Abstract
Purpose
To evaluate the efficacy of xylitol-sweetened milk as a caries preventive strategy.
Methods
In this nine-month prospective proof-of-principle trial, 153 Peruvian school children Peru were randomized to a milk group: 8g xylitol/200mL milk once/day, 4g xylitol/100mL milk twice/day, 8g sorbitol/200mL milk once/day, 4g sorbitol/100mL milk twice/day, or 8g sucrose/200mL milk once/day. The primary outcome was plaque mutans streptococci (MS) at nine-months. A secondary outcome was tooth decay incidence. We hypothesized children in the xylitol groups would have a greater MS decline and lower tooth decay incidence.
Results
One-hundred-thirty-five children were included in the intent-to-treat analyses. Children receiving xylitol had a greater reduction in MS than sucrose (P=0.02) but were not different from sorbitol (P=0.07). Tooth decay incidence for xylitol once/day or twice/day was 5.3±3.4 and 4.3±4.0 surfaces, respectively, compared to sorbitol once/day, sorbitol twice/day, or sucrose (4.1±2.8,3.7±4.2, and 3.2±3.4 surfaces, respectively). There were no differences in tooth decay incidence between xylitol and sucrose (Rate Ratio [RR]=1.51;95% confidence interval [CI]=0.88,2.59;P=0.13) or between xylitol and sorbitol (RR=1.28;95% CI=0.90,1.83;P=0.16).
Conclusion
Xylitol-sweetened milk significantly reduced MS levels compared to sucrose-sweetened milk, but we were unable to detect differences in caries incidence. ISRCTN34705772.
Xylitol efficacy has been evaluated in children1–4 and is known to lower mutans streptococci levels,5 but findings are mixed regarding caries prevention.6,7An unexplored modality is xylitol as a beverage sweetener.
Peru’s Vaso de Leche (Glass of Milk) is a nutrition program that provides low-income school children with milk.8,9 Plain milk protects against tooth decay.10 However, most children in Peru and other South American countries dislike plain milk. A common practice is using sweeteners like honey or sugar to encourage milk consumption,11,12 which increases risk for caries and other diseases like obesity.13 Sugar-sweetened milk is served to children at many schools participating in Vaso de Leche.
Milk sweetened with xylitol is well accepted by children,14 presenting a viable alternative to sugar as a sweetener.15 In this proof-of-principle trial, which is designed to identify the mechanism of a preventive agent as well as preliminary evidence of clinical efficacy15, we tested the hypotheses that children who consume xylitol milk at school would have lower mutans streptococci (MS) levels and lower rates of new tooth decay than other children at follow-up. These findings are expected to serve as preliminary data for a large-scale study evaluating the caries prevention efficacy of xylitol as a beverage sweetener.
Methods
Trial design
This was a nine-month, five-arm prospective parallel randomized clinical trial with equal allocation. Nine months is a short period to assess caries incidence,16 but was believed to be sufficient time to demonstrate an effect on mutans streptococci. The trial was registered with the ISRCTN registry (ISRCTN34705772).
Participants
We recruited children kindergarten to grade 5 from an elementary school in a low-income community in Arequipa, Peru (N=246). Exclusion criteria were: 1) milk allergy or lactose intolerance; 2) antibiotic use four weeks prior to the baseline examination to control for the effects on intraoral MS17; 3) inability to cooperate for an examination; and 4) no parent consent or child assent. Pre-study information sessions in Spanish were held for parents and teachers. Study staff explained the importance of oral health, use of xylitol and sorbitol as sweeteners, and study procedures. Questions were answered and commercial products containing xylitol were available to sample. Staff members screened for eligibility. All 246 children were eligible. We obtained parent consent and assent from children. The Institutional Review Boards at the University of Washington (Seattle, Washington, USA) and the Universidad Catolica de Santa Maria (Arequipa, Peru) approved the study.
Interventions
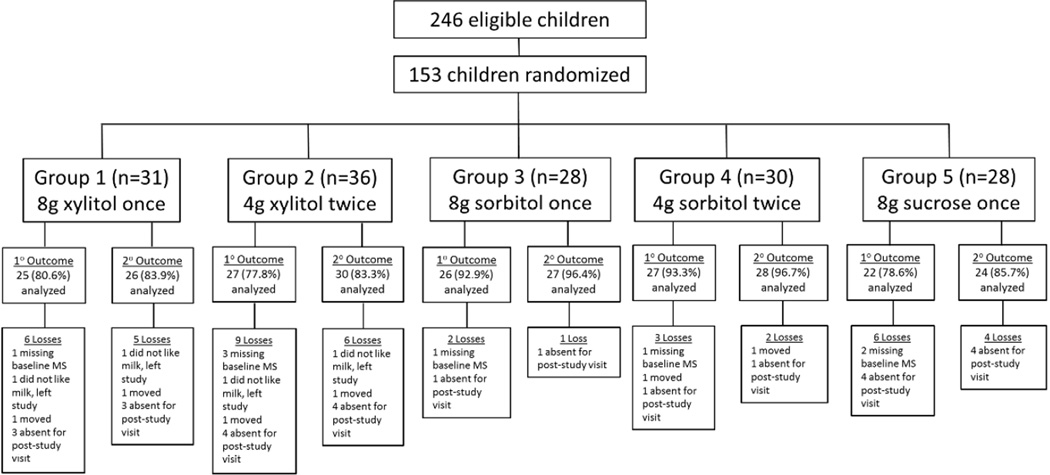
There were five arms (Figure 1). Children in the active experimental groups received whole milk (Gloria Ultra-High Temperature [UHT] Processed milk, Grupo Gloria, Arequipa, Peru) sweetened with xylitol (8g in 200mL milk once/day or 4g in 100mL milk twice/day) (XilySweet, Shandong Longlive Bio-Technology Co. Ltd., Yucheng City, Shandong, China). Dosing was based on evidence that 6–10g xylitol/day is needed for caries prevention.2 Children in the active treatment control groups received UHT milk sweetened with sorbitol (8g in 200mL milk once/day or 4g in 100mL milk twice/day) (NEOSORB®, Roquette, Lestrem, France). Children in the control group received the standard 8g of sucrose in 200mL UHT milk once/day (Complejo Agroindustrial Cartavio S.A.A., Cartavio, Peru). There was no plain milk control group because children at the school refuse unsweetened milk. School officials insisted on sweetened milk to ensure the study did not disrupt delivery of milk to children through Vaso de Leche.
Figure 1.
CONSORT Flowchart for RCT to Assess the Efficacy of Milk Sweetened with Xylitol for Elementary School Children in Peru’s Vaso de Leche Program
Study procedures
Sweeteners were pre-weighed, packaged in airtight bags, and labeled with colored stickers. Color-matched stickers on the sweetener bags, cups, and bracelets worn by children ensured participants received the assigned milk. Parent workers were trained on milk preparation and monitored to ensure proper preparation. Milk was served before breakfast. For children who received two milk servings, the second was served before lunch. Milk was distributed at the same times. The prepared milk was warmed to about 60°C, which is customary in Peru. Children were monitored to ensure cups were not traded and the milk was consumed.
Before collecting plaque and caries data, we assessed baseline hygiene using a modified plaque index based on supragingival plaque.18 Children were classified as having good (no visible plaque), fair (light plaque), or poor (heavy plaque) hygiene.
Outcomes measures
The primary outcome was change in mutans streptococci (MS) levels (log10). Pooled supragingival plaque samples were collected from the buccal surfaces of the maxillary and mandibular arches using two sterile microbrushes/child (Microbrush®, Microbrush International, Grafton, Wisconsin, USA),prior to toothbrushing and the examination. Samples were processed as previously described.19,20 MS enumeration included Streptococcus mutans and Streptococcus sobriunus.19,20 Negative cultures were defined as plates with no MS growth after seven days.
The secondary outcome was tooth decay incidence, measured at nine months post-baseline examination and defined as the number of new decayed, missing, or filled primary and permanent tooth surfaces. A single examiner was trained according to the U.S. National Institute of Dental and Craniofacial Research criteria.21 The examiner was blinded to allocation. Ten-percent of participants were randomly selected for a second examination to assess reliability. Examiner intrarater agreement (kappa coefficient=0.94; 95% confidence interval [CI]=0.92, 0.95) was good. Non-cavitated carious lesions were identified, recognizing that these lesions are difficult to assess reliably. Because there were no differences when non-cavitated lesions were included in the primary outcome, we reported all findings with non-cavitated lesions as part of the outcome. Teeth were cleaned with a dry toothbrush, dried with gauze, and examined with a disposable mouth mirror. No explorers were used. Examinations were conducted at baseline prior to randomization and at end-of-study at nine months.
Power calculations
We estimate sample sizes using previous estimates of 73% reduction in dmft in children observed between xylitol gum and sugar gum.22 With 25 subjects in the each of the five groups, the power was estimated to be 85% to demonstrate a reduction in the incremental dmft in the xylitol groups of 2/3 standard deviation compared to the sorbitol groups, based on a two-sided two-sample t-test (alpha=0.025). Power was 78% to demonstrate a ¾. standard deviation in the incremental dmft comparing xylitol and sucrose groups. In addition, power was 80% to detect a difference in the incremental dmft 0.8 standard deviation between the once/day versus twice/day xylitol groups. The goal was to enroll all eligible children. Because of the requirement for active parent consent, we expected 60% participation or about 150 participants.
Randomization and allocation
Each child was randomly assigned using computer-generated permuted blocks of varying block sizes and stratification on classroom to ensure balanced groups within classrooms. To prevent treatment assignment determination, blocks sizes were equal to five or ten, and were chosen randomly with three-fourth and one-fourth probability, respectively.23 The randomization list was prepared using the sample function of the R statistical software (R Foundation for Statistical Computing, Vienna, Austria, Version 3.0). After baseline data collection, the names of enrolled children were randomly sorted by classroom. Children were assigned according to the randomization list for their classroom that was maintained by the PI until the intervention start date to conceal allocation. Caries and plaque data were stored separately from the randomization list.
Blinding
Participating children, parents who prepared and distributed the milk, teachers, and laboratory staff were blinded to allocation. During baseline examinations, children had not yet been allocated to a group. The examiner did not know the child’s assignment during the post-study examinations.
Incentives
Each child received a toothbrush, one 31.9g tube of fluoridated toothpaste (Colgate Maxima Proteccion AntiCaries, 1450 ppm sodium monofluorophosphate and sodium fluoride, Colgate, Guanajuato, Mexico), and a t-shirt. At the study end, we sponsored a school-wide party that included oral health education delivered by cartoon characters.
Safety monitoring
Parents and teachers monitored children for potential side effects associated with the intervention (e.g., headache, stomachache, vomiting) and other side effects not normally associated with polyols. When side effects were observed or reported, milk distribution to the child was stopped. The child was monitored to ensure symptoms resolved and then the milk distribution was resumed.
Statistical analyses
The unit of analysis was the child. We generated descriptive statistics on the study population (e.g., grade, age, gender, baseline hygiene, attendance) and compared these characteristics across milk groups using the chi-square test, ANOVA, or Cochran-Mantel-Haenszel test. We compared characteristics of children included in the final analyses and children who were randomized but excluded due to drop out or missing data. We estimated baseline and post-study tooth decay across the five groups and by sweetener type.
For the primary outcome, MS levels were logarithmically transformed (log10). We assessed baseline and MS levels across the five groups and by sweetener type. For hypothesis testing, the two xylitol groups and two sorbitol groups were combined because of the small samples and similar outcomes. Unadjusted and covariate-adjusted linear regression models with generalized estimating equations (GEE) and robust standard error estimates were used to test the hypothesis that children in the xylitol groups would have a greater MS decline than children in the sorbitol or sucrose groups. As a supplemental analysis, we used the Spearman rank correlation to examine the associations between MS (baseline, post-study, and change) and tooth decay incidence. Analyses were completed using R statistical software (R Foundation for Statistical Computing, Vienna, Austria, Version 3.0) and SAS (SAS Institute, Cary, NC, Version 9.3) (two-sided α=0.05).
Similar to the primary outcome, the two xylitol groups and two sorbitol groups were combined for hypothesis testing. Unadjusted and covariate-adjusted log-linear regression models were used to evaluate the secondary hypothesis that children in the xylitol groups would have a lower tooth decay incidence than children in the sorbitol or sucrose groups. We used GEE and robust standard error estimates and calculated rate ratios and corresponding 95% confidence intervals.24 For teeth missing due to caries during the post-study examination, one-half of the corresponding baseline surfaces at risk for new decay were counted as new decay. An offset equal to the logarithm of the total number of tooth surfaces at risk for new decay was included in the regression model. Time-at-risk was set equal to one-half the follow-up time for surfaces present at baseline that by follow-up were erupted, carious, exfoliated, or missing due to reasons other than caries. For surfaces that erupted after baseline and developed caries by follow-up, time-at-risk was set equal to one-fourth of the follow-up time.
Results
Participant recruitment, flow, and allocation
Children were recruited in March 2014 and were followed up for nine months through December 2014.Of the 246 eligible children, we recruited, consented, and randomized 153 children (62.2%) (Figure 1). The largest proportion of participants was in grade 5 (22.2%) (Table 1). Most children were male (55.6%) and had poor hygiene (77.8%). There were no significant differences across the five groups on grade (P=0.97), age (P=0.23), gender (P=0.86), or oral hygiene (P=0.75).
Table 1.
Baseline Characteristics of Elementary School Children Randomized to 1 of 5 Milk Groups in a RCT to Assess the Efficacy of Milk Sweetened with Xylitol
| Characteristic | Group 1 8g xylitol once/day |
Group 2 4g xylitol twice/day |
Group 3 8g sorbitol once/day |
Group 4 4g sorbitol twice/day |
Group 5 8g sucrose once/day |
All Randomized Children |
|---|---|---|---|---|---|---|
| N=31 n (%) or mean±SD |
N=36 n (%) or mean±SD |
N=28 n (%) or mean±SD |
N=30 n (%) or mean±SD |
N=28 n (%) or mean±SD |
N=153 n (%) or mean±SD |
|
| Grade | ||||||
| Kindergarten (age 4) | 3 (9.7) | 3 (8.3) | 3 (10.7) | 2 (6.7) | 3 (10.7) | 14 (9.2) |
| Kindergarten (age 5) | 6 (19.4) | 6 (16.7) | 4 (14.3) | 4 (13.3) | 1 (3.6) | 21 (13.7) |
| 1 | 0 (0.0) | 4 (11.1) | 1 (3.6) | 5 (16.7) | 5 (17.9) | 15 (9.8) |
| 2 | 5 (16.1) | 7 (19.4) | 4 (14.3) | 3 (10.0) | 7 (25.0) | 26 (17.0) |
| 3 | 4 (12.9) | 3 (8.3) | 3 (10.7) | 6 (20.0) | 3 (10.7) | 19 (12.4) |
| 4 | 6 (19.4) | 6 (16.7) | 6 (21.4) | 3 (10.0) | 3 (10.7) | 24 (15.7) |
| 5 | 7 (22.6) | 7 (19.4) | 7 (25.0) | 7 (23.3) | 6 (21.4) | 34 (22.2) |
| Age | 6.9±1.8 | 7.1±1.9 | 8.0±2.3 | 7.1±1.9 | 6.9±1.9 | 7.2±2.0 |
| Gender | ||||||
| Female | 13 (41.9) | 16 (44.4) | 12 (42.9) | 12 (40.0) | 15 (53.6) | 68 (44.4) |
| Male | 18 (58.1) | 20 (55.6) | 16 (57.1) | 18 (60.0) | 13 (46.4) | 85 (55.6) |
| Oral Hygiene | ||||||
| Good | 3 (9.7) | 1 (2.8) | 1 (3.6) | 2 (6.7) | 0 (0.0) | 7 (4.6) |
| Fair | 5 (16.1) | 4 (11.1) | 3 (10.7) | 5 (16.7) | 5 (17.9) | 22 (14.4) |
| Poor | 22 (71.0) | 30 (83.3) | 23 (82.1) | 23 (76.7) | 21 (75.0) | 119 (77.8) |
| Missing | 1 (3.2) | 1 (2.8) | 1 (3.6) | 0 (0.0) | 2 (7.1) | 5 (3.3) |
Among randomized participants, there were five dropouts: three children moved and two withdrew because they disliked the milk (Figure 1). We had missing data for 21 children: 13 were absent for the end-of-study examination and eight were missing baseline MS data (plaque samples had been collected but were lost during transport to the laboratory). There were no significant differences in grade (P=0.40) between children with missing data and those included in the analyses. However, children with missing data were more likely to be male (77.8% vs. 52.6%; P=0.04) and to have poor hygiene (100% vs. 74.8%;P=0.03) (data not shown).
Primary outcome
We had complete MS data on 127 children (83.0%). Children in the five milk groups had similar MS levels at baseline (P=0.24) and at the end of the study (P=0.47) (Table 2). Children in the sorbitol twice/day and both xylitol groups exhibited declines in MS level whereas children in the sorbitol once/day and sucrose groups exhibited increases in MS. Across the combined groups, there were no differences in MS change between combined sorbitol and combined xylitol (P>0.05) or between combined sorbitol and sucrose (P>0.05). However, children in the combined xylitol group had a significant decrease in MS compared to children in the sucrose group, who exhibited an increase in MS level (P<0.05).
Table 2.
Baseline and Final Mutans Streptococci and Primary Outcome Data for Children Randomized to 1 of 5 Milk Groups and All Children in a RCT to Assess the Efficacy of Milk Sweetened with Xylitol
| Group 1 8g xylitol once/day |
Group 2 4g xylitol twice/day |
Groups 1 and 2 combined (xylitol) |
Group 3 8g sorbitol once/day |
Group 4 4g sorbitol twice/day |
Groups 3 and 4 combined (sorbitol) |
Group 5 8g sucrose once/day |
All Children Included in Primary Outcome Analyses |
||
|---|---|---|---|---|---|---|---|---|---|
| N=25 mean±SD |
N=27 mean±SD |
N=52 mean±SD |
N=26 mean±SD |
N=27 mean±SD |
N=53 mean±SD |
N=22 mean±SD |
N=127 mean±SD |
P-value1 | |
| Baseline Mutans Streptococci Level (log10) | 4.8±1.3 | 4.9±0.8 | 4.8±1.1 | 4.6±1.1 | 4.9±1.2 | 4.8±1.1 | 4.4±1.0 | 4.7±1.1 | 0.24 |
| Final Mutans Streptococci Level log10) | 4.7±1.3 | 4.8±0.8 | 4.7±1.0 | 5.1±1.3 | 4.8±1.0 | 4.9±1.1 | 4.9±1.1 | 4.9±1.1 | 0.47 |
| Primary Outcome | |||||||||
| Change in Mutans Streptococci Level (log10) | −0.1±1.2 | −0.1±1.2 | −0.1±1.02 | 0.5±1.1 | −0.1±1.4 | 0.2±1.33 | 0.5±0.8 | 0.11±1.1 | 0.01 |
Linear regression (unadjusted for other covariates), omnibus test (df=2) comparing Groups 1 and 2 combined (xylitol) versus Groups 3 and 4 combined (sorbitol) versus Group 5.
Children in the combined xylitol group had significantly lower change in MS level than children in the sucrose group (P<.05).
here were no significant differences in MS level change between children in the combined sorbitol group and children in the combined xylitol group (P>.05) or between children in the combined sorbitol group and children in the sucrose group (P>.05).
The linear regression models adjusted for age, gender, baseline MS levels, baseline tooth decay, and attendance indicated that at follow-up children in the xylitol groups had significantly lower MS levels than children in the sucrose group (coefficient=−0.46;P=0.02) but were not different from children in the sorbitol groups (coefficient=−0.34;P=0.07). In the covariate-adjusted model, there was no difference in MS level between sorbitol and sucrose (coefficient=−0.12; P=0.59). In the unadjusted analysis, the twice/day sorbitol group had a significant decline in MS levels as compared to the sucrose group (coefficient=−0.64;P=0.03), the difference was no longer significant in adjusted regression analysis (coefficient =−0.33;P=0.22)
Secondary outcome
The mean total tooth decay incidence for all children was 4.1±3.6 surfaces (Table 3). Tooth decay incidence for children in the xylitol once/day and twice/day was 5.3±3.4 and 4.3±4.0 surfaces, respectively, compared to sorbitol once/day, sorbitol twice/day, or sucrose (4.1±2.8, 3.7±4.2, and 3.2±3.4 surfaces, respectively) (Table 2). Similar trends existed for primary and permanent tooth decay incidence. In the intent-to-treat analyses adjusted only for time-at-risk (for the combined groups), there were no significant differences in tooth decay incidence when comparing xylitol and sucrose (rate ratio [RR]=1.51;95% CI=0.88,2.59;P=0.13) or xylitol and sorbitol (RR=1.28;95% CI=0.90,1.83;P=0.16). Findings were similar in the covariate-adjusted regression models including age, gender, baseline MS level, baseline tooth decay, and attendance. The covariate-adjusted rate ratio for tooth decay for xylitol versus sucrose was 1.23 (95% CI=0.67,2.23;P=0.49) and 1.00 for xylitol versus sorbitol (95% CI=0.77,1.32;P=0.95). There were no differences when the regression models were restricted to primary or permanent teeth.
Table 3.
Baseline Dental, Attendance, and Secondary Outcome Data for Children Randomized to 1 of 5 Milk Groups and All Children in a RCT to Assess the Efficacy of Milk Sweetened with Xylitol
| Group 1 8g xylitol once/day |
Group 2 4g xylitol twice/day |
Groups 1 and 2 combined (xylitol) |
Group 3 8g sorbitol once/day |
Group 4 4g sorbitol twice/day |
Groups 3 and 4 combined (sorbitol) |
Group 5 8g sucrose once/day |
Children Included in Secondary Outcome Analyses |
||
|---|---|---|---|---|---|---|---|---|---|
| N=26 mean±SD |
N=30 mean±SD |
N=56 mean±SD |
N=27 mean±SD |
N=28 mean±SD |
N=55 mean±SD |
N=24 mean±SD |
N=135 mean±SD |
P-value1 | |
| Baseline Number of Teeth | |||||||||
| Total (Primary+Permanent) | 22.3±1.8 | 22.6±2.6 | 22.4±2.2 | 22.7±2.5 | 22.3±1.7 | 22.5±2.1 | 22.3±1.9 | 22.4±2.1 | 0.86 |
| Primary | 14.5±4.7 | 12.8±5.9 | 13.6±5.4 | 11.4±6.5 | 13.1±5.5 | 12.2±6.0 | 13.5±5.6 | 13.0±5.7 | 0.42 |
| Permanent | 7.7±6.0 | 9.8±7.4 | 8.8±6.8 | 9.2±6.9 | 8.8±6.6 | 10.3±7.6 | 9.8±7.4 | 9.4±7.1 | 0.51 |
| Baseline Tooth Decay | |||||||||
| Total (dmft+DMFT) | 8.1±5.3 | 7.0±4.3 | 7.5±4.8 | 6.2±3.6 | 5.6±3.8 | 5.9±3.7 | 6.0±4.6 | 6.6±4.4 | 0.12 |
| dmft | 7.5±5.3 | 6.3±4.6 | 6.8±4.9 | 5.3±3.5 | 5.1±3.7 | 5.2±3.6 | 5.3±4.5 | 5.9±4.4 | 0.11 |
| DMFT | 0.7±1.2 | 0.7±1.4 | 0.7±1.3 | 0.9±1.9 | 0.5±1.4 | 0.7±1.7 | 0.7±1.2 | 0.7±1.4 | 0.98 |
| Attendance (number of days) | 154±14 | 144±29 | 149±232 | 132±44 | 145±28 | 139±373 | 160±8 | 147±29 | <.01 |
| Secondary Outcome | |||||||||
| Total tooth decay incidence (dmfs+DMFS) | 5.3±3.4 | 4.3±4.0 | 4.7±3.7 | 4.1±2.8 | 3.7±4.2 | 3.9±3.6 | 3.2±3.4 | 4.1±3.6 | 0.25 |
| dmfs | 3.9±3.4 | 3.1±3.8 | 3.5±3.6 | 2.3±2.5 | 2.5±3.4 | 2.4±3.0 | 2.4±3.5 | 2.9±3.3 | 0.18 |
| DMFS | 1.4±2.5 | 1.2±1.6 | 1.3±2.0 | 1.8±2.2 | 1.1±3.1 | 1.5±2.7 | 0.8±0.9 | 1.3±2.2 | 0.57 |
Log-linear regression (unadjusted for other covariates), omnibus test (d.f. = 2) comparing Groups 1 and 2 combined versus Groups 3 and 4 combined versus Group 5.
Attendance was significantly higher in the sucrose group compared to the combined xylitol group (P<0.05).
Attendance was significantly higher in the sucrose group compared to the combined sorbitol group (P<0.05)
Harms
Of the 153 randomized children, 13 children reported symptoms (8.5%). The most common side effect was stomachache (n=13) followed by headache (n=9) and/or vomiting (n=2). Of the 13 children reporting any symptoms, four were from the 8g sorbitol once/day group (14.3%), four from the 4g xylitol twice/day group (11.1%), two from the 4g sorbitol twice/day group (6.7%), two from the 8g xylitol once/day group (6.5%), and one from the sucrose group (3.6%). All reported symptoms self-resolved within 24–48 hours and no children withdrew because of side effects. There were no adverse effects.
Discussion
In this proof-of-principle trial, there were two main findings. First, children receiving xylitol-sweetened milk once or twice/day at a total dose of 8g for nine months had significantly lower MS levels compared to children who received milk with sucrose. Second, xylitol-sweetened milk did not appear to prevent tooth decay compared to sorbitol-or sucrose-sweetened milk.
Although the reduction was small, xylitol significantly reduced MS levels, which is consistent with published literature indicating a microbiological mechanism of action underlying xylitol.19,25 In our study, there was no difference in MS between one and two doses of xylitol, indicating that total xylitol dose (8g) may be more important than frequency in reducing intraoral bacteria. There appeared to be differences in MS levels by sorbitol frequency in which once/day was associated with an increased MS level whereas twice/day was associated with MS decrease. This indicates the potential importance of dosing frequency for sorbitol. However, these differences were not statistically significant in any of the models. Finally, children in the sucrose group exhibited increases in MS levels. One explanation is that constant sugar intake over time leads to a greater number of carious lesions that harbor MS colonies. Future work should continue to elucidate the microbiological impact of various sweeteners and the long-term effects of sweeteners on oral microflora levels.
Our second finding in consistent with two studies indicating no caries prevention benefit associated with xylitol administered over a short time period in high-risk children.4,26 There are three explanations. First, it is possible that a longer study period is needed to see benefits. The International Consensus Workshop on Caries Clinical Trials recommended that caries trials involving preventive agents should last 2–3 years.16 Long study periods are particularly important in populations with low caries to allow for sufficient caries cases to develop. Even though caries rates in our study were high, with 85.9% of children developing any new tooth decay during the nine-month period, a longer trial may have been needed. It is not clear how long teeth need to be exposed to preventive therapies like xylitol to begin seeing reductions in both MS levels and caries. Additional research is needed to determine optimal caries prevention trial duration for high-risk children.
A second explanation is that baseline tooth decay rates and MS levels in our population were high. A ceiling effect might have limited xylitol efficacy. Stronger topical chemotherapeutics like diammine silver fluoride might be needed to prevent caries in populations at a caries ceiling,27 followed by xylitol used as a maintenance therapy. Another approach would be to focus on younger children prior to teething. This could explain why xylitol syrup was shown to be efficacious among children ages 9–15 months in a similarly high-risk population.2 Future work should evaluate the efficacy of introducing stronger chemotherapeutics to be used in conjunction with xylitol as well as introduction of xylitol-sweetened beverages like milk in younger children during the pre-eruptive phase who are already being exposed to sugar-sweetened beverages.
A third explanation is that our control condition involved milk sweetened with sucrose. A plain (non-sweetened) milk group would have been ideal to include in the study, which may have allowed us to detect differences between xylitol and non-sweetened milk, particularly if the non-sweetened milk group exhibited lower caries incidence than the sucrose group. However, a no-treatment control group was not possible because school administrators believed children would refuse unsweetened milk. Future studies examining xylitol-sweetened beverages should include a non-sweetened beverage arm, if feasible, or minimize the amount of added sucrose. It may also be possible to recruit a comparison school without the milk program.
In populations with high caries rates like those participating in programs like Vaso de Leche, it is possible that xylitol alone is insufficient in preventing caries, a multifactorial disease linked to suboptimal behaviors and social factors. These populations may require comprehensive interventions that increase daily exposure to fluorides, reduce sugar-sweetened beverages and snacks through xylitol replacement, and improve access to preventive dental care like fluoride varnish and sealants. Few children saw a dentist and based on the oral hygiene assessment most were not brushing regularly with fluoridated toothpaste. In addition, we tested the school drinking water (likely to be the same source of water at the childrens’ homes) and found that fluoride levels were about 0.2ppm, which is below the recommended level of 0.7ppm. Equally important, the water pH was 5.5, which is the critical pH for demineralization and may expose children to acidogenic episodes.28 Another contextual observation was the sugar-sweetened snacks and beverages for sale at school snack carts. These carts are used to fundraise for the school. Insufficient fluoride exposure, acidic drinking water, and excess added sugar intake are important risk factors that could be offset through school-based fluoride rinse, toothbrushing, and varnish programs. Policies are needed to reduce the availability of sweets within schools.
The overall side effect rate was 8.5%, which is consistent with those reported in previous studies.2 Within the milk groups, children in the 8g sorbitol once/day exhibited the greatest rate (14.3%) followed by children in the 4g xylitol twice/day group (11.1%). The most common side effect was stomachache. Symptoms self-resolved within 48 hours. While these findings indicate that xylitol is safe, interventions involving polyols should include monitoring protocols and be prepared to implement polyols gradually.
There were three limitations. First, the analyses are likely to be underpowered. We did not conduct post-hoc power analyses based on previous recommendations.29 Future studies should use findings from the current study to estimate sample sizes, plan for loss to follow up, and ensure adequate power. Second, the study length was nine months, which may be insufficient time to detect clinical benefits associated with therapeutics like xylitol, even in populations with high caries rates. Third, the findings are generalizable to low-income children from a single elementary school. Nevertheless, children in Vaso de Leche are similar to low-income children in other counties. Additional studies should assess the benefits of xylitol as part of a comprehensive public health intervention.
Conclusion
This is the first known study to evaluate the caries preventive efficacy of milk sweetened with xylitol. The proof-of-principle trial indicates xylitol-sweetened milk reduces mutans streptococci levels, one of the known risk factors for dental caries, but does not reduce caries rates compared to milk sweetened with sorbitol or sugar. These findings suggest that xylitol-sweetened milk is a potentially promising strategy to prevent tooth decay in places where milk is sweetened. Additional studies are needed on use of xylitol-sweetened milk and beverages in school-aged children as part of a comprehensive caries preventive strategy.
Acknowledgments
We would like to thank all participating children, parents, and teachers. The study was funded by the IADR Colgate Community-Based Research Award for Caries Prevention, NIDCR Grant Number K08DE020856, and the William T. Grant Foundation Scholars Program.
References
- 1.Stecksén-Blicks C, Holgerson PL, Twetman S. Effect of xylitol and xylitol-fluoride lozenges on approximal caries development in high-caries-risk children. Int J Paediatr Dent. 2008;18(3):170–177. doi: 10.1111/j.1365-263X.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- 2.Milgrom P, Ly KA, Tut OK, et al. Xylitol pediatric topical oral syrup to prevent dental caries: a double-blind randomized clinical trial of efficacy. Arch Pediatr Adolesc Med. 2009;163(7):601–607. doi: 10.1001/archpediatrics.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhan L, Cheng J, Chang P, et al. Effects of xylitol wipes on cariogenic bacteria and caries in young children. J Dent Res. 2012;91(7):85S–90S. doi: 10.1177/0022034511434354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi DL, Tut O, Milgrom P. Cluster-randomized xylitol toothpaste trial for early childhood caries prevention. J Dent Child (Chic) 2014;81(1):27–32. [PMC free article] [PubMed] [Google Scholar]
- 5.Milgrom P, Soderling EM, Nelson S, Chi DL, Nakai Y. Clinical evidence for polyol efficacy. Adv Dent Res. 2012;24(2):112–116. doi: 10.1177/0022034512449467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rethman MP, Beltran-Aguilar ED, Billings RJ, et al. Nonfluoride caries-preventive agents: executive summary of evidence-based clinical recommendations. J Am Dent Assoc. 2011;142(9):1065–1071. doi: 10.14219/jada.archive.2011.0329. [DOI] [PubMed] [Google Scholar]
- 7.Riley P, Moore D, Ahmed F, Sharif MO, Worthington HV. Xylitol-containing products for preventing dental caries in children and adults. Cochrane Database Syst Rev. doi: 10.1002/14651858.CD010743.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cueto S. Breakfast and performance. Public Health Nutr. 2001;4(6):1429–1431. doi: 10.1079/phn2001233. [DOI] [PubMed] [Google Scholar]
- 9.Stifel D, Alderman H. The “Glass of Milk” Subsidy Program and Malnutrition in Peru. Policy Research Working Papers Series #3089. 2003 [Google Scholar]
- 10.Levy SM, Warren JJ, Broffitt B, Hillis SL, Kanellis MJ. Fluoride, beverages and dental caries in the primary dentition. Caries Res. 2003;37(3):157–165. doi: 10.1159/000070438. [DOI] [PubMed] [Google Scholar]
- 11.Pak-Gorstein S, Haq A, Graham EA. Cultural influences on infant feeding practices. Pediatr Rev. 2009;30(3):e11–e21. doi: 10.1542/pir.30-3-e11. [DOI] [PubMed] [Google Scholar]
- 12.Beck AL, Takayama JI, Halpern-Felsher B, Badiner N, Barker JC. Understanding how Latino parents choose beverages to serve to infants and toddlers. Matern Child Health J. 2014;18(6):1308–1315. doi: 10.1007/s10995-013-1364-0. [DOI] [PubMed] [Google Scholar]
- 13.Keller A, Bucher Della Torre S. Sugar-Sweetened Beverages and Obesity among Children and Adolescents: A Review of Systematic Literature Reviews. Child Obes. 2015;11(4):338–346. doi: 10.1089/chi.2014.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castillo JL, Milgrom P, Coldwell SE, Castillo R, Lazo R. Children's acceptance of milk with xylitol or sorbitol for dental caries prevention. BMC Oral Health. 2005;5:6. doi: 10.1186/1472-6831-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt B. Proof of Principle studies. Epilepsy Res. 2006;68(1):48–52. doi: 10.1016/j.eplepsyres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Pitts NB, Stamm JW. International Consensus Workshop on Caries Clinical Trials (ICW-CCT)— final consensus statements: Agreeing where the evidence leads. J Dent Res. 2004;83:C125–C128. doi: 10.1177/154405910408301s27. [DOI] [PubMed] [Google Scholar]
- 17.Dasanayake AP, Roseman JM, Caufield PW, Butts JT. Distribution and determinants of mutans streptococci among African-American children and association with selected variables. Pediatr Dent. 1995;17(3):192–198. [PubMed] [Google Scholar]
- 18.Silness J, Loe H. Periodontal Disease in Pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 19.Ly K, Milgrom P, Roberts MC, Yamaguchi DK, Rothen M, Mueller G. Linear response of mutans streptococci to increasing frequency of xylitol chewing gum use: a randomized controlled trial. BMC Oral Health. 2006;6:6. doi: 10.1186/1472-6831-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milgrom P, Ly K, Roberts MC, Rothen M, Mueller G, Yamaguchi DK. Mutans streptococci dose response to xylitol chewing gum. J Dent Res. 2006;85(2):177–181. doi: 10.1177/154405910608500212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization: Oral Health Surveys: Basic Methods. 2nd. Geneva: World Health Organization; 1979. [Google Scholar]
- 22.Makinen KK, Bennett CA, Hujoel PP, et al. Xylitol chewing gums and caries rates: a 40-month cohort study. J Dent Res. 1995;74(12):1904–1913. doi: 10.1177/00220345950740121501. [DOI] [PubMed] [Google Scholar]
- 23.Efird J. Block randomization with randomly selected block sizes. Int J Environ Res Public Health. 2011;8(1):15–20. doi: 10.3390/ijerph8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 25.Söderling EM. Xylitol, mutans streptococci, and dental plaque. Adv Dent Res. 2009;21(1):74–78. doi: 10.1177/0895937409335642. [DOI] [PubMed] [Google Scholar]
- 26.Lee W, Spiekerman C, Heima M, et al. The effectiveness of xylitol in a school-based cluster-randomized clinical trial. Caries Res. 2015;49(1):41–49. doi: 10.1159/000360869. [DOI] [PubMed] [Google Scholar]
- 27.Duangthip D, Chu CH, Lo CM. A randomized clinical trial on arresting dentine caries in preschool children by topical fluorides-18 month results. J Dent. 2015 May 30; doi: 10.1016/j.jdent.2015.05.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Dong YM, Pearce EI, Yue L, Larsen MJ, Gao XJ, Wang JD. Plaque pH and associated parameters in relation to caries. Caries Res. 1999;33(6):428–436. doi: 10.1159/000016547. [DOI] [PubMed] [Google Scholar]
- 29.Lenth RV. Statistical power calculations. J Anim Sci. 2007;85(13):E24–E29. doi: 10.2527/jas.2006-449. [DOI] [PubMed] [Google Scholar]