Abstract
Parkinson's disease (PD) is a common neurodegenerative disorder, which is characterized by the selective and progressive death of dopaminergic (DA) neurons in the substantia nigra. Increasing evidence suggests that inflammation is important in the degeneration of DA neurons. The purinergic receptor subtype P2X7 receptor (P2X7R) is key in the activation and proliferation of microglia. The present study aimed to examine whether inhibiting purinergic P2X7 receptors is neuroprotective in a rat model of PD, specifically via inhibiting p38 mitogen-activated protein kinase (MAPK). In an intranigral lipopolysaccharide (LPS) rat model of PD, immunohistochemical analysis revealed enhanced expression of P2X7R was observed in microglia. The administration of the P2X7R antagonist, brilliant blue G (BBG), reduced activation of the microglia and the loss of nigral DA neurons. In addition, immunohistochemistry and western blot analysis revealed the phosphorylation level of p38 MAPK increased in the microglia of the LPS-injected rats, which was inhibited by BBG treatment. The p38 MAPK inhibitor, SB203580, reduced microglial activation and the loss of DA neurons. Thus, these findings suggested that inhibition of P2X7R by BBG attenuated microglial activation and the loss of substantia nigra DA neurons via p38 MAPK in the rat LPS model of PD.
Keywords: Parkinson's disease, microglia, P2X7 receptor, brilliant blue G, p38 mitogen-activated protein kinase
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized by the selective and progressive loss of dopaminergic (DA) neurons in the substantia nigra. The majority of cases are sporadic and of unknown etiology. Several lines of evidence indicate that brain inflammation specifically activates microglia, leading to the pathogenesis of PD (1,2). The activated microglia secrete high levels of inflammatory mediators, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), eicosanoids, nitric oxide (NO) and reactive oxygen species (2–4). These inflammatory mediators impair neurons and further activate microglia, resulting in a vicious cycle, which promotes further inflammation and neurodegeneration (5–7). Microglial activation is an integral aspect of inflammatory processes in the brain. The molecular mechanism through which microglial activation occurs in patients with PD remains to be fully elucidated.
The P2X7 receptor (P2X7R) is a purinergic, ATP-binding receptor, which is expressed at high levels in cells of monocyte/macrophage lineages. This receptor is important in the innate immune system. In the central nervous system (CNS), the extensive functional expression of P2X7R is detected in microglia, which are resident macrophages of the brain (8). The leakage of ATP from damaged cells signals the proliferation and activation of microglia by binding P2X7R (9). As a unique member of the purinergic receptor family, P2X7R is strictly associated with the maturation and release of the IL-1β cytokine in microglia (10). The activation of P2X7R in microglia has been correlated with the production of proinflammatory cytokines and chemokines, including TNF-α (11) and CC-chemokine ligand 3 (12). In microglial cells, activated P2X7R also stimulates the production of superoxide and NO (13–15). Therefore, P2X7R is considered to be a key in eliciting an inflammatory response in microglia as it stimulates microglial activation. Thus, it has the potential to lead to a deleterious cycle of neuroinflammation and neurodegeneration (16,17).
The expression of P2X7R is enhanced in several types of brain pathology, in which the presence of activated microglia is a concurrent feature (18). The expression of P2X7R is not only upregulated in the brains of patients with PD (19), but also in animal models of various neurodegenerative diseases, including multiple sclerosis (MS), Alzheimer's disease (AD) and Huntington's disease (HD) (20–22). Whether P2X7R has a beneficial or detrimental role in the development of these diseases remains to be elucidated, although a number of studies have demonstrated that the inhibition or deficit of P2X7R has neuroprotective effects in animal models of MS (20), HD (22) and AD (21). Several lines of evidence have confirmed that the P2X7R pathway causes neuronal injury, leading to the progression of neurodegeneration (17,20). Although reports on the role of P2X7R in PD are rare and contradictory, previous studies have established that this receptor is upregulated in an animal model of PD (23,24), although a P2X7R antagonist was not associated with the reduction of DA cell loss (24,25). However, a previous study provided evidence supporting the suggestion that P2X7R antagonism attenuates the neuronal dysfunction and damage in an animal model of PD (23). This discrepancy in previous reports may be attributed to the use of different animal models. Animal models of PD, which are established through exposure of the animals to toxins, including 6-hydroxydopamine, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine or rotenone, are based on the destruction of DA neurons in the nigra. P2X7R may be mediating the microglial inflammatory responses in PD. By excessively activating microglia, P2X7R may be promoting DA neuron damage. The present study hypothesized that inhibiting this receptor may alleviate the progression of PD.
When P2X7Rs are stimulated, they activate mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinases, c-Jun N-terminal kinases and p38 MAPKs (26). p38 MAPKs have been implicated in the release of immune-associated cytotoxic factors, including NO and pro-inflammatory cytokines in neuroglia. Thus, p38 MAPK is pivotal in glial-mediated or inflammation-mediated neurotoxicity (27). Inhibition of p38 MAPK has been found to reduce the production of inflammatory factors following exposure to the bacterial endotoxin, lipopolysaccharide (LPS), in an animal model of PD (27,28). Thus, the expression of p38 MAPK appears to be regulated following activation of P2X7R in the substantia nigra. The intranigral LPS model is suitable for examining the potential neuroprotective effects of P2X7R antagonists on the neuroinflammatory processes leading to cell death. LPS-induced inflammation causes the loss of DA neurons and elicits a microglial inflammatory response in the PD rat model (29,30).
In the present study, the immunoreactivity of P2X7R in microglia present in the LPS-injured substantia nigra was investigated. Furthermore, immunohistochemical analysis was performed to determine whether the P2X7R antagonist, brilliant blue G (BBG) (31) prevents LPS-induced injury in nigrostriatal DA neurons (29,30). The present study aimed to test the hypothesis that P2X7R causes the loss of DA neurons in the substantia nigra by activating p38 MAPK in an LPS animal model of PD. It was found that selective inhibition of the P2X7R affords marked protection of DA neurons from LPS-induced cytotoxicity by suppressing p38 MAPK in the microglia of the substantia nigra in rats.
Materials and methods
LPS model of PD
Male Sprague-Dawley rats (n=42; 250–300 g) used in the present study were obtained from the Laboratory Animal Center of the China Medical University (Shenyang, China). Rats were housed in a room with the temperature maintained at 22±1°C. The relative humidity of the room was 55%. The rats were subjected to a 12-h light/dark cycle with free access to food and water. All animal experiments were performed in accordance with the guidelines by the Committee on Animal Research at the University of China Medical University (Shenyang, China). The experimental protocol was approved by the institutional ethics committee of China Medical University (Shenyang, China).
The rats were anesthetized with chloral hydrate (400 mg/kg) and positioned in a stereotactic apparatus. LPS (5.0 µl; 2 µg/µl) was purchased from Sigma-Aldrich; Merck Millipore (Darmstadt, Germany) and injected into the right substantia nigra (30,32,33). Injections were administered at the following locations: 5.5 mm posterior to the bregma, 2 mm lateral to the midline, 8.0 mm ventral to the dura. LPS was delivered over a period of 5 min, with the needle remaining in situ for 5 min prior to removal (1 mm/min), thus reflux was prevented along the injection tract. A total of 12 rats were treated only with intranigral injection of LPS. BBG and SB203580 groups (n=12 for each group) were treated with BBG or SB203580 following LPS administration. A total of six rats were included in the control group and were administered with 0.5 µl phosphate-buffered saline (PBS) injections.
The preparation and administration of BBG, the P2X7R antagonist, were performed according to previously described procedures (21). The BBG (Sigma-Aldrich; Merck Millipore) was dissolved in saline and injected intraperitoneally at a dose of 50 mg/kg 1 h prior to administering the LPS injection. The same dose was administered for 15 days (BBG injection administered 1 h prior to LPS). It has previously been reported that this BBG treatment protocol is effective at inhibiting LPS-induced inflammatory reactions in rat brains (34).
In order to investigate the role of the p38 MAPK signaling pathway in LPS-induced neuroprotection, SB203580 (Sigma-Aldrich; Merck Millipore), a selective p38 MAPK antagonist, was injected intracerebroventricularly. Immediately following LPS injection, a stainless steel guide cannula was lowered into the right lateral cerebral ventricle using standard stereotaxic procedures (1.0 mm posterior to the bregma, 1.5 mm lateral from the midline, 3.5 mm ventral to dura). SB203580 solution (1 mg/ml) was prepared in 3% dimethyl sulfoxide, and 10 µl of this solution was injected directly into the right lateral ventricle of the experimental rats via the stainless steel cannula connected with polyethylene tubing (35,36). The same dose was injected daily for 15 days.
Immunohistochemistry
At 15 days post-LPS injection, the animals were euthanized by chloral hydrate (600 mg/kg) and perfused with 100 ml of 0.9% NaCl followed by 4% paraformaldehyde, and their brains were processed into 30 µm slices via cryostat sectioning. Tyrosine hydroxylase (TH) immunoreactivity was determined using an avidin-biotin-peroxidase method. To inhibit endogenous peroxidase activity, the sections were incubated for 30 min in 1% H2O2 solution. These sections were then blocked with 5% (v/v) normal goat serum in PBS for 1 h. Subsequently; the sections were incubated overnight with rabbit anti-TH polyclonal antibody (cat. no. 2792; 1:1,000; Cell Signaling Technology, Beverly, MA, USA) at 4°C. The brain sections were then incubated with biotinylated goat anti-rabbit secondary antibody (cat. no. A0277; 1:500, Beyotime Institute of Biotechnology, Haimen, China) for 2 h at room temperature. Subsequently, the brain sections were incubated with avidin-conjugated horseradish peroxidase for 1 h at 37°C. The sections were then incubated with the peroxidase substrate, diaminobenzidine, to develop a stain of desired intensity observed by a light microscope at ×100 magnification (BX60; Olympus Corporation, Tokyo, Japan).
The microglia in the substantia nigra were identified by their immunoreactivity to the microglial marker anti-ionized calcium binding adapter molecule 1 (Iba-1). Double immunolabeling for P2X7R and Iba-1 through use of red and green fluorescence labeling was performed with the aim of determining the localization of P2X7R immunoreactivity on the microglia cells. Similarly, to determine the localization of phosphorylated (p-)p38 MAPK immunoreactivity in microglia cells, double immunolabeling of p-p38 MAPK and Iba-1 was performed. The sections were incubated overnight at 4°C with the following primary antibodies: Rabbit anti-P2X7R polyclonal antibody (cat. no. ab77413; 1:1,000; Abcam, Cambridge, UK), goat anti-Iba-1 polyclonal antibody (cat. no. ab5076; 1:500; Abcam), and rabbit anti-p-p38 MAPK monoclonal antibody (cat. no. 4511; 1:1,000; Cell Signaling Technology). After washing with 0.1 M PBS, the sections were incubated for 2 h at room temperature in a solution containing appropriate donkey secondary antibodies conjugated to Alexa Fluor 488 and 594 (cat. nos. A11055 and A21207; 1:500; Molecular Probes; Thermo Fisher Scientific, Inc., Waltham, MA, USA). For immunostaining controls, primary antibody was omitted in all staining procedures. Digitized images of the immunostained sections were captured using a Zeiss Axioplan-2 light microscope (Zeiss GmbH, Jena, Germany), which was equipped with a digital camera.
The number of TH-positive neurons in the substantia nigra was estimated using an optical fractionator technique and Stereo-Investigator™ software (MBF Bioscience, Inc., Williston, VT, USA). Every sixth section in the entire substantia nigra was selected for analysis (−4.56 to −6.48 mm posterior to the bregma). A fractionator sampling scheme was used comprising counting frames (80×40 mm), which were superimposed on sections at intervals of x=150 mm and y=200 mm (37).
Measurements of the expression of P2X7R and p-p38 MAPK, and microgliosis (Iba-1 marker) were performed on immunostained sections by quantifying the integrated optical density (IOD) of immunoreactivity using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Bethesda, MD, USA). In each stained section, four non-overlapping fields within the substantia nigra regions were selected. The nomenclature and boundaries of brain structures were in agreement with those described in the rat brain atlas of Paxinos and Watson (38). A fixed setting was used throughout the entire process, and the background reading was acquired from immune-negative regions of each section.
Western blot analysis
The rats were sacrificed by decapitation, following which the substantia nigra was isolated and immediately frozen in liquid nitrogen. The nigral tissue was lysed with ultrasound for <30 sec on ice and buffer containing the following reagents: 150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 5 mM EDTA, 1% sodium deoxycholate, 1% Triton X-100, 0.1% SDS and 1 mM PMSF. The homogenates were centrifuged at 12,000 × g for 20 min at 4°C and the supernatants were carefully removed. Protein concentration was determined using a bicinchoninic acid protein assay method with bovine serum albumin (BSA) as the standard. The samples were boiled in sodium dodecyl sulphate-polyacrylamide electrophoresis (SDS-PAGE) loading buffer for 5 min. Protein samples (40 µg/lane) were loaded and were separated by SDS-PAGE (12% polyacrylamide gels). Subsequently, the samples were electrotransferred onto nitrocellulose membranes. These membranes were blocked with 10% non-fat dry milk in Tris-buffered saline for 1 h at room temperature, and incubated overnight at 4°C with rabbit anti-p38 MAPK monoclonal antibody (cat. no. 8690; 1:1,000; Cell Signaling Technology), p-p38 MAPK (cat. no. 4511; 1:1,000; Cell Signaling Technology) and mouse monoclonal anti-tubulin (cat. no. AT819; 1:1,000; Beyotime Institute of Biotechnology) diluted in 2% BSA in PBS. The immunoblots were processed with appropriate horseradish peroxidase-conjugated secondary antibodies: Goat anti-mouse and goat anti-rabbit (cat. no. A0216 and A0208; 1:1,000; Beyotime Institute of Biotechnology) for 2 h at room temperature. The bands were visualized with an ECL Plus chemiluminescence reagent kit (Beyotime Institute of Biotechnology), and the density of immunoreactive bands was quantified using NIH ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The results are expressed as the mean ± standard error of the mean. Statistical analysis of the data was performed using one-way analysis of variance, followed by the least significant difference test and Student-Newman-Keuls test, which were performed using the Statistical Package for Social Sciences software (version 16.0; SPSS, Inc., Chicago, IL, USA). In all the cases, P<0.05 was considered to indicate a statistically significant difference.
Results
P2X7R antagonism and the inhibition of p38 MAPK reduce the damage of DA neurons in the LPS model of PD in rats
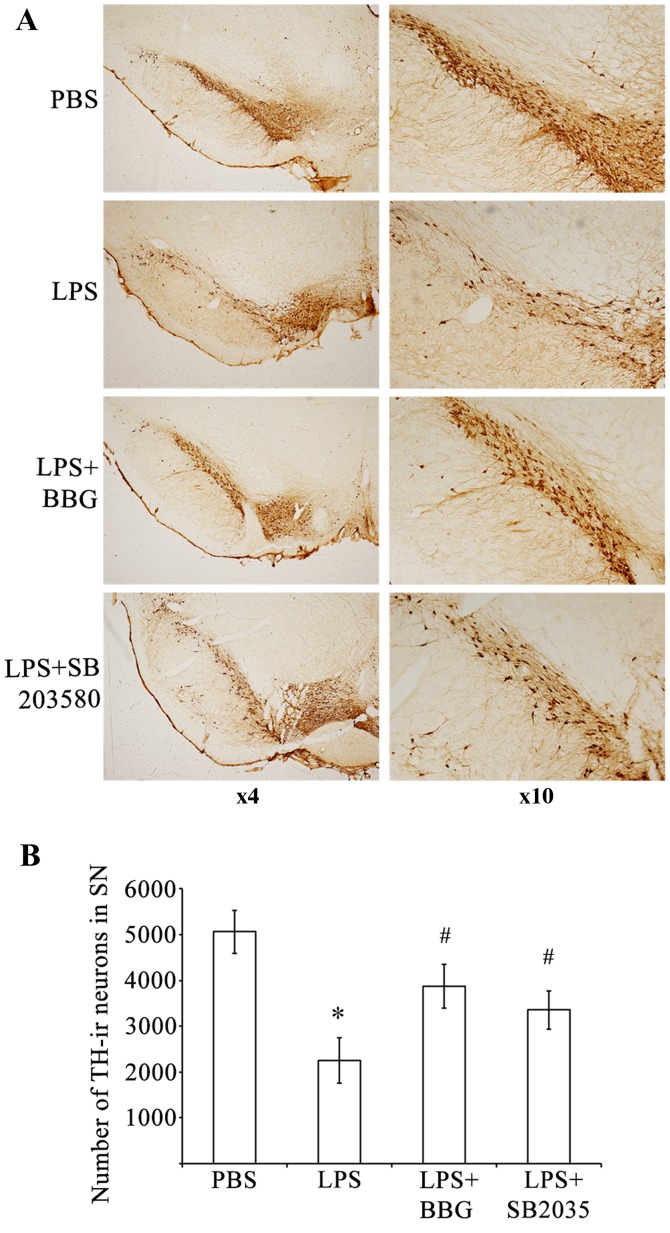
To determine whether P2X7R causes the loss of DA neurons, the present study evaluated the effects of the P2X7R antagonist, BBG, on the LPS-induced loss of DA neurons in the substantia nigra of a rat model of PD. The loss of DA neurons, which was caused by LPS injection, was assessed immunohistochemically by counting TH-immunoreactive (TH-ir) neurons in the substantia nigra (Fig. 1). Compared with the sham control group, there was a significant reduction (P<0.001) in the number of TH-ir nerve cells 15 days following injury of DA neurons in the rats treated with LPS (PBS group, 5,068±470; LPS group, 2,249±492). An intranigral injection of LPS reduced TH-ir neurons in the substantia nigra by ~55.6%, however, those in the adjacent ventral tegmental area were spared (Fig. 1A). Treating the LPS-treated animals with BBG partially reversed the reduction of TH-ir neurons on the lesion side. The loss of TH-ir neurons in the substantia nigra was initially 2,249±492 neurons following induction of the LPS-induced lesion. This loss was significantly reduced to 3,867±478 neurons in the LPS-injected rats, which were treated with BBG for 15 days following LPS (Fig 1B; P=0.039). Compared with the control animals treated with PBS, TH-ir cells were reduced by approximately 23.7% in the LPS-treated animals treated with BBG for 15 days. To ascertain whether p38 MAPK was involved in the LPS-induced loss of DA neurons, the p38 MAPK inhibitor, SB203580, was applied following injection with LPS in the substantia nigra of rats. The loss of TH-ir neurons was 2,249±492 when the rats were treated with LPS; however, SB203580 led to a significant reduction in the loss of TH-ir neurons to 3,353±424 neurons (Fig. 1B; P<0.001). Compared with the control animals treated with PBS, TH-ir cells were reduced by 33.8% in the LPS-treated animals exposed to SB203580, the p38 MAPK inhibitor. These results indicated that BBG, the specific P2X7R antagonist, and SB203580, the p38 MAPK inhibitor, offered neuroprotection to the rats in the LPS model of PD.
Figure 1.
BBG and SB203580 attenuate LPS-induced death of DA neurons in the SNpc of rats. (A) Representative examples of TH-stained sections of SNpc, adjacent VTA and SNr in rats of the PBS control group, LPS group, LPS+BBG group and LPS+SB203580 group. (B) Quantification of the number of TH-ir neurons in the SNpc of rats in the PBS, LPS+BBG and LPS+SB203580 groups. Data were obtained from six independent animals (n=6). Compared with the SNpc in the PBS control group, rats injected with LPS exhibited a significant reduction in the number of TH-positive cells in the SNpc region. BBG and SB203580 significantly reduced the size of the SNpc lesion induced by LPS. *P<0.001, compared with the PBS control; #P<0.001, compared with the LPS group. LPS, lipopolysaccharide; PBS, phosphate-buffered saline; DA, dopaminergic; BBG, brilliant blue G; TH-ir, tyrosine hydroxylase immunoreactive; SNpc, substantia nigra pars compacta.
LPS-induced upregulation of the expression of P2X7R and activation of microglia are attenuated by P2X7R antagonism and p38 MAPK inhibition in the substantia nigra of in the LPS model of PD
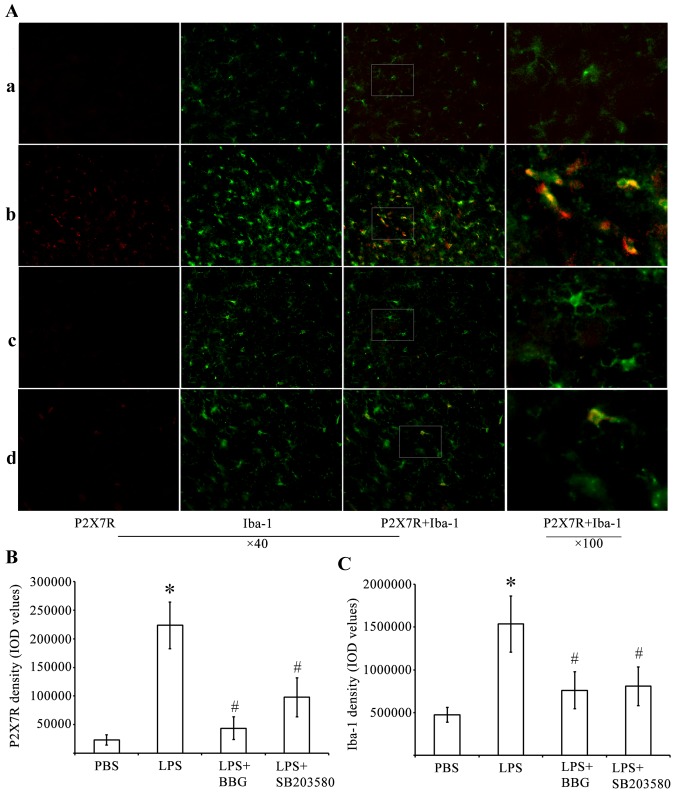
The present study performed immunohistochemical analysis to determine whether P2X7R had an active role in LPS-induced DA neuronal damage, which was mediated by microglia. For this analysis, antibodies against P2X7R and the microglia marker, Iba-1, were used. The analysis was performed in the substantia nigra tissue of the LPS-treated rats. Whereas few P2X7R-ir cells were present in the substantia nigra of the PBS-injected control animals (Fig. 2), cells expressing a high level of P2X7R were identified in the LPS-injured substantia nigra. Of note, the P2X7R antagonist, BBG, was effective in reducing the number of P2X7R-ir cells. The p38 MAPK inhibitor, SB203580, was also effective to a certain extent in reducing P2X7R-ir.
Figure 2.
Double immunofluorescence labeling of P2X7R and Iba-1 in the SNpc of rats in the LPS model of Parkinson's disease. (A) Immunofluorescence for P2X7R (red) and Iba-1, (green) are shown for the (a) PBS, (b) LPS, (c) LPS+BBG and (d) LPS+SB203580 groups. Co-immunolabeled P2X7R/Iba-1 microglia are yellowish in color. Marked microglial activation was observed in the SNpc of the LPS group. In the PBS group, microglia had an inactive morphology with small soma and thin processes, whereas microglia exposed to LPS had enlarged soma-lacking processes. BBG significantly decreased microglia activation. Immunostaining for P2X7R showed a marked increase in the LPS animals, compared with PBS animals. BBG was effective in attenuating P2X7R-ir in LPS-injected SNpc. Double-labeling immunohistochemistry identified P2X7R-ir predominantly in activated, Iba-1-ir microglia, following LPS treatment. SB203580 significantly inhibited the upregulation of P2X7R and microglia activation in SNpc. Quantitative analysis of IOD values of (B) P2X7R-ir and (C) Iba-1 in the groups (n=6) is shown. *P<0.05, compared with the PBS control; #P<0.05, compared with the LPS group. LPS, lipopolysaccharide; PBS, phosphate-buffered saline; BBG, brilliant blue G; SNpc, substantia nigra pars compacta; Iba-1, ionized calcium binding adapter molecule 1; -ir, immunoreactive; IOD, integrated optical density.
Double-labeling experiments were performed using antibodies against P2X7R and Iba-1. These experiments showed that P2X7R-ir was distributed predominantly within activated immunoreactive Iba-1 (Iba-1-ir) microglial cells of the LPS-treated substantia nigra pars compacta (SNpc; Fig. 2A). A higher intensity of staining of P2X7R in cells correlated with increased Iba-1-ir potency. LPS induced microglial activation by increasing P2X7R-ir in the substantia nigra. The activated microglial cells were characterized by minimum ramification, hypertrophy and proliferation. The activated microglia were positive for Iba-1, and were distributed in the ventral tegmental area, SNpc and substantia nigra pars reticulata (SNpr). In the substantia nigra of the PBS-injected control animals, Iba-1-ir cells had elongated nuclei and ramifying processes, which are typical of inactivated microglia.
By performing quantitative analysis of the IOD values of P2X7R (Fig. 2B) and Iba-1 (Fig. 2C), it was found that the IOD of P2X7R-ir was significantly increased by 962% in the substantia nigra of the LPS-treated rats. By contrast, the IOD values of P2X7R-ir decreased by 80.5 and 56% in the substantia nigra of BBG- and SB203580-treated LPS-injected rats, respectively (Fig. 2B; P<0.05). At 15 days post-LPS injection, the experimental rats exhibited a 3.25-fold increase in the density of Iba-1 in the substantia nigra, compared with the controls (Fig. 2C; P<0.05). BBG and SB203580 were effective in reducing microglial responses in the LPS-stimulated animals. The IOD of Iba-1 in the substantia nigra decreased by 51 and 47% in the BBG- and SB203580-treated rats, respectively, compared with the levels observed in LPS-treated rats (Fig. 2C; P<0.05).
LPS-induced upregulation of p-p38 MAPK is dependent on P2X7R
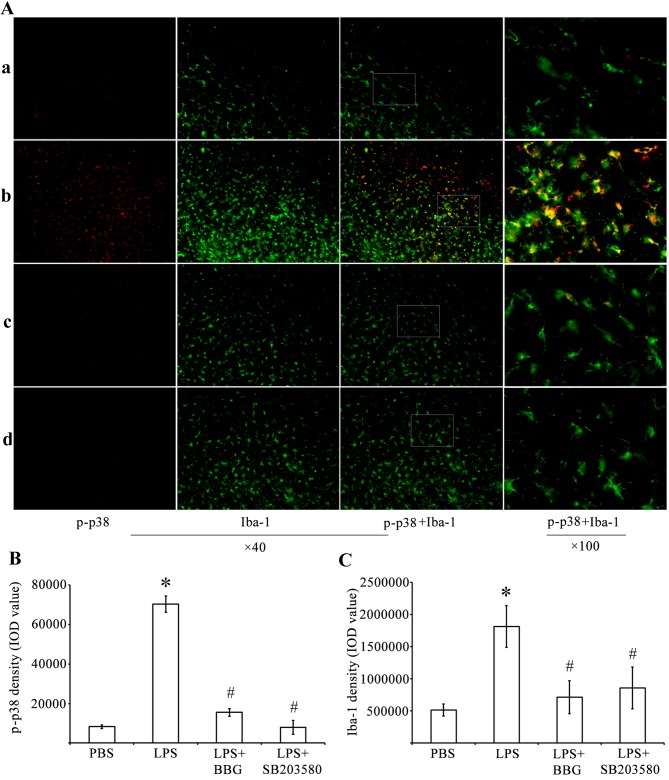
Immunohistochemistry and western blot analysis were performed to determine whether LPS treatment activated p38 MAPK. Compared with the control rats injected with PBS, the expression of p-p38 MAPK was increased in the substantia nigra of the experimental rats injected with LPS (Fig. 3). The immunoreactivity of p-p38 MAPK was decreased in the substantia nigra of BBG-treated and SB203580-treated rats, which were also injected with LPS. Double-labeling immunohistochemistry was performed using antibodies against p-p38 MAPK (Fig. 3A; red) and the microglial marker Iba-1 (Fig. 3A; green). The immunohistochemical analysis showed that, compared with the PBS-injected control animals, the activated microglia increased the expression levels of p-p38-MAPK in rats administered with LPS. Therefore, quantitative analysis of IOD values of p-p38 MAPK (Fig. 3B) was performed. The analysis revealed that the IOD for p-p38-ir was significantly increased by 845% in the substantia nigra of the LPS-treated rats, whereas the same parameter decreased by 77.9 and 88.7% in the BBG- and SB203580-treated LPS-induced rats, respectively (Fig. 3B; P<0.05). Compared with the controls, the density of Iba-1 in the substantia nigra of the experimental rats was increased by 3.62-fold 15 days post LPS injection (Fig. 3C; P<0.05). BBG and SB203580 were effective in reducing the microglial responses of the LPS-treated animals. Compared with the IOD of Iba-1 in the substantia nigra of the LPS-treated rats, the IOD of Iba-1 decreased by 60.6 and 52.8% in the BBG- and SB203580-treated LPS-injected rats, respectively (Fig. 3C; P<0.05).
Figure 3.
Double immunofluorescence labeling of p-p38 MAPK and Iba-1 in the SNpc of rats in the LPS model of Parkinson's disease. (A) Immunofluorescence for p-p38 MAPK (red) and Iba-1 (green) are shown for the (a) PBS, (b) LPS, (c) LPS+BBG and (d) LPS+SB203580 groups. Co-immunolabeled p-p38/Iba-1 microglia are a yellowish color. Compared with the PBS rats, LPS increased the expression of p-p38 MAPK. Quantitative analysis of IOD values of (B) p-p38 and (C) Iba-1 immunofluorescence in the SNpc of rats (n=6). *P<0.05, compared with the PBS control; #P<0.05, compared with the LPS group. MAPK, mitogen-activated protein kinase; LPS, lipopolysaccharide; PBS, phosphate-buffered saline; SNpc, substantia nigra pars compacta; Iba-1, ionized calcium binding adapter molecule 1; p-, phosphorylated; IOD, integrated optical density.
Similar results were obtained when western blot analysis was performed using antibodies against p-p38 MAPK (Fig. 4A). Quantification of the western blots revealed that, compared with the PBS-injected control group, the LPS-injected group showed a significant increase in the levels of p-p38 MAPK, but not p38 MAPK (Fig. 4B). In addition, BBG, the P2X7R antagonist, and SB203580, the p38 MAPK inhibitor, significantly reduced the activation of p38 MAPK in LPS-treated rats, as revealed by the quantification of p38 MAPK bands. This indicated that BBG and SB203580 offered protection against LPS-induced DA neuron death in the substantia nigra of the experimental rats.
Figure 4.
Effect of BBG treatment on p38 MAPK activation in substantia nigra in the LPS model of PD. (A) Representative western blot analysis of the expression of p-p38 and p38 MAPK in the substantia nigra of rats in the LPS model of PD. (B) Quantitative analysis of western blots (n=6) of the expression of p-p38 and p38 MAPK in the substantia nigra of rats in the PBS control group, LPS group, LPS+BBG and LPS+SB203580 groups. Protein bands were normalized to the expression levels of tubulin. *P<0.05, compared with the PBS control; #P<0.05, compared with the LPS group. PD Parkinson's disease; MAPK, mitogen-activated protein kinase; LPS, lipopolysaccharide; PBS, phosphate-buffered saline; BBG, brilliant blue G; SNpc, substantia nigra pars compacta; Iba-1, ionized calcium binding adapter molecule 1; p-, phosphorylated; IOD, integrated optical density.
Discussion
The chemical neuroanatomical analysis indicated that the expression of P2X7R was upregulated in the substantia nigra following LPS injection in experimental rats. When these LPS-injected rats were treated with the P2X7R antagonist, BBG, microglial activation was attenuated and a reduction in the loss of TH-ir DA neurons was observed in the substantia nigra. In addition, when the LPS-injected rats were treated with BBG, the activation of p38 MAPK was reversed, microglial activation was attenuated and a reduction in the loss of DA neurons was observed in the substantia nigra. Similarly, SB203580, the p38 MAPK antagonist, attenuated the activation of microglia, reduced the expression of P2X7R, and protected DA neurons from LPS-induced neuronal damage in the substantia nigra. These findings indicated that P2X7R activity, mediated by the p38 MAPK signaling pathway, contributed to the activation of microglia and the loss of DA neurons in LPS-injected rats. These data are consistent with the hypothesis that P2X7R contributes to the loss of DA neurons in the substantia nigra by activating microglia via the p38 MAPK pathway.
Previously, it has been shown that the expression and function of P2X7R are increased in patients with PD (19) and other neurodegenerative diseases (39). The gene expression of P2X7R has been found to be significantly upregulated in substantia nigra samples obtained from patients, who were clinically and neuropathologically diagnosed with idiopathic PD (19). In addition, genetic polymorphism of P2X7R can affect the occurrence and development of sporadic PD (40). Diseases, including amyotrophic lateral sclerosis (ALS) and MS, are inflammatory neurodegenerative disorders and, in tissue specimens of patients with MS and ALS specimens, significantly higher densities of P2X7R-ir microglial cells/macrophages have been found in the affected regions of the brain (20). Another previous study provided evidence that increased expression and function of P2X7R are associated with the microglia of patients with AD, and indicates that P2X7R is important in mediating microglial purinergic inflammatory responses in AD brains (39). The levels of P2X7R have been reported to be higher in the brains of HD mice, with P2X7R antagonism attenuating neuronal apoptosis (22). In addition, P2X7R antagonists ameliorated the motor performance of mice with experimental ALS (20). In the present study, it was found that P2X7R was upregulated in the substantia nigra microglia of rats in the LPS model of PD. In addition, BBG, a P2X7R antagonist, protected DA neurons from LPS-induced damage. Therefore, the expression of P2X7R in microglia appeared to be a critical factor in mediating microglial activity and stimulating the loss of DA neurons in PD. These observations are consistent with the suggestion that P2X7R is critical in neuroinflammation, which is observed during the pathogenesis of a variety of neurodegenerative diseases (14,17,18,20–23).
The mechanism through which P2X7R is upregulated in neurodegenerative diseases remains to be fully elucidated, however, it may be closely associated with the role of microglia in disease progression. P2X7R triggers the maturation and release of the IL-1β inflammatory cytokine from microglia (10). IL-1β is a crucial mediator in the pathogenesis of inflammatory diseases of the CNS. Among the rats included in the LPS model of PD, the LPS injection triggered inflammatory mechanisms, which caused the degeneration of DA neurons in the substantia nigra (29,33). LPS is as a potent stimulator of glial cells, particularly microglia, and has been a useful tool for modeling inflammation-mediated neurodegeneration of DA in rats in an LPS model of PD (30). At the molecular level, LPS requires activated Toll-like receptor 4 (TLR4) to induce its neurodegenerative effect, however, it also requires activated microglia (41). A previous study found that the production of microglial-derived IL-1β occurs through the following mechanism: The activation of multiple TLR isoforms (TLR2, TLR3 and TLR4) in the nervous system elevates the levels of extracellular ATP and subsequently activates P2X7R (42).
According to a previous in vivo study, when LPS injection was administered into the striatum, it markedly increased the expression of P2X7R in microglia, whereas the inhibition of P2X7R increased neuronal survival in the striatum (43). In addition, LPS stimulated the cultured human microglia to enhance the cellular expression of several proinflammatory factors, including cyclooxygenase-2, IL-1β, IL-6, IL-12 and TNF-α, which are inhibited by P2X7R antagonists (43). The double-labeling experiments in the present study showed an upregulation of P2X7R in activated IBA-1-ir microglial cells. BBG treatment provided protection to DA neurons and reduced the activation of microglia. This indicated that BBG exerted its neuroprotective effect by suppressing the activation of microglia and inhibiting the expression of P2X7R in activated microglia. These findings suggested that P2X7R enhances its neuroinflammatory nigral processes by activating microglia.
In the present study, it was also found that BBG prevented the LPS-induced loss of DA neurons in the substantia nigra. This finding supports an earlier study, which reported that P2X7R antagonists significantly prevent 6-hydroxydopamine-induced depletion of striatal DA stores (23,24). However, other studies have reported that P2X7R deficiency or inhibition is not effective against 1-methyl-4-phenylpyridinium or rotenone-induced DA loss in chemical PD models (25). The discrepancies in different PD models are attributed to the extent of the substantia nigra lesion induced in different paradigms and/or the duration of treatment with the P2X7R antagonist.
A previous study reported that P2X7R mediates the phosphorylation of p38 MAPK during the progression of subarachnoid hemorrhage (44). The significant activation of p38 MAPK has also been observed in the substantia nigra of other PD models (45), and p38 MAPK inhibitors have provided significant neuroprotection (28,45). Although LPS activates all the three major MAPK pathways (46), the p38 MAPK pathway appears to the most closely associated with the LPS-induced upregulation of inflammatory mediators (47). The p38 MAPK signaling pathway inhibitor, SB203580, downregulates the expression of pro-inflammatory mediators, including TNF-α and IL-1β (46). In glial cells, p38 MAPK induces NO synthase to stimulate the production of NO, which underlies the LPS-induced death of mesencephalic neurons (28). In the present study, it was found that LPS induced an increase in the levels of p-p38 MAPK. In addition, SB203580, the selective inhibitor of p38 MAPK, prevented the LPS-induced loss of DA neurons in the substantia nigra of experimental rats. BBG, a specific P2X7R antagonist, almost completely inhibited p38 MAPK activation. Therefore, the inhibition of P2X7R in the LPS-injected rats was neuroprotective as it reduced p38 MAPK activation and the loss of DA neurons.
Several studies have suggested that P2X7R is present in striatal DA terminals (23) and astroglial cells (48), indicating that P2X7R-mediated neurotoxicity is linked to microglial activation. Other studies have reported that microglia are a crucial contributing factor, which governs the ability of P2X7R in controlling neurotoxicity (23,24,42,43). In the present study, it was found that P2X7R was upregulated in microglial cells following LPS-induced microgliosis; however, BBG attenuated microgliosis. These findings support the hypothesis that the localization of P2X7R on microglia is linked to its ability to control the function of microglial cells.
In conclusion, the present study showed that the increased expression of p38 MAPK and P2X7R in the substantia nigra of rats caused the LPS-induced death of DA neurons. The results provided evidence that the inhibition of P2X7R by BBG reduced LPS-induced degeneration of DA neurons. Furthermore, there was a reduction in the regional activation of microglia, which express P2X7R protein. The interaction between P2X7R and the p38 MAPK signaling pathway may have contributed to the loss of DA neurons in the substantia nigra of the experimental PD rats. The results of the present study suggested that substantia nigra DA neurons were protected from neurodegeneration when P2X7R activity was inhibited in activated microglial cells. These findings may be exploited for developing neuroprotective therapies, which can be used in the treatment of various neurodegenerative diseases.
Acknowledgements
This study was funded by the China National Nature Science Fund (grant no. 81371421) and the Foundation of the Liaoning Educational Committee (grant nos. L202013136 and L2010560).
References
- 1.Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson's disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S210–S212. doi: 10.1016/S1353-8020(11)70065-7. [DOI] [PubMed] [Google Scholar]
- 2.Appel SH. Inflammation in Parkinson's disease: Cause or consequence? Mov Disord. 2012;27:1075–1077. doi: 10.1002/mds.25111. [DOI] [PubMed] [Google Scholar]
- 3.Qian L, Flood PM, Hong JS. Neuroinflammation is a key player in Parkinson's disease and a prime target for therapy. J Neural Transm (Vienna) 2010;117:971–979. doi: 10.1007/s00702-010-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ouchi Y, Yagi S, Yokokura M, Sakamoto M. Neuroinflammation in the living brain of Parkinson's disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S200–S204. doi: 10.1016/S1353-8020(09)70814-4. [DOI] [PubMed] [Google Scholar]
- 5.Anderson KM, Olson KE, Estes KA, Flanagan K, Gendelman HE, Mosley RL. Dual destructive and protective roles of adaptive immunity in neurodegenerative disorders. Transl Neurodegener. 2014;3:25. doi: 10.1186/2047-9158-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: Relevance to Parkinson's disease. J Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 7.Politis M, Su P, Piccini P. Imaging of microglia in patients with neurodegenerative disorders. Front Pharmacol. 2012;3:96. doi: 10.3389/fphar.2012.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaur C, Hao AJ, Wu CH, Ling EA. Origin of microglia. Microsc Res Tech. 2001;54:2–9. doi: 10.1002/jemt.1114. [DOI] [PubMed] [Google Scholar]
- 9.Monif M, Reid CA, Powell KL, Smart ML, Williams DA. The P2X7 receptor drives microglial activation and proliferation: A trophic role for P2X7R pore. J Neurosci. 2009;29:3781–3791. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: A key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kataoka A, Tozaki-Saitoh H, Koga Y, Tsuda M, Inoue K. Activation of P2X7 receptors induces CCL3 production in microglial cells through transcription factor NFAT. J Neurochem. 2009;108:115–125. doi: 10.1111/j.1471-4159.2008.05744.x. [DOI] [PubMed] [Google Scholar]
- 13.Gendron FP, Chalimoniuk M, Strosznajder J, Shen S, González FA, Weisman GA, Sun GY. P2X7 nucleotide receptor activation enhances IFN gamma-induced type II nitric oxide synthase activity in BV-2 microglial cells. J Neurochem. 2003;87:344–352. doi: 10.1046/j.1471-4159.2003.01995.x. [DOI] [PubMed] [Google Scholar]
- 14.Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- 15.Bartlett R, Yerbury JJ, Sluyter R. P2X7 receptor activation induces reactive oxygen species formation and cell death in murine EOC13 microglia. Mediators Inflamm. 2013;2013:271813. doi: 10.1155/2013/271813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monif M, Burnstock G, Williams DA. Microglia: Proliferation and activation driven by the P2X7 receptor. Int J Biochem Cell Biol. 2010;42:1753–1756. doi: 10.1016/j.biocel.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Skaper SD, Facci L, Culbert AA, Evans NA, Chessell I, Davis JB, Richardson JC. P2X(7) receptors on microglial cells mediate injury to cortical neurons in vitro. Glia. 2006;54:234–242. doi: 10.1002/glia.20379. [DOI] [PubMed] [Google Scholar]
- 18.Sperlágh B, Illes P. P2X7 receptor: An emerging target in central nervous system diseases. Trends Pharmacol Sci. 2014;35:537–547. doi: 10.1016/j.tips.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Durrenberger PF, Grünblatt E, Fernando FS, Monoranu CM, Evans J, Riederer P, Reynolds R, Dexter DT. Inflammatory pathways in Parkinson's Disease; A BNE microarray study. Parkinson's Dis. 2012;2012:214714. doi: 10.1155/2012/214714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryu JK, McLarnon JG. Block of purinergic P2X(7) receptor is neuroprotective in an animal model of Alzheimer's disease. Neuroreport. 2008;19:1715–1719. doi: 10.1097/WNR.0b013e3283179333. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Hernández M, Díez-Zaera M, Sánchez-Nogueiro J, Gómez-Villafuertes R, Canals JM, Alberch J, Miras-Portugal MT, Lucas JJ. Altered P2X7-receptor level and function in mouse models of Huntington's disease and therapeutic efficacy of antagonist administration. FASEB J. 2009;23:1893–1906. doi: 10.1096/fj.08-122275. [DOI] [PubMed] [Google Scholar]
- 23.Carmo MR, Menezes AP, Nunes AC, Pliássova A, Rolo AP, Palmeira CM, Cunha RA, Canas PM, Andrade GM. The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology. 2014;81:142–152. doi: 10.1016/j.neuropharm.2014.01.045. [DOI] [PubMed] [Google Scholar]
- 24.Marcellino D, Suárez-Boomgaard D, Sánchez-Reina MD, Aguirre JA, Yoshitake T, Yoshitake S, Hagman B, Kehr J, Agnati LF, Fuxe K, Rivera A. On the role of P2X(7) receptors in dopamine nerve cell degeneration in a rat model of Parkinson's disease: Studies with the P2X(7) receptor antagonist A-438079. J Neural Transm (Vienna) 2010;117:681–687. doi: 10.1007/s00702-010-0400-0. [DOI] [PubMed] [Google Scholar]
- 25.Hracskó Z, Baranyi M, Csölle C, Gölöncsér F, Madarász E, Kittel A, Sperlágh B. Lack of neuroprotection in the absence of P2X7 receptors in toxin-induced animal models of Parkinson's disease. Mol Neurodegener. 2011;6:28. doi: 10.1186/1750-1326-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenertz LY, Gavala ML, Zhu Y, Bertics PJ. Transcriptional control mechanisms associated with the nucleotide receptor P2X7, a critical regulator of immunologic, osteogenic, and neurologic functions. Immunol Res. 2011;50:22–38. doi: 10.1007/s12026-011-8203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeohn GH, Cooper CL, Wilson B, Chang RC, Jang KJ, Kim HC, Liu B, Hong JS. p38 MAP kinase is involved in lipopolysaccharide-induced dopaminergic neuronal cell death in rat mesencephalic neuron-glia cultures. Ann N Y Acad Sci. 2002;962:332–346. doi: 10.1111/j.1749-6632.2002.tb04078.x. [DOI] [PubMed] [Google Scholar]
- 29.Tufekci KU, Genc S, Genc K. The endotoxin-induced neuroinflammation model of Parkinson's disease. Parkinson's Dis. 2011;2011:487450. doi: 10.4061/2011/487450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutta G, Zhang P, Liu B. The lipopolysaccharide Parkinson's disease animal model: Mechanistic studies and drug discovery. Fundam Clin Pharmacol. 2008;22:453–464. doi: 10.1111/j.1472-8206.2008.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- 32.Sui Y, Stanić D, Tomas D, Jarrott B, Horne MK. Meloxicam reduces lipopolysaccharide-induced degeneration of dopaminergic neurons in the rat substantia nigra pars compacta. Neurosci Lett. 2009;460:121–125. doi: 10.1016/j.neulet.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 33.Herrera AJ, Castaño A, Venero JL, Cano J, Machado A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol Dis. 2000;7:429–447. doi: 10.1006/nbdi.2000.0289. [DOI] [PubMed] [Google Scholar]
- 34.Gourine AV, Poputnikov DM, Zhernosek N, Melenchuk EV, Gerstberger R, Spyer KM, Gourine VN. P2 receptor blockade attenuates fever and cytokine responses induced by lipopolysaccharide in rats. Br J Pharmacol. 2005;146:139–145. doi: 10.1038/sj.bjp.0706287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choe ES, McGinty JF. N-Methyl-D-aspartate receptors and p38 mitogen-activated protein kinase are required for cAMP-dependent cyclase response element binding protein and Elk-1 phosphorylation in the striatum. Neuroscience. 2000;101:607–617. doi: 10.1016/S0306-4522(00)00379-1. [DOI] [PubMed] [Google Scholar]
- 36.Zhu P, Zhan L, Zhu T, Liang D, Hu J, Sun W, Hou Q, Zhou H, Wu B, Wang Y, Xu E. The roles of p38 MAPK/MSK1 signaling pathway in the neuroprotection of hypoxic postconditioning against transient global cerebral ischemia in adult rats. Mol Neurobiol. 2014;49:1338–1349. doi: 10.1007/s12035-013-8611-7. [DOI] [PubMed] [Google Scholar]
- 37.Stanic D, Finkelstein DI, Bourke DW, Drago J, Horne MK. Timecourse of striatal re-innervation following lesions of dopaminergic SNpc neurons of the rat. Eur J Neurosci. 2003;18:1175–1188. doi: 10.1046/j.1460-9568.2003.02800.x. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The Rat Brain: In Stereotaxic Coordinates. Academic Press; Incorporated: 1998. [Google Scholar]
- 39.McLarnon JG, Ryu JK, Walker DG, Choi HB. Upregulated expression of purinergic P2X(7) receptor in Alzheimer disease and amyloid-beta peptide-treated microglia and in peptide-injected rat hippocampus. J Neuropathol Exp Neurol. 2006;65:1090–1097. doi: 10.1097/01.jnen.0000240470.97295.d3. [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Han X, Li Y, Zou H, Xie A. Association of P2X7 receptor gene polymorphisms with sporadic Parkinson's disease in a Han Chinese population. Neurosci Lett. 2013;546:42–45. doi: 10.1016/j.neulet.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 41.Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Facci L, Barbierato M, Marinelli C, Argentini C, Skaper SD, Giusti P. Toll-like receptors 2, −3 and −4 prime microglia but not astrocytes across central nervous system regions for ATP-dependent interleukin-1β release. Sci Rep. 2014;4:6824. doi: 10.1038/srep06824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi HB, Ryu JK, Kim SU, McLarnon JG. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci. 2007;27:4957–4968. doi: 10.1523/JNEUROSCI.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen S, Ma Q, Krafft PR, Chen Y, Tang J, Zhang J, Zhang JH. P2X7 receptor antagonism inhibits p38 mitogen-activated protein kinase activation and ameliorates neuronal apoptosis after subarachnoid hemorrhage in rats. Crit Care Med. 2013;41:e466–e474. doi: 10.1097/CCM.0b013e31829a8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu F, Wang Z, Gu JH, Ge JB, Liang ZQ, Qin ZH. p38(MAPK)/p53-Mediated Bax induction contributes to neurons degeneration in rotenone-induced cellular and rat models of Parkinson's disease. Neurochem Int. 2013;63:133–140. doi: 10.1016/j.neuint.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Pawate S, Shen Q, Fan F, Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77:540–551. doi: 10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- 47.Krishna M, Narang H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol Life Sci. 2008;65:3525–3544. doi: 10.1007/s00018-008-8170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franke H, Verkhratsky A, Burnstock G, Illes P. Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 2012;8:629–657. doi: 10.1007/s11302-012-9300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]