Abstract
Contrast-induced nephropathy (CIN) develops after the injection of iodinated contrast media. This is a post hoc analysis of the data obtained from the TRUST study, which was a prospective, multicentre, observational study conducted to evaluate the safety and tolerability of the contrast medium iopromide in patients undergoing cardiac catheterization from August 2010 to September 2011 in China, conducted to explore the current status, trends and risk predictors of hydration treatment. The status of hydration to prevent CIN in each patient was recorded. Of the total 17,139 patients from the TRUST study (mean age, 60.33 ± 10.38 years), the overall hydration usage was 46.1% in patients undergoing percutaneous coronary intervention (PCI) and 77.4%, 51.7%, and 48.5% in patients with pre-existing renal disease, diabetes mellitus, and hypertension, respectively. The proportion of hydration use increased from 36.5% to 55.5% from August 2010 to September 2011, which was independently associated with risk predictors like older age, pre-existing renal disease, hypertension, diabetes mellitus, prior myocardial infarction, ST segment elevation MI, high contrast dose, multi-vessel disease and reduced LVEF (<45%). Overall, the usage of intravenous hydration treatment for patients with a high risk of CIN following PCI was high in China.
It is estimated that an average of 23–46% patients with coronary artery disease (CAD) develop chronic kidney disease (CKD) or end-stage renal disease as a major complication, which accounts to increase hospitalization and mortality worldwide1,2,3. Contrast-induced nephropathy (CIN) or contrast-induced acute kidney injury (CI-AKI) can develop after the injection of iodinated contrast media4. The incidence of CIN is about 3.3% in general population5, whereas it can be 20% and up to 50% in patients with concomitant or previous history of severe cardiac4 and kidney6 diseases, respectively. In the past decade, the number of cardiac catheterizations, including coronary angiography (CAG) and percutaneous coronary intervention (PCI), has increased by 17 and 21 fold, respectively, in China, according to the China PEACE study7. As per the Chinese National data of coronary intervention, there were 567,583 cases of PCI in 2015, with an annual growth rate of 10–15%8. This increase in PCI cases led to a rise in CIN incidences, making it the third largest cause of hospitalization for AKI, which was associated with increased cases of renal replacement treatments, cardiovascular adverse events and high healthcare costs9,10,11.
Strategies to prevent CIN such as identifying high-risk patients who may develop CIN and restricting them to the procedure, reducing contrast agent volume and intensifying pre-procedural intravenous saline hydration, respectively, were established. The current PCI guidelines recommend pre- and post-procedural intravenous infusion of isotonic saline as a prevention strategy12,13. Moreover, the ACC/ESC PCI practice guidelines and most clinical studies have recommended saline hydration in patients with pre-existing renal disease or kidney dysfunction undergoing CAG/PCI at a rate of 1 mL/kg/h 12 h before and 12–24 h after the procedure12,13,14,15.
According to a cross-sectional survey conducted in China, about 120 million adults have CKD (estimated glomerular filtration rate [eGFR]: <60 mL/min/1.73 m2)16 and approximately 60% of the patients undergoing PCI have CKD (eGFR <90 mL/min/1.73 m2)17. Therefore, hydration treatment is essential, especially in patients with risk factors such as kidney disease, reduced ventricular function and diabetes. However, data on the status of hydration treatment use in China are scarce. Therefore, we conducted a post hoc data analysis from the safety and toleRability of UltraviSt in patients undergoing cardiac caTheterization (TRUST) study18 to provide evidence on hydration use and also the risk predictors for hydration administration.
Results
Baseline and procedural characteristics
The baseline characteristics of the enrolled patients are given in Table 1. Of the 17,513 patients invited to participate in the survey, 374 were excluded due to missing hydration records and 17,139 patients were enrolled in the survey (mean age, 60.33 ± 10.38 years; men, 64.3%). When stratified based on the hydration status, 7901/17,139 (46.1%) patients were found to have received hydration treatment peri-procedurally. The average hydration volume in the hydration group was 1105.25 ± 631.73 mL and there was no saline hydration use in the no hydration group. There were more comorbidities in the hydration group patients than in the non-hydration group patients, such as hypertension (58.5% vs. 53.1%, P < 0.0001), diabetes mellitus (22.5% vs. 18.0%, P < 0.0001), prior MI (8.2% vs. 5.9%, P < 0.0001) and pre-existing renal disease based on personal health history (2.3% vs. 0.6%, P < 0.0001). There was also a higher patient proportion diagnosed with STEMI, unstable angina, multi-vessel disease, left main lesion and left anterior descending branch disease in the hydration group (P < 0.0001). Furthermore, the hydration group patients had received larger doses of the contrast agent (140.38 ± 80.59 vs. 111.49 ± 62.57 mg of iodine/mL, P < 0.0001). There were no significant differences between the groups in terms of age, gender and history of prior catheterization.
Table 1. Baseline and procedural characteristics of patients in the hydration and non-hydration groups.
| Characteristics | Total (N = 17139) | Hydration (N = 7901) | Non-hydration (N = 9238) | P value |
|---|---|---|---|---|
| Age (years) | 60.33 ± 10.38 | 60.24 ± 10.31 | 60.41 ± 10.44 | 0.285 |
| Age >75 years, n (%) | 1139 (6.8) | 505 (6.5) | 634 (7.0) | 0.228 |
| Female, n (%) | 6117 (35.7) | 2829 (35.8) | 3288 (35.6) | 0.783 |
| Weight (kg) | 69.16 ± 10.71 | 70.05 ± 11.02 | 68.41 ± 10.38 | <0.0001 |
| Previous history | ||||
| History of hypertension, n (%) | 9528 (55.6) | 4619 (58.5) | 4909 (53.1) | <0.0001 |
| History of diabetes mellitus, n (%) | 3441 (20.1) | 1778 (22.5) | 1663 (18.0) | <0.0001 |
| Prior MI, n (%) | 1187 (6.9) | 646 (8.2) | 541 (5.9) | <0.0001 |
| Pre-existing renal disease, n (%) | 239 (1.4) | 185 (2.3) | 54 (0.6) | <0.0001 |
| Family history of CAD, n (%) | 1185 (6.9) | 660 (8.4) | 525 (5.7) | <0.0001 |
| Prior cardiac catheterization, n (%) | 1749 (10.2) | 864 (10.9) | 885 (9.6) | 0.003 |
| Systolic blood pressure (mmHg) | 134.20 ± 18.46 | 135.04 ± 18.66 | 133.47 ± 18.25 | <0.0001 |
| Diastolic blood pressure (mmHg) | 80.21 ± 11.48 | 81.31 ± 11.75 | 79.27 ± 11.16 | <0.0001 |
| Preoperative metformin, n (%) | 319 (1.9) | 168 (2.1) | 151 (1.6) | 0.018 |
| LVEF < 35%, n (%) | 158 (0.9) | 70 (0.9) | 88 (1.0) | 0.628 |
| LVEF <45%, n (%) | 717 (4.2) | 302 (3.8) | 315 (4.6) | 0.025 |
| Clinical presentation | ||||
| STEMI, n (%) | 1890 (11.0) | 1023 (12.9) | 867 (9.4) | <0.0001 |
| NSTEMI, n (%) | 833 (4.9) | 375 (4.7) | 458 (5.0) | 0.521 |
| Unstable angina, n (%) | 8689 (50.7) | 4610 (58.3) | 4079 (44.2) | <0.0001 |
| Stable angina, n (%) | 2039 (11.9) | 628 (7.9) | 1411 (15.3) | <0.0001 |
| Others, n (%) | 3912 (22.8) | 1388 (17.6) | 2524 (27.3) | <0.0001 |
| TC (mmol/L) | 4.51 ± 1.17 | 4.57 ± 1.17 | 4.46 ± 1.17 | <0.0001 |
| TG (mmol/L) | 1.69 ± 0.95 | 1.72 ± 0.98 | 1.66 ± 0.91 | <0.0001 |
| Contrast dose (mL) | 124.80 ± 72.88 | 140.38 ± 80.59 | 111.49 ± 62.57 | <0.0001 |
| Coronary artery features | ||||
| Normal, n (%) | 3044 (17.8) | 1036 (13.1) | 2008 (21.7) | <0.0001 |
| Single-vessel disease, n (%) | 6756 (39.4) | 2943 (37.2) | 3813 (41.3) | <0.0001 |
| Multi-vessel disease, n (%) | 7339 (42.8) | 3922 (49.6) | 3417 (37.0) | <0.0001 |
| Left main disease, n (%) | 1456 (8.5) | 809 (10.2) | 647 (7.0) | <0.0001 |
| Left anterior descending branch, n (%) | 11,142 (65.0) | 5434 (68.8) | 5708 (61.8) | <0.0001 |
| Left circumflex artery, n (%) | 6478 (37.8) | 3354 (42.5) | 3124 (33.8) | <0.0001 |
| Right coronary artery, n (%) | 6534 (38.1) | 3359 (42.5) | 3175 (34.4) | <0.0001 |
| Stents implanted, n (%) | 8287 (48.4) | 4078 (51.6) | 4209 (45.6) | <0.0001 |
| Number of stents implanted | 1.68 ± 0.76 | 1.69 ± 0.76 | 1.67 ± 0.76 | 0.302 |
Abbreviations: MI, myocardial infarction; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; STEMI, acute ST segment elevation myocardial infarction; NSTEMI, non-ST segment elevation acute myocardial infarction; TC, total cholesterol; TG, total triglyceride.
Proportion of saline hydration rates in high-risk patients
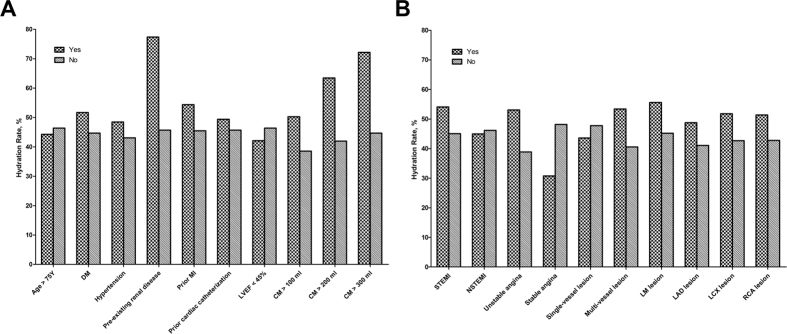
The hydration rate in patients with and without risk factors is shown in Fig. 1. Hydration was significantly higher in patients with pre-existing renal disease (77.4% vs. 45.7%), STEMI (54.1% vs. 45.1%), unstable angina (53.1% vs. 38.9%) and multi-vessel disease (53.4% vs. 40.6%) (all P < 0.001). In addition, the use of a higher dose of the contrast medium was associated with an increased hydration rate (P < 0.001). It is important to note that, compared with patients without concomitant diseases, the absolute saline volumes were significantly lower in patients with prior MI (915.25 ± 499.44 vs. 1122.16 ± 639.48 mL, P < 0.001), pre-existing renal disease (775.32 ± 680.36 vs. 1113.16 ± 628.44 mL, P < 0.001) and prior catheterization (901.60 ± 553.04 vs. 1130.25 ± 636.28 mL, P < 0.001), whereas it was higher in patients >75 years of age (1045.41 ± 566.61 vs. 1110.27 ± 635.64 mL, P = 0.014), in patients with hypertension (1143.47 ± 631.88 vs. 1051.44 ± 627.67 mL, P < 0.001), multi-vessel disease (1152.27 ± 619.52 vs. 1058.90 ± 640.24 mL, P < 0.001), left main lesion (1213.93 ± 704.35 vs. 1092.85 ± 621.76 mL, P < 0.001), left anterior descending branch lesion (1133.76 ± 626.63 vs. 1042.27 ± 638.45 mL, P < 0.001) and left circumflex branch lesion (1150.99 ± 626.74 vs. 1071.51 ± 633.34 mL, P < 0.001). There was no significant difference in the saline volumes between patients with and without reduced LVEF of <45%, single-vessel disease and right coronary lesion (Table 2).
Figure 1. Hydration rate status of patients.
(A) Hydration rate of patients with or without risk factors; (B) Hydration rate of patients with different coronary diseases. Abbreviations: DM, diabetes mellitus; MI, myocardial infarction; LVEF, left ventricular ejection fraction; CM, contrast medium; STEMI, ST segment elevation myocardial infarction; NSTEMI, non-ST segment elevation acute myocardial infarction; LM lesion, left main lesion; LAD lesion, left anterior descending branch lesion; LCX lesion, left circumflex branch lesion; RCA lesion, right coronary lesion.
Table 2. Hydration status of high-risk patients.
| Characteristics | Yes | No | P value |
|---|---|---|---|
| Age >75 years | 1045.41 ± 566.61 | 1110.27 ± 635.64 | 0.014 |
| Hypertension | 1143.47 ± 631.88 | 1051.44 ± 627.67 | <0.001 |
| Diabetes mellitus | 1112.49 ± 600.26 | 1103.15 ± 640.60 | 0.570 |
| Prior MI | 915.25 ± 499.44 | 1122.16 ± 639.48 | <0.001 |
| Pre-existing renal disease | 775.32 ± 680.36 | 1113.16 ± 628.44 | <0.001 |
| Prior cardiac catheterization | 901.60 ± 553.04 | 1130.25 ± 636.28 | <0.001 |
| LVEF <45% | 1104.78 ± 632.81 | 1131.75 ± 601.46 | 0.446 |
| STEMI | 1170.44 ± 674.98 | 1095.55 ± 624.51 | 0.001 |
| NSTEMI | 1177.60 ± 604.46 | 1101.64 ± 632.88 | 0.023 |
| Unstable angina | 1087.17 ± 642.63 | 1130.59 ± 615.33 | 0.002 |
| Stable angina | 1061.78 ± 574.41 | 1109.00 ± 636.33 | 0.050 |
| Contrast dose ≥100 mL | 1078.68 ± 660.64 | 1166.69 ± 554.46 | <0.001 |
| Contrast dose ≥200 mL | 1000.65 ± 610.97 | 1142.91 ± 634.88 | <0.001 |
| Contrast dose ≥300 mL | 1121.22 ± 632.90 | 1103.90 ± 631.65 | 0.515 |
| Single-vessel disease | 1098.69 ± 656.55 | 1109.14 ± 616.56 | 0.484 |
| Multi-vessel disease | 1152.27 ± 619.52 | 1058.90 ± 640.24 | <0.001 |
| Left main lesion | 1213.93 ± 704.35 | 1092.85 ± 621.76 | <0.001 |
| Left anterior descending branch lesion | 1133.76 ± 626.63 | 1042.47 ± 638.45 | <0.001 |
| Left circumflex branch lesion | 1150.99 ± 626.74 | 1071.51 ± 633.34 | <0.001 |
| Right coronary lesion | 1106.29 ± 593.58 | 1104.48 ± 658.58 | 0.898 |
Abbreviations: MI, myocardial infarction; LVEF, left ventricular ejection fraction; STEMI, ST segment elevation myocardial infarction; NSTEMI, non-ST segment elevation acute myocardial infarction.
Trend of increased hydration use
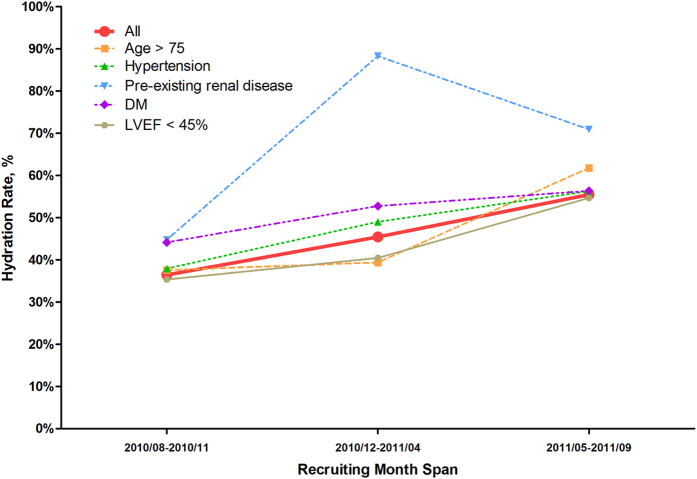
Figure 2 shows the trend of hydration use over time from August 2010 to September 2011. The overall rate of hydration use among patients undergoing CAG/PCI increased during the study period from 36.5% to 55.5%. Moreover, the rate of hydration therapy also increased over time in patients with one of the risk factors, such as pre-existing renal disease (44.7% to 70.9%), diabetes mellitus (44.1% to 56.3%), hypertension (37.9% to 56.3%), reduced LVEF (<45%; 35.3% to 54.7%) and older age (37.6% to 61.8%).
Figure 2. The trend of hydration rate over time in patients with different risk factors in three groups.
Abbreviations: DM, diabetes mellitus; LVEF, left ventricular ejection fraction.
Predictors of hydration
After adjusting for covariates described in baseline characteristics, hydration use was independently associated with older age, hypertension, diabetes mellitus, pre-existing renal disease, prior MI, STEMI, contrast dose, multi-vessel disease and LVEF <45% (Table 3). Patients aged >75 years (OR, 0.994; 95% CI, 0.991–0.997; P = 0.001) and with reduced LVEF (OR, 0.720; 95% CI, 0.614–0.844; P < 0.001) were less likely to receive hydration therapy, whereas patients with pre-existing renal disease (OR, 3.673; 95% CI, 2.694–5.008; P < 0.001) were at a higher risk of CIN and, hence, were more likely to receive hydration therapy. Similarly, patients with hypertension, diabetes mellitus, MI, STEMI, high contrast dose and multi-vessel disease were likely to receive hydration therapy due to a high risk of CIN.
Table 3. Multivariable analysis of factors associated with hydration therapy in patients undergoing CAG/PCI.
| Risk factors | Multivariate logistic regression | ||
|---|---|---|---|
| OR | 95% CI | P value | |
| Age >75 years | 0.994 | 0.991–0.997 | <0.001 |
| Hypertension | 1.192 | 1.118–1.270 | <0.001 |
| Diabetes mellitus | 1.182 | 1.093–1.278 | <0.001 |
| Pre-existing renal disease | 3.673 | 2.694–5.008 | <0.001 |
| Prior MI | 1.187 | 1.048–1.343 | 0.007 |
| STEMI | 1.168 | 1.056–1.292 | 0.003 |
| Contrast dose | 1.005 | 1.004–1.005 | <0.001 |
| Multi-vessel disease | 1.293 | 1.208–1.384 | <0.001 |
| LVEF <45% | 0.720 | 0.614–0.844 | <0.001 |
Abbreviations: MI, myocardial infarction; LVEF, left ventricular ejection fraction; STEMI, ST segment elevation myocardial infarction.
Discussion
To the best of our knowledge, this is the first study to provide contemporary information on the status of hydration use while undergoing CAG/PCI and its positive increase over time in Chinese patients, which is different from other retrospective study that analyzed the hydration practices in any patient undergoing coronary angiography or receiving specified contrast agent determined by cardiologists. The overall rate of hydration use remained relatively low (46.1%) in China. However, a significant increase in hydration use was observed over the study period in elderly patients and in patients with pre-existing renal diseases. In addition, multivariate analysis showed that hypertension, diabetes mellitus, pre-existing renal disease, prior MI, STEMI, contrast dose, multi-vessel disease and LVEF <45% were independent predictors of hydration use.
Different mechanisms are involved in the complex pathophysiology that underlies CIN. The direct toxic effects of the contrast medium would induce medullary vasoconstriction mediated by endothelin that reduce renal blood flow and damage the tubular epithelial cells and vascular endothelium, which lead to altered renal hemodynamics, regional hypoxia and the production of reactive oxygen radicals9,10,19. These may further increase tubular cell injury. In addition, the beneficial effects of saline hydration in salvaging CIN due to catheterization techniques are well documented20,21,22. The protective mechanisms underlying the salvaging effect have been investigated widely. Aurelio et al., reported that pre-procedural intravascular volume expansion could maintain medullary blood flow, enhance washout of the contrast medium through the kidney and shorten the time of contrast exposure, which might dilute the concentration of contrast medium, hampering the release of reactive oxygen species and cell necrosis factors that may damage the renal architecture. Moreover, hydration relieves regional hypoxia by preventing endothelial dysfunction and blood hyper viscosity by facilitating nitric oxide release9.
Despite the advancement in knowledge regarding CIN and hydration therapy, the awareness of the same in China remains weak. As mentioned previously, a low rate of hydration in patients of our study clearly suggests a weak understanding of both the disease and the therapy. A recent survey conducted by Prasad et al., to evaluate contemporary practice patterns with regard to prevention of CIN in patients undergoing invasive angiography from the Society of Cardiovascular Angiography and Intervention (SCAI) member cardiologists, revealed that 96.8% of the patients believe that the acute kidney injury (AKI) risk was always important; however, there were only 64.8% cardiologists who inclined to adopt the use of standardized volume expansion protocols to prevent the occurrence of CIN23.
The risk of CIN increases in patients with concomitant diseases. The patients with STEMI certainly have a higher risk of CIN24. Although current guidelines for the management of STEMI do not have a specific hydration strategy recommendation, a series of clinical trials have made some exploration. Jurado-Román et al., evaluated the effective role of peri-procedural intravenous hydration with saline in patients with STEMI undergoing primary PCI and observed an overall CIN incidence of 14% and reduction in the risk of CIN by 48% with the use of intravenous saline hydration peri-procedurally21. Furthermore, Maioli et al., suggested that the incidence of CIN would be lower with pre-procedural hydration strategy25. Previous studies have discovered that CIN occurred more frequently in patients treated with primary PCI than in those treated with elective procedures, with an incidence of up to 30%26. On the other hand, the rate and volume of hydration might be affected when patients presented active heart failure with pulmonary edema during acute coronary syndrome or STEMI. While in the present study, it was difficult to clarify this specific group of patients because we did not divide these patients before. Therefore, patients with STEMI should be emphasized on receiving hydration treatment. Our ongoing study (ATTEMPT, NCT02067195) aims to investigate the benefit and risk of aggressive hydration (faster and longer hydration) for patients with STEMI undergoing primary PCI27. The present study showed that 54.1% of the patients with STEMI received hydration, with a higher volume of hydration compared with patients without STEMI (mean, 1170 mL vs. 1096 mL). However, hydration was still deprived in 45.9% of the patients with STEMI, which warrants the need for awareness among clinicians and patients.
Furthermore, pre-existing renal disease based on personal health history is a major predictor of CIN in patients undergoing PCI/CAG procedures. The 2014 European Society of Cardiology Guidelines recommended patients with CKD to adopt hydration treatment 12 h before and at least 24 h after PCI13. Moreover, several clinical trials have proven that a higher volume of hydration efficiently reduces the risk of CIN and other adverse events28,29,30,31. In contrast, Liu et al., reported that excess hydration could not have a better effect on preventing CIN and may worsen heart failure17. The present study showed a higher hydration rate (77.4% vs. 45.7%) in high-risk patients with pre-existing renal disease than in patients with normal renal function, while the hydration volume was significantly lower. Our study also found that the volume of hydration was similar in patients with or without LVEF <45%, but a relatively lower hydration rate in patients with LVEF <45% (42.1% vs. 46.4%). In addition, hydration rate was increased with every 100-mL increase in the contrast dose used. These findings reflect the preliminary status of hydration therapy in Chinese patients undergoing CAG/PCI with different risk factors. However, the practice of hydration treatment is still not standardized and different from guideline recommendations at present in China.
Overall, the current study showed that the low rate of hydration treatment in patients undergoing CAG/PCI had gradually improved in China, especially in patients with risk factors such as pre-existing renal disease (44.7% to 70.9%, P < 0.001), diabetes mellitus (44.1% to 56.3%, P < 0.001), hypertension (37.9% to 56.3%, P < 0.001), reduced LVEF (35.3% to 54.7%, P < 0.001) and older age (37.6% to 61.8%, P < 0.001). This may be attributed to the promotion of coronary intervention technology and awareness of related complications, especially about CIN. Moreover, specialized CIN risk evaluation system, CIN education programs and hydration intensity monitoring have been established to improve awareness in patients undergoing CAG/PCI and clinicians on the benefits of hydration therapy.
Our study has several limitations: first, this was a post hoc observational analysis of a prospective observational study that focused on acute adverse drug reactions following iopromide administration without administration of prespecified hydration strategy; the study design may cause a bias due to the non-interventional nature and the variety of the quality of documentation of the patient’s hydration status across centers. Meanwhile, the prespecified contrast “iopromide” may increase the bias of strategy of prevention of CI-AKI and acute adverse drug reactions for operators. Second, several important indicators useful to evaluate the preventive effect of hydration on CIN, cannot be taken into account in a study design like that as additional lab tests are not conducted. Finally, most participating centers of this study were tertiary hospitals and therefore the data may not fully be representative of the whole country as clinical practice might differ to a large extend across hospitals in China.
In conclusion, the use of hydration among Chinese patients undergoing CAG/PCI which used to be relatively low in tertiary hospitals, including high-risk patients (e.g., pre-existing renal disease and older age) showed a significant increase in the study period. However, more awareness on hydration treatment and the risk of CIN in patients and clinicians along with an increase in hydration treatment compliance with guidelines among the high-risk patients are warranted in China.
Methods
Study design and participants
The present survey was a part of the TRUST study, which was a prospective, multicenter, observational study conducted on patients undergoing CAG or PCI procedure with the low-osmolar non-ionic contrast agent iopromide (Ultravist; Bayer, Berlin, Germany) at 63 hospitals in China from August 2010 to September 2011 (ClinicalTrials.gov identifier: NCT01206257). The objectives of the present analysis were to provide information on intravenous hydration practice in China, identify any changes over time and factors associated with hydration use, as well as to understand opportunities for further improvement. The study protocol conformed to the principles of the Declaration of Helsinki and was approved by the Chinese ethics committee of registering clinical trials (ChiECRCT), China (approval number ChiECRCT-2010018, 31st August 2010) as the leading ethics board and additionally was approved by the local ethics boards of each participating center.
The study was approved by local ethics committees and We conducted a post hoc analysis of patient data from the TRUST study18. Patients with coronary artery disease scheduled for CAG and/or PCI with iopromide (300 or 370 mg iodine/mL) were included in the study, and a written informed consent was procured from all the eligible patients. Pregnant and lactating women, patients with a contraindication to iopromide and/or cardiac catheterization were excluded from the study.
Study procedures
Hydration treatment
We recorded whether patients accepted intravenous isotonic saline hydration pre or post-procedurally or not and the total hydration volume through the case report form. All the enrolled patients were stratified into two groups based on their hydration status (those who received the hydration treatment and those who did not receive the hydration treatment).
Coronary angiography or PCI
Coronary angiography was performed using standard guide catheters, guide wires, balloon catheters and stents by the femoral or radial approach according to the standard clinical practice. The concentration and dose of intra-arterially injected iopromide were left to the discretion of the interventional cardiologist according to operative requirements.
Screening protocol and assessment criteria
Participants were asked to complete a questionnaire documenting their socio-demographic status (e.g., age and sex), personal and family health history (e.g., hypertension, diabetes and pre-existing renal disease), physical examination (e.g., weight and blood pressure) and laboratory examination (e.g., serum lipids) with the assistance of trained nurses. Medical records of the patients were also reviewed by the nurses to confirm hydration treatment during the perioperative period. Data testing and collection were conducted at the local central hospital.
Statistical analysis
All the analyses were conducted using the SPSS 22.0 software (IBM Corp, Armonk, New York, United States). Continuous variables are presented as mean ± standard deviation, and categorical variables are expressed as counts and percentages with 95% confidence intervals. Differences in demographic and socio-economic characteristics, presence of metabolic conditions, clinical presentation and features of coronary artery among participants were analyzed using two-tailed unpaired student t-tests for continuous variables and chi-square tests for categorical variables. A multivariable logistic regression model adjusted for age (>75 years), hypertension, diabetes mellitus, pre-existing renal disease, prior myocardial infarction (MI), ST-elevation MI (STEMI), contrast dose, multi-vessel disease and left ventricular ejection fraction (LVEF <45%) was used to identify factors independently associated with hydration use. A P value of <0.05 was considered to be statistically significant.
Additional Information
How to cite this article: Bei, W. et al. Post-Hoc Study: Intravenous Hydration Treatment in Chinese Patients with High Risk of Contrast-Induced Nephropathy Following Percutaneous Coronary Intervention. Sci. Rep. 7, 45023; doi: 10.1038/srep45023 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
The authors thank Ms. Navya Reddy and Dr. Amit Bhat (Indegene, Bangalore, India) for providing medical writing assistance.
Footnotes
The authors declare no competing financial interests.
Author Contributions All authors contributed substantially to the study. W.B., H.L., K.L., K.W. and S.C. designed the post hoc study for the TRUST study and collected the data with the help of other TRUST investigators. X.G., Y.L., N.T. and J.C. performed the analysis and developed the manuscript. All authors approved the final draft of the manuscript.
Contributor Information
TRUST investigators:
Xiangtai Yang, Xi Su, Zhimin Du, Qiutang Zeng, Zhenfei Fang, Yan Wang, Hong Jiang, Longgen Xiong, Yuqing Hou, Yong Yuan, Tianfa Li, Lang Hong, Yanqing Wu, Yin Liu, Wenhua Lin, Tiemin Jiang, Junhua Fu, Yi An, Bo Yu, Ye Tian, Yang Zheng, Bin Liu, Ping Yang, Xianyan Jiang, Hao Wang, Peng Qu, Lianqun Cui, Xueqi Li, Xiaoyong Qi, Zengcai Ma, Jifu Li, Lili Zhang, Shengquan Liu, Wenyue Pang, Yibo Li, Manguang Yang, Zheng Ji, Pitian Zhao, Lu Li, Junbo Ge, Huigen Jin, Weimin Pan, Yaoming Song, Jianmei Li, Jianming Xiao, Hanxiong Liu, Jianhong Tao, Zhongdong Wu, Buxiong Tuo, Wei Li, Yixian Xu, Zhaoqi Zhang, Yundai Chen, Lefeng Wang, Jinying Zhang, Fengling Wang, Yongping Jia, Bin Wang, Fakuan Tang, Qiang Tang, Wei Wang, Yuemin Sun, and Weiqing Su
References
- Anavekar N. S. et al. Relation between Renal Dysfunction and Cardiovascular Outcomes after Myocardial Infarction. N Engl J Med. 351, 1285–1295 (2004). [DOI] [PubMed] [Google Scholar]
- Ix J. H., Shlipak M. G., Liu H. H., Schiller N. B. & Whooley M. A. Association Between Renal Insufficiency and Inducible Ischemia in Patients with Coronary Artery Disease: The Heart and Soul Study. J Am Soc Nephrol. 14, 3233–3238 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlipak M. G., Smith G. L., Rathore S. S., Massie B. M. & Krumholz H. M. Renal Function, Digoxin Therapy, and Heart Failure Outcomes: Evidence From the Digoxin Intervention Group Trial. J Am Soc Nephrol. 15, 2195–2203 (2004). [DOI] [PubMed] [Google Scholar]
- Mehran R. & Nikolsky E. Contrast-Induced Nephropathy: Definition, Epidemiology, and Patients at Risk. Kidney Int Suppl. S11–S15 (2006). [DOI] [PubMed] [Google Scholar]
- Rihal C. S. et al. Incidence and Prognostic Importance of Acute Renal Failure after Percutaneous Coronary Intervention. Circulation. 105, 2259–2264 (2002). [DOI] [PubMed] [Google Scholar]
- Waybill M. M. & Waybill P. N. Contrast Media-Induced Nephrotoxicity: Identification of Patients at Risk and Algorithms for Prevention. J Vasc Interv Radiol. 12, 3–9 (2001). [DOI] [PubMed] [Google Scholar]
- Zheng X. et al. Coronary Catheterization and Percutaneous Coronary Intervention in China: 10-Year Results From the China PEACE-Retrospective CathPCI Study. Jama Intern Med. 176, 512–521 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y. 2015 China National Data of Coronary Intervention in the 19Th National Interventional Cardiology Forum (2015). [Google Scholar]
- Aurelio A. & Durante A. Contrast-Induced Nephropathy in Percutaneous Coronary Interventions: Pathogenesis, Risk Factors, Outcome, Prevention and Treatment. Cardiology. 128, 62–72 (2014). [DOI] [PubMed] [Google Scholar]
- McCullough P. A. Contrast-Induced Acute Kidney Injury. J Am Coll Cardiol. 51, 1419–1428 (2008). [DOI] [PubMed] [Google Scholar]
- Nash K., Hafeez A. & Hou S. Hospital-Acquired Renal Insufficiency. Am J Kidney Dis. 39, 930–936 (2002). [DOI] [PubMed] [Google Scholar]
- Levine G. N. et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 58, e44–e122 (2011). [DOI] [PubMed] [Google Scholar]
- Windecker S. et al. 2014 ESC/EACTS Guidelines On Myocardial Revascularization: The Task Force On Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the Special Contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 35, 2541–2619 (2014). [DOI] [PubMed] [Google Scholar]
- Marenzi G. et al. Contrast-Induced Nephropathy in Patients Undergoing Primary Angioplasty for Acute Myocardial Infarction. J Am Coll Cardiol. 44, 1780–1785 (2004). [DOI] [PubMed] [Google Scholar]
- Wijns W. et al. Guidelines On Myocardial Revascularization. Eur Heart J. 31, 2501–2555 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang L. et al. Prevalence of Chronic Kidney Disease in China: A Cross-Sectional Survey. Lancet. 379, 815–822 (2012). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Excessively High Hydration Volume May Not be Associated with Decreased Risk of Contrast-Induced Acute Kidney Injury after Percutaneous Coronary Intervention in Patients with Renal Insufficiency. J Am Heart Assoc. 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Y. et al. Safety and Tolerability of Iopromide in Patients Undergoing Cardiac Catheterization: Real-World Multicenter Experience with 17,513 Patients from the TRUST Trial. Int J Cardiovasc Imaging. 31, 1281–1291 (2015). [DOI] [PubMed] [Google Scholar]
- Homma K. Contrast-Induced Acute Kidney Injury. Keio J Med (2016). [DOI] [PubMed] [Google Scholar]
- Bader B. D. et al. What is the Best Hydration Regimen to Prevent Contrast Media-Induced Nephrotoxicity? Clin Nephrol. 62, 1–7 (2004). [DOI] [PubMed] [Google Scholar]
- Jurado-Roman A. et al. Role of Hydration in Contrast-Induced Nephropathy in Patients Who Underwent Primary Percutaneous Coronary Intervention. Am J Cardiol. 115, 1174–1178 (2015). [DOI] [PubMed] [Google Scholar]
- Trivedi H. S. et al. A Randomized Prospective Trial to Assess the Role of Saline Hydration On the Development of Contrast Nephrotoxicity. Nephron Clin Pract. 93, C29–C34 (2003). [DOI] [PubMed] [Google Scholar]
- Prasad A. et al. Contemporary Practice Patterns Related to the Risk of Acute Kidney Injury in the Catheterization Laboratory: Results From a Survey of Society of Cardiovascular Angiography and Intervention (SCAI) Cardiologists. Catheter Cardiovasc Interv. (2016). [DOI] [PubMed] [Google Scholar]
- Narula A. et al. Contrast-Induced Acute Kidney Injury After Primary Percutaneous Coronary Intervention: Results From the HORIZONS-AMI Substudy. Eur Heart J. 35, 1533–1540 (2014). [DOI] [PubMed] [Google Scholar]
- Maioli M., Toso A., Leoncini M., Micheletti C. & Bellandi F. Effects of Hydration in Contrast-Induced Acute Kidney Injury after Primary Angioplasty: A Randomized, Controlled Trial. Circ Cardiovasc Interv. 4, 456–462 (2011). [DOI] [PubMed] [Google Scholar]
- Chong E., Poh K. K., Liang S., Soon C. Y. & Tan H. C. Comparison of Risks and Clinical Predictors of Contrast-Induced Nephropathy in Patients Undergoing Emergency Versus Nonemergency Percutaneous Coronary Interventions. J Interv Cardiol. 23, 451–459 (2010). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Aggressive hydraTion in patients with ST-Elevation Myocardial infarction undergoing Primary percutaneous coronary intervention to prevenT contrast-induced nephropathy (ATTEMPT): Study design and protocol for the randomized, controlled trial, the ATTEMPT, RESCIND 1 (First study for REduction of contraSt-induCed nephropathy followINg carDiac catheterization) trial. Am Heart J. 172, 88–95 (2016). [DOI] [PubMed] [Google Scholar]
- Brar S. S. et al. Haemodynamic-Guided Fluid Administration for the Prevention of Contrast-Induced Acute Kidney Injury: The POSEIDON Randomised Controlled Trial. Lancet. 383, 1814–1823 (2014). [DOI] [PubMed] [Google Scholar]
- Kroneberger C. et al. Contrast-Induced Nephropathy in Patients with Chronic Kidney Disease and Peripheral Arterial Disease. Acta Radiol Open. 4, 2058460115583034 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenzi G. et al. Prevention of Contrast Nephropathy by Furosemide with Matched Hydration: The MYTHOS (Induced Diuresis with Matched Hydration Compared to Standard Hydration for Contrast Induced Nephropathy Prevention) Trial. Jacc Cardiovasc Interv. 5, 90–97 (2012). [DOI] [PubMed] [Google Scholar]
- Nakahashi H. et al. Combined Impact of Chronic Kidney Disease and Contrast-Induced Nephropathy On Long-Term Outcomes in Patients with ST-segment Elevation Acute Myocardial Infarction Who Undergo Primary Percutaneous Coronary Intervention. Heart Vessels (2016). [DOI] [PubMed] [Google Scholar]