Abstract
Nonoperative management of both blunt and penetrating injuries can be challenging. During the past three decades, there has been a major shift from operative to increasingly nonoperative management of traumatic injuries. Greater reliance on nonoperative, or “conservative” management of abdominal solid organ injuries is facilitated by the various sophisticated and highly accurate noninvasive imaging modalities at the trauma surgeon’s disposal. This review discusses selected topics in nonoperative management of both blunt and penetrating trauma. Potential complications and pitfalls of nonoperative management are discussed. Adjunctive interventional therapies used in treatment of nonoperative management-related complications are also discussed.
Republished with permission from:
Stawicki SPA. Trends in nonoperative management of traumatic injuries – A synopsis. OPUS 12 Scientist 2007;1(1):19-35.
Key Words: Blunt injury, complications, nonoperative management, penetrating injury, pitfalls, review
INTRODUCTION
During the past three decades, there has been a major shift from operative to selective nonoperative management of the injured patient. Traumatologists caring for adult patients began to follow the trend initiated by their pediatric counterparts after an increasing amount of evidence supported nonoperative management of great majority of pediatric splenic and hepatic injuries. It was subsequently shown that even the presence of hemoperitoneum and altered mental status do not seem to negate initial nonoperative management in blunt abdominal trauma (BAT), even in patients with higher grade injuries or those of older age.[1]
The increased utilization of nonoperative, or “conservative,” management of abdominal solid organ injuries is facilitated by the various sophisticated and highly accurate noninvasive imaging tools at the trauma surgeon's disposal. The nonoperative approach relies heavily on the availability of trauma-trained surgeons, advanced radiographic imaging (particularly computed tomography [CT]), accurate interpretation of such high quality radiographic images, and specialized institutional support and infrastructure.[1]
Despite some early resistance, selective nonoperative management of abdominal stab wounds has become routine in most trauma centers.[2] More recently, some trauma centers began to employ selective nonoperative management of gunshot wounds (GSW).[2,3] The increasing utilization of selective nonoperative approach for GSW follows the trends in nonoperative management of other types of traumatic injuries.
However, the feasibility and safety of the nonoperative approach to blunt or penetrating traumatic injury, especially in a limited-resource environment (such as limited availability of Intensive Care Unit [ICU] and advanced imaging/interventional techniques such as CT and angiography) remains a contentious issue.
HISTORICAL PERSPECTIVE
Nonoperative approaches to penetrating abdominal trauma remained the standard of care through most of the 19th century. This paradigm began to change after 1887, when the American Surgical Association recommended surgical approaches for civilian penetrating abdominal wounds.[2] Due to the high mortality associated with nonoperative management of penetrating abdominal injuries, surgical exploration became standard during World War I.[2] This dictum was further augmented by the fact that many surgeons coming back from the World War II advocated continued use of mandatory surgical exploration for all civilian GSWs.[3] This resulted in significant mortality reductions and remained the standard of care until the 1970s when a trend toward selective nonoperative management of abdominal stab wounds began.[2] Surgical exploration of all GSWs to the abdomen remained the standard practice until 1990's, when major urban trauma centers published their experiences on selective non operative management of penetrating abdominal injuries.[2]
Patients admitted with BAT are actually shown to have definite intra-abdominal injuries only in approximately one-third of the cases. On the other hand, the abdomen epitomizes the problem of missed injuries, with significant findings being absent in up to one-third of patients with BAT.[4] Of note, a missed splenic injury is the most common cause of preventable death in BAT patients. The masking effect of concurrent extra-abdominal injuries and altered sensorium from shock, head injury, or alcohol/drug intoxication, further compound the problem.[5,6] The propensity for low pressure bleeding from solid viscera makes it difficult to predict which injuries are likely to be self-limiting based solely on initial findings.[4]
The most common presenting features of intra-abdominal injury are abdominal pain, tenderness, guarding, and distention.[7] Other symptoms such as shortness of breath or chest pain may also be associated with significant abdominal injuries. One must remember, however, that 40% of patients with significant hemoperitoneum have no peritoneal signs.[8,9]
The trend in favor of nonoperative management of solid organ injuries has been clearly shown by studies of blunt hepatic, splenic, and renal injuries, and further aided by the increasing availability and accuracy of various advanced imaging techniques and hemodynamic monitoring technologies.[8,10,11] This resulted in a sharp decline in both therapeutic and nontherapeutic laparotomy (NTL) rates.
Whereas nonoperative management carries the inherent risks of a missed hollow visceral injury, delayed bleeding, and transfusion-related risks, laparotomy carries a different set of risks that are related to the surgeon, the anesthesia, the nature of operation and potential complications, and patient-related risk factors. Evidence seems to support the contention that the choice between the two modalities of management should be guided by hemodynamic considerations rather than the severity of organ injury.[9]
UNNECESSARY LAPAROTOMY
Trauma literature describes a category of laparotomies that reveal no pathologic findings and are termed “nontherapeutic.” NTL is also defined by some as a laparotomy for a minor injury that, in retrospect, required no surgical treatment.[2] It is partly because of this experience that the trauma surgeon's scope of practice has shifted toward selective nonoperative management of an entire spectrum of injuries.
The reported incidence of NTL for trauma can be as high as 40%, depending on the experience and practice patterns across a broad range of institutions.[2] In one prospective study of 938 laparotomies for blunt or penetrating injuries, 27% were deemed “unnecessary.”[12] In another study by Leppaniemi, a policy of mandatory operative exploration for stab wounds resulted in 37% negative laparotomy rate.[13] At the same time, trauma, centers employing a policy of selective nonoperative management reported a significantly lower rate of NTL (3.2%–10%).[14,15,16,17]
A collected review of over 8100 patients with penetrating abdominal trauma found the overall incidence of “unnecessary” operative explorations to be approximately 20%.[2] Of interest, the reported the incidence of NTL for blunt trauma is similar to that in penetrating trauma (approximately 20%).[18,19]
Nontherapeutic laparotomies are associated with significant morbidity and costs. The reported incidence of early laparotomy or anesthesia-related complications can be as high as 26%.[12,20] The reported overall incidence of late (or delayed) complications is approximately 5%.[13,21]
Both overall cost and hospital stay for patients undergoing NTL are also significantly greater than for patients successfully managed nonoperatively.[15,17] For example, mean hospital charges for patients who sustained abdominal GSWs and were managed nonoperatively were approximately $10,000 less than those for patients with NTLs.[15] Consequently, a policy of selective nonoperative management for abdominal GSWs has been shown to be associated with the reduction in both attributable hospital days and hospital-related charges.[17]
Benefits of selective nonoperative management should always be considered in the context of potential consequences of missed injuries and delayed diagnosis. In one review of patients with penetrating trauma, the overall incidence of delayed diagnosis was 3.4%, with no deaths attributed to the delayed treatment and morbidity comparable to that in patients receiving an early operation.[2] Similar results were reported in blunt traumatic injuries.[22] The time delay beyond which the morbidity increases is not precisely known, but some have suggested a time frame of 6 to 12 h.[2] The injured organ, the amount of blood loss, the length of delay, and/or the degree of peritoneal contamination all contribute to the incidence and severity of complications attributable to delayed management.
GENERAL PRINCIPLES OF NONOPERATIVE MANAGEMENT OF TRAUMATIC INJURIES
Physical examination remains the most important element of trauma triage. The presence of peritonitis and/or hemodynamic instability constitute strong indications for emergency celiotomy. Initial physical exam findings of significant traumatic injury can be subtle and the diagnosis of intra-abdominal injury may not be obvious. Infact, approximately 30% of patients with significant hemoperitoneum have a benign initial abdominal examination.[9]
The physical examination has other important limitations. For example, the older trauma patient taking antihypertensive medications may not manifest the signs of early shock.[2] Likewise, younger patients, including those with short prehospital transport times, may not exhibit overt signs or symptoms of shock despite the presence of significant internal hemorrhage.[2] Patients with severe traumatic brain or spinal injury may be difficult to assess. Altered sensorium due to alcohol or other substances may affect the accuracy of clinical assessment. Acutely intoxicated and combative patients pose further diagnostic dilemma, not only due to the lack of reliable clinical assessment, but also due to the potential danger to self, health care personnel, and lack of cooperation during the subsequent diagnostic work-up, which requires the patient to be cooperative.
Among the most difficult skills to master is the evaluation of a hemodynamically unstable patient with multiple injuries and “competing priorities” (i.e., concurrent head injury, aortic injury (AI), pelvic fractures, and extremity trauma). In such scenarios, the probability of co-existing injuries can be estimated from knowledge of the mechanism. Subsequent confirmation by CT scan may allow observation if the patient is hemodynamically stable. In patients for whom clinical examination is not reliable, special investigations can be crucial in early and accurate triage. The lack of reliable physical examination may constitute a relative contraindication to nonoperative management of traumatic injuries in patients who fall into this “indeterminate” zone. General principles of nonoperative management of trauma are summarized in Table 1.
Table 1.
Synopsis of general principles of nonoperative management

NONOPERATIVE MANAGEMENT OF BLUNT ABDOMINAL TRAUMA
Nonoperative management of blunt traumatic injuries is well-established, and strategies based on CT scan diagnosis and the hemodynamic stability of the patient are now being widely used in the treatment of solid organ injury, including the liver, the spleen, the kidneys, as well as pelvic injuries. In BAT, including severe solid organ injuries, selective nonoperative management has become the standard of care.[1]
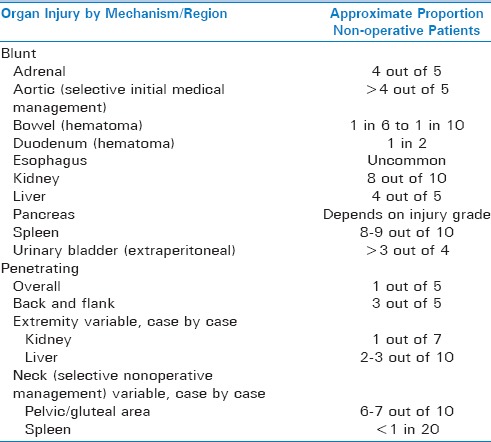
If the decision has been made to observe the patient and to pursue nonoperative management, close monitoring of vital signs and frequently repeated physical examinations are instituted. An increased temperature or respiratory rate can indicate a hollow viscus perforation or abscess formation. Pulse and blood pressure can also change with sepsis or intra-abdominal bleeding. Adjunctive laboratory assessments, such as serial determination of lactic acid, base deficit, hemoglobin/hematocrit levels, and white blood cell counts can also help guide the overall clinical management approach. The development of peritonitis on physical examination and lack of response to nonoperative treatment, constitute an indication for surgery. Table 2 summarizes reported success rates for non-operative management of blunt and penetrating injuries by anatomic location.
Table 2.
Success of nonoperative management strategy for different organ/anatomic location injuries (in alphabetical order) and mechanism of injury (blunt vs. penetrating)
TRAUMA IMAGING: DETERMINATION OF THE INITIAL TREATMENT COURSE
The hemodynamic stability of the patient and the specific pattern of suspected injury will determine the imaging approach that is most appropriate in the given situation. Entire assortment of radiographic studies is available to the modern trauma surgeon, including plain radiograms, CT, magnetic resonance (MR) imaging, and ultrasonography. Imaging has become essential in the early clinical decision-making process regarding the choice of an operation, endovascular intervention, close observation in the ICU, or admission to trauma unit.
The availability of the imaging equipment and the clinical capabilities of the supporting institutional environment should always be considered. Regardless of the type of radiographic study, the injured patient under the trauma team's care should always be monitored diligently. In addition, the trauma team should carefully plan the resuscitative sequence in order to minimize the loss of time and avoid radiographs that are technically impaired or have low diagnostic yield.
Although the overall value of traditional roentgenograms in the evaluation of BAT is limited, they can demonstrate clinically useful findings. Whenever hemodynamic stability allows, blunt trauma patients should have plain radiographs of the cervical spine, chest, and pelvis as part of their evaluation. The need for further imaging is based on the mechanism of injury and findings during the initial and secondary trauma assessments.
Plain films should be read carefully, in a systematic and standard fashion. The chest roentgenogram is useful in confirming or demonstrating suspected pneumothorax, hemothorax, and widened mediastinum. It provides direct evidence for initiation of immediate therapy (i.e., tube thoracostomy). The chest radiograph may also aid in the diagnosis of abdominal injuries such as ruptured hemidiaphragm (i.e., a nasogastric tube seen in the chest) or the presence of free intraperitoneal air.
The pelvic and/or chest radiograph can help detect fractures of the thoracic and/or lumbar spine. When identified, transverse fractures of the vertebral bodies (i.e., chance fractures) may be associated with blunt bowel or pancreatic injuries. At times, free intraperitoneal air, or trapped retroperitoneal air from duodenal or colonic injury, may be detectable. Penetrating injuries should have a radiographic marker (i.e., paper clip) placed over each wounding site and radiographs obtained to help elucidate injury trajectory and/or detect any retained projectiles or fragments.
Although by no means definitive, a negative initial plain radiographic workup allows the trauma physician to continue on the path of nonoperative management, keeping in mind that any deterioration in patient status or additional findings on subsequent imaging, physical examination, or laboratory investigations, may constitute an indication for change in therapeutic approach.
As an added benefit, the Focused Assessment with Sonography in Trauma (FAST) examination can be performed repeatedly, providing a useful adjunct to serial physical examinations during the post-injury clinical monitoring period.[23] At some institutions, the FAST examination has virtually replaced diagnostic peritoneal lavage (DPL) as the primary tool in the evaluation of trauma patients with hemodynamic instability. The American College of Surgeons included the use of ultrasound in the Advanced Trauma Life Support secondary survey since 1999.
The FAST examination is based on the evidence showing that clinically significant abdominal injuries are likely to cause hemoperitoneum. The “basic” FAST protocol includes four acoustic windows (pericardial, perihepatic, perisplenic, and pelvic – the four P's) with the patient supine. The detection of free intraperitoneal fluid is based on factors such as the body habitus, injury location, presence of blood clots, patient positioning, and the quantity of free fluid. The FAST examination is deemed positive if fluid is identified in any of the above-mentioned acoustic windows and is interpreted as negative if no fluid is found. An examination is termed indeterminate if any of the windows cannot be adequately assessed. The minimum threshold for detecting hemoperitoneum remains a subject of interest. Of note, even 30–70 mL of blood could be detected on FAST.[24,25] Moreover, a small anechoic stripe in the Morison's pouch represents approximately 250 mL of fluid, whereas 0.5 and 1 cm stripes represent approximately 500 mL and 1 L of peritoneal fluid, respectively.[25]
The pericardial view is obtained using a subcostal or transthoracic window. It provides a 4-chamber view of the heart and can detect the presence of hemopericardium, which is demonstrated by the separation of the visceral and parietal pericardial layers. The perihepatic view visualizes the liver, diaphragm, and right kidney. It reveals fluid in the Morison's pouch, the subphrenic space, and the right pleural space. The perisplenic view visualizes the spleen and the left kidney and reveals fluid in the splenorenal recess, the left pleural space, and the subphrenic space. The pelvic view uses the bladder as a sonographic window. This view is best obtained while the patient has a full bladder. In males, free fluid is seen as an anechoic area (sonographically black) in the rectovesicular pouch or cephalad to the bladder. In females, fluid accumulates in the pouch of Douglas, posterior to the uterus.
Throughout the literature, sensitivities and negative predictive values for FAST in detecting the hemoperitoneum are 78%–99% and 93%–99%, respectively. FAST examination relies on hemoperitoneum for injury identification. In one study of 772 blunt trauma patients undergoing FAST scans, 15/52 (29%) patients with confirmed abdominal injury had no hemoperitoneum on FAST or CT scan.[26] Hence, the finding of free peritoneal fluid as the best indicator of abdominal visceral injury limits the utility of FAST as a diagnostic screening tool in hemodynamically stable patients with BAT. In this setting, CT scanning may be necessary to further delineate exact injuries. To define the subsets of patients who would benefit the most from trauma bay FAST examinations, Rozycki et al. found that ultrasound was most sensitive and specific in patients with penetrating chest wounds or in hypotensive BAT patients (sensitivity and specificity nearly 100%).[27]
Hemodynamically stable patients with negative FAST results require close observation, serial abdominal examinations, and a follow-up FAST examination (if resources permit). However, one should strongly consider performing a CT scan, especially if the patient is intoxicated or has other associated injuries. Hemodynamically unstable patients with negative FAST constitute a diagnostic challenge. Other diagnostic options available in this situation include repeated FAST, DPL, laparoscopy, exploratory laparotomy, and, possibly, a CT scan after aggressive resuscitation.
Certain limitations of FAST ultrasonography, if ignored, can result in significant harm. For example, FAST does not identify retroperitoneal hemorrhage or hollow viscus perforations and may give false negative results in the cases of significant intraperitoneal bleeding.[2] While some studies report excellent sensitivity, ranging from 90% to 100%, other studies report significantly lower sensitivity and negative predictive value.[27,28,29,30,31] Given the wealth of literature available on FAST examinations, the consensus remains that although a positive FAST is a strong predictor of injury, a negative FAST does not rule out significant intra-abdominal injury or bleeding. Thus, additional diagnostic studies should be performed if FAST is negative.[2] Moreover, the role of FAST should be validated by each individual trauma center.
CT in trauma has advanced to the point of near-universal utilization during the initial trauma patient assessment, and is arguably the most valuable and most widely used tool in the initial evaluation of the hemodynamically stable patient with BAT. Contemporary, high-resolution CT features much greater speed and accuracy of image acquisition, which can now be performed in potentially unstable (metastable) patients previously to tolerate the long scanning times of the older generation imaging equipment. Increasing availability of CT scanners within modern emergency departments enables the speedy evaluation of critically injured patients.
CT can provide highly reliable information about the presence and quantity of peritoneal free fluid, the severity of many solid-organ injuries, and can visualize the retroperitoneum (the pancreas, the duodenum, and the genitourinary system) and many cases of hollow viscus perforation. The evolution of high-quality, multi-detector, fast CT scanning allows accurate assessment of organ injury grading, and ongoing bleeding (i.e., the presence of radiographic “blush”) [Figure 1].
Figure 1.
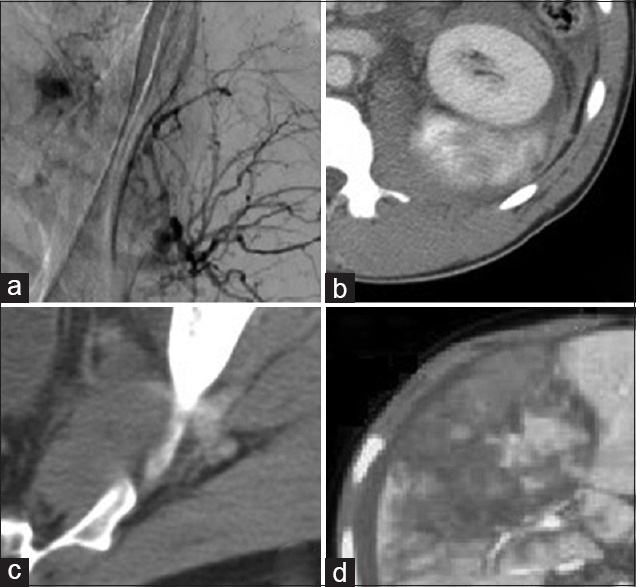
Angiographic (a) and computed tomographic (b-d) appearance of intravenous contrast extravasation or “blush.” Anatomic locations include pelvis (a and c), kidney (b) and liver (d)
The CT scan allows the detection and grading of solid organ injuries. In addition, CT can reveal other associated injuries, notably vertebral and pelvic fractures, as well as thoracic and extremity injuries. CT, unlike DPL or FAST, has the capability to fairly accurately determine the source of hemorrhage. Moreover, retroperitoneal injuries that may have been missed on DPL and FAST examinations, can be visualized on CT scans. Tomographic images can help quantitate the amount of blood in the abdomen and visualize individual organs with precision. Limitations of CT scans include marginal sensitivity for diagnosing diaphragmatic, pancreatic, and hollow visceral injuries. Furthermore, CT is relatively expensive and requires intravenous and/or oral contrast, which may cause adverse reactions.
In addition, the imaging accuracy of the CT, it can play a critical role in selecting the therapeutic modality in patients with solid organ injuries. This includes, for example, the determination of whether the trauma physician should resort to operative treatment versus angiographic embolization for selected injuries.
CT is very helpful in the evaluation of blunt thoracoabdominal injuries. Here, the chest can be evaluated for injury to the thoracic aorta, pulmonary contusion can be differentiated from hemothorax, and occult pneumothoraces can be visualized.[32,33] Coronal image reconstructions can help to determine the presence or absence of diaphragmatic injury as well as injuries of the bony skeleton. The role of radiography in nonoperative trauma management is summarized in Table 3.
Table 3.
Impact of radiography on nonoperative trauma management

BLUNT INJURIES: ORGAN- AND REGION-SPECIFIC APPROACHES TO NONOPERATIVE THERAPY
Management of blunt traumatic injuries can be complex, difficult, and depends on multiple clinical factors. Modern trauma surgeons depend on the conglomerate of clinical examination, laboratory tests, and radiographic studies in their decision-making process. With growing understanding of anatomic patterns of injury, we continue to refine our ability to appropriately apply nonoperative management strategies [Table 3]. The organ-and region-specific approach to injuries seems to be the most logical to follow in this review, making our discussion organized and systematic. We will begin with organ- and region-specific synopsis of blunt traumatic injury.
To exemplify the gradual transition from operative to nonoperative management strategy, hepatic trauma, which used to be a common reason to venture to the operative theatre, now is the staple in nonoperative management of BAT. Significant recent evidence for nonoperative management of splenic injuries has been inspired by the successes of the nonoperative hepatic injury management. Other organ injuries, including renal and pancreatic, are also increasingly being managed nonoperatively. Our discussion will begin with splenic injuries.
NONOPERATIVE MANAGEMENT OF SPLENIC INJURIES
Splenic injury is the most common indication for laparotomy following BAT. Blunt splenic injuries result from compression or deceleration due to a variety of mechanisms, from falls to motor vehicular accidents. The spleen receives approximately 5% of cardiac output, primarily through the splenic artery, making any splenic bleeding potentially life-threatening. The splenic artery usually bifurcates into superior and inferior polar arteries, and the spleen has an open microcirculation without endothelium.
Clinical presentation of splenic injury may vary considerably. Of importance is the presence of referred left shoulder pain (Kehr's sign) as well as the association of splenic injury with left lower rib fractures (ribs 9 through 12). In fact, up to 25% of patients with left lower rib fractures can have some degree of splenic injury.
In modern trauma practice, more and more splenic injuries are treated nonoperatively.[1] If hemodynamically stable, adult patients with lower grade splenic injuries (Grades I and II) can most often be treated nonoperatively. Grade III splenic injuries can be treated nonoperatively, based on patient stability and reliability of the physical examination. Even very severe splenic injuries, associated with significant hemoperitoneum, have been successfully managed nonoperatively [Figure 2].[34] There is evidence that nonoperative management of splenic injury that rapidly stabilizes with little fluid or blood replacement is successful in 80%–90% of cases.[34,35]
Figure 2.
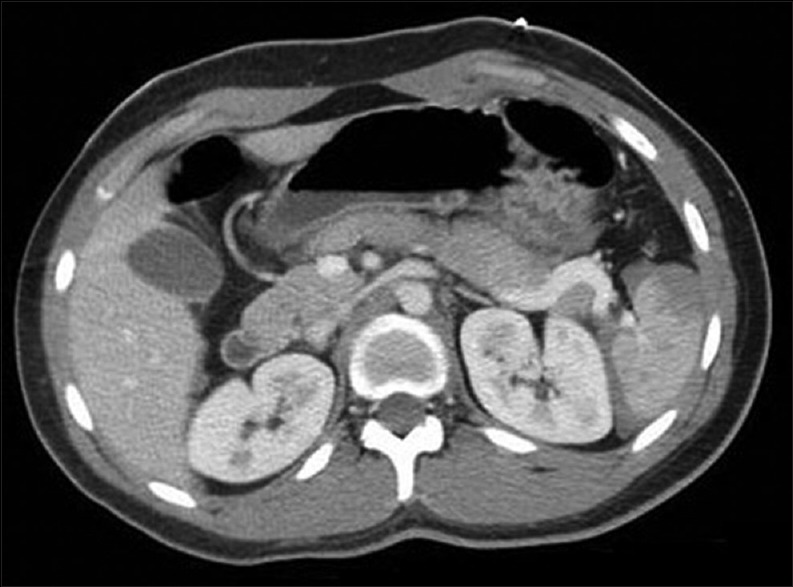
Computed tomographic appearance of a significant splenic injury in a hemodynamically stable patient. This injury was managed nonoperatively
CT findings that may predict failure of nonoperative therapy include the presence of large hemoperitoneum, as well as the presence of radiographic “blush.” Nonoperative treatment of splenic injuries fails in approximately 10%–20% of adults. Because of that, patients with significant splenic injuries treated nonoperatively should be observed in the ICU for 48 h and have immediate access to CT imaging, angiography, and/or the operating room.
NONOPERATIVE MANAGEMENT OF HEPATIC INJURIES
Alhough liver and spleen cumulatively represent the most common injuries in BAT, the liberal use of high resolution CT scanning has showed that the liver and not the spleen is the most frequently injured solid organ in BAT.[8,35]
Although the frequency of blunt hepatic and splenic injuries appears to be fairly constant, the percentage of patients managed nonoperatively has grown markedly since the 1990's.[1] Modern imaging techniques, more than ever, are able to demonstrate small, otherwise undetectable hepatic injuries. Because physical examination is often unreliable in the blunt trauma patient, up to 40% of liver injuries may be missed on physical examination. Therefore, in hemodynamically stable blunt trauma patients, CT is preferred. Most hemodynamically stable patients with hepatic trauma can be treated nonoperatively, provided that no other injuries that require laparotomy are present.[34] In fact, even AAST Grade IV or V hepatic injuries, with associated large-volume hemoperitoneum or limited of transfusion requirement, can be managed nonoperatively.[34,36] There is also evidence that nonoperative management of hepatic injury that rapidly stabilizes with little fluid or blood replacement is successful in over 80% of such instances.[34,35,37] Of importance, neither advanced patient age nor higher grade of injury appear to predict the incidence of failure of nonoperative approach.[1] Predictors of failure of nonoperative management include large hemoperitoneum, arterial “blush” or pooling of intravenous contrast, and the presence of high Grade (IV and V) injuries [Figure 3].
Figure 3.
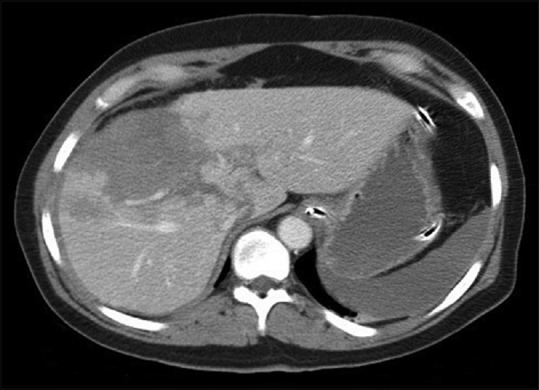
Severe liver injury that was successfully managed nonoperatively
Established criteria for nonoperative treatment of blunt hepatic trauma include hemodynamic stability, absence of peritonitis, reliable exam in a neurologically intact patient, delineation of the injury by CT and radiographic absence of other operative injuries, <2 units of packed red blood cells transfused for the injury, and CT documentation of stabilization or resolution of the injury. Finally, posterior right hepatic lobe and “split liver” injuries (where seemingly extensive injury occurs along a relatively avascular plane) can usually be managed without surgery.
Clinical monitoring of patients with blunt hepatic trauma depends largely on injury grade. Grade I and II injuries can generally be observed on the regular surgical/trauma floor. Injuries of Grades III and above should be observed in the ICU for the first 48 h. Observation includes serial hematocrit determinations, 3–5 days of bed rest, and a follow-up abdominal CT at approximately 48 h following admission for injury Grades III or higher. Most liver injuries treated nonoperatively heal within 8–12 weeks.
NONOPERATIVE TREATMENT OF BLUNT DUODENAL AND BOWEL INJURIES
Nonoperative approach to duodenal injuries is mainly limited to isolated blunt traumatic contusions. More commonly encountered in children, intramural duodenal hematomas are potentially amenable to nonoperative management as well. In these patients, a follow-up upper gastrointestinal imaging with gastrografin should be performed every 7 days if the obstruction persists clinically. Other components of therapy in nonoperatively treated duodenal injuries include nasogastric suction and intravenous alimentation. The usually accepted time limit to nonoperative management is 2–3 weeks. In one study, 57% of duodenal injuries were managed nonoperatively.[38]
Similar to duodenal injuries, small or large bowel injury that appears to be limited to a bowel wall hematoma on high-resolution imaging, can qualify for nonoperative management. Frequent clinical assessments should be performed, up to and including repeated CT studies and ultrasound exams. If any evidence of clinical worsening or peritonitis appears, the patient should be taken to the operating room promptly.
NONOPERATIVE TREATMENT OF BLUNT PANCREATIC INJURIES
Isolated blunt pancreatic injuries are rare. Fewer than 1 in 10 major trauma events result in injury of the pancreas, with majority being associated with other solid organ injuries. In fact, literature reports show a range of 1.6–4.5 non-pancreatic injuries co-occurring per patient. Seldom is the pancreas the sole organ injured.[39,40,41]
In terms of diagnostic imaging imaging, the best test of choice is a high-definition CT scan performed with intravenous contrast [Figure 4]. CT findings suggestive of pancreatic injury include: (a) Peripancreatic intra-and extra-peritoneal fluid; (b) fluid in the lesser sac; (c) pancreatic hematoma or laceration; (d) diffuse pancreas enlargement with pancreatitis or focal edema at injury site; (e) thickening of the left anterior renal fascia. These findings are often subtle, and patients with pancreatic injury rarely exhibit multiple findings.[42] CT imaging can also suggest disruption of the pancreatic duct. The presence of a complete fracture is usually, but not always, associated with a concomitant duct transection.[43,44,45] A finding which is easy to recognize, and can direct attention to additional subtle findings of pancreatic injury, is the presence of fluid interdigitating between the pancreas and the splenic vein.[46]
Figure 4.
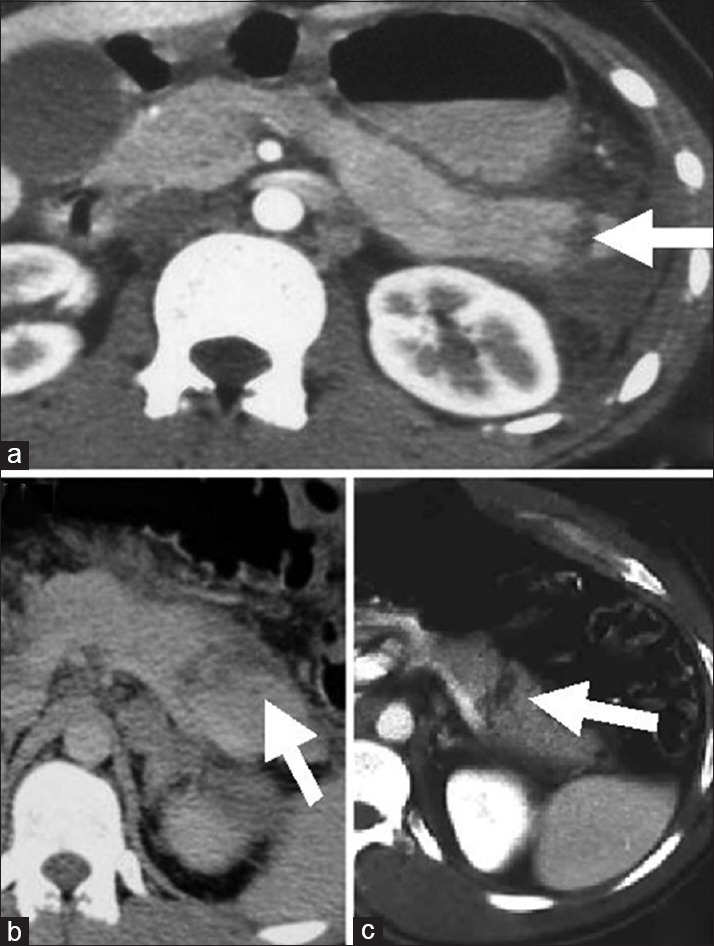
Examples of computed tomography appearance of traumatic pancreatic lacerations. Patients (a and c) required surgery. Patient (b) was treated nonoperatively
One proposed CT grading scheme mirrors the surgical classification by Moore: (a) Grade A, pancreatitis or superficial laceration (<50% pancreatic thickness); (b) Grade B1, deep laceration (>50% pancreatic thickness) of the pancreatic tail; (c) Grade B2, transection (entire thickness) of the pancreatic tail; (d) Grade C1, deep laceration of the pancreatic head; (e) and Grade C2, transection of the pancreatic head.[45] Due to broad range of early CT manifestations of pancreatic injury, trauma surgeons should exercise caution when entertaining nonoperative management. False negative results or underestimation of initial CT scan grading may be associated with unopacified bowel loops adjacent to the pancreas, motion and streak artifacts, as well as suboptimal bolus enhancement. In more severe injuries, the pancreatic fracture line is not easily detected when the separation of the fractured fragments is minimal or nonexistent.[44] CT may also overestimate the extent of trauma in Grade C injuries because deep lacerations though the proximal pancreas are not always associated with disruption of the proximal main duct, and transections through the proximal pancreas may only disrupt the minor duct.[45] CT also has great utility in diagnosing and characterizing complications such as abscesses, fistulae, pancreatitis, and pseudocysts.
There is a significant increase in pancreas-specific complications in patients who underwent delayed surgery.[47] This is further compounded by the fact that in the acute setting pancreatic injury can be asymptomatic in up to 20% of patients. Moreover, laboratory findings are often nonspecific (in particular, initial serum amylase levels may be normal in about 25% of patients), and underestimation of the severity of pancreatic injury on the initial CT is possible.[47,48] Finally, it is possible for low severity BAT to cause an isolated pancreatic injury.
Patients with pancreatic injuries can be broadly grouped into two general categories: (a) Those who require immediate operation and (b) those who qualify for clinical observation. While clinical management of other solid organ injuries (may be) is an accepted practice, nonoperative management of pancreatic injuries continues to be controversial. The involvement of the pancreatic duct is the principal determinant in the clinical decision making. Because operative management is the default in patients with penetrating injuries or multi-organ involvement, delay in diagnosis of a pancreatic ductal injury most commonly occurs in cases of isolated blunt pancreatic trauma. Some authors also advocate that CT grading of the degree of severity of blunt pancreatic trauma can be useful in predicting ductal integrity or disruption.[43,47,49]
In cases of isolated pancreatic trauma, serial physical examinations and repeated CTs are important when determining if nonoperative management is to be undertaken.[49] It is important to remember that the initial CT may provide false reassurance and will sometimes miss or underestimate pancreatic injuries that may require operative treatment.
NONOPERATIVE TREATMENT OF BLUNT RENAL INJURIES
Approximately 80% of renal injuries are due to blunt trauma. There is an associated 5% incidence of renal loss. Blunt kidney injury is associated with specific mechanisms of injury, the presence of hematuria, and characteristic physical findings. Computed tomography has evolved to a point where renal injury staging can be done almost entirely using established CT criteria [Figure 5].
Figure 5.
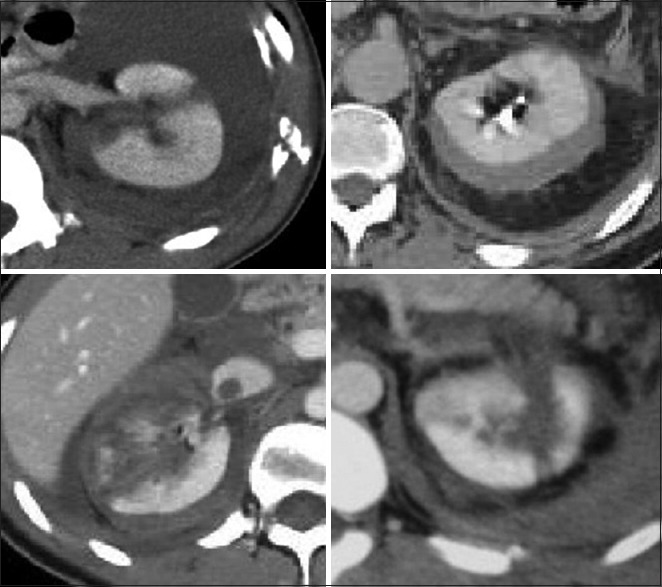
Computed tomographic examples of blunt renal injuries
In most cases of blunt kidney injury and some cases of penetrating renal trauma, nonoperative approach may be chosen, with careful patient selection being the most important determining factor. Approximately 85% of patients with blunt renal trauma can successfully be treated nonoperatively, with key determinant of the therapeutic course being the presence of other concurrent injuries.
The anatomy of the kidney makes it inherently amenable to nonoperative management in the setting of blunt trauma. The kidney's arterial blood supply has a segmental pattern of vessels supplying different “terminal” portions of renal parenchyma. Following blunt force trauma, renal tears tend to occur through the parenchyma. The associated hematoma may displace unaffected renal tissue, but the segmental vessels usually remain intact. Retroperitoneal tissues that surround the kidney also promote tamponade of bleeding renal injuries.
Nonoperative management of kidney injuries (Grades I to III) is now well accepted. As long as the injury is accurately staged, non-surgical approach is usually successful for contusions, contained lacerations, and even lesions with moderate amount of extravasation of urine or blood, provided that hemodynamic stability is maintained. Of note, the kidney is rich in tissue factor, further promoting hemostasis after injury via activation of the extrinsic coagulation pathway.
Traumatic renal artery thrombosis is associated with high rate of kidney loss. Although renal salvage has been reported with arterial repair when the warm ischemia time is <4 h, this is unusual without early operative intervention and repair. Nonoperative approach is recommended in stable individuals, with the understanding that delayed nephrectomy may be needed for sequelae of renal infarction.
Interventional radiology has further expanded the applicability of the nonoperative management of kidney injuries. Percutaneous drainage of urinomas and other fluid collections has been used to treat complications associated with nonoperative approaches. Furthermore, selective angiographic embolization and/or stenting has been useful in cases of isolated renal vascular trauma. Another way to optimize success rates of the nonoperative approach involves endourologic stenting.
NONOPERATIVE TREATMENT OF BLUNT URINARY BLADDER INJURIES
Blunt urinary bladder injuries are fortunately rare (e.g., <2% of abdominal injuries). This has been attributed to the relatively protected anatomic position of the bladder deep within the bony pelvis. This also makes bladder injury a marker for other severe injuries, often associated with significant morbidity and mortality.[50] Blunt urinary bladder injuries are strongly associated with fractures (e.g., >80% of patients with bladder injury experience pelvic fracture, and about 10% of patients with pelvic fractures have bladder injuries).[51,52]
The majority of patients with injury to the urinary bladder present with gross hematuria, although microscopic hematuria can also be seen.[50,51] The presence of gross blood is usually associated with greater extent of injury (i.e., bladder rupture) whereas microhematuria seems to be more common with bladder contusions or hematomas.[51]
The algorithm for management of suspected urinary tract injury starts with detailed history and physical examination. Lower abdominal pain, tenderness, and the presence of contusion over the lower abdomen and perineum may be present. Due to the high co-occurrence rate, these signs and symptoms may be hard to differentiate from the findings associated with pelvic fractures. For example, intraperitoneal bladder ruptures may not be recognized until a urethral catheter fails to return urine. When the diagnosis of bladder injury is delayed, fever, absence of voiding, peritoneal irritation, and elevated blood urea nitrogen may be observed. Consequently, patients with these signs and symptoms should have formal cystography to confirm or rule out bladder injury.
Inspection for blood at the urethral meatus should be performed during initial trauma evaluation, with this clinical finding being present is approximately half of significant urethral injuries.[53] Passage of a urinary catheter should not be attempted in such cases, and an immediate retrograde urethrography needs to be performed to rule out urethral injury. Approximately 10-20% of patients with bladder injuries will have associated urethral trauma.[51,53] If urethrography is normal, a urinary catheter can be placed. However, if urethrography demonstrates an injury, suprapubic urinary catheter, surgical exploration, and repair should be undertaken. In the absence of urethral injury, the determination of whether bladder rupture is present, and classifying it as intraperitoneal or extraperitoneal follows. Nonoperative management applies only to extraperitoneal ruptures, which can be drained with a urinary catheter. Retrograde cystography with plain abdominal roentgenographic imaging has been proven to be very accurate.[50] The technique has two important technical considerations: (a) The bladder needs to be filled completely; (b) a postdrainage film is essential. Approximately 300 mL of diluted contrast is instilled into the bladder by gravity.
More recently, CT cystography has become the study of choice for evaluation of bladder trauma. During the study, diluted contrast medium is infused into the bladder by gravity, and pelvic CT is then performed. As with traditional cystography, postdrainage film is crucial.
Intraperitoneal bladder ruptures are managed surgically and will not be discussed in this review. Extraperitoneal ruptures, which are isolated in approximately two-third of instances, may be amenable to nonoperative management.[50] These are commonly managed with urinary catheter drainage alone, although the presence of a bone fragment projecting into the rupture (very uncommon), open pelvic fracture, and rectal perforation may all be contraindications to nonoperative approach.[54] Open pelvic fractures and rectal perforations are associated with high risk of severe infection.[55] It has been postulated that if clots obstruct the urinary catheter within 48 h of injury, then surgical repair should be undertaken and a suprapubic detailed placed.[56] In addition, operative bladder repair may be considered in patients who undergo abdominal or pelvic surgical procedures for other indications.[56]
In cases of extraperitoneal bladder rupture, antibiotics are given on the day of injury and continued until about 72 h following urinary catheter removal.[56] Follow-up cystography in cases of extraperitoneal bladder rupture should be done between 10–14 days postinjury.[57] Most extraperitoneal ruptures will heal within 10 days, and nearly all heal within 21 days.[56,57] If the cystogram shows no extravasation, the catheter can be removed. Otherwise, cystography is repeated after approximately 3 weeks.[57] In summary, nonoperative management of extraperitoneal bladder rupture is both safe and effective.
NONOPERATIVE MANAGEMENT OF PENETRATING TRAUMA
Let us start the discussion of this topic on a contrarian note. When should penetrating trauma victim be operated on? These circumstances include hemodynamic instability, trajectory of the projectile suspicious for internal injuries, peritonitis, evisceration, pneumoperitoneum on imaging, massive hemothorax, hemopericardium, blood in the nasogastric tube, blood in the urinary catheter, and rectal bleeding.
The key to successful nonoperative management of penetrating injuries is to remember that such approach should be employed only in certain selected scenarios. Traditionally, penetrating trauma has been associated with significant incidence of nontherapeutic operative interventions. More evidence is emerging that observation and selective nonoperative approach may have been underutilized in the past. Surgeons have been reluctant to move toward nonoperative management because many consider a NTL to be relatively harmless. However, several studies clearly demonstrate that this point of view is far from optimal (see earlier section on NTL).
Nonoperative management of penetrating abdominal trauma invokes several important principles [Table 4]. First, adequate resources have to be present in order to provide continued and timely monitoring of the injured, with the ability to promptly detect failure of nonoperative therapy. Second, high-quality imaging modalities have to be readily available for the purpose of initial definition of the path of the projectile as well as for re-imaging (if appropriate) when failure of nonoperative management is suspected. Third, adequate resources (operating surgeon, operative suite, nursing staff, house staff) need to be in place to quickly institute operative therapy if needed.
Table 4.
Important considerations when treating penetrating trauma nonoperatively

When a patient is being evaluated for possible nonoperative management of penetrating injury, the treating physician must ask two questions. First, did the projectile enter the peritoneal, retroperitoneal, or pelvic cavity? And second, if it did, is there an injury that will require an operation? Accurate determination of the projectile trajectory can help answer both of these questions. Thus, the decision about whether a patient can undergo nonoperative management depends on the clinical evaluation and trajectory determination.[3] To simplify the decision-making process, hemodynamically unstable patients with penetrating injury automatically fail nonoperative management.
Selective nonoperative management is practiced widely in stab wounds and BAT, but routine laparotomy is still the standard of care in abdominal GSWs. Mandatory exploration is the standard approach to management of patients with GSWs to the abdomen and back. This policy can be associated with a high incidence of unnecessary laparotomies and significant morbidity. To simplify and systematize the decision-making algorithm for nonoperative management of penetrating injury, the following sections will be divided into mechanism-based, and regional anatomy-based considerations (as opposed to organ-specific considerations for blunt injury).
PENETRATING INJURIES: MECHANISMBASED CONSIDERATIONS
There are important differences between penetrating military and civilian wounds. Civilian injuries are usually caused by low-caliber handgun projectiles with muzzle velocities between 800 and 1400 feet/s [Figure 6].[58] Generally, these weapons cause relatively smaller wounds associated with relatively less injury from blast or cavitation effect. Tissue damage is limited to the area around the missile trajectory, and organ injuries occur mainly within the path of the projectile.[58]
Figure 6.

Examples of high-velocity military projectiles (left), low-velocity handgun projectiles (right upper) and high-velocity handgun projectiles (right lower)
Blast injury and cavitation are much more extensive with high-velocity missiles, such as those from rifles and military weapons, or from shotgun wounds.[59] There is much greater tissue destruction along a missile path, which tends to be significantly wider than the projectile itself. Associated tissue damage often may involve areas of necrosis, and the management of these wounds includes surgical exploration and debridement, even in the absence of peritonitis or obvious peritoneal penetration.
Stab wounds can be grouped into those due to wider blades (knives, bayonets, etc.), and those due to long punctate objects (ice picks, screwdrivers, etc.) [Figure 7]. Stab wounds cause direct damage along the path of injury, with less damage to surrounding tissues. Of great importance to the treating physician is the exact mechanism of stabbing and the suspected path (including depth) of the penetrating injury. This is because imaging techniques are note as useful in demonstrating tissue injury from stab wounds as they are in demonstrating bullet tract for gunshot wounds.
Figure 7.

Examples of weapons used in stabbing injuries. From left to right: bayonet, military knife, ice pick, screwdrivernegative
PENETRATING INJURIES: REGIONAL AND ANATOMIC CONSIDERATIONS
The approach to nonoperative management of penetrating traumatic injury should be based on regional and anatomic considerations of the projectile path as well as the patient's clinical stability. Subsequent sections will focus on regional anatomy in relation to injury in an attempt to systematize and simplify the overall approach to the penetrating trauma patient.
THE ABDOMEN
Published reports from the 1990's suggest that a subset of patients with abdominal GSWs can be safely and effectively treated in non-surgical fashion. According to one source, approximately 80% of patients with peritonitis were taken directly to the operating room, whereas 20% of patients without peritoneal signs were managed nonoperatively and did not require a laparotomy.[60] Within the observation group, eight patients had negative physical examinations, even with trajectory-based suspicion peritoneal penetration. The negative and non-therapeutic laparotomy rates for the two groups were 5% and 1%, respectively. Selection was based entirely on physical examination and traditional radiography. Advanced imaging (e.g., CT) was not utilized.
Demetriades et al. corroborated the importance of physical examination in evaluation of suspected abdominal injury in a prospective study of 146 patients with abdominal GSWs.[61] In this report, most patients (72%) had a positive clinical examination on admission, prompting an immediate laparotomy. Of the 41 (28%) patients who underwent clinical observation, seven (17%) eventually underwent delayed laparotomy, with no significant change in morbidity or mortality.
One of the largest reported series of nonoperative management of abdominal GSWs evaluated 1856 patients.[17] Of those, 792 (42%) patients were initially triaged to nonoperative arm of the study. Within this group, 712 were eventually discharged without an operation. Among the 80 patients who failed nonoperative management and received an a delayed laparotomy, 57 had injuries necessitating operative repair. Of note, while the primary triage tool was the clinical abdominal examination, most patients chosen for nonoperative approach also required a abdominal CT. The combined negative and NTL rate was 13% among patients taken for immediate laparotomy and 29% in those undergoing delayed surgical intervention. The authors reported five complications in the “operative delay” group, including three intraabdominal abscesses, one ileus, and one case of acute respiratory distress syndrome. These complications were attributed to a delay in treatment among the 80 patients in the delayed exploration group, with no associated deaths reported.. In aggregate, this and the other studies corroborate the importance of physical examination as a sensitive indicator of intraabdominal injury in the setting of penetrating trauma.
THORACOABDOMINAL WOUNDS
In terms of defining landmarks, the thoracoabdominal area (TAA) extends from the nipple line to the costal margins bilaterally. This region represents a space within which thoracic and abdominal contents can shift dynamically based on patient positioning, respiratory cycle dynamics, and other factors. The difficulty in determining penetrating wound trajectory the TAA region is partly caused by the diaphragmatic mobility and the dynamic relationship between internal organs and surface landmarks. The right side of TAA is mostly occupied by the liver, whereas the left side contains the spleen, stomach, portions of large bowel, and small intestinal loops. Due to the complexity of these anatomic relationships, injuries to the TAA are treated differently, depending on laterality, patient positioning, and mechanism of injury.[3]
Penetrating trauma to the right TAA usually involves the liver and the diaphragm. Whilst is has been proposed the right-sided diaphragmatic injuries are less severe because the liver acts to “protect” the defect and prevent delayed herniation of abdominal contents, this theory may not be universally true.[62] Overall, right-sided TAA gunshot injuries account for <7% of all abdominal GSWs.[63] Non-surgical approach to this particular injury complex can be attributed to the frustration caused by reportedly avoidable operations on nonbleeding injuries isolated to the liver. In one series of right TAA GSWs, the majority of patients were noted to have both a chest injury (hemothorax, lung contusion) and a solid organ (liver, kidney) trauma. Most of the thoracic injuries were treated chest tube drainage thoracostomy only, with the associated solid abdominal organ injuries being observed without the need for surgery.[63] In another series of 33 patients with right TAA GSWs treated nonoperatively, only one required a laparotomy due to clinical deterioration.[64] In another study, 16 hemodynamically stable patients with right TAA GSWs were managed nonoperatively after evaluation with abdominal CT.[65] Within this group, five underwent a delayed celiotomy for peritonitis or abdominal compartment syndrome. Among the 11 patients treated non-surgically, one patient developed a biloma that was drained using percutaneous techniques. At some institutions, penetrating injuries to right TAA region, with CT-proven trajectory through the liver and no clinical or radiographic evidence of other injuries, may be considered for selective nonoperative management.
Injuries to the left TAA pose a much higher risk of diaphragmatic and/or hollow viscus injury. Because abdominal CT lacks sensitivity/specificity for identification of diaphragmatic injury, other methods must be utilized.[66] It has been noted that the incidence of diaphragmatic injury with left TAA GSWs can be as high as 42%.[67] In fact, out of all the patients who underwent diagnostic laparoscopy to rule out diaphragmatic injury and were subsequently found to have one 31% had no signs of peritonitis and 40% had a normal chest x-rays. Consequently diagnostic laparoscopy is recommended for all patients with left TAA GSWs.
THE BACK AND FLANK
The flank is the anatomic region enclosed between the tips of the scapulae superiorly, the iliac crests inferiorly, the anterior axillary lines and the posterior axillary lines. The back is defined by the posterior axillary line bilaterally, the scapular tip superiorly, and the iliac crest inferiorly. Clinical assessment of trauma victims with penetrating injuries to these areas is traditionally challenging. There are insufficient data to firmly support nonoperative management of penetrating injuries to these anatomic areas, and existing studies suffer from small sample sizes. There is also the clinical conundrum of penetrating injuries involving only the retroperitoneum, which can be associated with delayed clinical manifestations due to lack of typical peritoneal irritation. Finally, commonly utilized diagnostic adjuncts, such as laparoscopy, FAST ultrasound and DPL, are known to be insufficient in detecting injuries to the retroperitoneum.
Abdominal CT has been shown to be sensitive and specific for defining injuries to the flank and back. Consequently, CT is currently the test of choice for wounds thought to be limited to the retroperitoneum. A variation of the standard CT scan utilizes “triple contrast” to better characterize retroperitoneal injuries. Here, the standard oral and intravenous contrast is supplemented by additional contrast material placed into the colon as an enema. Proponents of “triple contrast” CT postulate that it can help detect subtle injuries to the retroperitoneal colon. Disadvantages of “triple contrast” CT include the inconvenience, delay that the preparation-related delays, and added expense. Injuries to the colon associated with penetrating trauma to back or flank are fortunately very rare.[68,69]
In one of the largest clinical experiences with back and flank GSWs, 130 (69%) out of 206 patients studied were treated with clinical observation alone. There were 4 (3%) NTLs. The authors concluded that selective nonoperative management of back and flank GSWs is safe and effective.[16] Another study used abdominal CT to characterize 45 GSWs to the flank.[70] In that report, forty patients had negative abdominal CTs, were observed for 24 h, and were noted to have no delayed laparotomies or morbidity.
THE PELVIS
Due to the anatomically restricted pelvic space being occupied by numerous important anatomic structures, penetrating injuries to this region have an 85% chance of causing an internal organ injury.[71] Having said that, selective nonoperative management of penetrating trauma to the pelvis has been successfully implemented using criteria similar to those for abdominal, back, and flank wounds. Among diagnostic adjuncts useful in detecting pelvic injury are urinalysis (helpful in screening for urinary tract injuries) and rigid proctoscopy (used in detection of rectal injury). Hematuria or hematochezia carry a high predictive value for pelvic organ injury.[71]
In one study, a selective approach was employed to evaluate 59 patients with pelvic or gluteal GSWs.[72,73] Based on physical examination, adjunct examinations, and in some cases CT scan, 40 (67.8%) patients were selected for nonoperative treatment approach. There were no delayed explorations in this group, with the clinical examination alone being 100% sensitive and 95.3% specific for significant internal organ injuries after pelvic GSWs.
PENETRATING NECK INJURIES
Penetrating injuries to the neck pose a diagnostic and therapeutic challenge. Treatment of these injuries has varied historically. Before the World War II, few penetrating neck wounds underwent operative treatment unless major bleeding or deep injuries were obvious; reported mortality rates ranged between 11% and 18%.[74] Fogelman and Stewart in 1956, reported a mortality rate of 6% with prompt exploration versus 35% in cases with delayed or omitted operation.[75] This led to the widespread theory of treating penetrating neck injuries much like penetrating abdominal wounds, and the dictum of surgical exploration if platysma is violated. Using this methodology, Jones et al. reported a mortality of 3.6% in 1967.[76]
The very high density of important vascular, neurologic, and aerodigestive structures within the neck make the management of penetrating injuries difficult and contributes to the morbidity and mortality in this group of patients. Current management of penetrating neck injuries depends on several factors, which include patient stability, injury location, injury mechanism (GSWs carry higher risk of major injury), and the presence of findings suggesting aerodigestive injury.
Patients with signs of significant neck injury or those who are unstable should undergo prompt surgical exploration following rapid initial clinical assessment and airway control. Those who do not fall into this group will proceed further on the nonoperative management path, which will largely depend on injury location.
Zone I injuries pose potential danger to the great vessels, the trachea, the esophagus, thoracic duct, the upper mediastinum, and lung apices. Structures in Zone II include the carotid and vertebral arteries, jugular veins, pharynx, larynx, esophagus, and trachea. Of note, injuries to Zone II can be readily evaluated and exposed operatively. Important structures in Zone III include the distal extracranial carotid and vertebral arteries as well as segments of the jugular veins [Figure 8].
Figure 8.
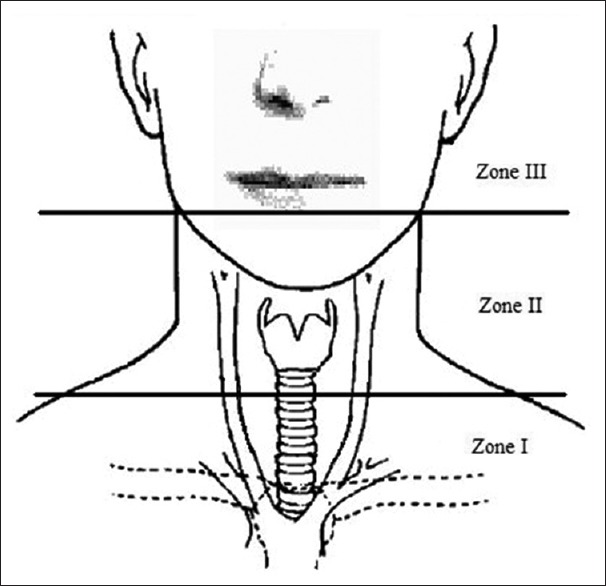
Schematic representation of the three zones of the neck
Today, the majority of penetrating neck injuries can be managed without surgical intervention, but meticulous attention to physical signs and clinical symptoms is crucial.[77,78,79] Previous dictum revolved around strong recommendation for early mandatory routine exploration of penetrating cervical trauma, especially in the “Zone II” region.[75] This paradigm was based on a review of 274 patients from the mid-1950s, where patients who underwent early exploration had an operative mortality of 6%; those explored late or not at all had a mortality of 35%.[75] More recently, Demetriades et al. have demonstrated that conservative nonoperative management of penetrating neck injuries can be performed safely if there are no clinical findings suggestive of vascular or aerodigestive injury.[80] This approach is consistent with the findings of Golueke et al., who prospectively randomized 160 patients with penetrating neck injuries and found no statistical difference in length of stay, morbidity or mortality between those patients who underwent routine or selective exploration of their injuries.[81] They concluded that surgeons should base their treatment on their own experience and the particular circumstances surrounding the patient.
While the evidence for nonoperative management of penetrating cervical injury is compelling, the extent of the diagnostic evaluation remains as crucial as ever. McConnell and Trunkey, in a meta-analysis of penetrating neck trauma, found that the incidence of isolated esophageal injury was 6.6%, isolated spinal cord damage was 3%, and the overall incidence of injury in the neck was proportional to the volume occupied by the organ.[82] In the presence of symptoms or signs of aerodigestive or vascular injury, advances imaging is clearly indicated. In addition, the signs and symptoms routinely assessed, the mechanism of injury must be considered. Gunshot wounds may not follow a predictable trajectory, and may travel in unexpected patterns if they impact upon bony structures.[83,84,85,86] In addition to the path of the bullet, two additional factors must be considered: The blast effect and the possibility of either bullet or bone fragments causing additional injury. Because of this, upper aerodigestive endoscopy and high-definition “thin cut” CT scans should be considered, and further supplemented by other endoscopic and radiographic techniques.
The significance of the aerodigestive injury may be magnified by the presence of other concurrent injuries, especially to the vascular or neurologic structures. In most cases, small perforations of the cervical esophagus do not lead to catastrophic consequences.[87] However, the combination of esophageal and dural injury can lead to neurologic complications including meningitis and quadriparesis.[88,89,90]
In summary, accumulated evidence points away from the zone-based approach to neck injuries and the practice of mandatory neck exploration. The new paradigm focuses on a combination of physiologic considerations and prompt determination of the presence of vascular and/or aerodigestive injury. High-definition radiographic testing and endoscopic techniques are crucial in this approach to neck injuries.
NONOPERATIVE MANAGEMENT OF PERIPHERAL VASCULAR INJURIES
Assessment for vascular injury must be performed in all traumatized extremities. The significance of missed vascular injury in the extremity translates into limb loss and/or functional loss and disability. Delay in recognition and treatment is the leading cause of preventable limb loss in the setting of peripheral vascular injury. Vascular injuries most commonly result from penetrating trauma, with injuries to the brachial vessels of the upper extremity and superficial femoral vessels of the lower extremity being most common in the civilian trauma practice. The management of peripheral arterial injuries is continuously evolving, and the traditional dictum of mandatory exploration for all potential vascular injuries has changed to one of selective evaluation and nonoperative management of “minimal” injuries.
Nonocclusive “minimal” arterial injuries are well documented to have a benign natural history. These injuries should be followed, rather than immediately operated on, if they are asymptomatic (i.e., absence of hard signs), the vessel is patent, and there is no gross extravasation. Most of these minor injuries never require surgery.
There is a well-defined subset of physical exam characteristics that help the trauma physician single out injuries that may fail selective nonoperative management. Detailed physical examination for suspected vascular injury includes assessment of pulses, Doppler signals, capillary refill, skin temperature, and motor and sensory function. Associated soft tissue and skeletal injuries should be noted, as these may alter clinical management. In addition, the use of “hard” signs of vascular injury as indicators of need for surgery yields an accuracy of over 95%. On the other hand, most clinically occult vascular injuries resolve on their own and never require surgery [Table 5].
Table 5.
“Hard” and “soft” signs associated with peripheral vascular injury

Increasing body of evidence advocates nonoperative management of vascular injuries with “minimal” findings on arteriography. These injuries are characterized by: (a) Minimal vessel irregularities, (b) small intimal flaps, (c) small pseudoaneurysm (<1 cm), (d) small arteriovenous fistulae. Also important, “proximity” alone is not an indication for arteriography. Interventional radiologic techniques such as stenting across injured arterial segments, are being employed more frequently, with acceptable results.[91,92,93]
Important diagnostic adjuncts in the setting of peripheral vascular injury include measurements of the ankle-brachial and the ankle-ankle index. In the absence of preexisting severe peripheral arterial disease, an ankle-brachial index (ABI) measurement of <0.40 constitutes an indication for operative intervention. ABI values between 0.40 and 0.90 warrant emergent arterial imaging.
SPECIAL CONSIDERATIONS IN NONOPERATIVE MANAGEMENT OF TRAUMA
Certain types of injuries to pharynx and esophagus can be amenable to nonoperative management. Nonoperative approach to these injuries generally applies to small injuries, with no free flow of contrast material into surrounding tissues. It is also important to determine that no other lesions requiring surgical intervention exist, and there continue to be minimal or no symptoms over time. Furthermore, there should be no evidence of active infection and antibiotic treatment should be initiated promptly after the injury. Adjuncts in successful nonoperative management of pharyngeal and esophageal injuries include nasogastric suction, adequate nutrition (either parenteral or via jejunostomy tube), antibiotic therapy, and nothing by mouth. Antibiotic coverage used in the setting of these injuries should adequately address oropharyngeal flora, with appropriate gram-negative anaerobic and antifungal coverage. Adequate enteral nutrition given via postpyloric route, may be associated with lower infection and mortality rates.
Adrenal gland trauma [Figure 9], a rare and largely coincidental finding during initial radiographic evaluation of patients who sustained severe trauma, has been associated with high injury severity and associated mortality of nearly 33% (5 times the mortality of patients without concomitant adrenal gland trauma).[94] Although nearly 80% of patients with documented adrenal gland trauma in one study were treated nonoperatively, these patients sustained significant other associated severe injuries, including liver injury (58%), rib fractures (51%), renal injury (41%), and splenic injury (33%). Pulmonary complications were most common, followed by infection/sepsis, and cardiovascular complications in this group.[94]
Figure 9.
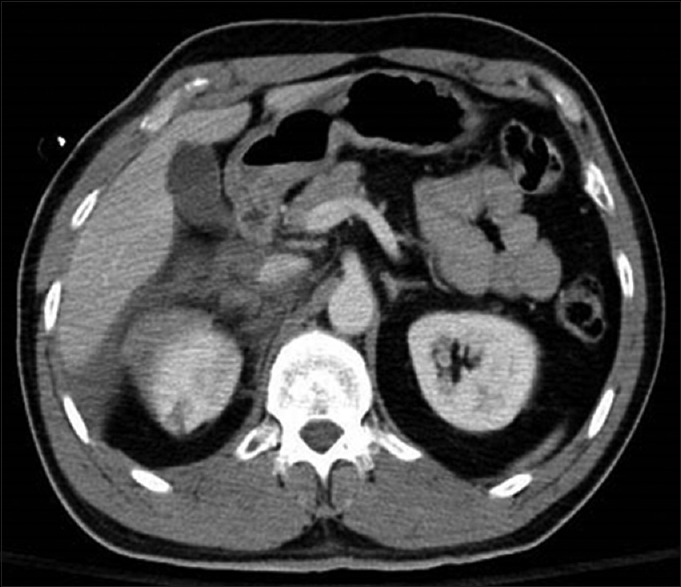
An example of right adrenal injury with concurrent right renal laceration and hemoperitoneum. This patient was managed nonoperatively
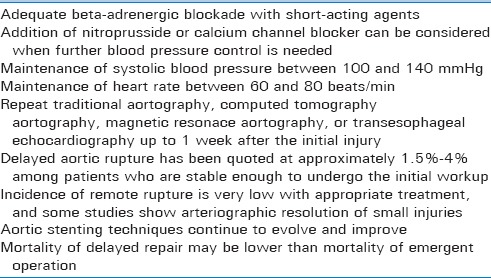
Nonoperative management of blunt AI is centered around adequate blood pressure and heart rate control [Table 6].[95,96] Appropriate beta-adrenergic blockade with short-acting beta-blockers is essential when nonoperative path of management for traumatic AI is chosen. The addition of nitroprusside and/or calcium channel blocker should be considered for blood pressure control, if needed. Different authors vary on the preferred target systolic blood pressure in the setting of blunt AI, with desired systolic blood pressure ranges between 100 and 140 mmHg, with evidence that most trauma patients can tolerate the lower limit of blood pressure quite well.[97,98,99] Heart rate goal in nonoperative blunt AI should be kept around 60–80 beats/min.[96] Patients should subsequently be followed by CT angiography, MR angiography, and/or transesophageal echocardiography done up to 1 week after the initial injury.[95] The delayed rupture of aortic injuries has been quoted at approximately 1.5%–4% among patients who are stable enough to undergo the initial workup, including CT scan and aortography.[95] Studies also demonstrate that the incidence of remote rupture of AI is uncommon with proper treatment, and that some patients have regression of AI with anti-hypertensive management.[100,101,102] For example, only 7% of patients with a history of acute AI developed chronic thoracic aneurysms in one study with median follow-up period of 22 years.[103] Aortic stenting can be successful, although operative repair is still the most reliable and proven treatment.[95] It may also be that a hybrid approach including initial nonoperative management followed by delayed surgical repair may be most beneficial, as the mortality of delayed operation has been quoted at 10% as opposed over 30% mortality for patients undergoing emergent operation.[98]
Table 6.
Key points regarding nonoperative management of blunt aortic injuries
Severe pelvic fractures resulting from blunt injury should undergo external fixation, and associated Zone III retroperitoneal hematomas should not be explored, as this will most commonly be disastrous. External fixation controls a significant amount of bleeding. Angiography and embolization may be required. These patients are best treated with supportive therapy in the ICU environment, and surgery is reserved only for significant arterial or visceral injuries associated with fractures.
THE ROLE OF INTERVENTIONAL RADIOLOGY
Interventional radiology has been one of the most important adjuncts in nonoperative management of trauma, especially in recent years. Angiography is a valuable modality in the nonoperative management of multitude of injuries. It has been successfully used as an adjunct to surgery and as the definitive treatment modality in selected blunt and penetrating trauma scenarios. Although traditionally viewed as an adjunct to surgical treatment of complex liver and pelvic injuries, over the past decade it has been used more aggressively for nonoperative control of bleeding due to other solid organ injuries. Angiographic techniques contribute significantly to the reduced number of operative interventions in modern trauma practice.
The selection of patients for angiographic intervention is based on clinical examination and CT scan findings. The patient must be hemodynamically stable or at least transiently responsive to resuscitation, with no peritoneal signs, and the CT scan must demonstrate solid-organ or pelvic injury with evidence of active bleeding. The presence of angiographic “blush” or pooling of contrast material in the parenchyma of a ruptured solid organ or in the pelvic region constitute a strong indication for angiographic embolization.[104,105,106] Angiographic intervention has been successful in selected cases of blunt splenic or renal injury, and even in patients with high-grade blunt liver injury.[104,105,107,108]
In fact, the success rate in renal injuries is significantly higher than in liver or splenic injuries, mainly because of the presence of the Gerota's fascia, which serves as a containment barrier. With appropriate institution-specific protocols and a dedicated team of interventional radiologists, many patients can be spared an operation and the potential complications associated with it [Table 7].
Table 7.
Types of minimally invasive procedural interventions in trauma patient management

Perhaps the most successful example of application of the angiographic approach to traumatic injury is in cases of severe pelvic hemorrhage. Life-threatening pelvic hemorrhage is primarily caused by arterial injury and most bleeding sites originate from branches of the hypogastric artery. An aggressive surgical approach may not be successful because bleeding sites are difficult to localize surgically, arterial ligation often does not achieve hemostasis, and opening of the retroperitoneum may negate the tamponading effect of the hematoma as well as increase the danger of infection. Angiography with selective embolization can successfully control the hemorrhage. Early embolization is very important in reducing transfusion requirements and associated complications.[109] More recently, angiographic intervention has been successful in the nonoperative management of penetrating injuries to the liver and kidneys, including GSWs.[110]
Most of the local complications following nonoperative management of traumatic injuries, including bilomas, urinomas, or abscesses, can be managed with percutaneous drainage. In addition, interventional radiology has helped trauma surgeons significantly with the placement of both permanent and retrievable vena caval filters in situations when anticoagulation (prophylactic or therapeutic) for deep venous thrombosis is not feasible.[111]
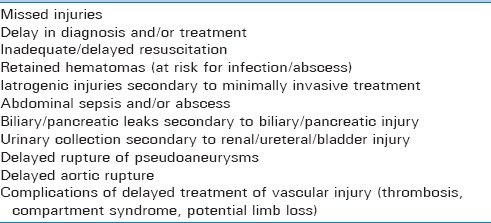
COMPLICATIONS OF NONOPERATIVE MANAGEMENT OF TRAUMATIC INJURIES
Complications associated with nonoperative management of blunt traumatic injuries include, but are not limited to, the following: Missed injuries, delays in diagnosis and/or treatment, potential iatrogenic injuries resulting from minimally-invasive techniques, intra-abdominal sepsis and abscess, inadequate resuscitation, delayed rupture of traumatic pseudoaneurysms (especially splenic). An example of a patient simultaneously treated with several percutaneous interventions is provided in Figure 10.
Figure 10.
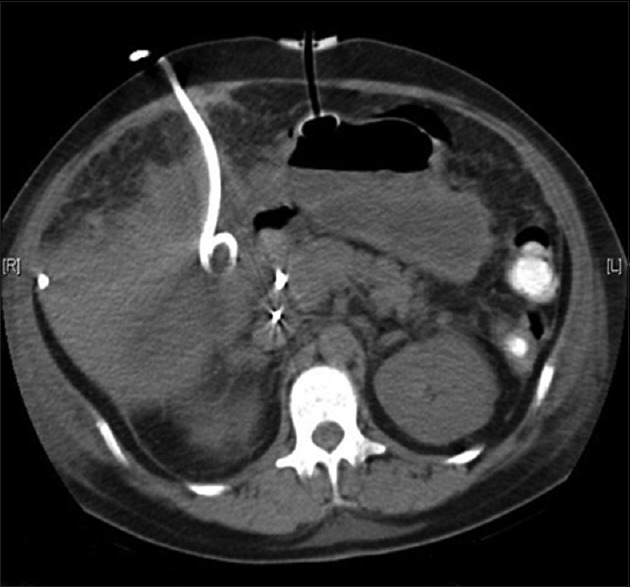
Computed tomographic image showing an example of simultaneous use of a percutaneous pigtail catheter to drain a bile collection (left) and a PEG tube (center) in the same patient
Infections prevail as one of the most common causes of morbidity in trauma patients. Infection has been one of the late consequences of nonoperative management of traumatic injuries, especially in the setting of critically ill patients who require prolonged ICU stays. Clinicians caring for severely injured patients who are being managed nonoperatively should have a high index of suspicion and should approach suspected infectious complications aggressively [Table 8].
Table 8.
Synopsis of complications associated with nonoperative management of traumatic injuries
Many of the complications of nonoperative management of traumatic injuries are amenable to percutaneous of endoscopic interventions and do not require formal surgical exploration. For example, bile leaks and bile collections following nonoperative management of hepatobiliary injuries can be effectively managed utilizing percutaneous cholangiographic techniques and endoscopic retrograde cholangiopancreatography (ERCP).[112]
Although many posttraumatic hematomas resolve on their own, percutaneous approaches may be of therapeutic and diagnostic value, and may help avoid surgery. Potential benefits of percutaneous management of hematomas have to be considered in the context of associated risks.
Certain complications of nonoperative approaches to pancreatic injuries may also require further invasive interventions. Within this group of morbidities are pancreatic fistulae, pancreatic ascites, and posttraumatic pancreatitis with potential for pseudocyst formation. While many of these injuries can be treated with a combination of ERCP and percutaneous approaches, some ultimately necessitate an operation.[113]
Complications of nonoperative management of urinary tract injuries include urinoma formation, which may be amenable to a combination of urologic endoscopic procedures (retrograde stenting) and interventional radiologic procedures (percutaneous drainage). Finally, various types of traumatic pseudoaneurysms may qualify for percutaneous interventions, with operative treatment reserved for failures of nonoperative or minimally invasive therapy.[114]
CONCLUSIONS
Nonoperative management of both blunt and penetrating traumatic injuries can be challenging. However, it can be quite satisfying to be able to successfully manage patients with severe and multiple traumatic injuries in nonoperative fashion. The advent of sophisticated imaging technologies and adjunctive minimally invasive techniques has somewhat lightened the trauma surgeon's operative burden. Despite that, more than ever, nothing surpasses the value of repeated clinical assessment by an experienced trauma surgeon, in guiding the ultimate therapeutic decisions. After all, the ultimate default pathway for severely injured trauma patients who failed nonoperative management is the operating theater.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Justifications for re-publishing this scholarly content include: (a) The phasing out of the original publication – the OPUS 12 Scientist and (b) Wider dissemination of the research outcome(s) and the associated scientific knowledge. Minor language editing for clarity was performed during the process.
REFERENCES
- 1.Schwab CW. Selection of nonoperative management candidates. World J Surg. 2001;25:1389–92. doi: 10.1007/s00268-001-0137-x. [DOI] [PubMed] [Google Scholar]
- 2.Demetriades D, Velmahos G. Technology-driven triage of abdominal trauma: The emerging era of nonoperative management. Annu Rev Med. 2003;54:1–15. doi: 10.1146/annurev.med.54.101601.152512. [DOI] [PubMed] [Google Scholar]
- 3.Pryor JP, Reilly PM, Dabrowski GP, Grossman MD, Schwab CW. Nonoperative management of abdominal gunshot wounds. Ann Emerg Med. 2004;43:344–53. doi: 10.1016/s0196-0644(03)00815-1. [DOI] [PubMed] [Google Scholar]
- 4.Feliciano DV. Diagnostic modalities in abdominal trauma. Peritoneal lavage, ultrasonography, computed tomography scanning, and arteriography. Surg Clin North Am. 1991;71:241–56. doi: 10.1016/s0039-6109(16)45377-6. [DOI] [PubMed] [Google Scholar]
- 5.Enderson BL, Maull KI. Missed injuries. The trauma surgeon's nemesis. Surg Clin North Am. 1991;71:399–418. doi: 10.1016/s0039-6109(16)45387-9. [DOI] [PubMed] [Google Scholar]
- 6.McAnena OJ, Moore EE, Marx JA. Initial evaluation of the patient with blunt abdominal trauma. Surg Clin North Am. 1990;70:495–515. doi: 10.1016/s0039-6109(16)45126-1. [DOI] [PubMed] [Google Scholar]
- 7.Davis JJ, Cohn I, Jr, Nance FC. Diagnosis and management of blunt abdominal trauma. Ann Surg. 1976;183:672–8. doi: 10.1097/00000658-197606000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knudson MM, Maull KI. Nonoperative management of solid organ injuries. Past, present, and future. Surg Clin North Am. 1999;79:1357–71. doi: 10.1016/s0039-6109(05)70082-7. [DOI] [PubMed] [Google Scholar]
- 9.McConnell DB, Trunkey DD. Nonoperative management of abdominal trauma. Surg Clin North Am. 1990;70:677–88. doi: 10.1016/s0039-6109(16)45137-6. [DOI] [PubMed] [Google Scholar]
- 10.Christmas AB, Wilson AK, Manning B, Franklin GA, Miller FB, Richardson JD, et al. Selective management of blunt hepatic injuries including nonoperative management is a safe and effective strategy. Surgery. 2005;138:606–10. doi: 10.1016/j.surg.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Rutledge R, Hunt JP, Lentz CW, Fakhry SM, Meyer AA, Baker CC, et al. A statewide, population-based time-series analysis of the increasing frequency of nonoperative management of abdominal solid organ injury. Ann Surg. 1995;222:311–22. doi: 10.1097/00000658-199509000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renz BM, Feliciano DV. Unnecessary laparotomies for trauma: A prospective study of morbidity. J Trauma. 1995;38:350–6. doi: 10.1097/00005373-199503000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Leppäniemi A, Salo J, Haapiainen R. Complications of negative laparotomy for truncal stab wounds. J Trauma. 1995;38:54–8. doi: 10.1097/00005373-199501000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Demetriades D, Rabinowitz B. Indications for operation in abdominal stab wounds. A prospective study of 651 patients. Ann Surg. 1987;205:129–32. doi: 10.1097/00000658-198702000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demetriades D, Velmahos G, Cornwell E, 3rd, Berne TV, Cober S, Bhasin PS, et al. Selective nonoperative management of gunshot wounds of the anterior abdomen. Arch Surg. 1997;132:178–83. doi: 10.1001/archsurg.1997.01430260076017. [DOI] [PubMed] [Google Scholar]
- 16.Velmahos GC, Demetriades D, Foianini E, Tatevossian R, Cornwell EE, 3rd, Asensio J, et al. A selective approach to the management of gunshot wounds to the back. Am J Surg. 1997;174:342–6. doi: 10.1016/s0002-9610(97)00098-6. [DOI] [PubMed] [Google Scholar]
- 17.Velmahos GC, Demetriades D, Toutouzas KG, Sarkisyan G, Chan LS, Ishak R, et al. Selective nonoperative management in 1,856 patients with abdominal gunshot wounds: Should routine laparotomy still be the standard of care? Ann Surg. 2001;234:395–402. doi: 10.1097/00000658-200109000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson VJ, Organ CH, Jr, Smith RS. Negative trauma celiotomy. Am Surg. 1993;59:365–70. [PubMed] [Google Scholar]
- 19.Miller FB, Cryer HM, Chilikuri S, Creech P, Richardson JD. Negative findings on laparotomy for trauma. South Med J. 1989;82:1231–4. doi: 10.1097/00007611-198910000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Hasaniya N, Demetriades D, Stephens A, Dubrowskiz R, Berne T. Early morbidity and mortality of non-therapeutic operations for penetrating trauma. Am Surg. 1994;60:744–7. [PubMed] [Google Scholar]
- 21.Weigelt JA, Kingman RG. Complications of negative laparotomy for trauma. Am J Surg. 1988;156:544–7. doi: 10.1016/s0002-9610(88)80549-x. [DOI] [PubMed] [Google Scholar]
- 22.Bensard DD, Beaver BL, Besner GE, Cooney DR. Small bowel injury in children after blunt abdominal trauma: Is diagnostic delay important? J Trauma. 1996;41:476–83. doi: 10.1097/00005373-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Scalea TM, Rodriguez A, Chiu WC, Brenneman FD, Fallon WF, Jr, Kato K, et al. Focused Assessment with Sonography for Trauma (FAST): Results from an international consensus conference. J Trauma. 1999;46:466–72. doi: 10.1097/00005373-199903000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Kawaguchi S, Toyonaga J, Ikeda K. Five point method: An ultrasonographic quantification formula of intra-abdominal fluid collection. Jpn J Acute Med. 1987;7:993–7. [Google Scholar]
- 25.Tiling T, Bouillon B, Schmid A, Schweins M, Steffens H. Ultrasound in blunt abdominothoracic trauma. In: Border JR, editor. Blunt Multiple Trauma: Comprehensive Pathophysiology and Care. New York: Marcel Dekker; 1990. pp. 415–33. [Google Scholar]
- 26.Chiu WC, Cushing BM, Rodriguez A, Ho SM, Mirvis SE, Shanmuganathan K, et al. Abdominal injuries without hemoperitoneum: A potential limitation of focused abdominal sonography for trauma (FAST) J Trauma. 1997;42:617–23. doi: 10.1097/00005373-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Rozycki GS, Ballard RB, Feliciano DV, Schmidt JA, Pennington SD. Surgeon-performed ultrasound for the assessment of truncal injuries: Lessons learned from 1540 patients. Ann Surg. 1998;228:557–67. doi: 10.1097/00000658-199810000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boulanger BR, Kearney PA, Tsuei B, Ochoa JB. The routine use of sonography in penetrating torso injury is beneficial. J Trauma. 2001;51:320–5. doi: 10.1097/00005373-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Udobi KF, Rodriguez A, Chiu WC, Scalea TM. Role of ultrasonography in penetrating abdominal trauma: A prospective clinical study. J Trauma. 2001;50:475–9. doi: 10.1097/00005373-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Coley BD, Mutabagani KH, Martin LC, Zumberge N, Cooney DR, Caniano DA, et al. Focused abdominal sonography for trauma (FAST) in children with blunt abdominal trauma. J Trauma. 2000;48:902–6. doi: 10.1097/00005373-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Thourani VH, Pettitt BJ, Schmidt JA, Cooper WA, Rozycki GS. Validation of surgeon-performed emergency abdominal ultrasonography in pediatric trauma patients. J Pediatr Surg. 1998;33:322–8. doi: 10.1016/s0022-3468(98)90455-9. [DOI] [PubMed] [Google Scholar]
- 32.Demetriades D, Gomez H, Velmahos GC, Asensio JA, Murray J, Cornwell EE, 3rd, et al. Routine helical computed tomographic evaluation of the mediastinum in high-risk blunt trauma patients. Arch Surg. 1998;133:1084–8. doi: 10.1001/archsurg.133.10.1084. [DOI] [PubMed] [Google Scholar]
- 33.Velmahos GC, Demetriades D, Chan L, Tatevossian R, Cornwell EE, 3rd, Yassa N, et al. Predicting the need for thoracoscopic evacuation of residual traumatic hemothorax: Chest radiograph is insufficient. J Trauma. 1999;46:65–70. doi: 10.1097/00005373-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Goan YG, Huang MS, Lin JM. Nonoperative management for extensive hepatic and splenic injuries with significant hemoperitoneum in adults. J Trauma. 1998;45:360–4. doi: 10.1097/00005373-199808000-00026. [DOI] [PubMed] [Google Scholar]
- 35.Meredith JW, Young JS, Bowling J, Roboussin D. Nonoperative management of blunt hepatic trauma: The exception or the rule? J Trauma. 1994;36:529–34. doi: 10.1097/00005373-199404000-00012. [DOI] [PubMed] [Google Scholar]
- 36.American Association for the Surgery of Trauma. AAST Injury Scaling and Scoring System, Daphne, AL. 1998. [Last accessed on 2007 Feb 25]. Available from: http://www.aast.org/injury/injury.html .
- 37.Croce MA, Fabian TC, Menke PG, Waddle-Smith L, Minard G, Kudsk KA, et al. Nonoperative management of blunt hepatic trauma is the treatment of choice for hemodynamically stable patients. Results of a prospective trial. Ann Surg. 1995;221:744–53. doi: 10.1097/00000658-199506000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huerta S, Bui T, Porral D, Lush S, Cinat M. Predictors of morbidity and mortality in patients with traumatic duodenal injuries. Am Surg. 2005;71:763–7. doi: 10.1177/000313480507100914. [DOI] [PubMed] [Google Scholar]
- 39.Akhrass R, Yaffe MB, Brandt CP, Reigle M, Fallon WF, Jr, Malangoni MA. Pancreatic trauma: A ten-year multi-institutional experience. Am Surg. 1997;63:598–604. [PubMed] [Google Scholar]
- 40.Glancy KE. Review of pancreatic trauma. West J Med. 1989;151:45–51. [PMC free article] [PubMed] [Google Scholar]
- 41.Ivatury RR, Nallathambi M, Rao P, Stahl WM. Penetrating pancreatic injuries. Analysis of 103 consecutive cases. Am Surg. 1990;56:90–5. [PubMed] [Google Scholar]
- 42.Gay SB, Sistrom CL. Computed tomographic evaluation of blunt abdominal trauma. Radiol Clin North Am. 1992;30:367–88. [PubMed] [Google Scholar]
- 43.Gupta A, Stuhlfaut JW, Fleming KW, Lucey BC, Soto JA. Blunt trauma of the pancreas and biliary tract: A multimodality imaging approach to diagnosis. Radiographics. 2004;24:1381–95. doi: 10.1148/rg.245045002. [DOI] [PubMed] [Google Scholar]
- 44.Soto JA, Alvarez O, Múnera F, Yepes NL, Sepúlveda ME, Pérez JM. Traumatic disruption of the pancreatic duct: Diagnosis with MR pancreatography. AJR Am J Roentgenol. 2001;176:175–8. doi: 10.2214/ajr.176.1.1760175. [DOI] [PubMed] [Google Scholar]
- 45.Wong YC, Wang LJ, Lin BC, Chen CJ, Lim KE, Chen RJ. CT grading of blunt pancreatic injuries: Prediction of ductal disruption and surgical correlation. J Comput Assist Tomogr. 1997;21:246–50. doi: 10.1097/00004728-199703000-00014. [DOI] [PubMed] [Google Scholar]
- 46.Lane MJ, Mindelzun RE, Sandhu JS, McCormick VD, Jeffrey RB. CT diagnosis of blunt pancreatic trauma: Importance of detecting fluid between the pancreas and the splenic vein. AJR Am J Roentgenol. 1994;163:833–5. doi: 10.2214/ajr.163.4.7503824. [DOI] [PubMed] [Google Scholar]
- 47.Bradley EL, 3rd, Young PR, Jr, Chang MC, Allen JE, Baker CC, Meredith W, et al. Diagnosis and initial management of blunt pancreatic trauma: Guidelines from a multiinstitutional review. Ann Surg. 1998;227:861–9. doi: 10.1097/00000658-199806000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leppäniemi AK, Haapiainen RK. Risk factors of delayed diagnosis of pancreatic trauma. Eur J Surg. 1999;165:1134–7. doi: 10.1080/110241599750007649. [DOI] [PubMed] [Google Scholar]
- 49.Akhrass R, Kim K, Brandt C. Computed tomography: An unreliable indicator of pancreatic trauma. Am Surg. 1996;62:647–51. [PubMed] [Google Scholar]
- 50.Carroll PR, McAninch JW. Major bladder trauma: Mechanisms of injury and a unified method of diagnosis and repair. J Urol. 1984;132:254–7. doi: 10.1016/s0022-5347(17)49581-4. [DOI] [PubMed] [Google Scholar]
- 51.Cass AS. The multiple injured patient with bladder trauma. J Trauma. 1984;24:731–4. doi: 10.1097/00005373-198408000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Hochberg E, Stone NN. Bladder rupture associated with pelvic fracture due to blunt trauma. Urology. 1993;41:531–3. doi: 10.1016/0090-4295(93)90099-v. [DOI] [PubMed] [Google Scholar]
- 53.Cass AS, Gleich P, Smith C. Simultaneous bladder and prostatomembranous urethral rupture from external trauma. J Urol. 1984;132:907–8. doi: 10.1016/s0022-5347(17)49940-x. [DOI] [PubMed] [Google Scholar]
- 54.Corriere JN, Jr, Sandler CM. Mechanisms of injury, patterns of extravasation and management of extraperitoneal bladder rupture due to blunt trauma. J Urol. 1988;139:43–4. doi: 10.1016/s0022-5347(17)42284-1. [DOI] [PubMed] [Google Scholar]
- 55.Cass AS, Luxenberg M. Management of extraperitoneal ruptures of bladder caused by external trauma. Urology. 1989;33:179–83. doi: 10.1016/0090-4295(89)90386-5. [DOI] [PubMed] [Google Scholar]
- 56.Kotkin L, Koch MO. Morbidity associated with nonoperative management of extraperitoneal bladder injuries. J Trauma. 1995;38:895–8. doi: 10.1097/00005373-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Corriere JN, Jr, Sandler CM. Management of extraperitoneal bladder rupture. Urol Clin North Am. 1989;16:275–7. [PubMed] [Google Scholar]
- 58.Bellamy RF, Zajtchuk R, editors. Conventional Warfare: Ballistic, Blast and Burn Injuries. Washington, DC: Office of the Surgeon General, Department of the Army; 1990. [Google Scholar]
- 59.Swan KG, Swan RC. Principles of ballistics applicable to the treatment of gunshot wounds. Surg Clin North Am. 1991;71:221–39. doi: 10.1016/s0039-6109(16)45376-4. [DOI] [PubMed] [Google Scholar]
- 60.Muckart DJ, Abdool-Carrim AT, King B. Selective conservative management of abdominal gunshot wounds: A prospective study. Br J Surg. 1990;77:652–5. doi: 10.1002/bjs.1800770620. [DOI] [PubMed] [Google Scholar]
- 61.Demetriades D, Charalambides D, Lakhoo M, Pantanowitz D. Gunshot wound of the abdomen: Role of selective conservative management. Br J Surg. 1991;78:220–2. doi: 10.1002/bjs.1800780230. [DOI] [PubMed] [Google Scholar]
- 62.Zierold D, Perlstein J, Weidman ER, Wiedeman JE. Penetrating trauma to the diaphragm: Natural history and ultrasonographic characteristics of untreated injury in a pig model. Arch Surg. 2001;136:32–7. doi: 10.1001/archsurg.136.1.32. [DOI] [PubMed] [Google Scholar]
- 63.Renz BM, Feliciano DV. The length of hospital stay after an unnecessary laparotomy for trauma: A prospective study. J Trauma. 1996;40:187–90. doi: 10.1097/00005373-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Chmielewski GW, Nicholas JM, Dulchavsky SA, Diebel LN. Nonoperative management of gunshot wounds of the abdomen. Am Surg. 1995;61:665–8. [PubMed] [Google Scholar]
- 65.Demetriades D, Gomez H, Chahwan S, Charalambides K, Velmahos G, Murray J, et al. Gunshot injuries to the liver: The role of selective nonoperative management. J Am Coll Surg. 1999;188:343–8. doi: 10.1016/s1072-7515(98)00315-9. [DOI] [PubMed] [Google Scholar]
- 66.Guth AA, Pachter HL, Kim U. Pitfalls in the diagnosis of blunt diaphragmatic injury. Am J Surg. 1995;170:5–9. doi: 10.1016/s0002-9610(99)80242-6. [DOI] [PubMed] [Google Scholar]
- 67.Murray JA, Demetriades D, Cornwell EE, 3rd, Asensio JA, Velmahos G, Belzberg H, et al. Penetrating left thoracoabdominal trauma: The incidence and clinical presentation of diaphragm injuries. J Trauma. 1997;43:624–6. doi: 10.1097/00005373-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Phillips T, Sclafani SJ, Goldstein A, Scalea T, Panetta T, Shaftan G. Use of the contrast-enhanced CT enema in the management of penetrating trauma to the flank and back. J Trauma. 1986;26:593–601. doi: 10.1097/00005373-198607000-00002. [DOI] [PubMed] [Google Scholar]
- 69.Kirton OC, Wint D, Thrasher B, Windsor J, Echenique A, Hudson-Civetta J. Stab wounds to the back and flank in the hemodynamically stable patient: A decision algorithm based on contrast-enhanced computed tomography with colonic opacification. Am J Surg. 1997;173:189–93. doi: 10.1016/s0002-9610(96)00010-4. [DOI] [PubMed] [Google Scholar]
- 70.Ginzburg E, Carrillo EH, Kopelman T, McKenney MG, Kirton OC, Shatz DV, et al. The role of computed tomography in selective management of gunshot wounds to the abdomen and flank. J Trauma. 1998;45:1005–9. doi: 10.1097/00005373-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 71.DiGiacomo JC, Schwab CW, Rotondo MF, Angood PA, McGonigal MD, Kauder DR, et al. Gluteal gunshot wounds: Who warrants exploration? J Trauma. 1994;37:622–8. [PubMed] [Google Scholar]
- 72.Velmahos GC, Demetriades D, Cornwell EE, Asensio J, Belzberg H, Berne TV. Gunshot wounds to the buttocks: Predicting the need for operation. Dis Colon Rectum. 1997;40:307–11. doi: 10.1007/BF02050420. [DOI] [PubMed] [Google Scholar]
- 73.Velmahos GC, Demetriades D, Cornwell EE., 3rd Transpelvic gunshot wounds: Routine laparotomy or selective management? World J Surg. 1998;22:1034–8. doi: 10.1007/s002689900512. [DOI] [PubMed] [Google Scholar]
- 74.Shires GT, Thal ER, Jones RC, Shires GT, III, Perry MO. Trauma. In: Schwartz SI, editor. Principles of surgery. 6th ed. New York, USA: McGraw-Hill; 1994. pp. 187–93. [Google Scholar]
- 75.Fogelman MJ, Stewart RD. Penetrating wounds of the neck. Am J Surg. 1956;91:581–93. doi: 10.1016/0002-9610(56)90289-6. [DOI] [PubMed] [Google Scholar]
- 76.Jones RF, Terrell JC, Salyer KE. Penetrating wounds of the neck: An analysis of 274 cases. J Trauma. 1967;7:228–37. doi: 10.1097/00005373-196703000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Demetriades D, Theodorou D, Cornwell E, Berne TV, Asensio J, Belzberg H, et al. Evaluation of penetrating injuries of the neck: Prospective study of 223 patients. World J Surg. 1997;21:41–7. doi: 10.1007/s002689900191. [DOI] [PubMed] [Google Scholar]
- 78.Demetriades D, Charalambides D, Lakhoo M. Physical examination and selective conservative management in patients with penetrating injuries of the neck. Br J Surg. 1993;80:1534–6. doi: 10.1002/bjs.1800801213. [DOI] [PubMed] [Google Scholar]
- 79.Demetriades D, Theodorou D, Cornwell E, 3rd, Weaver F, Yellin A, Velmahos G, et al. Penetrating injuries of the neck in patients in stable condition. Physical examination, angiography, or color flow Doppler imaging. Arch Surg. 1995;130:971–5. doi: 10.1001/archsurg.1995.01430090057019. [DOI] [PubMed] [Google Scholar]
- 80.Demetriades D, Theodorou D, Cornwell E, Asensio J, Belzberg H, Velmahos G, et al. Transcervical gunshot injuries: Mandatory operation is not necessary. J Trauma. 1996;40:758–60. doi: 10.1097/00005373-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 81.Golueke PJ, Goldstein AS, Sclafani SJ, Mitchell WG, Shaftan GW. Routine versus selective exploration of penetrating neck injuries: A randomized prospective study. J Trauma. 1984;24:1010–4. doi: 10.1097/00005373-198412000-00002. [DOI] [PubMed] [Google Scholar]
- 82.McConnell DB, Trunkey DD. Management of penetrating trauma to the neck. Adv Surg. 1994;27:97–127. [PubMed] [Google Scholar]
- 83.Ordog GJ, Wasserberger J, Balasubramaniam S. Shotgun wound ballistics. J Trauma. 1988;28:624–31. doi: 10.1097/00005373-198805000-00011. [DOI] [PubMed] [Google Scholar]
- 84.Ordog GJ, Wasserberger J, Prakash A, Balasubramaniam S. Civilian gunshot wounds: Determinants of injury. J Trauma. 1987;27:943–7. [PubMed] [Google Scholar]
- 85.Thal ER, Meyer DM. Penetrating neck trauma. Curr Probl Surg. 1992;29:3–86. doi: 10.1016/0011-3840(92)90031-w. [DOI] [PubMed] [Google Scholar]
- 86.Ledgerwood AM. The wandering bullet. Surg Clin North Am. 1977;57:97–109. doi: 10.1016/s0039-6109(16)41136-9. [DOI] [PubMed] [Google Scholar]
- 87.Winter RP, Weigelt JA. Cervical esophageal trauma. Incidence and cause of esophageal fistulas. Arch Surg. 1990;125:849–51. doi: 10.1001/archsurg.1990.01410190041007. [DOI] [PubMed] [Google Scholar]
- 88.Craig JB. Cervical spine osteomyelitis with delayed onset tetraparesis after penetrating wounds of the neck. A report of 2 cases. S Afr Med J. 1986;69:197–9. [PubMed] [Google Scholar]
- 89.Nakai S, Yoshizawa H, Kobayashi S, Miyachi M. Esophageal injury secondary to thoracic spinal trauma: The need for early diagnosis and aggressive surgical treatment. J Trauma. 1998;44:1086–9. doi: 10.1097/00005373-199806000-00024. [DOI] [PubMed] [Google Scholar]
- 90.Colachis S, 3rd, Murray KD. Esophageal perforation: A delayed complication following traumatic spinal cord injury. Case report. Paraplegia. 1992;30:449–53. doi: 10.1038/sc.1992.98. [DOI] [PubMed] [Google Scholar]
- 91.Werre A, van der Vliet JA, Biert J, Blankensteijn JD, Kool LJ. Endovascular management of a gunshot wound injury to the innominate artery and brachiocephalic vein. Vascular. 2005;13:58–61. doi: 10.1258/rsmvasc.13.1.58. [DOI] [PubMed] [Google Scholar]
- 92.Panetta T, Sclafani SJ, Goldstein AS, Phillips TF. Percutaneous transcatheter embolization for arterial trauma. J Vasc Surg. 1985;2:54–64. [PubMed] [Google Scholar]
- 93.Lupattelli T, Basile A, Iozzelli A, Quarenghi M, Nano G, Casana R, et al. Thrombolytic therapy followed by stenting for renal artery dissection secondary to blunt trauma. Emerg Radiol. 2005;11:164–6. doi: 10.1007/s10140-004-0390-z. [DOI] [PubMed] [Google Scholar]
- 94.Stawicki SP, Hoey BA, Grossman MD, Anderson HL, 3rd, Reed JF., 3rd Adrenal gland trauma is associated with high injury severity and mortality. Curr Surg. 2003;60:431–6. doi: 10.1016/S0149-7944(02)00796-1. [DOI] [PubMed] [Google Scholar]
- 95.Hirose H, Gill IS, Malangoni MA. Nonoperative management of traumatic aortic injury. J Trauma. 2006;60:597–601. doi: 10.1097/01.ta.0000205044.99771.44. [DOI] [PubMed] [Google Scholar]
- 96.Kepros J, Angood P, Jaffe CC, Rabinovici R. Aortic intimal injuries from blunt trauma: Resolution profile in nonoperative management. J Trauma. 2002;52:475–8. doi: 10.1097/00005373-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 97.Mattox KL, Wall MJ., Jr Historical review of blunt injury to the thoracic aorta. Chest Surg Clin N Am. 2000;10:167–82, x. [PubMed] [Google Scholar]
- 98.Kwon CC, Gill IS, Fallon WF, Yowler C, Akhrass R, Temes RT, et al. Delayed operative intervention in the management of traumatic descending thoracic aortic rupture. Ann Thorac Surg. 2002;74:S1888–91. doi: 10.1016/s0003-4975(02)04148-6. [DOI] [PubMed] [Google Scholar]
- 99.Pate JW, Fabian TC, Walker W. Traumatic rupture of the aortic isthmus: An emergency? World J Surg. 1995;19:119–25. doi: 10.1007/BF00316994. [DOI] [PubMed] [Google Scholar]
- 100.Fisher RG, Oria RA, Mattox KL, Whigham CJ, Pickard LR. Conservative management of aortic lacerations due to blunt trauma. J Trauma. 1990;30:1562–6. doi: 10.1097/00005373-199012000-00022. [DOI] [PubMed] [Google Scholar]
- 101.Katsumata T, Shinfeld A, Westaby S. Operation for chronic traumatic aortic aneurysm: When and how? Ann Thorac Surg. 1998;66:774–8. doi: 10.1016/s0003-4975(98)00519-0. [DOI] [PubMed] [Google Scholar]
- 102.Maggisano R, Nathens A, Alexandrova NA, Cina C, Boulanger B, McKenzie R, et al. Traumatic rupture of the thoracic aorta: Should one always operate immediately? Ann Vasc Surg. 1995;9:44–52. doi: 10.1007/BF02015316. [DOI] [PubMed] [Google Scholar]
- 103.Akins CW, Buckley MJ, Daggett W, McIlduff JB, Austen WG. Acute traumatic disruption of the thoracic aorta: A ten-year experience. Ann Thorac Surg. 1981;31:305–9. doi: 10.1016/s0003-4975(10)60955-1. [DOI] [PubMed] [Google Scholar]
- 104.Fang JF, Chen RJ, Wong YC, Lin BC, Hsu YB, Kao JL, et al. Classification and treatment of pooling of contrast material on computed tomographic scan of blunt hepatic trauma. J Trauma. 2000;49:1083–8. doi: 10.1097/00005373-200012000-00018. [DOI] [PubMed] [Google Scholar]
- 105.Poletti PA, Mirvis SE, Shanmuganathan K, Killeen KL, Coldwell D. CT criteria for management of blunt liver trauma: Correlation with angiographic and surgical findings. Radiology. 2000;216:418–27. doi: 10.1148/radiology.216.2.r00au44418. [DOI] [PubMed] [Google Scholar]
- 106.Velmahos GC, Chahwan S, Hanks SE, Murray JA, Berne TV, Asensio J, et al. Angiographic embolization of bilateral internal iliac arteries to control life-threatening hemorrhage after blunt trauma to the pelvis. Am Surg. 2000;66:858–62. [PubMed] [Google Scholar]
- 107.Hagiwara A, Yukioka T, Ohta S, Nitatori T, Matsuda H, Shimazaki S. Nonsurgical management of patients with blunt splenic injury: Efficacy of transcatheter arterial embolization. AJR Am J Roentgenol. 1996;167:159–66. doi: 10.2214/ajr.167.1.8659363. [DOI] [PubMed] [Google Scholar]
- 108.Hagiwara A, Sakaki S, Goto H, Takenega K, Fukushima H, Matuda H, et al. The role of interventional radiology in the management of blunt renal injury: A practical protocol. J Trauma. 2001;51:526–31. doi: 10.1097/00005373-200109000-00017. [DOI] [PubMed] [Google Scholar]
- 109.Panetta T, Sclafani SJ, Goldstein AS, Phillips TF, Shaftan GW. Percutaneous transcatheter embolization for massive bleeding from pelvic fractures. J Trauma. 1985;25:1021–9. [PubMed] [Google Scholar]
- 110.Velmahos GC, Chahwan S, Falabella A, Hanks SE, Demetriades D. Angiographic embolization for intraperitoneal and retroperitoneal injuries. World J Surg. 2000;24:539–45. doi: 10.1007/s002689910087. [DOI] [PubMed] [Google Scholar]
- 111.Hoff WS, Hoey BA, Wainwright GA, Reed JF, Ball DS, Ringold M, et al. Early experience with retrievable inferior vena cava filters in high-risk trauma patients. J Am Coll Surg. 2004;199:869–74. doi: 10.1016/j.jamcollsurg.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 112.Jaik NP, Stawicki SP, Hoey BA. Emerging role of ERCP in blunt extrahepatic hepatic injuries. Gastroenterology. 2006;130(Suppl 2):A864. [Google Scholar]
- 113.Jaik NP, Hoey BA, Stawicki SP. Evolving role of endoscopic retrograde cholangiopancreatography in management of extrahepatic hepatic ductal injuries due to blunt trauma: Diagnostic and treatment algorithms. HPB Surg. 2008;2008:259141. doi: 10.1155/2008/259141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stawicki SP, Hoey BA. Lower extremity arterial thrombosis following sonographically guided thrombin injection of a femoral pseudoaneurysm. J Clin Ultrasound. 2007;35:88–93. doi: 10.1002/jcu.20268. [DOI] [PubMed] [Google Scholar]


