Abstract
Introduction:
Scientific studies show that many factors related to lifestyles affect the reduction of bone mineral density and osteoporosis in postmenopausal women.
Goal:
The goal of this study was to determine whether smoking, drinking coffee and alcohol in menopausal women contribute to the reduction of bone mass and osteoporosis, as well as the impact of physical activity on bone mass.
Material and methods:
The study was carried out as case study and matched controls. The group of cases consisted of 100 females in postmenopausal age, in which by the DEXA method was newly diagnosed osteoporosis at the Clinic of Endocrinology, Diabetes and Metabolic Diseases, University Medical Center of RS during 2015-2016, while the control group consisted of 100 females in a postmenopausal age without diagnosed osteoporosis. The groups were matched by age (±2 years). In order to collect demographic data and information on risk factors for osteoporosis and lifestyle of patients was used the questionnaire Bone Mineral Density Questionnaire- Female of the Irish Association for osteoporosis.
Results:
Testing the significance of differences in terms of smoking showed that the studied groups are statistically significantly different in terms of smoking (χ2=24.025, p=0.000). In terms of consumption of coffee, a statistically significant difference was found between the group of cases and control group (χ2=0.615, p=0.735). When observing the obtained information about the consumption of alcohol, we find that this preventable risk factor in the present study did not show as significant for osteoporosis in postmenopausal women (χ2=4.35, p=0.114). Statistical analysis shows that there are significant differences between the group of cases and control group in terms of physical activity (χ2=7.30, p=0.026). Analysis of the data of our study by univariate logistic regressions showed that smoking (p=0.000) was statistically significantly associated with osteoporosis, while physical activity is a protective factor for bone mass (p=0.036). Results of multivariate logistic regression showed that the independent risk factors for osteoporosis in postmenopausal women is smoking (OR=1.665; p=0.006).
Conclusion:
The results of our study show that smoking is an independent risk factor for osteoporosis in postmenopausal women, and physical activity is a protective factor for bone mass retention. Through education and certain preventive measures should be stressed the importance of these factors on bone health from the earliest period.
Keywords: risk factors, osteoporosis, menopause
1. INTRODUCTION
Osteoporosis is a disease that can be characterized as a “silent epidemic” because of its wide presence throughout the world and the continuing increase in the number of patients deserves full attention and appropriate multidisciplinary approach, both in prevention and in treatment. Close to 10% of the world’s population and 30% of post-menopausal women suffering from osteoporosis (1, 2). The main complications of osteoporosis and a major cause of morbidity and mortality in the elderly population are fractures (3). Osteoporosis involves a number of factors which can be divided into variable and constant factors i.e. genetic factors and factors from the environment (4). They can individually or in synergy significantly contribute to the loss of bone mass leading to osteoporosis (5-7). Recent scientific findings indicate that factors associated with poor living habits are also one of the most important factors contributing to the rapid loss of bone mineral density in postmenopausal women (8).
2. GOAL
The goal of this study was to determine whether certain risk factors from the environment (smoking, drinking coffee and alcohol) contribute to the reduction of bone mineral density and occurrence of osteoporosis, and what is the impact of physical activity on bone mineral density in postmenopausal women.
3. MATERIAL AND METHODS
Working Group (group of cases) consisted of 100 females in postmenopausal age (for at least two years after the last menstrual period) found to have a newly diagnosed osteoporosis in the Cabinet for Osteodensitometry (Clinic of Endocrinology, Diabetes and Metabolic Diseases, University Medical Center of RS) determining bone mineral density by DEXA method at the level of lumbar spine (L2-L4), hip, and the upper part of the femur.
The control group (group of controls) consisted of 100 females in a postmenopausal age where after the determination of bone mineral density, by DEXA method, osteoporosis has not been determined.
Exclusion criteria were malignant diseases, diabetes, thyroid, parathyroid and adrenal glands, chronic renal failure, inflammatory arthritis, the use of statins in the treatment of dyslipidemia, corticosteroids, hormones and diuretics for more than three months, secondary osteoporosis due to endocrine diseases, diseases of the gastrointestinal tract (Crohn’s disease, malabsorption), operations peptic ulcer, chronic liver disease, and osteoporosis induced by drugs.
In order to collect demographic data and information on risk factors for osteoporosis and lifestyle of patients is used a questionnaire on Bone Mineral Density in Women of the Irish Association of Osteoporosis.
4. RESULTS
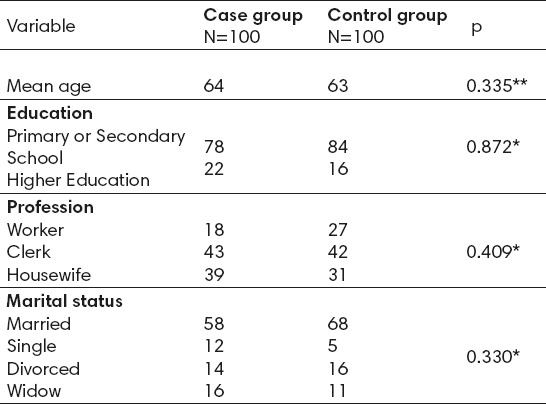
Looking at both groups, from a demographic point of view, the result shows that in the group of cases the mean age of respondents was 64 years, and in the control group 63 years which does not represent a statistically significant difference. In terms of education level in both groups dominated by women with primary or secondary education (in a group of cases 78% and in the control, group 84%), with no statistically significant difference between groups. Also in terms of occupation and marital status there were no statistically significant differences between groups (Table 1).
Table 1.
Independent indicators for the association of demographic data with osteoporosis. * X2 test ** t-test
Testing of the significance of differences in terms of smoking showed that the studied groups are statistically significantly different in terms of smoking (χ2=24.025, p=0.000). In the group of cases, smoking was present in 50%, and in the group of controls in 33% of the patients (Table 2).
Table 2.
The differences between group of cases and control group in terms of smoking, alcohol, coffee and physical activity
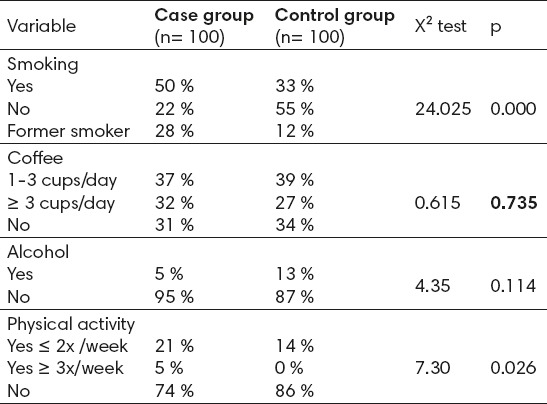
In terms of consumption of coffee, a statistically significant difference was not found between the group of cases and control group (χ2=0.615, p=0.735). It can be observed that roughly the same number of women in both groups consumed coffee ≥ 3 cups/day, in a group of cases, 32% in the control group 27%. Moderate, or 1-3 cups/day consumed 37% of the women in group of cases, and 39% of respondents in control group. Coffee did not drink 31% of the women in group of cases, and34% of the control group.
When observing the obtained information about the consumption of alcohol, we find that this preventable risk factor in the present study did not show as significant for osteoporosis in postmenopausal women (χ2=4.35, p=0.114). In both groups dominated by women who do not consume alcohol.
Statistical analysis shows that there are significant differences between the group of cases and control group in terms of physical activity (χ2=7.30, p=0.026) (Table 2).
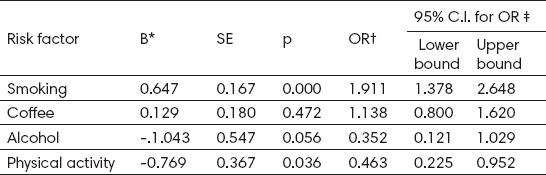
Testing of variable risk factors for osteoporosis in postmenopausal women by logistic regression included all studied factors. Univariate logistic regression analysis showed that smoking (p=0.000; OR=1.911) is significantly associated with the development of osteoporosis, and physical activity was protective factor for bone mineral density in women (p=0.036; OR=0.463). Coffee consumption ≥ 3 cups per day (p=0.472; OR=1.138) and alcohol >3 drinks per day (p=0.056; OR=0.352) were not significant risk factors for osteoporosis in postmenopausal women (Table 3).
Table 3.
Risk factors for osteoporosis identified by univariate logistic regression. * coefficient; † Odds Ratio; ‡ Confidence interval
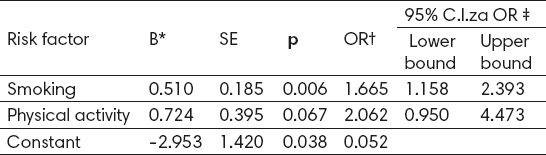
Results of multivariate logistic regression showed that only smoking is an independent risk factor for osteoporosis in postmenopausal women (OR=1.665; p=0.006) (Table 4).
Table 4.
Risk factors for osteoporosis as identified by multivariate logistic regression * coefficient; † Odds Ratio; ‡ Confidence interval
5. DISCUSSION
Osteoporosis is a metabolic bone disease which in developed countries represent a very important social and medical problem and it is getting more and more a form of epidemic, as it has a steady increase in the number of cases (9). After age of 30 years the reduction of bone mass is an inevitable process, and consequently, changes in the bone remodeling cycle leading to bone fragility and increased risk of bone fractures (10). In osteoporosis are involved numerous factors which can be classified in the group of risk factors that cannot be influenced (unchangeable factors) and the risk factors that can be affected (variable or preventable factors) (11).
Among the variable risk factors for osteoporosis, which are related to poor living habits, recent studies show that smoking has an important place because it leads to some changes in the level of microarchitecture of trabecular bone, which results in reduced bone resistance to mechanical stress and friction (12). Smokers, regardless of the gender have a higher risk of having osteoporotic fractures (13). Women who smoke are almost twice as likely affected by osteoporosis than women nonsmokers. Our results are consistent with other scientific studies, in which the prevalence of osteoporosis was much higher in the group of smokers (31.3%) compared to former smokers (28.6%) or non-smokers (7.5%) (14). The results of our study, in accordance with the previously imposed attitudes, have shown that smoking is a significant independent risk factor for osteoporosis (p=0.000, OR=1.911, 95% CI=1.378 to 2.648).
Scientific findings on the impact of alcohol on bone mineral density show different results. According to certain studies, nonhazardous alcohol use (1.0 drinks per day) has a slightly positive effect on bone density because alcohol contains certain substances that may have the estrogen-like stimulatory effect on bone (15). Ilic and colleagues have found that a low intake of alcohol, mostly wine, is positively correlated with bone mineral density at the level of lumbar spine in postmenopausal women (16), while Berg and colleagues have shown that people who consume 0.5 to 1.0 drinks a day have a lower risk of hip fracture compared to abstainers or hazardous consumers (17). On the other hand, excessive alcohol consumption has a negative impact on the mechanism of bone remodeling, osteoblastic proliferation and activity, and therefore the direct negative effect on bone homeostasis (18). The results of univariate logistic regression in our study did not indicate that alcohol consumption of >3 drinks a day is a significant risk factor for osteoporosis in postmenopausal women (OR=0.352, p=0.056). In both groups of respondents dominated abstainers which is consistent with other studies, according to which the majority of postmenopausal women do not consume alcohol. Nonhazardous alcohol consumption had 4% of the women in the group cases, and 8% in the control group, while we found hazardous alcohol consumption in 1% of respondents in the group of cases and 5% of the control group. Harmful drinking of alcohol did not report any of the respondents.
The results of our study are consistent with other studies that have demonstrated the negative impact of high doses of caffeine to osteoporosis and fractures, particularly in postmenopausal women, which is a reflection of direct or indirect harmful effects of caffeine on osteoblastic activity (19,20). In fact, caffeine from coffee can lead to increased excretion of calcium in the urine, and that this loss cannot be fully compensated even 24 hours later. Caffeine intake leads to a decrease in interstitial absorption of calcium, and high doses of caffeine (>300 mg/g or ≥4 cups a day) can accelerate bone loss at the level of lumbar spine in older postmenopausal women (21). Logistic regression analysis in our study did not point that coffee consumption of ≥3 cups a day is a risk factor for osteoporosis (32% in a group of cases versus 27% in the control group (OR=1.138, p=0.472)).
For healthy bone, it is necessary to regularly exercise and have physical activity, avoiding sedentary lifestyle, as if the bones are not energized and physical active, mechanoreceptors (osteocytes) do not receive signals about the need for remodeling, removal of damaged and synthesis of new bone, and so there is a gradual reduction of total bone mineral density (22). Physical activity or physical exercise in postmenopausal women have to provide the necessary voltage essential for maintaining bone density (23). Walking, according to the National Osteoporosis Foundation, is one of the most effective form of exercise for the maintenance or improvement of bone mineral density in postmenopausal women (24). It was found that engaging in recreational sports or active walk (30-60 minutes) more than twice a week reduces the risk of osteoporosis and fractures in older postmenopausal women (25). Exercises by load of its own weight or exercise against resistance are effective for increasing bone density and aerobic exercises increase the balance and functional activity of muscles thus reducing the risks of falls (26). Data from other studies also suggest that physical activity is essential for bone health and the prevention of osteoporosis (27). In our study, the patients took a large percentage of cases were physically inactive, 74% in the group of cases versus 86% in the control group, which agrees with the results of other studies. The results of univariate logistic regression showed that physical activity is an important protective factor for osteoporosis (OR=0.463, p=0.036), as were women who were physically active less frequently suffered from osteoporosis.
6. CONCLUSION
Many preventable risk factors from the environment can have a significant impact on bone mineral density in postmenopausal women. The results of our study show that smoking is an independent risk factor for osteoporosis in postmenopausal women, and physical activity is a protective factor for bone mineral density. Through education and certain preventive measures, it should be stressed the importance of these factors on bone health from the earliest period.
Footnotes
• Conflict of interest: none declared.
Author’s contributions: RB and JB performed the examination the patients. RB collected the data, analyzed them and wrote the text. SM assisted in writing the text including final editing and critical revision of the scientific content. All authors have read the text and approved the final manuscript.
REFERENCES
- 1.National osteoporosis foundation (NOF) Clinician’s guide to prevention and treatment of osteoporosis. Washington: NOF; 2013. [Google Scholar]
- 2.Alibasic E, Ramic E, Batic Mujanovic O, et al. Assessment of Osteoporosis in Family Medicine Obtained by Ultrasound Densitometry. Acta Inform Med. 2013;21(4):274–6. doi: 10.5455/aim.2013.21.274-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muftic M, Kucukalic Selimovic E, Miladinovic K. Osteoporosis - Comparative Study Between Quantitative Ultrasound of Calcaneus and DXA. Med Arh. 2013;67(4):289–91. doi: 10.5455/medarh.2013.67.289-291. [DOI] [PubMed] [Google Scholar]
- 4.Bijelic R, Balaban J, Milicevic S. Correlation of the Lipid Profile, BMI and Bone Mineral Density in Postmenopausal Women. Mater Sociomed. 2016;28(6):412–5. doi: 10.5455/msm.2016.28.412-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendosa-Romo MA, Ramirez-Arriola MC, Velasco-Chávez JF. Parity and menarche as risk factors for osteoporosis in postmenopausal women. Ginecol Obstet Mex. 2014;82(2):75–82. [PubMed] [Google Scholar]
- 6.González-Mercado A, Sánchez-López JY, Ibarra B. Risk factors for osteoporosis in postmenopausal women from Guadalajara, Jelisco. Salud Publica Mex. 2013;55(6):627–30. [PubMed] [Google Scholar]
- 7.Pasco A, Brennan SL, Kotowicz M. Morbid obesity in women on the rise: an observational, population- based study. BMC Public Helath. 2013;13:290. doi: 10.1186/1471-2458-13-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu K, Prince RL. Lifestyle and Osteoporosis. Curr Osteoporos Rep. 2015;13:52–59. doi: 10.1007/s11914-014-0248-6. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, McCloskey EV, Johansson H. Register, on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF) Osteoporos Int. 2013;24(1):23–57. [Google Scholar]
- 10.Alimanovic-Alagic R. Evaluation of Risk Factors for Osteoporosis in Postmenopausal Women. Mater Sociomed. 2010;22(3):157–61. [Google Scholar]
- 11.Guthrie JR, Ebeling PR, Dennerstein L. Risk factors for osteoporosis: prevalence, change, and association with bone density. Medscape Womens Health. 2000;5:E2. [PubMed] [Google Scholar]
- 12.Brook JS, Balka EB, Zhang C. The smoking patterns of women in their fortries: Their relationship to later osteoporosis. Psyholog Rep. 2012;110:351–62. doi: 10.2466/13.18.PR0.110.2.351-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korkor AB, Eastwood D, Bretzmman C. Effects of gender, alcohol, smoking, and dairy consumption on bone mass in Winsconsin adolescents. WMJ. 2009;108(4):181–8. [PubMed] [Google Scholar]
- 14.Øyin J, Gjesdal CG, Nyagård OK, Lie SA, Mayer HE, et al. Smoking and Body Fat Mass in Relation to Bone Mineral Density and Hip Fractures: The Hordaland Health Study. Plos One. 2014;9(6):e101335 doi. doi: 10.1371/journal.pone.0092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurel DB, Boisseau N, Benhamou CL, Jaffre C. Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int. 2012;23:1–16. doi: 10.1007/s00198-011-1787-7. [DOI] [PubMed] [Google Scholar]
- 16.Ilich JZ, Brownbill RA, Tamborini L, Crncevic-Orlic Z. To drink or not to drink: how are alcohol, caffeine and past smoking related to bone mineral density in erderly women? J Am Coll Nutr. 2002;21:536–44. doi: 10.1080/07315724.2002.10719252. [DOI] [PubMed] [Google Scholar]
- 17.Berg KM, Kunins HV, Jackson JL, et al. Association Between Alcohol Consumption and Bone Osteoporotic Fractures and Bone Density. Am J Med. 2008;121(5):406–18. doi: 10.1016/j.amjmed.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Yu Z, Yu M, Qu X. Alcohol consumption and hip fracture risk. Osteoporosis Int. 2015;26:531–42. doi: 10.1007/s00198-014-2879-y. [DOI] [PubMed] [Google Scholar]
- 19.Freedman ND, Park Y, Abnet CC. Association of coffee drinking with total and cause- specific mortality. N Engl J Med. 2012;366(20):1891–1904. doi: 10.1056/NEJMoa1112010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallström H, Byberg L, Glynn A, et al. Long-term Coffee Consuption in Relation to Fracture Risk and Bone Mineral Density in Women. Am J Epidemiol. 2013;50:324–34. doi: 10.1093/aje/kwt062. [DOI] [PubMed] [Google Scholar]
- 21.Shuai L, Zhipeng D, Qiang W. Effect of coffee intake on hip fracture: a meta- analysis of prospective cohort. Nutr J. 2015;14:38. doi: 10.1186/s12937-015-0025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Branković S, Vukojević P, Vujasinović-Stupar N, Palić-Obradović S. Role of exercise program on bone mass in matients with osteoporosis. Acta Rheuma Belgrade. 2009;30:34–8. [Google Scholar]
- 23.Gomez-Cabello A, Ara I, Gonzales-Augero WI, Casaus JA. Effects of training on bone mass in older adults: A systematic review. Sports Medicine. 2012;42:301–25. doi: 10.2165/11597670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.National Osteoporosis Foundation. Exercise for Strong Bones. Washington: National Osteoporosis Foundation; 2015. [Google Scholar]
- 25.Schmitt NM, Schmitt J, Doren M. The role of physical activity in the prevention of osteoporosis in postmenopausal women- an update. Maturitas. 2009;63:34–8. doi: 10.1016/j.maturitas.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Kemmler W, Häberle L, von Stengel S. Effects of exercise on fracture reduction in older adults: A systematic review and meta- analysis. Osteoporos Int. 2013;24:1937–50. doi: 10.1007/s00198-012-2248-7. [DOI] [PubMed] [Google Scholar]
- 27.Međedović B, Romanov R, Ðokić Z, Perić D, Ahmeović Z. Fizička aktivnost i mineralna gustina kostiju. TIMS Acta. 2015;9:63–74. [Google Scholar]


