Abstract
Dexmedetomidine (DXM) is a selective α2-adrenoceptor (α2-AR) and imidazoline receptor (IR) agonist that has been reported to regulate inflammatory responses mediated by diverse signaling pathways through α2-AR. The majority of the reported receptors or downstream molecules have been demonstrated to locate with caveolin-1, a protein suggested to participate in regulating Toll-like receptor 4 (TLR4)-mediated inflammatory responses and the pathogen endocytosis capability of macrophages. The present study hypothesized that DXM may influence these pathways by regulating the expression of caveolin-1 and mediating the subsequent effects. Using a cecal-ligation and puncture-induced rat sepsis model, it was initially observed that pre-emptive DXM is able to upregulate and stabilize the amount of caveolin-1 expression, which may be partly antagonized by both α2-AR and the IR antagonist atepamezole (APZ). The pathophysiological parameters indicated that DXM is able to inhibit secondary lung injury, in addition to the rise of body temperature and arterial lactate accumulation, however it marginally increased arterial glucose and the murine sepsis score, which can be largely antagonized by APZ. The overall effect was beneficial and improved the 24-h cumulative survival rate of rats with sepsis. In conclusion, preemptive clinical sedative doses of DXM may upregulate the expression of caveolin-1 downregulated by sepsis, which may contribute to the inhibition of inflammatory pathways such as TLR4-mediated pathways. Furthermore, DXM may favor the improvement of short-term outcomes by the regulation of other metabolic pathways.
Keywords: dexmedetomidine, caveolin-1, sepsis, inflammation, secondary lung injury, signaling crosstalk
Introduction
Sepsis is a systemic inflammatory response syndrome with a proven or suspected infectious etiology (1). The early stages of sepsis are classically characterized by fever and blood hyperdynamics, with rapidly developing secondary organ injury, and hypothermia and death as sepsis progresses (1,2). The mortality rate of sepsis remains high and the therapy remains intractable.
Dexmedetomidine (DXM) is a selective α2-adrenoceptor (α2-AR) and imidazoline receptor (IR) agonist (3), which was formally approved for sedation and adjunct analgesia for patients in the intensive care unit (ICU) by the US Food and Drug Administration in 1999, particularly recommended for patients with sepsis (4) Clinical studies of patients with severe sepsis and other critically ill patients identified that DXM may marginally improve the survival rate and significantly inhibit the release of pro-inflammatory factors in patients with sepsis (5–7). These beneficial effects were confirmed by an animal study (8), however the underlying signaling pathways reported from different animal and cytological models presented a wide range of pathways including: Toll-like receptor 4 (TLR4)/myeloid differentiation primary response gene 88 (MyD88)/nuclear factor-κB (NF-κB) or mitogen-activated protein kinase [c-Jun N-terminal kinase, extracellular signal-regulated kinases (ERK)1/2] (8,9), endothelial nitric oxide synthase (eNOS)/nitric oxide (10) and Janus kinase/signal transducers and activators of transcription (11). How DXM can affect so numerous signaling pathways or downstream molecules remains unclear.
Caveolae, typically flask-shaped membrane invaginations expressed in various cell types, are abundant in lung, muscle and adipose tissues, and have been demonstrated to participate in the regulation of lipid and glucose metabolism (12), in addition to the maturation of immunocytes and inflammation responses (13). Caveolin-1 is a protein that maintains the morphological and functional integrity of caveolae (12). Knockout of caveolin-1 decreases the survival rate in mice with cecal ligation and puncture (CLP)-induced sepsis (14). Jiao et al (15) identified that tyrosine (Tyr) 14 phosphorylation of caveolin-1 induced interaction with TLR4 and mediated TLR4/MyD88 signaling regulation. Additional studies have demonstrated that receptors including G-protein coupled receptors (GPCRs), α-ARs, β-ARs (16), tumor necrosis factor receptor (17) and downstream molecules including Gα subunits (Gs, Gi and Gq) (16), eNOS (18) and ERK1/2 (19), localized to the caveolae. GPCR activation led to the release and translocation of Gβγ subunits and subsequent Src-dependent Tyr phosphorylation of caveolin-1 (20). Following these studies, it was hypothesized that DXM may affect inflammatory pathways through the influence on caveolin-1.
The present study aimed to evaluate the effect of DXM on the most frequent clinical manifestations of sepsis and the expression of caveolin-1 in lung tissues, and to identify the association between inflammatory pathways and the characteristic receptors of DXM.
Materials and methods
Animals
A total of 170 male Sprague-Dawley rats aged 8 weeks (weight, 250–300 g) were obtained from the Animal Center of the Second XiangYa Hospital of Central South University (Changsha, China) and housed in the Animal Laboratory Center of the Second XiangYa Hospital of Central South University, in groups of three per cage. All rats were weighed and numbered on arrival and acclimated for 7 days, under 24°C and 48% humidity, a 12-h light/dark circadian cycle, and were provided with specific pathogen-free rodent diet and water ad libitum throughout all experiments. Animals were euthanized with an overdose of pentobarbital injected after the experiments. All animal procedures were approved by the Animal Care and Use Committee of XiangYa Medical College, Central South University (Changsha, China).
Materials
The small-animal-specialized digital thermometer (AT320; Da-Xiong Ltd., Shenzhen, China) and blood gas analyzer (GEM Premier 3000; Instrumentation Laboratory Co., Bedford, MA, USA) were used. The silk suture (Ethilon; Ethicon, Inc., Somerville, NJ, USA), sodium dodecyl sulfate (SDS) buffer and phosphate-buffered saline (PBS; Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) were donated by Dr Lili Jiang (Department of Anesthesiology, Affiliated Hospital of Qingdao University, Qingdao, China). DXM and ketamine (HengRui Medicine, Ltd., Nanjing, China) were purchased from Second XiangYa Hospital of Central South University. Pentobarbital sodium (Sigma-Aldrich; Merck Millipore, Darmstadt., Germany), atepamezole (APZ; Axon Medchem, Groningen, Netherlands), D46G3XP caveolin-1 rabbit monoclonal antibody [horseradish peroxidase (HRP)-conjugated; 1:10,000; catalog no. 3267S] and 13E5 β-actin rabbit monoclonal (1:1,000; catalog no. 4970S) antibodies (Cell Signaling Technology, Inc., Danvers, MA, USA) were purchased from respective companies. The HRP-conjugated secondary antibody (1:1,000; catalog no. CW0103M) was obtained from Beijing ComWin Biotech Co., Ltd. (Beijing, China). Enhanced bicinchoninic acid assay (BCA) protein assay kit was purchased from Beyotime Institute of Biotechnology (Shanghai, China).
CLP surgery
All rats were anesthetized with pentobarbital sodium [45 mg/kg, intra-peritoneal (i.p.)] and ketamine (1.5 mg/100 g, intravenous). Following a previous study (21), a severe polymicrobial septic model was induced by CLP. Rats were fixed on a surgical board following the shaving of abdominal hair. Surgical fields were covered with sterile drapes after disinfection with iodine complex (three times), then a 1–2 cm lower-midline laparotomy was conducted. The cecum was exposed and ligated tightly 25% distal to the ileocecal valve with a 6–0 silk suture, and perforated twice with an 18-gauge needle on the same side near and distal to the ligation, respectively. The cecum was squeezed gently to extrude two small amounts of feces after which the cecum was returned to abdominal cavity. The abdomen was closed with 3–0 silk sutures in two layers. Then 5 ml/100 g 37°C normal saline was injected subcutaneously using a 25G needle prior to the rat being transferred to a recovery cage. Perioperative body temperature was protected with temperature-adjusting blankets and monitored at 1 h intervals.
Experimental protocol
Rats were randomly divided into five groups: Control group (Control); sham surgery (Sham); CLP (CLP); DXM + CLP (DXM); and DXM + APZ + CLP (APZ). Control rats did not undergo any procedures. Sham surgery was conducted with laparotomy and cecum exposure without ligation and puncture. CLP was conducted as aforementioned. DXM (2 µg/100 g, 10 µg/ml) or an equal volume normal saline was injected i.p. 10 min prior to surgery. APZ (25 µg/100 g, 1 mg/ml) or an equal volume normal saline was injected i.p. simultaneously with pentobarbital injection.
Survival rate, anal temperature and disease severity monitoring, and arterial blood gas (ABG) analysis
A total of 65 rats were randomly divided into five groups: Control and Sham (10 per group), CLP, DXM and APZ (15 per group) as aforementioned. All rats were monitored up to 24 h or until death, and the time when an animal died was recorded. Left ventricular blood was collected when rats died or were euthanized at 24 h for ABG. Anal temperature and disease severity were determined at pre-set time-points (immediately prior to surgery, 4, 8, 12 and 24 h postoperatively) with a digital thermometer and a scoring system, murine sepsis score (MSS), developed by Shrum et al (22). The criteria in MSS (data not shown) include: Degree of piloerection, spontaneous activity, response to stimuli, level of consciousness, openness of eyes, posture and degree of labored breathing. Each criterion was scored from 0 to 4 and a total MSS score was calculated. Mortality rises as MSS increases.
Lung tissue collection
At each time-point (immediately prior to surgery, 4, 8, 12 and 24 h postoperatively), thoracotomy was performed on the rats (three rats per group in control and sham groups, five per group in the rest of the groups), with pulmonary transfusion of 0.9% normal saline under speed of 120 ml/h for 15 min with drainage of the blood from left atrium. Then both lungs were removed. The lower and middle lobes of right lung tissues were snap frozen in the liquid nitrogen and stored at −80°C for subsequent protein detection, and the whole left lung was perfused with 1.5 ml 10% formalin via the bronchus and stored in 10% formalin for hematoxylin and eosin (H&E) staining and pathological analyses.
Western blot analysis
Frozen rat lung tissues were homogenized and the lysates were prepared in ice-cold lysis buffer and centrifuged (10,000 × g, −4°C, 10 min). The supernatant was collected and normalized for equal amounts of total protein measured with the BCA method; 60 µg protein from each sample were separated on a 12% SDS-polyacrylamide gel (SDS-PAGE) and transferred to PVDF membranes (Merck Millipore). The membranes were blocked with 5% non-fat milk and incubated overnight with the primary anti-caveolin-1 antibody at 4°C, followed by incubation with the HRP-conjugated secondary antibody for 4 h. Cellular β-actin protein was immunodetected as the internal standard.
HE staining and histological injury scoring for lung tissues
Formalin-fixed lungs were embedded in paraffin and serially sectioned in toto, then stained with H&E. A total of five images per slide were captured with a microscope (Nikon Eclipse E200; Nikon Corporation, Tokyo, Japan) at magnifications of ×4 and ×40. Histological changes, including alveolar wall edema, congestion, hemorrhage and inflammatory cell infiltration under a magnification of ×40, were scored by a pathologist blinded to the present study, as previously described (8). Each criterion was scored between 0 (normal) and 5 (severe), and the overall pulmonary inflammation was categorized according to the sum of the score (0–5, normal to minimal inflammation; 6–10, mild inflammation; 11–15, moderate inflammation; 16–20, severe inflammation).
Statistical analysis
Data were presented as the mean ± standard deviation and analyzed with SPSS software, version 20.0 (SPSS, Inc., Chicago, IL, USA). Differences between groups were determined by analysis of variance, followed by a post hoc test (least significant difference method; LSD) in ABG and histological scoring data. The missing values of MSS and anal temperature due to mortality were interpolated with the maximal MSS score and the temperature measured immediately prior to mortality, respectively. Western blotting images were read and calculated with Image J2X software (http://imagej.net/ImageJ2). Then MSS, anal temperature and caveolin-1 expression data were analyzed with repeated analysis of variance, followed by post hoc analysis (LSD). The cumulative survival rates among groups were analyzed with log-rank χ2 test and the survival curve with the Kaplan-Meier method. P<0.05 was considered to indicate a statistically significant difference.
Results
Survival rate
As demonstrated in Fig. 1, the survival rate 24-h post-operatively was 100, 90, 20, 40 and 33.3, respectively, for the Control, Sham, CLP, DXM and APZ groups. No significant differences were identified between the Control and Sham groups. CLP surgery markedly decreased survival rate compared with the Sham group, and survival rate was notably improved by pre-emptive DXM treatment. The antagonism of APZ did not significantly decrease the protection of DXM.
Figure 1.

The effect of DXM and its antagonist APZ on survival rates of septic rats induced by CLP. Control, control group (n=10); Sham, sham operation group (n=10); CLP, CLP operation group (n=15); DXM, DXM pretreatment group (n=15); APZ, APZ antagonizing group (n=15). The survival rate 24 h after operation was analyzed. #P<0.05, vs. Sham, DXM and APZ groups, respectively. DXM, dexmedetomidine; APZ, atepamezole; CLP, cecal ligation and puncture.
The severity of disease
The MSS score, which was reported to be highly predictive of sepsis progression and mortality and was paralleled with systemic pro-inflammatory factors in CLP-induced mice models (22), was employed to evaluate the effect of DXM. Fig. 2A provides the MSS scoring at pre-set time-points after surgery from individual rats in different treatment groups without missing data interpolation. Control rats scored 0 at all time-points. Sham-operated rats scored similar to CLP-operated rats 4 h postoperatively and went back to control level 8 h postoperatively, with the exception of one whose cecum was in the right upper quadrant and kept high scores after surgery. Scores of CLP-operated rats increased continuously 8 h postoperatively. DXM pretreatment resulted in the rise of scoring, however inhibited the rising speed 4 h postoperatively compared with that of CLP; however, APZ partly antagonized this effect. Fig. 2B demonstrates the differences of MSS among groups with missing data interpolation. MSS scores significantly increased after Sham and CLP surgery and only returned to the control level 8 h after surgery in the Sham group. DXM markedly increased scores at 4 h and maintained a similar level until 12 h postoperatively compared with the CLP group. APZ significantly antagonized the effect of DXM and reduced the scores until 24 h postoperatively.
Figure 2.
The effect of DXM and its antagonist APZ on the MSS of septic rats induced by CLP. Control, control group (n=10); Sham, sham operation group (n=10); CLP, CLP group (n=15); DXM, DXM treatment group (n=15); APZ, APZ antagonizing group (n=15). The subjects represent each numbered rat in each group. (A) The MSS scoring at pre-set time-points after operation from individual rats in the different treatment groups without missing data interpolation. (B) Differences of MSS among groups with missing data interpolation were analyzed. *P<0.05, vs. control and CLP groups, respectively; #P<0.05, vs. DXM group. DXM, dexmedetomidine; APZ, atepamezole; MSS, murine sepsis scores; CLP, cecal ligation and puncture.
ABG analysis
As demonstrated in Table I, there was no significant difference of pH among the groups. Blood lactate and base excess significantly increased in the CLP group compared with the Sham group, DXM marginally ameliorated lactate accumulation, and APZ marginally antagonized these effects, however these were not significant. Blood glucose (Glu) significantly increased in the Sham, CLP and DXM groups, however remained higher in the DXM group compared with the CLP group but (not statistically significant). Glu was markedly antagonized by APZ.
Table I.
Arterial blood gas analysis data.
| Group | pH | Lac (mmol/ml) | BE (mmol/ml) | Glu (mmol/ml) |
|---|---|---|---|---|
| Control | 7.42±0.08 | 1.34±0.45 | 0.33±2.77 | 6.40±1.76 |
| Sham | 7.40±0.08 | 1.79±0.33a | −1.45±3.31a | 9.85±2.31 |
| CLP | 7.32±0.13 | 4.5±1.35 | −4.18±5.04 | 10.85±2.51 |
| DXM | 7.45±0.13 | 3.58±1.01 | −4.10±4.78 | 12.67±5.74 |
| APZ | 7.43±0.14 | 4.02±0.89 | −3.96±3.56 | 7.59±1.86b |
Control, control group (n=10); Sham, sham operation group (n=10); CLP, CLP operation group (n=15); DXM, DXM treatment group (n=15); APZ, APZ antagonizing group (n=15). The femoral blood collected when rats died naturally or were euthanized at 24 h were determined immediately after death and were analyzed.
P<0.001, vs. CLP group
P<0.05, vs. DXM group. Lac, blood lactate; BE, base excess; Glu, blood glucose; CLP, cecal ligation and puncture; DXM, dexmedetomidine; APZ, atepamezole.
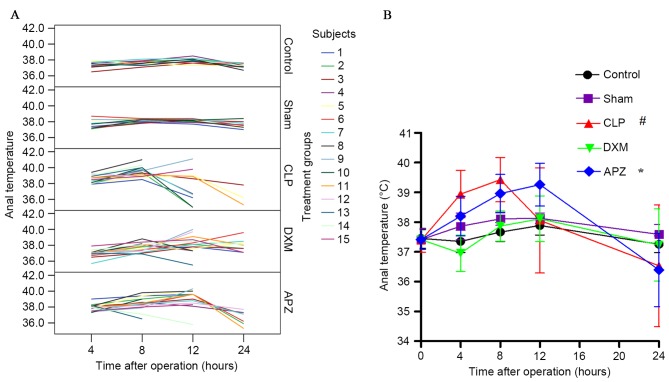
Anal temperature
Fig. 3A demonstrates the anal temperature (°C) of individual rats at pre-set time-points after surgery from different treatment groups without missing data interpolation. Sham-operated rats convergently maintained a higher temperature without influence on short-term survival. CLP surgery affected the temperature and survival of rats. DXM and APZ effectively improved survival rate, however DXM significantly inhibited the increase of temperature as sepsis progressed, which was effectively antagonized by APZ. Fig. 3B demonstrates the differences of anal temperature among groups with missing data interpolation. Normal anal temperatures fluctuated between 37.0–38.0°C and peaked at 12 h after surgery (corresponding to 22:00 h). CLP surgery caused significant fever and hypothermia at different stages of sepsis compared with the Sham group, and severely disturbed the circadian rhythm, which was significantly inhibited by DXM pretreatment. APZ significantly antagonized the effect of DXM.
Figure 3.
The effect of DXM and its antagonist APZ on temperature of rats with CLP-induced sepsis. Control, control group (n=10); Sham, sham operation group (n=10); CLP, CLP operation group (n=15); DXM, DXM treatment group (n=15); APZ, APZ antagonizing group (n=15). The subjects represent each numbered rat in each group. (A) The anal temperature (°C) of individual rats at pre-set time-points after operation from different treatment groups without missing data interpolation. (B) Differences of anal temperature among groups with missing data interpolation were analyzed. #P<0.05, vs. Sham and DEX groups; *P<0.05, vs. DXM group. DXM, dexmedetomidine; APZ, atepamezole; CLP, cecal ligation and puncture.
Histological changes of lung tissues
As demonstrated in Fig. 4, the histological changes due to procedures of tissue harvesting were minimal (Fig. 4A and F). Sham surgery caused minimal but significant lung injury compared with the Control group (Fig. 4B and F). CLP surgery induced mild to moderate injury (Fig. 4C and F), which was significantly reduced by DXM pretreatment to a minimal level (Fig. 4D and F). APZ antagonized the beneficial effect of DXM (Fig. 4E and F).
Figure 4.
Histological changes of rat lung tissues stained with hematoxylin and eosin (A) Control group (Control); (B) sham operation group (Sham); (C) CLP operation group (CLP); (D) DXM group (DXM); (E) APZ antagonizing group (APZ). Magnification, ×4. (F) The histological changes of lung tissue presented in A-E were magnified (magnification, ×40) and scored and the data were presented as the mean ± standard error. The CLP and APZ groups demonstrated mild to moderate injury, whilst Sham and DXM groups demonstrated minimal to mild injury. #P<0.01, vs. Control and CLP group. *P<0.001, vs. CLP and APZ group. CLP, cecal ligation and puncture; DXM, dexmedetomidine; APZ, atepamezole.
Western blot analysis for caveolin-1 expression in lung
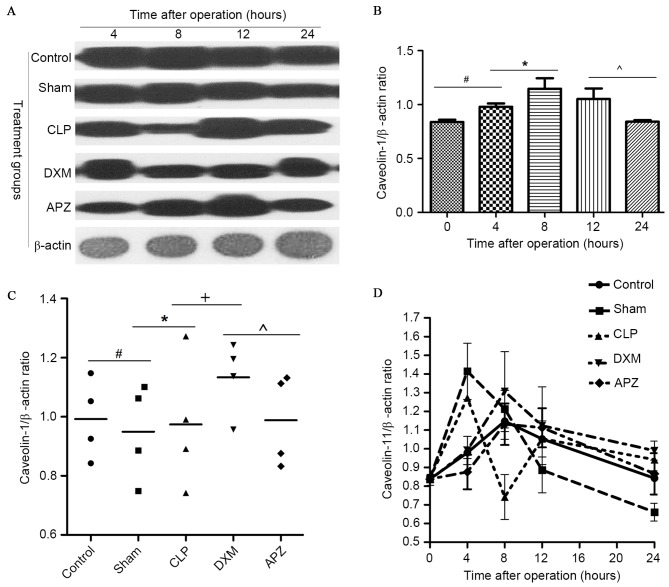
In order to identify whether DXM is able to affect caveolin-1 expression, the expression of caveolin-1 in lung tissues was examined. Fig. 5A demonstrates the caveolin-1 and β-actin bands determined by western blotting in different groups at pre-set time-points. Fig. 5B demonstrates the diurnal expression mode of caveolin-1 in the Control group. As indicated in Fig. 5A and B, rats in the Control group expressed a stable amount of caveolin-1 with peak expression at the 8-h time-point (corresponding to ~4:00 p.m.), and the lowest expression at pre- and 24-h time-point (corresponding to ~8:00 a.m.). Fig. 5C indicates the average level of caveolin-1 expression during one day in different treatment groups. Fig. 5D demonstrates the difference of caveolin-1 expression in different treatment groups. As indicated in Fig. 5A, C and D, caveolin-1 expression fluctuated in operated rats, with the exception of the DXM and APZ pretreated groups. DXM significantly upregulated the expression which was markedly inhibited by CLP surgery 4 h postoperatively. This effect was partly antagonized by APZ. DXM and APZ pretreatment effectively preserved the diurnal rhythm disturbed by CLP surgery.
Figure 5.
The effect of DXM and its antagonist APZ on caveolin-1 expression in lung tissues of rats with sepsis determined by western blotting. Control, control group (n=10); Sham, sham operation group (n=10); CLP, CLP operation group (n=15); DXM, DXM pretreatment group (n=15); APZ, APZ antagonizing group (n=15). β-actin was used as the internal standard protein. The grey value of protein band was read by Image J2X and presented as the mean ± standard deviation. (A) The caveolin-1 and β-actin expression determined by western blotting in different groups at pre-set time-points. (B) The diurnal expression mode of caveolin-1 in control group, the tissue was harvested at the time corresponding to the pre-set time-points in treated groups. #,*,^P<0.05. (C) The average level of caveolin-1 expression during a day in different treatment groups, #,*,+,^P<0.05. (D) The difference of caveolin-1 expression in different treatment groups. DXM, dexmedetomidine; APZ, atepamezole; CLP, cecal ligation and puncture.
Discussion
DXM was recommended as a sedative for septic patients in the ICU due to its anti-inflammatory effects, however its underlying mechanisms and long-term effects remain unclear. In the present study, it was identified that DXM pretreatment at a single sedative/hypnotic dose (20 µg/kg, i.p.) effectively decreased 24-h mortality and acute lung injury, and inhibited the increase of temperature, however it failed to reduce the increase of blood lactate, glucose and MSS scores. It was demonstrated that DXM can upregulate the expression and partly preserve the diurnal rhythm of caveolin-1 in lung tissues. APZ could partly neutralize the effects of DXM.
DXM pharmaceutically causes a dose-dependent inhibition of sympathetic tone in the central and peripheral nervous system and is used for sedation, analgesia, hypotension and hypothermia, its use resulting in a spontaneous motility decrease and induction of sleep. All these effects can be inhibited by prior or simultaneous delivery of APZ (23,24). The results of the present study agreed with this, even though it appeared that DXM marginally increased MSS scores while APZ decreased the scores. Due to the fact that the MSS scoring system gauged activity, consciousness, response to stimuli and eye opening as criteria, DXM could have increased these scores due to its sedative and analgesic activity, and APZ downgraded the scores through the neutralization of these effects. The present study also identified that DXM slightly decreased blood lactate and increased glucose levels, which can be significantly antagonized by APZ. To the best of our knowledge, stress can induce the rise of blood glucose, and tissue hypoxia can induce lactate generation. DXM may have reduced blood glucose due to the inhibition of stress through sedation and analgesia, however the effect of DXM on blood glucose and insulin secretion was more complex, since it can directly inhibit insulin secretion through activation of α2-AR and imidazoline receptors on pancreatic β cells (25), but also diminished activation of α1-AR and β-AR through reducing the sympathoadrenal output: The balance of these factors may have resulted in different glucose levels (26). These effects on peripheral sympathetic tone in addition with its central sympatholysis and vagal effect can significantly reduce systemic blood pressure. In addition, DXM was reported to enhance micturition through α2b-AR on the proximal tubule endothelia (27), which may further decrease circulatory volume and blood pressure when not under continuous fluid perfusion. These effects may have contributed to the failure of DXM to effectively reduce the hypoxia in tissues and the accumulation of lactate, even following the balance of its favorable effect in the preservation of capillary perfusion through attenuation of leukocyte-endothelial interaction and micro-emboli generation (28). Above all, DXM may be a promising sedative and antipyretic in patients with sepsis, and may be promising for clearing blood lactate after efficient fluid resuscitation. However, DXM may be detrimental to patients who survive sepsis, with the exception of the unclear effect on lactate, due to the fact that the effect of central hypothermia and glucose upregulation could bring patients into a chronic infectious or susceptible state (29). This indicates that the long-term effect of DXM on patients with sepsis or critically ill patients who survive should be further studied to assist clinical practice.
DXM was reported to inhibit lung injury and improve survival rate in CLP-induced rats (8), which was in agreement with the results of the present study. These results indicated that the anti-inflammatory action of DXM may be mediated by inhibition of the TLR4/MyD88/NF-κB signaling pathway. The TLR4-dependent signaling pathway mediates neutrophil sequestration into the lungs and causes secondary lung injury due to endotoxin or hyperinflation (30). Additional studies have implied that the receptors mediate the anti-inflammatory actions of DXM possibly through α2-AR and IRs, although one study used APZ as an antagonist in a renal ischemic-reperfusion injury (IRI) rat model (11) and the other utilized yohimbine in a lung IRI rats model (9). It is known that DXM binds to α2-AR and specifically activates the Gαi subunit, which has been demonstrated to bind with the N-terminus (Residual 82–101) of caveolin-1 prior to being activated, and to translocate away from caveolin-1, concomitantly releasing Gβγ subunits after activation (31). Studies have also demonstrated that Src Tyrosine kinases, H-ras and eNOS share the same binding domain of caveolin-1 (32), the Gβγ complex induces activation of Src after release (33) and the later phosphorylated caveolin-1 at Tyr14 induces interaction with TLR4 and mediates MyD88-dependent signaling (15). Deficiency of caveolin-1 would reduce TLR4 signaling (34). However, knockout studies have identified that caveolin-1 protects against sepsis by modulating inflammatory responses, alleviating bacterial burden and suppressing thymocyte apoptosis in rats with CLP-induced sepsis (35). In brief, caveolin-1 may biphasicly regulate the TLR4-dependent inflammatory responses.
In the present study, the expression of caveolin-1 in the lung was determined and it was identified that DXM can effectively inhibit the increase of caveolin-1 at 4 h and the decrease at 8 h postoperatively induced by CLP surgery, and promote the average expression amount. APZ only partly antagonized the upregulation effect of DXM. This implied that DXM could exert influence on the TLR4 pathway-mediated inflammatory responses and the promote sepsis survival rate through regulation of caveolin-1 expression, however the exact receptors and downstream molecular events require additional investigation.
In conclusion, preemptive clinical sedative doses of DXM may upregulate the expression of caveolin-1 downregulated by sepsis, and may contribute to the inhibition of inflammatory pathways such as the TLR4-mediated pathways. Furthermore, DXM may favor the improvement of short-term outcome by the regulation of other metabolic pathways.
Acknowledgements
The present study was supported by the Provincial Natural Science Foundation of Hunan, China (grant nos. 2013FJ4082 and 2015SK2085).
References
- 1.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Medicine. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infect Immun. 2006;74:5227–5235. doi: 10.1128/IAI.01220-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ernsberger P, Giuliano R, Willette RN, Reis DJ. Role of imidazole receptors in the vasodepressor response to clonidine analogs in the rostral ventrolateral medulla. J Pharmacol Exp Ther. 1990;253:408–418. [PubMed] [Google Scholar]
- 4.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit. Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 5.Pandharipande PP, Sanders RD, Girard TD, McGrane S, Thompson JL, Shintani AK, Herr DL, Maze M, Ely EW. MENDS investigators: Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: An apriori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14:R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Memiş D, Hekimoğlu S, Vatan I, Yandim T, Yüksel M, Süt N. Effects of midazolam and dexmedetomidine on inflammatory responses and gastric intramucosal pH to sepsis, in critically ill patients. Br J Anaesth. 2007;98:550–552. doi: 10.1093/bja/aem017. [DOI] [PubMed] [Google Scholar]
- 7.Fraser GL, Devlin JW, Worby CP, Alhazzani W, Barr J, Dasta JF, Kress JP, Davidson JE, Spencer FA. Benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adults: A systematic review and meta-analysis of randomized trials. Crit Care Med. 2013;41(Suppl 1):S30–S38. doi: 10.1097/CCM.0b013e3182a16898. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Liu Y, Huang H, Zhu Y, Zhang Y, Lu F, Zhou C, Huang L, Li X, Zhou C. Dexmedetomidine inhibits inflammatory reaction in lung tissues of septic rats by suppressing TLR4/NF-kB pathway. Mediators Inflamm. 20132013:562154. doi: 10.1155/2013/562154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang L, Li L, Shen J, Qi Z, Guo L. Effect of dexmedetomidine on lung ischemia-reperfusion injury. Mol Med Rep. 2014;9:419–426. doi: 10.3892/mmr.2013.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snapir A, Talke P, Posti J, Huiku M, Kentala E, Scheinin M. Effects of nitric oxide synthase inhibition on dexmedetomidine-induced vasoconstriction in healthy human volunteers. Br J Anaesth. 2009;102:38–46. doi: 10.1093/bja/aen316. [DOI] [PubMed] [Google Scholar]
- 11.Si Y, Bao H, Han L, Shi H, Zhang Y, Xu L, Liu C, Wang J, Yang X, Vohra A, Ma D. Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the JAK/STAT signaling activation. J Transl Med. 2013;11:141. doi: 10.1186/1479-5876-11-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, Ravid K, Pilch PF. Deletion of Cavin/PTRF causes global loss of caveolae, dislipidemia, and glucose intolerance. Cell Metab. 2008;8:310–317. doi: 10.1016/j.cmet.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu Y, Moore XL, Lee MK, Fernández-Rojo MA, Parat MO, Parton RG, Meikle PJ, Sviridov D, Chin-Dusting JP. Caveolin-1 plays a critical role in the differentiation of monocytes into macrophages. Arterioscler Thromb Vasc Biol. 2012;32:e117–e125. doi: 10.1161/ATVBAHA.112.254151. [DOI] [PubMed] [Google Scholar]
- 14.Feng H, Guo L, Song Z, Gao H, Wang D, Fu W, Han J, Li Z, Huang B, Li XA. Caveolin-1 protects against sepsis by modulating inflammatory response, alleviating bacterial burden, and supressing thymocyte apoptosis. J Biol Chem. 2010;285:25154–25160. doi: 10.1074/jbc.M110.116897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiao H, Zhang Y, Yan Z, Wang ZG, Liu G, Minshall RD, Malik AB, Hu G. Caveolin-1 Tyr14 phosphorylation induces interaction with TLR4 in endothelial cells and mediates MyD88-dependent signaling and sepsis-induced lung inflammation. J Immunol. 2013;191:6191–6199. doi: 10.4049/jimmunol.1300873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calizo RC, Scarlata S. A role for G-proteins in directing G-protein-coupled receptor-caveolae localization. Biochemistry. 2012;51:9513–9523. doi: 10.1021/bi301107p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Alessio A, Kluger MS, Li JH, Al-Lamki R, Bradley JR, Pober JS. Targeting of tumor necrosis factor receptor 1 to low density plasma membrane domains in human endothelial cells. J Biol Chem. 2010;285:23868–23879. doi: 10.1074/jbc.M110.122853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z, Bakhshi FR, Shajahan AN, Sharma T, Mao M, Trane A, Bernatchez P, van Nieuw Amerongen GP, Bonini MG, Skidgel RA, et al. Nitric oxide-dependent Src activation and resultant caveolin-1 phosphorylation promote eNOS/caveolin-1 binding and eNOS inhibition. Mol Biol Cell. 2012;23:1388–1398. doi: 10.1091/mbc.E11-09-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vetterkind S, Poythress RH, Lin QQ, Morgan KG. Hierarchical scaffolding of an ERK1/2 activation pathway. Cell Commun Signal. 2013;11:65. doi: 10.1186/1478-811X-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banquet S, Delannoy E, Agouni A, Dessy C, Lacomme S, Hubert F, Richard V, Muller B, Leblais V. Role of Gi/o-Src kinase-PI3K/Akt pathway and caveolin-1 in β2-adrenoceptor coupling to endothelial NO synthase in mouse pulmonary artery. Cell Signal. 2011;23:1136–1143. doi: 10.1016/j.cellsig.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Toscano MG, Ganea D, Gamero AM. cecal ligation puncture procedure. J Vis Exp. 2011:2860. doi: 10.3791/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrum B, Anantha RV, Xu SX, Donnelly M, Haeryfar SM, McCormick JK, Mele T. A robust scoring system to evaluate sepsis severity in an animal model. BMC Res Notes. 2014;7:233. doi: 10.1186/1756-0500-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virtanen R. Pharmacological profiles of medetomidine and its antagonist, atipamezole. Acta Vet Scand Suppl. 1989;85:29–37. [PubMed] [Google Scholar]
- 24.Madden CJ, Tupone D, Cano G, Morrison SF. α2 adrenergic receptor-mediated inhibition of thermogenesis. J Neurosci. 2013;33:2017–2028. doi: 10.1523/JNEUROSCI.4701-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi T, Kawano T, Eguchi S, Chi H, Iwata H, Yokoyama M. Effects of dexmedetomidine on insulin secretion from rat pancreatic β cells. J Anesth. 2015;29:396–402. doi: 10.1007/s00540-014-1943-2. [DOI] [PubMed] [Google Scholar]
- 26.Restitutti F, Raekallio M, Vainionpää M, Kuusela E, Vainio O. Plasma glucose, insulin, free fatty acids, lactate and cortisol concentrations in dexmedetomidine-sedated dogs with or without MK467: A peripheral α-2adrenoceptor antagonist. Vet J. 2012;193:481–485. doi: 10.1016/j.tvjl.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Cussac D, Schaak S, Gales C, Flordellis C, Denis C, Paris H. alpha(2B)-adrenergic receptors activate MAPK and modulate proliferation of primary cultured proximal tubule cells. Am J Physiol Renal Physiol. 2002;282:F943–F952. doi: 10.1152/ajprenal.0108.2001. [DOI] [PubMed] [Google Scholar]
- 28.Miranda ML, Balarini MM, Bouskela E. Dexmedetomidine attenuates the microcirculatory derangements evoked by experimental sepsis. Anesthesiology. 2015;122:619–630. doi: 10.1097/ALN.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 29.Drewry AM, Fuller BM, Skrupky LP, Hotchkiss RS. The presence of hypothermia within 24 hours of sepsis diagnosis predicts persistent lymphopenia. Crit Care Med. 2015;43:1165–1196. doi: 10.1097/CCM.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu G, Malik AB, Minshall RD. Toll-like receptor 4 mediates neutrophil sequestration and lung injury induced by endotoxin and hyperinflation. Crit care med. 2010;38:194–201. doi: 10.1097/CCM.0b013e3181bc7c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti M. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995;270:15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- 32.Li S, Couet J, Lisanti MP. Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem. 1996;271:29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shajahan AN, Tirupathi C, Minshall RD. Gbetagamma activation of Src induces caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279:48055–48062. doi: 10.1074/jbc.M308710200. [DOI] [PubMed] [Google Scholar]
- 34.Mirza MK, Yuan J, Gao XP, Garrean S, Brovkovych V, Malik AB, Tiruppathi C, Zhao YY. Caveolin-1 deficiency dampens Toll-like receptor 4 signaling through eNOS activation. Am J Pathol. 2010;176:2344–2351. doi: 10.2353/ajpath.2010.091088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng H, Guo L, Song Z, Gao H, Wang D, Fu W, Han J, Li Z, Huang B, Li XA. caveolin-1 protects against sepsis by modulating inflammatory response, alleviating bacterial burden, and supressing thymocyte apoptosis. J Biol Chem. 2010;285:25154–25160. doi: 10.1074/jbc.M110.116897. [DOI] [PMC free article] [PubMed] [Google Scholar]