Abstract
Background
Deinagkistrodon acutus envenomation is associated with severe hematological and wound complications but is rarely described.
Case presentation
Herein, we report three cases of victims bitten by D. acutus and indicate that rapid-onset severe coagulopathy and thrombocytopenia are distinct features of D. acutus snakebite, which are not observed in other crotaline snakebites (i.e., Trimeresurus stejnegeri and Protobothrops mucrosquamatus) in Taiwan. The toxic effects could occur as early as 2 to 3 h following D. acutus envenomation and persist if the administration of specific antivenom is delayed or even not commenced. Based on our findings, 2 to 4 vials of specific antivenom as the first dose should be administered to victims and repeated at 6 to 8 h intervals if coagulopathy or thrombocytopenia persists. Fresh frozen plasma or platelet replacement is probably safe as an adjunct therapy for D. acutus bite in the presence of venom-induced consumptive coagulopathy.
Conclusion
Severe coagulopathy and thrombocytopenia could occur as early as 2 to 3 h after D. acutus envenomation. The current recommendation for antivenom is 2 to 4 vials as the first dose and repeated every 6– to 8 h if coagulopathy or thrombocytopenia persists. These cases studied may be helpful to first-line medical personnel in the early diagnosis and management of D. acutus envenomation among other crotaline snakebites in Taiwan.
Keywords: Coagulopathy, Thrombocytopenia, Envenomation, Deinagkistrodon acutus, Snakebite
Background
Envenomation caused by snakebite comprises a worldwide public health problem, especially in Asia [1]. The venoms of snakes are a fascinating mix that allow the design of new drugs for use in medicine, as well as being a challenge for researchers in the development of specific antivenoms [2–5]. In Taiwan, six major venomous snakes are found, namely: Trimeresurus stejnegeri, Protobothrops mucrosquamatus, Deinagkistrodon acutus and Daboia siamensis in the family Viperidae, and Naja atra and Bungarus multicinctus in the family Elapidae [6]. D. acutus – also known as the hundred pacer, five pacer or Chinese moccasin – is the largest snake (80–155 cm) of the subfamily Crotalinae on the island [6]. It is additionally distributed throughout south China, Vietnam and possibly Laos [7]. D. acutus envenomation is rare; however, it is considered the most lethal and can result in life- or limb-threatening complications after envenomation [6, 8]. Although severe coagulopathy and thrombocytopenia, defined as an international normalized ratio (INR) of prothrombin time (PT) > 9 and platelet count < 50,000/mm3, are considered the hallmarks of D. acutus envenomation, little is known about the timing of onset and treatment with specific antivenom [9–13]. In the present study, we summarize the clinical manifestations and treatment of three cases of D. acutus envenomation admitted to Taichung Veterans General Hospital (VGH-TC) with the aim of improving the diagnosis and management of D. acutus envenomation.
Case Presentation
Case 1
A 36-year-old previously healthy man was bitten on his right hand by a snake during cleaning work around his home. He was immediately sent to a local hospital, with the dead snake identified as D. acutus. However, only two vials of antivenom for T. stejnegeri and P. mucrosquamatus were administered, because specific antivenom for D. acutus was not available and because the treating physician believed that cross-neutralization would occur. He received right upper limb fasciotomy on day 2 for suspected compartment syndrome. On day 3, he was transferred to VGH-TC due to worsening of his general condition and bleeding tendency. On arrival, the patient’s blood pressure (BP) was 109/47 mmHg, pulse 119/min, respiratory rate 20/min, and body temperature 38.5 °C.
Physical examination revealed continuous oozing from the wound and venous catheter insertion site, multiple hemorrhagic bullae, swelling extending up to the shoulder and gross hematuria in the urinary bag. Laboratory examination revealed a hemoglobin level of 5.8 g/dL (reference range 14–18 g/dL), platelet count 17,000/mm3 (reference range 150,000–400,000/mm3), fibrinogen 130 mg/dL (reference range 200–400 mg/dL), D-dimer > 1 μg/mL (reference range < 0.55 μg/mL), fibrinogen degradation products (FDPs) > 40 μg/mL (reference range < 10 μg/mL), and incoagulable blood [PT > 169 s; activated partial thromboplastin time (aPTT) > 224 s] (Table 1).
Table 1.
Initial blood laboratory data of the three patients at Taichung Veterans General Hospital
| Laboratory data (On arrival, day 1) | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| White blood cell count (4,500–11,000/mm3) | 4,700 | 12,300 | 9,300 |
| Differential count (%) | |||
| Neutrophil | 76.9 | 84.7 | 44 |
| Lymphocyte | 15.2 | 7.6 | 45.2 |
| Hemoglobin (male: 14–18, female 12–16 g/dL) | 5.8 | 13.1 | 15.4 |
| Platelet count (150,000–400,000/mm3) | 17,000 | 14,000 | 17,000 |
| Sodium (mEq/L) | 136 | 142 | 144 |
| Potassium (mEq/L) | 4.0 | 4.0 | 3.7 |
| Blood urea nitrogen (5–25 mg/dL) | 12 | 14 | 24 |
| Creatinine (0.7–1.4 mg/dL) | 0.7 | 1.4 | 0.9 |
| Alanine aminotransferase (male: 10–50, female: 10–35 U/L) | 28 | 26 | 21 |
| Aspartate aminotransferase (8–38 U/L) | 30 | 43 | 26 |
| Total bilirubin (0.2–1.6 mg/dL) | 1.3 | 0.8 | – |
| Direct bilirubin (0–0.3 mg/dL) | 0 | 0.2 | – |
| Lactate dehydrogenase (120–240 U/L) | 206 | 181 | 242 |
| Creatine kinase (10–160 U/L) | 855 | 745 | 155 |
| Prothrombin time (seconds) | >169 | >164 | >164 |
| Activated partial thromboplastin time (s) | >224 | >224 | >224 |
| Fibrinogen (200–400 mg/dL) | 130 | – | 149.1 |
| D-dimer (<0.55 μg/mL) | >1 | – | – |
| Fibrinogen degradation products (FDPs < 10 μg/mL) | >40 | – | >40 |
Three vials of monovalent antivenom for D. acutus and blood components [600 mL of packed red blood cells, 600 mL of fresh frozen plasma (FFP), and 300 mL of platelet concentrate] were immediately administered, resulting in a good response (Fig. 1). The patient’s blood coagulation normalized at 6 h after administration of the antivenom and oozing from the wound stopped. A follow-up coagulation profile did not reveal recurrent coagulopathy on days 4, 10 and 13. He had intermittent fever, and extensive wound necrosis that required repetitive debridement on days 8, 12 and 17 during hospitalization. Deep tissue cultures obtained during surgery grew Pseudomonas aeruginosa, Morganella morganii, Staphylococcus aureus and Enterococcus spp. Although thrombocytopenia persisted throughout days 4 to 10 (22,000–116,000/mm3), it was amenable to platelet transfusion and medical treatment. The bite wound improved gradually after antibiotic therapy and debridement. Staged wound closure was performed 3 weeks post-bite, and he was transferred to another hospital for a rehabilitation program 1 month later.
Fig. 1.
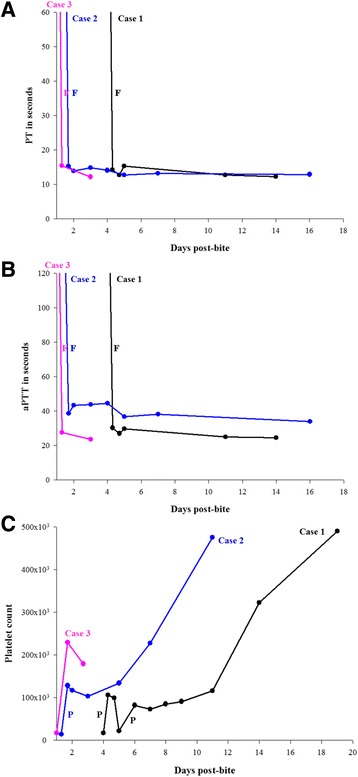
a Trend of PT in three patients. b Trend of aPTT in three patients. c Trend of platelet level in three patients. F: fresh frozen plasma transfusion. P: platelet concentrate transfusion
Case 2
A 41-year-old previously healthy woman was bitten on the left ankle by a snake while she collected herbs in northwestern Taiwan. She was sent to a local hospital 3 h later, where thrombocytopenia (3000/mm3) and incoagulable blood (PT > 100 s) were noted. The snake was identified as D. acutus by the patient through a picture; however, two vials of antivenom for T. stejnegeri and P. mucrosquamatus were administered for an unknown reason. She was then referred to VGH-TC 7.5 h post-bite.
On arrival, her BP was 172/94 mmHg, pulse 108/min, respiratory rate 20/min, and body temperature 39.3 °C. Physical examination revealed many hemorrhagic bullae scattered along the calf, continuous oozing from the fang marks, and painful swelling extending to the knee region. Laboratory examination disclosed thrombocytopenia (14,000/mm3) and PT of > 169 s and aPTT of > 224 s. Four vials of monovalent antivenom for D. acutus and blood components (200 mL of FFP and 300 mL of platelet concentrate) were administered at 8.5 h post-bite. Her coagulation profiles normalized at 15 h post-bite. On day 2, her platelet count increased to 158,000/mm3. Due to prolonged aPTT (43.3–44.6 s), another seven vials of antivenom (2 to 3 vials every 6 to 8 h) were sequentially administered without measurable responses.
The leg wound was complicated with necrotizing fasciitis that required repetitive debridement on days 21 and 31 and supplemental hyperbaric oxygen therapy. The wound cultures obtained during surgery revealed S. aureus, Enterococcus spp. and Bacteroides fragilis. After antibiotic treatment, her wound infection improved and split-thickness skin grafting was performed after debridement on day 31. The patient was discharged on day 47 post-bite with good functional recovery of the leg.
Case 3
A 69-year-old previously healthy woman was bitten on the left middle finger by a snake while collecting firewood. Painful swelling, tissue ecchymosis, and oozing from the wound developed a few minutes later. The patient was sent to VGH-TC 2 h post-bite. The dead snake brought in by the patient was identified as D. acutus. On arrival, her BP was 200/120 mmHg, pulse 95/min, respiratory rate 22/min, and body temperature 36.7 °C. Physical examination revealed swelling of left hand. A low platelet count (17,000/mm3), PT > 169 s, aPTT > 224 s, fibrinogen level 149.1 mg/dL and FDPs > 40 μg/mL were noted in laboratory analyses.
Four vials of monovalent antivenom for D. acutus and 1000 mL of FFP were administered. Seven hours post-bite, both PT and aPTT normalized, and the platelet count increased to 230,000/mm3. Because of recurrent oozing from the wound, another four vials of antivenom (two at 12 h intervals) were administered without examination of PT and aPTT levels in the following 24 h. Although the surgeon recommended partial finger amputation due to finger necrosis, the patient declined and insisted on being discharged against medical advice on day 5. No recurrent coagulopathy was noted prior to discharge; however, the patient did not return for follow-up.
Discussion
T. stejnegeri and P. mucrosquamatus account for more than 70% of snakebite cases each year in Taiwan, which share similar clinical manifestations as well as the same treatment with bivalent specific antivenom [6, 8]. Sporadic cases with INR above 1.67 and platelet counts of 36,000/mm3 were reported in P. mucrosquamatus envenomation, and none manifested systemic bleeding [11, 14]. In contrast, severe coagulopathy and thrombocytopenia are the main laboratory findings of D. acutus envenomation in addition to serious wound complications and systemic bleeding [11–13]. Valenta et al. [15] reported a victim of D. acutus bite who developed incoagulable blood between 1.5 and 7 h post-bite. Hung et al. [13] reported the case of a man with persistent thrombocytopenia 44 h after envenomation; his platelet level was normal 30 min post-bite. In our observation, rapid-onset and severe coagulopathy and thrombocytopenia developed as early as 2 to 3 h post-bite may persist if correct antivenom is not administered.
D. acutus venom is composed of several hemotoxins, including the thrombin-like enzymes (TLEs), anticoagulant toxins, platelet aggregation inhibitors, hemorrhagins, and enzymes that facilitate venom spreading [16–19]. The anticoagulation effect of TLEs occurs rapidly, and circulating fibrinogen levels start to fall within 30 min, reaching 9% of the normal value within 2 h [16]. Anticoagulant toxins inactivate prothrombin, tissue factor and coagulation factors V and IX/X, resulting in a transient but marked prolongation of blood coagulation within 5 min after injection [16, 20, 21]. The platelet inhibitors, mainly adenosine diphosphatase, inhibit platelet aggregation in the presence of adenosine diphosphate or collagen [17]. The hemorrhagins (e.g., snake venom metalloproteinases) cause extensive vascular damage and increase vascular permeability [22]. These effects may explain the remarkable bleeding tendency and wound complications in D. acutus envenomation.
The Taiwan government produces four types of antivenom against the six major venomous snakebites [8]. Concerning the monovalent antivenom for D. acutus, each vial roughly neutralizes 52 mg of venom. The Taiwan Poison Control Center thus recommends that 2 to 4 vials of antivenom be administered in a envenomed patient because the mean amount of D. acutus venom injected is 105.1 mg [8]. However, this recommendation was not validated and there is no standard dosing regimen available in Taiwan. According to our findings, the current recommendation is 2 to 4 vials of antivenom to be administered as the first dose and repeated at 6 to 8 h intervals if coagulopathy or thrombocytopenia persists (e.g., INR > 3, aPTT > 50s, and platelet level < 20,000/mm3) [23, 24]. Although cross-neutralization occurs between bivalent antivenom for T. stejnegeri and P. mucrosquamatus and monovalent antivenom for D. acutus, substitution should be avoided as the bivalent antivenom has only a weak cross-reactivity with the D. acutus venom [13].
All of our cases had a rapid improvement in coagulopathy after receiving specific antivenom. In case 2, s slightly prolonged aPTT persisted for 4 days without clinical thrombosis or bleeding tendency. Although a workup for prolonged aPTT was not performed in the case, it was probably unrelated to the venom because no apparent response was observed after repetitive antivenom administration [25]. In case 1, thrombocytopenia did not show improvement after antivenom therapy, which was probably attributable to uncontrolled infection. Nevertheless, Valenta et al. [15] reported a single case of D. acutus envenomation who manifested severe coagulopathy in the absence of thrombocytopenia. Therefore, the exact mechanism of thrombocytopenia in D. acutus snakebite remains unclear.
Evidence suggests that, when compared with antivenom alone, early FFP replacement therapy shortens coagulopathy in patients suffering from hemotoxic snake envenomation and, theoretically, lower the risk of major bleeding [26]. The bleeding risk may be pronounced in cases of severe thrombocytopenia [10]. However, in cases of venom-induced thrombotic microangiopathy, platelet transfusion may be problematic [27]. In our study, no elevation of blood bilirubin or lactate dehydrogenase was found, and the first two cases had received platelet concentrate transfusions, resulting in good responses. It appears to be safe to use the blood component therapy during D. acutus envenomation. In case 3, the platelet count spontaneously normalized after 24 h with FFP and specific antivenom administration alone. This effect may have occurred because of the relatively modest injury in this case. However, the patient received a higher dosage (eight vials) of antivenom, which was sufficient to counteract the venom effects on platelets.
Conclusion
Severe coagulopathy and thrombocytopenia could occur as early as 2 to 3 h after D. acutus envenomation. The current recommendation for antivenom therapy is 2 to 4 vials as the first dose and repeat it every 6 to 8 h if coagulopathy or thrombocytopenia persists. Our observation may be helpful to first-line medical personnel in the timely diagnosis and management of D. acutus bites.
Acknowledgments
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Funding
Not applicable.
Authors’ contributions
The first two authors, CLC and YCM interpreted the clinical findings and drafted the manuscript. The third to the fifth authors, PYL, LCC, and SCL provided professional opinions in bacteriology of snakebite and snake venomics and antivenomics, and revised the manuscript. The correspondent author CCY designed this study, interpreted the clinical findings and revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patients for publication of this case report.
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of Taichung Veterans General Hospital (IRB, CE14202A).
Abbreviations
- aPTT
activated partial thromboplastin time
- BP
Blood pressure
- FDPs
Fibrinogen degradation products
- FFP
Fresh frozen plasma
- INR
International normalized ratio
- PT
Prothrombin time
- TLEs
Thrombin-like enzymes
- VGH-TC
Taichung Veterans General Hospital
Contributor Information
Chin-Lung Cheng, Email: tony5720269@gmail.com.
Yan-Chiao Mao, Email: doc1385e@gmail.com.
Po-Yu Liu, Email: idfellow@gmail.com.
Liao-Chun Chiang, Email: axe956956@gmail.com.
Shu-Chen Liao, Email: ermdsusan@gmail.com.
Chen-Chang Yang, Phone: 886 2 2875 7525, Email: ccyang@vghtpe.gov.tw.
References
- 1.World Health Organization (WHO). Snake antivenoms. Fact sheet N° 337. Reviewed February 2015. Retrieved from http://www.who.int/mediacentre/factsheets/fs337/en/. Accessed 23 Oct 2016.
- 2.Wei CB, Chen J, Li JH. Acutolysin C, a weak hemorrhagic toxin from the venom of Agkistrodon acutus with leucoagglutination activity. J Venom Anim Toxins incl Trop Dis. 2011;17(1):34–41. doi: 10.1590/S1678-91992011000100005. [DOI] [Google Scholar]
- 3.Wei CB, Chen J. A novel lipocalin homologue from the venom gland of Deinagkistrodon acutus similar to mammalian lipocalins. J Venom Anim Toxins incl Trop Dis. 2012;18(1):16–23. [Google Scholar]
- 4.Chieh-Fan C, Tzeng-Jih L, Wen-Chi H, Hua-Wei Y. Appropriate antivenom doses for six types of envenomations caused by snakes in Taiwan. J Venom Anim Toxins incl Trop Dis. 2009;15(3):479–90. doi: 10.1590/S1678-91992009000300009. [DOI] [Google Scholar]
- 5.Ratanabanangkoon K, Tan KY, Eursakun S, Tan CH, Simsiriwong P, Pamornsakda T, et al. A simple and novel strategy for the production of a pan-specific antiserum against elapid snakes of Asia. PLoS Negl Trop Dis. 2016;10(4):e0004565. doi: 10.1371/journal.pntd.0004565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao YC, Hung DZ. Epidemiology of snake envenomation in Taiwan. Clinical Toxinology in Asia Pacific and Africa. 2015. p. 3–22. http://link.springer.com/referenceworkentry/10.1007/978-94-007-6386-9_45. Accessed 21 Feb 2017.
- 7.Uetz P, Hošek J. The Reptile Database. Retrieved from http://www.reptile-database.org/. Accessed 8 Dec 2014.
- 8.Mao YC, Hung DZ. Management of snake envenomation in Taiwan. Clinical Toxinology in Asia Pacific and Africa. 2015. p. 23–52. http://link.springer.com/referenceworkentry/10.1007/978-94-007-6386-9_43. Accessed 21 Feb 2017.
- 9.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133(6_Suppl):160S–98. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 10.Williamson DR, Albert M, Heels-Ansdell D, Arnold DM, Lauzier F, Zarychanski R, et al. Thrombocytopenia in critically ill patients receiving thromboprophylaxis: frequency, risk factors, and outcomes. Chest. 2013;144(4):1207–15. doi: 10.1378/chest.13-0121. [DOI] [PubMed] [Google Scholar]
- 11.Shen MC. Afibrinogenemia and thrombocytopenia following crotalid snake bites in Taiwan. J Formos Med Assoc. 1983;82(2):239–44. [PubMed] [Google Scholar]
- 12.Rao DS. Clinical observation of 21 cases of Agkistrodon acutus (Guenther) bite complicated by disseminated intravascular coagulation (author’s transl) Zhonghua Nei Ke Za Zhi. 1981;20(11):670–2. [PubMed] [Google Scholar]
- 13.Hung DZ, Wu TC, Deng JF. The painful experience of inappropriate therapy of snake bites: a report of two cases. Zhonghua Yi Xue Za Zhi (Taipei) 1997;60(6):326–30. [PubMed] [Google Scholar]
- 14.Chen YW, Chen MH, Chen YC, Hung DZ, Chen CK, Yen DHT, et al. Differences in clinical profiles of patients with Protobothrops mucrosquamatus and Viridovipera stejnegeri envenoming in Taiwan. Am J Trop Med Hyg. 2009;80(1):28–32. [PubMed] [Google Scholar]
- 15.Valenta J, Stach Z, Michalek P. Envenoming by Crotalid Snake Chinese Moccasin Agkistrodon Acutus Bite - A Case Report. Prague Med Rep. 2015;116(2):155–60. doi: 10.14712/23362936.2015.53. [DOI] [PubMed] [Google Scholar]
- 16.Ouyang C, Teng CM. In vivo effects of the purified thrombin-like and anticoagulant principles of Agkistrodon acutus (Hundred pace snake) venom. Toxicon. 1978;16(6):583–93. doi: 10.1016/0041-0101(78)90186-1. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang C, Huang TF. Platelet aggregation inhibitors from Agkistrodon acutus snake venom. Toxicon. 1986;24(11–12):1099–106. doi: 10.1016/0041-0101(86)90136-4. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Wang C, Liu J, Lu Z. Purification and characterization of hemorrhagic components from Agkistrodon acutus (hundred pace snake) venom. Toxicon. 1981;19(5):633–44. doi: 10.1016/0041-0101(81)90101-X. [DOI] [PubMed] [Google Scholar]
- 19.Xu X, Wang XS, Xi XT, Liu J, Huang JT, Lu ZX. Purification and partial characterization of hyaluronidase from five pace snake (Agkistrodon acutus) venom. Toxicon. 1982;20(6):973–81. doi: 10.1016/0041-0101(82)90099-X. [DOI] [PubMed] [Google Scholar]
- 20.Ouyang C, Teng CM. The effect of the purified anticoagulant principle of Agkistrodon acutus venom on blood coagulation. Toxicon. 1973;11(3):287–92. doi: 10.1016/0041-0101(73)90057-3. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang C, Teng CM, Huang TF. Characterization of the purified principles of Formosan snake venoms which affect blood coagulation and platelet aggregation. J Formos Med Assoc. 1982;81(7):781–90. [PubMed] [Google Scholar]
- 22.Kamiguti AS. Platelets as targets of snake venom metalloproteinases. Toxicon. 2005;45(8):1041–9. doi: 10.1016/j.toxicon.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med. 2002;347(5):347–56. doi: 10.1056/NEJMra013477. [DOI] [PubMed] [Google Scholar]
- 24.Boyer LV, Seifert SA, Cain JS. Recurrence phenomena after immunoglobulin therapy for snake envenomations: Part 2. Guidelines for clinical management with crotaline Fab antivenom. Ann Emerg Med. 2001;37(2):196–201. doi: 10.1067/mem.2001.113134. [DOI] [PubMed] [Google Scholar]
- 25.Chng WJ, Sum C, Kuperan P. Causes of isolated prolonged activated partial thromboplastin time in an acute care general hospital. Singapore Med J. 2005;46(9):450–6. [PubMed] [Google Scholar]
- 26.Isbister GK, Buckley NA, Page CB, Scorgie FE, Lincz LF, Seldon M, et al. A randomized controlled trial of fresh frozen plasma for treating venom-induced consumption coagulopathy in cases of Australian snakebite (ASP-18) J Thromb Haemost. 2013;11(7):1310–8. doi: 10.1111/jth.12218. [DOI] [PubMed] [Google Scholar]
- 27.Isbister GK. Snakebite doesn’t cause disseminated intravascular coagulation: coagulopathy and thrombotic microangiopathy in snake envenoming. Semin Thromb Hemost. 2010;36(4):444–51. doi: 10.1055/s-0030-1254053. [DOI] [PubMed] [Google Scholar]


