Abstract
Background
Arthritis is a set of inflammatory conditions that induce aching, stiffness, swelling, pain and may cause functional disability with severe consequences to the patient’s lives. These are multi-mediated pathologies that cannot be effectively protected and/or treated. Therefore, the aim of this study was to establish a new model of acute arthritis, using a Lys49-PLA2 (Bothrops asper myotoxin II; MT-II) to induce articular inflammation.
Methods
The articular inflammation was induced by MT-II (10 μg/joint) injection into the left tibio-tarsal or femoral-tibial-patellar joints. Cellular influx was evaluated counting total and differential cells that migrated to the joint. The plasma extravasation was determined using Evans blue dye. The edematogenic response was evaluated measuring the joint thickness using a caliper. The articular hypernociception was determined by a dorsal flexion of the tibio-tarsal joint using an electronic pressure-meter test. The mediators involved in the articular hypernociception were evaluated using receptor antagonists and enzymatic inhibitors.
Results
Plasma extravasation in the knee joints was observed 5 and 15 min after MT-II (10 μg/joint) injection. MT-II also induced a polymorphonuclear cell influx into the femoral-tibial-patellar joints observed 8 h after its injection, a period that coincided with the peak of the hyperalgesic effect. Hyperalgesia was inhibited by the pretreatment of the animals with cyclooxygenase inhibitor indomethacin, with type-2 cyclooxygenase inhibitor celecoxib, with AACOCF3 and PACOCF3, inhibitors of cytosolic and Ca2+-independent PLA2s, respectively, with bradykinin B2 receptor antagonist HOE 140, with antibodies against TNFα, IL-1β, IL-6 and CINC-1 and with selective ET-A (BQ-123) and ET-B (BQ-788) endothelin receptors antagonists. The MT-II-induced hyperalgesia was not altered by the lipoxygenase inhibitor zileuton, by the bradykinin B1 receptor antagonist Lys-(Des-Arg9,Leu8)-bradykinin, by the histamine and serotonin antagonists promethazine and methysergide, respectively, by the nitric oxide inhibitor LNMMA and by the inhibitor of matrix 1-, 2-, 3-, 8- and 9- metalloproteinases GM6001 (Ilomastat).
Conclusion
These results demonstrated the multi-mediated characteristic of the articular inflammation induced by MT-II, which demonstrates its relevance as a model for arthritis mechanisms and treatment evaluation.
Keywords: Lys49-PLA2, Myotoxin II, Arthritis, Bothrops asper, Phospholipase
Background
Articular inflammations or arthritis are pathological conditions that affect around 54 million adults (23% of the population) only in USA [1]. Arthritis comprises more than 100 different diseases and conditions, being rheumatoid arthritis and osteoarthritis the two most common types. Other frequently occurring forms of arthritis include lupus and gout [2]. Rheumatoid arthritis and osteoarthritis are the most common inflammatory joint diseases, and their symptoms include aching, stiffness, and swelling in or around the joints, having pain and functional disability as their main consequences [2–4].
Articular inflammation is a multi-mediated condition, implying a role for mediators such as interleukin (IL)-1β, IL-6, tumour necrosis factor (TNF), platelet activating factor (PAF), and prostaglandin E2 (PGE2) [5]. Besides these and other mediators present in this pathology, the participation of phospholipases A2 (PLA2) in this process is also well documented [6].
The PLA2 superfamily includes 16 groups comprising six main types comprising the secreted (sPLA2), cytosolic (cPLA2), calcium-independent (iPLA2), platelet-activating factor acetylhydrolase (PAF-AH) also known as lipoprotein-associated (LpPLA2), lysosomal (LPLA2), and adipose (AdPLA) enzymes [7]. It has been demonstrated the presence of high levels of PLA2 in the synovial fluid of inflamed joints of animals and humans, being the PLA2activity increased in correlation with the severity of arthritis [8–11].
Many new therapies and strategies to control arthritis are currently being investigated, raising hopes for a better future for patients with this disease [12, 13]. In this context, experimental models that allow the study of the mechanisms underlying these inflammatory and pain conditions are of great clinical relevance.
PLA2s are widespread in nature, and can be found in a great diversity of tissues and fluids, including mammalian cells. These enzymes are notoriously abundant in venoms from snakes, bees, the Heloderma lizard, and the marine snail Conodipina sp [14–19].
Four myotoxins with PLA2 structure have been isolated from the venom of the viperid snake Bothrops asper, named MT-I to MT-IV [20]. Despite high homology among these proteins, MT-II and MT-IV (which present a Lys instead of the canonical Asp residue at position 49) lack catalytic activity, whereas MT-I and MT-III (which contain an Asp residue at position 49) display high enzymatic activity [21, 22]. These PLA2s comprise approximately 30% of the venom proteins in this venom, and play a relevant role in its myotoxic, pro-inflammatory and hyperalgesic activities [18, 22, 23].
Regardless of their catalytic activity, both MT-II and III induce marked local inflammation and pain. Despite few differences in kinetics of release, both MT-II (Lys49-PLA2) and MT-III (Asp49-PLA2) are able to stimulate the production and release of inflammatory mediators such as IL-1 and IL-6, TNFα, LTB4, TXA2, PGE2 and PGD2 at the site of their injection as well as under in vitro conditions [24–27]. Concerning their hyperalgesic activity, both MT-II and MT-III cause significant local hyperalgesia in the rat hind paw after intraplantar injection, of rapid onset and similar time-course [28]. The mediators involved in the nociceptive process induced by both myotoxins are almost the same, differing in the level of the pain threshold [27–29]. These results indicate that enzymatic activity is not a strict requirement for the induction of nociception, but is important for determination of the intensity of the nociceptive phenomenon.
Therefore, the aim of this study was to establish a new model of myotoxin-induced joint acute arthritis to investigate the role of PLA2s in this process. For this purpose, MT-II was used because, not being itself catalytically-active, allows for the study of the phenomenon without the interference of exogenous enzymatic phospholipid degradation.
Our results demonstrated that in spite of its enzymatic inactivity, MT-II induces a multi-mediated acute articular inflammation that shares many of the features observed in humans arthritis. Thus, MT-II can be considered a suitable model for the determination of cellular and molecular mechanisms involved in arthritis process as well as a useful assay to evaluate new possible therapeutic compounds.
Methods
Isolation of Myotoxin II (MT-II)
MT-II, an enzymatically-inactive Lys49 PLA2, was isolated from Bothrops asper venom obtained from adult specimens collected in the Caribbean region of Costa Rica, by ion-exchange chromatography on CM-Sephadex C-50, as previously described [30]. Salt-free, lyophilized MT-II was stored at −20 °C until use.
Animals
Male Wistar rats (170–190 g) were used throughout this study. Animals were housed in a temperature-controlled (21 ± 2 ° C) and light-controlled (12/12 h light/dark cycle) room with standard food and water available ad libitum.
Induction of articular inflammation
The articular inflammation was induced by administration of MT-II, in different doses, into the left tibio-tarsal or femoral-tibial-patellar joints, depending on the experimental protocol used, in rats lightly anesthetized by inhalation of halothane (Cristália Ltda, Brazil). MT-II was diluted in sterile PBS solution (NaCl 0.14 M; KCl 2.7 mM; Na2HPO4 8.0 mM; KH2PO4 1.5 mM) and injected in a volume of 25 or 50 μL into the tibio-tarsal or femoral-tibial-patellar joints, respectively, using an insulin syringe (0.5 mL, needle 5/16” 30G) inserted into the joint. For the femoral-tibial-patellar joint inflammation, carrageenin was used as positive control (200 μg/50 μL) and PBS (50 μL) was used as a control [31, 32]; while for the tibio-tarsal joint inflammation the control groups were constituted by animals that received zymosan (30 μg/ 25 μL, used as positive control) or bovine serum albumin (BSA, 20 μg/25 μL, used as a control of the protein content injected in the joint) or PBS (25 μL) [33–35].
Determination of the cellular influx to the articulation
The cellular influx was evaluated using two methods.
Total and differential counts
To evaluate the cellular influx to the femoral-tibial-patellar articulation, the animals were terminally anaesthetized (halothane inhalation), killed by cervical dislocation and ex-sanguinated by sectioning the cervical vessels 1, 4, 8 and 12 h after MT-II (5, 10, 15 and 20 μg/joint) injection. The synovial cavity of the knee joints was then washed with 50 μL of PBS containing 4 mM of ethylenediaminetetraacetic acid. The synovial exudates were collected by aspiration and total and differential cell counts were performed using a Neubauer chamber (1:20 dilution v:v) and stained smears (violet crystal 0.5%), respectively. A total of 100 cells were counted on a light microscope.
Measurement of myeloperoxidase (MPO) activity
The tibio-tarsal joint region was separated from the tibio-tarsal bone complex at 8 h after MT-II (10 μg/joint) administration. The neutrophil migration to the tibio-tarsal joint region of rats was evaluated by the myeloperoxidase (MPO) kinetic-colorimetric assay as described previously [36]. Samples of joint tissue were collected and kept at −80 °C until use. Samples were placed in CTAB solution (hexadecyl trimethylammonium bromide 0.5%, prepared in 50 mM K2HPO4 buffer, pH 6.0) at 37 ° C, homogenized and centrifuged at 4,200 g for 10 min at 4 ° C. Briefly, 20 μL of the supernatant was mixed with 130 μL of ODP solution (o-Phenylene diamine, 10 mg, dissolved in 10 mL of phosphate buffer containing 1 μmol of hydrogen peroxide); and the mixture was assayed spectrophotometrically for MPO activity determination at 492 nm.
The determination of the cellular influx, assessed by the measurement of MPO activity was performed 8 h after intra-articular injection of MT-II (10 μg) or PBS, in animals pre-treated or not with fucoidan (5 mg/kg, i.v.), a sulfated polysaccharide that binds to L-selectin, 15 min prior to myotoxin.
Trypan blue exclusion test of cell viability
Cell viability was determined using polymorphonuclear cells collected from peritoneal cavity by the Trypan blue exclusion method. Peritoneal cell migration was induced by i.p. injection of glycogen (10 mL). Four hours later, animals were euthanized in a CO2 chamber, ex-sanguinated by sectioning the cervical vessels and had the peritoneal cavity washed with 10 mL of cold PBS [37–39]. After gentle massage of the abdominal wall, the peritoneal fluid containing cells was collected. Cells were kept (1 × 106 cells/mL) in RPMI 1640 medium with or without MT-II (5, 10, 15 and 20 μg/mL) for 1 h in a 37o CO2 incubator. The dye exclusion counting was performed in a Neubauer’s hemocytometer using 1% Trypan blue. A total of 100 cells were counted by light microscopy.
Plasma extravasation in the knee joint induced by myotoxin
The plasma extravasation was determined according to the protocol described by Lam and Ferrell [40]. Evans Blue dye (75 mg/kg) was injected i.v. 20 min before joint excision. MT-II was injected by intra-articular route and 5, 15, 30, 60, 240 and 360 min afterwards, animals were euthanized by cervical dislocation, exsanguinated by sectioning the cervical vessels and the knee joint capsules were dissected. These samples were weighed, cut into smaller pieces and mixed in a solution containing acetone and 1% aqueous solution of sodium sulphate (7:3 proportion). Samples were kept in continuous mild shaking for 24 h at room temperature. Each preparation was then centrifuged at 2000 rpm for 10 min. The supernatant was collected and the amount of dye recovered was calculated by comparing the absorbance of the supernatant at 620 nm (Labsystems MuItiscan) with that of a standard curve prepared with known concentrations of Evans blue.
As Evans blue dye binds to plasma proteins normally restricted to the vascular compartment, its presence in the capsule provides an index of altered vascular permeability. In this experiment, the control group was constituted of animals that received Ringer-Lock solution injected by intra-articular route. The amount of tissue obtained from each animal was small, thus requiring the pooling of the samples. Then, for each experimental procedure, four groups of three rats were used. Results are expressed as μg Evans blue/mL.
Evaluation of edema
The edematogenic response induced by myotoxin was evaluated in both tibio-tarsal and femoral-tibial-patellar joints. MT-II (10 g/articulation) was diluted in 25 (tibio-tarsal articulation) or 50 μL (femoral-tibial-patellar articulation) of PBS. The same volume of PBS was injected in the contralateral articulation. The increase in the articulation was determined by measuring joint thickness using a caliper at 0 (time before injections), 1, 2, 4, 8 and 24 h after MT-II or PBS injection. Results were calculated by the difference in thickness of both joints, and edema was expressed as the percentage increase in joint thickness as compared to the control.
Evaluation of articular hypernociception
The articular hypernociception was determined by a dorsal flexion of the tibio-tarsal joint, evaluated using a modified electronic pressure-meter test, as previously described [34]. Rats were placed in acrylic cages with a wire grid floor 20 min before testing for environmental adaptation. A tilted mirror was placed below the grid floor to provide a clear view of the hind paw. Stimulations were performed only when animals were quiet, did not display exploratory movements or defecation, and were not resting on their paws. In these experiments, an electronic pressure meter was used. It consists of a hand-held force transducer fitted with a polypropylene tip (Insight Ltda, Brazil) with a large tip (4.15 mm2) adapted to the probe.
In this test, an increasing perpendicular force is applied to the central area of the plantar surface of the hind paw to induce flexion of the tibio-tarsal joint, and this force is automatically interrupted when the animal reacts by withdrawing the paw. The electronic pressure-meter apparatus automatically recorded the intensity of the force necessary to induce this animal reaction. The test was repeated until three measurements with less than 1 g of variation were obtained. The flexion-elicited mechanical threshold was expressed in grams (g). The test was applied before and in different times after the intra-articular injection of MT-II (10 μg) or BSA (20 μg), zymosan (30 μg) and PBS, used as controls.
Pharmacological treatments
In order to investigate the mechanisms involved in the articular hypernociception induced by MT-II, receptor antagonists and enzymatic inhibitors were used:
To evaluate the contribution of the cellular influx to the joint to the hypernociceptive effect, fucoidan (5 mg/kg, i.v.), a sulfated polysaccharide that binds to L-selectin, was injected 15 min prior to MT-II [41].
To investigate the involvement of arachidonate metabolites in this phenomenon, different groups of rats were treated with the cyclooxygenase inhibitor indomethacin (4 mg/kg, 30 min before myotoxin), with the type-2 cyclooxygenase inhibitor celecoxib (10 mg/kg, 60 min before myotoxin) or with the 5-lipoxygenase inhibitor zileuton (100 mg/kg, 60 min before myotoxin) [28, 42].
In order to assess the involvement of endogenous PLA2 activity to the myotoxin-induced hypernociception, rats were treated with arachidonyl trifluoromethil ketone (AACOCF3, 200 μg/joint), a potent and selective inhibitor of cPLA2, or palmitoyl trifluoromethyl ketone (PACOCF3, 1 μg/joint), an inhibitor of iPLA2, 30 min before myotoxin administration [43, 44].
To evaluate the participation of bradykinin in the algogenic effect of myotoxin, a bradykinin B1 receptor antagonist Lys-(Des-Arg9,Leu8)-bradykinin (Lys-BK, 10 and 40 nmol) and a bradykinin B2 receptor antagonist icatibant (HOE 140, 0.75 μmol) were injected by the intra-articular route 20 min before myotoxin administration [28, 45].
To evaluate the contribution of cytokines, animals were treated with an anti-TNF-α antibody (0.5 μg/joint), with an anti-interleukin-1β antibody (1.5 μg/joint), with an anti-interleukin-6 antibody (4.0 μg/joint) or with an anti-CINC-1 antibody (5.0 μg/joint), 30 min before myotoxin. Carragenin (200 μg/joint) was used as positive control of the antibody doses used since carragenin-induced hypernociception is abrogated by these antibodies.
To examine the participation of histamine and serotonin, animals were injected with promethazine or methysergide (5 mg/kg, i.p.) 30 min before myotoxin injection [28].
To explore the effect of endothelin, BQ-123 and BQ-788 (10 and 20 nmol/joint), selective antagonists of ET-A and ET-B endothelin receptors, were injected 30 min before myotoxin administration [46].
In order to investigate the participation of metalloproteinases in the MT-II effects, Ilomastat (GM6001, 27 and 71 nM/joint), a potent broad-spectrum hydroxamate inhibitor of matrix metalloproteinases (inhibitor of 1-, 2-, 3-, 8- and 9-MMPs) was injected 30 min before myotoxin administration. Zymosan (30 μg/joint) was used as positive control of GM6001 doses since it is capable of increasing the mRNA expression to MMPs-2, −3 and −9 in the synovial tissue [47].
In order to investigate the participation of nitric oxide (NO) on myotoxin-induced hypernociception, rats were treated with the inhibitor of nitric oxide synthase (NOS), L-NMMA (50 μg/joint), 60 min before myotoxin injection [48].
Indomethacin was diluted in Tris buffer (1 M, pH 8.0 at 37o C) and PBS. Celecoxib and zileuton were dissolved in CMC 1%. HOE 140, Lys-(Des-Arg9,Leu8)-bradykinin, anti-IL-1β, anti-IL-6, anti-TNFα and anti-CINC-1 antibodies were diluted in PBS. BQ-123 and BQ-788 were diluted in distilled water. GM6001, AACOCF3 and PACOCF3 were dissolved in DMSO. LNMMA, promethazine, methysergide and fucoidan were diluted in saline. In all experiments, control groups were constituted of animals treated with MT-II plus the specific diluents of each drug.
Drugs used
Anti-IL-1β, anti-IL-6, anti-TNFα and anti-CINC-1 antibodies were supplied by R&D Systems Inc. (USA). Indomethacin, AACOCF3 and PACOCF3 were purchased from Biomol Research Laboratories (USA). GM6001 was supplied by USBiological (USA); whereas L-NMMA, HOE 140, Lys-(Des-Arg9,Leu8)-bradykinin, promethazine, methysergide, BQ-123, BQ-788 and fucoidan were purchased from Sigma-Aldrich Co. (USA). Celecoxib was supplied by Searle and Co (Puerto Rico). Zileuton was purchased from Abbott Laboratories (Zyflo®, USA). Carrageenin was purchased from Marine Colloids.
Statistical analysis
Results are presented as mean ± S.E.M. Statistical evaluation of data was carried out by analysis of variance (ANOVA) and sequential differences among means were compared according to Tukey contrast analysis at p < 0.05 [49].
Results
Cellular migration induced by myotoxin II
An increase in the total influx of cells into the femoral-tibial-patellar joints of animals was noticed 8 h after intra-articular injection of myotoxin, only with the dose of 10 μg/joint. This increase was comparable to the cell influx induced by carrageenin, used as positive control, and is due to an increase in the numbers of polymorphonuclear cells (Table 1). When animals were treated with other doses of myotoxin (5, 15 and 20 μg/joint) or BSA, used as a control of the quantity of protein injected in the joint, no statistically significant difference was noted for cell migration values when compared to groups treated with PBS (Table 1).
Table 1.
Myotoxin-induced cell migration to the joint
| Treatment | Groups (n = 6) | Total cells (× 106/mL) | Mononuclear cells (× 106/mL) | PMN cells (× 106/mL) | |||
|---|---|---|---|---|---|---|---|
| 1 h | 4 h | 8 h | 12 h | 8 h | 8 h | ||
| PBS | 6 | 2.12 ± 0.70 | 3.72 ± 1.80 | 1.09 ± 0.61 | 0.24 ± 0.14 | 0.25 ± 0.13 | 1.65 ± 0.77 |
| Myotoxin 5 μg/joint | 6 | 2.07 ± 0.6 | 83.44 ± 25.2 | 52.27 ± 23.67 | 5.20 ± 1.54 | 7.97 ± 4.65 | 44.30 ± 19.07 |
| Myotoxin 10 μg/joint | 6 | 18.71 ± 4.10 | 73.16 ± 22.10 | 163.08 ± 48.04a | 28.12 ± 7.57 | 8.15 ± 2.87 | 154.93 ± 34.16a |
| Myotoxin 15 μg/joint | 6 | 6.12 ± 1.80 | 48.5 ± 22.70 | 66.38 ± 21.50 | 21.32 ± 8.38 | 3.91 ± 0.86 | 62.47 ± 13.73 |
| Myotoxin 20 μg/joint | 6 | 10.90 ± 12.75 | 41.37 ± 20.10 | 43.15 ± 5.53 | 12.75 ± 3.40 | 2.79 ± 0.85 | 40.36 ± 4.88 |
| Carrageenin | 6 | 4.74 ± 1.10 | 49.43 ± 7.20 | 109.14 ± 30.31a | 15.64 ± 3.65 | 7.21 ± 2.36 | 101.93 ± 28.22a |
| BSA | 6 | 0.50 ± 0.30 | 1.00 ± 0.5 | 1.50 ± 3.40 | 2.00 ± 0.50 | / | / |
Total and differential cellular influx to the femoral-tibial-patellar articulation, evaluated 1, 4, 8 and 12 h after myotoxin II (5, 10, 15 and 20 μg/joint) injection. Total and differential cell counts were performed using a Neubauer chamber (1:20 dilution v:v) and stained smears (violet crystal 0.5%), respectively. A total of 100 cells were counted on a light microscope
aSignificantly different from mean values of control group (BSA)
Trypan blue exclusion test of cell viability
Since the increase in the cell influx was observed just for the dose of 10 μg/joint of myotoxin, we used the dye exclusion test to determine the number of viable cells collected from peritoneal cavity after treatment with MT-II.
After 1 h exposure, trypan blue exclusion assay revealed that cell viability of groups treated with PBS, 5 μg of myotoxin and 10 μg of myotoxin was 100%, while in the groups treated with 15 and 20 μg the cell viability was 50 and 20%, respectively. Based on these findings, and in agreement with the results obtained in the cellular migration assay, the dose of 10 μg/joint of myotoxin/joint was chosen for subsequent tests.
Plasma extravasation in the knee joint induced by myotoxin
Plasma extravasation in the knee joints was determined 5, 15, 30, 60, 240 and 360 min after myotoxin injection. Results demonstrated an increase of 25 and 57% in the concentrations of Evans blue dye in the samples from animal treated with myotoxin 5 and 15 min after injection, respectively, when compared with animal treated with Ringer-Lock solution. No statistically significant difference was noted for plasma extravasation values in the subsequent times.
Characterization of articular hypernociception and edema
The intraplantar injection of myotoxin II (10 μg/joint) into the rat tibio-tarsal joint caused a significant decrease in pain threshold (Fig. 1). The hypernociception was detected from 4 to 8 h, decreasing thereafter and completely disappearing within 24 h. Zymosan (30 μg/joint) used as positive control, induced hypernociception with same intensity of myotoxin, observed 8 h after its injection (Fig. 1) [34]. The injection of saline or BSA (control groups) did not modify the pain threshold of the animals (Fig. 1).
Fig. 1.
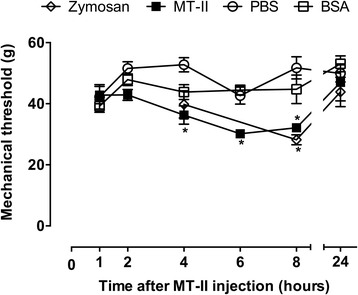
MT-II-induced articular hyperalgesia. MT-II (10 μg/joint) or PBS (vehicle) was injected in tibio-tarsal articulation (25 μL). Pain threshold was determined by a dorsal flexion of the tibio-tarsal joint using a modified electronic pressure-meter test before (time 0 – basal) and 1, 2, 4, 6, 8 e 24 h after MT-II injection, and was represented as force (in g). Zymosan (30 μg) and BSA (20 μg) were used as controls. Each point represents the mean ± SEM of six animals. *p < 0.05 indicate statistically significant differences when compared with PBS group (vehicle)
In agreement, the injection of myotoxin caused a time-dependent edema, observed in both tibio-tarsal (Fig. 2a) and femoral-tibial-patellar (Fig. 2b) joints. In both joints, the maximum increase in hind-paw swelling occurred 1 h after MT-II injection, decreasing thereafter and completely disappearing within 24 h (Fig. 2).
Fig. 2.
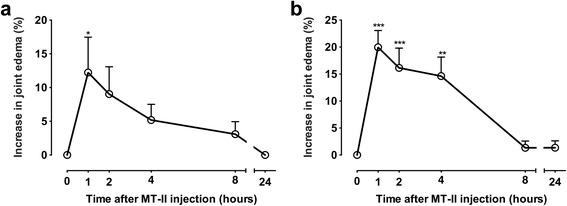
Edema induced by MT-II in (a) tibio-tarsal and (b) femoral-tibial-patellar rat joints. MT-II (10 μg/articulation) was injected in 25 (tibio-tarsal articulation) or 50 μL (femoral-tibial-patellar articulation) of PBS (vehicle). The same volume of PBS was injected in the contralateral articulation. The increase in the articulation was determined by measuring the joint edema using a caliper at 0 (time before injections) or 1, 2, 4, 8 and 24 h after MT-II or PBS injection. Results are expressed as the percentage in the increase in joint thickness of MT-II group in relation to the PBS group. Each point represents the mean ± SEM of six animals. *p < 0.05, **p < 0.01 and ***p < 0.001 indicate statistically significant differences when compared with baseline (time 0)
Contribution of the cellular influx to the joint to the hypernociceptive effect of myotoxin
The treatment with fucoidan, a sulfated polysaccharide that binds to L-selectin, prevented the hyperalgesia induced by myotoxin (Fig. 3a). The efficacy of fucoidan in decrease the cellular influx to the joint was confirmed in the MPO activity assay (Fig. 3b).
Fig. 3.
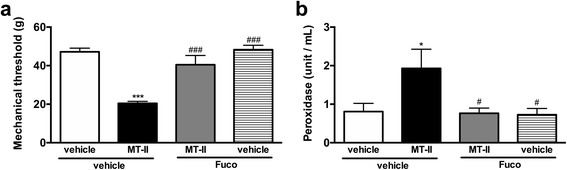
Effect of a L-selectin binder on MT-II- induced articular hyperalgesia. MT-II (10 μg/joint) or PBS (vehicle) was injected in tibio-tarsal articulation (25 μL). Fucoidan (fuco), a L-selectin binder (5 mg/kg, i.v.) or saline (vehicle) was injected 15 min prior to MT-II. a Pain threshold was determined using a modified electronic pressure-meter test 8 h after MT-II injection, and represented as force (in g). b The neutrophil migration to the tibio-tarsal joint region of mice was evaluated by the myeloperoxidase (MPO) kinetic-colorimetric assay, tested 8 h after MT-II injection. Each point represents the mean ± SEM of six animals. *p < 0.05 and ***p < 0.001 indicate statistically significant differences when compared with control group (vehicle + vehicle). #p < 0.05 and ###p < 0.001 indicate statistically significant differences when compared with MT-II group (MT-II + vehicle)
Mediation of myotoxin-induced hypernociceptive effect
Participation of eicosanoids and endogenous phospholipases A2
Pretreatment with the cyclooxygenase inhibitor indomethacin (Fig. 4a) or type 2 cyclooxygenase inhibitor celecoxib (Fig. 4b) significantly reduced the hyperalgesia caused by myotoxin. The lipoxygenase inhibitor zileuton did not modify the hyperalgesic response (Table 2).
Fig. 4.
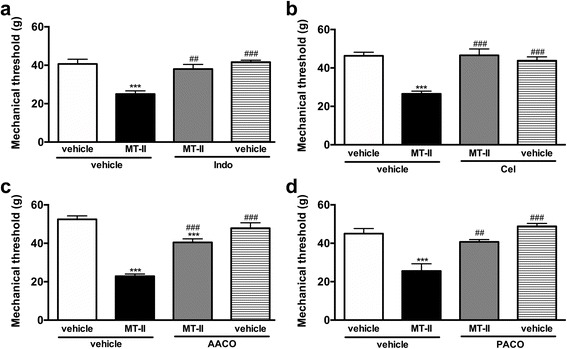
Involvement of eicosanoids and endogenous phospholipases A2 on MT-II- induced articular hyperalgesia. MT-II (10 μg/joint) or PBS (vehicle) was injected in tibio-tarsal articulation (25 μL). Pain threshold was determined using a modified electronic pressure-meter test 8 h after MT-II injection, and represented as force (in g). a Indomethacin, a cyclooxygenase inhibitor (Indo, 4 mg/kg, 30 min before MT-II) or (b) celecoxib, a type-2 cyclooxygenase inhibitor (Cel, 10 mg/kg, 60 min before MT-II) or (c) arachidonyl trifluoromethil ketone, a selective inhibitor of cPLA2 (AACO, 200 μg/joint, 30 min before MT-II) or (d) palmitoyl trifluoromethyl ketone, an inhibitor of iPLA2 (PACO, 1 μg/joint, 30 min before MT-II) was injected prior to MT-II. Each point represents the mean ± SEM of six animals. ***p < 0.001 indicate statistically significant differences when compared with control group (vehicle + vehicle). ##p < 0.01 and ###p < 0.001 indicate statistically significant differences when compared with MT-II group (MT-II + vehicle)
Table 2.
Evaluation of histamine, serotonin, nitric oxide and metalloproteinases in the myotoxin-induced hypernociceptive effect
| Treatment | Force in grams evaluated 8 h after myotoxin injection |
|---|---|
| Saline + PBS | 49.90 ± 1.72 |
| DMSO + PBS | 49.60 ± 2.11 |
| Saline + Myotoxin | 25.58 ± 0.59a |
| DMSO + Myotoxin | 23.64 ± 0.79a/NS |
| Zyleuton + Myotoxin | 29.82 ± 3.03a/NS |
| Methysergide + Myotoxin | 26.72 ± 0.85a/NS |
| Promethazine + Myotoxin | 24.74 ± 1.04a/NS |
| L-NMMA + Myotoxin | 29.14 ± 1.72a/NS |
| GM6001 + Myotoxin | 26.06 ± 0.69a/NS |
| Methysergide + PBS | 49.82 ± 1.31 |
| Promethazine + PBS | 50.06 ± 2.18 |
| L-NMMA + PBS | 48.26 ± 3.87 |
| GM6001 + PBS | 44.54 ± 2.33 |
Articular hyperalgesia induced by MT-II in rats in the presence or in the absence of different pharmacological treatments. The articular hypernociception was determined by a dorsal flexion of the tibio-tarsal joint using a modified electronic pressure-meter test and was represented as force (in g), observed 8 h after MT-II injection. Zyleuton: 5-lipoxygenase inhibitor; methysergide: antagonist of H1 histaminergic receptor; promethazine: antagonist of serotoninergic receptors; L-NMMA: inhibitor of nitric oxide synthase; GM6001: a potent broad-spectrum hydroxamate inhibitor of matrix metalloproteinases (inhibitor of 1-, 2-, 3-, 8- and 9-MMPs)
NS Not significantly different from mean values of myotoxin group
aSignificantly different from mean values of control group (Saline or DMSO + PBS)
Since it was demonstrated that both cyclooxygenase and type 2 cyclooxygenase inhibitors blocked the hyperalgesic effect of myotoxin and considering that this myotoxin is an enzymatically-inactive PLA2, we investigated the possible participation of endogenous phospholipases in this effect, since myotoxin cannot hydrolyze membrane phospholipids directly.
Results demonstrated the both AACOCF3 (Fig. 4c) and PACOCF3 (Fig. 4d) prevented the hypernociception induced by myotoxin, suggesting the participation of cytosolic and Ca2+-independent PLA2s in this effect.
Participation of bradykinin
Myotoxin-induced hyperalgesia was abolished by treating the animals with the bradykinin B2 receptor antagonist HOE 140 (Fig. 5a), but it was not altered by bradykinin B1 receptor antagonist Lys-(Des-Arg9,Leu8)-bradykinin (Fig. 5b).
Fig. 5.
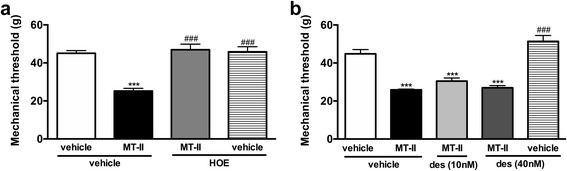
Involvement of bradykinin on MT-II-induced articular hyperalgesia. MT-II (10 μg/joint) or PBS (vehicle) was injected in tibio-tarsal articulation (25 μL). Pain threshold was determined using a modified electronic pressure-meter test 8 h after MT-II injection, and represented as force (in g). (a) A bradykinin B2 receptor antagonist icatibant (HOE 140, 0.75 μmol) or (b) a bradykinin B1 receptor antagonist Lys-(Des-Arg9,Leu8)-bradykinin (des, 10 and 40 nmol) was injected by intra-articular route 20 min prior to MT-II. Each point represents the mean ± SEM of six animals. ***p < 0.001 indicate statistically significant differences when compared with control group (vehicle + vehicle). ###p < 0.001 indicate statistically significant differences when compared with MT-II group (MT-II + vehicle)
Participation of cytokines
Pretreatment with antibodies against TNFα (Fig. 6a), IL-1β (Fig. 6b) and IL-6 (Fig. 6c) blocked the hypernociceptive effect of myotoxin. Antibodies against CINC-1 partially reduced this effect (Fig. 6d).
Fig. 6.
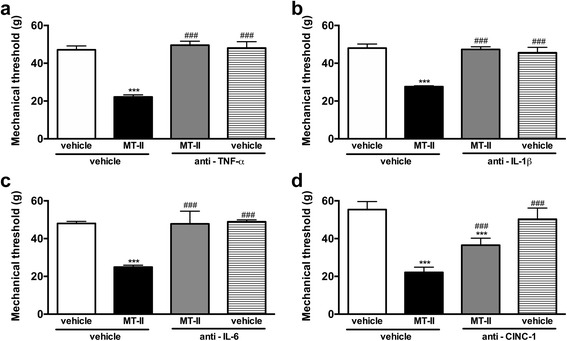
Involvement of cytokines on MT-II-induced articular hyperalgesia. MT-II (10 μg/joint) or PBS (vehicle) was injected in tibio-tarsal articulation (25 μL). Pain threshold was determined using a modified electronic pressure-meter test 8 h after MT-II injection, and represented as force (in g). a anti-TNFα antibody (0.5 μg/joint) or (b) anti-IL-1β antibody (1.5 μg/joint) or (c) anti-IL-6 antibody (4.0 μg/joint) or (d) anti-CINC-1 antibody (5.0 μg/joint) was injected 30 min before MT-II. Each point represents the mean ± SEM of six animals. *** p < 0.001 indicate statistically significant differences when compared with control group (vehicle + vehicle). ### p < 0.001 indicate statistically significant differences when compared with MT-II group (MT-II + vehicle)
Participation of endothelin
The hypernociceptive effect induced by myotoxin was partially reversed by the pretreatment with BQ-123 and BQ-788, selective antagonists of ET-A (Fig. 7a) and ET-B (Fig. 7b) endothelin receptors respectively.
Fig. 7.
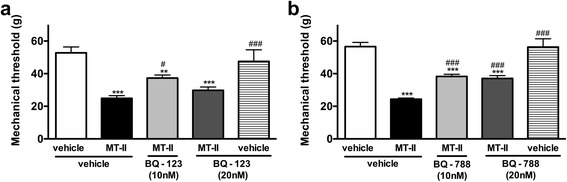
Involvement of endothelin on MT-II-induced articular hyperalgesia. MT-II (10 μg/joint) or PBS (vehicle) was injected in tibio-tarsal articulation (25 μL). Pain threshold was determined using a modified electronic pressure-meter test 8 h after MT-II injection, and represented as force (in g). a BQ-123 or (b) BQ-788 (10 and 20 nmol/joint, selective antagonists of ET-A and ET-B endothelin receptors, respectively) were injected 30 min before MT-II. Each point represents the mean ± SEM of six animals. **p < 0.01 and ***p < 0.001 indicate statistically significant differences when compared with control group (vehicle + vehicle). #p <0.05 and ### p < 0.001 indicate statistically significant differences when compared with MT-II group (MT-II + vehicle)
Participation of histamine, serotonin, nitric oxide and metalloproteinases
The histamine and serotonin antagonists promethazine and methysergide, respectively, the nitric oxide inhibitor LNMMA and the inhibitor of matrix metalloproteinases 1-, 2-, 3-, 8- and 9- GM6001 (Ilomastat) did not interfere with the effect of myotoxin (Table 2).
Discussion
Although outstanding efforts have been performed by clinicians and researchers to find effective strategies to treat and to restore joint function in articular inflammatory conditions such as osteoarthritis and rheumatoid arthritis, effective and/or protective treatments are still a challenge [50, 51]. For this reason, animal models that share the same characteristics of human arthritis are useful for the characterization of these conditions and for the study of new therapies.
The multi-mediated characteristic of articular inflammatory diseases and the importance of PLA2s and cyclooxygenase-derived mediators to these conditions have been well documented [5, 6]. Interestingly, studies performed using MT-II, a catalytically inactive PLA2 homologue, demonstrated that its enzymatic activity is not essential for its proinflammatory effects, since it is able to induce eicosanoid production through the stimulation of endogenous cytosolic and Ca2+-independent phospholipases A2 [26, 52, 53]. Herein it was demonstrated that in spite of lacking enzymatic activity, MT-II can induce acute arthritis, allowing the study of mediators involved in this condition. Our observations indicate that this is a multi-mediated process that involves the participation of eicosanoids (through the activation of endogenous PLA2s), bradykinin, cytokines, endothelin and is dependent on the cellular influx to the joint.
Both MT-II (Lys49-PLA2) and MT-III (Asp49-PLA2) induce hyperalgesia, allodynia, edema, plasma extravasation and H2O2 production by isolated macrophages [24, 28, 29, 54, 55]. The difference among the myotoxins is the intensity of their effects, since in all of them the effect observed with MT-II is weaker than that of MT-III. The Lys49-PLA2 was chosen for the present study since the lack of enzymatic activity eliminates the possibility that exogenous PLA2 degradation of phospholipids may contribute to the genesis of the inflammation, thus allowing the study of the role of endogenous, inflammatory PLA2s in this phenomenon.
The kinetics of the articular inflammation induced by MT-II was characterized. MT-II induced a rapid plasma extravasation in the knee joints observed 5 min after its injection, which peaked at 15 min. A time-dependent edema was observed in both tibio-tarsal and femoral-tibial-patellar joints, reaching its maximum increase 1 h after myotoxin injection. The inflammatory response reached its peak 8 h after MT-II injection, a time when the cell influx and hyperalgesic effect reached their maximum. In these studies, the selected dose (10 μg) was not cytotoxic. Previous studies already demonstrated that MT-II induces prominent leukocyte infiltration to the peritoneal cavity 6 h after its injection, composed predominantly of polymorphonuclear leukocytes [24]. This same cell migration profile was obtained in the present study using carrageenan and is in agreement with previous studies [56], confirming articular MT-II injection as a suitable model for articular inflammation evaluation.
According to the World Health Organization rheumatoid arthritis and osteoarthritis are included in the group of conditions having the greatest impact on society, being osteoarthritis one of the ten most disabling diseases in developed countries [57]. In addition, pain can be considered one of the most prominent symptoms in people suffering of arthritis, being the most important cause of disability and loss of joint function in patients with osteoarthritis [57, 58]. Considering this, the hyperalgesic effect of articularly injected MT-II was investigated and the role of several inflammatory mediators in this process was determined.
MT-II induced significant hyperalgesia which peaked 8 h after injections. The hyperalgesic effect of both MT-II and MT-III was previously investigated after intraplantar injection of the toxins [28]. These authors demonstrated that MT-II induced hyperalgesia that peaked 1 h later after intraplantar injection, decreasing afterwards. Differences in the experimental conditions between that study and our present report, particularly regarding the site of injection, could explain the differences described. In our case, it is interesting to note that the peak of the hyperalgesic response of the animals coincided with the peak of cell influx.
The cellular traffic between the blood and the tissues is regulated by adhesion molecules expressed on the blood and endothelial cell surface [59]. Among the major adhesion molecules involved in cell transmigration is L-selectin, a molecule indispensable for adhesion, diapedesis and subsequent cell migration to the tissue [60, 61]. Thus, the importance of cell influx to the hyperalgesic effect induced by MT-II was investigated using fucoidan, a binder of L-selectin which is able to inhibit cell migration into the tissue in a dose that does not affect the number of circulating leukocytes [41]. Our data showing that fucoidan fully reverted the hyperalgesia induced by MT-II confirmed the importance of cell influx to the joint to MT-II-induced hyperalgesia. The reduction in cell migration into the joint cavity was confirmed by myeloperoxidase assay.
It is important to point out that previous studies demonstrated that fucoidan significantly inhibited both cytotoxic and myotoxic effects of MT-II and that this inhibition is due to a rapid formation of complexes between fucoidan and myotoxins [62]. Regardless this interference of fucoidan in MT-II-induced myotoxicity, it probably does not explain the inhibition of MT-II-induced hyperalgesia observed in our results, because this interference was observed only when fucoidan was incubated with MT-II or when they were injected simultaneously at the same site [62, 63]. In contrast, MT-II-induced muscle necrosis was not inhibited when fucoidan was administered by i.v. route, immediately after i.m. toxin injection [63]. Therefore, considering that in our studies fucoidan was administered by i.v. route and MT-II directly in the joint, it is possible to consider that the inhibition of MT-II-induced hyperalgesia was a consequence of the decrease in leukocytes migration into joint articulation.
This hyperalgesic effect clearly involves the participation of type 2 cyclo-oxygenase-derived mediators, since both indomethacin and celecoxib inhibited this effect. The lipoxygenase inhibitor zileuton did not modify the hyperalgesic response, suggesting that leukotrienes are not likely to be involved in this phenomenon. These results are in agreement with Chacur et al. [28], who had previously demonstrated the involvement of prostaglandins and the absence of leukotrienes on MT-II-induced hyperalgesia using the intraplantar injection model. Considering that MT-II is a PLA2-like protein devoid of catalytic activity and, therefore, cannot hydrolyze membrane phospholipids directly, the participation of cytosolic and Ca2+-independent endogenous PLA2s was presently investigated.
The combined activities of sPLA2 and endogenous cPLA2 or Ca2+-independent PLA2 to induce eicosanoid formation in different cells has already been proposed [64, 65]. In addition, previous works have demonstrated the ability of MT-II to induce inflammation through endogenous PLA2s activation. Moreira et al. [26] demonstrated that MT-II is able to induce PGD2 and PGE2 release and expression of COX-2 in macrophages in culture, being these phenomena decreased by the inhibition of cytosolic PLA2 but not Ca2+ independent PLA2. Giannotti et al. [52], investigated the ability of MT-II to induce, in isolated macrophages, the formation of lipid droplets (LD), which are key elements of inflammatory responses. It was demonstrated that iPLA2, but not cPLA2, signaling pathways are involved in this LD formation. Corroborating these data, our results showed that, in the joint, both cytosolic and Ca2+-independent phospholipases are involved in MT-II-induced articular hyperalgesia.
The role of several mediators on MT-II PLA2-induced hyperalgesia was presently investigated using inhibitors of specific pathways or receptor antagonists. It was observed that this effect involves the participation of bradykinin, acting through B2 receptors, indicating the importance of kinins to the hyperalgesic effect. Bradykinin is an inflammatory mediator involved in both pain and nociceptor sensitization [66, 67]. It was already demonstrated that in some inflammatory conditions, bradykinin may induce the release of several mediators that act in a cascade fashion, causing both pain and nociceptors sensitization. These are considered multi-mediated processes that involve participation of biogenic amines, cytokines (TNFα, IL-6, IL-1β and IL-8), prostanoids and sympathomimetic amines [66, 68–72]
The importance of bradykinin to the onset of pain in articular inflammatory conditions has also been highlighted. Severe acute pain is considered the most important clinical symptom in patients suffering from crystal-induced arthritis (CIA). Ramonda et al. [73], evaluating this phenomenon, demonstrated that bradykinin can be included as one of the most important molecules to induce pain, together with prostaglandins, cytokines (in particular, interleukin-1β) and substance P, exerting their effects through different receptors present in both peripheral sensory neurons and in the spinal cord. De Falco et al. [74] reviewed the importance of bradykinin to osteoarthritis and described the action of B2 receptor antagonists to this condition, presenting these antagonists as promising agents to the osteoarthritis treatment.
In spite of the fact that (i) bradykinin-induced pain partly depends on the release of inflammatory mediators by mast cells [75]; (ii) the release of vasoactive amines from mast cells incubated with venom cationic PLA2s has been previously detected [76, 77] and (iii) Chacur et al. [28] demonstrated that the hyperalgesic effect of MT-II injected in the rat paw is partially mediated by histamine and serotonin; these mediators do not seem to be involved in the MT-II-induced articular hyperalgesia, since both histamine and serotonin antagonists did not interfere with the hyperalgesic effect of MT-II. In addition, nitric oxide inhibitor LNMMA and the inhibitor of matrix 1-, 2-, 3-, 8- and 9-metalloproteinases GM6001 (Ilomastat) did not interfere with the effect of myotoxin. Although the importance of these mediators to inflammatory conditions is well stablished, it is suggested that they are not contributing to the observed hyperalgesic effect [47, 78–81].
The role of cytokines in hyperalgesic and inflammatory processes, including arthritis, is well documented [82–84]. The sensitization of nociceptors by cytokines is a multi-mediated process that involves the release of prostaglandins and sympathomimetic amines [68, 69, 72, 85, 86]. In addition, the release of cytokines induced by both Bothrops asper venom or isolated Lys49 PLA2 has already been described [28, 29, 55, 87, 88]. In agreement with these data, our results confirmed the importance of cytokines to the articular inflammation induced by MT-II, since antibodies against TNFα, IL-1β, IL-6 and CINC-1 interfered with the effects induced by MT-II.
Endothelins are peptides implicated in pain transmission in both humans and animals, which contribute to sensory changes associated with inflammatory and neuropathic pain [89–91]. In addition, these peptides have been involved in articular inflammatory conditions, including osteoarthritis, where endothelin signaling may play a role in destruction of bone-cartilage unit [92]. Thus, the participation of endothelin acting on ET-A or ET-B receptors in MT-II induced articular pain was investigated. Our results demonstrated that both ET-A and ET-B antagonists partially reversed the hyperalgesic effect of MT-II, even when both antagonists were associated (data not shown). These results underscore the involvement of endothelin in the MT-II-induced pain and suggest that the mediators involved in this pain signaling are not released in a sequencial manner, but probably through parallel pathways.
Conclusion
In conclusion, our work demonstrated that MT-II, a catalytically-inactive Lys49-PLA2, induces an acute multi-mediated inflammatory articular process that includes most of the important mediators described in articular chronic conditions. Considering that arthritis is a pathological condition that has no cure, more in vivo animal models and clinical studies are needed to better understand the cellular and molecular mechanisms involved in this process as well as the efficacy and tolerability of new therapeutic compounds. In this context, MT-II-induced articular inflammation can be considered a valuable model for arthritis pathology and treatment evaluation.
Acknowledgments
Thanks are due to the Center for the Study of Venoms and Venomous Animals (CEVAP) of UNESP for enabling the publication of this paper (Edital Toxinologia CAPES no. 063/2010, Process no. 230.38.006285/2011–21, AUXPE Toxinologia 1219/2011).
Funding
This work was supported by the Brazilian agencies State of São Paulo Research Foundation (FAPESP – grant number 2006/03879–0) and Coordination for the Improvement of Higher Education Personnel (CAPES).
Authors’ contributions
RGD performed the behavioral experiments under the supervision of GP and YC and the in vitro assays under the supervision of SCS. They designed the assays and analyzed the results. FQC supervised the articular hypernociception evaluation. JMG and BL purified MT-II. MBS prepared the figures, revised the statistical analysis, helped with the manuscript preparation and text formatting. YC idealized the project. GP is the corresponding author and prepared the manuscript for publication. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval
All procedures were in accordance with the guidelines for the ethical use of conscious animals in pain research published by the International Association for the Study of Pain [93] and were approved by the Institutional Animal Care Committee of the Butantan Institute (Protocol number 240/05).
Abbreviations
- CIA
Crystal-induced arthritis
- cPLA2
Cytosolic phospholipase A2
- i.m.
Intramuscular
- i.p.
Intraperitoneal
- i.v.
Intravenous
- IL
Interleukin
- iPLA2
Calcium-independent phospholipase A2
- LD
Lipid droplets
- MPO
Myeloperoxidase
- MT-II
Myotoxin II
- PAF
Platelet activating factor
- PAF-AH
Platelet-activating factor acetylhydrolase
- PGE2
Prostaglandin E2
- PLA2
Phospholipase A2
- sPLA2
Secreted phospholipase A2
- TNF
Tumour necrosis factor
Contributor Information
Renata Gonçalves Dias, Email: rgdvet@yahoo.com.br.
Sandra Coccuzzo Sampaio, Email: sandra.coccuzzo@butantan.gov.br.
Morena Brazil Sant’Anna, Email: morena.santanna@butantan.gov.br.
Fernando Queiroz Cunha, Email: fdqcunha@fmrp.usp.br.
José María Gutiérrez, Email: jose.gutierrez@ucr.ac.cr.
Bruno Lomonte, Email: bruno.lomonte@ucr.ac.cr.
Yara Cury, Email: yara.cury@butantan.gov.br.
Gisele Picolo, Phone: +55 11 2627-9897, Email: gisele.picolo@butantan.gov.br.
References
- 1.Centers for Disease Control and Prevention (CDC) Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation--United States, 2010–2012. Morb Mortal Wkly Rep. 2013;62(44):869–73. [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Arthritis Improving the Quality of Life for People With Arthritis. At a glance 2016. Natl Cent Chronic Dis Prev Heal Promot. GA, USA. 2016. https://www.cdc.gov/chronicdisease/resources/publications/aag/pdf/2016/aag-arthritis.pdf.
- 3.Niedermeier M, Pap T, Korb A. Therapeutic opportunities in fibroblasts in inflammatory arthritis. Best Pract Res Clin Rheumatol. 2010;24(4):527–40. doi: 10.1016/j.berh.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Choi SI, Brahn E. Rheumatoid arthritis therapy: advances from bench to bedside. Autoimmunity. 2010;43(7):478–92. doi: 10.3109/08916931003674717. [DOI] [PubMed] [Google Scholar]
- 5.Rahmati M, Mobasheri A, Mozafari M. Inflammatory mediators in osteoarthritis: A critical review of the state-of-the-art, current prospects, and future challenges. Bone. 2016;85:81–90. doi: 10.1016/j.bone.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Murakami M, Taketomi Y, Sato H, Yamamoto K. Secreted phospholipase A2 revisited. J Biochem. 2011;150(3):233–55. doi: 10.1093/jb/mvr088. [DOI] [PubMed] [Google Scholar]
- 7.Mouchlis VD, Dennis EA. Membrane and inhibitor interactions of intracellular phospholipases A2. Adv Biol Regul. 2016;61:17–24. doi: 10.1016/j.jbior.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hegen M, Sun L, Uozumi N, Kume K, Goad ME, Nickerson-Nutter CL, et al. Cytosolic Phospholipase A2α-deficient Mice Are Resistant to Collagen-induced Arthritis. J Exp Med. 2003;197(10):1297–302. doi: 10.1084/jem.20030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leistad L, Feuerherm AJ, Ostensen M, Faxvaag A, Johansen B. Presence of secretory group IIa and V phospholipase A2 and cytosolic group IVα phospholipase A2 in chondrocytes from patients with rheumatoid arthritis. Clin Chem Lab Med. 2004;42(6):602–10. doi: 10.1515/CCLM.2004.104. [DOI] [PubMed] [Google Scholar]
- 10.Tai N, Kuwabara K, Kobayashi M, Yamada K, Ono T, Seno K, et al. Cytosolic phospholipase A2 alpha inhibitor, pyrroxyphene, displays anti-arthritic and anti-bone destructive action in a murine arthritis model. Inflamm Res. 2010;59(1):53–62. doi: 10.1007/s00011-009-0069-8. [DOI] [PubMed] [Google Scholar]
- 11.Kumar LD, Karthik R, Gayathri N, Sivasudha T. Advancement in contemporary diagnostic and therapeutic approaches for rheumatoid arthritis. Biomed Pharmacother. 2016;79:52–61. doi: 10.1016/j.biopha.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Gavrilă BI, Ciofu C, Stoica V. Biomarkers in Rheumatoid Arthritis, what is new? J Med Life. 2016;9(2):144–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss PF. Update on enthesitis-related arthritis. Curr Opin Rheumatol. 2016;28(5):530–6. doi: 10.1097/BOR.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 14.Welker S, Markert Y, Koditz J, Mansfeld J, Ulbrich-Hofmann R. Disulfide bonds of phospholipase A2 from bee venom yield discrete contributions to its conformational stability. Biochimie. 2011;93(2):195–201. doi: 10.1016/j.biochi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: Classification and characterization. Biochim Biophys Acta. 2000;1488(1–2):1–19. doi: 10.1016/S1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 16.Huang TF, Chiang HS. Effect on human platelet aggregation of phospholipase A2 purified from Heloderma horridum (beaded lizard) venom. Biochim Biophys Acta. 1994;1211(1):61–8. doi: 10.1016/0005-2760(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 17.Mansfeld J. Plant phospholipases A2: perspectives on biotechnological applications. Biotechnol Lett. 2009;31(9):1373–80. doi: 10.1007/s10529-009-0034-1. [DOI] [PubMed] [Google Scholar]
- 18.Santos LD, Pieroni M, Menegasso ARS, Pinto JRAS, Palma MS. A new scenario of bioprospecting of Hymenoptera venoms through proteomic approach. J Venom Anim Toxins incl Trop Dis. 2011;17(3):364–77. [Google Scholar]
- 19.Ramírez-Avila J, Quevedo BE, López E, Renjifo JM. Purification and partial characterization of phospholipases A2 from Bothrops asper (barba amarilla) snake venom from Chiriguaná (Cesar, Colombia) J Venom Anim Toxins incl Trop Dis. 2004;10(3):242–59. doi: 10.1590/S1678-91992004000300005. [DOI] [Google Scholar]
- 20.Angulo Y, Lomonte B. Biochemistry and toxicology of toxins purified from the venom of the snake Bothrops asper. Toxicon. 2009;54(7):949–57. doi: 10.1016/j.toxicon.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Lomonte B, Carmona E. Individual expression patterns of myotoxin isoforms in the venom of the snake Bothrops asper. Comp Biochem Physiol B. 1992;102:325–9. doi: 10.1016/0305-0491(92)90129-f. [DOI] [PubMed] [Google Scholar]
- 22.Gutiérrez JM, Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon. 1995;33(11):1405–24. doi: 10.1016/0041-0101(95)00085-Z. [DOI] [PubMed] [Google Scholar]
- 23.Teixeira C, Cury Y, Moreira V, Picolo G, Chaves F. Inflammation induced by Bothrops asper venom. Toxicon. 2009;54(1):67–76. doi: 10.1016/j.toxicon.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Zuliani JP, Fernandes CM, Zamuner SR, Gutiérrez JM, Teixeira CF. Inflammatory events induced by Lys-49 and Asp-49 phospholipases A2 isolated from Bothrops asper snake venom: role of catalytic activity. Toxicon. 2005;45(3):335–46. doi: 10.1016/j.toxicon.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Moreira V, Gutiérrez JM, Soares AM, Zamunér SR, Purgatto E, Teixeira CF. Secretory phospholipases A2 isolated from Bothrops asper and from Crotalus durissus terrificus snake venoms induce distinct mechanisms for biosynthesis of prostaglandins E2 and D2 and expression of cyclooxygenases. Toxicon. 2008;52(3):428–39. doi: 10.1016/j.toxicon.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Moreira V, de Castro Souto PC, Ramirez Vinolo MA, Lomonte B, Gutiérrez JM, Curi R, et al. A catalytically-inactive snake venom Lys49 phospholipase A2 homolog induces expression of cyclooxygenase-2 and production of prostaglandins through selected signaling pathways in macrophages. Eur J Pharmacol. 2013;708(1–3):68–79. doi: 10.1016/j.ejphar.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 27.Teixeira CF, Landucci EC, Antunes E, Chacur M, Cury Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon. 2003;42(8):947–62. doi: 10.1016/j.toxicon.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Chacur M, Longo I, Picolo G, Gutiérrez JM, Lomonte B, Guerra JL, et al. Hyperalgesia induced by Asp49 and Lys49 phospholipases A2 from Bothrops asper snake venom: pharmacological mediation and molecular determinants. Toxicon. 2003;41(6):667–78. doi: 10.1016/S0041-0101(03)00007-2. [DOI] [PubMed] [Google Scholar]
- 29.Chacur M, Milligan ED, Sloan EM, Wieseler-Frank J, Barrientos RM, Martin D, et al. Snake venom phospholipase A2s (Asp49 and Lys49) induce mechanical allodynia upon peri-sciatic administration: involvement of spinal cord glia, proinflammatory cytokines and nitric oxide. Pain. 2004;108(1–2):180–91. doi: 10.1016/j.pain.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Lomonte B, Gutiérrez JM. A new muscle damaging toxin, myotoxin II, from the venom of the snake Bothrops asper (terciopelo) Toxicon. 1989;27(7):725–33. doi: 10.1016/0041-0101(89)90039-1. [DOI] [PubMed] [Google Scholar]
- 31.Tonussi CR, Ferreira SH. Rat knee-joint carrageenin incapacitation test: an objective screen for central and peripheral analgesics. Pain. 1992;48(3):421–7. doi: 10.1016/0304-3959(92)90095-S. [DOI] [PubMed] [Google Scholar]
- 32.Ekundi-Valentim E, Santos KT, Camargo EA, Denadai-Souza A, Teixeira SA, Zanoni CI, et al. Differing effects of exogenous and endogenous hydrogen sulphide in carrageenan-induced knee joint synovitis in the rat. Br J Pharmacol. 2010;159(7):1463–74. doi: 10.1111/j.1476-5381.2010.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerrero AT, Verri WA, Cunha TM, Silva TA, Schivo IR, Dal-Secco D, et al. Involvement of LTB4 in zymosan-induced joint nociception in mice: participation of neutrophils and PGE2. J Leukoc Biol. 2008;83(1):122–30. doi: 10.1189/jlb.0207123. [DOI] [PubMed] [Google Scholar]
- 34.Guerrero AT, Verri WA, Jr, Cunha TM, Silva TA, Rocha FA, Ferreira SH, et al. Hypernociception elicited by tibio-tarsal joint flexion in mice: a novel experimental arthritis model for pharmacological screening. Pharmacol Biochem Behav. 2006;84(2):244–51. doi: 10.1016/j.pbb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Freysdottir J, Hardardottir I, Gizurarson S, Vikingsson A. Mucosal tolerance to KLH reduces BSA-induced arthritis in rats--an indication of bystander suppression. J Clin Immunol. 2007;27(3):284–93. doi: 10.1007/s10875-007-9081-3. [DOI] [PubMed] [Google Scholar]
- 36.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78(3):206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 37.Sampaio SC, Brigatte P, Sousa-e-Silva MC, dos-Santos EC, Rangel-Santos AC, Curi R, et al. Contribution of crotoxin for the inhibitory effect of Crotalus durissus terrificus snake venom on macrophage function. Toxicon. 2003;41(7):899–907. doi: 10.1016/S0041-0101(03)00069-2. [DOI] [PubMed] [Google Scholar]
- 38.Sampaio SC, Santos MF, Costa EP, Rangel-Santos AC, Carneiro SM, Curi R, et al. Crotoxin induces actin reorganization and inhibits tyrosine phosphorylation and activity of small GTPases in rat macrophages. Toxicon. 2006;47(8):909–19. doi: 10.1016/j.toxicon.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Rosenfeld G. Método rápido de coloração de esfregaços de sangue. Noções práticas sobre corantes pancrômicos e estudos de diversos fatores. Mem Inst Butantan. 1947;20:315–28. [Google Scholar]
- 40.Lam FY, Ferrell WR. Inhibition of carrageenan induced inflammation in the rat knee joint by substance P antagonist. Ann Rheum Dis. 1989;48(11):928–32. doi: 10.1136/ard.48.11.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zambelli VO, Sampaio SC, Sudo-Hayashi LS, Greco K, Britto LR, Alves AS, et al. Crotoxin alters lymphocyte distribution in rats: Involvement of adhesion molecules and lipoxygenase-derived mediators. Toxicon. 2008;51(8):1357–67. doi: 10.1016/j.toxicon.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto T, Nozaki-Taguchi N. The role of cyclooxygenase-1 and −2 in the rat formalin test. Anesth Analg. 2002;94(4):962–7. doi: 10.1097/00000539-200204000-00035. [DOI] [PubMed] [Google Scholar]
- 43.Lucas KK, Svensson CI, Hua XY, Yaksh TL, Dennis EA. Spinal phospholipase A2 in inflammatory hyperalgesia: role of group IVA cPLA2. Br J Pharmacol. 2005;144(7):940–52. doi: 10.1038/sj.bjp.0706116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaeffer EL, Gattaz WF. Requirement of hippocampal phospholipase A2 activity for long-term memory retrieval in rats. J Neural Transm (Vienna) 2007;114(3):379–85. doi: 10.1007/s00702-006-0585-4. [DOI] [PubMed] [Google Scholar]
- 45.Cruwys SC, Garrett NE, Perkins MN, Blake DR, Kidd BL. The role of bradykinin B1 receptors in the maintenance of intra-articular plasma extravasation in chronic antigen-induced arthritis. Br J Pharmacol. 1994;113(3):940–4. doi: 10.1111/j.1476-5381.1994.tb17083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daher JB, Souza GE, D’Orléans-Juste P, Rae GA. Endothelin ETB receptors inhibit articular nociception and priming induced by carrageenan in the rat knee-joint. Eur J Pharmacol. 2004;496(1–3):77–85. doi: 10.1016/j.ejphar.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 47.van de Loo FAJ, Bennink MB, Arntz OJ, Smeets RL, Lubberts E, Joosten LAB, et al. Deficiency of NADPH Oxidase Components p47phox and gp91phox Caused Granulomatous Synovitis and Increased Connective Tissue Destruction in Experimental Arthritis Models. Am J Pathol. 2003;163(4):1525–37. doi: 10.1016/S0002-9440(10)63509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Picolo G, Cury Y. Peripheral neuronal nitric oxide synthase activity mediates the antinociceptive effect of Crotalus durissus terrificus snake venom, a δ- and κ-opioid receptor agonist. Life Sci. 2004;75(5):559–73. doi: 10.1016/j.lfs.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 49.Gad SC, Weil CS. Statistics for toxicologists. Principles and Methods of Toxicology. Edited by A. Wallace Hayes. 2nd ed. New York: Raven Press; 1989. p. 435–83.
- 50.Correa D, Lietman SA. Articular cartilage repair: current needs, methods and research directions. Semin Cell Dev Biol. 2016;S1084-9521(16):30208–7. doi: 10.1016/j.semcdb.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 51.Dekkers JS, Schoones JW, Huizinga TW, Toes RE, van der Helm-van Mil AH. Possibilities for preventive treatment in rheumatoid arthritis? Lessons from experimental animal models of arthritis: a systematic literature review and meta-analysis. Ann Rheum Dis. doi: 10.1136/annrheumdis-2016-209830 [DOI] [PubMed]
- 52.Giannotti KC, Leiguez E, Moreira V, Nascimento NG, Lomonte B, Gutiérrez JM, et al. A Lys49 phospholipase A2, isolated from Bothrops asper snake venom, induces lipid droplet formation in macrophages which depends on distinct signaling pathways and the C-terminal region. Biomed Res Int. 2013;2013:807982. doi: 10.1155/2013/807982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moreira V, Gutiérrez JM, Amaral RB, Lomonte B, Purgatto E, Teixeira C. A phospholipase A2 from Bothrops asper snake venom activates neutrophils in culture: Expression of cyclooxygenase-2 and PGE2 biosynthesis. Toxicon. 2011;57(2):288–96. doi: 10.1016/j.toxicon.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Chaves F, León G, Alvarado VH, Gutiérrez JM. Pharmacological modulation of edema induced by Lys-49 and Asp-49 myotoxic phospholipases A2 isolated from the venom of the snake Bothrops asper (terciopelo) Toxicon. 1998;36(12):1861–9. doi: 10.1016/S0041-0101(98)00107-X. [DOI] [PubMed] [Google Scholar]
- 55.Zuliani JP, Gutiérrez JM, Silva LL C e, Sampaio SC, Lomonte B, Pereira Teixeira CF. Activation of cellular functions in macrophages by venom secretory Asp-49 and Lys-49 phospholipases A2. Toxicon. 2005;46(5):523–32. doi: 10.1016/j.toxicon.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 56.Yang Y, Leech M, Hutchinson P, Holdsworth SR, Morand EF. Antiinflammatory effect of lipocortin 1 in experimental arthritis. Inflammation. 1997;21(6):583–96. doi: 10.1023/A:1027330021479. [DOI] [PubMed] [Google Scholar]
- 57.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–56. [PMC free article] [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention (CDC) Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation --- United States, 2007–2009. Morb Mortal Wkly Rep. 2010;59(39):1261–5. [PubMed] [Google Scholar]
- 59.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272(5258):60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 60.Ley K, Bullard DC, Arbonés ML, Bosse R, Vestweber D, Tedder TF, et al. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med. 1995;181(2):669–75. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74(3):541–54. doi: 10.1016/0092-8674(93)80055-J. [DOI] [PubMed] [Google Scholar]
- 62.Angulo Y, Lomonte B. Inhibitory effect of fucoidan on the activities of crotaline snake venom myotoxic phospholipases A2. Biochem Pharmacol. 2003;66(10):1993–2000. doi: 10.1016/S0006-2952(03)00579-3. [DOI] [PubMed] [Google Scholar]
- 63.Azofeifa K, Angulo Y, Lomonte B. Ability of fucoidan to prevent muscle necrosis induced by snake venom myotoxins: comparison of high- and low-molecular weight fractions. Toxicon. 2008;51(3):373–80. doi: 10.1016/j.toxicon.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Wijewickrama GT, Kim JH, Kim YJ, Abraham A, Oh Y, Ananthanarayanan B, et al. Systematic evaluation of transcellular activities of secretory phospholipases A2: High activity of group V phospholipases A2 to induce eicosanoid biosynthesis in neighboring inflammatory cells. J Biol Chem. 2006;281(16):10935–44. doi: 10.1074/jbc.M512657200. [DOI] [PubMed] [Google Scholar]
- 65.Chakraborti S. Phospholipase A2 isoforms: A perspective. Cell Signal. 2003;15(7):637–65. doi: 10.1016/S0898-6568(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 66.Dray A, Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993;16(3):99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- 67.Petho G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699–775. doi: 10.1152/physrev.00048.2010. [DOI] [PubMed] [Google Scholar]
- 68.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107(3):660–4. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cunha FQ, Lorenzetti BB, Poole S, Ferreira SH. Interleukin-8 as a mediator of sympathetic pain. Br J Pharmacol. 1991;104(3):765–7. doi: 10.1111/j.1476-5381.1991.tb12502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334(6184):698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- 71.Nakamura M, Ferreira SH. A peripheral sympathetic component in inflammatory hyperalgesia. Eur J Pharmacol. 1987;135(2):145–53. doi: 10.1016/0014-2999(87)90606-6. [DOI] [PubMed] [Google Scholar]
- 72.Poole S, Lorenzetti BB, Cunha JM, Cunha FQ, Ferreira SH. Bradykinin B1 and B2 receptors, tumour necrosis factor alpha and inflammatory hyperalgesia. Br J Pharmacol. 1999;126(3):649–56. doi: 10.1038/sj.bjp.0702347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramonda R, Oliviero F, Galozzi P, Frallonardo P, Lorenzin M, Ortolan A, et al. Molecular mechanisms of pain in crystal-induced arthritis. Best Pract Res Clin Rheumatol. 2015;29(1):98–110. doi: 10.1016/j.berh.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 74.De Falco L, Fioravanti A, Galeazzi M, Tenti S. Bradykinin and its role in osteoarthritis. Reumatismo. 2013;65(3):97–104. doi: 10.4081/reumatismo.2013.97. [DOI] [PubMed] [Google Scholar]
- 75.Paterson KJ, Zambreanu L, Bennett DLH, McMahon SB. Characterisation and mechanisms of bradykinin-evoked pain in man using iontophoresis. Pain. 2013;154(6):782–92. doi: 10.1016/j.pain.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Landucci EC, Toyama M, Marangoni S, Oliveira B, Cirino G, Antunes E, et al. Effect of crotapotin and heparin on the rat paw oedema induced by different secretory phospholipases A2. Toxicon. 2000;38(2):199–208. doi: 10.1016/S0041-0101(99)00143-9. [DOI] [PubMed] [Google Scholar]
- 77.Landucci EC, Castro RC, Pereira MF, Cintra AC, Giglio JR, Marangoni S, et al. Mast cell degranulation induced by two phospholipase A2 homologues: Dissociation between enzymatic and biological activities. Eur J Pharmacol. 1998;343(2–3):257–63. doi: 10.1016/S0014-2999(97)01546-X. [DOI] [PubMed] [Google Scholar]
- 78.Guzzo ML, Farsky SH, De Nucci G, Antunes E, Silva MA, Mello SB. Role of kinins and nitric oxide on the rabbit arthritis induced by Bothrops jararaca venom. Toxicon. 2000;38(11):1535–46. doi: 10.1016/S0041-0101(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 79.Ahlawat A, Rana A, Goyal N, Sharma S. Potential role of nitric oxide synthase isoforms in pathophysiology of neuropathic pain. Inflammopharmacology. 2014;22(5):269–78. doi: 10.1007/s10787-014-0213-0. [DOI] [PubMed] [Google Scholar]
- 80.Lee AS, Ellman MB, Yan D, Kroin JS, Cole BJ, van Wijnen AJ, et al. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527(2):440–7. doi: 10.1016/j.gene.2013.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rose BJ, Kooyman DL. A Tale of Two Joints : The Role of Matrix Metalloproteases in Cartilage Biology. Dis Markers. 2016;2016:4895050. doi: 10.1155/2016/4895050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thompson C, Davies R, Choy E. Anti cytokine therapy in chronic inflammatory arthritis. Cytokine. 2016;86:92–9. doi: 10.1016/j.cyto.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 83.Guidelli GM, Barskova T, Brizi MG, Lepri G, Parma A, Talarico R, et al. One year in review: novelties in the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2015;33(1):102–8. [PubMed] [Google Scholar]
- 84.Mifflin KA, Kerr BJ. Pain in autoimmune disorders. J Neurosci Res. 2016 doi: 10.1002/jnr.23844. [DOI] [PubMed] [Google Scholar]
- 85.Cunha JM, Cunha FQ, Poole S, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br J Pharmacol. 2000;130(6):1418–24. doi: 10.1038/sj.bjp.0703434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferreira SH, Lorenzetti BB, Poole S. Bradykinin initiates cytokine-mediated inflammatory hyperalgesia. Br J Pharmacol. 1993;110(3):1227–31. doi: 10.1111/j.1476-5381.1993.tb13946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zamuner SR, Zuliani JP, Fernandes CM, Gutiérrez JM, Pereira Teixeira CF. Inflammation induced by Bothrops asper venom: release of proinflammatory cytokines and eicosanoids, and role of adhesion molecules in leukocyte infiltration. Toxicon. 2005;46(7):806–13. doi: 10.1016/j.toxicon.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 88.Lomonte B, Tarkowski A, Hanson LA. Host response to Bothrops asper snake venom: Analysis of edema formation, inflammatory cells, and cytokine release in a mouse model. Inflammation. 1993;17(2):93–105. doi: 10.1007/BF00916097. [DOI] [PubMed] [Google Scholar]
- 89.Zarpelon AC, Cunha TM, Alves-Filho JC, Pinto LG, Ferreira SH, McInnes IB, et al. IL-33/ST2 signalling contributes to carrageenin-induced innate inflammation and inflammatory pain: role of cytokines, endothelin-1 and prostaglandin E2. Br J Pharmacol. 2013;169(1):90–101. doi: 10.1111/bph.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith TP, Haymond T, Smith SN, Sweitzer SM. Evidence for the endothelin system as an emerging therapeutic target for the treatment of chronic pain. J Pain Res. 2014;7:531–45. doi: 10.2147/JPR.S65923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Forner S, Martini AC, de Andrade EL, Rae GA. Neuropathic pain induced by spinal cord injury: Role of endothelin ETA and ETB receptors. Neurosci Lett. 2016;617:14–21. doi: 10.1016/j.neulet.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 92.Sin A, Tang W, Wen CY, Chung SK, Chiu KY. The emerging role of endothelin-1 in the pathogenesis of subchondral bone disturbance and osteoarthritis. Osteoarthritis Cartilage. 2015;23(4):516–24. doi: 10.1016/j.joca.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 93.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


