Abstract
The adipokine nicotinamide phosphoribosyltransferase (Nampt), also known as pre-B-cell colony-enhancing factor or the insulin-mimetic hormone visfatin, has a crucial role in the conversion of nicotinamide to nicotinamide mononucleotide during biosynthesis of the coenzyme nicotinamide adenine dinucleotide. Previous reports have demonstrated the inhibitory effects of Nampt on osteoclast formation from human peripheral blood mononuclear cells and CD14+ monocytes. However, the effects of Nampt on bone marrow macrophage (BMM)-derived osteoclastogenesis and its precise role in the process remain unclear. The present in vitro study used recombinant Nampt and BMMs as osteoclast precursors demonstrated that Nampt suppresses receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclastogenesis by decreasing the phosphorylation of various early signal transducers, including c-Jun N-terminal kinase, Akt, glycogen synthase kinase-3 β, Bruton's tyrosine kinase and phospholipase C γ-2. In addition, western blotting and reverse transcription-quantitative polymerase chain reaction analysis indicated that Nampt downregulates the mRNA and protein expression levels of c-Fos and nuclear factor of activated T cells, cytoplasmic 1, leading to a decrease in the expression of osteoclast-specific genes including tartrate-resistant acid phosphatase, osteoclast-associated receptor and cathepsin K. However, the bone-resorbing activity of mature osteoclasts treated with Nampt was similar to untreated control osteoclasts. This finding indicates that Nampt exerts its anti-osteoclastogenic activity by targeting osteoclast precursor cells rather than mature osteoclasts. Consequently, the present study demonstrated that Nampt acts as a negative regulator of RANKL-mediated differentiation of BMMs into osteoclasts, suggesting the potential therapeutic targets to treat bone-related disorders such as osteoporosis.
Keywords: nicotinamide phosphoribosyltransferase, adipokine, osteoclast, osteoporosis
Introduction
Nicotinamide phosphoribosyltransferase (Nampt) is a novel adipokine, which has been reported to be expressed in adipose tissue, chondrocytes in the articular cartilage matrix and peripheral blood mononuclear cells (PBMCs) (1–4). It has been revealed that Nampt is closely associated with various biological processes, including nicotinamide adenine dinucleotide (NAD) biosynthesis, cellular metabolism and immunomodulatory responses. In the process of NAD biosynthesis, Nampt regulates the activity of the NAD-dependent deacetylase silent information regulator 2 (Sir2) through increasing the cellular level of NAD, and subsequently promoting Sir2 transcriptional activity in mammalian cells (5). The potent Nampt inhibitor FK866 negatively regulates glycolysis by altering the initial steps in glucose oxidation and leads to changes in carbohydrate metabolism in cancer cells (6). Furthermore, Nampt is an essential catabolic mediator of osteoarthritis, which is the most common form of inflammatory arthritis, and regulates hypoxia-inducible factor 2α-mediated matrix metalloproteinase (MMP) expression in chondrocytes, leading to the destruction of osteoarthritic cartilage (7).
For the treatment of metabolic bone diseases, including osteoporosis, adipokines are considered to be a therapeutic target via their effects on two types of bone cell, osteoclasts and osteoblasts (8). Osteoclasts are well-characterized cells that are required for bone resorption and excessive osteoclast differentiation is a predominant indicator of osteoporosis. Osteoblasts are responsible for bone formation. A previous study indicated that osteoblast proliferation and differentiation are enhanced in vitro, and that acceleration of bone formation and mineral apposition rates are observed in vivo in the absence of the adipokine apelin, which is a ligand of the Gi-G protein-coupled receptor APJ. These data suggest a crucial role of apelin in bone homeostasis as a physiological antianabolic factor (9). Another adipokine, visceral adipose tissue-derived serine protease inhibitor (vaspin), has been reported to suppress receptor activator of nuclear factor-κB ligand (RANKL)-mediated differentiation of RAW264.7 cells and bone marrow cells (BMCs) into mature osteoclasts by reducing the expression of nuclear factor of activated T cells, cytoplasmic 1 (NFATc1) and the subsequent induction of osteoclast-specific gene markers, such as MMP-9 and cathepsin K (10). Adiponectin is an important adipokine that regulates energy homeostasis, which also inhibits RANKL-induced osteoclastogenesis by decreasing the expression of several osteoclastogenic factors, including NFATc1, tumor necrosis factor receptor-associated factor 6, cathepsin K and tartrate-resistant acid phosphatase (TRAP), and induces apoptosis in mature osteoclasts (11). It has previously been demonstrated that Nampt attenuates osteoclast differentiation derived from PBMCs in patients with multiple myeloma and human CD14+ monocytes; however, the role of Nampt in the differentiation of murine bone marrow macrophages (BMMs) into osteoclasts and its underlying mechanisms have not yet been revealed (12,13).
The present study investigated the effects of Nampt on RANKL-mediated osteoclast differentiation and functional bone-resorbing activity. In addition, the present study determined whether Nampt is involved in RANKL-dependent intracellular signaling pathways and the expression of osteoclast-specific gene markers.
Materials and methods
Preparation of Nampt and reagents
Recombinant mouse Nampt (visfatin/pre-B-cell colony-enhancing factor) was purchased from Adipogen International, Inc. (San Diego, CA, USA). Recombinant soluble human macrophage colony-stimulating factor (M-CSF) and human RANKL were obtained from PeproTech EC Ltd. (London, UK). Anti-p38 (cat. no. 9212), anti-phosphorylated (p)-p38 (cat. no. 9211), anti-extracellular signal-regulated protein kinases (ERK) 1/2 (cat. no. 9102), anti-p-ERK 1/2 (cat. no. 9101), anti-c-Jun N-terminal kinase (JNK; cat. no. 9252), anti-p-JNK (cat. no. 9251), anti-Akt (cat. no. 9272), anti-p-Akt (cat. no. 9271), anti-glycogen synthase kinase-3 β (GSK3β; cat. no. 9315), anti-p-GSK3β (cat. no. 9323) and anti-Bruton's tyrosine kinase (Btk; cat. no. 3533) antibodies were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). Anti-c-Fos (cat. no. sc-7202), anti-NFATc1 (cat. no. sc-7294), anti-phospholipase C γ-2 (PLCγ2; cat. no. sc-5283) and anti-p-PLCγ2 (cat. no. sc-101785) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Anti-p-Btk (cat. no. GTX61792) and monoclonal anti-β-actin (cat. no. GTX109639) antibodies were obtained from GeneTex, Inc. (Irvine, CA, USA) and Sigma-Aldrich (Merck Millipore; Darmstadt, Germany), respectively. Fetal bovine serum (FBS), α-minimum essential medium (α-MEM) and penicillin/streptomycin were purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). All other chemicals were of analytical grade or complied with the standards required for cell culture experiments.
Mouse BMM preparation and osteoclast differentiation
A total of 10 male ICR strain mice (age, 5 weeks; weight, 30±2 g) were purchased from Samtako (Osan, Korea). During the experimental period, the mice were maintained in a temperature- and humidity-controlled environment at 22–24°C and 55–60% humidity, with a 12-h light/dark cycle and access to sterilized water and standard rodent chow (Samtako) ad libitum. All experiments were conducted according to the guidelines of the Institutional Animal Care and Use Committee of Wonkwang University (WKU-14-23; Iksan, Korea). BMMs from mice were cultured as described previously (14). Briefly, to obtain BMMs, BMCs were cultured in α-MEM supplemented with 10% FBS and M-CSF (10 ng/ml) for 1 day. Non-adherent cells were further cultured in the presence of M-CSF (30 ng/ml) for 3 days. Subsequently, the adherent cells were used as BMMs. BMMs were cultured in 48-well plates at 37°C in 5% CO2 for 4 days in the condition of M-CSF (30 ng/ml) and RANKL (100 ng/ml), and pretreated with Nampt (100, 250 or 500 ng/ml). The cells were fixed in 3.7% formalin, permeabilized with 0.1% Triton X-100, and stained with TRAP solution. The stained multinucleated cells (MNCs) with >5 nuclei were counted to determine the level of osteoclast differentiation.
Cell viability assay, western blotting, reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis and bone resorption assay
The XTT cell viability assay, western blot analysis, RT-qPCR analysis and the resorption pit assay were performed as described previously (14). Resorption pits were imaged and analyzed using Image Pro-Plus version 4.5 (Media Cybernetics, Inc., Rockville, MD, USA). Primers used for PCR are summarized in Table I. The western blots were analyzed using ImageJ (imagej.nih.gov/).
Table I.
Primer sequences used for reverse transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer sequence (5′-3′) |
|---|---|
| GAPDH | |
| Forward | 5′-TCAAGAAGGTGGTGAAGCAG-3′ |
| Reverse | 5′-AGTGGGAGTTGCTGTTGAAGT-3′ |
| c-Fos | |
| Forward | 5′-GGTGAAGACCGTGTCAGGAG-3′ |
| Reverse | 5′-TATTCCGTTCCCTTCGGATT-3′ |
| NFATc1 | |
| Forward | 5′-GAGTACACCTTCCAGCACCTT-3′ |
| Reverse | 5′-TATGATGTCGGGGAAAGAGA-3′ |
| TRAP | |
| Forward | 5′-TCATGGGTGGTGCTGCT-3′ |
| Reverse | 5′-GCCCACAGCCACAAATCT-3′ |
| OSCAR | |
| Forward | 5′-GGAATGGTCCTCATCTCCTT-3′ |
| Reverse | 5′-TCCAGGCAGTCTCTTCAGTTT-3′ |
| DC-STAMP | |
| Forward | 5′-TCCTCCATGAACAAACAGTTCCA-3′ |
| Reverse | 5′-AGACGTGGTTTAGGAATGCAGCTC-3′ |
| Atp6vOd2 | |
| Forward | 5′-GACCCTGTGGCACTTTTTGT-3′ |
| Reverse | 5′-GTGTTTGAGCTTGGGGAGAA-3′ |
| Cathepsin K | |
| Forward | 5′-CCAGTGGGAGCTATGGAAGA-3′ |
| Reverse | 5′-CTCCAGGTTATGGGCAGAGA-3′ |
| αv-integrin | |
| Forward | 5′- ACAAGCTCACTCCCATCACC-3′ |
| Reverse | 5′- ATATGAGCCTGCCGACTGAC-3′ |
| β3-integrin | |
| Forward | 5′-GGAGTGGCTGATCCAGATGT-3′ |
| Reverse | 5′-TCTGACCATCTTCCCTGTCC-3′ |
| CTR | |
| Forward | 5′-TCCAACAAGGTGCTTGGGAA-3′ |
| Reverse | 5′-CTTGAACTGCGTCCACTGGC-3′ |
| Nampt | |
| Forward | 5′-ATCCAGGAGGCCAAAGAAGT-3′ |
| Reverse | 5′-CGGGAGATGACCATCGTATT-3′ |
CTR, calcitonin receptor; DC-STAMP, dendritic cell-specific transmembrane protein; Nampt, nicotinamide phosphoribosyltransferase; NFATc1, nuclear factor of activated T cells, cytoplasmic 1; OSCAR, osteoclast-associated receptor; TRAP, tartrate-resistant acid phosphatase.
Retroviral gene transfection
Packaging of the retroviral vectors pMX-IRES-EGFP, pMX-cFos-IRES-EGFP and pMX-NFATc1-IRES-EGFP was performed using transient transfection of these pMX vectors (Cell Biolabs, Inc., San Diego, CA, USA) into platinum-E (plat-E) retroviral packaging cells (Cell Biolabs, Inc.) using X-tremeGENE 9 (Roche, Nutley, NJ, USA) according to the manufacturer's protocol. Following incubation at 37°C in fresh medium for 2 days, the culture supernatants of the retrovirus-producing cells were collected. For retroviral infection, non-adherent BMCs were cultured in M-CSF (30 ng/ml) for 2 days. The BMMs were incubated with viral supernatant medium of pMX-IRES-EGFP, pMX-cFos-IRES-EGFP and pMX-NFATc1-IRES-EGFP virus-producing plat-E cells together with polybrene (10 ng/ml) and M-CSF (30 ng/ml) for 6 h. The infection efficiency of the retrovirus was determined by green fluorescent protein (GFP) expression and was always >80%. Post-infection, the BMMs were induced to differentiate in the presence of M-CSF (30 ng/ml) and RANKL (100 ng/ml) for 4 days. The expression of each construct was detected using a fluorescence microscope and osteoclast formation was determined by fixing in 3.7% formalin, permeabilizing with 0.1% Triton X-100, and staining with TRAP solution.
Statistical analysis
Each experiment was performed at least three times and all quantitative data are presented as the mean ± standard deviation. All statistical analyses were performed using SPSS (Korean version 14.0; SPSS, Inc., Chicago, IL, USA). Student's t-test was used to compare the parameters between two groups, whereas the one-way analysis of variance test, followed by the Tukey post hoc test, was used to compare the parameters among three groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Nampt inhibits RANKL-mediated osteoclast formation in a dose-dependent manner with no cytotoxicity
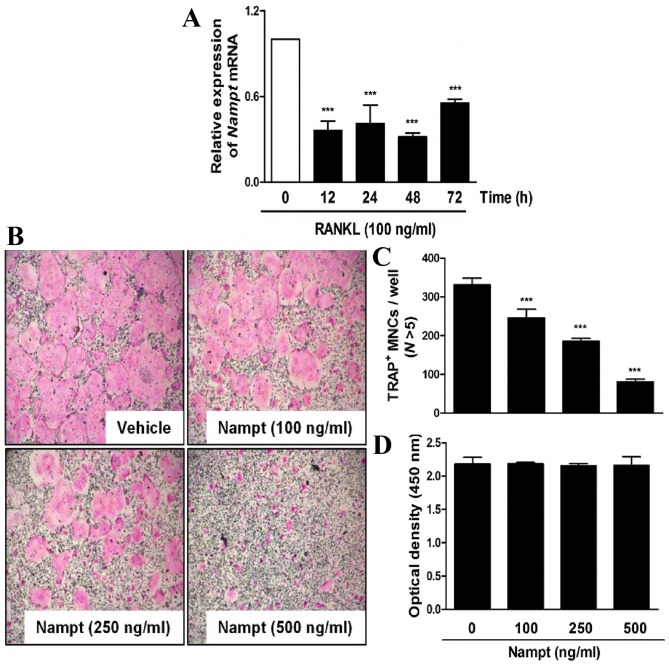
The present study analyzed the expression of Nampt in BMM cultures treated with M-CSF (30 ng/ml) and RANKL (100 ng/ml). As shown in Fig. 1A, the mRNA expression levels of Nampt were reduced in the presence of RANKL. To validate the effects of Nampt on osteoclast differentiation, mouse primary BMMs were treated with M-CSF (30 ng/ml) and RANKL (100 ng/ml) in the presence or absence of various concentrations of Nampt. As expected, the control untreated group generated TRAP-positive (TRAP+) osteoclasts. However, the presence of Nampt suppressed the formation of TRAP+ multinucleated osteoclasts in a dose-dependent manner (Fig. 1B and C). Subsequently, XTT cell viability assays were conducted to ascertain whether Nampt induced cytotoxicity during RANKL-induced osteoclast differentiation. The addition of Nampt did not affect cell viability at any of the concentrations used in the present study (Fig. 1D).
Figure 1.
Nampt attenuates TRAP-positive osteoclast formation without cytotoxicity. (A) BMMs were cultured in the presence of M-CSF (30 ng/ml) and then stimulated with RANKL (100 ng/ml) for the indicated durations. Total RNA was isolated from cells using QIAzol reagent and Nampt mRNA levels were evaluated by reverse transcription-quantitative polymerase chain reaction. ***P<0.001 vs. control group. (B) BMMs were cultured for 3 days in the presence of M-CSF (30 ng/ml) and RANKL (100 ng/ml), with or without the indicated concentrations of Nampt. Cells were fixed, permeabilized and stained with TRAP solution. Images of TRAP+ cells were captured under a light microscope (magnification, ×5). (C) BMMs were seeded into a 96-well plate and cultured for 3 days in the presence of M-CSF (30 ng/ml) with the indicated concentrations of Nampt (100, 250 or 500 ng/ml). After 3 days, cell viability was analyzed by the XTT assay. (D) TRAP+ MNCs with >5 nuclei were counted as osteoclasts. ***P<0.001 vs. control group. BMMs, bone marrow macrophages; M-CSF, macrophage colony-stimulating factor; MNCs, mononucleated cells; N, nuclei; Nampt, nicotinamide phosphoribosyltransferase; RANKL, receptor activator of nuclear factor-κB ligand; TRAP, tartrate-resistant acid phosphatase.
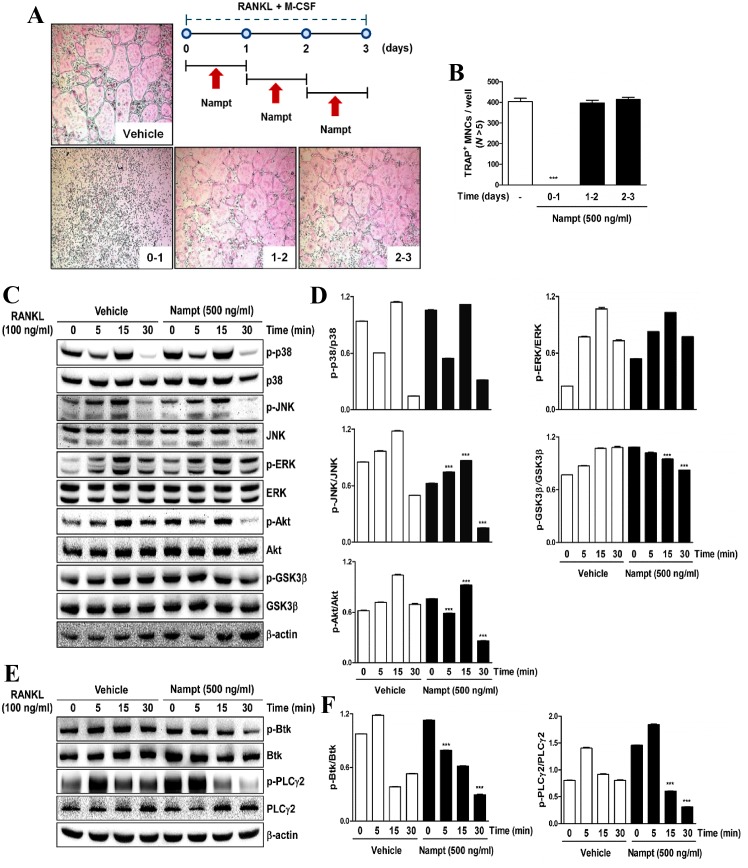
Nampt regulates osteoclastogenesis via mediating RANKL-dependent early signaling pathways
To elucidate a molecular mechanism that underlies the inhibitory effects of Nampt on osteoclastogenesis, Nampt was added to BMM cultures treated with M-CSF (30 ng/ml) and RANKL (100 ng/ml), at three different time points after RANKL treatment. The results indicated that Nampt (500 ng/ml) significantly blocked osteoclast differentiation when the cells were exposed on days 0–1 after RANKL treatment but not on days 1–2 or 2–3 (Fig. 2A and B). As shown in Fig. 2C and D, Nampt negatively affected the phosphorylation of JNK, Akt and GSK3β. In addition, Nampt downregulated the phosphorylation of Btk and PLCγ2, which are required for calcium signaling during osteoclast differentiation (Fig. 2E and F). These results indicated that Nampt is involved in the early stages of osteoclast differentiation by inducing dephosphorylation of JNK, Akt and its downstream target GSK3β, Btk and PLCγ2.
Figure 2.
Nampt inhibits the early stages of osteoclastogenesis by downregulating RANKL-dependent early signaling pathways. (A) BMMs were cultured for 3 days in the presence of M-CSF (30 ng/ml) and RANKL (100 ng/ml) with or without Nampt (500 ng/ml) for the indicated durations. Cells were then stained with TRAP solution and images of TRAP+ cells were captured under a light microscope (magnification, 5x). The diagram shows the time period of Nampt treatment. (B) TRAP+ MNCs with >5 nuclei were counted as osteoclasts. ***P<0.001 vs. control group. (C) BMMs were pretreated with or without Nampt (500 ng/ml) for 1 h in the presence of M-CSF (30 ng/ml) prior to RANKL (100 ng/ml) stimulation at the indicated time points. Whole-cell lysates were analyzed by western blotting with the indicated antibodies. β-actin was used as the internal control. (D) Semi-quantification of blots was performed using ImageJ. ***P<0.001 vs. the control group (E) BMMs were pretreated with or without of Nampt (500 ng/ml) for 1 h in the presence of M-CSF (30 ng/ml) prior to RANKL (100 ng/ml) stimulation at the indicated time points. Whole-cell lysates were analyzed by western blotting with the indicated antibodies. β-actin was used as the internal control. (F) Semi-quantification of western blot bands was performed using ImageJ. ***P<0.001 vs. the control group. BMMs, bone marrow macrophages; Btk, Bruton's tyrosine kinase; ERK, extracellular signal-regulated protein kinases; GSK3β, glycogen synthase kinase-3 β; JNK, c-Jun N-terminal kinase; M-CSF, macrophage colony-stimulating factor; MNCs, mononucleated cells; N, nuclei; Nampt, nicotinamide phosphoribosyltransferase; p-, phosphorylated; PLCγ2, phospholipase C γ-2 RANKL, receptor activator of nuclear factor-κB ligand; TRAP, tartrate-resistant acid phosphatase.
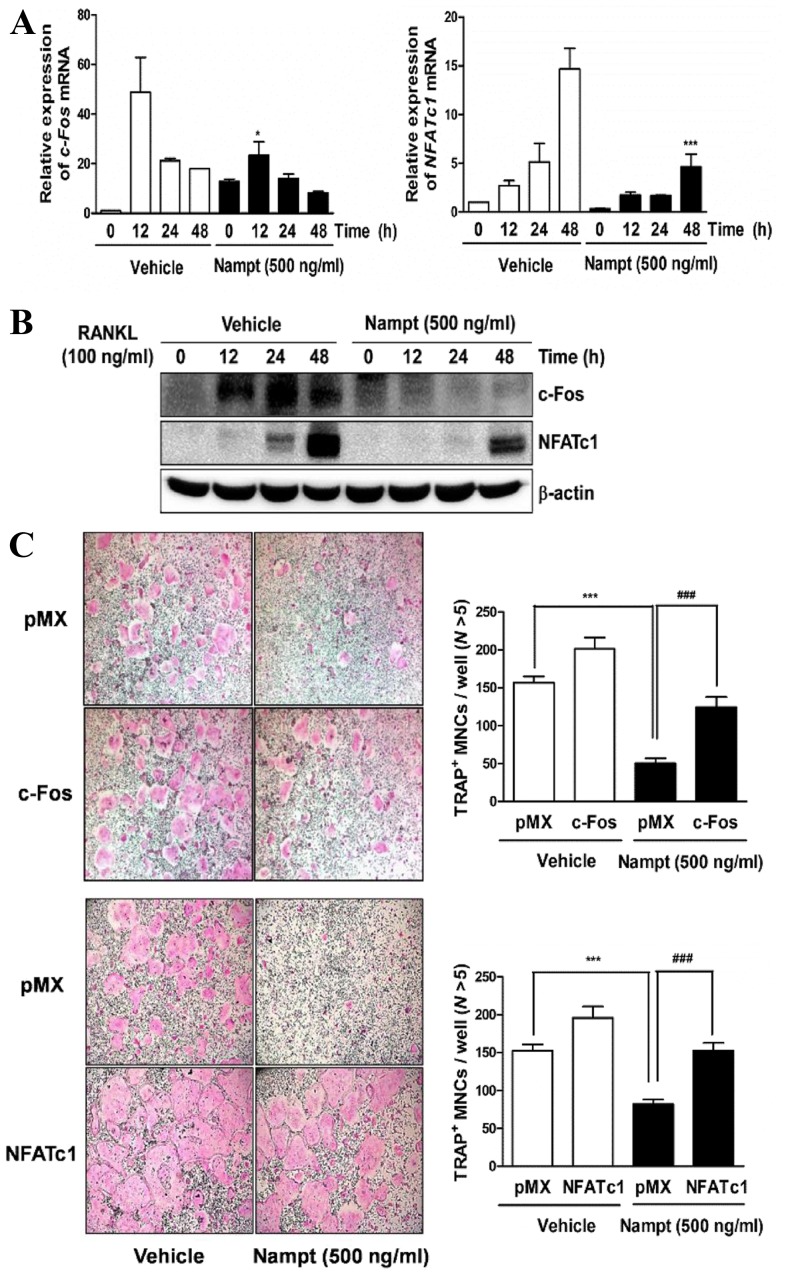
Nampt downregulates the expression levels of c-Fos, NFATc1 and osteoclast-specific marker genes
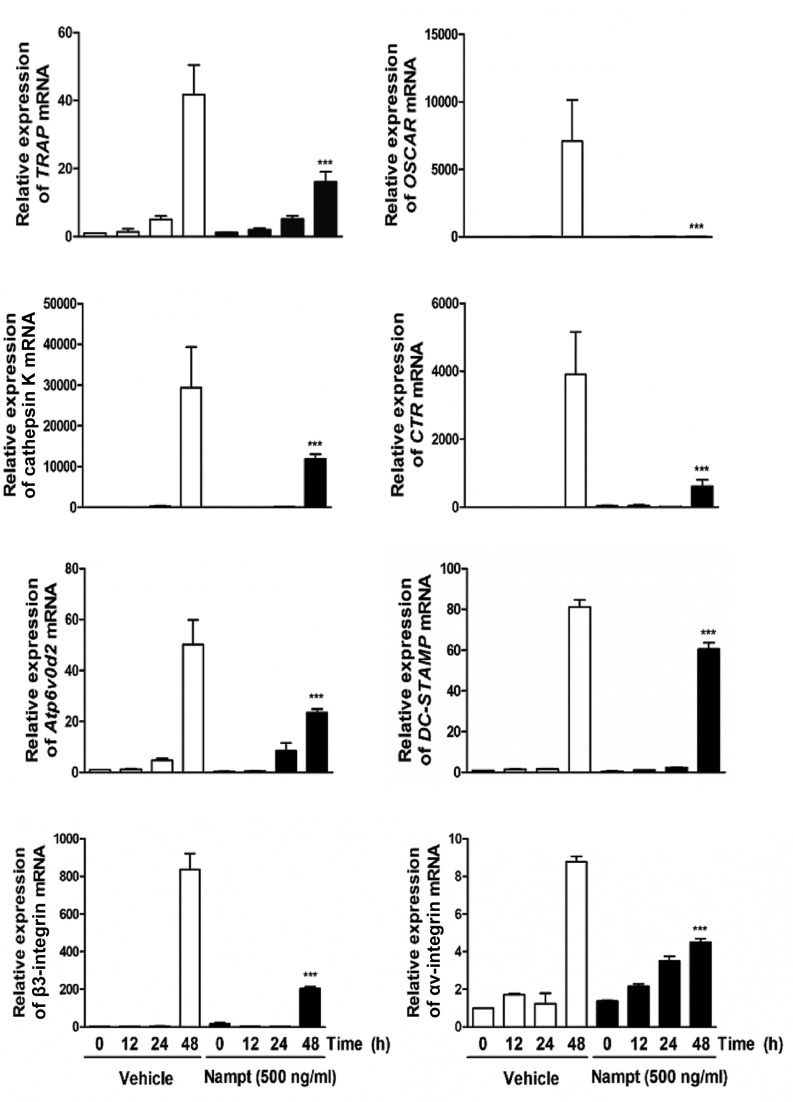
To examine whether Nampt regulates RANKL-induced osteoclast differentiation by downregulating the activation of c-Fos and NFATc1, the present study evaluated the effects of Nampt on RANKL-induced c-Fos and NFATc1 expression. When BMMs were stimulated for 12–48 h with RANKL (100 ng/ml), the mRNA expression levels of c-Fos and NFATc1 were increased in the control group, whereas Nampt treatment reduced their expression (Fig. 3A). Similarly, western blot analysis demonstrated that Nampt significantly reduced the protein levels of c-Fos and NFATc1 (Fig. 3B). Subsequently, the present study examined whether ectopic expression of c-Fos or NFATc1 is sufficient to rescue the inhibitory effects of Nampt on osteoclastogenesis using a retroviral system. BMMs were infected with c-Fos or NFATc1-encoding retroviruses and cultured with M-CSF (30 ng/ml) and RANKL (100 ng/ml) in the presence or absence of Nampt (500 ng/ml). Indeed, the overexpression of c-Fos or NFATc1 rescued the anti-osteoclastogenic effect of Nampt (Fig. 3C). In addition, it was determined whether Nampt regulates the mRNA expression of various osteoclast-specific transcription factors, including TRAP, osteoclast associated receptor (OSCAR), cathepsin K, calcitonin receptor (CTR), Atp6v0d2, dendritic cell-specific transmembrane protein (DC-STAMP), αv-integrin and β3-integrin. These genes are associated with osteoclast formation and function during RANKL-induced osteoclast differentiation. The mRNA expression levels of TRAP, OSCAR, cathepsin K, CTR, Atp6v0d2, DC-STAMP, αv-integrin and β3-integrin were significantly decreased by Nampt (Fig. 4). These results suggested that Nampt efficiently inhibits c-Fos and NFATc1 activation, leading to the downregulation of osteoclast marker gene expression during RANKL-mediated osteoclast differentiation.
Figure 3.
Nampt reduces the expression of c-Fos and NFATc1. (A) BMMs were pretreated with or without Nampt (500 ng/ml) for 1 h in the presence of M-CSF (30 ng/ml), and then stimulated with RANKL (100 ng/ml) for the indicated times. The mRNA expression levels of c-Fos and NFATc1 were analyzed by reverse transcription-quantitative polymerase chain reaction. *P<0.05, ***P<0.001 vs. control group at the indicated time points. (B) The effects of Nampt on protein levels of c-Fos and NFATc1 were evaluated by western blot analysis with the indicated antibodies. β-actin was used as the internal control. (C) BMMs were infected with retroviruses expressing pMX-IRES-EGFP (pMX), pMX-cFos-IRES-EGFP (c-Fos) or pMX-NFATc1-IRES-EGFP (NFATc1). Infected BMMs were cultured with or without Nampt (500 ng/ml) in the presence of M-CSF (30 ng/ml) and RANKL (100 ng/ml) for 4 days. After culturing, the cells were stained with TRAP solution. Images of TRAP+ cells were captured under a light microscope (magnification, 5x). TRAP+ MNCs with >5 nuclei were counted as osteoclasts. ***P<0.001 vs. control group and ###P<0.001 vs. Nampt group. BMMs, bone marrow macrophages; M-CSF, macrophage colony-stimulating factor; MNCs, mononucleated cells; N, nuclei; Nampt, nicotinamide phosphoribosyltransferase; NFATc1, nuclear factor of activated T cells, cytoplasmic 1; RANKL, receptor activator of nuclear factor-κB ligand; TRAP, tartrate-resistant acid phosphatase.
Figure 4.
Nampt reduces the expression of osteoclast-specific genes. BMMs were pretreated with or without Nampt (500 ng/ml) for 1 h in the presence of M-CSF (30 ng/ml) and then stimulated with RANKL (100 ng/ml) for the indicated times. Total RNA was isolated from cells using QIAzol reagent and the mRNA expression levels of TRAP, OSCAR, cathepsin K, CTR, Atp6vOd2, DC-STAMP, β3-integrin and αv-integrin were evaluated by reverse transcription-quantitative polymerase chain reaction. ***P<0.001 vs. control group at the indicated time. BMMs, bone marrow macrophages; CTR, calcitonin receptor; DC-STAMP, dendritic cell-specific transmembrane protein; M-CSF, macrophage colony-stimulating factor; Nampt, nicotinamide phosphoribosyltransferase; OSCAR, osteoclast-associated receptor; RANKL, receptor activator of nuclear factor-κB ligand; TRAP, tartrate-resistant acid phosphatase.
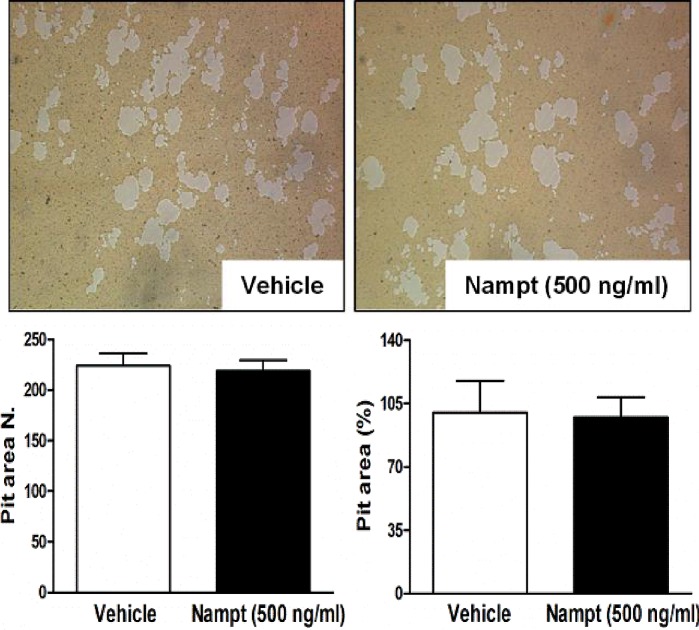
Nampt is not associated with the bone-resorbing activity of mature osteoclasts
The present study subsequently examined whether Nampt regulates osteoclastic bone-resorptive functions. To investigate this role, mature osteoclasts were seeded onto the top of hydroxyapatite-coated plates in the presence or absence of Nampt (500 ng/ml). However, the number and area of resorption pits were unaffected by Nampt treatment (Fig. 4), suggesting that Nampt does not have a role in the resorbing activity of mature osteoclasts.
Discussion
The present study demonstrated that Nampt attenuated RANKL-mediated differentiation of primary mouse BMMs into TRAP+ MNCs in a dose-dependent manner without cytotoxic effects. During this process, Nampt decreased the phosphorylation of various early signal transducers, including JNK and Akt, and its downstream target, GSK3β, as well as calcium-dependent signaling pathways, including PLCγ2 and Btk. Furthermore, the mRNA and protein expression levels of two master regulators of osteoclastogenesis, c-Fos and NFATc1, were significantly decreased by Nampt treatment, leading to decreased expression levels of various key transcription factors in osteoclast differentiation including TRAP, OSCAR, cathepsin K, CTR, Atp6vOd2, DC-STAMP, β3- and αv-integrin.
The differentiation of monocyte/macrophage lineage precursors into bone-resorbing osteoclasts is initiated in response to two important cytokines, M-CSF and RANKL, resulting in the activation of early downstream pathways (15). During this process, the phosphorylation of numerous signal transducers, including mitogen-activated protein kinases (MAPKs), which are comprised of p38, ERK and JNK; nuclear factor-κB; phosphatidylinositol 3-kinase/Akt; PLCγ2 and Btk occurs (16–19). RANKL-mediated activation of JNK is known to have an anti-apoptotic function in osteoclastogenesis, and Akt is a potent inducer of osteoclast differentiation by promoting the formation of an inactive form of GSK3β (p-GSK3β) and the nuclear translocation of NFATc1 (20,21). In addition, it has been well established that calcium signaling is crucial for RANKL-dependent osteoclastogenesis. PLCγ2 activation requires phosphorylation of its tyrosine residues to induce calcium oscillations and the translocation of NFATc1 by forming a complex with regulatory adapter molecule GRB2-associated binding protein 2 and modulating its recruitment to RANK (22,23). PLCγ2 is regulated by the upstream tyrosine kinase Btk, which is involved in osteoclast differentiation by linking RANK and immunoreceptor tyrosine-based activation motif signaling, with subsequent regulation of the formation of Btk/BLNK-containing complex and activation of PLCγ2-dependent calcium signaling (19). The results of the present study revealed that Nampt suppressed RANKL-induced osteoclast differentiation by interfering with survival-related signaling pathways that contain JNK and Akt, as well as Btk-PLCγ2-dependent intracellular calcium signaling. Since Nampt affected several signals associated with the early stages of osteoclastogenesis, the present study examined whether Nampt is involved in the expression of late-stage transcription factors, c-Fos and NFATc1. A previous report indicated that c-Fos knock-out mice exhibit morphological characteristics of osteopetrosis, owing to osteoclast malfunction, whereas impaired osteoclastogenesis in murine BMMs is completely rescued by exogenous overexpression of c-Fos (24,25). In response to the activation of c-Fos, another master regulator, NFATc1, serves a crucial role in osteoclast differentiation. NFATc1 inhibition in embryonic stem cells suppresses their ability to differentiate into normal osteoclasts, and this phenomenon is reversed by ectopic expression of NFATc1 even in the absence of RANKL (26,27). This c-Fos-NFATc1 activation cascade leads to the elevated expression of osteoclast-specific gene markers, such as TRAP, OSCAR, CTR, cathepsin K, DC-STAMP and β3-integrin. The present data revealed that Nampt expression significantly decreased mRNA and protein levels of c-Fos and NFATc1 compared with the control, resulting in the downregulation of various transcription factors associated with osteoclast formation and function.
Although the relationship between bone regulation and Nampt has previously been reported, the present study is the first, to the best of our knowledge, to identify the effects of Nampt on mouse BMM-derived osteoclastogenesis and its molecular mechanisms. In conclusion, the present study demonstrated that the adipokine Nampt effectively interferes with RANKL-mediated osteoclast differentiation by inactivating several early signal transducers. These signaling molecules include JNK, Akt and GSK3β, as well as Btk-PLCγ2-calcium signaling. Furthermore, Nampt treatment decreases c-Fos and NFATc1 mRNA and protein levels, resulting in the downregulation of various target gene mRNA levels. Nampt does not influence the bone-resorbing activity of mature osteoclasts; instead, Nampt exerts its anti-osteoclastogenic effects by targeting osteoclast precursors rather than mature multinucleated osteoclasts. Although further studies are required to reveal the restorative effect of Nampt on osteoporotic bone loss in mouse models, it may be suggested that increased Nampt is a potential target for the treatment of metabolic bone diseases, such as osteoporosis, by suppressing osteoclast differentiation and function.
Figure 5.
Nampt does not affect the bone-resorbing activity of mature osteoclasts. Mature osteoclasts were seeded on hydroxyapatite-coated plates and treated for 24 h with Nampt (500 ng/ml). Attached cells were removed and images were captured under a light microscope (magnification, ×5). Pit areas on the hydroxyapatite plate were quantified using the Image Pro-Plus (version 4.5) software. Nampt, nicotinamide phosphoribosyltransferase.
Acknowledgments
The present study was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant no. NRF-2016R1D1A1B03933712).
References
- 1.Samal B, Sun Y, Steams G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/MCB.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: A novel mediator of innate immunity. J Leukoc Biol. 2006;83:804–816. doi: 10.1189/jlb.0807581. [DOI] [PubMed] [Google Scholar]
- 3.Gosset M, Berenbaum F, Salvat C, Sautet A, Pigenet A, Tahiri K, Jacques C. Crucial role of visfatin/pre-B cell colony-enhancing factor in matrix degradation and prostaglandin E2 synthesis in chondrocytes: Possible influence on osteoarthritis. Arthritis Rheum. 2008;58:1399–1409. doi: 10.1002/art.23431. [DOI] [PubMed] [Google Scholar]
- 4.Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 5.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 6.Tan B, Dong S, Shepard RL, Kays L, Roth KD, Geeganage S, Kuo MS, Zhao G. Inhibition of nicotinamide phosphoribosyltransferase (NAMPT), an enzyme essential for NAD+ biosynthesis, leads to altered carbohydrate metabolism in cancer cells. J Biol Chem. 2015;290:15812–15824. doi: 10.1074/jbc.M114.632141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S, Ryu JH, Oh H, Jeon J, Kwak JS, Kim JH, Kim HA, Chun CH, Chun JS. NAMPT (visfatin), a direct target of hypoxia-inducible factor-2α, is an essential catabolic regulator of osteoarthritis. Ann Rheum Dis. 2015;74:595–602. doi: 10.1136/annrheumdis-2013-204355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Song CY, Wu SS, Liang QH, Yuan LQ, Liao EY. Novel adipokine and bone metabolism. Int J Endocrinol. 2013;2013:895045. doi: 10.1155/2013/895045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wattanachanya L, Lu WD, Kundu RK, Wang L, Abbott MJ, O'Carroll D, Quertermous T, Nissenson RA. Increased bone mass in mice lacking the adipokine apelin. Endocrinology. 2013;154:2069–2080. doi: 10.1210/en.2012-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamio N, Kawato T, Tanabe N, Kitami S, Morita T, Ochiai K, Maeno M. Vaspin attenuates RANKL-induced osteoclast formation in RAW264.7 cells. Connect Tissue Res. 2013;54:147–152. doi: 10.3109/03008207.2012.761978. [DOI] [PubMed] [Google Scholar]
- 11.Tu Q, Zhang J, Dong LQ, Saunders E, Luo E, Tang J, Chen J. Adiponectin inhibits osteoclastogenesis and bone resorption via APPL1-mediated suppression of Akt1. J Biol Chem. 2011;286:12542–12553. doi: 10.1074/jbc.M110.152405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkateshaiah SU, Khan S, Ling W, Bam R, Li X, van Rhee F, Usmani S, Barlogie B, Epstein J, Yaccoby S. NAMPT/PBEF1 enzymatic activity is indispensable for myeloma cell growth and osteoclast activity. Exp Hematol. 2013;41:547.e2–557.e2. doi: 10.1016/j.exphem.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moschen AR, Geiger S, Germer R, Tilg H. Pre-B cell colony enhancing factor/NAMPT/visfatin and its role in inflammation-related bone disease. Mutat Res. 2010;690:95–101. doi: 10.1016/j.mrfmmm.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Kim JY, Cheon YH, Oh HM, Rho MC, Erkhembaatar M, Kim MS, Lee CH, Kim JJ, Choi MK, Yoon KH, et al. Oleanolic acid acetate inhibits osteoclast differentiation by downregulating PLCγ2-Ca(2+)-NFATc1 signaling, and suppresses bone loss in mice. Bone. 2014;60:104–111. doi: 10.1016/j.bone.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10:1165–1177. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Udagawa N, Itoh K, Suda K, Murase Y, Nishihara T, Suda T, Takahashi N. p38 MAPK-mediated signals are required for inducing osteoclast differentiation but not for osteoclast function. Endocrinology. 2002;143:3105–3113. doi: 10.1210/endo.143.8.8954. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, Inoue J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001;20:1271–1280. doi: 10.1093/emboj/20.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gingery A, Bradley E, Shaw A, Oursler MJ. Phosphatidylinositol 3-kinase coordinately activates the MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival. J Cell Biochem. 2003;89:165–179. doi: 10.1002/jcb.10503. [DOI] [PubMed] [Google Scholar]
- 19.Shinohara M, Koga T, Okamoto K, Sakaguchi S, Arai K, Yasuda H, Takai T, Kodama T, Morio T, Geha RS, et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132:794–806. doi: 10.1016/j.cell.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda F, Matsubara T, Tsurukai T, Hata K, Nishimura R, Yoneda T. JNK/c-Jun signaling mediates an anti-apoptotic effect of RANKL in osteoclasts. J Bone Miner Res. 2008;23:907–914. doi: 10.1359/jbmr.080211. [DOI] [PubMed] [Google Scholar]
- 21.Moon JB, Kim JH, Kim K, Youn BU, Ko A, Lee SY, Kim N. Akt induces osteoclast differentiation through regulating the GSK3β/NFATc1 signaling cascade. J Immunol. 2012;188:163–169. doi: 10.4049/jimmunol.1101254. [DOI] [PubMed] [Google Scholar]
- 22.Wilde JI, Watson SP. Regulation of phospholipase C gamma isoforms in haematopoietic cells: Why one, not the other? Cell Signal. 2001;13:691–701. doi: 10.1016/S0898-6568(01)00191-7. [DOI] [PubMed] [Google Scholar]
- 23.Mao D, Epple H, Uthgenannt B, Novack DV, Faccio R. PLCgamma2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J Clin Invest. 2006;116:2869–2879. doi: 10.1172/JCI28775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wisdon R, Verma IM. Transformation by Fos proteins requires a C-terminal transactivation domain. Mol Cell Biol. 1993;13:7429–7438. doi: 10.1128/MCB.13.12.7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. c-Fos: A key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 26.Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007;1116:227–237. doi: 10.1196/annals.1402.071. [DOI] [PubMed] [Google Scholar]
- 27.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]