Abstract
The β1-adrenergic receptor (AR) is the primary β-AR subtype in the heart and is the target of metoprolol (Met), which is commonly used to treat angina and hypertension. Previous studies have revealed a positive correlation between the methylation levels of the adrenoreceptor β1 gene (Adrb1) promoter in the myocardium with the antihypertensive activity of Met in spontaneously hypertensive rats (SHR), which affects β1-AR expression in H9C2 cells. The aim of the present study was to investigate the effects of myocardial β1-AR downregulation using short-hairpin RNA (shRNA) against Adrb1 on the antihypertensive activity of Met in SHR. Recombinant adeno-associated virus type 9 (rAAV9) vectors carrying Adrb1 shRNA (rAAV9-Adrb1) or a negative control sequence (rAAV9-NC) were generated and used to infect rat hearts via the pericardial cavity. The results of reverse transcription-quantitative polymerase chain reaction, immunohistochemistry and western blotting analyses demonstrated that cardiac β1-AR expression in the rAAV9-Adrb1 group was significantly downregulated when compared with the rAAV9-NC group (P<0.001, P<0.001 and P=0.032, respectively). In addition, a greater reduction in systolic blood pressure (SBP) was observed in the rAAV9-NC group compared with the rAAV9-Adrb1 group following Met treatment (P=0.035). Furthermore, downregulation of myocardial β1-AR was associated with a significant decrease in SBP (P<0.001). In conclusion, these data suggest that suppression of β1-AR expression in the myocardium reduces SBP and sensitivity to Met in SHR.
Keywords: β1 adrenergic receptor, myocardium, metoprolol, recombinant adeno-associated virus type 9 vector, blood pressure, spontaneously hypertensive rats
Introduction
Hypertension is a multifactorial disease that results from the combined effects of genetic, environmental and behavioral factors (1). Data obtained from the Chinese National Center for Cardiovascular Diseases (Ministry of Health, Beijing, China) indicate that approximately 18.8% of Chinese adults (aged ≥18 years) were diagnosed with hypertension in 2015 (2). In addition, data obtained from a suburban town of Shanghai demonstrated that the prevalence, awareness, treatment and control rates of hypertension in an elderly Chinese population (aged ≥60 years) from 2006 to 2008 were 59.4, 72.5, 65.8 and 24.4%, respectively (3). In addition, the Global Burden of Disease Study 2010 revealed that blood pressure is one of the leading risk factors for the global disease burden (4). In Africa, hypertension is the leading cause of heart failure (5). Globally, hypertension is responsible for >50% of mortalities due to stroke (5). Therefore, studies that aim to identify effective treatments for hypertension are important for the prevention of heart failure and stroke. β-blockers are commonly prescribed for the treatment of cardiovascular diseases, such as hypertension. The selective β-blocker, known as metoprolol (Met), is considered to be an effective therapy as it has been demonstrated to reduce mortality rates and adverse cardiac events among patients with coronary artery disease, heart failure and hypertension (6,7). However, only ~50% of patients with hypertension respond to β-blocker treatment (8).
In previous studies, polymorphisms in the adrenoreceptor β1 gene (Adrb1) and cytochrome P4502D6 (CYP2D6) genes are reportedly involved in the inter-individual differences in the response to Met treatment (9,10). Approximately 70–80% of Met is known to be metabolized by CYP2D6, and CYP2D6 polymorphisms have been demonstrated to affect the rate of Met metabolism (11). Previous studies investigating Adrb1 polymorphisms have focused primarily on the Ser49Gly and Arg389Gly polymorphisms. For instance, Wu et al (12) demonstrated that Chinese patients with essential hypertension that were homozygous for a mutant Adrb1 genotype (Arg389), exhibited an increased sensitivity to Met treatment. By contrast, an additional study demonstrated that subjects with the same CYP2D6 and Adrb1 genotypes exhibited varying responses to Met, which suggests that additional mechanisms responsible for the inter-individual differences in Met responses may exist (13).
The β1 adrenergic receptor (β1-AR) is the primary β-AR subtype in the heart and is the target of Met (14). A previous study conducted by our group demonstrated a correlation between reduced Adrb1 promoter methylation levels in the myocardium and enhanced Met-mediated antihypertensive activity in spontaneously hypertensive rats (SHR), and increased β1-AR expression in H9C2 cells (15). Thus, the authors hypothesized that the expression levels of myocardial β1-AR may affect the antihypertensive activity of Met, and may be responsible for the observed inter-individual variations in the response to Met treatment in patients with hypertension (16).
In the present study, a recombinant adeno-associated virus type 9 (rAAV9) vector containing a short-hairpin RNA (shRNA) sequence against Adrb1, was used to inhibit β1-AR expression in the myocardium, in order to investigate the effects of reduced cardiac β1-AR expression on the antihypertensive efficacy of Met in SHR. The data suggest a novel mechanism underlying the observed inter-individual differences in the response to Met treatment.
Materials and methods
Plasmids and cell culture
Plasmids containing shRNA sequences against rat β1-AR or non-targeting controls were purchased from Changsha Yingrun Biotechnology Co., Ltd. (Changsha, China). Adeno-associated virus (AAV) packaging mixtures were obtained from the Institute of Biomedicine and Biotechnology (Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China). The HEK293 human embryonic kidney cell line was obtained from Vector Gene Technology Company Ltd. (Beijing, China) and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS).
Preparation of rAAV9 vectors
rAAV9 vectors containing Adrb1 shRNA (rAAV9-Adrb1-shRNA-ZsGreen) or negative control (rAAV9-NC-ZsGreen) shRNA sequences were prepared in HEK293 cells as described previously (17,18). Briefly, polyethylenimine (1 µg/µl; Helixgen, Co., Ltd., Guangzhou, China) was added to the AAV Packaging Plasmid, target plasmid and Ad Helper (1.5 ml; 20 µg: 10 µg: 30 µg), mixed and incubated at room temperature for 15 min to generate the transfection mixture. The transfection mixture was then added to HEK293 cells (9×106) and incubated for 8–12 h. The medium was replaced with fresh 10% FBS-DMEM and incubated for 72 h, before cells and rAAV9 virus particles were harvested. The mixture was purified using chloroform, precipitated using polyethylene glycol/NaCl and extracted with chloroform as described previously (19). The purity of rAAV-ZsGreen was evaluated by gel electrophoresis using 12% SDS-PAGE gels. To achieve this, the viral stock solution was mixed with loading buffer (2X; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and incubated in boiling water for 10 min. Samples (15 µl) were then loaded and run at 20 mA for 2.5 h. The gel was stained with Coomassie Brilliant Blue R250 (Amresco, LLC, Solon, OH, USA) for 1 h and destained, until clear bands with a low background were evident. The viral titer, expressed as viral genomes (v.g.)/ml, was determined using dot blot hybridization as described previously (20). The efficiency of infection was validated by infecting HEK293 cells. Briefly, 10 µl purified virus was added to HEK293 cells (5×105) and incubated for 48 h at 37°C. Fluorescence signals were then visualized using an Olympus IX71 fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Experimental animals
A total of 36 male SHR (age, 20 weeks; weight, ~300 g) were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Rats were acclimated to laboratory conditions for one week before experimental procedures were performed according to the Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health, publication 86–23, revised 1986) and the animal regulations of the Department of Science and Technology of Hunan Province (Changsha, China). Rats were maintained at the Animal Experiment Center of Central South University (Changsha, China), and housed in individual cages at 22±2°C and 55±5% humidity with 12-h environmental light/dark cycles with free access to standard laboratory chow and tap water during the experimental period.
The rats were randomly divided into the following three groups of 12: i) Sham-operated; ii) rAAV9-NC; and iii) rAAV9-Adrb1. Rats in the rAAV9-NC and rAAV9-Adrb1groups were injected with a single dose of 1×1012 v.g./kg rAAV9-NC or rAAV9-Adrb1 into the myocardium via the pericardial cavity using the methods described previously (21,22). Briefly, SHR were anesthetized with 10% chloral hydrate (300 mg/kg), and each rat was placed in a supine position before connecting to an endotracheal tube. The tidal volume was set at 10 ml, and the ventilator rate was set at 75-breaths/min. A 2-cm skin incision was made over the fourth intercostal space, which separated the subcutaneous tissue and the underlying muscle, thereby permitting entrance to the thorax via the fourth intercostal space. The pericardial cavity was injected with 200 µl vector using an insulin syringe. The muscle and the skin were closed following injection. The rats received penicillin for three days via intramuscular injections to prevent infection. 50% of rats in each group received saline (10 ml/kg), while the remaining rats received Met (50 mg/kg/day) via oral gavage twice a day for 4 weeks. The systolic blood pressure (SBP) of the rats was monitored weekly using a tail blood pressure meter. The rats were sacrificed by intraperitoneal injection of 10% chloral hydrate (300 mg/kg; Hunan Xiangya Medicine Co., Ltd., Changsha, China) 4 weeks after the initial treatment.
Transfection efficiency of rAVV9 vectors
The transfection efficiency of rAAV9-shRNA-Adrb1-ZsGreen was determined at 4 weeks following rAAV9 injection, at which point the hearts were harvested and myocardial tissue (150 mg) was collected. The heart tissue was washed with ice-cold saline, immersed in 4% paraformaldehyde overnight, dehydrated in consecutive 15% and 30% sucrose solutions and dried with filter paper. Tissue sections were then embedded in paraffin at room temperature, and divided into 10-µm frozen sections. Cell nuclei were visualized by staining tissue sections with DAPI, and blue and green fluorescence signals were visualized using an Olympus IX-71 fluorescence microscope (Olympus Corporation).
Evaluation of β1-AR mRNA expression using reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Following excision of the heart from rats in each experimental group, a portion of the heart was used to measureβ1-AR mRNA expression levels by RT-qPCR. Briefly, total RNA was extracted from each heart using the RNA-Solv Reagent (Omega Bio-Tek, Inc., Norcross, GA, USA) according to the manufacturer's instructions. Total RNA (1 µg) was then reverse transcribed to generate cDNA using the to Revert Aid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). qPCR reaction mixtures (10 µl) consisted of 5 µl SYBR Green (Omega Bio-Tek, Inc.), 0.8 µl cDNA and 0.2 µl each primer. The following primers were used: Adrb1 (Rattus norvegicus) forward, 5′-CGCTGCCCTTTCGCTACCAG-3′ and reverse, 5′-CCGCCACCAGTGCTGAGGAT-3′; β-actin forward, 5′-CGTAAAGACCTCTATGCCAA-3′, and reverse, 5′-GGTGTAAAACGCAGCTCAGT-3′ (Nanjing GenScript Biotechnology Co., Ltd., Nanjing, China). The thermal cycling parameters were as follows: 95°C for 15 min, followed by 40 cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. Target gene expression levels were quantified relative to β-actin expression using the 2-ΔΔCq method (23).
Evaluation of β1-AR protein expression by western blot analysis
Heart tissue (50 mg) was lysed in lysis buffer consisting of phenylmethylsulfonyl fluoride and radioimmunoprecipitation assay buffer (1:100; Applygen Technologies Inc., Beijing, China) and centrifuged at 16.2 × g for 40 min at 4°C, before total protein was quantified using a bicinchoninic acid assay kit (Beijing Kawin Biotech Co., Ltd., Beijing, China). For the western blot analysis, 80 µl sample (~50 µg protein) was separated by 8% SDS-PAGE, and proteins were transferred to polyvinylidene difluoride membranes. After blocking with 5% non-fat milk in phosphate-buffered saline for 5 h at room temperature, membranes were incubated with polyclonal rabbit antibodies against Adrb1 (dilution, 1:1,000; cat. no. ab3442; Abcam, Cambridge, MA, USA) or GAPDH (dilution, 1:3,000; cat. no. 10494-1-AP, ProteinTech Group, Inc., Chicago, IL, USA) overnight at 4°C. Membranes were then incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (dilution, 1:8,000; cat. no. 10285-1-AP; ProteinTech Group, Inc.) for 1 h at room temperature. Chemiluminescence was detected using the ECL Western Blotting Substrate kit (Abnova Corporation, Taipei, Taiwan), and the relative intensity of the bands of interest were analyzed using ImageJ image analysis software (version, 1.44p; National Institutes of Health, Bethesda, MD, USA). The expression of β1-AR was calculated relative to GAPDH.
Evaluation of β1-AR protein expression by immunohistochemical analysis
Following excision of rat hearts, they were fixed in 4% paraformaldehyde and embedded in paraffin. Heart tissue sections were blocked using 5 ml horse serum (ZSGB-Bio, Beijing, China) at room temperature for 1 h. β1-AR expression was detected by staining with a polyclonal rabbit anti-β1-AR antibody (dilution, 1:100; cat. no. 10285-1-AP; ProteinTech Group Inc.) overnight at 4°C, followed by staining with hematoxylin. The integrated optical density of the positive expression area of the myocardial tissue sections was calculated using Image-Pro Plus software (version, 6.0; Media Cybernetics, Inc., Rockville, MD, USA).
Measurement of heart rate and SBP
The heart rate and SBP in conscious, resting rats was measured weekly using a tail-cuff method with an electrosphygmomanometer attached to a computerized recorder (Shanghai Alcott Biotech Co., Ltd., Shanghai, China) from 14:30-17:30 p.m. by the same investigator (Miss Xiao-Li Liu, The Second Affiliated Hospital of Hunan University of Traditional Chinese Medicine, Changsha, China). Each rat was held in a silent, dark and sizeable cylinder. Blood pressure measurements were performed in a blind manner, and the mean of three repeated measurements for each rat during each session was recorded.
Statistical analysis
Data are expressed as mean ± standard deviation. Levene's test was used to assess the equality of variances. One-way analysis of variance with the least significant difference test was used to compare the differences among the experimental and control groups. Data analyses were performed using the SPSS software program (version, 17.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Validating the purity and titer of rAAV9 vectors and the transfection efficiency in HEK293 cells
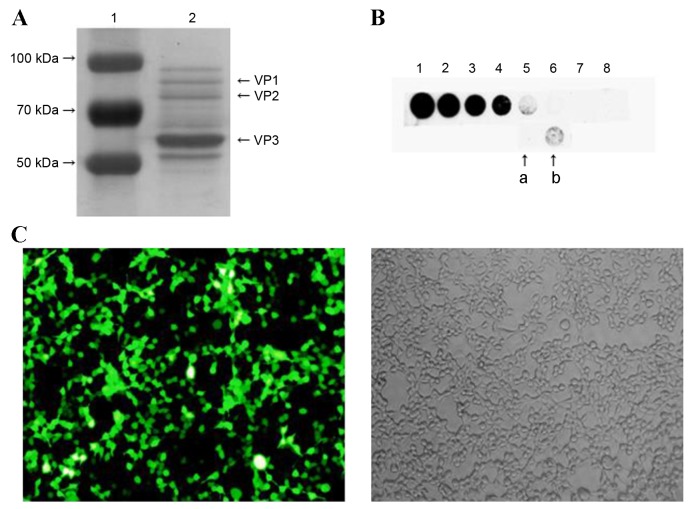
The purity of rAVV9-shRNA-Adrb1-ZsGreen plasmid vectors was assessed using SDS-PAGE followed by Coomassie brilliant blue (CBB) staining. Three characteristic protein bands for viral protein (VP) 1, VP2 and VP3, with molecular weights of 87, 73 and 62 KDa, respectively, were observed, indicating the purity of the rAAV9 vector (Fig. 1A). Dot blot results demonstrated that the vector titer was 1.5×1012 v.g./ml (Fig. 1B). Transfection efficiency was assessed by infecting HEK293 cells and visualizing green fluorescence signals with a fluorescence microscope. As shown in Fig. 1C, approximately 90% of the cells exhibited green fluorescence.
Figure 1.
The purity, titer and transfection efficiency of rAAV9-shRNA-Adrb1-ZsGreen vectors in HEK293 cells. (A) The purity of rAAV9-shRNA-Adrb1-ZsGreen vectors as determined by SDS-PAGE followed by Coomassie Brilliant Blue staining. The left lane indicates the protein maker (1), and the right lane indicates the purified vector sample (2). (B) The titer of the purified vectors. Spots 1–8 indicate 50, 25, 12.5, 6.25, 3.125, 1.56, 0.78, and 0.39×109 v.g./ml purified vector, respectively (a, 2-fold dilution of purified vectors; b, purified vectors). (C) Fluorescence (left) and light (right) microscope images of the transfection efficiency of rAAV9-shRNA-Adrb1-ZsGreen vectors in HEK293 cells (magnification, ×200). rAAV9, recombinant adeno-associated virus type 9 vector; shRNA, short-hairpin RNA; Adrb1, adrenergic receptor β1; v.g., viral genomes.
Determination of rAAV9-Adrb1-shRNA-ZsGreen expression in vivo
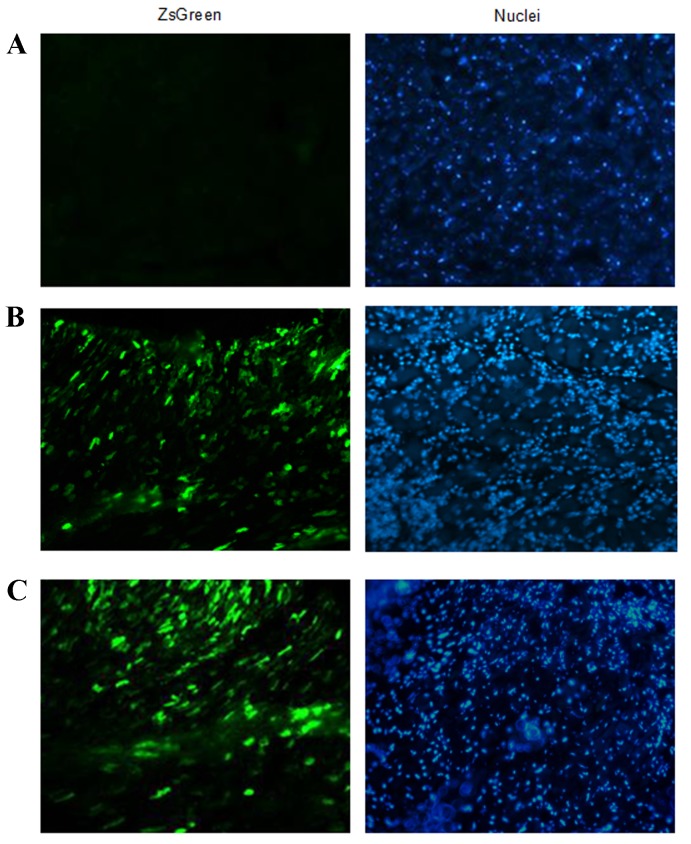
In order to investigate the efficiency of rAAV9-Adrb1-shRNA-ZsGreen delivery in vivo, the expression of ZsGreen in heart tissue sections of rats from the sham-operated, rAAV9-Adrb1 and rAAV9-NC groups at 4 weeks following injection of rAAV9-Adrb1-shRNA-ZsGreen and rAAV9-NC-ZsGreen vectors into the myocardium was examined by fluorescence microscopy. As shown in Fig. 2B and C, green fluorescence was observed in heart tissues of rAAV9-NC and rAAV9-Adrb1 groups. By contrast, no ZsGreen expression was detected in the heart tissues from the sham group (Fig. 2A).
Figure 2.
Determination of the efficiency of viral vector delivery in vivo. Fluorescence microscope images (magnification, ×200) of ZsGreen expression (left panel) and DAPI staining (right panel) in frozen heart tissues sections derived from rats in the (A) sham-operated (B) rAAV9-NC and (C) rAAV9-Adrb1 groups at 4 weeks following injection with saline, rAAV9-NC-ZsGreen and rAAV9-shRNA-Adrb1-ZsGreen vectors, respectively. rAAV9, recombinant adeno-associated virus type 9 vector; NC, negative control; Adrb1, adrenergic receptor β1; shRNA, short-hairpin RNA.
Inhibitory effects of rAAV9-Adrb1-shRNA-ZsGreen on β1-AR expression
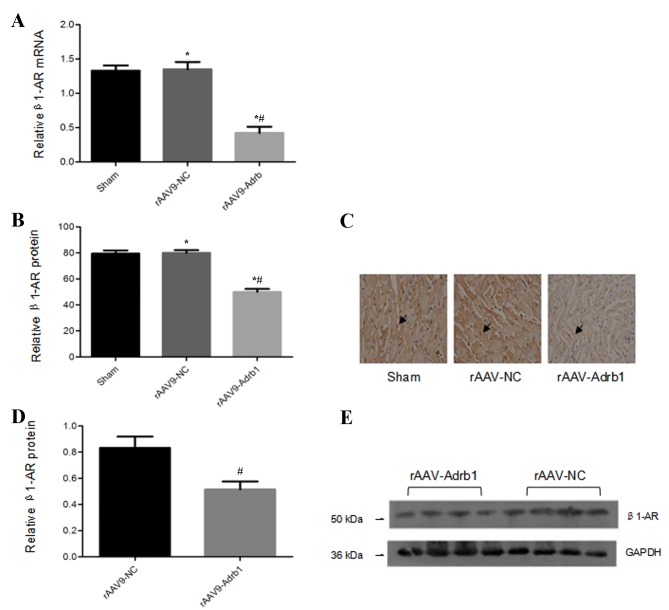
In order to assess the inhibitory effects of the injection of rAAV9-Adrb1-shRNA-ZsGreen vectors on β1-AR expression in the myocardium, the β1-AR mRNA expression levels in all experimental groups were examined. Compared with the rAAV9-NC group, cardiomyocytes in the rAAV9-Adrb1 group exhibited a significant decrease in β1-AR mRNA expression (0.42±0.06 vs. 1.3±0.08; P<0.001), whereas no significant difference in β1-AR mRNA expression was observed between the sham and rAAV9-NC groups (1.3±0.08 vs. 1.3±0.06; P>0.05; Fig. 3A). Immunohistochemical and western blot analyses revealed that cardiomyocytes in the rAAV9-Adrb1 group displayed a significant reduction in β1-AR protein expression levels when compared with the rAAV9-NC group (immunohistochemistry, 50.02±4.4 vs. 80.05±3.7, P<0.001; western blot, 0.52±0.12 vs. 0.83±0.17, P=0.032; Fig. 3B-E). Western blot analysis demonstrated no significant difference between β1-AR protein expression levels between the rAAV9-NC and sham groups (80.05±3.7 vs. 79.30±2.8; P>0.05; Fig. 3D and E). These data indicated that β1-AR expression in the myocardium of SHR was downregulated upon infection with rAAV9-Adrb1-shRNA-ZsGreen but not rAAV9-NC.
Figure 3.
β1-AR mRNA and protein expression levels in the myocardium of SHR from sham, rAAV9-NC and rAAV9-Adrb1 groups at 4 weeks following injection with saline, rAAV9-NC-ZsGreen or rAAV9-shRNA-Adrb1-ZsGreen vectors, respectively. (A) β1-AR mRNA levels as determined by RT-qPCR. (B) The integrated optical density of β1-AR protein levels as determined by immunohistochemical analysis. (C) Representative microscope images (magnification, ×400) of β1-AR protein levels as determined by immunohistochemical analysis. The black arrows indicate positive protein particles. (D) Relative β1-AR protein levels as determined by western blot analysis. (E) Representative western blot image of β1-AR protein levels. GAPDH was used as a loading control. *P<0.05 vs. sham group; #P<0.05 vs. rAAV9-NC group. β1-AR, β1-adrenergic receptor; SHR, spontaneously hypertensive rats; rAAV9, recombinant adeno-associated virus type 9 vector; NC, negative control; Adrb1, adrenergic receptor β1; shRNA, short-hairpin RNA; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Downregulating β1-AR expression in the myocardium results decreased sensitivity to Met
In order to investigate the effects of β1-AR downregulation on the response to Met treatment in SHR, the SBP of rats in the sham, rAAV9-NC and rAAV9-Adrb1 groups was examined using a tail blood pressure meter. As shown in Fig. 4, no significant difference in blood pressure was observed among all three experimental groups prior to Met treatment (P>0.05). Following 4 weeks of Met treatment, a decrease in SBP was observed in all experimental groups (Fig. 4). In addition, a significantly greater reduction in SBP was observed in the sham and rAAV9-NC groups when compared with the rAAV9-Adrb1 group (ΔSBP, 22.84±1.7, 28.78±2.7 and 30.16±5.7 mmHg for rAAV9-Adrb1, rAAV9-NC and sham groups, respectively; P=0.035; Fig. 4). As shown in Table I, no significant differences in heart rate among the three experimental groups was observed before or after 4 weeks of Met treatment (P>0.05). However, the heart rate of rats in all groups significantly decreased following 4 weeks of Met treatment (P<0.001; Table I).
Figure 4.
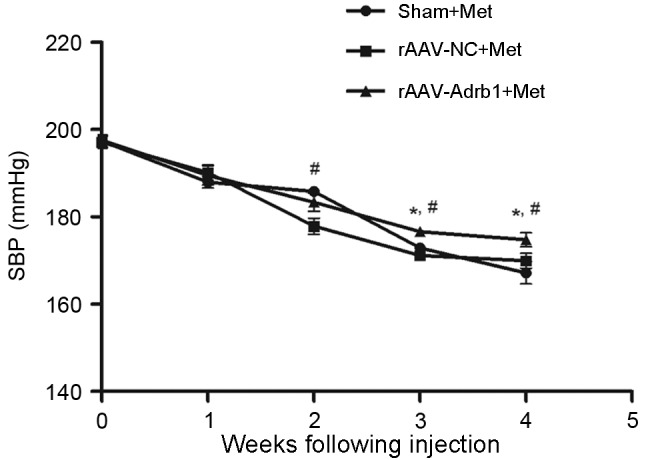
Effect of cardiac β1-AR downregulation on the SBP in SHR from sham, rAAV9-NC and rAAV9-Adrb1 groups up to 4 weeks following injection with saline, rAAV9-NC-ZsGreen and rAAV9-shRNA-Adrb1-ZsGreen vectors, respectively, and treatment with Met. *P<0.05 vs. rAAV9-NC+Met group; #P<0.05 vs. the sham+Met group. β1-AR, β1-adrenergic receptor; SBP, systolic blood pressure; rAAV9, recombinant adeno-associated virus type 9 vector; NC, negative control; Adrb1, adrenergic receptor β1; shRNA, short-hairpin RNA; Met, metoprolol.
Table I.
Effect of Met on the heart rate of SHR following downregulation of cardiac β1-AR expression.
| Group | No. of samples | Heart rate prior to Met intervention (bmp) | Heart rate following Met intervention (bmp) |
|---|---|---|---|
| Sham+Met | 4 | 461.7±3.7 | 435.7±3.3a |
| rAAV9-NC+Met | 6 | 459.9±6.0 | 434.1±2.6a |
| rAAV9-Adrb1+Met | 6 | 459.0±3.0 | 437.2±2.3a |
P<0.001 vs. each group prior to Met intervention. Met, metoprolol; SHR, spontaneously hypertensive rats; β1-AR, β1-adrenergic receptor; rAAV9, recombinant adeno-associated virus type 9 vector; NC, negative control; Adrb1, adrenergic receptor β1; bmp, beats per minute.
Downregulation of β1-AR in the myocardium reduces blood pressure
In addition to the assessment of Met treatment responses, the authors observed an unexpected phenomenon regarding the effects of cardiac β1-AR downregulation on blood pressure in SHR. As shown in Fig. 5, SBP of SHR in the rAAV9-Adrb1 group was significantly lower than that of the rAAV9-NC group at 4 weeks following injection (178.1±6.3 vs. 199.4±3.1 mmHg; P<0.001). By contrast, no significant difference in SBP was observed between the rAAV9-NC and sham groups (199.4±3.1 vs. 198.3±4.5 mmHg; P>0.05; Fig. 5). As shown in Table II, no significant differences in heart rate of SHR among all treatment groups were observed at 0 or 4 weeks following injection of saline or the rAAV9 vectors (P>0.05). In addition, no significant difference in the SBP of SHR in the rAAV9-Adrb1 group was observed following treatment with Met or saline at 4 weeks following injection of rAAV9-shRNA-Adrb1-ZsGreen vectors (174.8±3.9 vs. 178.1±6.3 mmHg; P>0.05; Fig. 6).
Figure 5.
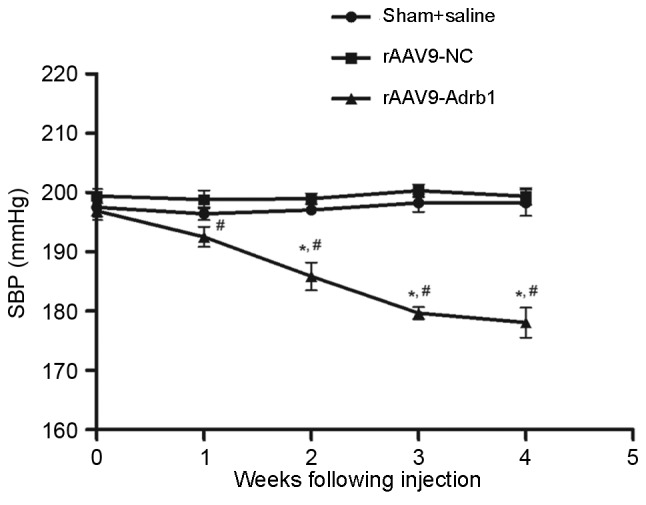
Effect of cardiac β1-AR downregulation on the SBP in SHR from sham+saline, rAAV9-NC and rAAV9-Adrb1 groups up to 4 weeks following injection with saline, rAAV9-NC-ZsGreen and rAAV9-shRNA-Adrb1-ZsGreen vectors, respectively. *P<0.05 vs. rAAV9-NC group; #P<0.05 vs. sham+saline group. β1-AR, β1-adrenergic receptor; SBP, systolic blood pressure; SHR, spontaneously hypertensive rats; rAAV9, recombinant adeno-associated virus type 9 vector; NC, negative control; Adrb1, adrenergic receptor β1; shRNA, short-hairpin RNA.
Table II.
Effect of cardiac β1-AR suppression on heart rate in SHR.
| Groups | No. of samples | Heart rate prior to β1-AR suppression (bmp) | Heart rate following β1-AR suppression (bmp) |
|---|---|---|---|
| Sham+saline | 4 | 461.7±3.7 | 460.4±3.3 |
| rAAV9-NC | 5 | 459.9±6.0 | 455.1±2.6 |
| rAAV9-Adrb1 | 6 | 459.0±3.0 | 456.5±2.3 |
β1-AR, β1-adrenergic receptor; SHR, spontaneously hypertensive rats; rAAV9, recombinant adeno-associated virus type 9 vector; NC, negative control; Adrb1, adrenergic receptor β1; bmp, beats per minute.
Figure 6.
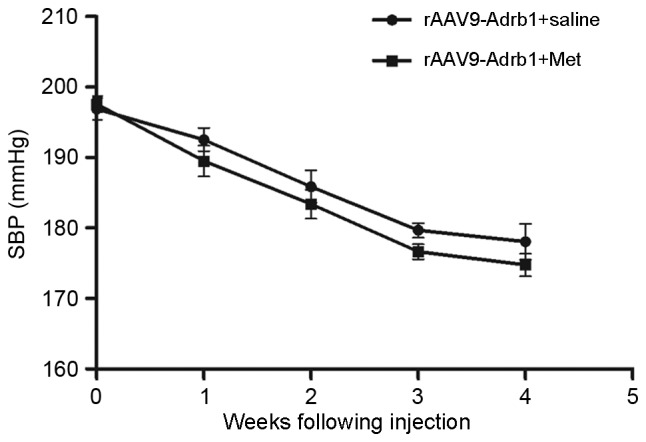
Effect of cardiac β1-AR gene knockdown on SBP in SHR from the rAAV9-Adrb1 group up to 4 weeks following injection with the rAAV9-shRNA-Adrb1-ZsGreen vector and following saline or Met treatment. β1-AR, β1-adrenergic receptor; SBP, systolic blood pressure; SHR, spontaneously hypertensive rats; rAAV9, recombinant adeno-associated virus type 9 vector; Adrb1, adrenergic receptor β1; shRNA, short-hairpin RNA; Met, metoprolol.
Discussion
Met is commonly prescribed for the treatment of hypertension. Although its efficacy is extremely variable among patients, the presence of CYP2D6 and Adrb1 polymorphisms only provide a partial explanation for this variability (10–12). A previous study investigating Adrb1 promoter methylation suggested that the level of Adrb1 promoter methylation influences the efficacy of Met via the regulation of β1-AR expression (14). In addition, β1-AR is the target of Met and its expression has been demonstrated to vary among individuals (23,24). Therefore, the authors of the present study hypothesized that the expression of myocardial β1-AR may present a novel mechanism underlying the inter-individual differences in response to Met treatment (15). In order to test this notion, the effect of downregulated cardiac β1-AR expression in the response to Met treatment in SHR was investigated in the present study. The results indicated that SHR injected with rAAV9-Adrb1 vectors into the myocardium, exhibited a lower reduction in SBP following Met treatment when compared with negative controls. Naya et al (24) demonstrated that patients with idiopathic dilated cardiomyopathy and decreased myocardial β-AR expression exhibited a greater sensitivity to the antiadrenergic drug carvedilol in clinical trails. Together, these findings suggest that alterations in myocardial β-AR expression levels are associated with differential responses to β-blockers.
A number of factors, including age, drugs and comorbidities are known to influence β1-AR expression (25,26). Oliver et al (27) revealed that β1-AR expression levels were increased in the circulating lymphocytes of hypertensive patients. Lymphocytes are considered to be a practical surrogate for myocardial or vascular cells. Patients with heart failure exhibit a downregulation and desensitization of β1-AR, which leads to a markedly diminished β1-AR-mediated contractile response (28). In this way, β1-AR expression is altered under different physiological conditions, and may potentially be used as a marker of response to different treatments. Therefore, if the hypothesis presented by the authors (15) is accepted by future clinical trials, β1-AR expression may be considered as a potential biomarker when establishing a quantitative pharmacological model of patient response to Met for individual therapy.
In addition to the original study objective, the results of the present study demonstrated an unexpected effect of suppressed β1-AR expression on blood pressure in SHR. shRNA-mediated reduction of cardiac β1-AR expression was associated with a significant decrease in SBP of SHR when compared with negative and sham-treated controls. In addition, this effect on SBP was comparable to Met-treated rAAV9-Adrb1 rats. Several studies have demonstrated that β1-AR expression serves a role in the development of cardiovascular diseases, such as hypertension (29–31). The intravenous injection of β1-AR antisense-oligodeoxy nucleotides delivered in cationic liposomes in an animal model of hypertension, demonstrated a significant reduction in cardiac β1-AR density of ~30–50% for 18 days, and a reduction in the blood pressure of SHR for 20 days, with a maximum decrease of 38 mmHg (30). Arnold et al (29) developed a small interfering RNA targeted to β1-AR that significantly reduced β1-AR mRNA levels to 33.3% of control levels, and lowered diastolic blood pressure in SHR by a maximum of 30 mmHg for >12 days. Using gene knockout technology, the 24 h mean artery pressure was decreased by 10% (102.02±1.81 vs. 92.11±2.62 mmHg) in β1/β2-AR−/− compared with wild-type mice (31). Consistent with these results, a decrease in cardiac β1-AR expression was associated with a reduction in SBP in the present study.
Since β1-AR is a member of the autonomic nervous system, the effect of cardiac β1-AR expression on blood pressure regulation may be comprehensible. The β1-AR subtype is often classified as the ‘cardiac’ β-AR subtype, due to the observation that the in vivo stimulation of this receptor by its agonists increases heart rate and contractility. It is known that stimulation of β1-AR induces robust chronotropic and inotropic effects via the Gs-protein adenylate cyclase-cyclic adenosine monophosphate-protein kinase A signaling pathway (32). Following stimulation of β1-AR, protein kinase A is activated and L-type Ca2+ channels in ventricular myocytes are phosphorylated, which leads to increased myocardial contractility through increased Ca2+ influx and the release of Ca2+ from the sarcoplasmic reticulum. Zhang et al (30) observed a marked attenuation of the β1-AR-mediated positive inotropic response in isolated perfused hearts in vitro and in conscious SHR following myocardial β1-AR downregulation. Through the targeted deletion of β1-AR, mice lacking β1-AR were demonstrated to exhibit increased heart rate variability and decreased resting heart rate (33). Therefore, myocardial β1-AR expression may influence blood pressure regulation by affecting heart rate and cardiac output through altering cardiac inotropy and chronotropy.
In the present study, decreasing β1-AR expression in the myocardium decreased blood pressure but had no significant effect on heart rate in SHR. Which is consistent with several previous studies (29–30). It is therefore possible that β2-AR may serve a more important role in regulating heart rate compared with β1-AR (29). However, a previous study revealed that β1-AR may also function to control heart rate (33). Ecker et al (33) demonstrated that resting heart rate (in beats/min) was decreased in β1-knockout (KO) and β1/β2-double KO mice when compared with wild-type mice. The possible reasons underlying these contradictory results require further investigation in future studies.
The present study was limited by the small sample size and β1-AR was downregulated only in the myocardium by injection of lentiviral shRNA vectors directly into the pericardial cavity (34). The results demonstrated that β1-AR expression in the myocardium affects the efficacy of Met in SHR. In addition, cardiac β1-AR expression may be associated with blood pressure regulation, however, the effect of β1-AR expression on cardiac physiology was not investigated in the present study. Therefore, the clinical applications or mechanisms involved in β1-AR-mediated regulation of blood pressure and response to Met treatment warrant attention in future studies. The rAAV9 vector, used to deliver shRNA to the myocardium in vivo in the present study, is a valuable, safe and efficacious means of cardiac gene transfer (18). However, its potential effects on cardiac function and the immune system were not examined. This will be an important aim in future studies.
Acknowledgements
The present study was supported by grants from The National Natural Science Foundation of China (grant no. 81470535), The National Science and Technology Major Projects for ‘Major New Drugs Innovation and Development’ (grant no. 2012ZX09303014001) and The National Key Technology Research and Development Program (grant no. 2012BAI137B05).
References
- 1.Chen S. Essential hypertension: Perspectives and future directions. J Hypertens. 2012;30:42–45. doi: 10.1097/HJH.0b013e32834ee23c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinese National Center for Cardiovascular Diseases (CNCCD), corp-author Report on Cardiovascular Diseases in China. 2013 [Google Scholar]
- 3.Sheng CS, Liu M, Kang YY, Wei FF, Zhang L, Li GL, Dong Q, Huang QF, Li Y, Wang JG. Prevalence, awareness, treatment and control of hypertension in elderly Chinese. Hypertens Res. 2013;36:824–828. doi: 10.1038/hr.2013.57. [DOI] [PubMed] [Google Scholar]
- 4.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tibazarwa KB, Damasceno AA. Hypertension in developing countries. Can J Cardiol. 2014;30:527–533. doi: 10.1016/j.cjca.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 7.McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: Beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–794. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 8.Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, Hamburger RJ, Fye C, Lakshman R, Gottdiener J. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med. 1993;328:914–921. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JA, Liggett SB. Cardiovascular pharmacogenomics of adrenergic receptor signaling: Clinical implications and future directions. Clin Pharmacol Ther. 2011;89:366–378. doi: 10.1038/clpt.2010.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bijl MJ, Visser LE, van Schaik RH, Kors JA, Witteman JC, Hofman A, Vulto AG, van Gelder T, Stricker BH. Genetic variation in the CYP2D6 gene is associated with a lower heart rate and blood pressure in beta-blocker users. Clin Pharmacol Ther. 2009;85:45–50. doi: 10.1038/clpt.2008.172. [DOI] [PubMed] [Google Scholar]
- 11.Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part II. Clin Pharmacokinet. 2009;48:761–804. doi: 10.2165/11318030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Wu D, Li G, Deng M, Song W, Huang X, Guo X, Wu Z, Wu S, Xu J. Associations between ADRB1 and CYP2D6 gene polymorphisms and the response to β-blocker therapy in hypertension. J Int Med Res. 2015;43:424–434. doi: 10.1177/0300060514563151. [DOI] [PubMed] [Google Scholar]
- 13.Yuan H, Huang Z, Yang G, Lv H, Sang H, Yao Y. Effects of polymorphism of the beta(1) adrenoreceptor and CYP2D6 on the therapeutic effects of metoprolol. J Int Med Res. 2008;36:1354–1362. doi: 10.1177/147323000803600624. [DOI] [PubMed] [Google Scholar]
- 14.Rockman HA, Koch WJ, Lefkowitz RJ. Seven- transmembrane-spanningreceptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Q, Yuan H, Xing X, Liu J, Huang Z, Du X. Methylation of adrenergic β1 receptor is a potential epigenetic mechanism controlling antihypertensive response to metoprolol. Indian J Biochem Biophys. 2011;48:301–307. [PubMed] [Google Scholar]
- 16.Liu H, Xing X, Huang L, Huang Z, Yuan H. The expression level of myocardial β1-adrenergic receptor affects metoprolol antihypertensive effects: A novel mechanism for interindividual difference. Med Hypotheses. 2013;81:71–72. doi: 10.1016/j.mehy.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Vandenberghe LH, Xiao R, Lock M, Lin J, Korn M, Wilson JM. Efficient serotype-dependent release of functional vector into the culture medium during adeno-associated virus manufacturing. Hum Gene Ther. 2010;21:1251–1257. doi: 10.1089/hum.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller C, Ratner D, Zhong L, Esteves-Sena M, Gao G. Production and discovery of novel recombinant adeno-associated viral vectors. Curr Protoc Microbiol. 2012 doi: 10.1002/9780471729259.mc14d01s26. Chapter 14: Unit14D.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu XB, Dong XY, Wu ZJ, Cao H, Niu DB, Qu JG, Wang H, Hou YD. A novel method for purification of recombinant adenoassociated virus vectors on a large scale. Chinese Sci Bull. 2001;46:pp485–488. doi: 10.1007/BF03187263. [DOI] [Google Scholar]
- 20.McClure C, Cole KL, Wulff P, Klugmann M, Murray AJ. Production and titering of recombinant adeno-associated viral vectors. J Vis Exp. 2011:e3348. doi: 10.3791/3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bish LT, Sweeney HL, Müller OJ, Bekeredjian R. Adeno-associated virus vector delivery to the heart. Methods Mol Biol. 2011;807:219–237. doi: 10.1007/978-1-61779-370-7_9. [DOI] [PubMed] [Google Scholar]
- 22.Katz MG, Swain JD, Tomasulo CE, Sumaroka M, Fargnoli A, Bridges CR. Current strategies for myocardial gene delivery. J Mol Cell Cardiol. 2011;50:766–776. doi: 10.1016/j.yjmcc.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Naya M, Tsukamoto T, Morita K, Katoh C, Nishijima K, Komatsu H, Yamada S, Kuge Y, Tamaki N, Tsutsui H. Myocardial beta-adrenergic receptor density assessed by 11C-CGP12177 PET predicts improvement of cardiac function after carvedilol treatment in patients with idiopathic dilated cardiomyopathy. J Nucl Med. 2009;50:220–225. doi: 10.2967/jnumed.108.056341. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara N, Komici K, Corbi G, Pagano G, Furgi G, Rengo C, Femminella GD, Leosco D, Bonaduce D. β-adrenergic receptor responsiveness in aging heart and clinical implications. Front Physiol. 2014;4:396. doi: 10.3389/fphys.2013.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellgren I, Sylven C, Magnusson Y. Study of the beta1 adrenergic receptor expression in human tissues: Immunological approach. Biol Pharm Bull. 2000;23:700–703. doi: 10.1248/bpb.23.700. [DOI] [PubMed] [Google Scholar]
- 27.Oliver E, Rovira E, Montó F, Valldecabres C, Julve R, Muedra V, Ruiz N, Barettino D, D'Ocon P. beta-Adrenoceptor and GRK3 expression in human lymphocytes is related to blood pressure and urinary albumin excretion. J Hypertens. 2010;28:1281–1289. doi: 10.1097/HJH.0b013e3283383564. [DOI] [PubMed] [Google Scholar]
- 28.Hamdani N, Linke WA. Alteration of the beta-adrenergic signaling pathway in human heart failure. Curr Pharm Biotechnol. 2012;13:2522–2531. doi: 10.2174/138920112804582998. [DOI] [PubMed] [Google Scholar]
- 29.Arnold AS, Tang YL, Qian K, Shen L, Valencia V, Phillips MI, Zhang YC. Specific beta1-adrenergic receptor silencing with small interfering RNA lowers high blood pressure and improves cardiac function in myocardial ischemia. J Hypertens. 2007;25:197–205. doi: 10.1097/01.hjh.0000254374.73241.ab. [DOI] [PubMed] [Google Scholar]
- 30.Zhang YC, Bui JD, Shen L, Phillips MI. Antisense inhibition of beta(1)-adrenergic receptor mRNA in a single dose produces a profound and prolonged reduction in high blood pressure in spontaneously hypertensive rats. Circulation. 2000;101:682–688. doi: 10.1161/01.CIR.101.6.682. [DOI] [PubMed] [Google Scholar]
- 31.Kim SM, Huang Y, Qin Y, Mizel D, Schnermann J, Briggs JP. Persistence of circadian variation in arterial blood pressure in beta1/beta2-adrenergic receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1427–R1434. doi: 10.1152/ajpregu.00074.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoo B, Lemaire A, Mangmool S, Wolf MJ, Curcio A, Mao L, Rockman HA. Beta1-adrenergic receptors stimulate cardiac contractility and CaMKII activation in vivo and enhance cardiac dysfunction following myocardial infarction. Am J Physiol Heart Circ Physio. 2009;l297:H1377–H1386. doi: 10.1152/ajpheart.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ecker PM, Lin CC, Powers J, Kobilka BK, Dubin AM, Bernstein D. Effect of targeted deletions of beta1- andbeta2-adrenergic-receptor subtypes on heart rate variability. Am J Physiol Heart Circ Physiol. 2006;290:H192–H199. doi: 10.1152/ajpheart.00032.2005. [DOI] [PubMed] [Google Scholar]
- 34.Chen SJ, Johnston J, Sandhu A, Bish LT, Hovhannisyan R, Jno-Charles O, Sweeney HL, Wilson JM. Enhancing the utility of adeno-associated virus gene transfer through inducible tissue-specific expression. Hum Gene Ther Methods. 2013;24:270–278. doi: 10.1089/hgtb.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]