Abstract
Introduction:
Advanced age has been traditionally associated with worse traumatic brain injury (TBI) outcomes. Although prompt neurosurgical intervention (NSI, craniotomy or craniectomy) may be life-saving in the older trauma patient, it does not guarantee survival and/or return to preinjury functional status. The aim of this study was to determine whether a simple score, based entirely on the initial cranial computed tomography (CCT) is predictive of the need for NSI and key outcome measures (e.g., morbidity and mortality) in the older (age 45+ years) TBI patient subset. We hypothesized that increasing number of categorical CCT findings is independently associated with NSI, morbidity, and mortality in older patients with severe TBI.
Methods:
After IRB approval, a retrospective study of patients 45 years and older was performed using our Regional Level 1 Trauma Center registry data between June 2003 and December 2013. Collected variables included patient demographics, Injury Severity Score (ISS), Abbreviated Injury Scale Head (AISh), brain injury characteristics on CCT, Glasgow Coma Scale (GCS), Intensive Care Unit (ICU) and hospital length of stay (LOS), all-cause morbidity and mortality, functional independence scores, as well as discharge disposition. A novel CCT scoring tool (CCTST, scored from 1 to 8+) was devised, with one point given for each of the following findings: subdural hematoma, epidural hematoma, subarachnoid blood, intraventricular blood, cerebral contusion/intraparenchymal blood, skull fracture, pneumocephalus, brain edema/herniation, midline shift, and external (skin/face) trauma. Descriptive statistics and univariate analyses were conducted with 30-day mortality, in-hospital morbidity, and need for NSI as primary end-points. Secondary end-points included the length of stay in the ICU (ICULOS), step-down unit (SDLOS), and the hospital (HLOS) as well as patient functional outcomes, and postdischarge destination. Factors associated with the need for NSI were determined using matched NSI (n = 310) and non-NSI (n = 310) groups. All other analyses examined the combined patient sample (n = 620). Variables achieving a significance level of P < 0.20 were included in the logistic regression. Receiver operating characteristic curves, with corresponding area under the curve (AUC) determinations, were also analyzed. Statistical significance was set at α = 0.05. Data are presented as percentages, mean ± standard deviation, or adjusted odds ratios (AORs) with 95% confidence intervals (95% CIs).
Results:
A total of 620 patients were analyzed, including 310 patients who underwent NSI and 310 age- and ISS-matched non-NSI controls. Average patient age was 72.8 ± 13.4 years (64.1% male, 99% blunt trauma, mean ISS 25.1 ± 8.68, and mean AISh/GCS of 4.63/10.9). CCTST was the only variable independently associated with NSI (AOR 1.23, 95% CI 1.06–1.42) and was inversely proportional to initial GCS and functional outcome scores on discharge. Increasing CCTST was associated with greater mortality, morbidity, HLOS, SDLOS, ICULOS, and ventilator days. On multivariate analysis, factors independently associated with mortality included AISh (AOR 2.70, 95% CI 1.21–6.00), initial GCS (AOR 1.14, 1.07–1.22), and CCTST (AOR 1.31, 1.09–1.58). Variables independently associated with in-hospital morbidity included CCTST (AOR 1.16, 1.02–1.34), GCS (AOR 1.05, 1.01–1.09), and NSI (AOR 2.62, 1.69–4.06). Multivariate models incorporating factors independently associated with each respective outcome displayed good overall predictive characteristics for mortality (AUC 0.787) and in-hospital morbidity (AUC 0.651). Finally, modified CCTST demonstrated good overall predictive ability for NSI (AUC 0.755).
Conclusion:
This study found that the number of discrete findings on CCT is independently associated with major TBI outcome measures, including 30-day mortality, in-hospital morbidity, and NSI. Of note, multivariate models with best predictive characteristics incorporate both CCTST and GCS. CCTST is easy to calculate, and this preliminary investigation of its predictive utility in older patients with TBI warrants further validation, focusing on exploring prognostic synergies between CCTST, GCS, and AISh. If independently confirmed to be predictive of clinical outcomes and the need for NSI, the approach described herein could lead to a shift in both operative and nonoperative management of patients with TBI.
Key Words: Clinical outcomes, cranial computed tomography, neurosurgical intervention, prognostic factors, traumatic brain injury scoring
INTRODUCTION
In the United States, trauma is among the leading causes of death in all age groups,[1] with traumatic brain injury (TBI) prominently contributing to both mortality and disability.[2] Advanced age has been traditionally associated with worse TBI outcomes. Current prognostication methods for patients with TBI are limited, relying largely on a combination of clinical (Glasgow Coma Scale [GCS]), anatomic (Abbreviated Injury Score Head [AISh]),[3] and radiographic (cranial computed tomography [CCT]) criteria.[4] It is well known that age is an important modulator of TBI-related mortality prediction at any given level of GCS and AISh.[5,6] There are many variables that place the older trauma patient at higher risk for TBI. These factors include increased risk for falls, gait imbalance, visual impairment, and polypharmacy.[7,8,9] Moreover, prolonged clinical recovery may be related to various comorbidities, including cardiovascular and neurodegenerative diseases with variable clinical baselines, as well as the use of anticoagulant and/or antiplatelet therapies.[10,11] There are several TBI classification systems. The GCS alone has limitations and does not take into account important factors such as cranial imaging, duration of loss of consciousness, or posttraumatic amnesia.[12,13,14] Despite a substantial body of knowledge, much remains to be learned regarding the multifactorial interplay between patient characteristics, TBI severity, and clinical outcomes.
Existing research shows that although both GCS and AISh predict mortality associated with TBI, these two scores do not necessarily correlate well.[5,15] This, in turn, suggests that additional information may be required to better explain inconsistencies between these two assessment paradigms. Although information used to estimate AISh is most frequently derived from the initial CCT, an important level of specificity may be lost as findings are assigned into predefined AISh categories.[16] Because current predictors of neurosurgical intervention (NSI) are ill-defined, it has been proposed that more detailed information based on computed tomography (CT) imaging may provide added value in this context.[4,17,18] The importance of imaging impacts surgical planning by providing anatomic direction and navigation information, determining extracranial sites to help determine the location of incision, and guiding placement of burr holes or other instrumentation when necessary.[19] In the acute setting, CT imaging findings can provide important prognostic information, thereby directing the aggressiveness of surgical versus medical management.[20,21,22] Evidence shows that specific findings on CCT are strongly associated with poor outcomes, both before[4] and after[23] NSI (craniotomy or craniectomy).
Although prompt NSI may be life-saving in the older trauma patient, it does not guarantee survival and/or return to preinjury functioning levels. The aim of this study was to determine whether a simple score, called CCT scoring tool (CCTST) and based entirely on the initial CCT, is predictive of the need for NSI, in-hospital morbidity, all-cause mortality, and other outcomes in older (age 45+ years) patients with severe TBI. We hypothesized that increasing number of discrete CCT findings may be independently associated with need for NSI, morbidity, and mortality in the above-defined population subset.
METHODS
Following IRB approval, a retrospective study of patients 45 years and older was performed using single institution registry data from our Level 1 Regional Trauma Center between June 2003 and December 2013. Abstracted data included patient demographics (age, gender); Injury Severity Score (ISS); AISh; brain injury characteristics on initial CCT; GCS; Intensive Care Unit (ICU) length of stay (LOS), hospital (HLOS), and step-down LOS (SDLOS); in-hospital morbidity; all-cause 30-day mortality; Functional Independence Measure (FIM™) score at discharge; as well as discharge disposition (home versus non-home destination). For the purposes of this study, in-hospital morbidity was classified as the presence any complication recorded in the trauma registry during the hospitalization period for each respective patient.
A novel CCT Scoring Tool (CCTST, scored from 1 to 8+) was devised by our research group for patients with TBI. Based on a combination of previously published information on anatomic, clinical, and imaging predictive factors,[4,24,25,26] it was determined that within the CCTST paradigm, one point will be given for each of the following findings: Subdural hematoma, epidural hematoma, subarachnoid hemorrhage, intraventricular hemorrhage, cerebral contusion/intraparenchymal hemorrhage, skull fracture, brain edema/herniation, midline shift, and external (skin/face) trauma. A detailed listing of CCTST subcomponents is provided in Table 1. Of note, no duplications for >1 item in each category were allowed (e.g., a patient with bilateral subdural hematomas would only receive 1 point for the presence of “subdural hematoma”). With the knowledge that a small number of patients with significant “bilateral” findings could have their injury magnitude underestimated, this determination was intended to reduce potential biases related to the large number of patients with bilateral lesions of “little to no clinical significance” (e.g., bilateral cerebral contusions or small bilateral subarachnoid hemorrhages). Finally, the determination to use an eight-point system was based on the sample size of each CCTST stratum, with minimal acceptable number of patients per group set at ten. Because of small subgroup sizes for scores >8, a single CCTST sub-group of “8+” was formed. This paradigm can, however, be extended further if sufficient number of patients at specific level of CCTST is present (e.g., at least ten patients with a score of nine could extend the current system).
Table 1.
Computed tomography findings by category

To help determine factors associated with the need for NSI, a control group of patients who did not undergo NSI but had known TBI based on their initial workup was matched with patients who underwent NSI. Matching criteria were as follows: (a) presentation within 6 months for each case–control pair, (b) gender, (c) age within 5 years for each case–control pair, and (d) ISS within three points for each matched pair. Of note, no matching was performed for either GCS or AISh.
Descriptive statistics and univariate analyses were subsequently conducted with all-cause 30-day mortality and in-hospital morbidity as primary end-points for the combined (NSI and non-NSI) cohort. Secondary end-points for the combined (NSI and non-NSI) group included HLOS, SDLOS, ICULOS, ventilator days, functional status (FIM™) at discharge, and postdischarge destination. Primary inter-group (NSI versus non-NSI) comparison involved the determination of clinical factors associated with the need for NSI. Variables achieving statistical significance of P < 0.20 were subsequently included in multivariate logistic regression for all three key study endpoints (all-cause mortality, in-hospital morbidity, and need for NSI). Potential clinical utility determination and predictive model optimization were performed using receiver operating characteristic (ROC) curves to determine the corresponding area under the curve (AUC) for all-cause 30-day mortality, in-hospital morbidity, and NSI. Statistical significance was set at α = 0.05. Unless otherwise specified, all data are presented as either percentages, mean ± standard deviation, or adjusted odds ratios (AORs) with 95% confidence intervals (95% CIs).
RESULTS
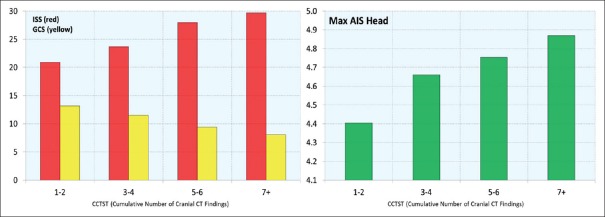
A total of 620 patients with TBI were included in the current analysis. Within the combined group, there were 310 patients who underwent NSI and 310 age- and ISS-matched non-NSI controls. Average patient age was 72.8 ± 13.4 years. The majority of patients (64.1%) were male, and the vast majority (98.7%) sustained blunt trauma, with mean ISS of 25.1 ± 8.68 (median 26, interquartile range [IQR] 10–36). In terms of TBI characteristics, the mean AISh was 4.63 ± 1.17 (median 5, IQR 4–5) and the initial mean GCS was 10.9 ± 5.07 (median 14, IQR 5–15). This was indicative of severe overall degree of brain injury in this sample. The corresponding mean CCTST was 3.64 ± 1.74 (median 3, IQR 2–6). For the combined (NSI and non-NSI) sample, the CCTST was inversely proportional to initial GCS and directly proportional to both ISS and AISh [Figure 1]. Increasing CCTST was associated with greater mortality, in-hospital morbidity, and ventilator days [Table 2]. In addition, higher CCTST scores were inversely proportional to patient FIM™ scores on discharge, and the percentage of patients discharged to home [Table 2]. Finally, increasing CCTST was associated with longer intensive care, step-down, and hospital stays [Figure 2].
Figure 1.
Relationship between Cranial Computed Tomography Scoring Tool and Injury Severity Score (red bars on left), Glasgow Coma Scale (yellow bars on left), and AISh (green bars on right); AIS Head = Abbreviated Injury Scale Head; CT = Computed tomography; CCTST = Cranial Computed Tomography Scoring Tool; GCS = Glasgow Coma Scale; ISS = Injury Severity Score
Table 2.
Cranial computed tomography scoring tool versus key clinical study outcomes
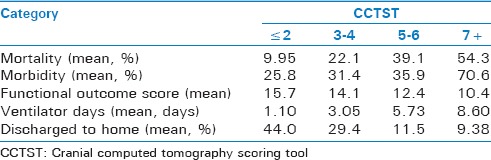
Figure 2.
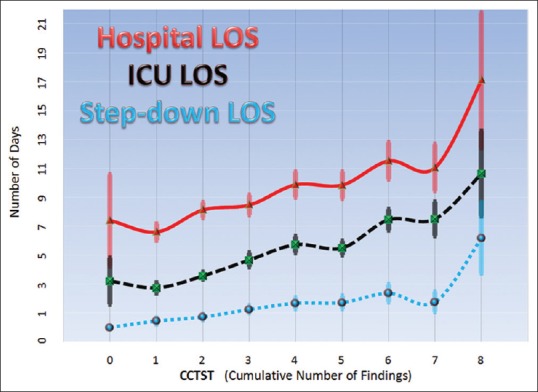
Relationships between cranial computed tomography scoring tool and hospital (red line – top), Intensive Care Unit (black dashed line – middle), and step-down (blue dashed line – bottom) lengths of stay are shown. Data are displayed as means with standard error bars
Mortality
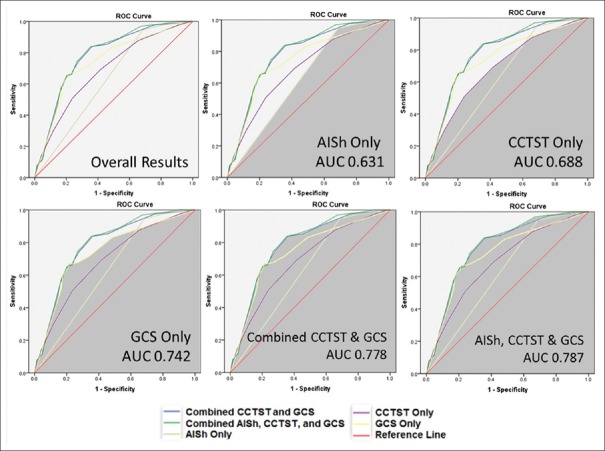
On univariate analyses of the combined 620-patient sample for 30-day mortality, variables achieving predetermined threshold (P < 0.20) for inclusion in multivariate analysis were patient age (70 years for survivors vs. 74 years for nonsurvivors, P < 0.20), AISh (4.54 survivors vs. 4.92 nonsurvivors, P < 0.01), CCTST (3.23 survivors vs. 4.73 nonsurvivors, P < 0.01), in-hospital morbidity (27% survivors, 36% nonsurvivors, P < 0.18), initial GCS (12.4 survivors vs. 7.41 nonsurvivors, P < 0.01), ISS (22.9 survivors vs. 28.7 nonsurvivors, P < 0.01), and gender (26.4% male vs. 17.6% female mortality, P < 0.02). Of note, there was no significant difference in mortality between NSI (23.6%) and non-NSI (22.9%) groups. On multivariate analysis, factors independently associated with 30-day mortality for the combined NSI and non-NSI cohort included AISh (AOR 2.698, 95% CI 1.214–5.996), initial GCS (AOR 1.14, 95% CI 1.07–1.22), and CCTST (AOR 1.31, 95% CI 1.09–1.58). Taking the current analysis a step further, ROC curves for 30-day mortality demonstrated that a model incorporating all three components (AISh, CCTST, and GCS) outperformed its subcomponents in terms of mortality prediction (AUC for the combined model was 0.787 compared to 0.742 for GCS, 0.688 for CCTST, and 0.631 for AISh) [Figure 3].
Figure 3.
Graphical representation of receiver operating characteristic curves, with the corresponding areas under the curve for key variables – alone or in combination – for mortality as the primary end-point. All results were statistically significant (P < 0.01)
In-hospital morbidity
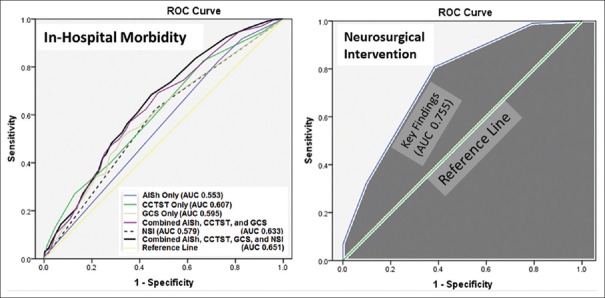
On univariate analyses for factors associated with in-hospital complications, parameters achieving our predetermined threshold (P < 0.20) for inclusion in multivariate analysis included patient age (74 years for the complication group [CG] vs. 69 years for patients without complications [NCG], P < 0.01), AISh (4.85 for CG vs. 4.55 for NCG, P < 0.01), CCTST (3.95 for CG vs. 3.30 for NCG, P < 0.01), GCS (9.61 for CG vs. 11.4 for NCG, P < 0.01), and ISS (26.8 for CG vs. 24.4 for NCG, P < 0.01). Of note, patients undergoing NSI had higher complication rate (35.2%) than patients in non-NSI group (16.6%, P < 0.01). On multivariate analysis, factors independently associated with in-hospital complications for the combined (NSI and non-NSI) cohort included CCTST (AOR 1.158, 95% CI 1.019–1.335), NSI (AOR 2.617, 95% CI 1.689–4.056), and GCS (AOR 1.046, 95% CI 1.002–1.093). Neither ISS (AOR 1.019, 95% CI 0.992–1.048) nor AISh (AOR 1.202, 95% CI 0.978–1.477) were independently associated with morbidity in the current model. When examining ROC curves for in-hospital morbidity prediction, the best performing model incorporated four components (AISh, CCTST, GCS, and NSI) and outperformed any of its subcomponents in terms of mortality prediction (AUC for the combined model was 0.651 compared to 0.579 for NSI, 0.595 for GCS, 0.607 for CCTST, and 0.553 for AISh) [Figure 4].
Figure 4.
(Left) receiver operating characteristic curves for in-hospital morbidity. Although none of the variables studied demonstrated good predictive ability for in-hospital complications, the combined neurosurgical intervention, AISh, cranial computed tomography scoring tool, and Glasgow Coma Scale had the highest area under the curve value. Results achieved statistical significance (P < 0.01) for all variables except for AISh (P = 0.06); (Right) receiver operating characteristic curve for modified cranial computed tomography scoring tool consisting of the following components (“Key Findings”): Subdural hematoma, epidural hematoma, brain edema, and midline shift. The overall predictive ability of the model is good, with area under the curve of 0.755 (P < 0.01)
Cranial computed tomography characteristics by neurosurgical intervention designation
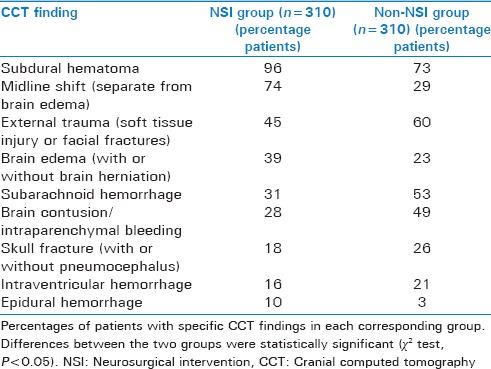
CCT findings differed significantly between NSI and non-NSI groups [Table 3]. As shown, subdural hematomas, midline shift, brain edema, and epidural hematoma were significantly more common in the NSI group. At the same time, external trauma, subarachnoid blood, cerebral contusion, skull fracture, and intraventricular bleeding were more commonly seen in the non-NSI group. These differences in TBI characteristics between NSI and non-NSI groups were utilized during the subsequent process of designing a clinically relevant, modified CCTST model geared specifically toward predicting NSI.
Table 3.
Cranial computed tomography findings grouped by neurosurgical intervention versus non-neurosurgical management
Neurosurgical intervention
In terms of factors associated with NSI, univariate analyses demonstrated that AISh (4.62 versus 4.53, P < 0.19), amount of midline shift (0.96 cm vs. 0.36 cm, P < 0.01), time to initial CCT (78.9 min vs. 56.8 min, P < 0.04), GCS (11.2 vs. 10.6, P < 0.16), presence of anticoagulation (35.6% vs. 26.2%, P < 0.08), and CCTST (3.59 vs. 3.26, P < 0.11) all met the predetermined threshold (e.g., P < 0.20) for inclusion in subsequent logistic regression. On multivariate analysis, only CCTST was independently associated with NSI (AOR 1.225, 95% CI 1.059–1.416). Of note, AISh (AOR 1.41, 95% CI 0.98–2.03), GCS (AOR 1.04, 95% CI 0.99–1.08), and the presence of anticoagulation (AOR 1.14, 95% CI 0.98–1.32) were not independently associated with NSI in our model.
When evaluating the ability of CCTST to predict the need for NSI, we found that the AUC for CCTST in its original format was only 0.521 (not shown). Further evaluation of specific subcomponents of CCTST demonstrated that modification of the original score to include a smaller subset of “neurosurgically specific” CCT findings – subdural hematoma, epidural hematoma, brain edema, and presence of midline shift – resulted in greatly improved ability to predict the need for NSI [AUC 0.755, Figure 4].
DISCUSSION
TBI continues to be one of the leading causes of death among older injured patients. Despite its admittedly preliminary nature, the current study strongly suggests that CCTST adds substantial level of granularity to the prevailing GCS-AISh paradigm. CCTST appears helpful in estimating 30-day mortality, in-hospital morbidity, and may be useful in determining the need for NSI. Our data demonstrate that predictive models incorporating all three variables (CCTST, GCS, and AISh) produced the best results for both mortality and morbidity, and that modified CCTST was the most optimal predictor of NSI.
A number of previous attempts have been made at designing and implementing classification and risk stratification schemes for moderate to severe TBI, with many such paradigms taking into consideration a combination of GCS, AISh, and CCT imaging characteristics.[24,25,26] There is also evidence showing that CCT findings may correlate with important clinical factors even in the setting of mild (GCS 13–15) TBI.[27,28] According to Lingsma et al., the prognostic value of “CT characteristics” in the context of global TBI assessment seems to be second only to “clinical severity” in terms of overall predictive contribution.[26] Finally, it is well established that extracranial head trauma is strongly associated with intracranial injuries,[29] supporting the use of anatomic scoring systems such as AISh in the setting of TBI.
Although all three neurologic trauma scores examined in our study correlated with 30-day mortality and in-hospital morbidity, only the CCTST was independently associated with NSI. This is not surprising, especially when one considers that high-resolution CCT has long been the foundation of prompt identification of life-threatening neurological injuries that require surgical therapy.[30] For example, one prospective multicenter study demonstrated that CCT findings most strongly associated with elevated intracranial pressure and mortality in TBI included midline shift, compression or obliteration of mesencephalic cisterns, and the presence of subarachnoid blood.[31] Parenchymal cerebral displacement across dural surface, with resultant pressure on nearby vascular structures, accounts for most posttraumatic infarction syndromes. The two most common infarction areas include the occipital lobe region (due to compression of the posterior cerebral artery against the tentorial incisura by the herniating temporal lobe) and the frontal lobe region (due to anterior cerebral artery occlusion from cingulate gyrus herniation against the falx cerebri). Frank global herniation and severe cerebral edema can occur with infarction in the middle cerebral artery territories.[32,33,34] Fearnside et al.[25] reported that CCT findings most predictive of TBI outcomes included subarachnoid blood, intracerebral hematoma, and brain contusion. Moreover, the determination that trauma-associated hypoxia and/or hypotension are associated with diffuse hemispheric swelling on CCT lends further credence to the prognostic utility of advanced brain imaging, regardless of primary or secondary nature of the neurologic insult.[31] Our analysis demonstrated that subdural hematomas, midline shift, brain edema, and epidural hematoma were all significantly more common in the NSI group. At the same time, external trauma, subarachnoid blood, cerebral contusion, skull fracture, pneumocephalus, and intraventricular blood were more commonly seen in the non-NSI group.
In terms of outcome prognostication, it has been suggested that anatomic injury assessment (e.g., AISh) may slightly outperform GCS as a predictor.[3] It is therefore not surprising that CCTST, an inherently “anatomic” measure of TBI, correlated well with the functional outcome measures in the current series. The strong relationship between CCTST, lengths of stay, discharge destination, and functional status on discharge supports the utility of this novel score as a valuable clinical prognostic tool, with implications beyond mortality, morbidity, and NSI. The importance of CCT in prognostication of TBI outcomes is not new, with Lobato et al., reporting in the early 1980s that patients with a diffuse brain injury pattern on imaging had worse outcomes than those with more focal or isolated findings.[35] Effacement of the surface sulci and basilar subarachnoid spaces is one of the earliest and most consistent neuroradiological signs of diffuse cerebral swelling. Classically, there is the appearance of decreased attenuation in a homogeneous fashion on CCT, with concurrent loss of the “gray-white” matter differentiation and the presence of generalized cerebral edema.[33] In another study, the Marshall CT classification (based on the appearance of basal cisterns, midline shift, traumatic subarachnoid or intraventricular hemorrhage, and the presence of different types of mass lesions) correlated well with 6-month mortality for a large cohort of TBI patients.[4] Based on these studies, the clinical utility of CCTST can therefore be attributable, at least in part, to the overall “quantity” of discrete traumatic brain lesions. For mortality, CCTST appears to perform similarly to the Marshall CT classification in terms of its discriminative characteristics (AUC 0.67 for unmodified Marshall CT score versus AUC 0.69 for unmodified CCTST).[4] The appeal of CCTST is that it employs a much simpler methodology, provides higher granularity (8+ vs. 6 maximum possible points), and requires significantly less radiographic and analytical expertise than the more complicated Marshall CT score (as well as another similar and highly correlated Rotterdam score).[4]
In all three domains examined in this study – mortality, morbidity, and NSI – CCTST was found to be independently associated with each respective end-point. In terms of mortality and morbidity prediction, CCTST demonstrated synergistic relationship with both GCS and AISh. Finally, the only area where both GCS and AISh added no predictive value, but CCTST demonstrated a small amount of benefit, was the determination of need for NSI. Moreover, after removing traditionally “nonoperative” components from CCTST and reducing it to a core of traditional “operative” variables, the predictive ability of this modified score increased dramatically. This approach is somewhat similar to that introduced by Maas et al.[4] and yields similar results in terms of the improvement of clinical predictive ability of the derived score when compared to the primary score. It is, therefore, consistent with previously published data that our CCTST variant modified for surgically-relevant “key findings” demonstrated a very good predictive ability for NSI [Figure 4].
It has been demonstrated that the presence of a low GCS score (e.g., <8) is associated with poorer outcomes and has traditionally been used to guide clinical TBI management and NSI.[36,37,38] The paradigm presented in this manuscript builds on the existing framework by creating a potentially more reliable predictive system that capitalizes on unique synergies that may be present when CCTST, GCS, and AISh are used together. In addition, utilizing a subset of CCTST constituent variables that are most strongly associated with NSI (subdural hematoma, midline shift, brain edema, and epidural hematoma) may help guide surgical decision-making and operative management of patients with severe TBI. Given the above observations, it is reasonable to propose that in the era of near-universal acquisition of CCT in the setting of trauma, the use of CCTST seems both practical and cost-effective.
There are several important limitations of this study. First, its retrospective nature may be associated with biases. Second, ours is a single-center experience, and significant differences may be seen between different trauma institutions in terms of clinical approaches to TBI. Consequently, our findings may have limited generalizability pending further validation. Third, the relatively small sample size and observed effect sizes suggest that both GCS and AISh could potentially be independently associated with NSI if our comparison groups were larger. Fourth, individual provider patterns may have played a role in the choice of ultimate management strategy, with medical versus surgical management not being standardized over the entire study interval. This consideration is also important in the context of temporal bias, with substantial risk of bias due to evolving management strategies over the multiyear study duration period. However, it should be noted that differences between- and within-individual provider patterns are not unique to this institution. Of importance, CT imaging has evolved considerably over the 10-year period of the study. Nevertheless, the presence or absence of major findings would not be significantly affected by imaging granularity, and only very minor findings would be likely to be missed on early scans. Further limitations of CCT include phenomena such as “partial volume errors” and “beam hardening” artifacts, especially in the posterior fossa, temporal and frontal regions.[39] However, despite these potential limitations, CCT remains the ideal initial diagnostic choice in the acute trauma setting due to its widespread availability and very short acquisition times. This is especially critical when approaching ventilated, agitated, or unstable trauma patients.[39]
CONCLUSION
This study demonstrates that the number of discrete findings on CCT significantly correlates with key TBI outcome measures, including 30-day mortality, in-hospital morbidity, and the need for NSI. In general, our findings are well supported by previously published reports in this area of research. However, we believe that the simplicity of CCTST and the near-universal use of CCT make the paradigm presented herein highly attractive to the mainstream trauma practitioner without specialty training in advanced imaging. In addition, there seems to be a significant prognostic synergy when CCTST and GCS are used together with AISh (for mortality prediction) or NSI (for morbidity prediction). The CCTST is easy to calculate and this preliminary investigation of its predictive utility warrants further validation, focusing on exploring the potential for clinical prognostic synergy between CCTST, GCS, and AISh.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, et al. Increasing trauma deaths in the United States. Ann Surg. 2014;260:13–21. doi: 10.1097/SLA.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 2.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: A brief overview. J Head Trauma Rehabil. 2006;21:375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Foreman BP, Caesar RR, Parks J, Madden C, Gentilello LM, Shafi S, et al. Usefulness of the abbreviated injury score and the injury severity score in comparison to the Glasgow Coma Scale in predicting outcome after traumatic brain injury. J Trauma Acute Care Surg. 2007;62:946–50. doi: 10.1097/01.ta.0000229796.14717.3a. [DOI] [PubMed] [Google Scholar]
- 4.Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: A comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57:1173–82. doi: 10.1227/01.neu.0000186013.63046.6b. [DOI] [PubMed] [Google Scholar]
- 5.Demetriades D, Kuncir E, Murray J, Velmahos GC, Rhee P, Chan L. Mortality prediction of head Abbreviated Injury Score and Glasgow Coma Scale: Analysis of 7,764 head injuries. J Am Coll Surg. 2004;199:216–22. doi: 10.1016/j.jamcollsurg.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Mosenthal AC, Lavery RF, Addis M, Kaul S, Ross S, Marburger R, et al. Isolated traumatic brain injury: Age is an independent predictor of mortality and early outcome. J Trauma Acute Care Surg. 2002;52:907–11. doi: 10.1097/00005373-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Stawicki SP, Kalra S, Jones C, Justiniano CF, Papadimos TJ, Galwankar SC, et al. Comorbidity polypharmacy score and its clinical utility: A pragmatic practitioner's perspective. J Emerg Trauma Shock. 2015;8:224–31. doi: 10.4103/0974-2700.161658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sterling DA, O'Connor JA, Bonadies J. Geriatric falls: Injury severity is high and disproportionate to mechanism. J Trauma Acute Care Surg. 2001;50:116–9. doi: 10.1097/00005373-200101000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Owsley C, McGwin G, Jr, Ball K. Vision impairment, eye disease, and injurious motor vehicle crashes in the elderly. Ophthalmic Epidemiol. 1998;5:101–13. doi: 10.1076/opep.5.2.101.1574. [DOI] [PubMed] [Google Scholar]
- 10.Ohm C, Mina A, Howells G, Bair H, Bendick P. Effects of antiplatelet agents on outcomes for elderly patients with traumatic intracranial hemorrhage. J Trauma Acute Care Surg. 2005;58:518–22. doi: 10.1097/01.ta.0000151671.35280.8b. [DOI] [PubMed] [Google Scholar]
- 11.Mina AA, Knipfer JF, Park DY, Bair HA, Howells GA, Bendick PJ. Intracranial complications of preinjury anticoagulation in trauma patients with head injury. J Trauma Acute Care Surg. 2002;53:668–72. doi: 10.1097/00005373-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Stuss DT, Binns MA, Carruth FG, Levine B, Brandys CF, Moulton RJ, et al. Prediction of recovery of continuous memory after traumatic brain injury. Neurology. 2000;54:1337–44. doi: 10.1212/wnl.54.6.1337. [DOI] [PubMed] [Google Scholar]
- 13.Demetriades D, Kuncir E, Brown CV, Martin M, Salim A, Rhee P, et al. Early prediction of mortality in isolated head injury patients: A new predictive model. J Trauma Acute Care Surg. 2006;61:868–72. doi: 10.1097/01.ta.0000219135.33398.f3. [DOI] [PubMed] [Google Scholar]
- 14.Sherer M, Struchen MA, Yablon SA, Wang Y, Nick TG. Comparison of indices of traumatic brain injury severity: Glasgow Coma Scale, length of coma and post-traumatic amnesia. J Neurol Neurosurg Psychiatry. 2008;79:678–85. doi: 10.1136/jnnp.2006.111187. [DOI] [PubMed] [Google Scholar]
- 15.Gennarelli TA, Champion HR, Copes WS, Sacco WJ. Comparison of mortality, morbidity, and severity of 59,713 head injured patients with 114,447 patients with extracranial injuries. J Trauma Acute Care Surg. 1994;37:962–8. doi: 10.1097/00005373-199412000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Walder AD, Yeoman PM, Turnbull A. The abbreviated injury scale as a predictor of outcome of severe head injury. Intensive Care Med. 1995;21:606–9. doi: 10.1007/BF01700170. [DOI] [PubMed] [Google Scholar]
- 17.Toutant SM, Klauber MR, Marshall LF, Toole BM, Bowers SA, Seelig JM, et al. Absent or compressed basal cisterns on first CT scan: Ominous predictors of outcome in severe head injury. J Neurosurg. 1984;61:691–4. doi: 10.3171/jns.1984.61.4.0691. [DOI] [PubMed] [Google Scholar]
- 18.Jeret JS, Mandell M, Anziska B, Lipitz M, Vilceus AP, Ware JA, et al. Clinical predictors of abnormality disclosed by computed tomography after mild head trauma. Neurosurgery. 1993;32:9–15. doi: 10.1227/00006123-199301000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Lee B, Newberg A. Neuroimaging in traumatic brain imaging. NeuroRx. 2005;2:372–83. doi: 10.1602/neurorx.2.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chesnut RM. Implications of the guidelines for the management of severe head injury for the practicing neurosurgeon. Surg Neurol. 1998;50:187–93. doi: 10.1016/s0090-3019(98)00075-5. [DOI] [PubMed] [Google Scholar]
- 21.Bullock R, Chesnut RM, Clifton G, Ghajar J, Marion DW, Narayan RK, et al. Guidelines for the management of severe head injury. Brain Trauma Foundation. Eur J Emerg Med. 1996;3:109–27. doi: 10.1097/00063110-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216–22. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Lubillo S, Bolaños J, Carreira L, Cardeñosa J, Arroyo J, Manzano J. Prognostic value of early computerized tomography scanning following craniotomy for traumatic hematoma. J Neurosurg. 1999;91:581–7. doi: 10.3171/jns.1999.91.4.0581. [DOI] [PubMed] [Google Scholar]
- 24.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–41. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 25.Fearnside MR, Cook RJ, McDougall P, McNeil RJ. The Westmead Head Injury Project outcome in severe head injury. A comparative analysis of pre-hospital, clinical and CT variables. Br J Neurosurg. 1993;7:267–79. doi: 10.3109/02688699309023809. [DOI] [PubMed] [Google Scholar]
- 26.Lingsma HF, Roozenbeek B, Steyerberg EW, Murray GD, Maas AI. Early prognosis in traumatic brain injury: From prophecies to predictions. Lancet Neurol. 2010;9:543–54. doi: 10.1016/S1474-4422(10)70065-X. [DOI] [PubMed] [Google Scholar]
- 27.Gómez PA, Lobato RD, Ortega JM, De La Cruz J. Mild head injury: Differences in prognosis among patients with a Glasgow Coma Scale score of 13 to 15 and analysis of factors associated with abnormal CT findings. Br J Neurosurg. 1996;10:453–60. doi: 10.1080/02688699647078. [DOI] [PubMed] [Google Scholar]
- 28.Nagy KK, Joseph KT, Krosner SM, Roberts RR, Leslie CL, Dufty K, et al. The utility of head computed tomography after minimal head injury. J Trauma Acute Care Surg. 1999;46:268–70. doi: 10.1097/00005373-199902000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Alvi A, Doherty T, Lewen G. Facial fractures and concomitant injuries in trauma patients. Laryngoscope. 2003;113:102–6. doi: 10.1097/00005537-200301000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman RA, Bilaniuk LT, Gennarelli T, Bruce D, Dolinskas C, Uzzell B. Cranial computed tomography in diagnosis and management of acute head trauma. AJR Am J Roentgenol. 1978;131:27–34. doi: 10.2214/ajr.131.1.27. [DOI] [PubMed] [Google Scholar]
- 31.Eisenberg HM, Gary HE, Jr, Aldrich EF, Saydjari C, Turner B, Foulkes MA, et al. Initial CT findings in 753 patients with severe head injury. A report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1990;73:688–98. doi: 10.3171/jns.1990.73.5.0688. [DOI] [PubMed] [Google Scholar]
- 32.Krieger DW, Demchuk AM, Kasner SE, Jauss M, Hantson L. Early clinical and radiological predictors of fatal brain swelling in ischemic stroke. Stroke. 1999;30:287–92. doi: 10.1161/01.str.30.2.287. [DOI] [PubMed] [Google Scholar]
- 33.Osborn AG. Diagnostic Neuroradiology. Vol. xvii. St. Louis: Mosby; 1994. p. 936. [Google Scholar]
- 34.Server A, Dullerud R, Haakonsen M, Nakstad PH, Johnsen UL, Magnaes B. Post-traumatic cerebral infarction. Neuroimaging findings, etiology and outcome. Acta Radiol. 2001;42:254–60. doi: 10.1080/028418501127346792. [DOI] [PubMed] [Google Scholar]
- 35.Lobato RD, Cordobes F, Rivas JJ, de la Fuente M, Montero A, Barcena A, et al. Outcome from severe head injury related to the type of intracranial lesion. A computerized tomography study. J Neurosurg. 1983;59:762–74. doi: 10.3171/jns.1983.59.5.0762. [DOI] [PubMed] [Google Scholar]
- 36.Asikainen I, Kaste M, Sarna S. Predicting late outcome for patients with traumatic brain injury referred to a rehabilitation programme: A study of 508 Finnish patients 5 years or more after injury. Brain Inj. 1998;12:95–107. doi: 10.1080/026990598122737. [DOI] [PubMed] [Google Scholar]
- 37.Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling: Indications and results. J Neurosurg. 1999;90:187–96. doi: 10.3171/jns.1999.90.2.0187. [DOI] [PubMed] [Google Scholar]
- 38.Harad FT, Kerstein MD. Inadequacy of bedside clinical indicators in identifying significant intracranial injury in trauma patients. J Trauma Acute Care Surg. 1992;32:359–61. doi: 10.1097/00005373-199203000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Coles JP. Imaging after brain injury. Br J Anaesth. 2007;99:49–60. doi: 10.1093/bja/aem141. [DOI] [PubMed] [Google Scholar]