Abstract
Background
The authors used a large community sample of methamphetamine users to verify the patterns and severity of dental disease and establish a hierarchy of caries susceptibility by tooth type and tooth surface.
Methods
Using a stratified sampling approach, 571 MA users received comprehensive oral examinations and psychosocial assessments. Three calibrated dentists characterized the dental and periodontal disease using National Health and Nutrition Examination Survey (NHANES) protocols. Data were also collected on substance-use history and other attributes linked to dental disease
Results
On all dental outcome measures, MA users evidenced very high dental and periodontal disease, with older (> 30 years) and moderate/heavy MA users disproportionately affected. Women had higher rates of tooth loss and caries as well as a greater prevalence of anterior caries. Current smokers were more likely to manifest 5 or more anterior surfaces with untreated caries and 3 or more teeth involved with root caries. Nearly 7% were edentulous and a significant percentage (40%) indicated embarrassment with their dental appearance.
Conclusions
MA users have very high rates of dental and periodontal disease, and manifest a dose-response relationship with greater levels of MA use associated with higher rates of dental disease. Women and current cigarette smokers are disproportionally impacted. The intraoral patterns and hierarchy of caries susceptibility in MA users are distinctive.
Practical Implications
The prevalence and patterns of dental and periodontal disease could be used to alert dentists to possible covert MA use as well as to plan treatment. Concerns about dental appearance have potential as triggers for behavioral interventions.
Keywords: dental disease, methamhetamine use, patterns, severity
INTRODUCTION
Accelerated and unusual dental disease patterns have been associated with the use of methamphetamine (MA), a popular and highly addictive stimulant drug. Although there have been sporadic reports on the dental effects of methamphetamine and its derivatives since the 1960’s, a couple of Emergency Department (ED) physicians were the first to alert the dental and medical communities to the peculiar and extreme patterns of dental destruction manifest in MA users.1 Their article stimulated a flurry of reports corroborating the severe dental consequences encountered in MA users.2–7 Along the way, the patterns of MA-associated dental disease described by Richards and Brofeldt (2000)1 acquired the moniker “meth mouth,” which began to seep into public awareness due to extensive, and sometimes sensational, media coverage. Interestingly, the moniker “meth mouth” originated from a perfunctory statement in a press release by the Academy of General Dentistry (AGD) publicizing a report8 in its December 2003 newsletter on the management of substance users in the dental office. Neither the AGD report nor the accompanying press release corroborated the depiction of a “sudden, massive onset of tooth decay, gum disease and worn down teeth” with any supporting data or research findings.9 Remarkably, the only incidental evidence available to support the 2003 AGD narrative of the “meth mouth” phenomenon was composed of nine articles on amphetamine-related dental disease, primarily brief case reports and case series.1,7,10,11,12,13,14,15
In an effort to anchor the accumulating anecdotal evidence in a scientifically rigorous framework, we had previously16 utilized the infrastructure of a large multisite clinical study (Methamphetamine Treatment Project or MTP) to systematically examine the oral health consequences of chronic MA use in a prospectively collected sample of users. Participating physician examiners carried out brief dental evaluations as part of comprehensive medical assessments conducted in a cohort of 301 MA users. The dental findings were compared to the dental status of a sociodemographically similar group of non-MA using participants enrolled in the National Health and Nutrition Examination Survey (NHANES). Our main finding was that dental disease was one of the most prevalent (41.3 percent) medical comorbidities in chronic MA users who otherwise tended to be generally healthy.16 On average, MA users had significantly more missing teeth than demographically comparable individuals in the general population (4.58 versus 1.96, P < .001) and were more likely to report having oral health problems. The findings from the physician-conducted oral assessments supported the prevailing assertion of higher rates of dental disease in MA users.
Building on our precursor findings, we conducted a follow-up study involving a new and larger sample of MA users with a range of MA-use behaviors. To provide greater granularity and validity, the oral examinations were performed by experienced dentists who were rigorously trained and calibrated by the reference dental examiner for NHANES. All decayed, missing, and filled teeth (DMFT) and periodontal evaluations were carried out by the dental examiners using well-articulated assessment protocols supported by a Quality Assurance (QA) program.17 Our overarching goal was to characterize the patterns of dental caries and periodontal disease in MA users and use the data to inform dental management strategies. Moreover, a methodical understanding of relevant behavioral underpinnings would facilitate the development of Screening, Brief Interventions, and Referral to Treatment (SBIRT) approaches applicable to dental settings.18 The specific objectives of this study were to (a) characterize the patterns and severity of dental disease in MA users, and (b) establish a hierarchy of caries susceptibility by tooth type and tooth surface.
METHODS
STUDY DESIGN
We conducted a cross-sectional study of a broad sample of community-based MA users using snowball sampling approaches.19 To maximize power to perform key statistical tests, we recruited MA users balanced across substance use patterns stratified into mild, moderate or heavy use. Because we reached our recruitment target for mild MA users first, we oversampled users in the moderate and heavy use categories relative to their representation in the local population of MA users. Important confounding variables (age, gender, other drug use) were controlled through matching or other statistical adjustments.
STUDY SETTING
The study was conducted in Los Angeles County, one of the largest and most populous urban areas in the USA and beset with high rates of MA use20,21. Between February 9, 2011 and August 26, 2013, 571 MA users recruited from local communities underwent comprehensive oral examinations and psychosocial assessments at dental clinics associated with two large community health centers: a) the AIDS Project, Los Angeles (APLA) center that primarily serves a sociodemographically diverse group of individuals with HIV/AIDS, and b) the Mission Community Hospital (Mission) in the San Fernando Valley that caters to a large, underserved migrant population. The study sites were chosen to provide access to a diverse cohort of Angelenos with a broad range of MA-use behaviors.
PARTICIPANTS
Participants were recruited using a combination of street outreach (e.g., posting flyers within the community, distributing advertising matchboxes in bars and restaurants), Craigslist postings, newspaper advertisements, referrals from local drug treatment centers, and word of mouth. Individuals were eligible to enroll in the study if they were 18 years of age or older, spoke either English or Spanish, had used MA in the past 30 days, able to undergo a detailed dental exam and psychosocial assessments, and willing to provide a urine sample. Of the 1,793 potential participants who contacted the research team, 1,120 were found eligible, 576 enrolled in the study and 571 completed the assessments. The informed consent process and the assessments were accomplished according to procedures reviewed and approved by the UCLA Institutional Review Board. A Federal Certificate of Confidentiality ensured unconditional confidentiality to the interviews, thus minimizing participant concerns regarding the disclosure of sensitive drug-use behaviors. Each participant received $60 as recompense for taking part in the study.
ASSESSMENTS
The main oral health outcome variables were the rates and patterns of dental caries and the periodontal disease status of the subjects. To maximize comparability with national datasets, assessments for dental caries and periodontal status adhered to NHANES examination protocols, which have been described in greater detail elsewhere.22,23 Dental caries were assessed at the surface-level using the NIDR criteria.24 Dental caries experience (DMFT) was calculated as the number of decayed (D), missing (M), and filled (F) teeth (T). The extent of untreated dental caries was calculated as the number of decayed surfaces (DS). A subcategory of the decayed component (Dx) was also calculated to indicate the severity of the decay (i.e., only crown shell or residual root tips remained). Periodontal disease status was assessed using the case definitions recommended for periodontitis surveillance by the CDC/AAP Periodontitis Workgroup.25 The CDC/AAP case definitions require information from two interproximal sites (DF, MF, ML, and/or DL) and are not dependent upon the presence of an adjacent tooth. Gingival recession and pocket depth measures were made at four sites per tooth, specifically the disto-facial (D), mid-facial (B), mesio-facial (M), and the disto-lingual (DL) sites. An algorithm calculated loss of attachment from the information on gingival recession and pocket depth. All four quadrants were examined and third molars were excluded from the periodontal exam.
Participants also completed a set of interviewer-facilitated questionnaires covering various psychological, substance-use, medications, and dietary attributes linked to the development of dental disease. Instruments eliciting information on medications as well as substance use history and behaviors included items from the Xerostomia Inventory26 and the UCLA Natural History Interview.27 Information on oral health quality of life was captured through select items from the Oral Health Impact Profile,28 and psychological symptoms through the Brief Symptom Inventory.29 Dietary intake, particularly the consumption of sugary sodas, was evaluated using two standard dietary assessments methods: the Food Frequency Questionnaire (FFQ)30 and the 24-hour Dietary Recall.31 Finally, the veracity of the drug use reports was verified by random urine drug tests carried out in a subset of the participants.
METHAMPHETAMINE -USE PATTERNS
Based on the self-reported history and patterns of MA-use (quantity, frequency, mode and duration of use) over the past 30 days, participants were clustered into 3 groups: light, moderate and heavy use. Participants who indicated that they had used methamphetamine for less than 10 days of the past 30 days at the time of screening were classified as being “light” MA users. Moderate use was defined as 10–15 days of MA use over the past 30 days, and high use as 16 or more days of MA use. Study participants in the moderate and heavy use groups were subsequently grouped together and classified as “moderate +” users for data analysis purposes.
DATA COLLECTION AND MANAGEMENT
Three trained dental examiners conducted all the oral examinations, with the data recorded by trained dental assistants. In conjunction with the dental exam, a bilingual (English and Spanish) interviewer conducted the comprehensive drug use and psychosocial assessments. The interviewer, skilled in working with substance-using populations and comfortable with drug use vocabulary, used simple, direct questions and clearly defined timeframes to elicit the specifics of the drug use behaviors. To ensure standardization and quality assurance in data collection and processing, all dental and psychosocial data were collected directly on a laptop computer using a web-based data-management system developed and maintained by the UCLA-Semel Institute Statistics Core (SIStat). Data collected through the user-friendly graphical interface on the laptop was encrypted and transmitted to be stored centrally in a secure server with firewall protection. Built-in logic and data-range checks allowed data verification to prevent invalid data. The real-time input verification facilitated the timely identification and resolution of any problems in data collection and processing. Automated reports and dashboards allowed the investigators and project manager to monitor the quality of the data collected at each clinical site by generating a variety of summary reports on data completeness and questionable values.
TRAINING AND QUALITY ASSURANCE
A combination of rater training and calibration, electronic data capture methods, trial monitoring, and statistical monitoring of performance indicators was used to ensure conformance and comparability with standard NHANES practices.32 The national trainer and reference examiner for the NHANES (Dr. Bruce Dye) supervised the training and Quality Assurance (QA) using procedures described previously.32 A local dental epidemiologist, trained and calibrated by the NHANES reference examiner to perform repeat examinations on the study participants, served as the local reference examiner and provided ongoing monitoring of the three dental examiners, evaluating their assessments and providing remediation when necessary. Approximately 9% of the 571 enrolled participants received a repeat dental caries and periodontal examination conducted by the reference examiner. There was good to excellent concordance between the reference examiner and the site examiners for identification of untreated dental disease (Kappa statistic values: 0.57 – 0.75, percent agreement 83% – 88%). For identification of untreated caries on at least 5 surfaces of anterior teeth, the Kappas ranged from 0.77 to 0.87, and percent agreement from 94% to 97%. The intra-class coefficients (ICCs) ranged from 0.87–0.89 for attachment loss across all periodontal sites assessed and the ICCs ranged from 0.79–0.81 for pocket depth. More details on the quality assurance program, confirming the procedural adherence and the quality and the reliability of the data collected, has been described elsewhere.17
VARIABLES
Key socio-demographic and behavioral covariates included age, sex, race/ethnicity, education, and smoking history. Caries-related outcome measures were computed from the DMFS examination excluding third molars. The dichotomous outcomes included having all 28 teeth present, having fewer than 10 teeth present, having at least five anterior surfaces classified as decayed, presence of any root caries, and presence of root caries on at least three teeth. The caries experience was defined as having at least one tooth classified as decayed, missing, or filled, and the presence of untreated caries was defined by having at least one tooth classified as decayed. The continuous caries outcomes were comprised of the number of non-missing teeth, the DMFT score (number of decayed, missing, or filled teeth), and the DFT score (number of decayed or filled teeth). Mean attachment loss (in mm), pocket depth (in mm), and recession (in mm) were calculated across all measured sites within an individual participants mouth. The presence of any sites with attachment loss and pocket depth above certain thresholds (4 mm/6 mm and 5 mm/7 mm respectively) was also reported.
STATISTICAL METHODS
SAS software (Version 9.3; SAS Institute Inc., Cary, NC, USA) was used for statistical analysis and data handling. Percent prevalence, means, and related standard errors were calculated for the main dental outcome variables.
Odds ratios for the presence of at least five surfaces of anterior decay, three or more teeth with root caries, and severe periodontal disease were computed separately for subgroups based on demographic characteristics (unadjusted odds ratios) along with odds ratios adjusting for the other covariates. Multiple logistic regression was used to produce adjusted odds ratios for dichotomous outcomes adjusting for covariates along 95% confidence intervals for the adjusted odds ratios.
RESULTS
PARTICIPANT CHARACTERISTICS
Of the 571 study participants, 19 were completely edentulous (dentate subjects = 552). Table 1 encapsulates the sociodemographic and substance-use characteristics of all study participants (n = 571). In general, participants were predominantly male, African-American and Hispanics (42.2% and 31.2% respectively), older than 30 years (Mean age = 44.4 years, SD = 9.5), and most had completed high school (mean = 12.5 years of education, SD = 1.6). Many of the MA users were current cigarette smokers (68.9%). Based on the patterns of MA use over the past month, over half of the participants could be classified as moderate/heavy MA users. On average, participants reported MA use on 4.5 days of the preceding 30 days (SD = 8.6) and the preferred route of MA administration was by smoking (64.2 percent, n = 190). Most subjects (75%) self-rated the conditions of their teeth and gums as fair or poor; nearly 40% indicated that they were often self-conscious or embarrassed because of the condition of their teeth or dentures.
TABLE 1.
SOCIODEMOGRAPHICS AND METHAMPHETAMINE-USE PATTERNS
| Subjects (n=571) |
|
|---|---|
| Age | |
| <30 years | 48 (8.4%) |
| ≥ 30 years | 523 (91.6%) |
| Sex | |
| Male | 460 (80.6%) |
| Female | 111 (19.4%) |
| Race/ethnicity | |
| Hispanic | 178 (31.2%) |
| African-American | 241 (42.2%) |
| Caucasian | 109 (19.1%) |
| Other | 43 (7.5%) |
| Education | |
| Less than High School | 170 (29.8%) |
| High School Graduate | 201 |
| More than High School | 200 (35.2%) |
| Cigarette Smoking History | |
| Current smoker | 392 (68.9%) |
| Former smoker | 54 (9.6%) |
| Never smoked | 124 (21.5%) |
| Methamphetamine Use | |
| Light | 253 (44.4%) |
| Moderate/Heavy | 318 (55.6%) |
DENTAL DISEASE AND T OOTH LOSS IN MA -USERS
Table 2 summarizes the dental caries and dentition status for the dentate subjects (n = 552) as a function of age, gender, ethnicity, education, smoking status, and methamphetamine use. Among the 522 dentate subjects, the percentage with 28 permanent teeth present was nearly 3 times higher for those less than 30 years (60.4%) compared to those older than 30 years (19.6%). Mean DMFT and DFT scores were generally lower for younger subjects (< 30 years). The frequency of root caries was higher for subjects over the age of 30. Compared to males, females appeared to have a higher caries experience and evidenced higher rates of tooth loss. The percentage of Hispanic MA users with no tooth loss was nearly twice as high compared to African-American MA users (31.8% vs 16.7%). The percentage of Caucasian and African-American MA users with untreated caries was similar (62 – 63%), but Hispanics (50%) manifested a lower prevalence of untreated caries. Similarly, the percentage of root caries was higher among African-Americans (50%) compared to Hispanic (38.6%) MA users. In general, MA users who were high school graduates showed higher tooth retention rates (27.8%) compared to those with no high school degree (18%). The rates of tooth loss appeared to be strongly influenced by the cigarette smoking history of the MA users. Only 17.5% of current cigarette smokers had all 28 teeth present when compared to non-smokers (41.3%). Similarly, the proportion of smokers with untreated caries, anterior caries, and root caries was higher compared to non-smokers.
TABLE 2.
TOOTH RETENTION AND DENTAL CARIES PATTERNS FOR DENTATE SUBJECTS
| All Teeth Present |
DMFT Score |
D | M | F | DX | Root Caries | Root Caries ≥ 3 teeth |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | CI | mean | CI | mean | CI | mean | CI | mean | CI | mean | CI | % | CI | % | CI | |
| Age | ||||||||||||||||
| <30 years (n=48) | 60.4% | (46.5%, 74.3%) | 8.0 | (6.3, 9.7) | 1.25 | (0.6, 1.9) | 1.13 | (0.4, 1.8) | 5.67 | (4.3, 7.1) | 6.91 | (5.5, 8.3) | 27.1% | (14.4%, 39.8%) | 14.6% | (4.6%, 24.6%) |
| ≥ 30 years (n=504) | 19.6% | (16.1%, 23.1%) | 13.1 | (12.5, 13.7) | 2.35 | (2.1, 2.6) | 5.38 | (4.9, 5.9) | 5.40 | (5.0, 5.8) | 7.75 | (7.3, 8.2) | 46.8% | (42.5%, 51.1%) | 23.2% | (19.5%, 26.9%) |
| Sex | ||||||||||||||||
| Male (n=446) | 24.2% | (20.3%, 28.1%) | 12.1 | (11.5, 12.8) | 2.13 | (1.8, 2.4) | 4.80 | (4.3, 5.3) | 5.18 | (4.8, 5.6) | 7.30 | (6.9, 7.8) | 43.0% | (38.5%, 47.5%) | 20.6% | (16.9%, 24.3%) |
| Female (n=106) | 18.9% | (11.5%, 26.4%) | 15.1 | (13.8, 16.4) | 2.79 | (2.0, 3.6) | 5.90 | (4.7, 7.1) | 6.44 | (5.5, 7.4) | 9.24 | (8.2, 10.2) | 53.8% | (44.2%, 63.4%) | 30.2% | (21.4%, 39.0%) |
| Race/ethnicity | ||||||||||||||||
| Hispanic (n=176) | 31.8% | (24.9%, 38.7%) | 11.5 | (10.5, 12.5) | 1.97 | (1.5, 2.5) | 3.45 | (2.8, 4.1) | 6.13 | (5.4, 6.8) | 8.10 | (7.3, 8.9) | 38.6% | (31.4%, 45.9%) | 17.0% | (11.5%, 22.5%) |
| White (n=103) | 23.3% | (15.1%, 31.5%) | 12.8 | (11.4, 14.2) | 2.19 | (1.5, 2.8) | 5.23 | (4.0, 6.5) | 5.41 | (4.5, 6.3) | 7.60 | (6.6, 8.6) | 43.7% | (34.1%, 53.3%) | 22.3% | (11.5%, 22.5%) |
| African-American (n=234) |
16.7% | (12.0%, 21.4%) | 13.3 | (12.4, 14.2) | 2.19 | (1.8, 2.6) | 6.13 | (5.4, 6.9) | 4.99 | (4.5, 5.5) | 7.18 | (6.6, 7.8) | 50.0% | (43.5%, 56.5%) | 25.2% | (19.7%, 30.7%) |
| Other (n=39) | 23.1% | (9.8%, 36.4%) | 13.6 | (11.0, 16.2) | 4.05 | (2.2, 6.0) | 4.74 | (2.9, 6.6) | 4.85 | (3.6, 6.1) | 8.90 | (7.1, 10.7) | 48.7% | (32.8%, 64.6%) | 30.8% | (16.1%, 30.3%) |
| Education | ||||||||||||||||
| Less than HS (n=161) |
18.0% | (12.1%, 23.9%) | 13.6 | (12.4, 14.8) | 2.59 | (1.9, 3.3) | 5.66 | (4.7, 6.6) | 5.32 | (4.7, 6.0) | 7.91 | (7.1, 8.7) | 43.5% | (35.9%, 51.1%) | 22.3% | (15.8%, 28.8%) |
| HS Grad (n=197) | 22.8% | (16.9%, 28.7%) | 12.6 | (11.6, 13.6) | 2.24 | (1.7, 2.8) | 5.32 | (4.5, 6.1) | 5.12 | (4.5, 5.7) | 7.36 | (6.7, 8.0) | 45.2% | (38.1%, 52.3%) | 20.8% | (15.1%, 26.5%) |
| More than HS (n=194) |
27.8% | (21.5%,34.1% | 12.0 | (11.0, 13.0) | 1.99 | (1.6, 2.4) | 5.16 | (4.5, 5.9) | 5.81 | (5.2, 6.4) | 7.80 | (7.1, 8.5) | 46.4% | (39.3%, 53.5%) | 24.2% | (18.1%, 30.3%) |
|
Cigarette Smoking History |
||||||||||||||||
| Current smoker (n=377) |
17.5% | (13.6%, 21.4%) | 13.1 | (12.4, 13.8) | 2.48 | (2.1, 2.9) | 5.66 | (5.1, 6.3) | 5.00 | (4.6, 5.4) | 7.48 | (7.0, 8.0) | 47.5% | (42.4%, 52.6%) | 24.9% | (20.6%, 29.2%) |
| Former smoker (n=53) |
22.6% | (11.2%, 34.0%) | 12.4 | (10.6, 14.2) | 2.11 | (1.2, 3.0) | 4.34 | (3.1, 5.6) | 5.98 | (4.8, 7.1) | 8.10 | (6.7, 9.5) | 47.2% | (33.7%, 60.7%) | 22.6% | (11.2%, 34.0%) |
| Never smoked (n=121) |
41.3% | (32.5%, 50.1%) | 11.2 | (10.0, 12.5) | 1.45 | (1.0, 1.9) | 3.29 | (2.4, 4.2) | 6.52 | (5.6, 7.4) | 7.97 | (7.0, 9.0) | 36.4% | (27.8%, 45.0%) | 14.0% | (7.7%, 20.3%) |
|
Methamphetamin e Use |
||||||||||||||||
| Low (n=245) | 25.3% | (19.8% ,30.8%) | 12.2 | (11.4, 13.0) | 1.81 | (1.4, 2.2) | 4.67 | (4.0, 5.3) | 5.72 | (5.2, 6.3) | 7.53 | (6.9, 8.2) | 41.6% | (35.3%, 47.9%) | 18.8% | (13.9%, 23.7%) |
| Moderate / Heavy (n=307) |
21.5% | (17.0%, 26.0%) | 13.1 | (12.3, 13.9) | 2.61 | (2.2, 3.0) | 5.28 | (4.6, 6.0) | 5.18 | (4.7, 5.7) | 7.79 | (7.2, 8.3) | 47.9% | (42.2%, 53.6%) | 25.4% | (20.5%, 30.3%) |
| Total (n=552) | 23.2% | (19.7%, 26.7%) | 12.7 | (12.1, 13.3) | 2.25 | (2.0, 2.5) | 5.01 | (4.5, 5.5) | 5.42 | (5.1, 5.8) | 7.67 | (7.3, 8.1) | 45.1% | (41.0%, 49.2%) | 22.5% | (19.0%, 26.0%) |
Table 3 shows the distribution of various periodontal outcomes by age, gender, ethnicity, education, cigarette smoking status, and methamphetamine use. The percentage of MA users with severe periodontitis was roughly twice as high for African Americans (36.5%) compared to Caucasians (17.6%). Among current cigarette smokers, 32% had severe periodontitis and 89% manifested periodontitis. In contrast, 22% of non-smokers had severe periodontitis and 75% had any periodontitis. The prevalence of severe periodontitis dropped further in low-use MA users where only 25% had severe periodontitis. Mean gingival recession was higher among current cigarette smokers compared to former or nonsmokers.
TABLE 3.
PREVALENCE OF PERIODONTAL DISEASE
| Total Periodontitis |
Moderate Periodontitis |
Severe Periodontitis |
||||
|---|---|---|---|---|---|---|
| % | CI | % | CI | % | CI | |
| Age | ||||||
| <30 years (n=48) | 68.8% | (55.5%, 82.1%) | 50.0% | (35.7%, 64.3%) | 8.3% | (0.5%, 16.1%) |
| ≥ 30 years (n=498) | 90.4% | (87.9%, 92.9%) | 57.6% | (53.3%, 61.9%) | 31.1% | (27.0%, 35.2%) |
| Sex | ||||||
| Male (n=441) | 89.1% | (86.2%, 92.0%) | 57.8% | (53.1%, 62.5%) | 29.5% | (25.2%, 33.8%) |
| Female (n=105) | 85.7% | (79.0%, 92.4%) | 53.3% | (43.7%, 62.9%) | 27.6% | (19.0%, 26.2%) |
| Race/ethnicity | ||||||
| Hispanic (n-174) | 86.8% | (81.7%, 91.9%) | 58.0% | (50.6%, 65.4%) | 25.9% | (19.4%, 32.4%) |
| African-American (n=233) | 91.0% | (87.3%, 94.7%) | 53.2% | (46.7%, 59.7%) | 36.5% | (30.2%, 42.8%) |
| Caucasian (n=102) | 86.3% | (79.6%, 93.0%) | 65.7% | (56.5%, 74.9%) | 17.6% | (10.2%, 25.0%) |
| Other (n=37) | 86.5% | (75.3%, 97.7%) | 51.4% | (35.1%, 67.7%) | 29.7% | (14.8%, 44.6%) |
| Education | ||||||
| Less than HS (n=159) | 90.6% | (86.1%, 95.1%) | 56.0% | (48.4%, 63.6%) | 32.7% | (25.4%, 40.0%) |
| HS (n=194) | 88.1% | (83.6%, 92.6%) | 57.7% | (50.6%, 64.8%) | 29.4% | (22.9%, 35.9%) |
| More than HS (n=193) | 87.0% | (82.3%, 91.7%) | 57.0% | (49.9%, 64.1%) | 25.9% | (19.6%, 32.2%) |
| Smoking History | ||||||
| Current smoker (n=372) | 89.5% | (86.4%, 92.6%) | 55.4% | (50.3%, 60.5%) | 32.0% | (27.3%, 36.7%) |
| Former smoker (n=52) | 88.5% | (79.7%, 97.3%) | 63.5% | (50.4%, 76.6%) | 23.1% | (11.5%, 34.7%) |
| Never smoked (n=121) | 75.1% | (68.8%, 81.4%) | 59.5% | (50.7%, 68.3%) | 22.3% | (14.9%, 29.7%) |
| Methamphetamine Use | ||||||
| Low (n=242) | 85.1% | (80.6%, 89.6%) | 56.2% | (49.9%, 62.5%) | 24.8% | (19.3%, 30.3%) |
| Moderate/High (n=304) | 91.1% | (88.0%, 94.2%) | 57.6% | (52.1%, 63.1%) | 32.6% | (27.3%, 37.9%) |
| Total (n=546) | 88.5% | (85.8%, 91.2%) | 57.0% | (52.9%, 61.1%) | 29.1% | (25.4%, 32.8%) |
Table 4 summarizes the association between anterior caries, root caries, and severe periodontitis by key demographic and behavioral factors. Being a current cigarette smoker and having medium/high MA use were both significant predictors of the unadjusted odds of having 5 or more surfaces of untreated caries. After adjusting for all risk indicators, MA users who were current cigarette smokers were roughly twice as likely to have 5 or more anterior surfaces of untreated caries compared to non-smokers (OR=1.94; 95% CI=1.05, 3.57). When evaluating for root caries, in unadjusted models, only current cigarette smokers had an elevated likelihood of having 3 or more teeth with root caries. In a full multivariate model, none of the candidate risk indicators was associated with 3 or more teeth having root caries. In models assessing for severe periodontitis, being older, African-American, or a smoker were associated with severe periodontitis. After adjusting for all risk indicators, being older (OR=2.26; 95% CI=1.32, 3.87) and African American (OR=1.50; 95%CI=1.08, 2.04) remained associated with the presence of severe periodontitis among all MA users.
TABLE 4.
ORAL HEALTH INDICATORS AMONG METHAMPHETAMINE USERS.
| Anterior Caries (unadjusted) DS ≥ 5 |
Anterior Caries (adjusted) DS ≥ 5 |
Root Caries (unadjusted) ≥ 3 teeth |
Root Caries (adjusted) ≥ 3 teeth |
Severe Periodontitis (unadjusted) |
Severe Periodontitis (adjusted) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | CI | OR | CI | OR | CI | OR | CI | OR | CI | OR | CI | |
| Age | ||||||||||||
| <30 yearsR | ||||||||||||
| ≥ 30 years | 1.84 | (0.55,6.13) | 1.30 | (0.69,2.45) | 1.76 | (0.77,4.02) | 1.28 | (0.84,1.65) | 4.94 | (1.74,13.99) | 2.26 | (1.32,3.87) |
| Sex | ||||||||||||
| Male | 0.43 | (0.24,0.79) | 0.69 | (0.50,0.94) | 0.62 | (0.39,1.00) | 0.81 | (0.63,1.20) | 1.14 | (0.70,1.83) | 1.10 | (0.86,1.42) |
| FemaleR | ||||||||||||
| Race/ethnicity | ||||||||||||
| Hispanic | 0.56 | (0.23,1.40) | 0.47 | (0.27,0.88) | 0.72 | (0.39,1.31) | 0.74 | (0.49,1.03) | 1.63 | (0.88,3.00) | 1.03 | (0.71,1.49) |
| African-American | 1.37 | (0.64,2.91) | 1.07 | (0.69,1.66) | 1.17 | (0.68,2.03) | 1.06 | (0.76,1.89) | 2.68 | (1.51,4.76) | 1.50 | (1.08,2.04) |
| Other | 2.48 | (0.90,6.86) | 2.33 | (1.18,4.59) | 1.42 | (0.61,3.29) | 1.35 | (0.76,1.81) | 1.80 | (0.74,4.37) | 1.12 | (0.62,2.04) |
| CaucasianR | ||||||||||||
| Education | ||||||||||||
| Less than HS | 1.32 | (0.69,2.52) | 1.32 | (0.87,2.00) | 0.88 | (0.53,1.44) | 0.99 | (0.72,1.38) | 1.36 | (0.86,2.17) | 1.13 | (0.85,1.51) |
| HS | 0.82 | (0.42,1.62) | 0.79 | (0.52,1.20) | 0.82 | (0.51,1.32) | 0.90 | (0.67,1.24) | 1.19 | (0.76,1.86) | 1.01 | (0.77,1.32) |
| More than HSR | ||||||||||||
| Smoking History | ||||||||||||
| Current smoker | 2.93 | (1.22,7.02) | 1.94 | (1.05,3.57) | 2.03 | (1.16,3.57) | 1.21 | (0.87,1.97) | 1.64 | (1.01,2.65) | 1.30 | (0.95,1.79) |
| Former smoker | 0.75 | (0.15,3.85) | 0.58 | (0.21,1.61) | 1.78 | (0.79,4.07) | 1.18 | (0.72,1.46) | 1.04 | (0.48,2.27) | 0.85 | (0.53,1.37) |
| Never smokedR | ||||||||||||
|
Medium / High Methamphetamine Use |
||||||||||||
| Yes | 2.08 | (1.15,3.75) | 1.34 | (0.98,1.83) | 1.46 | (0.96,2.20) | 1.17 | (0.94,1.17) | 1.45 | (0.99,2.12) | 1.17 | (0.96,1.43) |
HIERARCHY OF CARIES SUSCEPTIBILITY
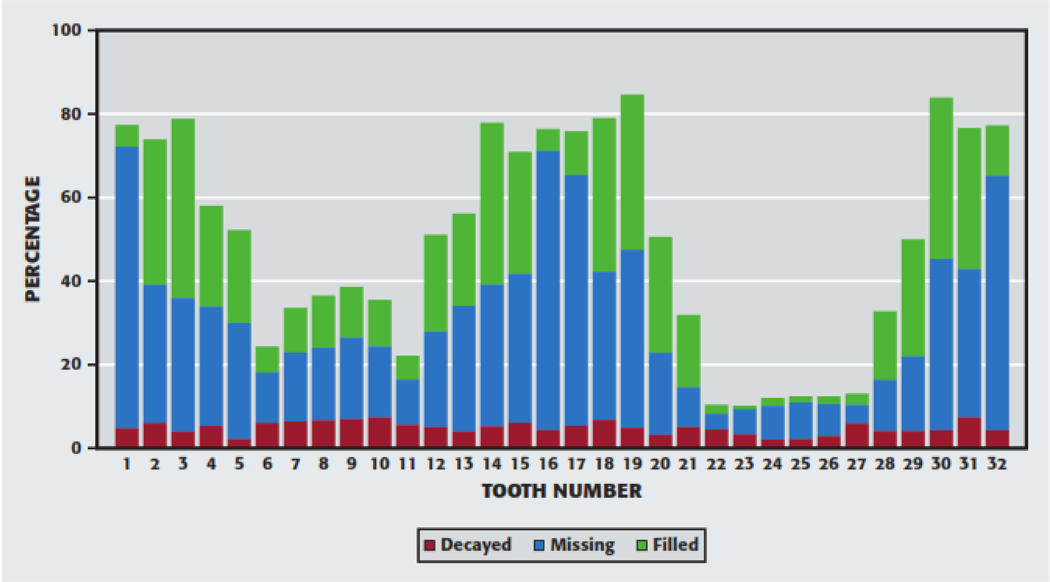
Figure 1 displays the distribution of the calculated DMF by each tooth. Caries experience was higher in posterior teeth and most were either restored or extracted. Caries experience was higher among maxillary anterior teeth compared to mandibular anterior teeth and more maxillary teeth were either restored or extracted compared to mandibular teeth. Based on DMF patterns, the teeth could be grouped, from most susceptible to least susceptible, as follows: mandibular second and first molars, maxillary second and first molars; maxillary first premolars, maxillary and mandibular second premolars; maxillary incisors; maxillary canines, mandibular first premolars; and mandibular central and lateral incisors, and mandibular canines.
Figure 1.
Decayed, missing, and filled teeth by tooth number.
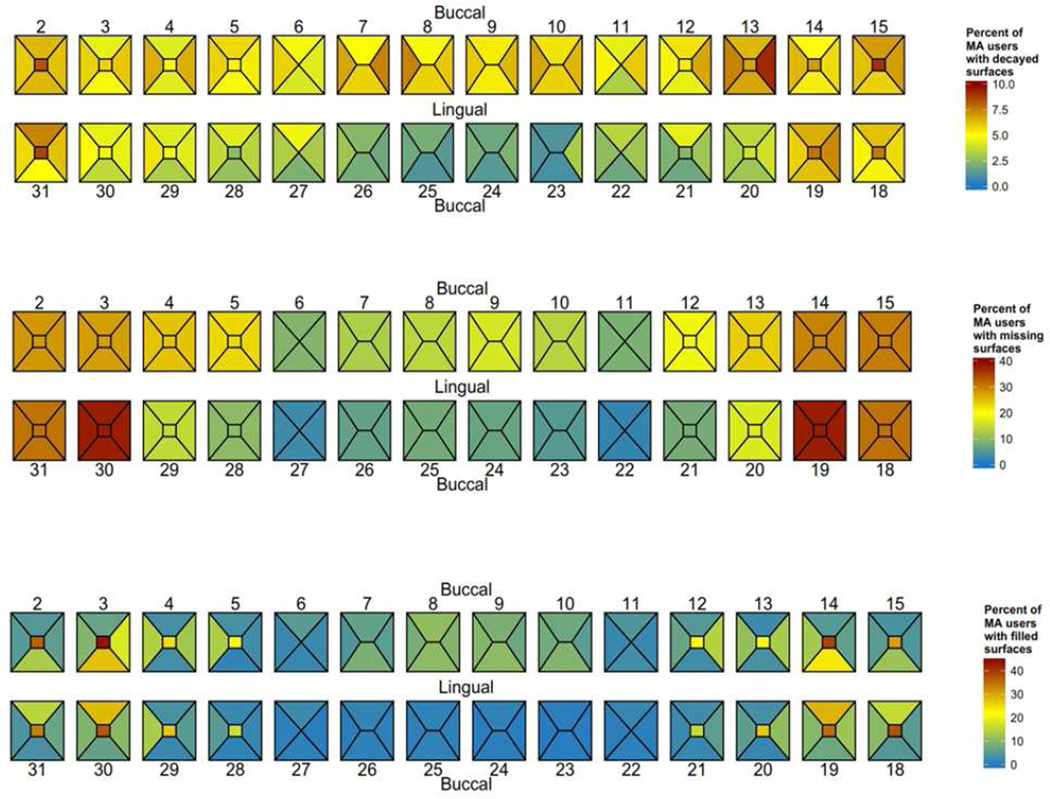
A graphical “heat map” representation of the proportion of the study sample with decayed, missing and filled tooth surfaces is provided in Figure 2. The occlusal surfaces of the molars had higher rates of being decayed or filled, with nearly 80% of the subjects having a decayed, missing or filled occlusal surface for each of the molars. In this sample, the most frequently missing teeth/surfaces were mandibular first molars, missing in approximately 40% of the sample. Overall, the pattern is consistent with a left:right symmetry with regards to the propensity for caries. However, there is a higher prevalence of surface decay on the distal surface of the maxillary right pre-molar (tooth 13) that is not present at the same prevalence on the corresponding left pre-molar (tooth 4).
Figure 2.
DISCUSSION
Although commonly accepted that MA use is associated with accelerated and extensive dental disease, our study is the first to present data, collected within a rigorous scientific framework, on the patterns and severity of dental disease in MA users or the differential susceptibility of their tooth surfaces to caries. Our study of a range of MA users, utilizing detailed oral examinations by trained dentists, corroborate our previous findings of high rates of dental disease in a physician-conducted investigation of the health consequences of MA-use.16 Across all observed dental outcomes, MA users were found to have substantial dental disease experience with older MA users (> 30 years) disproportionately affected. The disparate impact was manifest in the high rates of tooth loss and caries experience in the older MA users. Older MA users were less likely to have retained all their natural teeth and were more likely to have lost multiple teeth with more than 6% of the older MA users having less than 10 remaining teeth present. Older MA users had a high DMFT score with 97% having had dental caries in their lifetime, 59% having untreated dental caries, and 11% having caries involving their anterior teeth; teeth that are usually the least affected by dental decay.
Overall, 96% of the MA users in our study had experienced dental caries and 58% had untreated tooth decay. Only 23% retained all of their natural teeth. Although recent national estimates from 2011–2012 indicate a dental caries prevalence among adults that is similar to our MA user group, the untreated dental caries in our MA group was twice as high (27% vs. 58%).33 Furthermore, the tooth retention rates in the MA group was roughly half that of the US general population (23% vs. 48%). Given the relative youth (mean age = 44 years) of the cohort, it is striking that nearly 60% of the sample were missing one or more teeth and 7% were completely edentulous. Thirty-one percent of the MA-cohort had six or more missing teeth, a substantially higher portion than the 8.5% of adults in the general population who have six or more missing teeth.34 Our findings echo and reinforce the previous reports of Morio et al. (2008)35 who examined a small group of 18 MA users and determined that they had fewer molars and more dental decay than a corresponding group of age and sex-matched non-users. Although focusing on MA users, our findings confirm other research studies documenting worse oral-health status among substance-using populations.36,37,38
We found a dose-response relationship between greater levels of MA use and elevated rates of tooth decay. In our cohort of MA users, adults reporting medium/high MA use were twice as likely to have 5 or more surfaces involved with untreated dental caries compared to low MA users. Additionally, the data suggested that smoking conferred an increased risk of dental disease. Cigarette-smokers were nearly three times as likely to have untreated caries compared to non-smokers. After controlling for key socio-demographic and behavioural risk indicators, current smoking (and intensity) was found to be independently associated with greater rates of untreated caries. Beyond a larger proportion of untreated caries, the rates of anterior caries, root caries and tooth loss was generally higher in the smokers compared to non-smokers. Our finding supports the conclusion that smoking, a common underlying determinant of risk-behaving behaviors, acts as an effect modifier for caries severity among MA users. By extension, smoking may be construed as a modifiable risk factor for the increased rates of dental disease in MA users.
Overall, posterior teeth were most affected by dental caries in the MA users with occlusal surfaces being most commonly involved. The excessive involvement of the posterior teeth was manifest by the higher rates of missing teeth or the presence of multiple restored surfaces in the posterior regions. This finding is not surprising because the posterior teeth always have the highest prevalence of decay compared to the other teeth.41 Of note, the rates of tooth loss in women were higher compared to men. Although there was little difference in dental caries rates by gender, mandibular teeth in men were more likely to be involved by untreated caries whereas women had a dramatically higher prevalence of anterior caries. Root caries was very prevalent in this cohort, but none of the key socio-demographic and behavioral risk indicators were associated with extensive root caries (≥ 3 teeth) except for smoking. In general, the root caries experience was higher in the older MA group with the left mandibular first premolar and the right mandibular canine being the most commonly affected. Our findings in MA users contrasts with Hellyer et al. (1990)39 who have reported that maxillary canine were the most commonly affected by root caries in adult populations. Other investigators have found that mandibular molars were the most frequently attacked teeth, followed by the mandibular premolars and maxillary canines.40 Our findings suggest that the patterns of root caries in MA users are distinct and could be potentially used for identifying covert MA users.
A majority of the MA users reported unsatisfactory oral health status. Our finding is consistent with research that has found an association between substance use and perceptions of poor oral health.41,42 Equally important, a large subset of the MA subjects indicated that they were often self-conscious or embarrassed because of the condition of their teeth or dentures. The data suggest that the appearance of their teeth has a strong effect on both the MA users’ self-image and self-esteem. Several researchers have shown that perceived oral health is an important part of health-related quality of life and influences an individuals’ sense of general health and well-being.43, 44 Thus, the results underscore the importance of addressing MA users concerns about their oral health. In addition to using dental treatment to improve morale and self-esteem, the concerns about appearance could be used as the basis for brief behavioral interventions in dental settings.
Periodontal disease was unusually high in our study group. Whereas 37% of adults aged 35–49 in the US general population have total periodontitis,45 over 89% of the MA users showed total periodontitis. The risk indicators for severe periodontal disease in the MA users mostly mirrored the findings in the general US population but differed in other aspects. For instance, being African-American and older doubled the risk of severe periodontitis in our MA cohort, a finding common in the general US population. However, education (a proxy for socioeconomic status) and smoking were not significant in the MA cohort but are significant risk indicators in the general US population. Interestingly, current smoking status was not associated with severe periodontitis when adjusting for sociodemographic risk indicators and MA use levels although current smoking was associated with extensive untreated anterior dental caries and root caries. Though it is not clear why the effect of smoking was found to be significant for caries but not for periodontal disease in the MA users, one explanation may be that MA users who are current cigarette smokers may engage in behaviors that promote the initiation of dental caries at a higher rate. For example, they may consume more sugar-sweetened beverages or they may prefer a route of MA use (i.e., smoking) that promotes tooth decay.6
This study, which to our knowledge is the first broad-based, systematic study of the dental consequences of methamphetamine use, provides conclusive evidence of the elevated dental disease in MA users. Two compelling strengths of the study were the use of a calibrated measurement protocol to meticulously catalogue the nature and extent of dental disease and the availability of a large cohort of community individuals with varying methamphetamine-use behaviors. Furthermore, the study included several background and lifestyle variables considered important with regard to diet and oral health variability. Our results have important research as well as public health implications. Natural history studies of temporal changes in oral health in MA users would be very difficult to conduct and hybrid, case-study approach provides a valid alternative. Beyond scientific confirmation of the reported association between use of methamphetamines and poor oral health results, our data provide a clearer understanding of the rates and patterns of dental disease in a variety of MA users. In a follow up paper, we will compare the dental disease data to sociodemographically comparable, non-substance using individuals culled from the NHANES study. The exploration of age, gender, dental self-image and modifiable risk factors (e.g., smoking) as well as the rates and patterns of dental disease are essential for the development of screening, brief interventions and referral to treatment (SBIRT) strategies that can be applied in the setting. The unusual patterns of dental caries have potential utility as a screening indicator for covert MA users presenting for dental treatment. The overall poor rating of oral health in our sample indicates that MA-using individuals are worried about the health of their teeth and gums, a concern that could be used as the basis for motivational interventions. Our irrefutable finding of dental disease as a distinct comorbidity in MA users argues for the development of comprehensive treatment plans that address both MA use and oral health problems. Dentists should be trained to identify MA users presenting in their clinics and to pay particular attention to oral health among MA users. Additionally, general health providers and addiction specialists should be aware of oral health problems among MA users. Engaging this hard-to-reach population in addiction and medical care could be enabled by assisting MA users with their oral health needs and concerns.
Acknowledgments
This study was funded by NIH/National Institute on Drug Abuse (R01 DA026014: Dr. Vivek Shetty) which had no role in the design of the study, in the collection, analysis and interpretation of data, or in the writing of the report. Additional support for Dr. Belin was provided by a NIH/UCLA CTSI grant (UL1TR000124) The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute on Drug Abuse, the National Institutes of Health or the Centers of Disease Control and Prevention. We wish to acknowledge the efforts of Mr. Peter Cebezas (Interviewer) and Ms. Rachel Fintzy (Project Director) who were responsible for recruitment, study coordination and data collection. We gratefully acknowledge the participation and support of the subjects as well as the administrative and clinical staff of the clinics that participated in the study. This manuscript was prepared in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.46
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure. None of the authors reported any disclosures.
References
- 1.Richards JR, Brofeldt BT. Patterns of Tooth Wear Associated With Methamphetamine Use. Journal of Periodontology. 2000;71(8):1371–1374. doi: 10.1902/jop.2000.71.8.1371. [DOI] [PubMed] [Google Scholar]
- 2.Goodchild JH, Donaldson M, Mangini DJ. Methamphetamine abuse and the impact on dental health. Dent Today. 2007;26(5):124, 126, 128–131. quiz 131. [PubMed] [Google Scholar]
- 3.Mallatt ME. Meth mouth: a national scourge. J Indiana Dent Assoc. 2005;84(3):28–29. [PubMed] [Google Scholar]
- 4.Padilla R, Ritter AV. Meth mouth: methamphetamine and oral health. J Esthet Restor Dent. 2008;20(2):148–149. doi: 10.1111/j.1708-8240.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- 5.Rhodus NL, Little JW. Methamphetamine abuse and “meth mouth”. Northwest Dent. 2005;84(5):29, 31, 33–37. [PubMed] [Google Scholar]
- 6.Saini T, Edwards PC, Kimmes NS, Carroll LR, Shaner JW, Dowd FJ. Etiology of xerostomia and dental caries among methamphetamine abusers. Oral Health Prev Dent. 2005;3(3):189–195. [PubMed] [Google Scholar]
- 7.Shaner JW, Kimmes N, Saini T, Edwards P. “Meth mouth”: rampant caries in methamphetamine abusers. AIDS Patient Care STDS. 2006;20(3):146–150. doi: 10.1089/apc.2006.20.146. [DOI] [PubMed] [Google Scholar]
- 8.Diago S. When Your Patient is an Addict. AGD Impact. 2003;9 [Google Scholar]
- 9.Murakawa N. Toothless: The Methamphetamine Epidemic, Meth Mouth, and the Racial Construction of Drug Scares. Du Bois Review. 2011 Spring;2011:219–229. [Google Scholar]
- 10.Di Cugno F, Perec CJ, Tocci AA. Salivary secretion and dental caries experience in drug addicts. Archives of Oral Biology. 1981;26(5):363–367. doi: 10.1016/0003-9969(81)90031-5. [DOI] [PubMed] [Google Scholar]
- 11.Duxbury AJ. Ecstasy--dental implications. Br Dent J. 1993;175(1):38. doi: 10.1038/sj.bdj.4808200. [DOI] [PubMed] [Google Scholar]
- 12.Howe AM. Methamphetamine and childhood and adolescent caries. Aust Dent J. 1995;40(5):340. doi: 10.1111/j.1834-7819.1995.tb04825.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee CY, Heffez LB, Mohammadi H. Crystal methamphetamine abuse: a concern to oral and maxillofacial surgeons. J Oral Maxillofac Surg. 1992;50(10):1052–1054. doi: 10.1016/0278-2391(92)90489-m. [DOI] [PubMed] [Google Scholar]
- 14.Milosevic A, Agrawal N, Redfearn P, Mair L. The occurrence of toothwear in users of Ecstasy (3,4-methylenedioxymethamphetamine) Community Dent Oral Epidemiol. 1999;27(4):283–287. doi: 10.1111/j.1600-0528.1998.tb02022.x. [DOI] [PubMed] [Google Scholar]
- 15.Nixon PJ, Youngson CC, Beese A. Tooth surface loss: does recreational drug use contribute? Clin Oral Investig. 2002;6(2):128–130. doi: 10.1007/s00784-002-0159-2. [DOI] [PubMed] [Google Scholar]
- 16.Shetty V, Mooney LJ, Zigler CM, Belin TR, Murphy D, Rawson R. The Relationship Between Methamphetamine Use and Increased Dental Disease. JADA. 2010;141(3):307–318. doi: 10.14219/jada.archive.2010.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dye BA, Harrell L, Murphy DA, Belin TR, Shetty V. Performance of a Quality Assurance Program for Assessing Dental Health in Methamphetamine Users. BMC Oral Health. 2015;15(76) doi: 10.1186/s12903-015-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agerwala SM, McCance-Katz EF. Integrating screening, brief intervention, and referral to treatment (SBIRT) into clinical practice settings: a brief review. J Psychoactive Drugs. 2012;44(4):307–317. doi: 10.1080/02791072.2012.720169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atkinson R, Flint J. Accessing hidden and hard-to-reach populations: Snowball research strategies. Social Research Update. 2001;33(1):1–4. [Google Scholar]
- 20.Anglin MD, Burke C, Perrochet B, Stamper E, Dawud-Noursi S. History of the Methamphetamine Problem. Journal of Psychoactive Drugs. 2000;32(2):137–141. doi: 10.1080/02791072.2000.10400221. [DOI] [PubMed] [Google Scholar]
- 21.Gonzales R, Mooney L, Rawson RA. The Methamphetamine Problem in the United States. Annual Review of Public Health. 2010;31(1):385–398. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dye BA, Nowjack-Raymer R, Barker LK, et al. Overview and Quality Assurance for the Oral Health Component of the National Health and Nutrition Examination Survey (NHANES), 2003-04. Journal of Public Health Dentistry. 2008;68(4):218–226. doi: 10.1111/j.1752-7325.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- 23.Dye BA, Barker LK, Li X, Lewis BG, Beltrán-Aguilar ED. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2005-08. Journal of Public Health Dentistry. 2011;71(1):54–61. doi: 10.1111/j.1752-7325.2010.00202.x. [DOI] [PubMed] [Google Scholar]
- 24.Miller A, Brunelle J, Carlos J, Brown L, Loe H. Oral health of United States adults. The national survey of oral health in US employed adults and seniors. [Accessed May 19, 2015];National findings: 1985–1986. 1987 https://www.google.com/search?q=Ho%2C+Imai%2C+King%2C+Stuart+2011&ie=utf-8&oe=utf-8#q=Miller+1987+oral+health+of+united+states+adults&spell=1. [Google Scholar]
- 25.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the Case Definitions for Population-Based Surveillance of Periodontitis. Journal of Periodontology. 2012;83(12):1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomson WM, Chalmers JM, Spencer AJ, Williams SM. The Xerostomia Inventory: a multi-item approach to measuring dry mouth. Community Dent Health. 1999;16(1):12–17. [PubMed] [Google Scholar]
- 27.Murphy DA, Hser Y-I, Huang D, Brecht M-L, Herbeck DM. Self-report of Longitudinal Substance Use: A Comparison of the UCLA Natural History Interview and the Addiction Severity Index. J Drug Issues. 2010;40(2):495–516. doi: 10.1177/002204261004000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dent Health. 1994;11(1):3–11. [PubMed] [Google Scholar]
- 29.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 30.Wc W, Rd R, S C-H, L S, Ml B. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc. 1987;87(1):43–47. [PubMed] [Google Scholar]
- 31.Rl K, Lr K. Validity of the 24-hour dietary recall. J Am Diet Assoc. 1985;85(11):1437–1442. [PubMed] [Google Scholar]
- 32.Dye BA, Li X, Lewis BG, Iafolla T, Beltran-Aguilar ED, Eke PI. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2009–2010. J Public Health Dent. 2014 Jun; doi: 10.1111/jphd.12056. [DOI] [PubMed] [Google Scholar]
- 33.Dye BA, Thornton-Evans G, Li X, Iafolla T. Dental Caries and Tooth Loss in Adults in the United States, 2011–2012. [Accessed May 19, 2015];NCHS Data Brief. 2015 May; http://www.cdc.gov/nchs/data/databriefs/db197.htm. [PubMed]
- 34.Kapp JM, Austin Boren S, Yun S, LeMaster J. Diabetes and Tooth Loss in a National Sample of Dentate Adults Reporting Annual Dental Visits. [Accessed May 19, 2015];Prev Chronic Dis. 2007 4(3) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1955413/ [PMC free article] [PubMed] [Google Scholar]
- 35.Morio KA, Marshall TA, Qian F, Morgan TA. Comparing diet, oral hygiene and caries status of adult methamphetamine users and nonusers: a pilot study. J Am Dent Assoc. 2008;139(2):171–176. doi: 10.14219/jada.archive.2008.0133. [DOI] [PubMed] [Google Scholar]
- 36.Reece S. Dental Health In Addiction. Australian Dental Journal. 2009;54(2):185–186. doi: 10.1111/j.1834-7819.2009.01116_7.x. [DOI] [PubMed] [Google Scholar]
- 37.Brown C, Krishnan S, Hursh K, et al. Dental disease prevalence among methamphetamine and heroin users in an urban setting A pilot study. JADA. 2012;143(9):992–1001. doi: 10.14219/jada.archive.2012.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbins JL, Wenger L, Lorvick J, Shiboski C, Kral AH. Health and Oral Health Care Needs and Health Care-Seeking Behavior Among Homeless Injection Drug Users in San Francisco. J Urban Health. 2010;87(6):920–930. doi: 10.1007/s11524-010-9498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hellyer PH, Beighton D, Heath MR, Lynch EJ. Root caries in older people attending a general dental practice in East Sussex. Br Dent J. 1990;169(7):201–206. doi: 10.1038/sj.bdj.4807326. [DOI] [PubMed] [Google Scholar]
- 40.Wallace MC, Retief DH, Bradley EL. Prevalence of root caries in a population of older adults. Gerodontics. 1988;4(2):84–89. [PubMed] [Google Scholar]
- 41.Nathwani NS, Gallagher JE. Methadone: dental risks and preventive action. Dent Update. 2008;35(8):542–544. 547–548. doi: 10.12968/denu.2008.35.8.542. [DOI] [PubMed] [Google Scholar]
- 42.Titsas A, Ferguson MM. Impact of opioid use on dentistry. Aust Dent J. 2002;47(2):94–98. doi: 10.1111/j.1834-7819.2002.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 43.Gift HC, Atchison KA, Dayton CM. Conceptualizing oral health and oral health-related quality of life. Soc Sci Med. 1997;44(5):601–608. doi: 10.1016/s0277-9536(96)00211-0. [DOI] [PubMed] [Google Scholar]
- 44.Gift HC, Atchison KA. Oral health, health, and health-related quality of life. Med Care. 1995;33(11 Suppl):NS57–NS77. doi: 10.1097/00005650-199511001-00008. [DOI] [PubMed] [Google Scholar]
- 45.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ CDC Periodontal Disease Surveillance workgroup: James Beck (University of North Carolina, Chapel Hill, USA), Gordon Douglass (Past President, American Academy of Periodontology), Roy Page (University of Washin. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 46.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]