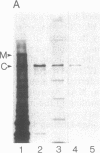
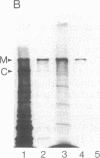
Abstract
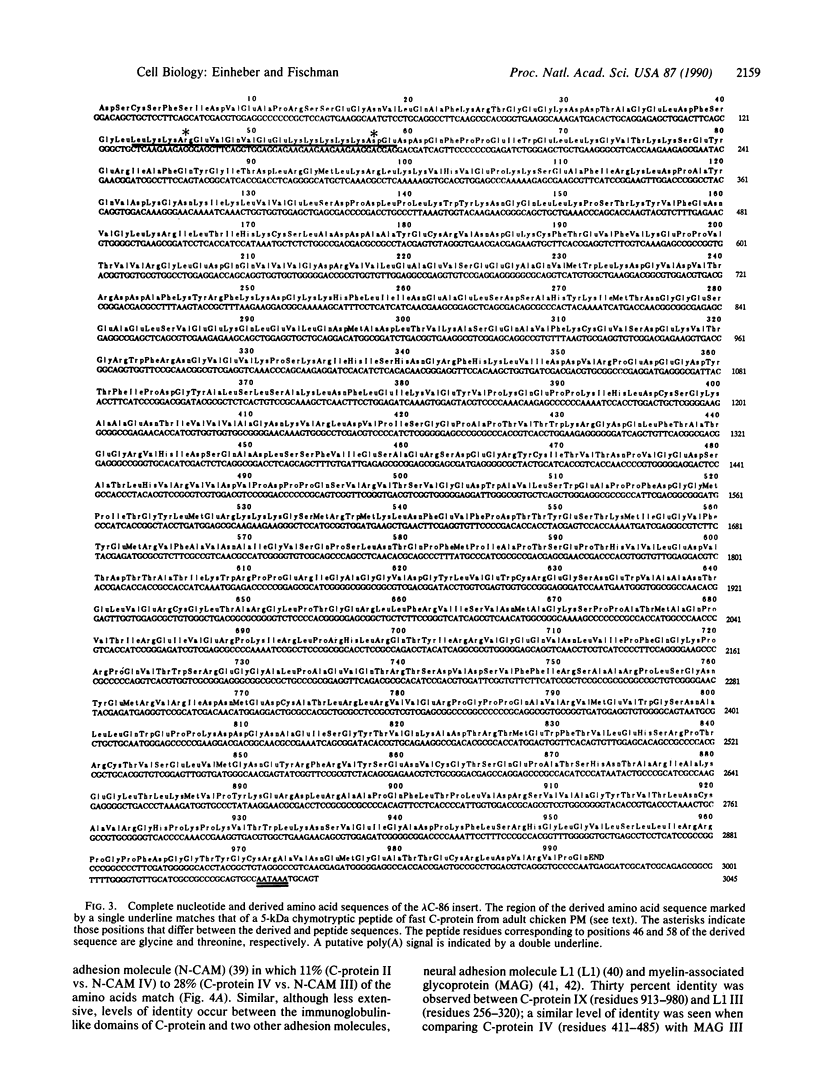
C-protein is a thick filament-associated protein located in the crossbridge region of vertebrate striated muscle A bands. Its function is unknown. To improve our understanding of its primary structure, we undertook the molecular cloning of C-protein mRNA. We describe the isolation and characterization of a cDNA clone, lambda C-86, that encodes approximately 80% of the fast isoform of C-protein in the chicken. Sequence analysis of the insert revealed that C-protein, although an intracellular, nonmembrane-associated protein, is a member of the immunoglobulin superfamily. Like several cell surface adhesion molecules that belong to this superfamily, C-protein contains sequence motifs that resemble immunoglobulin domains and fibronectin type III repeats. Computer searches using the C-protein sequence also lead to the identification of related domains in chicken smooth muscle myosin light chain kinase that have not been reported previously.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amzel L. M., Poljak R. J. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1979;48:961–997. doi: 10.1146/annurev.bi.48.070179.004525. [DOI] [PubMed] [Google Scholar]
- Arquint M., Roder J., Chia L. S., Down J., Wilkinson D., Bayley H., Braun P., Dunn R. Molecular cloning and primary structure of myelin-associated glycoprotein. Proc Natl Acad Sci U S A. 1987 Jan;84(2):600–604. doi: 10.1073/pnas.84.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader D., Masaki T., Fischman D. A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982 Dec;95(3):763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J. W., Erickson H. P., Hoffman S., Cunningham B. A., Edelman G. M. Topology of cell adhesion molecules. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1088–1092. doi: 10.1073/pnas.86.3.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benian G. M., Kiff J. E., Neckelmann N., Moerman D. G., Waterston R. H. Sequence of an unusually large protein implicated in regulation of myosin activity in C. elegans. Nature. 1989 Nov 2;342(6245):45–50. doi: 10.1038/342045a0. [DOI] [PubMed] [Google Scholar]
- Bennett P., Craig R., Starr R., Offer G. The ultrastructural location of C-protein, X-protein and H-protein in rabbit muscle. J Muscle Res Cell Motil. 1986 Dec;7(6):550–567. doi: 10.1007/BF01753571. [DOI] [PubMed] [Google Scholar]
- Bouché M., Goldfine S. M., Fischman D. A. Posttranslational incorporation of contractile proteins into myofibrils in a cell-free system. J Cell Biol. 1988 Aug;107(2):587–596. doi: 10.1083/jcb.107.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler M., Eppenberger H. M., Wallimann T. Novel thick filament protein of chicken pectoralis muscle: the 86 kd protein. II. Distribution and localization. J Mol Biol. 1985 Nov 20;186(2):393–401. doi: 10.1016/0022-2836(85)90113-5. [DOI] [PubMed] [Google Scholar]
- Callaway J. E., Bechtel P. J. C-protein from rabbit soleus (red) muscle. Biochem J. 1981 May 1;195(2):463–469. doi: 10.1042/bj1950463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R., Offer G. The location of C-protein in rabbit skeletal muscle. Proc R Soc Lond B Biol Sci. 1976 Mar 16;192(1109):451–461. doi: 10.1098/rspb.1976.0023. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Dennis J. E., Shimizu T., Reinach F. C., Fischman D. A. Localization of C-protein isoforms in chicken skeletal muscle: ultrastructural detection using monoclonal antibodies. J Cell Biol. 1984 Apr;98(4):1514–1522. doi: 10.1083/jcb.98.4.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. CAMs and Igs: cell adhesion and the evolutionary origins of immunity. Immunol Rev. 1987 Dec;100:11–45. doi: 10.1111/j.1600-065x.1987.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guerriero V., Jr, Russo M. A., Olson N. J., Putkey J. A., Means A. R. Domain organization of chicken gizzard myosin light chain kinase deduced from a cloned cDNA. Biochemistry. 1986 Dec 30;25(26):8372–8381. doi: 10.1021/bi00374a007. [DOI] [PubMed] [Google Scholar]
- Harrelson A. L., Goodman C. S. Growth cone guidance in insects: fasciclin II is a member of the immunoglobulin superfamily. Science. 1988 Nov 4;242(4879):700–708. doi: 10.1126/science.3187519. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Effects of phosphorylated and unphosphorylated C-protein on cardiac actomyosin ATPase. J Mol Biol. 1985 Nov 5;186(1):185–195. doi: 10.1016/0022-2836(85)90268-2. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Sale W. S. Structure of C protein purified from cardiac muscle. J Cell Biol. 1985 Jan;100(1):208–215. doi: 10.1083/jcb.100.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Titus L. Effects of cholinergic and adrenergic agonists on phosphorylation of a 165,000-dalton myofibrillar protein in intact cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):2111–2120. [PubMed] [Google Scholar]
- Kessler S. W. Use of protein A-bearing staphylococci for the immunoprecipitation and isolation of antigens from cells. Methods Enzymol. 1981;73(Pt B):442–459. doi: 10.1016/0076-6879(81)73084-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller J. S., Paterson B. M., Ricciardi R. P., Cohen L., Roberts B. E. Methods utilizing cell-free protein-synthesizing systems for the identification of recombinant DNA molecules. Methods Enzymol. 1983;101:650–674. doi: 10.1016/0076-6879(83)01046-0. [DOI] [PubMed] [Google Scholar]
- Moerman D. G., Benian G. M., Barstead R. J., Schriefer L. A., Waterston R. H. Identification and intracellular localization of the unc-22 gene product of Caenorhabditis elegans. Genes Dev. 1988 Jan;2(1):93–105. doi: 10.1101/gad.2.1.93. [DOI] [PubMed] [Google Scholar]
- Moos C., Feng I. N. Effect of C-protein on actomyosin ATPase. Biochim Biophys Acta. 1980 Oct 1;632(2):141–149. doi: 10.1016/0304-4165(80)90071-9. [DOI] [PubMed] [Google Scholar]
- Moos C. Fluorescence microscope study of the binding of added C protein to skeletal muscle myofibrils. J Cell Biol. 1981 Jul;90(1):25–31. doi: 10.1083/jcb.90.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos C., Mason C. M., Besterman J. M., Feng I. N., Dubin J. H. The binding of skeletal muscle C-protein to F-actin, and its relation to the interaction of actin with myosin subfragment-1. J Mol Biol. 1978 Oct 5;124(4):571–586. doi: 10.1016/0022-2836(78)90172-9. [DOI] [PubMed] [Google Scholar]
- Moos C., Offer G., Starr R., Bennett P. Interaction of C-protein with myosin, myosin rod and light meromyosin. J Mol Biol. 1975 Sep 5;97(1):1–9. doi: 10.1016/s0022-2836(75)80017-9. [DOI] [PubMed] [Google Scholar]
- Moos M., Tacke R., Scherer H., Teplow D., Früh K., Schachner M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988 Aug 25;334(6184):701–703. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Obinata T., Reinach F. C., Bader D. M., Masaki T., Kitani S., Fischman D. A. Immunochemical analysis of C-protein isoform transitions during the development of chicken skeletal muscle. Dev Biol. 1984 Jan;101(1):116–124. doi: 10.1016/0012-1606(84)90122-2. [DOI] [PubMed] [Google Scholar]
- Offer G., Moos C., Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973 Mar 15;74(4):653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Reinach F. C., Masaki T., Shafiq S., Obinata T., Fischman D. A. Isoforms of C-protein in adult chicken skeletal muscle: detection with monoclonal antibodies. J Cell Biol. 1982 Oct;95(1):78–84. doi: 10.1083/jcb.95.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986 Feb 28;44(4):517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Salzer J. L., Holmes W. P., Colman D. R. The amino acid sequences of the myelin-associated glycoproteins: homology to the immunoglobulin gene superfamily. J Cell Biol. 1987 Apr;104(4):957–965. doi: 10.1083/jcb.104.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr R., Offer G. The interaction of C-protein with heavy meromyosin and subfragment-2. Biochem J. 1978 Jun 1;171(3):813–816. doi: 10.1042/bj1710813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan R. C., Fischman D. A. Electron microscopy of C-protein molecules from chicken skeletal muscle. J Muscle Res Cell Motil. 1986 Apr;7(2):160–166. doi: 10.1007/BF01753417. [DOI] [PubMed] [Google Scholar]
- Takano-Ohmuro H., Goldfine S. M., Kojima T., Obinata T., Fischman D. A. Size and charge heterogeneity of C-protein isoforms in avian skeletal muscle. Expression of six different isoforms in chicken muscle. J Muscle Res Cell Motil. 1989 Oct;10(5):369–378. doi: 10.1007/BF01758433. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Moos C. The C-proteins of rabbit red, white, and cardiac muscles. J Biol Chem. 1983 Jul 10;258(13):8395–8401. [PubMed] [Google Scholar]
- Yamamoto K. The binding of skeletal muscle C-protein to regulated actin. FEBS Lett. 1986 Nov 10;208(1):123–127. doi: 10.1016/0014-5793(86)81545-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]