Abstract
Introduction:
chronic urticaria (CU) is a skin disorder characterized by transient, pruritic wheals persisting for longer than 6 weeks. The etiopathogenesis of the disease is still unclear, but there is evidence that autoimmunity and endocrine dysfunction may be involved.
Aim:
the aim of this study was to determine whether chronic urticaria is statistically associated with thyroid autoimmunity.
Patients and methods:
in a prospective case-control study, we compared the frequency of thyroid auto-antibodies (thyroglobulin antibody, anti-Tg and thyroid peroxidase antibody, anti-TPO) in 70 patients with chronic urticaria and in 70 healthy volunteers. Thyroid auto-antibodies and thyroid hormones (thyroxine (T4), triiodthyronine (T3) and thyroid stimulating hormone (TSH) were measured in all subjects.
Results:
thyroid functional abnormalities were found in 8 (11.43%) patients. Anti-Tg and anti-TPO were positive in 16 (23%) and 21 (30%) patients, respectively. In control group, only one subject (1.42%) had abnormalities in thyroid hormonal status, and two subjects (2.86%) had positive thyroid auto-antibodies. Compared with the control group, the frequency of both anti-Tg and anti-TPO was significantly higher in those with chronic urticaria (P < 0.05).
Conclusion:
this study shows a significant association between chronic urticaria and thyroid autoimmunity, and that tests to detect thyroid auto-antibodies are relevant in patients with chronic urticaria.
Keywords: autoantibodies, autoimmunity, thyroglobulin, urticaria
1. INTRODUCTION
Chronic urticaria (CU) is a skin disorder defined as the occurrence of daily, or almost daily wheals and itching for at least six weeks. It is a common disease affecting 0.5-1% of the general population (1). The pathophysiology of CU is not completely understood, although most agree that the central event is activation of cutaneous mast cells. This key pathophysiological event is predominant at the immediate phase of inflammation, which progresses to a complex interplay of varied proinflammatory mediators, cytokines, chemokines, and adhesion molecules that regulate vasoactivity and specific kinetics of cellular infiltration, ultimately evolving into a lymphocyte and granulocytes mediated hypersensitivity reaction, evident as urticarial wheals (2). The autoimmune origin is the most accepted hypothesis advanced to explain inappropriate activation and degranulation of mast cells in urticaria. This theory is supported by the clinical association of CU with various autoimmune disorders, the frequent detection of circulating autoantibodies, positive association with HLA subtypes DRB*04 and DQB1*0302 and therapeutic response to plasmapheresis and intravenous immunoglobulin (3-5).
The association of chronic urticaria with thyroid autoimmunity has been known since 1983 (6), but its frequency seems to vary in different reports. In literature reports, the prevalence of thyroid autoimmunity in CU patients varies from 4.3% to 57% and another 5-10% have clinically apparent thyroid disease (7-9).
The aim of this study was to determine whether chronic urticaria is statistically associated with thyroid autoimmunity.
2. PATIENTS AND METHODS
The study included 70 patients with chronic urticaria (40 female and 30 male). A detailed history and examination were taken in all study subjects, including patients age, age at onset, duration of disease, associated diseases, history of thyroid disorders and the extent and severity of disease. The diagnosis of chronic urticaria was made on clinical grounds. No patient was diagnosed before this study as having any type of thyroid dysfunction. The control group consisted of 70 volunteers (40 female and 30 male) who had skin diseases other then CU or autoimmune disorders. Blood samples were taken and a physical examination and thyroid sonography was performed. Thyroid auto-antibodies (thyroglobulin antibody, anti-Tg, and thyroid peroxidase antibody, anti-TPO) and thyroid hormones (thyroxine (T4), triiodthyronine (T3) and thyroid stimulating hormone (TSH) were measured in all subjects. Total T4 (normal range: 70-180 nmol/L) and total T3 (normal range: 1.3-3.3 nmol/L) were measured by use of radioimmunoassay (RIA); TSH (normal range: 0.3-4.2 mlU/L) was determined by use of immunoradiometric assay (IRMA) (BRAHMS Aktiengesellshaft, Hennigsdorf, Germany). Serum levels of anti-Tg (threshold value: 115 IU/mL) and anti-TPO (borderline value: 34 IU/mL) were measured by use of electrochemiluminiscence immunoassay (ECLIA) according to standard protocols (COBAS, Roche Diagnostics GmbH, Mannheim, Germany).
Baseline clinical characteristics for the two groups were compared with the use of Student’s t-test for continuous variables, the chi-square test or Fisher’s exact test (two-sided) for categorical variables, as appropriate. Data were considered statistically significant at P <0.05.
Statistical analyses were performed using MedCale for Windows, version 11.4.1.0 (MedCale Software, Mariakerke, Belgium).
3. RESULTS
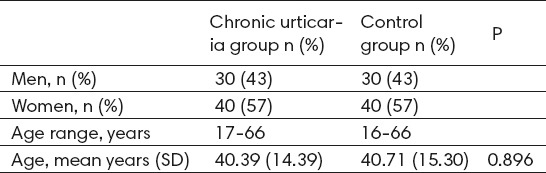
We performed a cross-sectional study in 70 consecutive patients with chronic urticaria and 70 age- and sex-matched controls. Demographic data of patients and controls are shown in Table 1. The mean (SD) age of the patient and control groups was 40.39 (±14.39) and 40.71 (±15.30), respectively (P = 0.896). The duration of CU ranged from 3 to 65 months. A family history of the same disease was present in 5 (7.14%) patients. Thyroid functional abnormalities were found in 8 (11.43%) patients. In the control group only one patient 8 (1.42%) had abnormalities in hormonal status.
Table 1.
Demographic data of patients (chronic urticaria group) and volunteers (Control group)
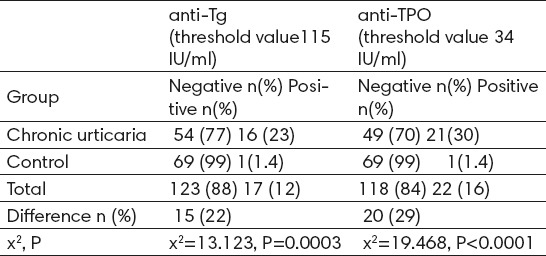
Hypoechogenic thyroid tissue was seen in 7 (10%) patients who all had elevated levels of thyroid autoantibodies. The thyroid gland was enlarged in 5 (7.14%) patients. Goitre was diagnosed in 4 (5.71%) cases. The ultrasound examination of the thyroid gland in control group was interpreted as normal in 64 (91.42%), and 6 (5.45%) volunteers had small simple goiter. Thyroid volume did not differ significantly between the study patients and the controls (p>0.05). In patients with chronic urticaria anti-Tg titers were ranging from 11.10 to 915.30 IU/mL and anti-TPO antibody titers from 5.10 to 714.40 IU/mL. In control group anti-Tg titers were ranging from 10.00 to 153.00 IU/mL, and anti-TPO antibody titers from 4.40 to 129.00 IU/mL. Anti-Tg antibody in 16 (22.85%) patients, anti-TPO antibody in 21 (30%) and both anti-Tg and anti-TPO antibodies in 13 (18.57%) were higher than the normal antibody titres. In the control group, one subject (1.42%) had positive anti-Tg and one volunteer (1.45%) had positive anti-TPO. The frequency of thyroid auto-antibodies was significantly higher in chronic urticaria patients than in control group (Table 2). A Chi-square test for independence (with Yates Continuity Correction) indicated significant association between higher values of anti-Tg (values more than 115 IU/ml) and chronic urticaria, χ2 (1, n=140)= 13.123, P=0.0003. A Chi-square test for independence (with Yates Continuity Correction) indicated significant association between higher values of anti-TPO (values more than 34 IU/ml) and chronic urticaria, χ2 (1, n=140)=19.468, P<0.0001.
Table 2.
The frequencies of positive detectable thyroid autoantibody (anti-Tg and anti-TPO)
4. DISCUSSION
Chronic urticaria has been reported in association with numerous endocrine disorders. One of the main association is with thyroid abnormalities. In the literature, the frequency of thyroid autoimmunity in patients with CU encompasses a vast range of values, varying from 4.3 % (7) to 57.4% (8). In accordance to previous studies, we also demonstrated that antithyroid autoantibodies were significantly increased in patients with CU in comparison to healthy subjects. We detected elevated anti-Tg in 16 (22.85%) and elevated anti-TPO in 21 (30%) of patients with CU. Usually about 5-10% of general population has positive antithyroid antibodies; in this study the prevalence of auto-antibodies in control group is much lower than expected. The difference it may partly be attributed to genetic factors. Compared with the control group, the frequency of both anti-Tg and anti-TPO antibodies was significantly higher in those with chronic urticaria. Our results are consistent with a clinical study performed by Palma-Carlos et colleagues (10). They performed a case-control study to evaluate thyroid antibodies in CU patients, and detected anti-Tg positivity in 22.2% and anti-TPO positivity in 26.8%. However, 93% of CU patients had normal thyroid functions. The authors concluded that thyroid antibody and function must be evaluated in all cases of CU. In contrast, Feibelmann et al. found that thyroid autoimmunity prevalence in CU patients is not greater than that in control group (11).
As regards the type of thyroid antibody that was more prevalent, our study shoved a higher prevalence of anti-TPO than anti-Tg. This is consistent with the results of Aamir et al. (8) who demonstrated that anti-TPO had higher prevalence than anti-Tg in his study group. Anti-TPO antibody, historically referred to as the antimicrosomal antibody, is established as a sensitive tool for the detection of early subclinical autoimmune thyroid diseases and identification of at-risk cases for autoimmune thyroid diseases (12). Nordyke et al. reported that anti-TPO antibody tends to have more correlation with thyroid dysfunction than does the anti-Tg antibody (13).
Although a specific mechanism linking the development of thyroid disease and CU has yet to be firmly elucidated, it is widely thought that both diseases occur because of a propensity within the patient to develop reaction to self. It has been hypothesized that thyroid disease may worsen urticaria through activation of the complement system (9). Kirpatric noted that C4a levels decrease when thyroid disease is treated, resulting in remission of CU (14). Therefore, while it is hypothesized that thyroid disease and CU may coexist due to a patient’s predilection for autoimmunity, thyroid disease may additionally exacerbate urticaria through direct mechanisms that result in complement activation. Contrary to this, the antithyroid IgG antibodies may not be directly involved in the mast cell degranulation and pathogenesis of the chronic urticaria, but only serve as indicators of autoimmunity (7). O’Donnell et al. observed that patients with positive anti-TPO antibodies were more likely to demonstrate histamine-releasing auto-antibodies as predicted by positive autologous intradermal skin testing and positive release in vitro of histamine from donor basophil leucocytes (15). Increased bcl-2 expression in activated T and B lymphocytes and increased CD40l expression on activated T cells have been found in these patients. It is conceivable but unproven that cellular immunity initiated in the thyroid gland could trigger development of the skin lesions.
In addition, Altrichter et al. reported that some of chronic spontaneous urticaria patients expressed IgE antibodies against TPO as a novel pathological mechanism of chronic urticaria (16). These IgE-anti-TPO autoantibodies, when bound and activated on the surface of mast cells, could cause “autoallergic” mast cell degranulation, a novel pathogenic pathway of urticaria induction. Later on, Shin and colleagues confirmed the presence of circulating serum specific IgE to TPO in CU patients and showed the direct role of serum specific IgE to TPO in effector cell with basophil activation test (17).
5. CONCLUSION
The study revealed a significant association between chronic urticaria and thyroid autoimmunity and showed the tests used to detect thyroid auto-antibodies to be relevant in patients with chronic urticaria.
Although cutaneous manifestations of autoimmune thyroid diseases are well described and thyroid hormone is known to regulate the development and function of skin, a better understanding of these processes is needed. It is a multidisciplinary problem requiring cooperation of specialists in different fields of medicine. Both dermatologists and endocrinologists have to inquire their patients about the family history of autoimmune diseases and to look for associated autoimmune disorders.
Footnotes
• Conflict of interest: none declared.
REFERENCES
- 1.Zuberbier T, Aberer W, Asero R, Bindslev-Jansen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69(7):868–87. doi: 10.1111/all.12313. [DOI] [PubMed] [Google Scholar]
- 2.Sanjiv J. Pathogenesis of chronic urticaria: an overview. Dermatol Res Pract. 2014:674709. doi: 10.1155/2014/674709. doi:10.1155/2014/674709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Donnell BF, O’Neill CM, Francis DM, Niimi N, Bar RM, Barlow RJ, et al. Human leucocyte antigen class II associations in chronic idiopathic urticaria. Br J Dermatol. 1999;140(5):853–8. doi: 10.1046/j.1365-2133.1999.02815.x. [DOI] [PubMed] [Google Scholar]
- 4.Grattan CE, Francis DM, Slater NG, Barlow RJ M, Graves MW. Plasmapheresis for severe, unremitting, chronic urticaria. Lancet. 1992;339(8801):1078–80. doi: 10.1016/0140-6736(92)90666-q. [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell BF, Barr RM, Black AK, Fransis DM, Kermani F, Niimi N, et al. Intravenous immunoglobulin in autoimmune chronic urticaria. Bri J Dermatol. 1998;138(1):101–6. doi: 10.1046/j.1365-2133.1998.02033.x. [DOI] [PubMed] [Google Scholar]
- 6.Leeznoff A, Josse RG, Denburg J, Dolovich J. Association of chronic urticaria and angioedema with thyroid autoimmunity. Arch Dermatol. 1983;119(8):636–40. [PubMed] [Google Scholar]
- 7.Levy Y, Segal N, Weintrob N, Danon YL. Chronic urticaria: association with thyroid autoimmunity. Arch Dis Child. 2003;88(6):517–9. doi: 10.1136/adc.88.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aamir IS, Tauheed S, Majid F, Atif A. Frequency of autoimmune thyroid disease in chronic urticaria. J Coll Physicians Surg Pak. 2010;20(3):158–61. [PubMed] [Google Scholar]
- 9.Sibbald RG, Cheema AS, Lozinski A, Tarlo S. Chronic urticaria-evaluation of role of physical, immunologic and other contributory factors. Int J Dermatol. 1991;30(6):381–6. doi: 10.1111/j.1365-4362.1991.tb03891.x. [DOI] [PubMed] [Google Scholar]
- 10.Palma-Carlos AG, Palma-Carlos MI. Chronic urticaria and thyroid autoimmunity. Eur Ann Allergy Clini Immunol. 2009;19(4):54–6. [PubMed] [Google Scholar]
- 11.Feibelmann TC, Goncalves FT, Daud MS, Jorge Ade S, Mentese SA, Jorge PT. Assessment of association between autoimmune thyroid disease and chronic urticaria. Arq Bras Endocrinol Metabol. 2007;51(7):1077–83. doi: 10.1590/s0004-27302007000700009. [DOI] [PubMed] [Google Scholar]
- 12.Kemp EH. Autoantibodies as diagnostic and predictive markers of vitiligo. Autoimmunity. 2004;37(4):287–90. doi: 10.1080/08916930410001710857. [DOI] [PubMed] [Google Scholar]
- 13.Nordyke RA, Gilbert FI, Miyamoto LA, Fleury KA. The superiority of antimicrosomal over antithyroglobulin antibodies for detecting Hashimoto’s thyroiditis. Arch Intern Med. 1993;153(7):862–5. [PubMed] [Google Scholar]
- 14.Kirpartick CH. A mechanism for urticaria/angioedema in patients with thyroid disease. J Allergy Clin Immunol. 2012;130(4):988–90. doi: 10.1016/j.jaci.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 15.O’Donnell BF, Francis DM, Swana GT, Seed PT, Kobza Black A, Greaves MW. Thyroid autoimmunity in chronic urticaria. Br J Dermatol. 2005;153(2):331–5. doi: 10.1111/j.1365-2133.2005.06646.x. [DOI] [PubMed] [Google Scholar]
- 16.Altricher S, Hans-Jurgen P, Pisarevskaja D, Metz M, Martus P, Marcus M. IgE mediated autoallergy against thyroid peroxidase - a novel pathomechanism of chronic spontaneous urticaria? PLoS One. 2011;6(4):e14794. doi: 10.1371/journal.pone.0014794. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin YS, Suh DH, Yang EM, Ye YM, Park HS. Serum specific IgE to thyroid peroxidase activates basophils in aspirin intolerant urticaria. J Korean Med Sci. 2015;30(6):705–9. doi: 10.3346/jkms.2015.30.6.705. [DOI] [PMC free article] [PubMed] [Google Scholar]


