involving free radical superoxide (O2*–) to form peroxynitrite (−OONO). Several studies investigated the controlled release of NO using nanoscale delivery systems. An optimal NO delivery system should have high loading capacity, prolonged time release, and low cytotoxicity.89,90 To that end, a pH- and light-responsive gatekeeper system was designed to modulate a spatiotemporal NO release for corneal wound-healing application.90 In another study, NO-releasing poly(lactic-co-glycolic acid) (PLGA)–polyethylenimine (PEI) nanoparticles were synthesized for assessment of healing activity in methicillin-resistant Staphylococcus aureus (MRSA) infected wounds.89,91 The system allowed for a sustained and prolonged NO release due to the incorporation of PEI/diazeniumdiolate (NONOate) into the hydrophobic PLGA nanoparticle matrix and inhibition of the NONOate group degradation. The antibacterial activity of the designed nanoparticles was complemented by the enhanced wound healing observed upon treating various skin infections with these nanoparticles.
Antibiotics- and Antioxidant-Containing Nanoparticles
Chen et al. functionalized gold nanodots with antimicrobial peptides, known as natural antibiotics, in order to inhibit the growth of drug-resistant bacteria and promote healing in a rodent wound model.92 In this system, the cyclic lipopeptide surfactin (SFT) self-assembled on gold nanodots by hydrophobically interacting with 1-dodecanethiol (DT) molecules that cap gold nanodots. The nanoparticles exhibited much higher antibacterial potency against Gram-negative and Gram-positive bacteria than free SFT. The enhanced inhibitory effect compared to the peptidic antibiotic alone was due to the ability of the designed nanoparticles to disintegrate the bacterial membrane. In an attempt to heal diabetic ulcer wounds, gold nanoparticles (AuNPs) were combined with the antioxidants epigallocatechine gallate (EGCG) and α-lipoic acid (ALA).43 These nanoparticles accelerated healing by regulating inflammation and angiogenesis. In addition, the application of AuNPs to the wounds allowed for an increase in the skin absorptivity of the nanoscale mixture.
The delivery of curcumin (CC) or diferuloylmethane via different types of nanomaterial-based vehicles has been investigated in wound healing given its antibiotic, antioxidant, and anti-infective properties.93 CC plays a major role in the proliferative phase of wound healing by increasing the granulation tissue and promoting the biosynthesis of several components of the ECM.94,95 Curcumin has also been encapsulated within a highly structured porous lattice of silane composite nanoparticles.96 The use of this nanoparticle matrix enabled topical delivery and controlled release of the drug overtime, thereby limiting curcumin’s degradation, increasing its bioavailability, and enhancing its performance for the treatment of infected burn wounds.
The majority of these nanoparticle carriers have been used for the delivery of therapeutic agents with antibacterial properties; nonetheless, other nanosystems such as solid lipid nanoparticles and liposomes were explored as nanocarriers of wound-healing drugs, chosen based on the needs of the wound.97 For example, liposomes were recently used to facilitate the delivery of madecassoside drug in order to promote cell growth, accelerate cutaneous wound healing, and reduce scar formation.98
Nanoengineered Scaffolds for Wound Healing
One significant aspect of the current therapeutic agents employed in wound healing involves engineering of nanopolymeric scaffolds to mimic the properties of ECM, i.e., its fibrous nature and nanoscale features. Several nanotechnology techniques are used for the formation of scaffolds, including electrospinning, self-assembly, and phase separation (Figure 3). Among these methods, electrospinning is the primary choice for nanofiber fabrication.32,51 Electrospinning is a well-established technique for engineering porous polymeric nanofibers, which has proven successful in generating nanoscaffolds that demonstrate physical and structural properties analogous to those of the ECM.99 Electrospun nanofibers derived from a combination of PLGA/silk fibroin SF were used as hybrid scaffolds to promote attachment and proliferation of fibroblasts for improved healing of diabetic wounds.48
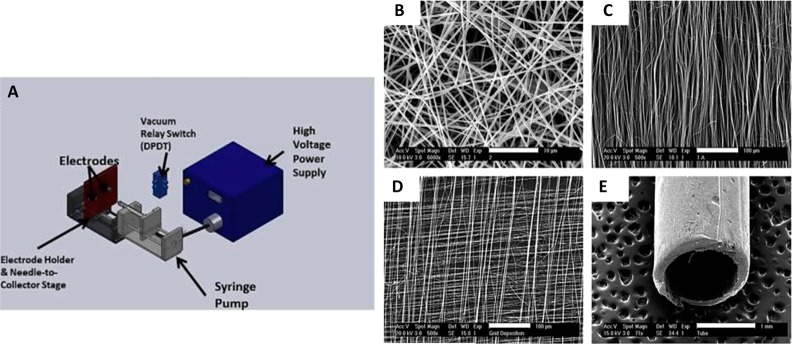
Figure 3.
(A) Diagrammatic representation of a custom-made electrospinning apparatus. (B) A representative scanning electron microscopy image of an electrospun scaffold with random fiber orientation, (C) with aligned fiber orientation, (D) with gridded fiber alignment, and (E) a tubular scaffold. Adapted with permission from ref (102). Copyright 2011 Acta Materialia Inc. Published by Elsevier Ltd.
Dendrimers (highly branched polymers with nanoscale dimensions) with anti-inflammatory properties were also incorporated into wound dressings.100 For instance, gelatin–dendrimer nanofibers were generated via electrospinning and treated the blends with polyethylene glycol (PEG) to form semi-interpenetrating networks (sIPNs).101 Silver was incorporated within the sIPNs network to add antibacterial properties to the network.
The nanotopography of a wound dressing scaffold has been the focus of interest of researchers aiming at minimizing scar formation while maintaining a high rate of wound closure.103 In this regard, silicon wafers with different polystyrene nanogroove patterns were designed to study cell adhesion and migration behavior for enhancement of wound closure.104 The 600 nm pitch grooves produced an enhanced initial osteoblast adhesion and a directional cell migration parallel to the grooves.104 Further work demonstrated that the orientation and density of nanogrooves are crucial for fibroblast migration during wound healing.103 Scale patterns of a few micrometers and a few hundred nanometers were also fabricated and contrasted to determine cell migration speed. The study showcased that geometrical nanotopography factors can alter the cell migration speed, division, ECM production, and proliferation rate,103 thus highlighting the importance of the nanotopography of the scaffold in wound healing.
Growth Factor Incorporated Nanomaterials
Growth factors play a pivotal role in modulating and coordinating cellular processes during all phases of wound healing (Figure 1).105,106 In addition to (FDA-approved) rhPDGF, many growth factors were brought for testing in patients, including recombinant human epidermal growth factor (rhEGF) and recombinant human vascular endothelial growth factor (rhVEGF).16,107,108 Although rhEGF has been shown to accelerate wound closure in patients, there is not enough evidence to support FDA approval at this time due to lack of large randomized controlled trials. Recombinant human fibroblast growth factor (rhFGF) has also been explored due to its beneficial effects on fibroblasts, which are crucial for proper wound bed restoration and ECM deposition.109
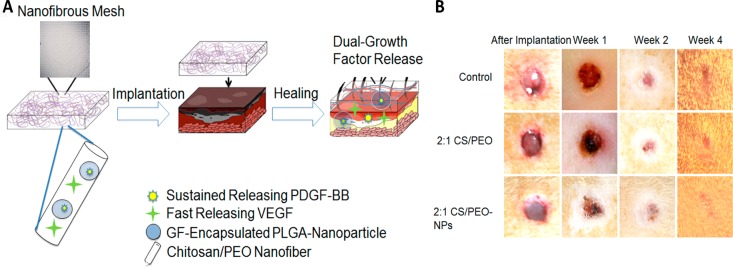
Major hurdles in designing growth factor therapy approach are protecting growth factors from enzymatic degradation in the proteolytic wound environment and achieving sustained release. Thus, formulations using nanoscale systems have been prepared.32 PLGA nanoparticles were used to deliver rhEGF to enhance the closure of full-thickness diabetic wounds.110 A sustained release of this growth factor was also achieved by incorporating it into chitosan nanoparticles within a fibrin gel. The encapsulation of rhEGF made it stable and assisted in keeping its bioactivity in simulated wound environments.111 Furthermore, solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) were developed for the sustained and effective release of rhEGF. The topical administration of rhEGF-loaded lipid nanoparticles was successful in terms of the encapsulation efficiency and increased rate of wound closure, in vivo.112 The growth factor rhVEGF has also been investigated for its application in wound healing due to its ability to promote angiogenesis.113 A hybrid composite of PLGA nanoparticles embedded in chitosan–poly(ethylene oxide) nanofibers was employed for the release of dual growth factor VEGF and PDGF.114 This nanoparticle-in-nanofiber system delivering both growth factors enhanced wound healing in vivo through VEGF-promoted angiogenesis and PDGF-mediated tissue regeneration and remodeling (Figure 4).
Figure 4.
(A) Schematic illustration of the nanoparticle-embedded electrospun nanofibers loaded with two growth factors VEGF and PDGF-BB for the wound healing and (B) representative macroscopic appearance of wound closure after treatment of rat wounds with control, 2:1 chitosan/PEO (CS/PEO) without growth factor, and 2:1 CS/PEO-NPs with nanoparticles and growth factors. Adapted with permission from ref (114). Copyright 2013 Acta Materialia Inc. Published by Elsevier Ltd.
Although highly promising in preclinical animal studies and small clinical trials, success of growth factor therapy has been so far hampered by the highly proteolytic chronic wound environment,4 which may be effectively addressed by nanotechnology-based design with incorporated protease inhibitors. However, multiple studies have shown downregulation of the corresponding growth factor receptors and signaling molecules in chronic wound tissue, limiting their applications.8,115
Gene, RNA Interference (RNAi), and Small Interfering RNA (siRNA) Nanotherapies
Gene-activated matrix therapy, which combines gene therapy and tissue engineering, has evolved as a means to increase or knock down the expression of a target gene responsible for regeneration of bone, cartilage, and skin.116 The advantage of using gene therapy for wound healing is the stability of DNA as compared to growth factor therapy.116,117 Naked and colloidal DNA have been delivered to wound sites by direct injection, gene gun, and electroporation. However, these techniques suffer from the need for repeated injections as well as inconsistent and short-term gene expression.117 These issues can be overcome by use of electrospun nanofibrous meshes as matrices for gene encapsulation and wound dressing material. Nucleic acids have been impregnated into these nanofibers to enhance tissue regeneration and eventually decrease scar formation in normal and diabetic wounds.117 More recently, electrospun scaffolds of a blend of poly(lactic acid) (PLA) and PCL were fabricated for localized delivery of DNA plasmid encoding for keratinocyte growth factor.54 The polyester scaffolds have also been shown to be a good option for treatment of cutaneous wounds.54,118
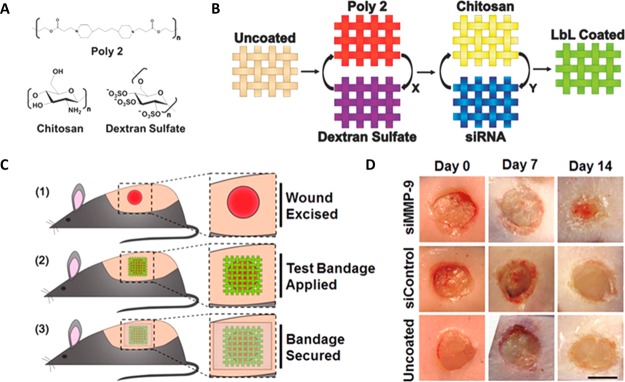
RNAi therapy permits silencing gene expression by selectively targeting molecules overexpressed in the chronic wound environment such as matrix metalloproteinases (MMP)s (Figure 5).118,119 Nanoparticle-based technology was implemented to protect the targeted delivery of siRNA from potential degradation of this effector molecule by intracellular RNases.120 A (MMP)-2 siRNA-incorporated linear polyethylenimine (LPEI) complex was immobilized onto a nanofibrous mesh via MMP cleavable peptides allowing for controlled release.121 This system efficiently suppressed MMP-2 levels and promoted wound healing in diabetic murine wounds. Spherical nucleic acid (SNA) gold nanoparticle conjugates have been also used for efficient siRNA delivery in vivo.55 A duplexed ganglioside-monosialic acid 3 synthase (GM3S) thiolated siRNA was attached to 13 nm diameter gold nanoparticles in order to engineer the GM3 SNA construct. The designed RNAi-based gene regulator, GM3S SNA, was capable of accelerating keratinocyte migration and proliferation by depleting the ganglioside GM3S and, consequently, accelerating wound healing in type 2 diabetic wound model. The key role of SNA nanotechnology is the ability of the SNAs to pass through the epidermal barrier, permitting topical therapeutic application. However, there is still a need for newer, more efficient, and refined RNAi-based technologies for wound healing. These technologies should be able to overcome the limitations of current RNA delivery by incorporating selective targeting and better retention, effectiveness, bioavailability, and safety, in addition to addressing the issues associated with this type of therapy in the presence of wound bacteria.120,122
Figure 5.
Layer-by-layer (LbL) coating for sustained release of siRNA and reduction of MMP-9 expression. (A) Chemical structures of polymers used for the preparation of LbL coating. (B) Hierarchical structure of LbL films into a single coating. (C) Application of bandages on full-thickness excisional wounds on the backs of mice. (D) Digital imaging of wounds immediately following surgery (day 0) and after 7 or 14 d of treatment. Adapted with permission from ref (118). Copyright 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Stem Cell Incorporated Nanoscaffolds
Therapeutic approaches involving stem cells (SC) have been investigated extensively for chronic wounds and shown to facilitate re-epithelialization and promote angiogenesis.123 Bone marrow derived mesenchymal stem cells (BM-MSCs), umbilical cord derived MSCs, adipose-derived stromal cells (ASCs), placenta-derived stem cells, bone marrow derived mononuclear cells (BM-MNCs), and bone marrow derived endothelial progenitor cells have all been highlighted as having therapeutic potential for wound-healing disorders.123−126 It is not fully clear what is the potential mechanism of action; however, it is believed that stem cell therapies provide a dynamic microenvironment (targeting multiple cell types simultaneously) through paracrine effects in order to facilitate process of healing.127−129
Direct delivery of MSCs to the wound can induce rapid cell death, and attempt of attenuation of this effect have been proposed by bioengineering of the therapeutic delivery platform.130 Current applications of SCs include local delivery to wounds using dressings, sprays, injections, and systemic administration. Nanotechnology-based approaches were explored to generate a nanomatrix with tailored biophysical properties to better control the differentiation of stem cells. An interesting strategy for use of MSCs is based on the production of nanoscaffolds that mimic the physical characteristics of human skin.131,132 The incorporation of SCs into this nanoscaffold resulted in an engineered wound dressing holding a “cocktail” of stem cells, growth factors, and the matrix itself.129,133 Polymeric nanofibers also had a recent surge in the tissue regeneration field. These nanofibers are biomimetic and can simulate native tissue, making for an ideal stem cell niche. BM-MSCs were attached to a collagen/PLGA nanofiber scaffold and demonstrated the ability of the nanoscaffold to secure a faster closure of cutaneous wounds.58 The surface nanotopography of the fibers, the presence of collagen, and the chemical conjugation of a CD29 antibody that will bind to CD29 antigen overexpressed on MSCs surface contributed to an enhanced stem cell adhesion. The nanoscaffold/BM-MSC composite was shown as a useful approach for wound healing and skin generation in acute full-thickness skin wounds. Despite significant advancements, no stem cell therapy for the treatment of chronic wounds has yet acquired FDA approval for efficacy.123 Viable treatments will likely use stem cells in accordance with other local and systemic therapies.
Nanotechnology-Based Targeted Delivery To Achieve Cell-Type Specificity
Targeted delivery of nanomaterials for wound-healing applications is still scarce in comparison to other nanotechnologies. Achieving targeted delivery of therapeutics is significant to improve efficacy, reduce any harmful side effects on the nontarget tissues, and reduce cost of therapy.120 A few known examples of targeted wound-healing therapeutics are discussed below.
Targeted delivery of the stromal cell derived factor 1 (SDF-1) to the wound site was performed by developing ROS stimulus responsive polymeric nanoparticles as delivery vehicles for SDF-1.134 The chemokine SDF-1 is involved in the migration of BMSCs to the sites of injury and subsequent induction of wound vascularization, which makes the presence of SDF-1 necessary for the stem cell based treatment of wound repair. The nanoparticles were composed of a biodegradable polymer poly(1,4-phenyleneacetone dimethylene thioketal), PPADT, that depolymerizes in the presence of a high concentration of ROS. Hence, the delivery of the macromolecular drug SDF-1 via the polymeric nanoparticles enhanced its bioavailability and provided a localized and specific targeting of the wound site. The intravenously injected nanoparticles were able to advance the healing of full-thickness wounds in mice.
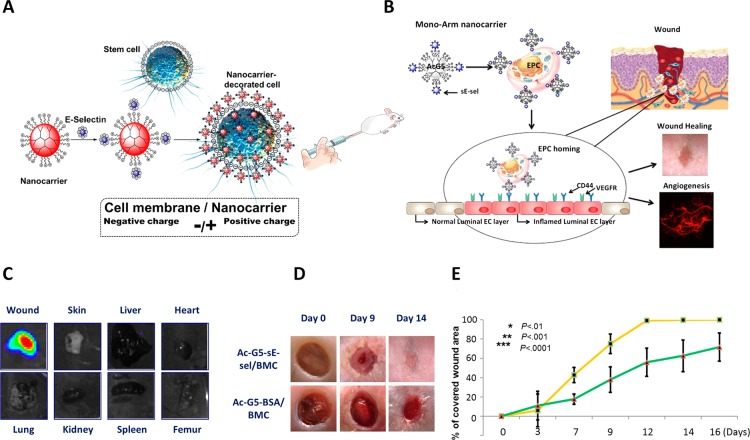
To date, the only example of targeted nanocarrier-mediated delivery of stem cells was designed by our team to achieve homing of the cells to the sites.135 This study demonstrates the competency of nanotechnology in overcoming the limitation associated with systemic stem cell therapy, which is the low number of viable cells that home to the target tissue. In this work, acetylated polyamido amine (PAMAM) dendrimeric nanocarriers armed with cellular molecular recognition moieties were used to coat BMC and MSC. The recognition molecule, typically a small peptide or protein, soluble part of E-selectin (sE-sel) that has a binding counterpart in the site of interest, was meticulously chosen to adhere to a receptor (CD44, SDF-1, I-CAM, P-CAM, etc.) highly overexpressed on the endothelium of wound bed capillaries, thus acting like a GPS to direct the cell complex with the surrounding nanocarriers to the site of injury. This nanocarrier-targeted delivery platform demonstrated biocompatibility and high efficiency of the designed nanocarriers to direct stem cells to the injured tissues, the eye and a dorsal wound, while enhancing the proangiogenic and prorepair functions of the cells without any toxicity or immunogenic effects (Figure 6). This nanocarrier platform is versatile as it can be tailored for the specific delivery of DNA, siRNA, small drugs, proteins, and cells to any location in the body by complexing the therapeutic agent to the dendrimer nanoparticle and choosing the right molecular recognition molecule to integrate within the nanoparticle.
Figure 6.
(A) Schematic illustration for the design of nanocarriers (Ac-G5 dendrimers complexed with sE-sel moiety) for stem cell coating. (B) Representative image of the “mono-arm”: Ac-G5-dendrimer-sE-sel, for bone marrow derived endothelial progenitor cell (EPC) coating; the adhesion moiety sE-sel interacts selectively with E-selectin ligand (CD44) expressed on inflamed luminal endothelial cells (EC) in wound tissues. (C) Bioluminescence imaging showing Ac-G5-dendrimer-sE-sel nanocarrier-coated Luciferase2+-MSC selectively homed to skin wound tissues but not other organs. (D, E) Healing of murine wound tissues (macroscopic images and wound-healing rate) upon systemic delivery of Ac-G5-sE-sel and Ac-G5-BSA nanocarrier-coated BMC. Adapted with permission from ref (135). Copyright 2016 PLOS.
Another approach that has been undertaken for targeted delivery is based on functionalization of lipid nanoparticles (LPNs) for restoration of oxidative metabolism in chronic ischemic wound edge tissue.136 AntihypoxamiR functionalized gramicidin LPNs (AFGLN nanostructures) were synthesized and loaded with a locked nucleic acid (LNA) based anti-miR-210 power inhibitor (AFGLNmiR-210). The intradermal delivery of AFGLNmiR-210 to ischemic wounds accelerated re-epithelialization, as the inhibition of hypoxamiR miR-210 is associated with maintaining keratinocyte cell proliferation. The advantage of the AFGLN design is that it is formulated using components previously approved by the FDA for human applications.136
Unmet Needs and Future Directions
Although more than seven million Americans are affected by chronic wounds annually, current research has shown limited success to produce more than a handful of therapeutic agents deemed efficacious by the FDA. Given the complexity of the chronic wound pathology and tissue repair process, it is not surprising that current therapeutic approaches failed to accumulate enough evidence in order to acquire FDA approval, which raises the need for alternative therapeutic approaches that are deemed viable and efficient for treatment of nonhealing wounds. There are several contributing elements that impede more rapid development of therapies for patients with chronic wounds including limited knowledge in understanding pathophysiology in patients; variability of patient population and comorbidities at the individual level (patient-to-patient) and the group level (diabetic foot to venous leg to pressure ulcers); complexity of clinical trial design and associated costs; alternative clinical end points; and lack of public awareness. The major challenge remains in developing appropriate combinational therapy able to target multiple dysfunctional cellular processes and identifying the optimal timing for delivery of each therapeutic agent. However, we are currently in the midst of the BD2K (Big Data to Knowledge) initiative whereby computational biomedicine stands to revolutionize medicine. BD2K programs and centers are rapidly developing tools and “ecosystems” for integration of large data sets, such as medical records, genomics, and genotyping with drug customization, or drug response data that may be accessible to public domains. One can envision a near future in which tailored therapies will be based on unique phenotype–genotype characteristics. This will provide major opportunities to adapt existing and create new nanotechnology approaches that will streamline and facilitate individualized treatment plans that arise from such platforms.
The emergence of various nanotechnologies, especially multifunctional systems, in wound healing is showcased by the high number of publications witnessed in recent years, which indicates the high expectations toward nanotherapeutic interventions in the wound-healing field. However, the hurdle lies in gathering enough information about the physicochemical properties of the nanoscale systems and their anticipated behavior and toxicity in the human body. In addition, the high purity of the scaffolds and nanoparticles mandated by the FDA for human use is also a challenge, as often the bulk preparation and purification of the polymers and nanoparticles prepared are not easy. Thus, there is an incessant need for better synthetic tools and analytical methods that will allow for the translation of nanotechnology-based approaches to the clinic. Extensive efforts are also vital to arm chronic wound therapies with site-specificity and targeting efficiency in order to avoid undesirable events and interferences that might hinder the nanosystems from their biological functions in the human body.
In the long term, further studies are indispensable to provide insights into how research findings about nanotechnology-based therapies can be applied in the clinical arena. It is expected that new and exciting nanotechnology platforms will arise; thus, additional research on these technologies is needed to develop international standards on biocompatibility and toxicology of nanotherapies. Overall, the current capabilities in advanced manufacturing and development of various nanosystems, together with the knowledge of chronic wounds, molecular pathology, and phenotype–genotype characteristics, with the help of BD2K, are projected to promote the design of the next generation of wound-healing nanotechnologies.
Acknowledgments
S. Daunert and S. Deo would like to thank the National Institutes of Health for financial support (R21AI124058-01 and R01GM047915). S. Daunert is grateful to the Miller School of Medicine of the University of Miami for the Lucille P. Markey Chair in Biochemistry and Molecular Biology. M. Tomic-Canic acknowledges funding from the National Institutes of Health (NR015649; DK098055; NR013881).
The authors declare no competing financial interest.
References
- Eming S. A.; Martin P.; Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr266–265sr266. 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastar I.; Stojadinovic O.; Yin N. C.; Ramirez H.; Nusbaum A. G.; Sawaya A.; Patel S. B.; Khalid L.; Isseroff R. R.; Tomic-Canic M. Epithelialization in wound healing: a comprehensive review. Adv. Wound Care 2014, 3, 445–464. 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwood K. S.; Williams L. V.; Schwarzbauer J. E. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004, 36, 1031–1037. 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Lindley L. E.; Stojadinovic O.; Pastar I.; Tomic-Canic M. Biology and Biomarkers for Wound Healing. Plast. Reconstr. Surg. 2016, 138, 18S–28S. 10.1097/PRS.0000000000002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem H.; Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Invest. 2007, 117, 1219–1222. 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojadinovic O.; Pastar I.; Nusbaum A. G.; Vukelic S.; Krzyzanowska A.; Tomic-Canic M. Deregulation of epidermal stem cell niche contributes to pathogenesis of nonhealing venous ulcers. Wound Repair Regen. 2014, 22, 220–227. 10.1111/wrr.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L.; Stone R. C.; Stojadinovic O.; Ramirez H.; Pastar I.; Maione A. G.; Smith A.; Yanez V.; Veves A.; Kirsner R. S.; Garlick J. A.; Tomic-Canic M. Integrative analysis of miRNA and mRNA paired expression profiling of primary fibroblast derived from diabetic foot ulcers reveals multiple impaired cellular functions. Wound Repair Regen. 2016, 24, 943–953. 10.1111/wrr.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem H.; Stojadinovic O.; Diegelmann R. F.; Entero H.; Lee B.; Pastar I.; Golinko M.; Rosenberg H.; Tomic-Canic M. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol. Med. 2007, 13, 30–39. 10.2119/2006-00054.Brem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem H.; Golinko M. S.; Stojadinovic O.; Kodra A.; Diegelmann R. F.; Vukelic S.; Entero H.; Coppock D. L.; Tomic-Canic M. Primary cultured fibroblasts derived from patients with chronic wounds: a methodology to produce human cell lines and test putative growth factor therapy such as GMCSF. J. Transl. Med. 2008, 6, 75–84. 10.1186/1479-5876-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche M.; Gardner S. E.; Kalan L.; Horwinski J.; Zheng Q.; Hodkinson B. P.; Tyldsley A. S.; Franciscus C. L.; Hillis S. L.; Mehta S.; Margolis D. J.; Grice E. A. Temporal Stability in Chronic Wound Microbiota Is Associated With Poor Healing. J. Invest. Dermatol. 2017, 137, 237–244. 10.1016/j.jid.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veves A.; Falanga V.; Armstrong D. G.; Sabolinski M. L. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers a prospective randomized multicenter clinical trial. Diabetes Care 2001, 24, 290–295. 10.2337/diacare.24.2.290. [DOI] [PubMed] [Google Scholar]
- Marston W. A.; Hanft J.; Norwood P.; Pollak R. The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers results of a prospective randomized trial. Diabetes Care 2003, 26, 1701–1705. 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- Driver V. R.; Lavery L. A.; Reyzelman A. M.; Dutra T. G.; Dove C. R.; Kotsis S. V.; Kim H. M.; Chung K. C. A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound Repair Regen. 2015, 23, 891–900. 10.1111/wrr.12357. [DOI] [PubMed] [Google Scholar]
- Smiell J. M.; Wieman T. J.; Steed D. L.; Perry B. H.; Sampson A. R.; Schwab B. H. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen. 1999, 7, 335–346. 10.1046/j.1524-475X.1999.00335.x. [DOI] [PubMed] [Google Scholar]
- Wei E. X.; Kirsner R. S.; Eaglstein W. H. End points in dermatologic clinical trials: A review for clinicians. J. Am. Acad. Dermatol. 2016, 75, 203–209. 10.1016/j.jaad.2016.01.052. [DOI] [PubMed] [Google Scholar]
- Barrientos S.; Brem H.; Stojadinovic O.; Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014, 22, 569–578. 10.1111/wrr.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond N. A.; Vivas A. C.; Kirsner R. S. Topical and biologic therapies for diabetic foot ulcers. Med. Clin. North Am. 2013, 97, 883–898. 10.1016/j.mcna.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Embil J. M.; Nagai M. K. Becaplermin: recombinant platelet derived growth factor, a new treatment for healing diabetic foot ulcers. Expert Opin. Biol. Ther. 2002, 2, 211–218. 10.1517/14712598.2.2.211. [DOI] [PubMed] [Google Scholar]
- Fonder M. A.; Lazarus G. S.; Cowan D. A.; Aronson-Cook B.; Kohli A. R.; Mamelak A. J. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206. 10.1016/j.jaad.2007.08.048. [DOI] [PubMed] [Google Scholar]
- Kalashnikova I.; Das S.; Seal S. Nanomaterials for wound healing: scope and advancement. Nanomedicine 2015, 10, 2593–2612. 10.2217/nnm.15.82. [DOI] [PubMed] [Google Scholar]
- Pereira R. F.; Bartolo P. J. Traditional therapies for skin wound healing. Adv. wound care 2016, 5, 208–229. 10.1089/wound.2013.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan K.; Tang J.; Escandon J.; Kirsner R. S. State of the art in topical wound-healing products. Plast. Reconstr. Surg. 2011, 127 (Suppl. 1), 44S–59S. 10.1097/PRS.0b013e3181fbe275. [DOI] [PubMed] [Google Scholar]
- Parani M.; Lokhande G.; Singh A.; Gaharwar A. K. Engineered Nanomaterials for Infection Control and Healing Acute and Chronic Wounds. ACS Appl. Mater. Interfaces 2016, 8, 10049–10069. 10.1021/acsami.6b00291. [DOI] [PubMed] [Google Scholar]
- Mogosanu G. D.; Grumezescu A. M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. 10.1016/j.ijpharm.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Marston W.; Tang J.; Kirsner R. S.; Ennis W. Wound Healing Society 2015 update on guidelines for venous ulcers. Wound Repair Regen. 2016, 24, 136–144. 10.1111/wrr.12394. [DOI] [PubMed] [Google Scholar]
- Dabiri G.; Damstetter E.; Phillips T. Choosing a wound dressing based on common wound characteristics. Adv. Wound Care 2016, 5, 32–41. 10.1089/wound.2014.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasiak J.; Cleland H.; Campbell F.; Spinks A. Dressings for superficial and partial thickness burns. Cochrane Database Syst. Rev. 2013, 1–42. 10.1002/14651858.CD002106.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B. K.; Siprashvili Z.; Khavari P. A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. 10.1126/science.1253836. [DOI] [PubMed] [Google Scholar]
- MacNeil S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- Borena B. M.; Martens A.; Broeckx S. Y.; Meyer E.; Chiers K.; Duchateau L.; Spaas J. H. Regenerative skin wound healing in mammals: state-of-the-art on growth factor and stem cell based treatments. Cell. Physiol. Biochem. 2015, 36, 1–23. 10.1159/000374049. [DOI] [PubMed] [Google Scholar]
- Duscher D.; Barrera J.; Wong V. W.; Maan Z. N.; Whittam A. J.; Januszyk M.; Gurtner G. C. Stem cells in wound healing: the future of regenerative medicine? A Mini-Review. Gerontology 2016, 62, 216–225. 10.1159/000381877. [DOI] [PubMed] [Google Scholar]
- Korrapati P. S.; Karthikeyan K.; Satish A.; Krishnaswamy V. R.; Venugopal J. R.; Ramakrishna S. Recent advancements in nanotechnological strategies in selection, design and delivery of biomolecules for skin regeneration. Mater. Sci. Eng., C 2016, 67, 747–765. 10.1016/j.msec.2016.05.074. [DOI] [PubMed] [Google Scholar]
- Mordorski B.; Rosen J.; Friedman A. Nanotechnology as an innovative approach for accelerating wound healing in diabetes. Diabetes Manage. 2015, 5, 329–332. 10.2217/dmt.15.28. [DOI] [Google Scholar]
- Tocco I.; Zavan B.; Bassetto F.; Vindigni V. Nanotechnology-Based Therapies for Skin Wound Regeneration. J. Nanomater. 2012, 2012, 1–11. 10.1155/2012/714134. [DOI] [Google Scholar]
- Tian J.; Wong K. K. Y.; Ho C. M.; Lok C. N.; Yu W. Y.; Che C. M.; Chiu J. F.; Tam P. K. H. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2007, 2, 129–136. 10.1002/cmdc.200600171. [DOI] [PubMed] [Google Scholar]
- Dunn K.; Edwards-Jones V. The role of Acticoat (TM) with nanocrystalline silver in the management of burns. Burns 2004, 30, S1–S9. 10.1016/S0305-4179(04)90000-9. [DOI] [PubMed] [Google Scholar]
- Tiwari M.; Narayanan K.; Thakar M. B.; Jagani H. V.; Rao J. V. Biosynthesis and wound healing activity of copper nanoparticles. IET Nanobiotechnol. 2014, 8, 230–237. 10.1049/iet-nbt.2013.0052. [DOI] [PubMed] [Google Scholar]
- Chigurupati S.; Mughal M. R.; Okun E.; Das S.; Kumar A.; McCaffery M.; Seal S.; Mattson M. P. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials 2013, 34, 2194–2201. 10.1016/j.biomaterials.2012.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C.; Chen X. F.; Miao G. H.; Lin C. Angiogenesis stimulated by novel nanoscale bioactive glasses. Biomed. Mater. 2015, 10, 025005. 10.1088/1748-6041/10/2/025005. [DOI] [PubMed] [Google Scholar]
- Barui A. K.; Veeriah V.; Mukherjee S.; Manna J.; Patel A. K.; Patra S.; Pal K.; Murali S.; Rana R. K.; Chatterjee S.; Patra C. R. Zinc oxide nanoflowers make new blood vessels. Nanoscale 2012, 4, 7861–7869. 10.1039/c2nr32369a. [DOI] [PubMed] [Google Scholar]
- Gao J.; Wang H.-L.; Iyer R. Suppression of proinflammatory cytokines in functionalized fullerene-exposed dermal keratinocytes. J. Nanomater. 2010, 2010, 1–9. 10.1155/2010/416408. [DOI] [Google Scholar]
- Archana D.; Dutta J.; Dutta P. K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. 10.1016/j.ijbiomac.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Chen S.-A.; Chen H.-M.; Yao Y.-D.; Hung C.-F.; Tu C.-S.; Liang Y.-J. Topical treatment with anti-oxidants and Au nanoparticles promote healing of diabetic wound through receptor for advance glycation end-products. Eur. J. Pharm. Sci. 2012, 47, 875–883. 10.1016/j.ejps.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Blecher K.; Martinez L. R.; Tuckman-Vernon C.; Nacharaju P.; Schairer D.; Chouake J.; Friedman J. M.; Alfieri A.; Guha C.; Nosanchuk J. D. Nitric oxide-releasing nanoparticles accelerate wound healing in NOD-SCID mice. Nanomedicine 2012, 8, 1364–1371. 10.1016/j.nano.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Chouake J.; Schairer D.; Kutner A.; Sanchez D. A.; Makdisi J.; Blecher-Paz K.; Nacharaju P.; Tuckman-Vernon C.; Gialanella P.; Friedman J. M.; Nosanchuk J. D.; Friedman A. J. Nitrosoglutathione generating nitric oxide nanoparticles as an improved strategy for combating Pseudomonas aeruginosa-infected wounds. J. Drugs Dermatol. 2012, 11, 1471–1477. [PubMed] [Google Scholar]
- Castangia I.; Nácher A.; Caddeo C.; Valenti D.; Fadda A. M.; Díez-Sales O.; Ruiz-Saurí A.; Manconi M. Fabrication of quercetin and curcumin bionanovesicles for the prevention and rapid regeneration of full-thickness skin defects on mice. Acta Biomater. 2014, 10, 1292–1300. 10.1016/j.actbio.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Nguyen T.-H.; Kim Y.-H.; Song H.-Y.; Lee B.-T. Nano Ag loaded PVA nano-fibrous mats for skin applications. J. Biomed. Mater. Res., Part B 2011, 96B, 225–233. 10.1002/jbm.b.31756. [DOI] [PubMed] [Google Scholar]
- Shahverdi S.; Hajimiri M.; Esfandiari M. A.; Larijani B.; Atyabi F.; Rajabiani A.; Dehpour A. R.; Gharehaghaji A. A.; Dinarvand R. Fabrication and structure analysis of poly(lactide-co-glycolic acid)/silk fibroin hybrid scaffold for wound dressing applications. Int. J. Pharm. 2014, 473, 345–355. 10.1016/j.ijpharm.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Dubský M.; Kubinová Š.; Širc J.; Voska L.; Zajíček R.; Zajícová A.; Lesný P.; Jirkovská A.; Michálek J.; Munzarová M. Nanofibers prepared by needleless electrospinning technology as scaffolds for wound healing. J. Mater. Sci.: Mater. Med. 2012, 23, 931–941. 10.1007/s10856-012-4577-7. [DOI] [PubMed] [Google Scholar]
- Han C. M.; Zhang L. P.; Sun J. Z.; Shi H. F.; Zhou J.; Gao C. Y. Application of collagen-chitosan/fibrin glue asymmetric scaffolds in skin tissue engineering. J. Zhejiang Univ., Sci., B 2010, 11, 524–530. 10.1631/jzus.B0900400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinern G. E. J.; Fawcett D.; Ng Y. J.; Ali N.; Brundavanam R. K.; Jiang Z. T. Nanoengineering a Biocompatible Inorganic Scaffold for Skin Wound Healing. J. Biomed. Nanotechnol. 2010, 6, 497–510. 10.1166/jbn.2010.1148. [DOI] [PubMed] [Google Scholar]
- Parkinson L. G.; Giles N. L.; Adcroft K. F.; Fear M. W.; Wood F. M.; Poinern G. E. The Potential of Nanoporous Anodic Aluminium Oxide Membranes to Influence Skin Wound Repair. Tissue Eng., Part A 2009, 15, 3753–3763. 10.1089/ten.tea.2008.0594. [DOI] [PubMed] [Google Scholar]
- Kwon M. J.; An S.; Choi S.; Nam K.; Jung H. S.; Yoon C. S.; Ko J. H.; Jun H. J.; Kim T. K.; Jung S. J. Effective healing of diabetic skin wounds by using nonviral gene therapy based on minicircle vascular endothelial growth factor DNA and a cationic dendrimer. J. Gene Med. 2012, 14, 272–278. 10.1002/jgm.2618. [DOI] [PubMed] [Google Scholar]
- Kobsa S.; Kristofik N. J.; Sawyer A. J.; Bothwell A. L.; Kyriakides T. R.; Saltzman W. M. An electrospun scaffold integrating nucleic acid delivery for treatment of full-thickness wounds. Biomaterials 2013, 34, 3891–3901. 10.1016/j.biomaterials.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randeria P. S.; Seeger M. A.; Wang X.-Q.; Wilson H.; Shipp D.; Mirkin C. A.; Paller A. S. siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 5573–5578. 10.1073/pnas.1505951112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chereddy K. K.; Lopes A.; Koussoroplis S.; Payen V.; Moia C.; Zhu H. J.; Sonveaux P.; Carmeliet P.; des Rieux A.; Vandermeulen G.; Preat V. Combined effects of PLGA and vascular endothelial growth factor promote the healing of non-diabetic and diabetic wounds. Nanomedicine 2015, 11, 1975–1984. 10.1016/j.nano.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Choi J. S.; Choi S. H.; Yoo H. S. Coaxial electrospun nanofibers for treatment of diabetic ulcers with binary release of multiple growth factors. J. Mater. Chem. 2011, 21, 5258–5267. 10.1039/c0jm03706k. [DOI] [Google Scholar]
- Ma K.; Liao S.; He L.; Lu J.; Ramakrishna S.; Chan C. K. Effects of nanofiber/stem cell composite on wound healing in acute full-thickness skin wounds. Tissue Eng., Part A 2011, 17, 1413–1424. 10.1089/ten.tea.2010.0373. [DOI] [PubMed] [Google Scholar]
- Tam K.; Cheyyatraviendran S.; Venugopal J.; Biswas A.; Choolani M.; Ramakrishna S.; Bongso A.; Fong C. Y. A nanoscaffold impregnated with human wharton’s jelly stem cells or its secretions improves healing of wounds. J. Cell. Biochem. 2014, 115, 794–803. 10.1002/jcb.24723. [DOI] [PubMed] [Google Scholar]
- Melaiye A.; Youngs W. J. Silver and its application as an antimicrobial agent. Expert Opin. Ther. Pat. 2005, 15, 125–130. 10.1517/13543776.15.2.125. [DOI] [Google Scholar]
- Silvestry-Rodriguez N.; Sicairos-Ruelas E. E.; Gerba C. P.; Bright K. R. Silver as a disinfectant. Rev. Environ. Contam. Toxicol. 2007, 191, 23–45. 10.1007/978-0-387-69163-3_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiyeh B. S.; Costagliola M.; Hayek S. N.; Dibo S. A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- James G. A.; Swogger E.; Wolcott R.; Pulcini E.; Secor P.; Sestrich J.; Costerton J. W.; Stewart P. S. Biofilms in chronic wounds. Wound Repair Regen. 2008, 16, 37–44. 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Kirker K. R.; Secor P. R.; James G. A.; Fleckman P.; Olerud J. E.; Stewart P. S. Loss of viability and induction of apoptosis in human keratinocytes exposed to Staphylococcus aureus biofilms in vitro. Wound Repair Regen. 2009, 17, 690–699. 10.1111/j.1524-475X.2009.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. P.; D’Arpa P.; Seth A. K.; Geringer M. R.; Jett M.; Xu W.; Hong S. J.; Galiano R. D.; Chen T.; Mustoe T. A. Dermal wound transcriptomic responses to Infection with Pseudomonas aeruginosa versus Klebsiella pneumoniae in a rabbit ear wound model. BMC Clin. Pathol. 2014, 14, 20–34. 10.1186/1472-6890-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastar I.; Nusbaum A. G.; Gil J.; Patel S. B.; Chen J.; Valdes J.; Stojadinovic O.; Plano L. R.; Tomic-Canic M.; Davis S. C. Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS One 2013, 8, e56846. 10.1371/journal.pone.0056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W.; Stewart P. S.; Greenberg E. P. Bacterial biofilms: a common cause of persistent infections. Science 1999, 284, 1318–1322. 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Bergin S. M.; Wraight P. Silver based wound dressings and topical agents for treating diabetic foot ulcers. Cochrane Database Syst. Rev. 2006, 1–19. 10.1002/14651858.CD005082.pub2. [DOI] [PubMed] [Google Scholar]
- Adhya A.; Bain J.; Ray O.; Hazra A.; Adhikari S.; Dutta G.; Ray S.; Majumdar B. K. Healing of burn wounds by topical treatment: A randomized controlled comparison between silver sulfadiazine and nano-crystalline silver. J. Basic Clin. Pharm. 2014, 6, 29–34. 10.4103/0976-0105.145776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S.; Tak Y. K.; Song J. M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelestemur S.; Kilic E.; Uslu Ü.; Cumbul A.; Ugur M.; Akman S.; Culha M. Wound healing properties of modified silver nanoparticles and their distribution in mouse organs after topical application. Nano Biomed. Eng. 2012, 4, 170–176. 10.5101/nbe.v4i4.p170-176. [DOI] [Google Scholar]
- do Nascimento E. G.; Sampaio T. B. M.; Medeiros A. C.; de Azevedo E. P. Evaluation of chitosan gel with 1% silver sulfadiazine as an alternative for burn wound treatment in rats. Acta Cir. Bras. 2009, 24, 460–465. 10.1590/S0102-86502009000600007. [DOI] [PubMed] [Google Scholar]
- Rice L. B. The clinical consequences of antimicrobial resistance. Curr. Opin. Microbiol. 2009, 12, 476–481. 10.1016/j.mib.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Peterson L. R. Bad Bugs, No Drugs: No ESCAPE Revisited. Clin. Infect. Dis. 2009, 49, 992–993. 10.1086/605539. [DOI] [PubMed] [Google Scholar]
- Kumar P. T. S.; Lakshmanan V. K.; Anilkumar T. V.; Ramya C.; Reshmi P.; Unnikrishnan A. G.; Nair S. V.; Jayakumar R. Flexible and Microporous Chitosan Hydrogel/Nano ZnO Composite Bandages for Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. 10.1021/am300292v. [DOI] [PubMed] [Google Scholar]
- P. T. S. K.; Lakshmanan V.-K.; Raj M.; Biswas R.; Hiroshi T.; Nair S. V.; Jayakumar R. Evaluation of Wound Healing Potential of β-Chitin Hydrogel/Nano Zinc Oxide Composite Bandage. Pharm. Res. 2013, 30, 523–537. 10.1007/s11095-012-0898-y. [DOI] [PubMed] [Google Scholar]
- Jamnongkan T.; Sukumaran S. K.; Sugimoto M.; Hara T.; Takatsuka Y.; Koyama K. Towards novel wound dressings: antibacterial properties of zinc oxide nanoparticles and electrospun fiber mats of zinc oxide nanoparticle/poly(vinyl alcohol) hybrids. J. Polym. Eng. 2015, 35, 575–586. 10.1515/polyeng-2014-0319. [DOI] [Google Scholar]
- Bondarenko O.; Juganson K.; Ivask A.; Kasemets K.; Mortimer M.; Kahru A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch. Toxicol. 2013, 87, 1181–1200. 10.1007/s00204-013-1079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soenen S. J.; Parak W. J.; Rejman J.; Manshian B. Intra)Cellular Stability of Inorganic Nanoparticles: Effects on Cytotoxicity, Particle Functionality, and Biomedical Applications. Chem. Rev. 2015, 115, 2109–2135. 10.1021/cr400714j. [DOI] [PubMed] [Google Scholar]
- Zhou Z. G.; Joslin S.; Dellinger A.; Ehrich M.; Brooks B.; Ren Q.; Rodeck U.; Lenk R.; Kepley C. L. A Novel Class of Compounds with Cutaneous Wound Healing Properties. J. Biomed. Nanotechnol. 2010, 6, 605–611. 10.1166/jbn.2010.1157. [DOI] [PubMed] [Google Scholar]
- Khan M. S.; Abdelhamid H. N.; Wu H.-F. Near infrared (NIR) laser mediated surface activation of graphene oxide nanoflakes for efficient antibacterial, antifungal and wound healing treatment. Colloids Surf., B 2015, 127, 281–291. 10.1016/j.colsurfb.2014.12.049. [DOI] [PubMed] [Google Scholar]
- Patrulea V.; Ostafe V.; Borchard G.; Jordan O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. 10.1016/j.ejpb.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Schwentker A.; Vodovotz Y.; Weller R.; Billiar T. R. Nitric oxide and wound repair: role of cytokines?. Nitric Oxide 2002, 7, 1–10. 10.1016/S1089-8603(02)00002-2. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J.Nitric oxide: biology and pathobiology; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Williams D. L. H. A chemist’s view of the nitric oxide story. Org. Biomol. Chem. 2003, 1, 441–449. 10.1039/b209748f. [DOI] [PubMed] [Google Scholar]
- Luo J. D.; Chen A. F. Nitric oxide: a newly discovered function on wound healing. Acta Pharmacol. Sin. 2005, 26, 259–264. 10.1111/j.1745-7254.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- Witte M. B.; Barbul A. Role of nitric oxide in wound repair. Am. J. Surg. 2002, 183, 406–412. 10.1016/S0002-9610(02)00815-2. [DOI] [PubMed] [Google Scholar]
- Han G.; Nguyen L. N.; Macherla C.; Chi Y.; Friedman J. M.; Nosanchuk J. D.; Martinez L. R. Nitric oxide–releasing nanoparticles accelerate wound healing by promoting fibroblast migration and collagen deposition. Am. J. Pathol. 2012, 180, 1465–1473. 10.1016/j.ajpath.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Nurhasni H.; Cao J. F.; Choi M.; Kim I.; Lee B. L.; Jung Y. J.; Yoo J. W. Nitric oxide-releasing poly(lactic-co-glycolic acid)-polyethylenimine nanoparticles for prolonged nitric oxide release, antibacterial efficacy, and in vivo wound healing activity. Int. J. Nanomed. 2015, 10, 3065–3080. 10.2147/IJN.S82199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. W.; Kim J.; Kim J.; Kim Y.; Song H. B.; Kim J. H.; Kim K.; Kim W. J. Light-Induced Acid Generation on a Gatekeeper for Smart Nitric Oxide Delivery. ACS Nano 2016, 10, 4199–4208. 10.1021/acsnano.5b07483. [DOI] [PubMed] [Google Scholar]
- Schairer D. O.; Martinez L. R.; Blecher K.; Chouake J. S.; Nacharaju P.; Gialanella P.; Friedman J. M.; Nosanchuk J. D.; Friedman A. J. Nitric oxide nanoparticles. Virulence 2012, 3, 62–67. 10.4161/viru.3.1.18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. Y.; Chang H. Y.; Lu J. K.; Huang Y. C.; Harroun S. G.; Tseng Y. T.; Li Y. J.; Huang C. C.; Chang H. T. Self-Assembly of Antimicrobial Peptides on Gold Nanodots: Against Multidrug-Resistant Bacteria and Wound-Healing Application. Adv. Funct. Mater. 2015, 25, 7189–7199. 10.1002/adfm.201503248. [DOI] [Google Scholar]
- Chereddy K. K.; Coco R.; Memvanga P. B.; Ucakar B.; des Rieux A.; Vandermeulen G.; Préat V. Combined effect of PLGA and curcumin on wound healing activity. J. Controlled Release 2013, 171, 208–215. 10.1016/j.jconrel.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Panchatcharam M.; Miriyala S.; Gayathri V. S.; Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell. Biochem. 2006, 290, 87–96. 10.1007/s11010-006-9170-2. [DOI] [PubMed] [Google Scholar]
- Sidhu G. S.; Mani H.; Gaddipati J. P.; Singh A. K.; Seth P.; Banaudha K. K.; Patnaik G. K.; Maheshwari R. K. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen. 1999, 7, 362–374. 10.1046/j.1524-475X.1999.00362.x. [DOI] [PubMed] [Google Scholar]
- Krausz A. E.; Adler B. L.; Cabral V.; Navati M.; Doerner J.; Charafeddine R. A.; Chandra D.; Liang H.; Gunther L.; Clendaniel A. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomedicine 2015, 11, 195–206. 10.1016/j.nano.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachuau L. Recent developments in novel drug delivery systems for wound healing. Expert Opin. Drug Delivery 2015, 12, 1895–1909. 10.1517/17425247.2015.1070143. [DOI] [PubMed] [Google Scholar]
- Li Z.; Liu M.; Wang H.; Du S. Increased cutaneous wound healing effect of biodegradable liposomes containing madecassoside: preparation optimization, in vitro dermal permeation, and in vivo bioevaluation. Int. J. Nanomed. 2016, 11, 2995–3007. 10.2147/IJN.S105035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvannasara P.; Praphairaksit N.; Muangsin N. Self-assembly of mucoadhesive nanofibers. RSC Adv. 2014, 4, 58664–58673. 10.1039/C4RA09329A. [DOI] [Google Scholar]
- Abdel-Sayed P.; Kaeppli A.; Siriwardena T.; Darbre T.; Perron K.; Jafari P.; Reymond J.-L.; Pioletti D. P.; Applegate L. A. Anti-Microbial Dendrimers against Multidrug-Resistant P. aeruginosa Enhance the Angiogenic Effect of Biological Burn-wound Bandages. Sci. Rep. 2016, 6, 22020. 10.1038/srep22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongargaonkar A. A.; Bowlin G. L.; Yang H. Electrospun Blends of Gelatin and Gelatin–Dendrimer Conjugates As a Wound-Dressing and Drug-Delivery Platform. Biomacromolecules 2013, 14, 4038–4045. 10.1021/bm401143p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero R. B.; Vial X.; Nguyen D. T.; Farhand S.; Reardon M.; Pham S. M.; Tsechpenakis G.; Andreopoulos F. M. bFGF-containing electrospun gelatin scaffolds with controlled nano-architectural features for directed angiogenesis. Acta Biomater. 2012, 8, 1778–1791. 10.1016/j.actbio.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. N.; Hong Y.; Kim M. S.; Kim S. M.; Suh K.-Y. Effect of orientation and density of nanotopography in dermal wound healing. Biomaterials 2012, 33, 8782–8792. 10.1016/j.biomaterials.2012.08.038. [DOI] [PubMed] [Google Scholar]
- Lamers E.; te Riet J.; Domanski M.; Luttge R.; Figdor C. G.; Gardeniers J. G. E.; Walboomers X. F.; Jansen J. A. Dynamic Cell Adhesion and Migration on Nanoscale Grooved Substrates. Eur. Cell. Mater. 2012, 23, 182–194. 10.22203/eCM.v023a14. [DOI] [PubMed] [Google Scholar]
- Hantash B. M.; Zhao L.; Knowles J. A.; Lorenz H. P. Adult and fetal wound healing. Front. Biosci., Landmark Ed. 2008, 13, 51–61. 10.2741/2559. [DOI] [PubMed] [Google Scholar]
- Barrientos S.; Stojadinovic O.; Golinko M. S.; Brem H.; Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Yang S.; Geng Z.; Ma K.; Sun X.; Fu X. Efficacy of Topical Recombinant Human Epidermal Growth Factor for Treatment of Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis. Int. J. Lower Extremity Wounds 2016, 15, 120–125. 10.1177/1534734616645444. [DOI] [PubMed] [Google Scholar]
- Gomez-Villa R.; Aguilar-Rebolledo F.; Lozano-Platonoff A.; Teran-Soto J. M.; Fabian-Victoriano M. R.; Kresch-Tronik N. S.; Garrido-Espíndola X.; Garcia-Solis A.; Bondani-Guasti A.; Bierzwinsky-Sneider G. Efficacy of intralesional recombinant human epidermal growth factor in diabetic foot ulcers in Mexican patients: A randomized double-blinded controlled trial. Wound Repair Regen. 2014, 22, 497–503. 10.1111/wrr.12187. [DOI] [PubMed] [Google Scholar]
- Robson M. C.; Hill D. P.; Smith P. D.; Wang X.; Meyer-Siegler K.; Ko F.; VandeBerg J. S.; Payne W. G.; Ochs D.; Robson L. E. Sequential cytokine therapy for pressure ulcers: clinical and mechanistic response. Ann. Surg. 2000, 231, 600–611. 10.1097/00000658-200004000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y.; Yu D.; Wang P.; Xu J.; Li D.; Ding M. Nanotechnology promotes the full-thickness diabetic wound healing effect of recombinant human epidermal growth factor in diabetic rats. Wound Repair Regen. 2010, 18, 499–505. 10.1111/j.1524-475X.2010.00612.x. [DOI] [PubMed] [Google Scholar]
- Zhou W.; Zhao M.; Zhao Y.; Mou Y. A fibrin gel loaded with chitosan nanoparticles for local delivery of rhEGF: preparation and in vitro release studies. J. Mater. Sci.: Mater. Med. 2011, 22, 1221–1230. 10.1007/s10856-011-4304-9. [DOI] [PubMed] [Google Scholar]
- Gainza G.; Pastor M.; Aguirre J. J.; Villullas S.; Pedraz J. L.; Hernandez R. M.; Igartua M. A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparticles: In vitro bioactivity and in vivo effectiveness in healing-impaired db/db mice. J. Controlled Release 2014, 185, 51–61. 10.1016/j.jconrel.2014.04.032. [DOI] [PubMed] [Google Scholar]
- Brem H.; Kodra A.; Golinko M. S.; Entero H.; Stojadinovic O.; Wang V. M.; Sheahan C. M.; Weinberg A. D.; Woo S. L.; Ehrlich H. P. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J. Invest. Dermatol. 2009, 129, 2275–2287. 10.1038/jid.2009.26. [DOI] [PubMed] [Google Scholar]
- Xie Z. W.; Paras C. B.; Weng H.; Punnakitikashem P.; Su L. C.; Vu K.; Tang L. P.; Yang J.; Nguyen K. T. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater. 2013, 9, 9351–9359. 10.1016/j.actbio.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastar I.; Stojadinovic O.; Krzyzanowska A.; Barrientos S.; Stuelten C.; Zimmerman K.; Blumenberg M.; Brem H.; Tomic-Canic M. Attenuation of the transforming growth factor beta-signaling pathway in chronic venous ulcers. Mol. Med. 2010, 16, 92–101. 10.2119/molmed.2009.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; Ma L.; Gao C. Design of gene-activated matrix for the repair of skin and cartilage. Polym. J. 2014, 46, 476–482. 10.1038/pj.2014.50. [DOI] [Google Scholar]
- Choi J. S.; Kim H. S.; Yoo H. S. Electrospinning strategies of drug-incorporated nanofibrous mats for wound recovery. Drug Delivery Transl. Res. 2015, 5, 137–145. 10.1007/s13346-013-0148-9. [DOI] [PubMed] [Google Scholar]
- Castleberry S. A.; Almquist B. D.; Li W.; Reis T.; Chow J.; Mayner S.; Hammond P. T. Self-Assembled Wound Dressings Silence MMP-9 and Improve Diabetic Wound Healing In Vivo. Adv. Mater. 2016, 28, 1809–1817. 10.1002/adma.201503565. [DOI] [PubMed] [Google Scholar]
- Gavrilov K.; Saltzman W. M. Therapeutic siRNA: Principles, Challenges, and Strategies. Yale J. Biol. Med. 2012, 85, 187–200. [PMC free article] [PubMed] [Google Scholar]
- Whittam A. J.; Maan Z. N.; Duscher D.; Wong V. W.; Barrera J. A.; Januszyk M.; Gurtner G. C. Challenges and Opportunities in Drug Delivery for Wound Healing. Adv. Wound Care 2016, 5, 79–88. 10.1089/wound.2014.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.; Yoo H. Matrix metalloproteinase-inspired suicidal treatments of diabetic ulcers with siRNA-decorated nanofibrous meshes. Gene Ther. 2013, 20, 378–385. 10.1038/gt.2012.49. [DOI] [PubMed] [Google Scholar]
- Charafeddine R. A.; Makdisi J.; Schairer D.; O’Rourke B. P.; Diaz-Valencia J. D.; Chouake J.; Kutner A.; Krausz A.; Adler B.; Nacharaju P.; Liang H.; Mukherjee S.; Friedman J. M.; Friedman A.; Nosanchuk J. D.; Sharp D. J. Fidgetin-Like 2: A Microtubule-Based Regulator of Wound Healing. J. Invest. Dermatol. 2015, 135, 2309–2318. 10.1038/jid.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeh N.; Pastar I.; Tomic-Canic M.; Stojadinovic O. Stem cells in skin regeneration, wound healing, and their clinical applications. Int. J. Mol. Sci. 2015, 16, 25476–25501. 10.3390/ijms161025476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbir A.; Cox A.; Rodriguez-Menocal L.; Salgado M.; Van Badiavas E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. 10.1089/scd.2014.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. R.; Patel K. K.; Stappenbeck T. S. The stem cell niche. J. Pathol. 2009, 217, 169–180. 10.1002/path.2474. [DOI] [PubMed] [Google Scholar]
- Blanpain C.; Lowry W. E.; Geoghegan A.; Polak L.; Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 2004, 118, 635–648. 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Metcalfe A. D.; Ferguson M. W. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc., Interface 2007, 4, 413–437. 10.1098/rsif.2006.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V. W.; Levi B.; Rajadas J.; Longaker M. T.; Gurtner G. C. Stem cell niches for skin regeneration. Int. J. Biomater. 2012, 2012, 1–8. 10.1155/2012/926059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartarini D.; Mele E. Adult Stem Cell Therapies for Wound Healing: Biomaterials and Computational Models. Front. Bioeng. Biotechnol. 2015, 3, 1–7. 10.3389/fbioe.2015.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; Zhao Y.; Hao H.; Han W.; Fu X. Mesenchymal stem cell–based therapy for nonhealing wounds: today and tomorrow. Wound Repair Regen. 2015, 23, 465–482. 10.1111/wrr.12304. [DOI] [PubMed] [Google Scholar]
- Altman A. M.; Matthias N.; Yan Y.; Song Y.-H.; Bai X.; Chiu E. S.; Slakey D. P.; Alt E. U. Dermal matrix as a carrier for in vivo delivery of human adipose-derived stem cells. Biomaterials 2008, 29, 1431–1442. 10.1016/j.biomaterials.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Chueng S.-T. D.; Yang L.; Zhang Y.; Lee K.-B. Multidimensional nanomaterials for the control of stem cell fate. Nano Convergence 2016, 3, 23–38. 10.1186/s40580-016-0083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura L. I.; Dias A. M.; Carvalho E.; de Sousa H. C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—a review. Acta Biomater. 2013, 9, 7093–7114. 10.1016/j.actbio.2013.03.033. [DOI] [PubMed] [Google Scholar]
- Tang T.; Jiang H.; Yu Y.; He F.; Ji S.-z.; Liu Y.-y.; Wang Z.-s.; Xiao S.-c.; Tang C.; Wang G.-Y. A new method of wound treatment: targeted therapy of skin wounds with reactive oxygen species-responsive nanoparticles containing SDF-1α. Int. J. Nanomed. 2015, 10, 6571. 10.2147/IJN.S88384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.-J.; Daftarian P.; Kovalski L.; Wang B.; Tian R.; Castilla D. M.; Dikici E.; Perez V. L.; Deo S.; Daunert S.; Velazquez O. C. Directing and Potentiating Stem Cell-Mediated Angiogenesis and Tissue Repair by Cell Surface E-Selectin Coating. PLoS One 2016, 11, e0154053. 10.1371/journal.pone.0154053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak S.; Li J.; Chan Y. C.; Gnyawali S. C.; Steen E.; Yung B. C.; Khanna S.; Roy S.; Lee R. J.; Sen C. K. AntihypoxamiR functionalized gramicidin lipid nanoparticles rescue against ischemic memory improving cutaneous wound healing. Nanomedicine 2016, 12, 1827–1831. 10.1016/j.nano.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]