Abstract
Brucellosis is diagnosed by detection of antibodies in the blood of animals and humans that are specific for two carbohydrate antigens, termed A and M, which are present concurrently in a single cell wall O-polysaccharide. Animal brucellosis vaccines contain these antigenic determinants, and consequently infected and vaccinated animals cannot be differentiated as both groups produce A and M specific antibodies. We hypothesized that chemical synthesis of a pure A vaccine would offer unique identification of infected animals by a synthetic M diagnostic antigen that would not react with antibodies generated by this vaccine. Two forms of the A antigen, a hexasaccharide and a heptasaccharide conjugated to tetanus toxoid via reducing and nonreducing terminal sugars, were synthesized and used as lead vaccine candidates. Mouse antibody profiles to these immunogens showed that to avoid reaction with diagnostic M antigen it was essential to maximize the induction of anti-A antibodies that bind internal oligosaccharide sequences and minimize production of antibodies directed toward the terminal nonreducing monosaccharide. This objective was achieved by conjugation of Brucella O-polysaccharide to tetanus toxoid via its periodate oxidized terminal nonreducing monosaccharide, thereby destroying terminal epitopes and focusing the antibody response on internal A epitopes. This establishes the method to resolve the decades-long challenge of how to create effective brucellosis vaccines without compromising diagnosis of infected animals.
Short abstract
We establish a method to resolve the decades-long challenge of how to create effective brucellosis vaccines without compromising diagnosis of infected animals.
Introduction
The World Health Organization ranks brucellosis among the top seven “neglected zoonoses”.1 The disease affects both domesticated (cattle, sheep, goats, and pigs) and wild (deer, bison, elk, moose, camels, caribou, and water buffalo) animals. Brucellosis causes abortions and infertility in these animals and undulant fever in humans, a grave disease that requires a long and costly antibiotic therapy.2 Where animal production systems involve close contact with human populations, as occurs widely in developing regions, brucellosis is an endemic, insidious and embedded disease. It impacts both human and animal health, with a significant detrimental economic effect that perpetuates poverty. Three vaccines are available for use in ruminants, but there is no human vaccine and there is no recognized vaccine for swine.3
Control of endemic brucellosis is achievable via mass vaccination of animals although this is fraught with many difficulties due to significant inadequacies in the current vaccines.4 They are all live vaccines and thus require refrigeration. They possess residual virulence in animals and can cause abortions. They may be excreted in milk and cause brucellosis in humans when consumed. Two of the three carry resistance to antibiotics that are important in the treatment of human brucellosis. However, chief among the shortfalls is that the most protective vaccines induce antibodies that react in serodiagnostic assays used to identify infected animals. Given that the available vaccines are most effective when combined with the removal of seropositive animals, these reactions are a major barrier to control and eradication. This frequently leads to campaign failure or reluctance to even initiate one.5
The most effective vaccines contain bacterial cell wall O-polysaccharide (OPS), but this defeats identification of infected animals because the reactive antibodies induced by these vaccines are primarily specific for the OPS and conventional serodiagnostic assays for these species rely upon the detection of anti-OPS antibodies. OPS is the outermost part of the lipopolysaccharide within the cell wall of these vaccines and the field strains of Brucella abortus, Brucella melitensis, and Brucella suis. Its presence gives the bacteria a smooth appearance on culture media, giving rise to the terms “smooth” strains and “smooth” (s) LPS. The OPS protrudes into the host environment, and antibodies against it dominate the humoral immune response.
Brucella is an intracellular pathogen, and effective protection requires the joint input of cell-mediated and humoral immunity. This is well documented in small animal models6−8 and also seems certain to be the case in small ruminants where rough vaccines lacking OPS have been insufficiently protective.9,10 A rough vaccine, B. abortus RB51, which provides protection in the target host under experimental conditions, is in field use for large ruminants and does not cause positive serology. However, its use is controversial11 and it is considered by many to be less effective at controlling endemic disease than the smooth vaccine B. abortus S19.12 It seems that maximum protection requires the presence of OPS. Thus, despite decades of research, efforts to develop vaccines that offer the combination of strong protection without diagnostic interference have been unsuccessful.
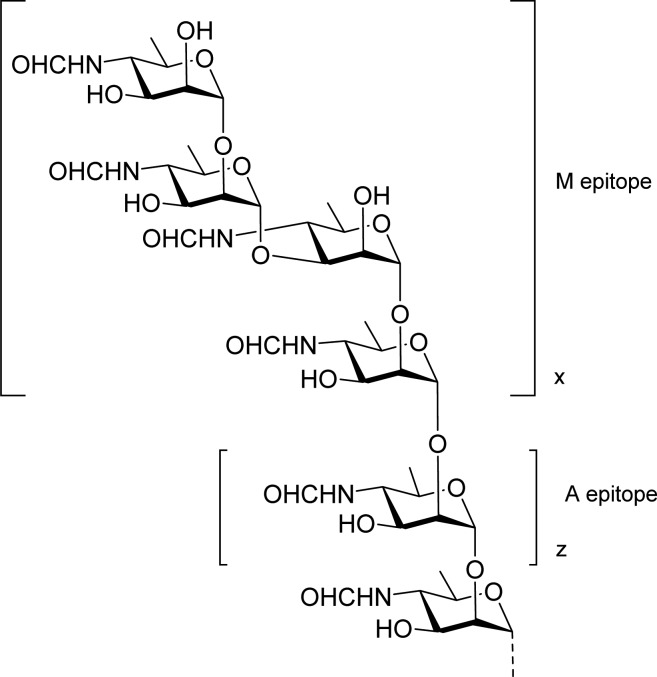
The inclusion of the native OPS in serodiagnostic assays to detect antibodies that recognize these antigens has been effective for the diagnosis of brucellosis for over 100 years.2,13 However, several problems occur. Some bacteria possess OPS that is structurally related to the A antigen, and infection by these bacteria can cause false positive diagnosis.14 It has been known since the 1930s that these two antigens could not be separated, but the precise molecular basis for this observation was only recently established.15,16Brucella OPS is a block copolymer of two distinct homopolysaccharide sequences composed of a single rare monosaccharide, 4-formamido-4,6-dideoxy-d-mannopyranose (Rha4NFo).17 A longer inner sequence of α1,2-linked residues constitutes the A antigen, and this is capped by a shorter sequence that creates the M antigen, which consists of one or several tetrasaccharide sequences containing an α1,3-linked residue (Figure 1).16
Figure 1.
O-antigen structure of B. abortus, B. melitensis, and B. suis (except biovar 2) showing the two elements M and A of the block copolymer.16 The capping tetrasaccharide containing a single 1,3-linkage defines the M epitope. The relative length of the M and A sections defined by the number of repeats, x and z, varies between strains. The OPS of B. suis biovar 218 is not terminated by a M tetrasaccharide.
We have shown previously that the capping M tetrasaccharide and an α1,3-linked disaccharide are sensitive and specific serodiagnostic antigens for the detection of antibodies in Brucella infected animals.19,20 We hypothesized that antibodies specific for the longer internal A motif, although also present in sera, are not detected by this disaccharide antigen and may be protective. Therefore, having access to this A antigen in a pure form should provide the basis of a protective vaccine.
Here we create these vaccine leads and diagnostics in pure form by chemical synthesis, conjugate them to protein, and demonstrate that a hexasaccharide representing the A antigen can induce A-specific antibodies that react only weakly with a disaccharide representing the M antigen. Using these insights, we were able to identify an approach that uses OPS glycoconjugates containing long sequences of exclusively α1,2-linked Rha4NFo units and wherein the terminal residues, containing the M antigen, have been chemically modified. This OPS-based vaccine lead in combination with chemically synthesized diagnostics19,20 permits differentiation of infected from vaccinated animals (DIVA) and breaks the decades-old scientific impasse for mass brucellosis vaccination in animals. These observations suggest key features for vaccines suitable for use in animals and humans that have the potential to reduce the estimated multibillion dollar annual economic losses and human suffering caused by the disease.21,22
Results and Discussion
Initially we selected a relatively large A type hexasaccharide conjugated to tetanus toxoid as a vaccine lead with the expectation that an immunogen of this type would induce antibodies reactive with the internal A antigen sequences and not with the terminal 1,3 linkage of the M antigen. We have described the syntheses of this A vaccine candidate 1 and 2 as well as its conjugate to BSA 3 as well as M type di- and tetrasaccharide BSA conjugates 6 and 7.19 In order to monitor the size of antibody combining sites in immune sera the 1,2-linked trisaccharide 4 and its BSA conjugate 5 were synthesized. These syntheses which closely follow previous work are referenced and described in the Supporting Information.
Immunization of mice with the A hexasaccharide 1 as its glycoconjugate 2 (Figure 2) gave a strong antibody response against the hexasaccharide epitope 1 when screened with the BSA conjugate 3.19 The sera also gave strong titers with the trisaccharide epitope 4 and exhibited strong cross reactivity with B. abortus LPS and weaker reactions with the LPS of B. melitensis and Yersinia enterocolitica O:9 (Figures 3 and S7). The O antigen of Y. enterocolitica O:9 LPS is an exclusively α1,2-linked polysaccharide of Rha4NFo23 and virtually identical to that of A dominant Brucella O antigen (for example B. abortus S99)17 but lacks the terminal M type tetrasacharide motif.16 M dominant O antigen, exemplified by B. melitensis 16M, contains several M repeats that cap the inner A antigen.16 The immune sera also exhibited cross reactions with the M disaccharide and tetrasaccharide antigens 6 and 7 (Figure 3) that were surprisingly high.
Figure 2.
Key oligosaccharides and their protein glycoconjugates used in this study. Additional glycoconjugates are shown in the Supporting Information and carry the prefix “S”: A type hexa- and trisaccharide 1 and 4, and their protein conjugates 2, 3, and 5; M type di- and tetrasaccharide BSA glycoconjugates 6 and 7; heptasaccharide 8 carries a terminal rhamnose residue equipped with an amino tether to effect attachment of an A type hexasaccharide repeat to protein via the terminal nonreducing end; as in BSA and tetanus toxoid conjugates 9 and 10.
Figure 3.
Comparison of the antibody binding profiles generated in mice immunized with 2 and 9 screened against synthetic antigens and Brucella LPS.
Because tetrasaccharide 7 is terminated by a 1,2-linked disaccharide, some of this reactivity could be expected. Screening of a monosaccharide conjugate (S28) was consistent with the presence of a population of antibodies that were able to bind the terminal monosaccharide. A wider panel of glycoconjugates of more complex structures, in which the terminal Rha4NFo residue was mono- or di-O-methylated (glycoconjugates S19–S21) or capped by d-mannose (S22–S24), blocked binding of this population of antibodies as evidenced by their correspondingly lower titers. This was consistent with a terminal epitope in which the formamido residue likely plays an immunodominant role.
To study this possibility in more detail, sera from six mice vaccinated with 2 and exhibiting high and intermediate titers against B. abortus LPS were studied by inhibition ELISA. Six pentasaccharide inhibitors were used in which Rha4NFo residues were replaced by d-Rha. These were originally designed to investigate antigen frame shifting for antibodies that recognize internal epitopes24,25 and are as follows: (where P = Rha4NFo and R = Rha): P4R; P2RP2; P2RPR; PRPRP, PR2PR; RP3R (Table S1). The most effective inhibition was achieved with P4R, P2RP2, and P2RPR, all of which were terminated by a Rha4NFo disaccharide. Two mouse sera showed weak inhibition (IC50 = 0.1 mM) against pentasaccharides (PRPRP, PR2PR) with a single Rha4NFo residue; however, RP3R, with a single terminal rhamnose, is inactive at 1 mM. Also noteworthy is that the antibody levels of the ten mice were tightly clustered with BSA–hexasaccharide conjugate 3, but the responses when measured against the LPS and conjugates 5–7 varied over a log range of 2.5. Glycoconjugate 2 would clearly fail to differentiate infected from vaccinated animals (DIVA) as it generates anti-O-antigen serum antibodies with low but significant reactivity with the M disaccharide antigen 6 and tetrasaccharide 7.
The observation that, despite the absence of any 1,3-linkages in glycoconjugate 2, the substantial titers observed for tetrasaccharide 7, M disaccharide 6, and the monosaccharide S28 prompted consideration that the terminal d-Rha4NFo residue may be particularly influential. As discussed above, this proved to be the case. Similar antibodies that target the nonreducing end have been described before, although this has previously been associated with heteropolymers (Francisella tularensis)26 or unique terminal residues that differ from those within the polymer (Vibrio cholera O1 serotype Ogawa).27
A seldom, if ever, used approach with synthetic glycoconjugate vaccines and one that could avoid the induction of antibodies to the terminal capping residue of an A type epitope involves location of a tether at the nonreducing end as shown in heptasaccharide 8 (Figure 2). Its synthesis employing the key intermediates 11–13 is outlined in Scheme 1, and syntheses of synthons (S1–S17) using known chemistry19 are outlined in the Supporting Information. Thioglycoside 11 serves as the glycosyl donor for a series of iterative glycosylations that begin with glycosylation of the known methyl glycoside S12(28) to afford disaccharide 14. Transesterification provides the disaccharide alcohol 15, which serves as the acceptor for a second glycosylation reaction. Sequential glycosylation by 11 followed by de-O-acetylation of the resultant oligosaccharide was repeated four times to provide, in succession, fully protected oligosaccharides 16, 18, 20, and 22 (Scheme 1) all in yields of 89% or higher. The selectively protected hexasaccharide alcohol 23 was glycosylated by the rhamnopyranosyl donor 13 equipped with a protected 5-aminopentanyl tether. The resulting heptasaccharide 25 was transformed to 8 by a series of previously described reactions involving azide reduction, N-formylation, and deprotection reactions (Scheme 1).19 The amine 8 was activated with disuccinimidyl glutarate or dibutyl squarate and covalently linked to tetanus toxoid and BSA to provide glycoconjugates 9 and 10.
Scheme 1. Synthesis of 'A' Type Heptasaccharide 8 with a Terminal Nonreducing Tether.
Conditions: (a) TMSOTf, NIS, 4 Å MS, CH2Cl2 −20 °C to rt, 3 h; (b) NaOMe, MeOH, rt, 6 h; (c) H2S, Py/H2O, 40 °C, 16 h; (d) (HCO)2O,MeOH, −20 °C, 3 h; (e) H2, Pd(OH)2, MeOH/H2O, rt, 16 h.
Mice were immunized with 9 using a protocol identical to that employed with 2. After three injections, very high titers were observed for the sera of all ten mice measured against the immunizing hapten, BSA conjugate 10 (Figure 3). A similarly wide distribution of titers against the three LPS antigens (B. abortus, B. melitensis and Y. enterocolitica O:9; Figure S7) was observed although the median titer for the ten mice was 2–3-fold lower than the mice vaccinated with 2. Most striking was the markedly confined distribution of titers against the M disaccharide and tetrasaccharide antigenic determinants 6 and 7. Whereas titers from sera of mice vaccinated with glycoconjugate 2 spanned a three-log range against 6 and 7, sera of mice vaccinated with 9 were restricted to a single log span and furthermore were 1–2 logs lower. This low titer against the diagnostic M antigen compound 6 comes closer to fulfilling the objective for a DIVA vaccine.
High titers against the immunizing haptens 1 and 8 for mice vaccinated with 2 or 9 compared to the corresponding titers against the 1,2-linked trisaccharide 4 suggested a significant proportion of nonreducing end-specific antibodies generated by 2 and a modest to good level against internal 1,2-linked Rh4NFo residues in the group immunized with 9. We reasoned that a glycoconjugate similar to 9 but with a larger A epitope should increase the titers against internal 1,2-linked Rh4NFo residues. If the terminal epitope was also eliminated from this structure, the presence of antibodies that bind M antigens (6 and 7) would be reduced further.
This objective could be achieved by conjugating the exclusively α1,2-linked O antigen produced by B. suis biovar 2.18 Kubler-Kielb and Vinogradov reported the structure of the Brucella OPS and the oligosaccharide through which it is attached to the inner core KDO (Scheme 2).29 There are two primary sites susceptible to mild periodate oxidation: the terminal Rha4NFo residue and the reducing end KDO residue, which remains attached to the OPS after the mild acid hydrolysis to cleave lipid A from the lipopolysaccharide molecule. Reductive amination of aldehydes created at these sites followed by reaction with disuccinimidyl glutarate allows conjugation of the activated polysaccharide to tetanus toxoid (Scheme 2). The scheme also shows how the terminal Rh4NFo is degraded by the process of oxidation followed by amination. Therefore, regardless of whether the OPS is conjugated to the protein via the terminal or reducing end, the modified OPS does not possess a tip epitope. The OPS from B. suis biovar 2 (strain Thomsen) and B. abortus strain S99, with 98% α1,2- and 2% α1,3-linkages, were conjugated to tetanus toxoid in this manner to yield respectively OPS–TT2 and OPS–TTS99. Notably, these α1,3-linkages occur near the modified nonreducing end of the OPS.16 However, they are sufficiently accessible on the OPS–TTS99 to bind the anti-M specific monoclonal antibody BM4030 as shown by Western blot (Figure S1). This antibody can bind the disaccharide 7, tetrasaccharide 6, and hexasaccharide 3, but not the exclusively α1,2-linked hexasaccharide S37 BSA conjugate (unpublished data).
Scheme 2. Conjugation of B. suis Biovar 2 OPS to Tetanus Toxoid.
Conditions: (a) 10 mM NaIO4, 50 mM NaOAc, pH 5.5, 4 °C 1 h; (b) 0.5 M NH4Cl, 0.1 M NaCNBH3, 37 °C; (c) aminated OPS 5 mg/mL in PBS containing 10% DMSO and 5 mg/mL disuccinimidal glutarate (dsg) 45 min, rt; (d) activated OPS, tetanus toxoid 2.5 mg/mL PBS.
Mice were immunized three times with each OPS tetanus toxoid conjugate (OPS–TT2 and OPS–TTS99) (Scheme 2). All eight mice immunized with the modified B. abortus OPS conjugate produced titers against both B. abortus S99 and B. melitensis 16M sLPS, despite the latter being M dominant, having OPS with 20% α1,3-links (Figure 4). Sera from all eight mice also reacted against B. abortus S99 and B. suis (Thomsen) whole cells although three did not react against the B. melitensis 16M whole cells. Not only was there was no response against the disaccharide 6 but there was also no response against the tetrasaccharide 7.
Figure 4.
Final bleed titers from eight CD1 mice immunized with modified Brucella OPS TT conjugates (left panel, B. abortus S99 OPS–TTS99; right panel, B. suis bv2 OPS–TT2). The antigens used for antibody detection are shown on the x-axis.
Binding to the hexasaccharide antigen 3 compared to that of the 1,3-linked hexasaccharide S37 shows that antibodies to the A epitope dominate. The lack of antibodies against the M epitope (as detected by binding to conjugates 6 and 7), even though 2% of the linkages are of type α1,3, may be due to the occurrence of these linkages near the site of terminal conjugation resulting in their reduced accessibility to the immune system. Antibodies that are induced against this region may also have epitopes that overlap with the modified terminal residue and fail to bind in its absence, or the antibodies require epitopes that are longer than four Rha4NFo units.
The absence of antibodies that bind to singular M epitopes would account for the average 5-fold reduction in binding of the antibodies to the M dominant (B. melitensis 16M sLPS) O antigen compared to the A dominant (B. abortus S99 sLPS) O antigen. Despite this reduction, the average titer was still approximately 1/7,000. Most of the sera also reacted against whole cell antigens although with lower titers. Importantly the results show that the OPS tetanus toxoid conjugate can induce antibodies that bind to M dominant cells via the A epitope of their OPS. It has also been shown previously that antibodies with anti-A specificity can provide protection against challenge with M dominant strains of Brucella.31
Immunization with OPS–TT2 gave lower titers than the OPS–TTS99 conjugate although five of the eight mice developed antibodies against B. abortus S99 sLPS and four against B. melitensis 16M sLPS. Notably, those mice with low or nondetectable antibody response to the sLPS antigens were those that also showed a lower, yet still substantial, response against the tetanus toxoid. The lower average titers generated by OPS–TT2 are most likely due to less efficient conjugation of OPS. Evaluation of the glycoconjugates by SDS–PAGE (Figure S3) and MALDI-ToF (Figures S4–S6) are consistent with this interpretation. More efficient conjugation may also address the higher variability of titers observed with the OPS–TT2 conjugate.
Antibodies against whole cell antigens were also generated. As with the OPS–TTS99, no antibodies were detectable that bound disaccharide and tetrasaccharide M antigens 6 and 7. Although the antibody titers generated by OPS–TT2 are lower than that induced by OPS–TTS99, the results demonstrate that antibodies that bind linear epitopes and are induced by exclusively 1,2-linked Rha4NFo can bind to these internal epitopes present in M dominant OPS. The binding to the M dominant OPS presumably occurs toward the reducing end of this block copolymer where longer stretches of α1,2-linked Rha4NFo units exist.
The development of the antibody response required a boost before all mice demonstrated an anti-sLPS or TT response (Figure 5). A second boost increased the antibody titers further until the mean anti-Brucella A dominant OPS antibody titer (demonstrated by reaction against B. abortus S99 sLPS) was in excess of 1/30,000. At no point was there a response to the disaccharide 7 antigen, even at a 1/32 dilution (101.5).
Figure 5.
Antibody binding end point titer (y-axis) of sera from eight CD1 mice immunized with TT–B. suis biovar 2 modified OPS conjugate evaluated against different antigens at four different time points on the x-axis (shown in days postimmunization [PI]). Central horizontal bar shows the mean titer. The range of titers tested was log10 2 to 4.5, except for the final bleed evaluation of the disaccharide 6.
Conclusion
Polysaccharide conjugates such as the one as described here create a long-sought Brucella vaccine component that is able to produce the anti-O-antigen antibodies that are considered to be a vital element of the most protective vaccines. Unlike the various whole cell live vaccines, these glycoconjugates produce a dominant immune response against only the α1,2-linked Rha4NFo units that make up the A epitope present in the Brucella OPS antigen. Thus, diagnostics based upon the M and terminal epitopes, including disaccharide and tetrasaccharide conjugates (6 and 7), provide a gold standard universal diagnostic that can discriminate infected from vaccinated animals. We have shown elsewhere the excellent sensitivity and discriminator power of this diagnostic test.20 The existence of anti-Brucella OPS antibodies that do not bind these M and tip epitope-specific conjugates (6 and 7) has been shown previously.32 We have now demonstrated the means to reliably and exclusively induce high titers of polyclonal antibodies with these particular characteristics; i.e., no cross-reactivity to the M and terminal epitopes. As shown in both cases, OPS tetanus toxoid conjugate induced antibodies can bind whole cell antigens of A and M serotype.33 The combination of diagnostic test and a vaccine that does not generate antibodies that bind to short M type conjugates establishes the important principles of a viable DIVA.
One could envisage various scenarios for a successful, cost-effective vaccine for ruminants with diverse immune systems. Combining anti-Brucella OPS antibody induction with an effective cell-mediated immune response will give the most effective protection against brucellosis.34 This could be achieved via OPS conjugation to or inclusion of a relevant Brucella protein, of which many have been reported.35 Avoiding growth of Brucella to harvest OPS which mandates level 3 containment could be achieved with conjugate vaccines derived from Y. enterocolitica O:9, which shares the same Rha4NFo polymer structure as B. suis biovar 218,23 but requires less stringent containment and likely represents the most cost-effective route to a human vaccine.
Brucellosis is a zoonotic infection that is passed to humans by contact with infected animals but is not spread by human-to-human contact. Eradication of human disease can only be achieved by mass vaccination of animals combined with test and slaughter.36 Our work is the first to establish a ground-breaking concept by which an OPS-based brucellosis vaccine can be applied in a DIVA format. Moreover, this work has described the main elements of a safe, viable and thermostable glycoconjugate vaccine that could protect humans against the disease. Finally, the study identifies principles that can guide biotechnology approaches for creation of a genetically engineered live DIVA vaccine that exploits known OPS biosynthetic mechanisms.37−39
Acknowledgments
All animal work in the U.K. was carried out in accordance with the UK Animal (Scientific Procedures) Act 1986 under appropriate licenses. The study protocol was approved by the APHA Animal Use Ethics Committee (UK PCD No. 70/6905). Animal work at the University of Alberta was performed by protocols approved by the Animal Care Committee, Faculty of Medicine, according to CCAC guidance.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.7b00019.
Details of chemical syntheses, glycoconjugation, spectroscopic proof of structure including mass spectrometry, immunization, and ELISA protocols (PDF)
Author Present Address
§ N.V.G.: Toronto Research Chemicals, 2 Brisbane Rd., Toronto, Ontario, Canada M3J 2J8.
Author Contributions
J.M. and D.R.B. jointly conceived and secured funding for the study, designed the experiments, and prepared the manuscript; S.S.M. and N.V.G. synthesized oligosaccharides and their glycoconjugates; L.D., S.S., and P.J.H. performed immunology and ELISA experiments, and L.D. and L.H. prepared polysaccharide conjugates and performed mass spectrometry experiments on polysaccharide and polysaccharide protein conjugates. All authors have given approval to the final version of the manuscript.
This work was funded in part by a grant from the Bill & Melinda Gates Foundation through the Grand Challenges Exploration Initiative. It was also supported by the Department for Environment, Food, and Rural Affairs (United Kingdom) under Contract SE0316 and by a Discovery grant to D.R.B. from the Natural Science and Engineering Research Council of Canada (NSERC).
The authors declare the following competing financial interest(s): None of the authors hold a direct financial interest in the work but disclose that a joint patent application between the University of Alberta and the APHA has been filed for the glycoconjugation process and glycoconjugate products described in this manuscript. Consequently, it is possible that they may benefit from patent licensing.
Supplementary Material
References
- Maudlin I.; Weber S.. The control of neglected zoonotic diseases: a route to poverty alleviation; WHO/SDE/ FOS/2006.1, Geneva; 2006.www.who.int/zoonoses/Report_Sept06.pdf.
- Corbel M. J.; Elberg S. S.; Cosivi O.. Brucellosis in humans and animals; World Health Organization: Geneva, 2006. www.who.int/csr/resources/publications/Brucellosis.pdf. [Google Scholar]
- Goodwin Z. I.; Pascual D. W. Brucellosis vaccines for livestock. Vet. Immunol. Immunopathol. 2016, 181, 51–58. 10.1016/j.vetimm.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Olsen S. C. Recent developments in livestock and wildlife brucellosis vaccination. Rev. Sci. Technol. 2013, 32, 207–217. 10.20506/rst.32.1.2201. [DOI] [PubMed] [Google Scholar]
- Olsen S. C.; Stoffregen W. S. Essential role of vaccines in brucellosis control and eradication programs for livestock. Expert Rev. Vaccines 2005, 4, 915–928. 10.1586/14760584.4.6.915. [DOI] [PubMed] [Google Scholar]
- González D.; Grilló M.-J.; De Miguel M.-J.; Ali T.; Arce-Gorvel V.; Delrue R.-M.; Conde-Álvarez R.; Muñoz P.; López-Goñi I.; Iriarte M.; Marín C.-M.; Weintraub A.; Widmalm G.; Zygmunt M.; Letesson J.-J.; Gorvel J.-P.; Blasco J.-M.; Moriyón I. Brucellosis vaccines: assessment of Brucella melitensis lipopolysaccharide rough mutants defective in core and O-polysaccharide synthesis and export. PLoS One 2008, 3, e2760. 10.1371/journal.pone.0002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A. J.; Duncan J. R.; Santisteban C. G.; Douglas J. T.; Adams L. G. Capacity of passively administered antibody to prevent establishment of Brucella abortus infection in mice. Infect. Immun. 1989, 57, 3438–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitry M.-A.; Hanot Mambres D.; De Trez C.; Akira S.; Ryffel B.; Letesson J.-J.; Muraille E. Humoral Immunity and CD4+ Th1 Cells Are Both Necessary for a Fully Protective Immune Response upon Secondary Infection with Brucella melitensis. J. Immunol. 2014, 192, 3740–3752. 10.4049/jimmunol.1302561. [DOI] [PubMed] [Google Scholar]
- Barrio M. B.; Grilló M. J.; Munoz P. M.; Jacques I.; González D.; de Miguel M. J.; Marín C. M.; Barberán M.; Letesson J.-J.; Gorvel J.-P.; Moriyón I.; Blasco J. M.; Zygmunt M. S. Rough mutants defective in core and O-polysaccharide synthesis and export induce antibodies reacting in an indirect ELISA with smooth lipopolysaccharide and are less effective than Rev 1 vaccine against Brucella melitensis infection of sheep. Vaccine 2009, 27, 1741–1749. 10.1016/j.vaccine.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Elzer P. H.; Enright F. M.; McQuiston J. R.; Boyle S. M.; Schurig G. G. Evaluation of a rough mutant of Brucella melitensis in pregnant goats. Res. Vet. Sci. 1998, 64, 259–260. 10.1016/S0034-5288(98)90135-7. [DOI] [PubMed] [Google Scholar]
- Moriyón I.; Grilló M. J.; Monreal D.; González D.; Marín C.; López-Goñi I.; Mainar-Jaime R. C.; Moreno E.; Blasco J. M. Rough vaccines in animal brucellosis: Structural and genetic basis and present status. Vet. Res. 2004, 35, 1–38. 10.1051/vetres:2003037. [DOI] [PubMed] [Google Scholar]
- Herrera-López E.; Suárez-Güemes F.; Hernández-Andrade L.; Córdova-López D.; Díaz-Aparicio E. Epidemiological study of Brucellosis in cattle, immunized with Brucella abortus RB51 vaccine in endemic zones. Vaccine 2010, 28, F59–F63. 10.1016/j.vaccine.2010.03.057. [DOI] [PubMed] [Google Scholar]
- Nielsen K. Diagnosis of brucellosis by serology. Vet. Microbiol. 2002, 90, 447–459. 10.1016/S0378-1135(02)00229-8. [DOI] [PubMed] [Google Scholar]
- McGiven J. A. New developments in the immunodiagnosis of brucellosis in livestock and wildlife. Rev. Sci. Technol. 2013, 32, 163–176. 10.20506/rst.32.1.2205. [DOI] [PubMed] [Google Scholar]
- Meikle P. J.; Perry M. B.; Cherwonogrodzky J. W.; Bundle D. R. Fine structure of A and M antigens from Brucella biovars. Infect. Immun. 1989, 57, 2820–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler-Kielb J.; Vinogradov E. Reinvestigation of the structure of Brucella O-antigens. Carbohydr. Res. 2013, 378, 144–147. 10.1016/j.carres.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff M.; Bundle D. R.; Perry M. B.; Cherwonogrodsky J. W.; Duncan J. R. Antigenic S-type lipopolysaccharide of Brucella abortus 1119–3. Infect. Immun. 1984, 46, 384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccheus M. V.; Ali T.; Cloeckaert A.; Zygmunt M. S.; Weintraub A.; Iriarte M.; Moriyón I.; Widmalm G. The Epitopic and Structural Characterization of Brucella suis Biovar 2 O-Polysaccharide Demonstrates the Existence of a New M-Negative C-Negative Smooth Brucella Serovar. PLoS One 2013, 8, e53941. 10.1371/journal.pone.0053941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh V. N.; Sadowska J. M.; Sarkar S.; Howells L.; McGiven J.; Bundle D. R. Molecular Recognition of Brucella A and M Antigens Dissected by Synthetic Oligosaccharide Glycoconjugates Leads to a Disaccharide Diagnostic for Brucellosis. J. Am. Chem. Soc. 2014, 136, 16260–16269. 10.1021/ja5081184. [DOI] [PubMed] [Google Scholar]
- McGiven J.; Howells L.; Duncombe L.; Stack J.; Ganesh N.; Guiard J.; Bundle D. R. Improved Serodiagnosis of Bovine Brucellosis by Novel Synthetic Oligosaccharide Antigens Representing the Capping M Epitope Elements of Brucella O-Polysaccharide. J. Clin. Microbiol. 2015, 53, 1204–1210. 10.1128/JCM.03185-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R. L.; Martins T. M.; Borges Á. M.; Paixão T. A. Economic losses due to bovine brucellosis in Brazil. Pesq. Vet. Bras. 2013, 33, 759–764. 10.1590/S0100-736X2013000600012. [DOI] [Google Scholar]
- Samartino L. E. Brucellosis in Argentina. Vet. Microbiol. 2002, 90, 71–80. 10.1016/S0378-1135(02)00247-X. [DOI] [PubMed] [Google Scholar]
- Caroff M.; Bundle D. R.; Perry M. B. Structure of the O-chain of the phenol-phase soluble cellular lipopolysaccharide of Yersinia enterocolitica serotype O:9. Eur. J. Biochem. 1984, 139, 195–200. 10.1111/j.1432-1033.1984.tb07994.x. [DOI] [PubMed] [Google Scholar]
- Kihlberg J.; Eichler E.; Bundle D. R. The design and synthesis of antibody binding site probes: three pentasaccharide analogues of the Brucella A antigen prepared by in situ activation of thioglycosides with bromine. Carbohydr. Res. 1991, 211, 59–75. 10.1016/0008-6215(91)84146-6. [DOI] [PubMed] [Google Scholar]
- Kihlberg J.; Bundle D. R. The synthesis of antibody binding site probes: A hexasaccharide and two pentasaccharides related to the Brucella A antigen prepared by in situ activation of thioglycosides with bromine. Carbohydr. Res. 1992, 216, 67–78. 10.1016/0008-6215(92)84151-H. [DOI] [PubMed] [Google Scholar]
- Lu Z.; Rynkiewicz M. J.; Yang C.-Y.; Madico G.; Perkins H. M.; Wang Q.; Costello C. E.; Zaia J.; Seaton B. A.; Sharon J. The binding sites of monoclonal antibodies to the non-reducing end of Francisella tularensis O-antigen accommodate mainly the terminal saccharide. Immunology 2013, 140, 374–389. 10.1111/imm.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve S.; Boutonnier A.; Mulard L. A.; Fournier J. M. Immunochemical characterization of an Ogawa-Inaba common antigenic determinant of Vibrio cholerae O1. Microbiology 1999, 145, 2477–2484. 10.1099/00221287-145-9-2477. [DOI] [PubMed] [Google Scholar]
- Bundle D. R.; Gerken M.; Peters T. Synthesis of antigenic determinants of the Brucella A antigen utilizing methyl 4-azido-4,6-dideoxy-α-D-mannopyranoside efficiently derived from D-mannose. Carbohydr. Res. 1988, 174, 239–251. 10.1016/0008-6215(88)85094-8. [DOI] [PubMed] [Google Scholar]
- Kubler-Kielb J.; Vinogradov E. The study of the core part and non-repeating elements of the O-antigen of Brucella lipopolysaccharide. Carbohydr. Res. 2013, 366, 33–37. 10.1016/j.carres.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiser-Wilke I.; Moennig V. Monoclonal antibodies and characterization of epitopes of smooth Brucella lipopolysaccharides. Ann. Inst. Pasteur/Microbiol. 1987, 138, 549–560. 10.1016/0769-2609(87)90040-8. [DOI] [PubMed] [Google Scholar]
- Cloeckaert A.; Jacques I.; de Wergifosse P.; Dubray G.; Limet J. N. Protection against Brucella melitensis or Brucella abortus in mice with immunoglobulin G (IgG), IgA, and IgM monoclonal antibodies specific for a common epitope shared by the Brucella A and M smooth lipopolysaccharides. Infect. Immun. 1992, 60, 312–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt M. S.; Bundle D. R.; Ganesh N. V.; Guiard J.; Cloeckaert A. Monoclonal Antibody-Defined Specific C Epitope of Brucella O-Polysaccharide Revisited. Clin. Vaccine Immunol. 2015, 22, 979–982. 10.1128/CVI.00225-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloeckaert A.; Weynants V.; Godfroid J.; Verger J.-M.; Grayon M.; Zygmunt M. S. O-Polysaccharide epitopic heterogeneity at the surface of Brucella spp. studied by enzyme-linked immunosorbent assay and flow cytometry. Clin. Diagn. Lab. Immunol. 1998, 5, 862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilló M. J.; Manterola L.; de Miguel M. J.; Muñoz P. M.; Blasco J. M.; Moriyón I.; López-Goñi I. Increases of efficacy as vaccine against Brucella abortus infection in mice by simultaneous inoculation with avirulent smooth bvrS/bvrR and rough wbkA mutants. Vaccine 2006, 24, 2910–2916. 10.1016/j.vaccine.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Oliveira S. C.; Giambartolomei G. H.; Cassataro J. Confronting the barriers to develop novel vaccines against brucellosis. Expert Rev. Vaccines 2011, 10, 1291–1305. 10.1586/erv.11.110. [DOI] [PubMed] [Google Scholar]
- Roth F.; Zinsstag J.; Orkhon D.; Chimed-Ochir G.; Hutton G.; Cosivi O.; Carrin G.; Otte J. Human health benefits from livestock vaccination for brucellosis: case study. Bull. W. H. O. 2003, 81, 867–876. [PMC free article] [PubMed] [Google Scholar]
- Whitfield C.; Trent M. S. Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 2014, 83, 99–128. 10.1146/annurev-biochem-060713-035600. [DOI] [PubMed] [Google Scholar]
- Feldman M. F.; Wacker M.; Hernandez M.; Hitchen P. G.; Marolda C. L.; Kowarik M.; Morris H. R.; Dell A.; Valvano M. A.; Aebi M. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 3016–3021. 10.1073/pnas.0500044102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.; Valentine J. L.; Huang C. J.; Endicott C. E.; Moeller T. D.; Rasmussen J. A.; Fletcher J. R.; Boll J. M.; Rosenthal J. A.; Dobruchowska J.; Wang Z.; Heiss C.; Azadi P.; Putnam D.; Trent M. S.; Jones B. D.; DeLisa M. P. Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E3609–E3618. 10.1073/pnas.1518311113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.