Abstract
Background
Sophorae Flos (SF) is a composite of flowers and buds of Styphnolobium japonicum (L.) Schott and has been used in traditional Korean and Chinese medicine for the treatment of hemostasis and inflammation. Previous studies reported that SF possesses anti-obesity properties, as well as anti-allergic, anti-proliferative, and anti-inflammatory activities. However, the effect of SF in bone resorption has not been studies. In this study, we examined the potential of SF extract (SFE) to inhibit receptor activator of NF-κB ligand (RANKL) -induced osteoclast differentiation in cultured mouse-derived bone marrow macrophages (BMMs).
Methods
BMMs, that act as osteoclast precursors, were cultured with M-CSF (50 ng/ml) and RANKL (100 ng/ml) for 4 days to generate osteoclasts. Osteoclast differentiation was measured by tartrate-resistant acidic phosphatase (TRAP) staining and the TRAP solution assay. Osteoclast differentiation marker genes were analyzed by the quantitative real-time polymerase chain reaction analysis. RANKLs signaling pathways were confirmed through western blotting.
Results
SFE significantly decreased osteoclast differentiation in a dose-dependent manner. SFE inhibited RANKL-induced osteoclastogenesis by suppressing NF-κB activation. By contrast, SFE did not affect phospholipase C gamma 2 or subsequent cAMP response element binding activation. SFE inhibited the RANKL-induced expression of nuclear factor of activated T cells c1 (NFATc1).
Conclusions
SFE attenuated the RANKL-mediated induction of NF-κB through inhibition of IκBα phosphorylation, which contributed to inhibiting of RANKL-induced osteoclast differentiation through downregulation of NFATc1.
Keywords: Sophorae Flos (SF), Osteoclast, NF-κB, NFATc1, PLCγ2
Background
Osteoclasts differentiate from monocyte/macrophage lineage hematopoietic precursor cells at various stages including proliferation, migration, fusion, and activation [1]. Osteoclasts are specialized as the only bone-resorbing cell type and increased numbers are implicated in the development of bone loss-accompanied diseases such as osteoporosis, periodontitis, rheumatoid arthritis, osteosarcoma, and bone cancer metastases [2–4]. Pharmaceutical inhibition of osteoclast differentiation is a current therapeutic approach for the treatment of bone loss-related diseases.
Macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL) are two cytokines secreted mainly by osteoclast. Both are differentiation factors [5, 6]. RANKL binds specifically to receptor activator of nuclear factor (NF)-κB (RANK) that mediates osteoclastogenesis by subsequent signal transduction to intracellular molecules through the TRAF6 adaptor protein. Thereafter, the RANKL/RANK interaction activates extracellular signal-regulated kinase (ERK), p38, c-Jun N-terminal kinase (JNK), Akt, and NF-κB [7–9]. Ultimately, these signal transduction pathways lead to the expression and activation of transcription factors such as nuclear factor of activated T cells c1 (NFATc1) and activator protein-1 (AP-1), both of which are involved in the expression of genes specific to osteoclasts [9–11].
Sophorae Flos (SF), the dried flower buds of Styphnolobium japonicum (L.) Schott, is a well-known herb in traditional Chinese medicine. It has been used in the treatment of bleeding-related disorders such as hematochezia, hemorrhoidal bleeding, dysfunctional uterine bleeding, and diarrhea [12]. Several phytochemical investigations have revealed that natural products from S. japonicum (L.) Schott fruit extracts contain various flavonoids, including sophoricoside, genistin, genistein, kaempferol, rutin, and quercetin [13, 14]. In both pharmacological studies and clinical practice, S. japonicum (L.) Schott exhibits anti-tumor, anti-inflammatory, anti-platelet, and anti-obesity activities [15–18].
Previous studies indicate that pro-inflammatory cytokines, including IL-17, TNF-α, IL-1, IL-4, and IFN-γ, that are induced during T-cell-mediated immune responses, directly control the expression of RANKL on osteoblasts and that inflammation affects bone metabolism [1, 19, 20]. Although S. japonicum (L.) Schott has anti-inflammatory activity, effect on bone metabolism has been studie infrequently. The exceptions are studies showing that dichloromethane extracts of Sophora japonica L. stimulate osteoblast differentiation in mesenchymal stem cells [21]. In addition, recent studies show that such extracts prevent bone loss, partly by inhibiting osteoclastic activity [21, 22]. However, the potential anti-osteoclast differentiation mechanisms of SF have not been defined clearly.
In our study, we confirmed the inhibitory effects of SF extract (SFE) on RANKL-mediated osteoclast differentiation, provided molecular mechanisms for its activity, and suggested possibilities for the use of SF as a traditional medicine against bone disorders, such as osteoporosis, RA, and periodontitis.
Methods
Experimental animals
BALB/c mice (Orient Bio, SeungNam, Korea) were used for all experiments, including osteoclast generation. All mouse studies were performed using protocols approved by the Animal Care and Use Committee of Wonkwang University.
Reagents
Recombinant murine sRANK Ligand and M-CSF were purchased from PeproTech (Rocky Hill, NJ, USA). Fetal bovine serum, α-minimal essential medium, and supplements were obtained from Gibco (Rockford, IL, USA).
Preparation of SFEs
Flower buds of S. japonicum (L.) Schott were purchased from Kwangmyungdang Medicinal Herbs (Ulsan, Korea) and authenticated by Prof. G.S. Lee. SF was extracted from 50 g of S. japonicum flower buds using the reflux method with ethanol. The extract was evaporated and then freeze-dried. The yield of the final extract was 2.56% (w/w).
Cell viability assay
In 96-well plates, bone marrow-derived macrophages (BMMs) were treated with different concentrations of SFE (0, 25, 50, 100, and 200 μg/ml) for 1 day, or with 100 μg/ml SFE under M-CSF treatment for 4 days. Next, cells were then incubated with EZ-Cytox reagent (Itsbio, Korea) for 4 h at 37 °C. After incubation, cell viability was measured using a microplate reader (Sunrise™, Tecan, Switzerland) at 450 nm.
In vitro osteoclast differentiation
Collected from mice tibiae and femur, BMMs were cultured with M-CSF (30 ng/ml). After 3 days, attached BMMs were used as osteoclast precursor. To form osteoclasts, BMMs were treated with M-CSF (50 ng/ml) and RANKL (100 ng/ml) and cultured for 4 days [23]. For TRAP staining, cells were fixed with 10% formalin and stained. Total TRAP activity was measured at an absorbance of 405 nm using p-nitrophenyl phosphate (Sigma Aldrich, St. Louis, MO, USA) as a substrate.
Real-time quantitative polymerase chain reaction (qRT-PCR)
Total RNA was isolated from cells using the Trizol reagent (Invitrogen, Carlsbad, CA, USA). One microgram of total RNA was synthesized to first strand cDNA using a PrimeScript™ RT reagent kit (TaKaRa Bio, Shiga, Japan). qRT-PCR was performed using the SYBR Green and the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). All results were normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (Gapdh). PCR primers used were: mouse Acp5 (sense: 5′-CTG GAG TGC ACG ATG CCA GCG ACA-3′ and antisense: 5′-TCC GTG CTC GGC GAT GGA CCA GA-3′); Oscar (sense: 5′-GGG GTA ACG GAT CAG CTC CCC AGA-3′ and antisense: 5′-CCA AGG AGC CAG AAC GTC GAA ACT-3′); CtsK (sense: 5′-ACG GAG GCA TTG ACT CTG AAG ATG-3′ and antisense: 5′-GTT GTT CTT ATT CCG AGC CAA GAG-3′); Tm7sf4 (sense: 5′-TGG AAG TTC ACT TGA AAC TAC GTG-3′ and antisense: 5′-CTC GGT TTC CCG TCA GCC TCT CTC-3′); Atp6v0d2 (sense: 5′-TCA GAT CTC TTC AAG GCT GTG CTG-3′ and antisense: 5′-GTG CCA AAT GAG TTC AGA GTG ATG-3′); Nfatc1 (sense: 5′-CTC GAA AGA CAG CAC TGG AGC AT-3′ and antisense: 5′-CGG CTG CCT TCC GTC TCA TAG-3′); and Gapdh (sense: 5′-TGC CAG CCT CGT CCC GTA GAC-3′ and antisense: 5′-CCT CAC CCC ATT TGA TGT TAG-3′).
Western blot analysis
Cells were lysed with RIPA Lysis buffer (Pierce Biotechnology, Rockford, IL, USA). The protein concentration in the supernatants was determined using the Bradford method [24]. Protein samples (30 μg) were separated in sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene fluoride membranes (GE, Buckinghamshire, UK) using a western blot apparatus. Each membrane was blocked in blocking buffer (2% bovine serum albumin or 5% skim milk) and then incubated with primary antibody ( phospholipase C gamma 2 (PLCγ2), p-ERK, p-JNK, p-p38, cAMP response element binding (CREB), p-IκBα, p-PLCγ2, ERK, JNK, p38, CREB (Cell signaling Technology, Danvers, MA, USA), NFATc1, c-fos (Santa Cruz Biotechnology, Santa Cruz, CA, USA), β-actin (Sigma-Aldrich)). Horseradish peroxidase-conjugated IgG (1:2000 dilutions) was used as the secondary antibody. Immunoreactivity was detected using a Mini HD6 image analyzer (Uvitec Cambridge, UK).
Statistical analysis
Results were analyzed using Student’s two-tailed t-test. Data are presented as means ± standard deviation (SD). P-values less than 0.05 were considered significant.
Results
Cytotoxic effects of SFE
To investigate the cytotoxicity of SFE on BMMs (osteoclast precursor), it treated with several concentrations of SFE (0, 25, 50, 100, and 200 μg/ml) for 1 day. Concentrations of SFE, up to 100 μg/ml, did not cause any significant change in cell viability (Fig. 1a). BMMs were also treated with 100 μg/ml SFE and cell viability was measured daily for 4 days. There were no significant differences in viability between control and SFE at any day (Fig. 1b).
Fig. 1.
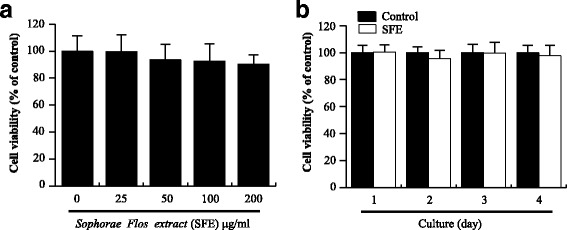
Effects of Sophorae Flos extract (SFE) on bone marrow marcrophages. a BMMs were cultured with various concentrations of SFE for 1 day. b BMMs were cultured with or without (control) 100 μg/ml SFE for 4 days. Cell viability was measured as described in methods. Data from three independent experiments are expressed as relative proliferation (% of control) ± SD
SFE treatment suppressed RANKL-mediated osteoclastogenesis in a dose-dependent manner
To investigate the effects of SFE on osteoclast differentiation, RANKL-stimulated BMMs were treated with SFE at the indicated concentrations for 4 days (Fig. 2a). After incubation, osteoclast differentiation and formation were measured by TRAP staining and the total TRAP activity assay. TRAP+ multimuclear cells present in each well were identified by the presence of more than three nuclei and a cell size larger than 100 μm in diameter, and were counted. Total TRAP activity was measured in fused and non-fused cells. SFE treatment markedly inhibited RANKL-induced osteoclast formation from BMMs in a dose-dependent manner (Fig. 2a, b). Total TRAP activity of osteoclasts was reduced significantly by SFE in a concentration dependent manner (Fig. 2c). These results suggest that SFE is able to repress osteoclast differentiation.
Fig. 2.
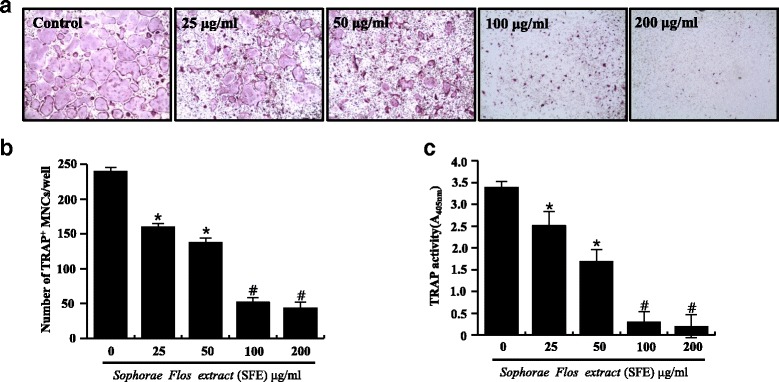
Effects of Sophorae Flos extract (SFE) on osteoclast differentiation. Bone marrow macrophages were cultured with various concentrations of SFE and treated with RANKL (100 μg/ml) and M-CSF (50 μg/ml) for 4 days. a Osteoclasts stained for tartrate-resistant acidic phosphatase (TRAP). b TRAP+ multinuclear cells (MNCs) with more than three nuclei were considered to be mature osteoclasts. c Total TRAP activity from TRAP+ mono-, di-, and multinuclear cells was quantified as described in methods. Data from three independent experiments are expressed as mean ± SD. *P < 0.05, # P < 0.01 versus control (0 μg/ml SFE)
Abrogation of differentiation-related gene expression by SFE
Acp5 (TRAP), Oscar, Ctsk, Tm7sf4 (dendritic cell-specific transmembrane protein, DC-STAMP), and Atp6v0d2 are well-known as osteoclast differentiation-related marker genes crucial for cell motility, fusion, and bone resorptive activities. To evaluate the inhibitory effect of SFE on RANKL-induced osteoclast differentiation, the expression of these marker genes and a major transcription factor Nfatc1, was measured during RANKL-induced osteoclast differentiation. SFE dramatically inhibited the expression of all tested osteoclast differentiation-related marker genes, as well as Nfatc1 (Fig. 3). These results suggest that SFE affects the differentiation-mediating signal pathway from an early-stage of osteoclastogenesis.
Fig. 3.
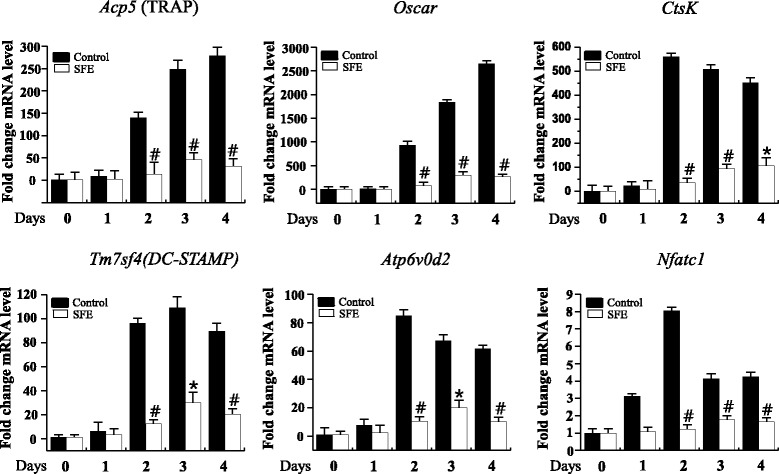
Effects of Sophorae Flos extract (SFE) on the expression of osteoclast differentiation marker genes. Bone marrow macrophages were cultured with RANKL and M-CSF treatment in the presence or absence of SFE (100 μg/ml) for 4 days. The expression of marker genes of osteoclast differentiation was measured by real-time quantitative PCR. Target gene mRNA levels were normalized to GAPDH and are presented as fold change from control (0 μg/ml SFE). Data are expressed as mean ± SD and are representative of at least three independent experiments. *P < 0.05, #P < 0.01 versus control
Suppression of NFATc1 expression by SFE-mediated NF-κB inactivation
Osteoclast differentiation is regulated by the recruitment of multiple downstream signaling molecules, including PLCγ2, p38, JNK, and ERK, as well as the transcription factors NF-κB, NFATc1, and c-fos, in response to RANKL and its binding to RANK [25]. We confirmed the effects of SFE on RANKL-induced early signaling pathways. First, we confirmed the activation of mitogen-activated protein kinases (MAPKs) and NF-κB by western blotting (Fig. 4). SFE showed had no effect on MAPK activation. By contrast, IκBα phosphorylation, proportional to the NF-κB pathway activity, was diminished in SFE-treated compared with control cells (Fig. 4a). Next, we investigated the expression levels of NFATc1 and c-fos proteins. In SFE-treated cells, the expression of c-fos protein was unchanged during RANKL-induced osteoclast differentiation (Fig. 4b). However, the expression of NFATc1 protein was inhibited dramatically by SFE (Fig. 4c). Concomitantly, we found that SFE failed to inhibit the activation of PLCγ2 and CREB during RANKL-induced osteoclast differentiation (Fig. 5a, b). These results suggest that SFE affects the regulation of RANKL-induced osteoclast differentiation through the NF-κB pathway.
Fig. 4.
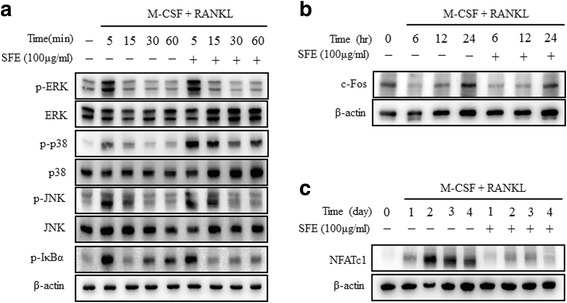
Effects of Sophorae Flos extract (SFE) on RANKL-induced intracellular signaling and expression of c-fos and NFATc1 in osteoclasts. Bone marrow macrophages were cultured with RANKL and MCSF in the presence or absence of SFE (100 μg/ml) for 4 days. Protein expression levels were evaluated by western blot analysis. a Activation of MAPKs and NF-κB measured using by their respective antibodies. Expression of (b) c-fos and (c) NFATc1 detected by the respective antibodies. All data are representative of at least three independent experiments
Fig. 5.
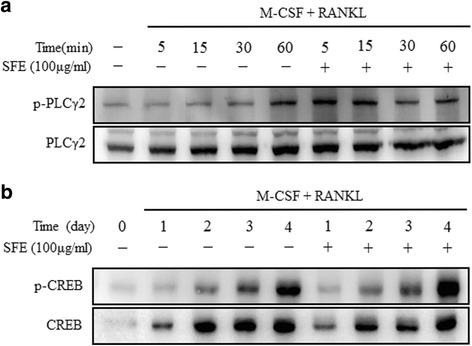
Sophorae Flos extract (SFE) does not inhibit RANKL-mediated activation of PLCγ2 and CREB in osteoclasts. Bone marrow macrophages were cultured with M-CSF and RANKL in presence or absence of SFE (100 μg/ml) for 4 days. Lysate (30 μg protein) was subjected to SDS-PAGE and the phosphorylation of (a) PLCγ2 and (b) CREB analyzed by western blotting. All data are representative of at least three independent experiments
Discussion
SF has been used commonly in traditional medicine because of its various hemostatic, anti-inflammatory, and anti-oxidative effects [26]. Additionally, recent studies indicated that S. japonicum (L.) Schott extracts showed preventive effects against bone loss, partly by inhibiting osteoclastic activity [19, 20]. However, the inhibitory potential and molecular mechanisms of SF on RANKL-induced osteoclast differentiation have not been elucidated. Here, we demonstrated that the inhibitory effect of SFE was elicited through a reduction of NFATc1 expression during the differentiation of osteoclasts, cells responsible for bone destruction and associated with inflammation-related bone diseases.
BMMs are precursor cells that differentiate into osteoclasts in response to RANKL, which is expressed in osteoblasts, osteocytes, and T cells, and a critical factor in osteoclastogenesis [27].
The RANKL/RANK interaction recruits multiple intracellular signaling molecules that regulate osteoclast differentiation, including MAPK, NF-κB, AP-1, TRAFs, NFATc1, and ionized calcium, with NF-κB being the most important factor [1, 28].
NF-κB is transcription factor and an inducible dimeric protein consisting of two subunits, p65 and p50 [29]. In unstimulated cells, NF-κB is located in the cytoplasm in a dormant form complexed with its inhibitory factor, IκB. Various inducers can dissociate this complex, presumably by phosphorylating IκB, resulting in the release of NF-κB. NF-κB then translocates into the nucleus, where it binds to specific DNA sites to regulate gene transcription. In several studies, NF-κB was shown to play a key role in osteoclastogenesis [30, 31], and its suppression affected NFATc1 expression. Takatsuna et al. have shown that the NF-κB inhibitor, (-)-dehydroxymethylepoxyquinomicin regulates RANKL-induced osteoclastogenesis through downregulation of NFATc1 [32]. In the current study, we found that IκBα phosphorylation was inhibited by SFE, whereas the induction of MAPK was unaffected (Fig. 4).
Multiple previous studies have established that NFATc1 is a master executor of RANKL-mediated osteoclast differentiation and activation. Stimulation of BMM s by RANKL increases the expression of NFATc1 through c-Fos and auto amplification [11, 23, 33–35]. NFATc1 also gradually induces the expression of osteoclast-specific genes, including Acp5 (encoding TRAP), Oscar, Tm7sf4 (encoding DC-STAMP), Atp6v0d2, and Ctsk [11, 33, 34, 36]. The present data suggest that SFE suppressed the induction of Acp5, Oscar, Tm7sf4, Atp6v0d2, and Ctsk (Fig. 3). In addition, SFE suppressed the RANKL-mediated induction of NFATc1 mRNA and protein expression, although the expression of c-fos was not unaffected (Figs. 3 and 4).
RANKL/RANK binding activates PLCγ2 and induces calcium ion signaling, followed by CREB phosphorylation [23, 25, 37]. CREB is crucial factor for the RANKL-stimulated induction of NFATc1 and c-Fos in osteoclast precursors [37]. Nevertheless, in our study, SFE failed to inhibit PLCγ2 and CREB activation, which are essential signaling molecules for osteoclast differentiation, through the repression of c-Fos and NFATc1 expression. These results indicate that SFE is an effective inhibitory agent of osteoclast differentiation through its control of NF-κB induction following RANKL/RANK binding.
Conclusions
Our results demonstrate that SFE reduces the RANKL-mediated induction of the NF-κB pathway by inhibiting of IκBα phosphorylation. This effect, in turn, contributes to the downregulation of NFATc1 and inhibition of RANKL-induced osteoclast differentiation. These findings reveal SFE as an effective traditional therapeutic medicine for the treatment of inflammatory bone diseases, such as osteoporosis, rheumatoid arthritis, and periodontitis.
Acknowledgements
The present study was supported by Wonkwang University in 2014.
Funding
Not applicable.
Availability of data and materials
The data and materials of this article are included within the article.
Authors’ contributions
JHL, YRL conceived and designed the study. JMK, DRG performed the experiments. EMN, HKS analyzed the data. KBK and YRL drafted the manuscript. SHL provided comments and editorial review of the manuscript. GSL provided the Sophorae Flos extract. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval
All mouse studies were performed using protocols approved by the Animal Care and Use Committee of Wonkwang University.
Abbreviations
- AP-1
Activator protein-1
- BMMs
Bone marrow macrophages
- BMs
Bone marrow cells
- DC-STAMP
Dendritic cell-specific transmembrane protein
- ERK
Extracellular signal-regulated kinase
- FBS
Fetal bovine serum
- JNK
c-Jun N-terminal kinase
- M-CSF
Macrophage colony-stimulating factor
- NFATc1
Nuclear factor of activated T cells c1
- PBS
Phosphate-buffered saline
- PLCγ2
Phospholipase C gamma 2
- RA
Rheumatoid arthritis
- RANK
Receptor activator of NF-κB
- RANKL
RANK ligand
- SD
Standard deviation
- SF
Sophorae flos
- SFE
SF extract
- TRAP+ MNCs
TRAP positive-multinuclear cells
- α-MEM
α-minimal essential medium
Contributor Information
Jeong-Mi Kim, Email: wjdal4219@hanmail.net.
Jung-Han Lee, Email: milpaso@wku.ac.kr.
Guem-San Lee, Email: rasfin@wku.ac.kr.
Eun-mi Noh, Email: jingle1234@hanmail.net.
Hyun-Kyung Song, Email: gusrud1654@naver.com.
Dong Ryun Gu, Email: mrwonsin@naver.com.
Seoung Hoon Lee, Email: leesh2@naver.com.
Kang-Beom Kwon, Phone: +82-63-850-6917, Email: desson@wku.ac.kr.
Young-Rae Lee, Phone: +82-63-850-6996, Email: mindyr@wku.ac.kr.
References
- 1.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 2.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–14. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama T, Dass CR, Choong PF. Novel therapeutic strategy for osteosarcoma targeting osteoclast differentiation, bone-resorbing activity, and apoptosis pathway. Mol Cancer Ther. 2008;7:3461–9. doi: 10.1158/1535-7163.MCT-08-0530. [DOI] [PubMed] [Google Scholar]
- 4.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 5.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 6.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darnay BG, Haridas V, Ni J, Moore PA, Aggarwal BB. Characterization of the intracellular domain of receptor activator of NF-kappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappab and c-Jun N-terminal kinase. J Biol Chem. 1998;273:20551–5. doi: 10.1074/jbc.273.32.20551. [DOI] [PubMed] [Google Scholar]
- 8.Lee SY, Reichlin A, Santana A, Sokol KA, Nussenzweig MC, Choi Y. TRAF2 is essential for JNK but not NF-kappaB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:703–13. doi: 10.1016/S1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 9.Lee ZH, Kim HH. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochem Biophys Res Commun. 2003;305:211–4. doi: 10.1016/S0006-291X(03)00695-8. [DOI] [PubMed] [Google Scholar]
- 10.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–9. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 11.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 12.Ishida H, Umino T, Tsuji K, Kosuge T. Studies on the antihemostatic substances in herbs classified as hemostatics in traditional Chinese medicine. I. On the antihemostatic principles in Sophora japonica L. Chem Pharm Bull (Tokyo) 1989;37:1616–8. doi: 10.1248/cpb.37.1616. [DOI] [PubMed] [Google Scholar]
- 13.Tang YP, Li YF, Hu J, Lou FC. Isolation and identification of antioxidants from Sophora japonica. J Asian Nat Prod Res. 2002;4:123–8. doi: 10.1080/10286020290027407. [DOI] [PubMed] [Google Scholar]
- 14.Liu R, Qi Y, Sun A, Xie H. Isolation and purification of chemical constituents from the pericarp of Sophora japonica L. by chromatography on a 12% cross-linked agarose gel. J Sep Sci. 2007;30:1870–4. doi: 10.1002/jssc.200600528. [DOI] [PubMed] [Google Scholar]
- 15.Abe M, Suzuki O, Tasaki K, Tominaga K, Wakasa H. Analysis of lectin binding properties on human Burkitt’s lymphoma cell lines that show high spontaneous metastasis to distant organs in SCID mice: the binding sites for soybean agglutinin lectin masked by sialylation are closely associated with metastatic lymphoma cells. Pathol Int. 1996;46:977–83. doi: 10.1111/j.1440-1827.1996.tb03577.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim BH, Chung EY, Min BK, Lee SH, Kim MK, Min KR, Kim Y. Anti-inflammatory action of legume isoflavonoid sophoricoside through inhibition on cyclooxygenase-2 activity. Planta Med. 2003;69:474–6. doi: 10.1055/s-2003-39712. [DOI] [PubMed] [Google Scholar]
- 17.Kim JM, Yun-Choi HS. Anti-platelet effects of flavonoids and flavonoid-glycosides from Sophora japonica. Arch Pharm Res. 2008;31:886–90. doi: 10.1007/s12272-001-1242-1. [DOI] [PubMed] [Google Scholar]
- 18.Park KW, Lee JE, Park KM. Diets containing Sophora japonica L. prevent weight gain in high-fat diet-induced obese mice. Nutr Res. 2009;29:819–24. doi: 10.1016/j.nutres.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 20.Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocr Rev. 2008;29:403–40. doi: 10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon HJ, Seo CR, Kim M, Kim YJ, Song NJ, Jang WS, Kim BJ, Lee J, Hong JW, Nho CW, Park KW. Dichloromethane extracts of Sophora japonica L. stimulate osteoblast differentiation in mesenchymal stem cells. Nutr Res. 2013;33:1053–62. doi: 10.1016/j.nutres.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Kim KW, Kim HK, Chae SW, Jung JC, Kwon SH, Rheu CH. The effect of Rexflavone (Sophorae fructus extract) on menopausal symptoms in postmenopausal women: a randomized double-blind placebo controlled clinical trial. Arch Pharm Res. 2010;33:523–30. doi: 10.1007/s12272-010-0405-0. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Kim T, Jeong D, Kim N, Choi Y. The tec family tyrosine kinase Btk Regulates RANKL-induced osteoclast maturation. J Biol Chem. 2008;283:11526–34. doi: 10.1074/jbc.M708935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Kite GC, Veitch NC, Boalch ME, Lewis GP, Leon CJ, Simmonds MS. Flavonol tetraglycosides from fruits of Styphnolobium japonicum (Leguminosae) and the authentication of Fructus Sophorae and Flos Sophorae. Phytochemistry. 2009;70:785–94. doi: 10.1016/j.phytochem.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Sims NA, Walsh NC. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 2012;10:109–17. doi: 10.1007/s11914-012-0096-1. [DOI] [PubMed] [Google Scholar]
- 28.Gohda J, Akiyama T, Koga T, Takayanagi H, Tanaka S, Inoue J. RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J. 2005;24:790–9. doi: 10.1038/sj.emboj.7600564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thanos D, Maniatis T. NF-kappa B: a lesson in family values. Cell. 1995;80:529–32. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 30.Boyce BF, Xing L, Franzoso G, Siebenlist U. Required and nonessential functions of nuclear factor-kappa B in bone cells. Bone. 1999;25:137–9. doi: 10.1016/S8756-3282(99)00105-2. [DOI] [PubMed] [Google Scholar]
- 31.Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–96. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takatsuna H, Asagiri M, Kubota T, Oka K, Osada T, Sugiyama C, Saito H, Aoki K, Ohya K, Takayanagi H, Umezawa K. Inhibition of RANKL-induced osteoclastogenesis by (-)-DHMEQ, a novel NF-kappaB inhibitor. J Bone Miner Res. 2005;20:653–62. doi: 10.1359/JBMR.041213. [DOI] [PubMed] [Google Scholar]
- 33.Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–9. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim K, Lee SH, Ha Kim J, Choi Y, Kim N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP) Mol Endocrinol. 2008;22:176–85. doi: 10.1210/me.2007-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JH, Kim N. Regulation of NFATc1 in osteoclast differentiation. J Bone Metab. 2014;21:233–41. doi: 10.11005/jbm.2014.21.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–63. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 37.Sato K, Suematsu A, Nakashima T, Takemoto-Kimura S, Aoki K, Morishita Y, Asahara H, Ohya K, Yamaguchi A, Takai T, Kodama T, Chatila TA, Bito H, Takayanagi H. Regulation of osteoclast differentiation and function by the CaMK-CREB pathway. Nat Med. 2006;12:1410–6. doi: 10.1038/nm1515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials of this article are included within the article.


