Abstract
Background:
Among the most important factors in wound healing pathways are transforming growth factor beta1 and vascular endothelial growth factor. Fibroblasts are the main cell in all phases wound closure. In this study, the extracts of plant materials such as Adiantum capillus-veneris, Commiphora molmol, Aloe vera, and henna and one mixture of them were used to treatment of normal mouse skin fibroblasts.
Methods:
Cytotoxic effects of each extract and their mixture were assessed on mouse skin fibroblasts cells using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. We performed migration assays to assess migration properties of mouse skin fibroblasts cells in response to the extracts. Changes in the gene expression of the Tgfβ1 and Vegf-A genes were monitored by real-time polymerase chain reaction.
Results:
A. capillus-veneris, C. molmol and henna extract improved the expression of Tgfβ1 gene. All used extracts upregulated the expression of Vegf-A gene and promoted the migration of mouse fibroblast cells in vitro.
Conclusions:
The present study demonstrated that the mentioned herbal extracts might be effective in wound healing, through the improvement in the migration of fibroblast cells and regulating the gene expression of Tgfβ1 and Vegf-A genes in fibroblast cells treated with extracts.
Keywords: Fibroblast, herbal, transforming growth factor-β1, vascular endothelial growth factor, wound healing
INTRODUCTION
Wound healing process can be divided into four overlapping phases: Homeostasis, inflammation, proliferation, and remodeling.[1] The renovation of tissue veracity is due to cell–cell and cell–matrix interactions.[2] These interactions are controlled by multiple cytokines and growth factors, as well as transforming growth factor-beta 1 (TGF-β1), and vascular endothelial growth factor (VEGF). Several cells such as platelets, macrophages, and T cells produce TGF-β1, which is an effective incentive of fibroblasts.[3,4]
TGF-β1 recruits neutrophils and fibroblasts to the site of damage at the inflammatory phase of wound healing.[5] TGF-β1 suppresses severe inflammation and may therefore endorse the switch to a reparative phase. The effect of cytokine is almost never limited to one single phase of the healing progression. Fibroblasts are the main cell in all phases particularly, the proliferative phase of wound closure. TGF-β1 also contributes to the migration, growth, diversity, and motivation of fibroblasts.[6] TGF-β1 stimulates fibroblasts, which differentiates into myofibroblasts. Fibroblasts collaborate with myofibroblasts to produce extracellular matrix (ECM), collagen[7] and matrix proteins, such as fibronectin.[8] TGF-β1 is accompanied by VEGF and basic fibroblast growth factor which motivate angiogenesis.[9]
VEGF-A (also known as VEGF) is created by several cells as well as endothelial cells, fibroblasts, smooth muscle cells, platelets, neutrophils, and macrophages.[10]
Previous studies have recommended that VEGF plays an important role in angiogenesis, epithelization and collagen deposition during wound closure.[2,11] In the remodeling step, fibroblasts deposit collagen and other ECM proteins modify the immature collagen matrix into mature scar tissue. VEGF can stimulate skin fibroblasts and promote scar tissue formation by means of various mechanisms.[12,13]
Therapeutic plants have been utilized in different populations as remedial for injuries; hence, the advantage of it is their slight toxicity and availability.[14] Several studies reported that herbal extracts can be utilized in the management of wound healing.[15]
Aloe vera (Liliaceae) is a therapeutic herb that acts as a cathartic in food to remedy burns and wounds, and also contains antifungal, antimicrobial, antidiabetic, and hypoglycemic properties.[16,17] Commiphora molmol (myrrh) is a plant that produces resin and contains antibacterial, antifungal, and antidiabetic properties.[18] It has been utilized to tend wounds and also for intestinal disorders, diarrhea, coughs, inflammation, and chest ailments.[19,20]
Adiantum capillus-vernis has a long history of medicinal use. It has anti-inflammatory, anti-diabetic, anti-infective, antimicrobial, and antioxidant properties.[21] A. capillus veneris has significant angiogenic properties and improves wound healing in vitro.[22] These properties indicate that local administration of A. capillus-veneris can decrease and heal wounds. Henna (Lawsonia inermis) is a well-known medicinal plant widely utilized to treat headaches, boils, diseases of the spleen, and skin disease.[23] Experimental and clinical studies have reported that henna is an antibacterial and antifungal that supports wound healing.[24]
Since the cells of mouse fibroblast cell line always have been to use it as model eukaryotic cells are similar to human fibroblasts. In this study, cell lines C147 purchased from cell bank of Iran Pasteur Institute and the necessary tests were performed on it.
The aims of this study were to further explore the fibroblast proliferation and migration properties of these plant extracts and their mixture, to assess their wound healing activity by means of normal mouse skin fibroblasts.
METHODS
Collection of plant materials
Fresh leaves of A. vera were collected and identified from the botanic garden of Ahvaz Jondishapour University of Medical Sciences and the Department of Horticulture of the Faculty of Agriculture. A voucher specimen (No. 93) was deposited at the herbarium in the Faculty of Pharmacy. Shoots of A. capillus-veneris (Adiantaceae) were collected from Lorestan Province in Iran (no. 1661). Fresh henna leaves were collected from Kerman city in Iran (KF 1408). The oleo gum resin of C. molmol was obtained from Saudi Arabia. The origin of plant materials were systemically identified and approved at the herbarium of Shahid Chamran University of Ahvaz, Iran. After the collection of plants, fresh leaves of henna and A. vera, and the shoots of A. capillus-veneris were washed twist and dried at 60°C in an oven. The dried leaves and resin of the myrrh were then grinded in a blender into a fine powder.
Preparation of plant extracts
A total of 30g of powdered A. vera was macerated with ethanol at room temperature for 72 h, filtered through Whatman No. 1 paper filter, and then separated part was evaporated at 65°C in rotary until complete dryness.
Fifty grams of the powdered leaves of henna was soaked in 500 mL of 70% ethanol, macerated for 24 h and filtered (Whatman No. 185); the filtrate was then evaporated at 65°C in rotary evaporator until complete dryness.
Fifty grams of the powdered shoots of A. capillus-veneris was soaked in 300 mL of methanol at room temperature for 72 h and then filtered through with Whatman No. 1 filter paper. The filtrates were collected in separate flasks and were evaporated at 65°C in rotary evaporator until complete dryness.
Fifty grams of the dried powder of C. molmol oleo-gum-resin was soaked in 200 mL of methanol with continuous shaking for 24h at 40°C. The crude extracts were filtered by means of Whatman No. 1 filter paper. The filtrates were collected in separate flasks and were evaporated at 65°C in rotary evaporator until complete dryness. Dried extracts were powdered and kept at 4°C.
Cell culture
The normal mouse skin fibroblast line (c147) employed in this investigation were obtained from a National Cell Bank of Iran, Pasteur Institute of Iran, Tehran, Iran and were cultured according to the source's guidelines. Fibroblast cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 (Biosera, France) + fetal bovine serum (FBS) 10% (Gibco, USA) medium, 100 U/mL penicillin and 100 µg/mL streptomycin (Bio-Idea, Iran). Cells were kept under standard culture conditions at 37°C and 5% CO2. All cells were used between passages 5 and 6. Trypsin 0.025%-ethylenediaminetetraacetic acid 0.02% (Sigma-Aldrich, USA) in phosphate-buffered saline was used to separate fibroblast cells from the flasks.
Fibroblast 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays
The fibroblast cell viabilities and the cytotoxic effects of the each extract were scanned via the reduction of yellow tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan by viable cells, in part by the action of dehydrogenase enzymes. Subsequently, intracellular formazan can be solubilized and distinguished by spectrophotometric means.[25,26]
Six passages of fibroblast cells were trypsinized, suspended in RPMI + FBS 10%, and centrifuged. The supernatant was discarded and fibroblast cells were seeded (5 × 103 cells/well) in a 96 well plate, RPMI + FBS 10% (200 µL) was added and incubated in 5% CO2 and 37°C for 24 h. All herbal extracts were liquefied in RPMI medium following filtration through a 0.2 µm filter to avoid cell contamination. About 50 µL of each extract and mixture of them in different concentrations were added to form a final concentration of 5, 50, 250, 500, 1000, 1500, and 2000 µg/mL.
Following the 24, 48 and 72 h incubation, the medium was replaced with 50 µL MTT (5 mg/mL) and incubated at 37°C for 4 h. The MTT was then discarded, and the formazan crystals were dissolved in 100 µL dimethyl sulfoxide (Bio-Idea, Iran). The optical density (OD) of cells was measured at 570 nm using an enzyme-linked immunosorbent assay (ELISA) reader (Bio-Rad, USA).[27]
This procedure was repeated in triplicate and to calculate the toxicity effect of each extract on fibroblast cell survival; the following formula was used:
In this formula, OD experimental and OD control represents the absorbance of treated cells and nontreated cells (control).
Cytotoxicity of extract % = [(OD control group – OD experimental group)/(OD control group)] × 100.
Cell migration assay
The in vitro scratch assay was utilized to detect the influence of each mentioned plant extracts and their mixture on the migration of mouse skin fibroblast cells. Fibroblasts were seeded at high density on a 24-well plate in RPMI 1640 medium containing 100 U/mL penicillin and 100 µg/mL streptomycin and 10% FBS. After 24 h, fibroblasts were attached and spread to form a confluent monolayer. Cell monolayer was scraped with a tip. Parted cells were removed and the attached cells were incubated with 500 μL of RPMI medium containing 5% FBS, 50 μg/mL of A. vera, A. capillus-veneris and C. molmol extract and 5 μg/mL of henna and 20 μg/mL of mixture of all above extracts was added and incubated at 37°C, 5% CO2 and 90% humidity. Wound closure was examined by the quantity of transferred fibroblasts from the edge of the nick in extract treated wells in comparison to the control wells for 24, 48, and 72 h in four separate fields.[28]
Expression analysis
Total RNA of fibroblast cells was isolated using TriReagent (Invitrogen). First strand complementary DNA (cDNA) was prepared by reverse transcription using PrimeScript™ RT Reagent kit (Takara, Japan) according to manufacturer instructions. The obtained cDNA was then used for real-time polymerase chain reaction (PCR) using master SYBR Green I (Takara Bio, Japan) on ABI 7900HT. Real-time PCR was executed at 95°C for 10 s, 62°C for 15 s, and 72°C for 8 s using the primers for the normalizing Gapdh gene against the Tgfβ1 and Vegf-A target genes. Primers were designed by Gen Script according to the cDNA sequences of mouse Tgfβ1, Vegf-A and Gapdh in Gene Bank as shown in Table 1. Real-time PCR was performed in triplicate for every cDNA. Expression in fibroblast cells was treated with each extract and the mixtures at 24, 48, and 72 h after treatment were compared with the control (nontreated cells) after normalization with Gapdh.
Table 1.
Sequence of designed primers for each gene is shown as forward and reverse

We used relative gene expression, to identifying the increase or decrease of a transcript of target gene in treated sample versus control sample via normalizing with a housekeeping gene. To determine the difference of the gene expression between groups, the data were analyzed using the Relative Expression Software Tool (REST; version 2009). REST calculates the relative expression of group means for target genes Tgfβ1 and Vegf-A versus the normalizing Gapdh gene.
Statistical analysis
Statistical analysis was performed with SPSS (version 18) software. All data were presented as mean ± standard deviation. Kolmogorov–Smirnov test was utilized to examine parametric features of all statistics. One-way analysis of variance followed by Dunnett's post hoc comparison was used for multiple between-group comparisons in MTT analysis. Student's t-test was used to examine the difference in migration assay. To determine the difference of gene expression, the data were analyzed utilizing the REST; version 2009. REST calculates the relative expression of target genes Tgfβ1 and Vegf-A versus the normalizing Gapdh gene. For all statistical tests, the level of statistical significance was set at P < 0.05. REST software performed the standard method called ΔCt analysis using the Ct values for each gene. The difference between the two ΔCt values ΔCt, represents the corrected shift of the target gene in treated sample versus control samples. This is a standard and published method and allowed us to determine orders of magnitude change. In the diagram, the relative changes in the expression of each gene expressed under the influence of herbal extracts were shown.
RESULTS
Fibroblasts 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
The results of this study demonstrated significant (P < 0.05) difference in the toxicity of ethanolic extract of A. vera between doses 1000, 1500 and 2000 μg/mL in comparison to the control (nontreated) cells, moreover, there was no significant (P > 0.05) difference at doses of 5, 50, 250 and 500 μg/mL, in comparison to the control cells at 24, 48, and 72 h after treatment [Figures 1–3 and Table 2].
Figure 1.
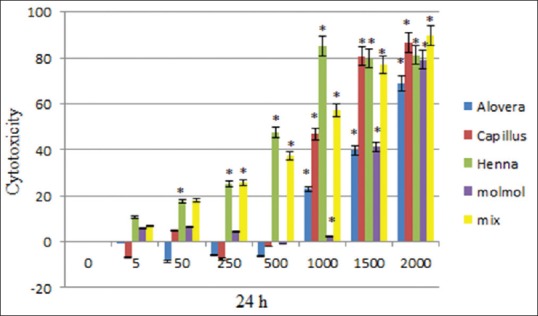
Cytotoxicity of Aloe vera, Adiantum capillus-veneris, henna, Commiphora molmol extracts and mixture of all extracts after 24 h treatment on mouse fibroblast cell line
Figure 3.
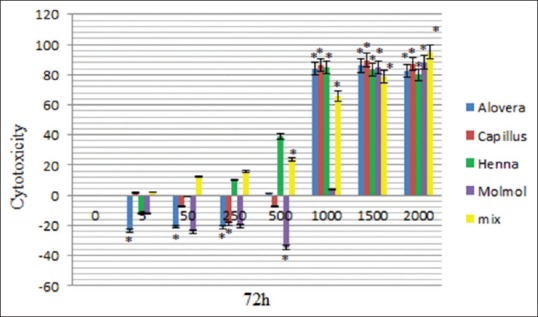
Cytotoxicity of Aloe vera, Adiantum capillus-veneris, henna, Commiphora molmol extracts and mixture of all extracts after 72 h treatment on mouse fibroblast cell line
Table 2.
Cytotoxicity of ethanolic extract Aloe vera, after 24, 48 and 72 h treatment on mouse fibroblast cell line
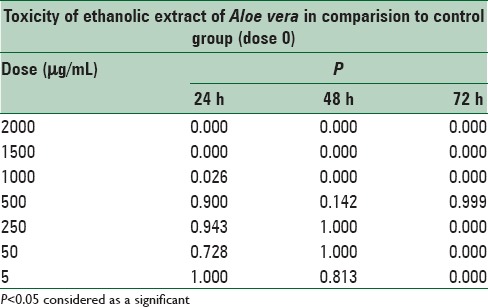
Figure 2.
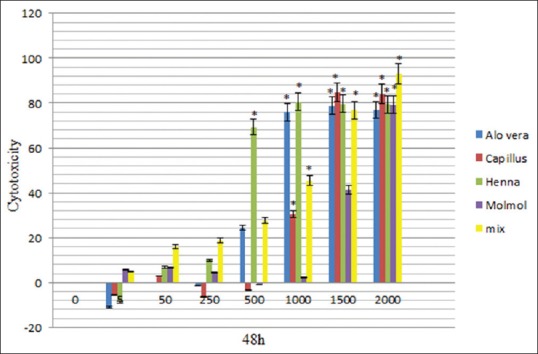
Cytotoxicity of Aloe vera, Adiantum capillus-veneris, henna, Commiphora molmol extracts and mixture of all extracts after 48 h treatment on mouse fibroblast cell line
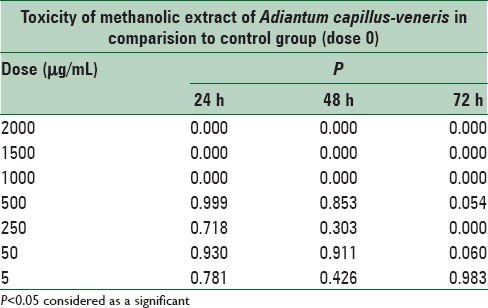
The data showed significant (P < 0.05) difference in the toxicity of methanolic extract of A. capillus-veneris between doses 1000, 1500, and 2000 μg/mL in comparison to the control cells, and there was no significant (P > 0.05) difference at doses of 5, 50, 250, and 500 μg/mL, in comparison to the cells group at 24, 48, and 72 h after treatment [Figures 1–3 and Table 3].
Table 3.
Cytotoxicity of methanolic extract Adiantum capillus-veneris, after 24, 48 and 72 h treatment on mouse fibroblast cell line
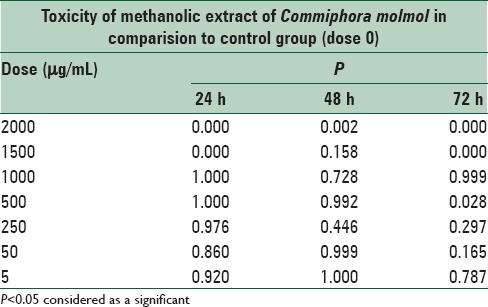
There was a significant difference in the toxicity of methanolic extract of C. molmol between doses 1500 and 2000 μg/mL in comparison to the control cells, whereas there was no significant (P > 0.05) difference at doses at 5, 50, 250, 500, and 1000 μg/mL in comparison to the control cells at 24, 48, and 72 h after treatment [Figures 1–3 and Table 4].
Table 4.
Cytotoxicity of methanolic extract Commiphora molmol, after 24, 48 and 72 h treatment on mouse fibroblast cell line
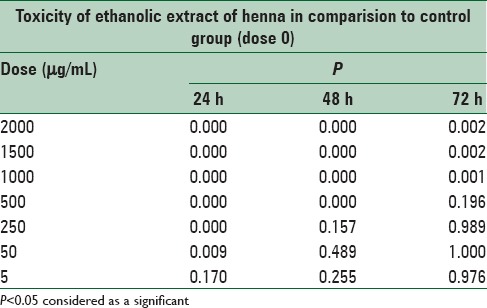
There was a significant difference (P < 0.05) in the toxicity of ethanolic extract of henna between doses of 50, 250, 500, 1000, 1500, and 2000 μg/mL in comparison to control cells, but there was no significant (P > 0.05) difference at doses 5 μg/mL in comparison to the control cells at 24 h after treatment. There was a significant difference (P < 0.05) in the toxicity of ethanolic extract of henna between doses of 500, 1000, 1500, and 2000 μg/mL in comparison to control cells, but there was no significant (P > 0.05) difference at doses 5, 50, and 250 μg/mL in comparison to the control cells at 48, 72 h after treatment [Figures 1–3 and Table 5].
Table 5.
Cytotoxicity of ethanolic extract henna, after 24, 48 and 72 h treatment on mouse fibroblast cell line
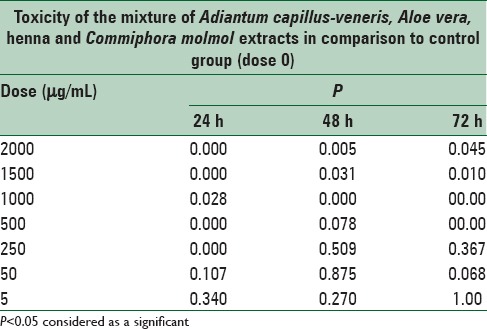
There is a significant differences (P < 0.05) in the in the toxicity of mixture of all extracts between doses of 50, 250, 500, 1000, 1500, and 2000 μg/mL in comparison to control cells, there are no significant (P > 0.05) difference at doses 5 and 50 to the control cells at 24, 48, 72 h after treatment [Figures 1–3 and Table 6].
Table 6.
Cytotoxicity of mixture of Aloe vera, Adiantum capillus-veneris, henna, Commiphora molmol extracts after 24, 48 and 72 h treatment on mouse fibroblast cell line
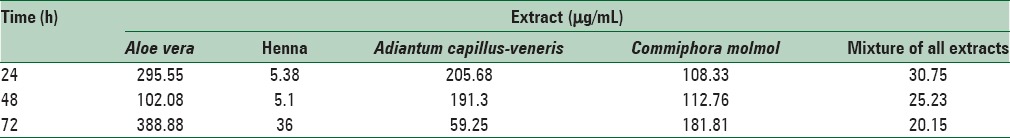
The 50% inhibitory concentration values of each used extracts for 24, 48, and 72 h treatment were calculated using linear regression as shown in Table 7. According to the results of the MTT test, the concentration of 50 μg/mL for A. vera, A. capillus-veneris and C. molmol extracts and 5 μg/mL for henna and 20 μg/mL of a mixture of all extracts that had low effects on cytotoxicity and fibroblast cells were selected.
Table 7.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay showing 50% inhibitory concentration of each extract at 24, 48 and 72 h after treatment
Gene expression analysis
The present investigation determined changes in the expression of Tgfβ1 and Vegf-A genes by real-time-PCR in fibroblast cells with each extract and their mixture at 24, 48, and 72 h after treatment. Before data analysis, melting curves were obtained for each gene. The curves confirmed the accuracy of the peak corresponding to the gene of interest and strings of primer dimer. A standard curve was plotted to evaluate the efficiency of the reaction using different dilutions of cDNA before performing real-time PCR. The relative expression of Tgfβ1 and Vegf-A gene in fibroblast cells treated with the mentioned extracts and their mixture in comparison to control cells (nontreated) at 24, 48, and 72 h after treatment are shown in Figures 4 and 5.
Figure 4.
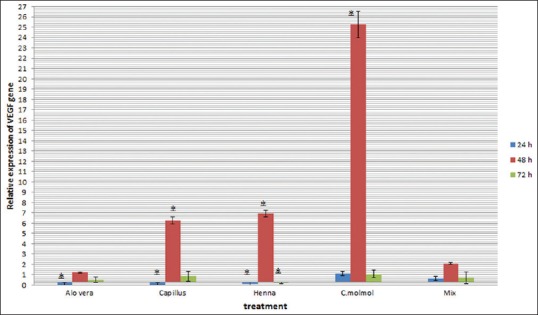
Changes in expression of Vegf-A gene in mouse fibroblast cells treated with Aloe vera, Adiantum capillus-veneris, henna, Commiphora molmol extracts and mixture of them after 24, 48 and 72 h (*P < 0.05 vs. control group)
Figure 5.
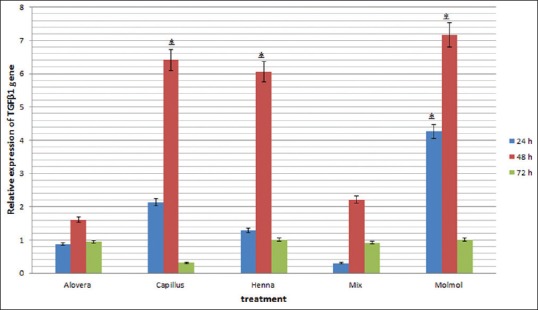
Changes in expression of Tgfβ1gene in mouse fibroblast cells treated with Aloe vera, Adiantum capillus-veneris, henna, Commiphora molmol extracts and mixture of them after 24, 48 and 72 h (*P < 0.05 vs. control group)
Fibroblast migration assay
At the start of in vitro scratch test, there was little or no cells inside the scratch region as shown in Figure 6. Migration of fibroblast cell was improved after 72 h of treatment with an ethanolic extract of A. vera when compared to control (P = 0.000) and mixture of all other extracts (P = 0.001) [Figure 7]. Migration of Fibroblast cell was significantly (P = 0.009) improved after 72 h of treatment with methanolic extract of C. molmol compared to control and at 48h (P = 0.049) and 72 h (P = 0.010) treatment with mixture of all other extracts. Migration of fibroblast cell was significantly (P = 0.000) improved after 24 h of treatment with ethanolic extract of henna compared to control and at 24 h (P = 0.002) and 48 h (P = 0.014) treatment with mixture of all other extracts [Figure 7]. This study showed that there was no significant (P > 0.05) difference in the migration of fibroblast cell treated with methanolic extract of A. capillus-veneris compared to control cells or cells treated with mixture.
Figure 6.
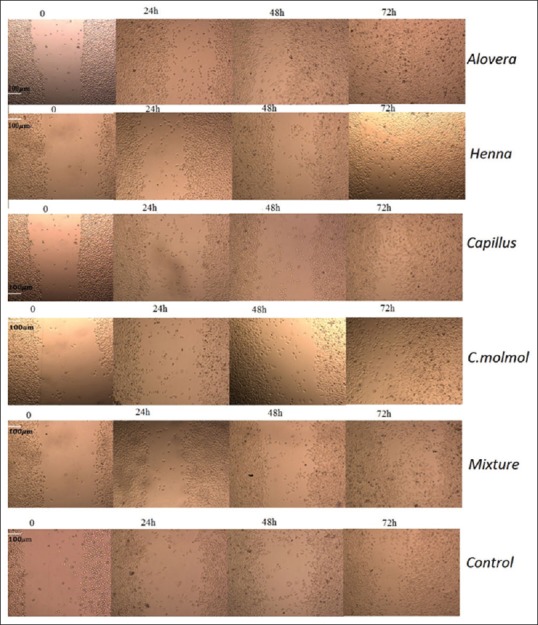
Migration of fibroblast cell at the start of in vitro scratch assay and after 24, 48 and 72 h of treatment with Aloe vera, Adiantum capillus-veneris, henna, Commiphora molmol extracts and mixture of them. Scale bar 100 μm
Figure 7.
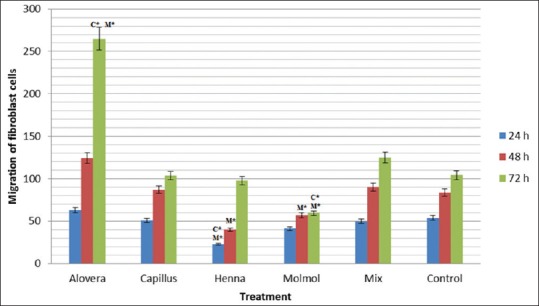
Migration of mouse fibroblast cells treated with Aloe vera, Adiantum capillus-veneris, henna, Commiphora molmol extracts and mixture of them after 24, 48 and 72 h
DISCUSSION
In recent years, the investigation on herbal treatment has improved worldwide. Various herbal extracts have shown beneficial properties as indicated in some studies.[21,25] We examined A. vera, henna, A. capillus-veneris and C. molmol extracts and their mixture for treatment of normal mouse skin fibroblast line. It was demonstrated that methanolic extract of A. capillus-veneris and C. molmol and ethanolic extract of hennasignificantly improved the expression of Tgfβ1 and Vegf-A genes at 48 h after treatment of fibroblast cells. Migration and proliferation of fibroblasts are essential during wound closure.[29,30]
Interestingly, previous studies showed that nonhealing wounds often display a loss of TGF-β1 signaling.[31,32] In the present study, significant up-regulation of the expression of Tgfβ1 occurred in treated fibroblast cells through methanolic extract of A. capillus-veneris, C. molmol and ethanolic extract of henna in comparison to control cells at 48 h posttreatment.
Coppé et al.[11] reported that hypoxia is a characteristic of wound that increases VEGF expression in different cells such as fibroblasts, keratinocytes, myocytes, and endothelial cells. Brem et al.[33] reported that in vitro administration of VEGF encourages keratinocytes and fibroblasts cells migration, and increases wound closure. Romana-Souza et al.[34] reported that VEGF and TGF-β1 increased the proliferation of keratinocytes in vitro.
Cell migration is an extremely coordinated, multi-step course that organizes embryonic morphogenesis, tissue healing, and redevelopment.[35,36] Fibroblasts migrate to the site of wound 48–72 h after injury. The migration of fibroblasts in the scratch area is likely to be a result of absolute cellular migration, proliferation, and cell death.[28] We intended a wound healing assay to measure the influence of moistened extracts on fibroblast cells migration as one of the key steps in the healing process. Fibroblasts are very important during all stages of wound healing. Our study showed that fibroblast cells’ migration was obviously increased when exposed to methanolic extract of C. molmol at 72 h compared to control cells and after 48 and 72 h compared to treated cells with mixture of herbal extracts. Previously, investigations have demonstrated that the properties of C. molmol can be attributed to terpenoids (exclusively furanoses quiterpenes), the active compounds existing in myrrh.[20,37] Phenolic compounds, alkaloids and saponins have also been detected in extracts of C. molmol. Manjula et al. demonstrated that C. molmol resin has anti-inflammatory properties in vitro via inhibition of interferon-γ, interleukin-12 (IL-12), TNF-α, IL-1β, and nitric oxide levels.[38] Tipton et al.[39] reported that myrrh oil have anti-inflammatory effects on human gingival fibroblasts and epithelial cells in vitro.
The migration assay significantly increased the migration of fibroblast cells after treatment with an ethanolic extract of henna at 24 h compared to control cells and at 24 and 48 h compared to treated cells with the mixture of herbal extracts. The leaves of the henna plant contain phytochemical ingredients such as tannin, gallic acid, glucose, mannitol, fat, resin, flavonoids, coumarins, and anthraquinones.[40,41] Habbal et al. showed that henna leaf extracts are efficient in preventing infections by inhibiting the growth of microorganisms.[42] It seems the strong cytotoxic properties of this extract could be attributed to its high antioxidant activities.
This study showed that there was no significant difference in the migration of fibroblast cell treated with methanolic extract of A. capillus-veneris compared to control cells or cells treated with the mixture of all other extracts. Nilforoushzadeh et al. reported that A. capillus-veneris promoted angiogenic effects of endothelial cells and proliferation fibroblast cells in vitro.[22] Antioxidant and anti-inflammatory activity of A. capillus-veneris could be attributed to polyphenolic and flavonoid activity.[43,44,45]
Data showed that the migration assay obviously improved fibroblast cells’ migration in exposure to ethanolic extract of A. vera at 72 h compared to control cells and herbal extract-treated cells. A. vera contains substantial amounts of phenol, saponin, and anthraquinones responsible for antibacterial, antiviral, and antifungal activity.[46] Acemannan is the main carbohydrate element obtained from the A. vera leaf that has antiviral and anticancer effects and stimulates the immune system and macrophages.[47] Jettanacheawchankit et al. investigated the influence of acemannan on the production of keratinocyte growth factor-1, VEGF, and Type I collagen production and reported that acemannan is important for oral wound healing.[48]
Histological study revealed that A. vera enhances vascularity of the wound, which removes the dead tissue and increases the health of the wound. Collagen is the main extracellular protein in the homeostasis and granulation tissue of a healing wound.[49] The results obtained from this study revealed that ethanolic extract of A. vera had no significant effects on Vegf-A and Tgfβ1 expression in fibroblast cells. A. vera leaves extract with high cytotoxicity effects could exhibit good in vitro antitumor activity. Kumar et al.[50] showed that A. vera stimulated fibroblast proliferation and migration and that these properties of A. vera could help wound healing.
CONCLUSIONS
The results of this study showed that the mentioned herbal extract can be effective during wound healing. The aim of this study was not to isolate the composition of the extract and the role of each of them. Further studies for considering and confirming the in vitro properties of each of the mentioned extracts are necessary.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors hereby express thank to the Shahid Chamran University of Ahvaz for providing grant for this study. The authors would like to thank Narges Laboratory in Khuzestan, Iran, for technical assistance.
REFERENCES
- 1.Deonarine K, Panelli MC, Stashower ME, Jin P, Smith K, Slade HB, et al. Gene expression profiling of cutaneous wound healing. J Transl Med. 2007;5:11. doi: 10.1186/1479-5876-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olczyk P, Mencner L, Komosinska-Vassev K. The role of the extracellular matrix components in cutaneous wound healing. Biomed Res Int 2014. 2014:747584. doi: 10.1155/2014/747584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med. 2006;23:594–608. doi: 10.1111/j.1464-5491.2006.01773.x. [DOI] [PubMed] [Google Scholar]
- 4.Darby IA, Bisucci T, Hewitson TD, MacLellan DG. Apoptosis is increased in a model of diabetes-impaired wound healing in genetically diabetic mice. Int J Biochem Cell Biol. 1997;29:191–200. doi: 10.1016/s1357-2725(96)00131-8. [DOI] [PubMed] [Google Scholar]
- 5.Khalil N, Greenberg AH. The role of TGF-beta in pulmonary fibrosis. Ciba Found Symp. 1991;157:194–207. doi: 10.1002/9780470514061.ch13. [DOI] [PubMed] [Google Scholar]
- 6.Nirodi CS, Devalaraja R, Nanney LB, Arrindell S, Russell S, Trupin J, et al. Chemokine and chemokine receptor expression in keloid and normal fibroblasts. Wound Repair Regen. 2000;8:371–82. doi: 10.1111/j.1524-475x.2000.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 8.Maroni D, Davis JS. Transforming growth factor Beta 1 stimulates profibrotic activities of luteal fibroblasts in cows. Biol Reprod. 2012;87:127. doi: 10.1095/biolreprod.112.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khaheshi I, Keshavarz S, Imani Fooladi AA, Ebrahimi M, Yazdani S, Panahi Y, et al. Loss of expression of TGF-ßs and their receptors in chronic skin lesions induced by sulfur mustard as compared with chronic contact dermatitis patients. BMC Dermatol. 2011;11:2. doi: 10.1186/1471-5945-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–58. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppé JP, Kauser K, Campisi J, Beauséjour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281:29568–74. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 12.Zhang D, Wan A, Chiu AP, Wang Y, Wang F, Neumaier K, et al. Hyperglycemia-induced secretion of endothelial heparanase stimulates a vascular endothelial growth factor autocrine network in cardiomyocytes that promotes recruitment of lipoprotein lipase. Arterioscler Thromb Vasc Biol. 2013;33:2830–8. doi: 10.1161/ATVBAHA.113.302222. [DOI] [PubMed] [Google Scholar]
- 13.Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, Dipietro LA. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2008;88:579–90. doi: 10.1038/labinvest.2008.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phan TT, See P, Lee ST, Chan SY. Protective effects of curcumin against oxidative damage on skin cells in vitro: Its implication for wound healing. J Trauma. 2001;51:927–31. doi: 10.1097/00005373-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Villegas LF, Fernández ID, Maldonado H, Torres R, Zavaleta A, Vaisberg AJ, et al. Evaluation of the wound-healing activity of selected traditional medicinal plants from Perú. J Ethnopharmacol. 1997;55:193–200. doi: 10.1016/s0378-8741(96)01500-0. [DOI] [PubMed] [Google Scholar]
- 16.Atiba A, Ueno H, Uzuka Y. The effect of Aloe vera oral administration on cutaneous wound healing in type 2 diabetic rats. J Vet Med Sci. 2011;73:583–9. doi: 10.1292/jvms.10-0438. [DOI] [PubMed] [Google Scholar]
- 17.Ajabnoor MA. Effect of aloes on blood glucose levels in normal and alloxan diabetic mice. J Ethnopharmacol. 1990;28:215–20. doi: 10.1016/0378-8741(90)90031-n. [DOI] [PubMed] [Google Scholar]
- 18.Nomicos EY. Myrrh: Medical marvel or myth of the Magi? Holist Nurs Pract. 2007;21:308–23. doi: 10.1097/01.HNP.0000298616.32846.34. [DOI] [PubMed] [Google Scholar]
- 19.Massoud A, El Sisi S, Salama O, Massoud A. Preliminary study of therapeutic efficacy of a new fasciolicidal drug derived from Commiphora molmol (myrrh) Am J Trop Med Hyg. 2001;65:96–9. doi: 10.4269/ajtmh.2001.65.96. [DOI] [PubMed] [Google Scholar]
- 20.Tonkal AM, Morsy TA. An update review on Commiphora molmol and related species. J Egypt Soc Parasitol. 2008;38:763–96. [PubMed] [Google Scholar]
- 21.Ishaq MS, Hussain MM, Afridi MS, Ali G, Khattak M, Ahmad S, et al. In vitro phytochemical, antibacterial, and antifungal activities of leaf, stem, and root extracts of Adiantum capillus veneris. ScientificWorldJournal 2014. 2014:269793. doi: 10.1155/2014/269793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilforoushzadeh MA, Javanmard SH, Ghanadian M, Asghari G, Jaffary F, Yakhdani AF, et al. The effects of Adiantum capillus-veneris on wound healing: An experimental in vitro evaluation. Int J Prev Med. 2014;5:1261–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Nayak BS, Isitor G, Davis EM, Pillai GK. The evidence based wound healing activity of Lawsonia inermis Linn. Phytother Res. 2007;21:827–31. doi: 10.1002/ptr.2181. [DOI] [PubMed] [Google Scholar]
- 24.Mikhaeil BR, Badria FA, Maatooq GT, Amer MM. Antioxidant and immunomodulatory constituents of henna leaves. Z Naturforsch C. 2004;59:468–76. doi: 10.1515/znc-2004-7-803. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Li S. Dandelion extracts protect human skin fibroblasts from UVB damage and cellular senescence. Oxid Med Cell Longev 2015. 2015:619560. doi: 10.1155/2015/619560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martínez A, Conde E, Moure A, Domínguez H, Estévez RJ. Protective effect against oxygen reactive species and skin fibroblast stimulation of Couroupita guianensis leaf extracts. Nat Prod Res. 2012;26:314–22. doi: 10.1080/14786411003752094. [DOI] [PubMed] [Google Scholar]
- 27.Houghton PJ, Hylands PJ, Mensah AY, Hensel A, Deters AM. In vitro tests and ethnopharmacological investigations: Wound healing as an example. J Ethnopharmacol. 2005;100:100–7. doi: 10.1016/j.jep.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Chen HH, Zhou HJ, Fang X. Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacol Res. 2003;48:231–6. doi: 10.1016/s1043-6618(03)00107-5. [DOI] [PubMed] [Google Scholar]
- 29.Wu XB, Luo XQ, Gu SY, Xu JH. The effects of Polygonum cuspidatum extract on wound healing in rats. J Ethnopharmacol. 2012;141:934–7. doi: 10.1016/j.jep.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 30.Wang XJ, Han G, Owens P, Siddiqui Y, Li AG. Role of TGF beta-mediated inflammation in cutaneous wound healing. J Investig Dermatol Symp Proc. 2006;11:112–7. doi: 10.1038/sj.jidsymp.5650004. [DOI] [PubMed] [Google Scholar]
- 31.Brown RL, Ormsby I, Doetschman TC, Greenhalgh DG. Wound healing in the transforming growth factor-beta-deficient mouse. Wound Repair Regen. 1995;3:25–36. doi: 10.1046/j.1524-475X.1995.30108.x. [DOI] [PubMed] [Google Scholar]
- 32.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 33.Brem H, Kodra A, Golinko MS, Entero H, Stojadinovic O, Wang VM, et al. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J Invest Dermatol. 2009;129:2275–87. doi: 10.1038/jid.2009.26. [DOI] [PubMed] [Google Scholar]
- 34.Romana-Souza B, Nascimento AP, Monte-Alto-Costa A. Propranolol improves cutaneous wound healing in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2009;611:77–84. doi: 10.1016/j.ejphar.2009.03.053. [DOI] [PubMed] [Google Scholar]
- 35.Mirzaaghaei S, Akrami H, Mansouri K. Ferulago angulata flower and leaf extracts inhibit angiogenesis in vitro through reducing VEGF-A and VEGFR-2 genes expression. Arch Iran Med. 2014;17:278–85. [PubMed] [Google Scholar]
- 36.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–94. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 37.Dolara P, Corte B, Ghelardini C, Pugliese AM, Cerbai E, Menichetti S, et al. Local anaesthetic, antibacterial and antifungal properties of sesquiterpenes from myrrh. Planta Med. 2000;66:356–8. doi: 10.1055/s-2000-8532. [DOI] [PubMed] [Google Scholar]
- 38.Manjula N, Gayathri B, Vinaykumar KS, Shankernarayanan NP, Vishwakarma RA, Balakrishnan A. Inhibition of MAP kinases by crude extract and pure compound isolated from Commiphora mukul leads to down regulation of TNF-alpha, IL-1beta and IL-2. Int Immunopharmacol. 2006;6:122–32. doi: 10.1016/j.intimp.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Tipton DA, Lyle B, Babich H, Dabbous MKh. In vitro cytotoxic and anti-inflammatory effects of myrrh oil on human gingival fibroblasts and epithelial cells. Toxicol In Vitro. 2003;17:301–10. doi: 10.1016/s0887-2333(03)00018-3. [DOI] [PubMed] [Google Scholar]
- 40.Kirkland D, Marzin D. An assessment of the genotoxicity of 2-hydroxy-1,4-naphthoquinone, the natural dye ingredient of Henna. Mutat Res. 2003;537:183–99. doi: 10.1016/s1383-5718(03)00077-9. [DOI] [PubMed] [Google Scholar]
- 41.Jeyaseelan EC, Jenothiny S, Pathmanathan MK, Jeyadevan JP. Antibacterial activity of sequentially extracted organic solvent extracts of fruits, flowers and leaves of Lawsonia inermis L. from Jaffna. Asian Pac J Trop Biomed. 2012;2:798–802. doi: 10.1016/S2221-1691(12)60232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Habbal O, Hasson SS, El-Hag AH, Al-Mahrooqi Z, Al-Hashmi N, Al-Bimani Z, et al. Antibacterial activity of Lawsonia inermis Linn (Henna) against Pseudomonas aeruginosa. Asian Pac J Trop Biomed. 2011;1:173–6. doi: 10.1016/S2221-1691(11)60021-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Askari G, Ghiasvand R, Feizi A, Ghanadian SM, Karimian J. The effect of quercetin supplementation on selected markers of inflammation and oxidative stress. J Res Med Sci. 2012;17:637–41. [PMC free article] [PubMed] [Google Scholar]
- 44.Calderón-Montaño JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011;11:298–344. doi: 10.2174/138955711795305335. [DOI] [PubMed] [Google Scholar]
- 45.Ibraheim ZZ, Ahmed AS, Gouda YG. Phytochemical and biological studies of Adiantum capillus-veneris L. Saudi Pharm J. 2011;19:65–74. doi: 10.1016/j.jsps.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi SW, Son BW, Son YS, Park YI, Lee SK, Chung MH. The wound-healing effect of a glycoprotein fraction isolated from Aloe vera. Br J Dermatol. 2001;145:535–45. doi: 10.1046/j.1365-2133.2001.04410.x. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka M, Misawa E, Ito Y, Habara N, Nomaguchi K, Yamada M, et al. Identification of five phytosterols from Aloe vera gel as anti-diabetic compounds. Biol Pharm Bull. 2006;29:1418–22. doi: 10.1248/bpb.29.1418. [DOI] [PubMed] [Google Scholar]
- 48.Jettanacheawchankit S, Sasithanasate S, Sangvanich P, Banlunara W, Thunyakitpisal P. Acemannan stimulates gingival fibroblast proliferation; expressions of keratinocyte growth factor-1, vascular endothelial growth factor, and type I collagen; and wound healing. J Pharmacol Sci. 2009;109:525–31. doi: 10.1254/jphs.08204fp. [DOI] [PubMed] [Google Scholar]
- 49.Morgan C, Nigam Y. Naturally derived factors and their role in the promotion of angiogenesis for the healing of chronic wounds. Angiogenesis. 2013;16:493–502. doi: 10.1007/s10456-013-9341-1. [DOI] [PubMed] [Google Scholar]
- 50.Kumar MS, Datta PK, Dutta Gupta S. In vitro evaluation of UV opacity potential of Aloe vera L. gel from different germplasms. J Nat Med. 2009;63:195–9. doi: 10.1007/s11418-008-0299-z. [DOI] [PubMed] [Google Scholar]


