Abstract
Mutations in epidermal growth factor receptor (EGFR) play critical roles in the pathogenesis of non-small cell lung cancer (NSCLC), and they are highly associated with sensitivity to tyrosine kinase inhibitors (TKIs). While the pathogenic and pharmacological characteristics of common mutations in EGFR have been thoroughly investigated, those of uncommon mutations remain to be elucidated. Traditional approaches to study common mutations by randomized controlled trials are not feasible for uncommon mutations owing to their rarity. Therefore, by systematically reviewing laboratory and clinical studies of the G719X mutation, one of the uncommon mutations, we concluded that the G719X mutation was intermediately sensitive to TKIs, with an average response rate of 35.1% (47/134). Moreover, accordingly, we proposed a comprehensive model to investigate uncommon mutations in EGFR. The model involves both basic and clinical components, composed of structural analyses, functional alterations, cell viabilities and animal models with various types of clinical studies. In this review, we systematically reviewed studies of the G719X mutation and put forward a research model that could be generalized to explore uncommon mutations in diseases associated with gene mutations.
Keywords: epidermal growth factor receptor, non-small cell lung cancer, uncommon mutations, G719X mutation, tyrosine kinase inhibitor, tyrosine kinase inhibitor sensitivity, targeted therapy, methodology
1. Introduction
Lung cancer is the leading cause of death among all cancer deaths (1). It has the highest morbidity and mortality among all malignancies worldwide (1,2). NSCLC accounts for 70–85% of all lung cancers, and most cases are of advanced stage or metastatic condition when diagnosed (3,4). As a targeted therapy, EGFR tyrosine kinase inhibitors (EGFR-TKIs) have been approved by the FDA for the treatment of advanced NSCLC since 2003 (5). EGFR-TKIs have produced encouraging results by postponing tumor progression and prolonging the progression-free survival (PFS) of advanced NSCLC patients for approximately 5 months compared to platinum-based doublet chemotherapy (6,7).
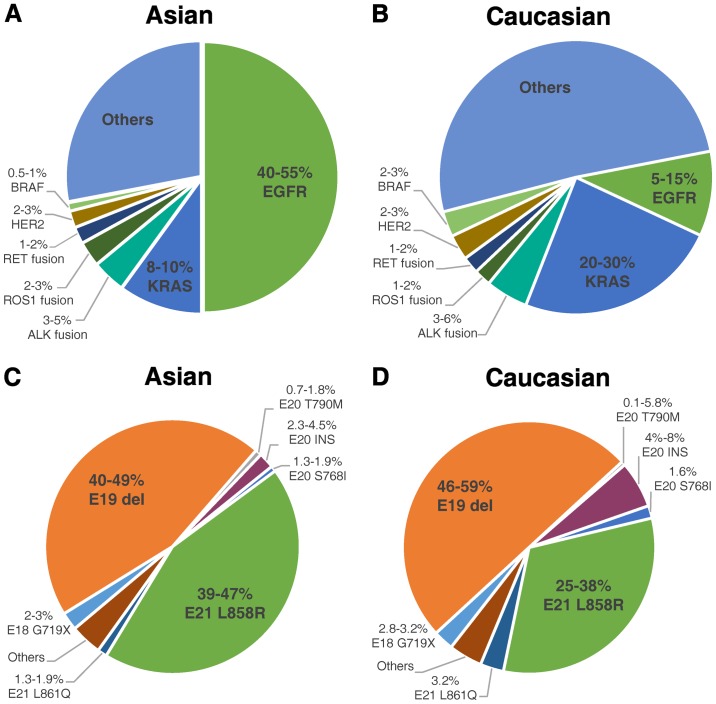
However, only 15% of NSCLC patients responded to TKI (8), and clinical trials on gefitinib or erlotinib failed in unselected patient populations, as they were not able to significantly prolong patient overall survival (OS) compared to traditional chemotherapy (9–11). Based on known EGFR mutations, researchers eventually discovered the association between TKI sensitivity and EGFR mutations (12). Moreover, they also observed considerable ethnic differences in the frequencies of EGFR mutations in NSCLC patients. EGFR mutations were detected in approximately 50% of Asian patients with NSCLC, but only in 10% of patients in the western world (13–15) (Fig. 1). Two types of EGFR mutations, in-frame deletions in exon 19 (Del19) and point mutations in exon 21 causing a leucine-to-arginine substitution at codon 858 (L858R), which are established to be definitely sensitive to TKIs, comprise approximately 90% of all EGFR mutations (15–18). The remaining 10% of EGFR mutations are defined as uncommon mutations (Fig. 1). Therefore, for NSCLC patients with uncommon EGFR mutations of unknown clinical significance, it is dubious whether they can benefit from TKI targeted therapy.
Figure 1.
Mutation frequency and distribution in Asian and Caucasian populations. (A and B) Frequencies of various driver mutations in NSCLC patients of Asian and Caucasian populations; the data are referred from Kohno 2015 (14). (C and D) Distribution of different mutations among EGFR mutations in NSCLC patients of Asian and Caucasian populations; the data were generated by summarizing the results from previous studies (14–22).
To answer this important question, there is an urgent need to determine the clinical significance of uncommon mutations in EGFR, particularly their sensitivity to TKIs. Although they only account for a small proportion of patients with EGFR mutations, they are still a large population due to the high incidence of NSCLC.
It seems apparent that we could try to pattern the methodologies of the Del19 and L858R mutations, which have been proven to be sensitive, mostly by means of clinical randomized controlled trials (RCTs) with large sample sizes. However, this is not a practical approach for uncommon mutations, as cases of uncommon mutations are too rare to conduct RCTs. Therefore, we have to take advantage of basic studies or other types of clinical studies. In other words, the ultimate problem is how we determine the sensitivity of uncommon EGFR mutations. Then, we can make clinical decisions regarding whether to apply TKI targeted therapy to NSCLC patients with certain uncommon mutations.
The G719X mutation in EGFR refers to point mutations that result in substitutions of the glycine at position 719 to other residues, primarily alanine (G719A), cysteine (G719C) and serine (G719S). The G719X mutation accounts for approximately 3% among all EGFR mutations in both Asian and Caucasian populations (14–22) (Fig. 1). It is the most commonly seen and most thoroughly studied EGFR uncommon mutation, and it is considered a sensitive mutation.
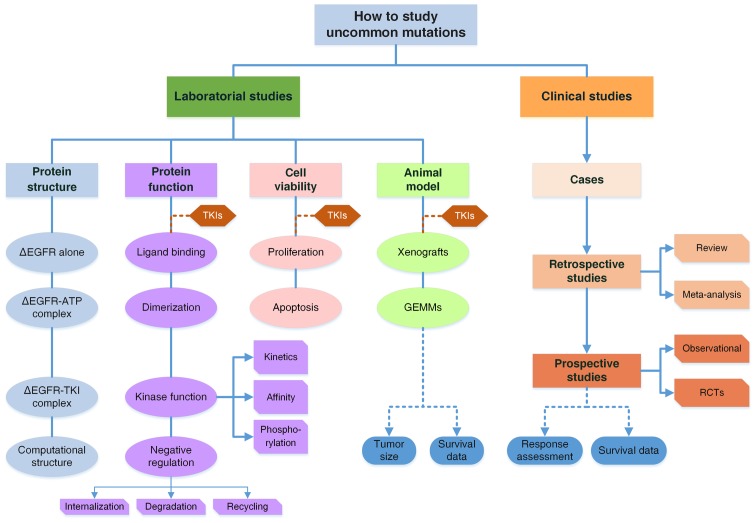
In this study, by reviewing studies of the G719X mutation, we propose a comprehensive research model to explore the laboratory and clinical characteristics of uncommon mutations (Fig. 3). We also systematically summarized the studies of the G719X mutation and discovered the missing components to form a complete research system. The conclusions regarding the G719X mutation would be much more convincing if the evidence was complete. More importantly, the research model can be generalized to direct researchers to explore other uncommon mutations in patients with diseases associated with gene mutations and to ascertain their pharmacologic properties efficiently.
Figure 3.
A comprehensive model to study uncommon mutations in EGFR. The system is comprised of both basic and clinical studies. Clinical studies include three parts: Case reports and series; Retrospective studies are reviews and meta-analyses to combine the data of RR and survival of patients with the G719X mutation; Prospective studies of observational ones or randomized controlled trials. There are two major issues in clinical studies: response to TKIs and survival data of patients. Laboratory studies are organized from mutant protein structures to functional changes, cell viability and animal models. Structural analyses by crystal diffraction or computational simulation determine the structures of mutant EGFR, EGFR-ATP complexes and EGFR-TKI complexes, to elucidate functional changes. Functional studies are categorized into four parts from the perspective of EGFR activation, including ligand biding, dimerization, kinase activity and downregulation, while the latter two are further subdivided as illustrated. For cell viability, the two aspects of proliferation and apoptosis are considered. Animal models involve GEMMs and tumor-cell inoculated xenografts including PDX models, which are discussed in terms of tumor sizes and animal survival. TKI treatment is introduced into all experiments on three levels. With basic and clinical studies integrated together, a complete evidence system is formed to draw conclusions regarding the pathogenic and pharmacological properties of uncommon mutations with high reliability. ∆EGFR, mutant EGFR.
2. Current studies of the G719X mutation in EGFR in NSCLC
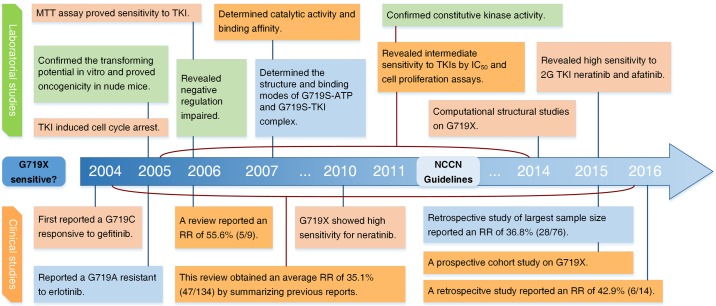
The first observation of the G719X mutation in EGFR in NSCLC patients was reported by Lynch et al in 2004 (23). The patient harbored a G719C mutation and presented with partial response to gefitinib, with an OS of 17.9 months. Based on studies conducted over the following two years, the NCCN guidelines for NSCLC (version 2.2011) described the G719X mutation in EGFR as associated with response to TKIs. This conclusion was supported by subsequent investigations in general. Herein, the studies of the G719X mutation are reviewed comprehensively from both clinical and laboratory perspectives. The history of studies of the G719X mutation in EGFR is presented in Fig. 2.
Figure 2.
The history of studies of G719X mutation in EGFR. 2G TKI, second generation of tyrosine kinase inhibitor; RR, response rate; wt, wild-type EGFR. Green, oncogenicity; red, TKI sensitive; orange, TKI intermediately sensitive; blue, TKI resistant.
Clinical studies of the G719X mutation in NSCLC
Case reports and retrospective studies
Since Lynch reported the first case (23), more and more cases have been reported either in the form of case reports or retrospective studies. However, most of them involved no more than ten patients. Only one retrospective study by Chiu et al (42) in 2015 enrolled a relatively large sample size of 76 patients with the G719X mutation, of which 28 responded to TKIs, indicating a response rate (RR) of 36.8%. To overcome the limitation of sample size, we summarized all of these studies and combined the results to obtain an average RR. We enrolled 22 relative studies from 2004 to 2016 and excluded all reviews to avoid possible data overlap (18,23–43). Then, we had a total of 134 G719X patients, of which 47 patients responded to 1st generation EGFR-TKIs (Table I). The average RR is 35.1% (47/134), indicating that G719X is a mutation of intermediate sensitivity, which is in accordance with previous reviews (16,44–46) (Table II).
Table I.
Summary of studies of G719X responses to 1G-TKIs.a
| Study | Mutation | TKI | Total | Response | RR/% | Sensitivity | Ref. |
|---|---|---|---|---|---|---|---|
| Lynch 2004 | G719C | G | 1 | 1 | 100 | Sensitive | 23 |
| Han 2005 | G719A | G | 2 | 1 | 50 | Intermediate | 24 |
| Takano 2005 | G719X | G | 2 | 1 | 50 | Intermediate | 25 |
| Eberhard 2005 | G719A | E | 1 | 0 | 0 | Resistant | 26 |
| Janne 2006 | G719C | G | 1 | 1 | 100 | Sensitive | 27 |
| Ichihara 2007 | G719X | G | 1 | 0 | 0 | Resistant | 28 |
| Pallis 2007 | G719D | G | 1 | 0 | 0 | Resistant | 29 |
| Sequist 2008 | G719A | G | 1 | 0 | 0 | Resistant | 30 |
| Wu 2008 | G719A | E/G | 2 | 1 | 50 | Intermediate | 31 |
| Wu 2011 | G719X | E/G | 8 | 4 | 50 | Intermediate | 32 |
| De Pas 2011 | G719S | E | 1 | 1 | 100 | Sensitive | 33 |
| Takahashi 2012 | G719A | G | 1 | 0 | 0 | Resistant | 34 |
| Lee 2013 | G719A | E | 1 | 0 | 0 | Resistant | 35 |
| Umekawa 2013 | G719A | E | 1 | 0 | 0 | Resistant | 36 |
| Locatelli-Sanchez 2013 | G719A | E/G | 1 | 1 | 0 | Resistant | 37 |
| Keam 2014 | G719A | G | 1 | 0 | 0 | Resistant | 38 |
| Beau-Faller 2014 | G719X | E/G | 10 | 1 | 10 | Resistant | 18 |
| Guan 2014 | G719A | E | 1 | 0 | 0 | Resistant | 39 |
| Watanabe 2014 | G719X | G | 3 | 0 | 0 | Resistant | 40 |
| Fukihara 2014 | G719A | E/G | 4 | 1 | 25 | Intermediate | 41 |
| Chiu 2015 | G719X | E/G | 76 | 28 | 36.8 | Intermediate | 42 |
| Xu 2016 | G719X | E/G/I | 14 | 6 | 42.9 | Intermediate | 43 |
| Total | 134 | 47 | 35.1 | Intermediate |
1G TKIs, 1st generation EGFR-TKIs mainly refers to gefitinib, erlotinib and icotinib. Data were extracted from corresponding studies. G, gefitinib; E, erlotinib; I, icotinib; RR, response rate. Sensitivity cut-off values: ≥0 RR<25%, resistant; ≥25% RR <75%, intermediately sensitive; ≥75% RR ≤100%, sensitive.
Table II.
Summary of clinical studies of the G719X mutation in EGFR.
| Research type | Article | Mutation | TKI | Total cases | Response | RR/% | Sensitivity | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Retrospective studies | |||||||||
| Summary of cases | This article | G719X | E/G | 134 | 47 | 35.1% | Intermediate | Table I | |
| Reviews | Mistudomi 2006 and 2007 | G719X | E/G | 9 | 5 | 55.6 | Intermediate | 44,45 | |
| Kobayashi 2015 | G719X | G | 3 | 2 | 66.7 | Intermediate | 16 | ||
| Klughammer 2016 | G719X | E | 2 | 1 | 50 | Intermediate | 46 | ||
| Meta-analysis | Not found | ||||||||
| Prospective studies | |||||||||
| Observational | Arrieta 2015 | G719X | E/G/A | 11 | NAa | NA | Not known | 47 | |
| Clinical trials | Not found |
The response rate of the G719X mutation were not discussed separately. G, gefitinib; E, erlotinib; A, Afatinib; RR, response rate; NA, not accessible. Sensitivity cut-off values: ≥0 RR <25%, resistant; ≥25% RR <75%, intermediately sensitive; ≥75% RR ≤100%, sensitive.
Reviews and meta-analyses
Four reviews concerned responses of G719X to TKIs, with response rates of 50–66.7% (16,44–46) (Table II). Still, in these reviews, the numbers of cases were too small to be convincing. No meta-analyses were found.
Prospective studies
Due to the rarity of uncommon mutations, we could not enroll enough patients to conduct a prospective randomized controlled trial. Observational studies are probably a feasible way to investigate the sensitivity of uncommon mutations in a prospective manner. Arrieta et al analyzed 188 NSCLC patients in their cohorts and found 11 patients with the G719X mutation who received TKIs, including a single G719X mutation and complex mutations. Although G719X was not discussed separately, they found the rare mutation group to be intermediately sensitive with an RR of 32.4% (47).
Summary of clinical studies
All clinical studies enrolled are summarized in Table II. As stated above, because of limitations in sample size, it is not adequately convincing to determine the sensitivity of the G719X mutation based only on clinical studies. Given the circumstances, it is necessary to seek supporting evidence from laboratory studies. With both clinical and basic studies to form a complete evidence system and logic network, we could have sufficient cause to consider G719X a sensitive mutation.
Laboratory studies of the G719X mutation in EGFR in NSCLC
In general, the laboratory studies mainly focused on alterations caused by the G719X mutation, regarding the protein structure, protein function, cell viability and animal experiments. Thus, the laboratory studies were reviewed in these four perspectives.
Functional alterations
The activation of EGFR is initiated after binding to its ligand, epidermal growth factor (EGF) or transforming growth factor-α (TGF-α). The receptor changed its conformation and then dimerized with another ligand-bound EGFR or other ErbB family members to form homodimers or heterodimers, respectively. The dimer harbored kinase activity and would phosphorylate itself at specific sites (48,49), which could act as catalytic sites to activate downstream signaling pathways, such as MAPK or PI3K/Akt, by phosphorylation of the corresponding molecules. Afterwards, the activated EGFRs were internalized into the cell plasma by endocytosis, and then they were either recycled onto the cell membrane or degraded by fusion with lysosomes (50). This is one way of negative regulation in EGFR signaling pathway. A series of studies revealed the influences of the G719X mutation and TKI treatment on all of the functional processes.
Ligand binding and dimerization
Choi et al explored how the G719S mutation affected ligand binding using a 125I-labelled EGF binding assay. Moreover, they also used antibodies against the EGFR extracellular region to label EGFR, and they observed dimerization of receptors with immunofluorescence microscopy. No differences were discovered in ligand binding or dimerization between G719S mutants and wild-type (wt), while they indeed observed EGF-independent dimerization of EGFRs in Del19 and L858R mutants (51).
Kinase activity
Greulich et al systematically investigated the kinase activity of G719S mutants (52). Using immunoblotting, they discovered the ligand-independent constitutive phosphorylation activity on both the receptor itself and on downstream signal molecules, such as Shc, STAT3 and Akt (52). Chen and Choi further compared the auto-phosphorylation levels of G719S with that of Del19 and L858R. They found that the auto-phosphorylation level of G719S was lower, indicating that the oncogenicity of G719S was weaker than that of the other two common mutants (51,53). Subsequent studies further confirmed the conclusions using western blotting and immunofluorescence staining (51,53–57).
As the transforming potential of G719X was determined, the question arose as to what extent EGFR-TKI can inhibit the uncontrolled kinase activity of G719X mutant EGFR. Jiang et al investigated gefitinib in their kinase assay of G719X and found that gefitinib was able to inhibit the auto-phosphorylation of G719S in a dose-dependent manner. However, compared with L858R, G719S required a higher concentration of gefitinib (54). Their conclusions were further validated by subsequent studies using other techniques (53,57–59). In general, based on the studies mentioned above, G719X was found to have moderate oncogenicity and intermediate sensitivity to TKIs regarding kinase function.
Kinetics and binding affinity
Why would the G719X mutation cause weaker oncogenicity and lower sensitivity to TKIs than L858R? Is it because the mutation affected the catalytic properties of the kinase and the interactions between EGFR and TKIs? As TKIs are competitive inhibitors of ATP, studies comparing the binding affinities of TKI-EGFR complexes with ATP-EGFR complexes may answer these questions.
Yun et al investigated the kinetics and affinities of G719X mutants using a continuous colorimetric assay and a fluorescence-quenching assay. The mutation in the tyrosine kinase domain was found to dramatically elevate catalytic activity by approximately 50-fold in L858R and 10-fold in G719S compared to wild-type (60). In terms of affinities, they determined the dissociation constants (Kd) of EGFR-gefitinib complexes and EGFR-AMPPNP (an analogue of ATP) complexes. Although the G719S mutation decreased the affinity to gefitinib, it lowered the affinity to ATP much more, indicating that the inhibiting potential (KdTKI/KmATP) of gefitinib was 5-fold stronger than wt, while L858R was approximately 100 times stronger (60). This might explain why G719S mutants were less sensitive to gefitinib than L858R mutants.
Negative regulation
There are mainly two ways to negatively regulate the activated wild-type EGFR after the activated receptor has done its job to trigger the downstream signaling. Once internalized by endocytosis, the receptors would either be recycled back to the cell membrane or be degraded by fusion with lysosomes mediated by ubiquitination (50,53). Along with persistent positive activation, impaired downregulation might take part in the oncogenicity of G719X mutants as well.
Few studies focused on negative regulation
Chen et al discovered that the G719S mutants were refractory to ubiquitination and had more sustained tyrosine phosphorylation than wild-type (53). A similar phenomenon was observed in Del19 and L858R mutants (61). Furthermore, internalization was also found to be impaired in Del19 mutants (61).
Structure determination
The determination of kinetic and binding parameters provided clues to help us understand the mechanisms of the pathogenic and pharmacological effects of the mutation. Nonetheless, to further elucidate why the mutation affects oncogenicity and sensitivity requires determination of the structures of mutated EGFRs and EGFR-TKI complexes.
Yun et al determined the 3D structures of the G719S-AMPPNP complex by crystal diffraction (60). The comparison of the structures between G719S and wild-type EGFR indicated that the G719S mutation destabilized the inactive conformation and thus promoted the active conformation of the kinase (60). This explained the oncogenic potential of G719S mutants on the basis of structures.
The inactive conformation of EGFR requires the C-helix to be rotated outward to be displaced from the active site. The glycine residue at position 719 interacts with several hydrophobic residues flanking L858, including F723, L747 and L862. Packing together with the N-terminal portion of the activation loop, the hydrophobic cluster changed into a helical turn, causing the C-helix to rotate outward by steric hindrance. Therefore, any substitution of a glycine residue at position 719 would sabotage the stable hydrophobic interactions, which are essential for the receptor to adopt the inactive conformation (60).
Furthermore, they also elucidated the binding mode of the G719S-gefitinib complex. Unfortunately, they were unable to resolve the differences in the binding modes among G719S-gefitinib, L858R-gefitinib and wt-gefitinib complexes, thus failing to explain the different binding affinities of various EGFRs and the distinct sensitivities to TKIs (61).
Another structural study of G719X was performed by Doss et al in 2014, with computational structure simulation, a new approach to study the structures of proteins. They simulated the real-time conformational alterations in G719S mutants to discover that the G719S mutation increased the distance between residues L718 and G796, forming a wider opening for TKIs to get into the ATP-binding pocket than wild-type, indicating that this mutation should respond to TKIs (62). However, they did not include Del19 or L858R mutants in their study.
Cell viability
Constitutive kinase activity of G719X mutants will persistently activate downstream signaling, resulting in EGFR-signaling-pathway-mediated cell proliferation in a ligand-independent manner. Will we observe uncontrolled cell proliferation when the G719X mutation is introduced into certain cell lines? And to what extent can TKI inhibit cell proliferation?
Elevated cell viability in G719S transformed NIH-3T3 and Ba/F3 cell lines were observed in several studies, but it was lower than that of Del19 and L858R mutants (51,52,56). Moreover, it can be abrogated by gefitinib, yet is somehow more resistant than L858R (52). To be more quantitative, the 50% inhibiting concentration (IC50) of gefitinib in various mutants was further measured in series of studies. The IC50 of gefitinib in G719S mutants was between that of gefitinib in wild-type cells and Del19/L858R mutants, implying an intermediate sensitivity of G719S (16,52,54,55,57,63).
The inhibition of cell viability by TKIs was observed, but the mechanisms for the abrogation remained to be elucidated. Jiang et al focused on the impact on the cell cycle of the mutant cells resulting from TKI treatment. Using FACS and immunoblotting, they found that gefitinib induced cell cycle arrest in the G1 phase by downregulating the level of D-type cyclins and CDK4 in G719S mutant Ba/F3 cell lines (54). However, they detected no apoptotic cells in G719S mutants (54), while apoptosis induced by gefitinib was observed in L858R mutants (48,64).
In conclusion, TKIs inhibit the proliferation of transformed cells to various extents in different mutants. The transformed cells with the G719S mutation exhibited intermediate sensitivity to TKI inhibition compared with Del19 and L858R mutants.
Animal models
The cell culture experiments are still too far from the authentic situation, thus it is necessary to investigate the role of the G719X mutation in animal models and to determine whether TKI treatment would be effective in animals with tumors driven by the G719X mutation.
Greulich et al injected the G719S transformed cells into immuno-compromised mice and observed tumorigenesis (52). Moreover, tumor size varied among the different mutation groups. The average diameter of tumors in the G719S group was half of that in the L858R group (52). They confirmed the oncogenic potential of the G719S mutation in an animal model; however, their experiment might be more complete if they took the survival condition of the inoculated animals and TKI treatment into account.
Summary of basic studies
The laboratory studies of G719X are summarized in Table III, with a brief overview of the results and the corresponding methods of the experiments. The high level of consistency in the results of the laboratory studies including protein function experiments, cell viability experiments and animal experiments further authenticate the oncogenicity and sensitivity of the G719X mutation. However, we still need a better understanding of the structure leading to intermediate sensitivity to further complete this research system.
Table III.
Summary of laboratory studies of the G719X mutation in EGFR.
| Research type | Conclusion | Main method | Ref. |
|---|---|---|---|
| Protein structure | Elucidated the mechanism of constitutive kinase activity of G719S. | Crystal diffraction | 60 |
| Determined the binding mode of TKI-G719S complex: same as wt and L858R. | Crystal diffraction | 60 | |
| Computational structural studies revealed that G719 caused TKI to move closer to the binding site and TKI easier to get into the ATP-binding pocket. | Molecular dynamic simulation | 62 | |
| Protein function | |||
| Ligand binding and dimerization | Basically the same between G719S and wt, while EGF-independent dimerization found in Del19 and L858R. | 125I-labelled EGF binding assay | 51 |
| Kinetics | Compare the catalytic activity among EGFRs: L858R>G719S>wt. | Continuous colorimetric assay and fluorescence-quenching assay | 60 |
| Affinity | Compare the binding affinity for TKI versus ATP to EGFRs: L858R>G719S>wt. | Continuous colorimetric assay | 60 |
| Kinase activity | Confirmed constitutive kinase activity in autophosphorylation and downstream signaling: Wt<G719X<L858R/Del19. | Immunoblotting | 52,54,55 |
| Western blotting | 51,53,56 | ||
| Immunofluorescence staining | 57 | ||
| TKI inhibition | Determined IC50 of TKI able to block constitutive kinase activity of mutants: wt>G719X>L858R/Del19 | Immunoblotting | 54 |
| Western blotting | 53 | ||
| BRET assay | 58 | ||
| Continuous colorimetric assay | 59 | ||
| YFP-EGFR-ICD relocation assay | 57 | ||
| Negative regulation | Negative regulation of kinase activity is impaired in G719S. | Western blotting | 53 |
| Cell proliferation | Confirmed the transforming potential of G719S mutants. | Colony formation assay | 52 |
| 3H thymidine incorporation assay | 51 | ||
| eGFP+ cell FACS | 56 | ||
| G719X transformed cells showed intermediate sensitivity to TKI. | Colony formation assay | 52,56 | |
| Cell viability assay by trypan/MTT/MTS staining | 54,55,56,63 | ||
| Colorimetric assay | 16 | ||
| G719X transformed cells show strong sensitivity to TKI. | Cell viability assay by MTT staining | 53 | |
| TKI induced cell cycle arrest in G719S transformed cells. | FACS and immunoblotting | 54 | |
| Animal model | Injection of G719S transformed cells cause tumorigenesis. | Nude mice injection | 52 |
wt, wild-type.
3. A comprehensive model for studying uncommon mutations
Based on the reviewed studies of the G719X mutation in EGFR in NSCLC, we propose a systematic model to investigate the pathogenic and pharmacological characteristics of an uncommon mutation at both the laboratory and clinical levels (Fig. 3). Due to the small number of patients with uncommon mutations, it is hardly possible to enroll enough patients to carry out RCTs, which would provide the most convincing evidence for the clinical features of a particular uncommon mutation. For this reason, we need a comprehensive experimental system.
The laboratory experiments are comprised of studies of protein structure, studies of functional alterations, cell viability assays and animal experiments, providing an understanding of the features of the mutations from the level of molecules to cells and then to animal models, microcosmically to macroscopically. In terms of clinical studies, although it is not feasible to perform RCTs, prospective observational studies might be a possible way to enroll more patients. On the other hand, summarizing cases retrospectively enables us to obtain the average response rates and survival data from a relatively large sample. The results would be more reliable if we enrolled adequate studies of low heterogeneity to conduct meta-analyses.
This model was organized logically. Structure determination elucidates the mechanisms of the functional alterations and pharmacologic effects of TKIs, which are further verified in cells and animals, and even in patients in clinical settings, thus providing sufficient evidence to determine the oncogenicity and sensitivity to TKI of uncommon mutations in EGFR in NSCLC.
4. Discussion
Basic studies of G719X are far from enough
Laboratory studies of G719X have included almost all processes in the EGFR signaling pathway; however, they are still far from being complete or satisfactory.
Dimerization of G719S differs from that of Del19 and L858R mutants in terms of the dependence on its ligand EGF, indicating a lower activating level of G719S. This may explain its lower oncogenicity. However, it remains to be investigated whether mutations alter the mode of dimerization and how TKI will affect ligand binding and dimerization.
Regarding negative regulation, the effects of TKI on impaired negative regulation were not explored. If TKI treatment could reverse the impairment of negative regulation by the G719S mutation, this could be another explanation of the sensitivity to TKIs. However, we found no studies regarding the internalization or recycling of G719S mutated EGFR. In addition, the negative regulation of a pathogenic mutation might be a new target for drug development.
For structural studies, apart from real 3D structure determination by crystal diffraction, computational structure studies are another more economical and efficient way to study the structures of proteins and the effects of mutations on protein structure. Computational structure studies can be used to determine the structures of mutants using real-time computation and can simulate the conformational alterations caused by specific mutations without having to purify the mutated proteins and obtain crystals. However, neither type of study explained the lower sensitivity of G719X compared to Del19 and L858R mutants.
The structural analyses by either crystal diffraction or computational structural studies provide a structural basis for oncogenicity and sensitivity to TKIs. Moreover, fully understanding the structures of mutants and the binding features of drug-mutant complexes will help us to predict the sensitivity of a mutation to a particular potential targeted drug, and our ultimate purpose is to design a tailored targeted drug for people with specific gene mutation-related diseases using computational biology and bioinformatics. These are important goals of precision medicine.
Cell viabilities are determined by both cell proliferation and apoptosis. Tumorigenesis and response to TKIs could also be studied according to these two measures. Proliferation mainly refers to experiments concerning the cell cycle, and apoptosis is basically about the caspase-mediated apoptotic pathway. The studies of proliferation are relatively clear; however, few studies focused on apoptosis in G719X mutants. The L858R mutation was found to enable cells to escape from apoptosis (48), yet no similar studies on G719X were found. Furthermore, G719X and L858R mutants showed distinct responses to TKIs in terms of apoptosis (48,54,64), providing a possible mechanism for the difference in their sensitivities. Unlike common mutations, there are no cell lines bearing a single G719X mutation that are established for research on cell viability, while plenty of cell lines harboring Del19 or L858R mutations are available to be used directly, such as H3255 and PC-9 (65–68). This might be the reason for the scarcity of relative studies of the G719X mutation concerning cell viability. Establishing cell lines harboring uncommon mutations would provide a foundation for studying these mutations.
For xenograft-type models, apart from these transformed cell-inoculated animals, the patient-derived xenograft (PDX) model, also known as ‘Mouse Avatars’, is a new type of animal model used to study neoplasms (69). The PDX model has demonstrated substantial clinical potential in predicting drug sensitivity, including sensitivity to TKI targeted therapies. As the tumor sample is obtained from the actual patient, it is the closest representation of the individual's authentic situation.
Another type of animal model is genetically engineered mouse model (GEMM). Distinct from xenografts, they are generated using genetic engineering techniques to harbor specific mutations for driver mutations. To the best of our knowledge, there are no GEMMs for the G719X mutation in EGFR available for basic or clinical studies. Gazdar et al discussed the comparison between these two types (70).
Is G719X a TKI-sensitive mutation
The NCCN guidelines for NSCLC first mentioned G719X as significantly associated with response to TKIs in 2011 (version 2.2011) and then referred to G719X as a drug-sensitive mutation in 2012 (version 2.2012), mainly based on laboratory studies of Greulich et al (52) and clinical studies of Lynch et al and Han et al (23,24), which each reported a single case of G719X with partial response to gefitinib. The newest NCCN guidelines for NSCLC (version 4.2016) (71) remained almost the same. In a word, the G719X mutation in EGFR is basically considered to be sensitive according to the NCCN guidelines.
Nevertheless, based on subsequent clinical studies involving more cases and a series of laboratory experiments, we have drawn the conclusion that it would be more accurate to define the G719X mutation as intermediately sensitive to first-generation TKIs. The high heterogeneity of NSCLC might explain the ambiguity of the G719X mutation in terms of sensitivity to TKIs.
Moreover, we still need to integrate some deeper and broader studies of G719X into the model to form a complete evidence system in order to be more confident about its sensitivity. Based on the research model, the following issues remain to be investigated. In the laboratory, the conformational differences between complexes of TKIs with various mutants requires more analysis to explain the differences in sensitivity between mutants on the basis of 3D structure. The influences of the G719X mutation and TKI treatment on negative regulation are far from clear. In terms of cell viability, more studies are required to determine whether G719X mutants could escape from apoptosis such as L858R mutants. In terms of animal experiments, we still need more data regarding tumor sizes and survival data from TKI treatment on animals with tumors driven by the G719X mutation or in PDX models from patients with the G719X mutation. Still, there is an urgent need to establish cell lines harboring the G719X mutation. At the clinical level, the major problem and difficulty lies in the sample sizes of the corresponding studies. It would be feasible to systematically review the clinical data in large-scale trial projects to retrieve information about responses and survival of patients with the G719X mutation (72). Based on these data, we could further conduct a meta-analysis on the sensitivity of the G719X mutation.
Complex mutations
Another interesting phenomenon to be noted is that G719X often occurs along with other mutations in EGFR, especially with S768I and L861Q with frequencies of 24.5 and 8.2% in all G719X mutations (15). They were also found to co-exist with mutations in other genes, for instance KRAS, BRAF and PIK3CA (15). It is referred to as complex mutation. Other uncommon mutations also tend to occur in the form of complex mutations. A possible explanation is that these uncommon mutations harbor inadequate tumor-driving ability and, therefore, must co-occur with another mutation to initiate tumorigenesis. Compared with the G719X single mutation, complex mutations are scarcer and their sensitivities to TKI are more obscure. Our research model provides practical approaches for studying these complex mutations.
Second generation EGFR-TKIs and G719X
New generations of EGFR-TKIs are developed to improve selectivity and efficacy and, thus, to reduce toxicity. First generation-TKIs (1G-TKIs) refer to reversible TKIs such as gefitinib, erlotinib and icotinib, while afatinib, dacomtinib and neratinib are categorized as irreversible 2G-TKIs (73,74). 3G-TKIs, including AZD-9291 (Osimertinib) and CO-1686 (75), are highly specific irreversible inhibitors for mutated receptors only, which are primarily used to target the secondary resistant mutation T790M (75,76). The G719X mutation showed intermediate sensitivity to 1G-TKIs; however, it was notable that 2G-TKIs demonstrated markedly high efficacy in patients with the G719X mutation. Although neratinib showed barely satisfactory efficacy in treating NSCLC patients in its phase 2 clinical trial (77), it exhibited great potential in patients with the G719X mutation. Three out of four patients with the G719X mutation exhibited a partial response with the tumor shrinking more than 50% in diameter, and one exhibited stable disease, indicating an RR of 75% and a DCR (disease control rate) of 100% (77). In addition, Yang et al reviewed patients with the G719X mutation who were treated with afatinib in the LUX-LUNG trial series and found that the overall response rate was 77.8% (14/18) (78). In laboratory studies, the G719A mutation exhibited higher sensitivity than Del19 in terms of both kinase activity and cell viability (16).
Although 2G-TKIs are not widely used clinically due to their high toxicity, some studies did explore their potential in treating patients with mutations in exon 18, especially the G719X mutation. These findings also shed light on TKI selection in patients with uncommon mutations. Although some types of TKIs failed to treat NSCLC patients with common sensitive mutations, they might have great potential against uncommon mutations.
Innovations and limitations
We systematically reviewed studies of G719X from both laboratory and clinical settings, and we sorted out the history of these studies. The basic studies are summarized regarding conclusions and corresponding experimental techniques. In terms of the clinical studies, we summarized 22 studies (18,23–43) investigating the response of the G719X mutation and determined an average response rate based on a relatively larger sample size. To the best of our knowledge, this is one of the most comprehensive systematic reviews of the G719X mutation in EGFR in NSCLC. Furthermore, the comprehensive research model we proposed provides researchers with a practical approach to determine the clinical significance of uncommon mutations of NSCLC or other diseases associated with gene mutation.
Nevertheless, there are some limitations of this review. Although we have included considerable studies of G719X in our review, it is inevitable that we may still have omitted some important studies. Moreover, due to the heterogeneity of the studies that we enrolled to calculate the average response rate, there might be a bias in the result. The heterogeneities of the studies mainly consist of demographic features, stage and histological classification of the tumor, different treatment lines and inconsistency in the criteria for response assessment. Additionally, the exclusion of 3 studies of which the original data were inaccessible in our calculation might also cause bias (47,79,80). Although we combined several studies to increase the sample size, it was still far from enough to draw a convincing conclusion on the sensitivity. By properly combining the response and survival data extracted from several large-scale trial projects, we can obtain more accurate and persuasive results.
Acknowledgements
We would like to thank Jie Zhou, Dongdong Wang, Jun Chen, Yuan Xu and Chao Guo from Peking Union Medical College Hospital for their generous help. We would also like to express our gratitude to American Journal Experts for their language assistance.
Glossary
Abbreviations
- Del19
in-frame deletions in exon 19 of EGFR
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- G719X
point mutations that result in substitutions of the glycine at position 719 to other residues
- L858R
point mutations that result in substitutions of the leucine at position 858 to arginine
- NSCLC
non-small cell lung cancer
- RCT
randomized controlled trial
- RR
response rate
- TKI
tyrosine kinase inhibitor
- wt
wild-type
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- 4.Parums DV. Current status of targeted therapy in non-small cell lung cancer. Drugs Today (Barc) 2014;50:503–525. doi: 10.1358/dot.2014.50.07.2185913. [DOI] [PubMed] [Google Scholar]
- 5.Herbst RS, Fukuoka M, Baselga J. Gefitinib - a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4:956–965. doi: 10.1038/nrc1506. [DOI] [PubMed] [Google Scholar]
- 6.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, et al. North-East Japan Study Group: Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 7.Lee CK, Wu YL, Ding PN, Lord SJ, Inoue A, Zhou C, Mitsudomi T, Rosell R, Pavlakis N, Links M, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: A meta-analysis. J Clin Oncol. 2015;33:1958–1965. doi: 10.1200/JCO.2014.58.1736. [DOI] [PubMed] [Google Scholar]
- 8.Miller VA, Kris MG, Shah N, Patel J, Azzoli C, Gomez J, Krug LM, Pao W, Rizvi N, Pizzo B, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:1103–1109. doi: 10.1200/JCO.2004.08.158. [DOI] [PubMed] [Google Scholar]
- 9.Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, Shinkai T, Negoro S, Imamura F, Eguchi K, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol. 2008;26:4244–4252. doi: 10.1200/JCO.2007.15.0185. [DOI] [PubMed] [Google Scholar]
- 10.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 11.Lee CK, Brown C, Gralla RJ, Hirsh V, Thongprasert S, Tsai CM, Tan EH, Ho JC, Chu T, Zaatar A, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: A meta-analysis. J Natl Cancer Inst. 2013;105:595–605. doi: 10.1093/jnci/djt072. [DOI] [PubMed] [Google Scholar]
- 12.Kris MG. How today's developments in the treatment of non-small cell lung cancer will change tomorrow's standards of care. Oncologist. 2005;10(Suppl 2):23–29. doi: 10.1634/theoncologist.10-90002-23. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch FR, Bunn PA., Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol. 2009;10:432–433. doi: 10.1016/S1470-2045(09)70110-X. [DOI] [PubMed] [Google Scholar]
- 14.Kohno T, Nakaoku T, Tsuta K, Tsuchihara K, Matsumoto S, Yoh K, Goto K. Beyond ALK-RET ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res. 2015;4:156–164. doi: 10.3978/j.issn.2218-6751.2014.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Li L, Zhu Y, Huang C, Qin Y, Liu H, Ren-Heidenreich L, Shi B, Ren H, Chu X, et al. Coexistence of EGFR with KRAS or BRAF or PIK 3CA somatic mutations in lung cancer: A comprehensive mutation profiling from 5125 Chinese cohorts. Br J Cancer. 2014;110:2812–2820. doi: 10.1038/bjc.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi Y, Togashi Y, Yatabe Y, Mizuuchi H, Jangchul P, Kondo C, Shimoji M, Sato K, Suda K, Tomizawa K, et al. EGFR exon 18 mutations in lung cancer: Molecular predictors of augmented sensitivity to afatinib or neratinib as compared with first- or third-generation TKIs. Clin Cancer Res. 2015;21:5305–5313. doi: 10.1158/1078-0432.CCR-15-1046. [DOI] [PubMed] [Google Scholar]
- 17.Han B, Tjulandin S, Hagiwara K, Normanno N, Wulandari L, Konstantinovich L Konstantin, Hudoyo A, Ratcliffe M, McCormack R, Reck M. Determining the prevalence of EGFR mutations in Asian and Russian patients (PTS) with advanced non-small-cell lung cancer (aNSCLC) of adenocarcinoma (ADC) and non-ADC histology: Ignite study. Ann Oncol. 2015;26(Suppl 1):i29–i30. doi: 10.1093/annonc/mdv050.01. [DOI] [Google Scholar]
- 18.Beau-Faller M, Prim N, Ruppert AM, Nanni-Metéllus I, Lacave R, Lacroix L, Escande F, Lizard S, Pretet JL, Rouquette I, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: A multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol. 2014;25:126–131. doi: 10.1093/annonc/mdt418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An SJ, Chen ZH, Su J, Zhang XC, Zhong WZ, Yang JJ, Zhou Q, Yang XN, Huang L, Guan JL, et al. Identification of enriched driver gene alterations in subgroups of non-small cell lung cancer patients based on histology and smoking status. PLoS One. 2012;7:e40109. doi: 10.1371/journal.pone.0040109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gahr S, Stoehr R, Geissinger E, Ficker JH, Brueckl WM, Gschwendtner A, Gattenloehner S, Fuchs FS, Schulz C, Rieker RJ, et al. EGFR mutational status in a large series of Caucasian European NSCLC patients: Data from daily practice. Br J Cancer. 2013;109:1821–1828. doi: 10.1038/bjc.2013.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G, Yang PC. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9:154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skov BG, Høgdall E, Clementsen P, Krasnik M, Larsen KR, Sørensen JB, Skov T, Mellemgaard A. The prevalence of EGFR mutations in non-small cell lung cancer in an unselected Caucasian population. APMIS. 2015;123:108–115. doi: 10.1111/apm.12328. [DOI] [PubMed] [Google Scholar]
- 23.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 24.Han SW, Kim TY, Hwang PG, Jeong S, Kim J, Choi IS, Oh DY, Kim JH, Kim DW, Chung DH, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005;23:2493–2501. doi: 10.1200/JCO.2005.01.388. [DOI] [PubMed] [Google Scholar]
- 25.Takano T, Ohe Y, Sakamoto H, Tsuta K, Matsuno Y, Tateishi U, Yamamoto S, Nokihara H, Yamamoto N, Sekine I, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:6829–6837. doi: 10.1200/jco.2005.23.16_suppl.7032. [DOI] [PubMed] [Google Scholar]
- 26.Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, Ince WL, Jänne PA, Januario T, Johnson DH, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 27.Jänne PA, Borras AM, Kuang Y, Rogers AM, Joshi VA, Liyanage H, Lindeman N, Lee JC, Halmos B, Maher EA, et al. A rapid and sensitive enzymatic method for epidermal growth factor receptor mutation screening. Clin Cancer Res. 2006;12:751–758. doi: 10.1158/1078-0432.CCR-05-2047. [DOI] [PubMed] [Google Scholar]
- 28.Ichihara S, Toyooka S, Fujiwara Y, Hotta K, Shigematsu H, Tokumo M, Soh J, Asano H, Ichimura K, Aoe K, et al. The impact of epidermal growth factor receptor gene status on gefitinib-treated Japanese patients with non-small-cell lung cancer. Int J Cancer. 2007;120:1239–1247. doi: 10.1002/ijc.22513. [DOI] [PubMed] [Google Scholar]
- 29.Pallis AG, Voutsina A, Kalikaki A, Souglakos J, Briasoulis E, Murray S, Koutsopoulos A, Tripaki M, Stathopoulos E, Mavroudis D, et al. ‘Classical’ but not ‘other’ mutations of EGFR kinase domain are associated with clinical outcome in gefitinib-treated patients with non-small cell lung cancer. Br J Cancer. 2007;97:1560–1566. doi: 10.1038/sj.bjc.6604068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sequist LV, Martins RG, Spigel D, Grunberg SM, Spira A, Jänne PA, Joshi VA, McCollum D, Evans TL, Muzikansky A, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–2449. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 31.Wu SG, Gow CH, Yu CJ, Chang YL, Yang CH, Hsu YC, Shih JY, Lee YC, Yang PC. Frequent epidermal growth factor receptor gene mutations in malignant pleural effusion of lung adenocarcinoma. Eur Respir J. 2008;32:924–930. doi: 10.1183/09031936.00167407. [DOI] [PubMed] [Google Scholar]
- 32.Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY, Yang PC. Effectiveness of tyrosine kinase inhibitors on ‘uncommon’ epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17:3812–3821. doi: 10.1158/1078-0432.CCR-10-3408. [DOI] [PubMed] [Google Scholar]
- 33.De Pas T, Toffalorio F, Manzotti M, Fumagalli C, Spitaleri G, Catania C, Delmonte A, Giovannini M, Spaggiari L, De Braud F, et al. Activity of epidermal growth factor receptor-tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring rare epidermal growth factor receptor mutations. J Thorac Oncol. 2011;6:1895–1901. doi: 10.1097/JTO.0b013e318227e8c6. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi R, Hirata H, Tachibana I, Shimosegawa E, Inoue A, Nagatomo I, Takeda Y, Kida H, Goya S, Kijima T, et al. Early [18F]fluorodeoxyglucose positron emission tomography at two days of gefitinib treatment predicts clinical outcome in patients with adenocarcinoma of the lung. Clin Cancer Res. 2012;18:220–228. doi: 10.1158/1078-0432.CCR-11-0868. [DOI] [PubMed] [Google Scholar]
- 35.Lee JK, Shin JY, Kim S, Lee S, Park C, Kim JY, Koh Y, Keam B, Min HS, Kim TM, et al. Primary resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with non-small-cell lung cancer harboring TKI-sensitive EGFR mutations: An exploratory study. Ann Oncol. 2013;24:2080–2087. doi: 10.1093/annonc/mdt127. [DOI] [PubMed] [Google Scholar]
- 36.Umekawa K, Kimura T, Kudoh S, Suzumura T, Oka T, Nagata M, Mitsuoka S, Matsuura K, Nakai T, Yoshimura N, et al. Plasma RANTES IL-10, and IL-8 levels in non-small-cell lung cancer patients treated with EGFR-TKIs. BMC Res Notes. 2013;6:139. doi: 10.1186/1756-0500-6-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Locatelli-Sanchez M, Couraud S, Arpin D, Riou R, Bringuier PP, Souquet PJ. Routine EGFR molecular analysis in non-small-cell lung cancer patients is feasible: Exons 18–21 sequencing results of 753 patients and subsequent clinical outcomes. Lung. 2013;191:491–499. doi: 10.1007/s00408-013-9482-4. [DOI] [PubMed] [Google Scholar]
- 38.Keam B, Kim DW, Park JH, Lee JO, Kim TM, Lee SH, Chung DH, Heo DS. Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non-small cell lung cancer. Int J Clin Oncol. 2014;19:594–600. doi: 10.1007/s10147-013-0602-1. [DOI] [PubMed] [Google Scholar]
- 39.Guan Y, Zhao H, Meng J, Yan X, Jiao S. Dramatic response to high-dose icotinib in a lung adenocarcinoma patient after erlotinib failure. Lung Cancer. 2014;83:305–307. doi: 10.1016/j.lungcan.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe S, Minegishi Y, Yoshizawa H, Maemondo M, Inoue A, Sugawara S, Isobe H, Harada M, Ishii Y, Gemma A, et al. Effectiveness of gefitinib against non-small-cell lung cancer with the uncommon EGFR mutations G719X and L861Q. J Thorac Oncol. 2014;9:189–194. doi: 10.1097/JTO.0000000000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukihara J, Watanabe N, Taniguchi H, Kondoh Y, Kimura T, Kataoka K, Matsuda T, Yokoyama T, Hasegawa Y. Clinical predictors of response to EGFR tyrosine kinase inhibitors in patients with EGFR-mutant non-small cell lung cancer. Oncology. 2014;86:86–93. doi: 10.1159/000357129. [DOI] [PubMed] [Google Scholar]
- 42.Chiu CH, Yang CT, Shih JY, Huang MS, Su WC, Lai RS, Wang CC, Hsiao SH, Lin YC, Ho CL, et al. Epidermal growth factor receptor tyrosine kinase inhibitor treatment response in advanced lung adenocarcinomas with G719X/L861Q/S768I mutations. J Thorac Oncol. 2015;10:793–799. doi: 10.1097/JTO.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 43.Xu J, Jin B, Chu T, Dong X, Yang H, Zhang Y, Wu D, Lou Y, Zhang X, Wang H, et al. EGFR tyrosine kinase inhibitor (TKI) in patients with advanced non-small cell lung cancer (NSCLC) harboring uncommon EGFR mutations: A real-world study in China. Lung Cancer. 2016;96:87–92. doi: 10.1016/j.lungcan.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 44.Mitsudomi T, Kosaka T, Yatabe Y. Biological and clinical implications of EGFR mutations in lung cancer. Int J Clin Oncol. 2006;11:190–198. doi: 10.1007/s10147-006-0583-4. [DOI] [PubMed] [Google Scholar]
- 45.Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007;98:1817–1824. doi: 10.1111/j.1349-7006.2007.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klughammer B, Brugger W, Cappuzzo F, Ciuleanu T, Mok T, Reck M, Tan EH, Delmar P, Klingelschmitt G, Yin AY, et al. Examining treatment outcomes with erlotinib in patients with advanced non-small cell lung cancer whose tumors harbor uncommon EGFR mutations. J Thorac Oncol. 2016;11:545–555. doi: 10.1016/j.jtho.2015.12.107. [DOI] [PubMed] [Google Scholar]
- 47.Arrieta O, Cardona AF, Corrales L, Campos-Parra AD, Sánchez-Reyes R, Amieva-Rivera E, Rodríguez J, Vargas C, Carranza H, Otero J, et al. CLICaP: The impact of common and rare EGFR mutations in response to EGFR tyrosine kinase inhibitors and platinum-based chemotherapy in patients with non-small cell lung cancer. Lung Cancer. 2015;87:169–175. doi: 10.1016/j.lungcan.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 49.Hynes NE, Lane HA. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 50.Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol. 2005;1:2005.0010. doi: 10.1038/msb4100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi SH, Mendrola JM, Lemmon MA. EGF-independent activation of cell-surface EGF receptors harboring mutations found in gefitinib-sensitive lung cancer. Oncogene. 2007;26:1567–1576. doi: 10.1038/sj.onc.1209957. [DOI] [PubMed] [Google Scholar]
- 52.Greulich H, Chen TH, Feng W, Jänne PA, Alvarez JV, Zappaterra M, Bulmer SE, Frank DA, Hahn WC, Sellers WR, et al. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med. 2005;2:e313. doi: 10.1371/journal.pmed.0020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y-R, Fu Y-N, Lin C-H, Yang ST, Hu SF, Chen YT, Tsai SF, Huang SF. Distinctive activation patterns in constitutively active and gefitinib-sensitive EGFR mutants. Oncogene. 2006;25:1205–1215. doi: 10.1038/sj.onc.1209159. [DOI] [PubMed] [Google Scholar]
- 54.Jiang J, Greulich H, Jänne PA, Sellers WR, Meyerson M, Griffin JD. Epidermal growth factor-independent transformation of Ba/F3 cells with cancer-derived epidermal growth factor receptor mutants induces gefitinib-sensitive cell cycle progression. Cancer Res. 2005;65:8968–8974. doi: 10.1158/0008-5472.CAN-05-1829. [DOI] [PubMed] [Google Scholar]
- 55.Kancha RK, von Bubnoff N, Peschel C, Duyster J. Functional analysis of epidermal growth factor receptor (EGFR) mutations and potential implications for EGFR targeted therapy. Clin Cancer Res. 2009;15:460–467. doi: 10.1158/1078-0432.CCR-08-1757. [DOI] [PubMed] [Google Scholar]
- 56.Kancha RK, Peschel C, Duyster J. The epidermal growth factor receptor-L861Q mutation increases kinase activity without leading to enhanced sensitivity toward epidermal growth factor receptor kinase inhibitors. J Thorac Oncol. 2011;6:387–392. doi: 10.1097/JTO.0b013e3182021f3e. [DOI] [PubMed] [Google Scholar]
- 57.Furuyama K, Harada T, Iwama E, Shiraishi Y, Okamura K, Ijichi K, Fujii A, Ota K, Wang S, Li H, et al. Sensitivity and kinase activity of epidermal growth factor receptor (EGFR) exon 19 and others to EGFR-tyrosine kinase inhibitors. Cancer Sci. 2013;104:584–589. doi: 10.1111/cas.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schiffer HH, Reding EC, Fuhs SR, Lu Q, Piu F, Wong S, Littler PL, Weiner DM, Keefe W, Tan PK, et al. Pharmacology and signaling properties of epidermal growth factor receptor isoforms studied by bioluminescence resonance energy transfer. Mol Pharmacol. 2007;71:508–518. doi: 10.1124/mol.106.027656. [DOI] [PubMed] [Google Scholar]
- 59.Yoshikawa S, Kukimoto-Niino M, Parker L, Handa N, Terada T, Fujimoto T, Terazawa Y, Wakiyama M, Sato M, Sano S, et al. Structural basis for the altered drug sensitivities of non-small cell lung cancer-associated mutants of human epidermal growth factor receptor. Oncogene. 2013;32:27–38. doi: 10.1038/onc.2012.21. [DOI] [PubMed] [Google Scholar]
- 60.Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, Meyerson M, Eck MJ. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: Mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11:217–227. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shtiegman K, Kochupurakkal BS, Zwang Y, Pines G, Starr A, Vexler A, Citri A, Katz M, Lavi S, Ben-Basat Y, et al. Defective ubiquitinylation of EGFR mutants of lung cancer confers prolonged signaling. Oncogene. 2007;26:6968–6978. doi: 10.1038/sj.onc.1210503. [DOI] [PubMed] [Google Scholar]
- 62.Doss GP, Rajith B, Chakraborty C, NagaSundaram N, Ali SK, Zhu H. Structural signature of the G719S-T790M double mutation in the EGFR kinase domain and its response to inhibitors. Sci Rep. 2014;4:5868. doi: 10.1038/srep05868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuza Y, Glatt KA, Jiang J, Greulich H, Minami Y, Woo MS, Shimamura T, Shapiro G, Lee JC, Ji H, et al. Allele-dependent variation in the relative cellular potency of distinct EGFR inhibitors. Cancer Biol Ther. 2007;6:661–667. doi: 10.4161/cbt.6.5.4003. [DOI] [PubMed] [Google Scholar]
- 64.Tracy S, Mukohara T, Hansen M, Meyerson M, Johnson BE, Jänne PA. Gefitinib induces apoptosis in the EGFRL858R non-small-cell lung cancer cell line H3255. Cancer Res. 2004;64:7241–7244. doi: 10.1158/0008-5472.CAN-04-1905. [DOI] [PubMed] [Google Scholar]
- 65.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 66.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 67.Helfrich BA, Raben D, Varella-Garcia M, Gustafson D, Chan DC, Bemis L, Coldren C, Barón A, Zeng C, Franklin WA, et al. Antitumor activity of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor gefitinib (ZD1839, Iressa) in non-small cell lung cancer cell lines correlates with gene copy number and EGFR mutations but not EGFR protein levels. Clin Cancer Res. 2006;12:7117–7125. doi: 10.1158/1078-0432.CCR-06-0760. [DOI] [PubMed] [Google Scholar]
- 68.Sharma SV, Gajowniczek P, Way IP, Lee DY, Jiang J, Yuza Y, Classon M, Haber DA, Settleman J. A common signaling cascade may underlie ‘addiction’ to the Src, BCR-ABL, and EGF receptor oncogenes. Cancer Cell. 2006;10:425–435. doi: 10.1016/j.ccr.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malaney P, Nicosia SV, Davé V. One mouse, one patient paradigm: New avatars of personalized cancer therapy. Cancer Lett. 2014;344:1–12. doi: 10.1016/j.canlet.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gazdar AF, Hirsch FR, Minna JD. From mice to men and back: An assessment of preclinical model systems for the study of lung cancers. J Thorac Oncol. 2016;11:287–299. doi: 10.1016/j.jtho.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.National Comprehensive Cancer Network, corp-author. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Non-Small Cell Lung Cancer (Version 4. 2016) 2016 2016 Jul 5; [Google Scholar]
- 72.Sebastian M, Schmittel A, Reck M. First-line treatment of EGFR-mutated nonsmall cell lung cancer: Critical review on study methodology. Eur Respir Rev. 2014;23:92–105. doi: 10.1183/09059180.00008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Subramaniam D, He AR, Hwang J, Deeken J, Pishvaian M, Hartley ML, Marshall JL. Irreversible multitargeted ErbB family inhibitors for therapy of lung and breast cancer. Curr Cancer Drug Targets. 2015;14:775–793. doi: 10.2174/1568009614666141111104643. [DOI] [PubMed] [Google Scholar]
- 74.Stasi I, Cappuzzo F. Second generation tyrosine kinase inhibitors for the treatment of metastatic non-small-cell lung cancer. Transl Respir Med. 2014;2:2. doi: 10.1186/2213-0802-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cross DAE, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ, et al. AZD9291, an irreversible EGFR TKI overcomes T 790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jänne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 77.Sequist LV, Besse B, Lynch TJ, Miller VA, Wong KK, Gitlitz B, Eaton K, Zacharchuk C, Freyman A, Powell C, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: Results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:3076–3083. doi: 10.1200/JCO.2009.27.9414. [DOI] [PubMed] [Google Scholar]
- 78.Yang JC, Sequist LV, Geater SL, Tsai CM, Mok TS, Schuler M, Yamamoto N, Yu CJ, Ou SH, Zhou C, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: A combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16:830–838. doi: 10.1016/S1470-2045(15)00026-1. [DOI] [PubMed] [Google Scholar]
- 79.Mitsudomi T, Kosaka T, Endoh H, Horio Y, Hida T, Mori S, Hatooka S, Shinoda M, Takahashi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23:2513–2520. doi: 10.1200/JCO.2005.00.992. [DOI] [PubMed] [Google Scholar]
- 80.Baek JH, Sun JM, Min YJ, Cho EK, Cho BC, Kim JH, Ahn MJ, Park K. Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated non-small cell lung cancer except both exon 19 deletion and exon 21 L858R: A retrospective analysis in Korea. Lung Cancer. 2015;87:148–154. doi: 10.1016/j.lungcan.2014.11.013. [DOI] [PubMed] [Google Scholar]