Abstract
Cardiac tumor is a rare cause of cerebral embolic infarction which has no established therapeutic strategies. Although some cases were successfully treated by recombinant tissue-plasminogen activator (rt-PA), this article indicates the effectiveness of emergency mechanical thrombectomy for tumorous type of emboli which do not respond to rt-PA. A 34-year-old man presented with ischemic stroke and right middle cerebral artery (MCA) occlusion by cardiac tumor originating emboli. Intravenous rt-PA therapy was ineffective, but mechanical endovascular thrombectomy using Merci Retriever was successful. His neurological deficit began to improve and good outcome was obtained at discharge. The embolus was histologically identical to a cardiac myxoma, confirmed and treated successfully by surgery later. We report the first successfully treated tumorous embolic stroke case with cardiac tumor by using Merci Retriever. Emergency mechanical thrombectomy would be an option for elastic hard myxoma emboli.
Keywords: cardiac tumor, cerebral infarction, mechanical endovascular thrombectomy, thrombectomy, myxoma
Introduction
Cardiac myxoma is a benign tumor and rarely becomes an embolic source.1) Emboli originating from cardiac myxoma are thought to be tumor emboli and/or thrombi around the tumor.2) Although some cases were successfully treated by recombinant tissue-plasminogen activator (rt-PA) for acute embolic stroke associated with cardiac myxoma,3–12) thrombolytic therapies did not seem to be effective for tumorous type of emboli. Garcia et al.13) treated two cases with cardiac myxoma emboli by mechanical endovascular thrombectomy using Solitaire FR Device (Ev3, Plymouth, Minnesota, USA) and Trevo (Concentric Medical, Mountain View, California, USA). Although their retrieved emboli were thrombi, they speculated on the usefulness of the Merci Retriever (Concentric Medical, Mountain View, California, USA) for hard thrombus originating from such cardiac tumor. To our knowledge, there is no report of embolic stroke case treated by Merci Retriever.
We treated a case with cerebral embolic stroke associated with cardiac myxoma, the embolus was successfully retrieved and the cardiac tumor was extirpated surgically. In this case, the retrieved embolus was histologically identical to the resected cardiac tumor.
Case Report
A 34-year-old right-handed man complained of sudden-onset left side weakness and difficulty of speaking. He had no relevant past history. He was transported to our hospital 34 minutes after the onset. On his arrival, Glasgow coma scale (GCS) was 14 (E3V5M6) and Japan coma scale (JCS) was 20. His blood pressure was 119/89 mmHg, and heart rate was regular. Neurological examinations revealed left hemiparesis and dysarthria. The National Institutes of Health Stroke Scale (NIHSS) score was 9. He was a current smoker, 1 pack/day. His laboratory data and electrocardiogram (ECG) were normal.
The brain computed tomography (CT) showed “early CT signs” in the right temporal and insular cortical areas with “hyperdense middle cerebral artery sign (HMCAS)” (Fig. 1). The brain magnetic resonance imaging (MRI) showed high signal intensity at the right middle cerebral artery (MCA) territory on diffusion-weighted imaging (DWI), mainly located in the temporal lobe, insular cortex, and corona radiata. MR angiography (MRA) visualized occlusion of the right MCA proximal portion (Fig. 1). Alberta Stroke Program Early CT Score (ASPECTS)14) was 8 and ASPECTS-DWI15) was 5.
Fig. 1.
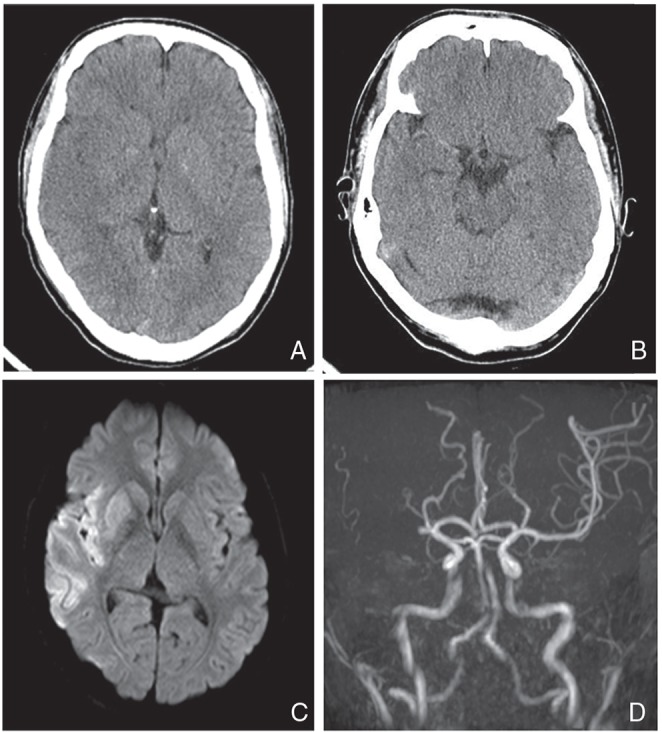
A CT scan showing loss of definition at the gray-white interface in the right temporal lobe and the insular cortex (A) with HMCAS (B). MRI showing high signal lesion in the right MCA territory on DWI (C). MRA indicates right M1 proximal occlusion (D). CT: computed tomography, HMCAS: hyperdense middle cerebral artery sign, MRI: magnetic resonance imaging, MCA: middle cerebral artery, DWI: diffusion-weighted imaging, MRA: magnetic resonance angiography.
For acute cerebral infarction, intravenous infusion (IV) of alteplase (0.6 mg/kg of rt-PA) was started at 113 minutes after the onset, but he did not improve neurologically and endovascular treatment was decided.
Cerebral angiography showed the right MCA proximal occlusion without cerebral aneurismal lesions (Fig. 2). Mechanical endovascular thrombectomy was carried out with Merci L5 Retriever (three passes: 2.5 soft × 2 and 3.0 firm). Each procedure was carried out under proximal flow control using 9Fr Optimo balloon-guiding catheter (Tokai Medical Products, Kasugai, Aichi) with continuous aspiration. Successful revascularization was obtained as achieving Thrombolysis in Cerebral Infarction (TICI)16) grade 3 (Fig. 2), and an elastic white embolus was retrieved by the third procedure using 3.0 firm Merci L5 Retriever (Fig. 3). Postoperative heparinization was not carried out.
Fig. 2.
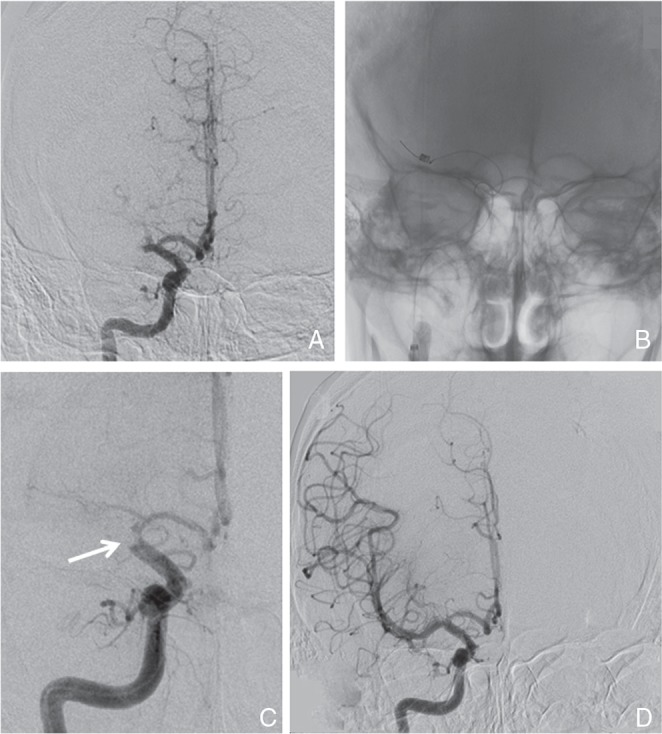
A: Right internal carotid angiography after IV rt-PA showing no recanalization of the right M1 occlusion and B: location of Merci L5 Retriever at the distal portion of occluded site. C: During the procedure, an embolus was retrieved to the terminal portion of the internal carotid artery showing as a round-shaped defect (arrow). D: Successful recanalization (TICI grade 3) was obtained. IV rt-PA: intravenous infusion recombinant tissue-plasminogen activator, TICI: thrombolysis in cerebral infarction.
Fig. 3.
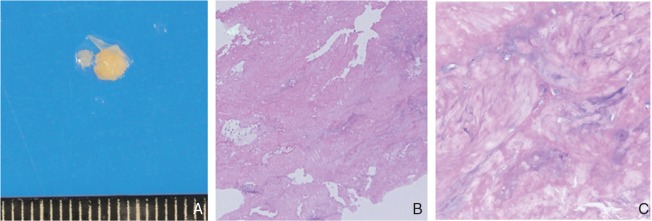
The retrieved embolus is round, whitish, and elastic hard (3 × 3 mm) (A). Specimen of the retrieved embolus demonstrates spindle cells with fibrinoid degeneration in myxoid matrix, not including red blood cell components, most like cardiac myxoma (B: H&E 10×, C: H&E 40×). H&E: hematoxylin and eosin.
After the procedure, patient’s consciousness and hemiparesis began to improve gradually. NIHSS score after 24 hours improved to 6. On the second day, brain CT showed asymptomatic intracerebral hemorrhage in the basal ganglia. Holter ECG showed no atrial fibrillation, and MRA showed no arteriosclerotic lesions of the carotid artery. The histology of retrieved embolus suggested tumorous tissue originating from a cardiac tumor (Fig. 3). In the test conducted subsequently, transesophageal echocardiography (TEE) visualized a mass lesion adherent to the anterior cusp of the mitral valve, which indicated cardiac myxoma (Fig. 4). Serum interleukin-6 (IL-6) concentration was elevated to 6.3 pg/ml. Anticoagulation therapy with warfarin (prothrombin time-international normalized ratio: PT-INR 2.0–3.0) was continued to prevent recurrent embolic strokes until surgical removal of the cardiac tumor.
Fig. 4.
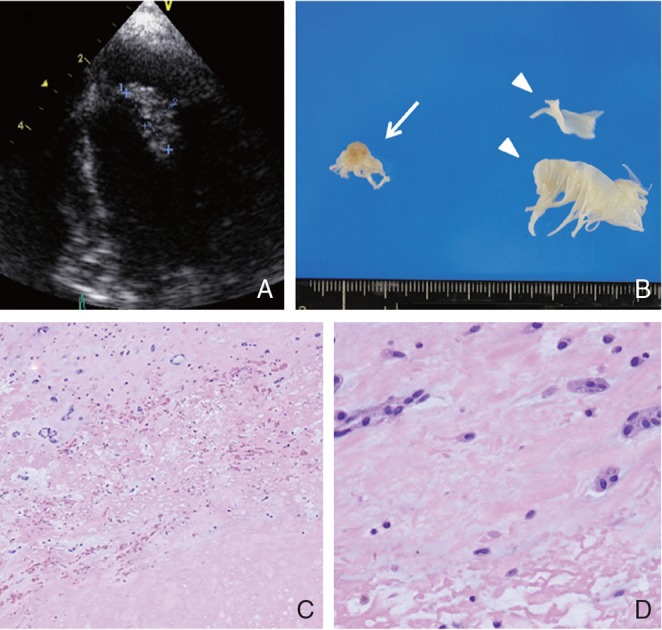
TEE visualizing a mass lesion (15 × 6 mm) adherent to the anterior cusp of the mitral valve indicating left atrial myxoma (A). Macroscopic findings of cardiac tumor (14 × 8 mm) removed by cardiosurgery (arrow), and normal anterior cusp of mitral valve (arrowhead) removed by open heart surgery (B). Specimen of cardiac tumor demonstrates paucicellular mesenchymal tumor with spindle cells in an abundant myxoid matrix, cardiac myxoma (C: H&E 40×, D: H&E 400×). TEE: transesophageal echocardiography, H&E: hematoxylin and eosin.
One month after the stroke, the cardiac tumor at the anterior cusp of mitral valve was removed with replacement of the mitral valve. The histology of the tumor was myxoma, which demonstrated spindle cells with marked fibrinoid degeneration in an abundant myxoid matrix that was identical to the embolus obtained by the thrombectomy from the MCA (Fig. 4). The histology of the anterior cusp of the mitral valve was normal. In our case, the embolus was composed of spindle cells with fibrinoid degeneration and was not mixed with thrombus. Both histopathological specimens showed almost similar characteristics. Patient’s 90 days NIHSS score was 1 and modified Rankin Scale (mRS) score was 2.
Discussion
This is the first case of successful mechanical endovascular thrombectomy using Merci Retriever for tumorous embolic MCA occlusion due to cardiac myxoma. Cardiac myxoma is a benign tumor and is a rare cause of cerebral embolism.1) The emboli originating from cardiac myxoma are thought to be tumor emboli and/or thrombus around the tumor.2)
Thrombolytic therapy for main cerebral arterial embolic occlusions associated with cardiac myxoma was reported in 12 cases, of which 8 cases were by IV rt-PA,3,6–12) 2 cases by IA rt-PA,4,5) and 2 cases by Urokinase.17,18) Out of these 12 cases, the treatment was effective in 8 of them3–6,8,10,11,17) and not in 4.7,9,12,18) Although, the tumorous embolus was suggested not to be indicated for rt-PA treatment,6) favorable outcomes were reported in some of these cases. In our case, within the histology of cardiac myxoma removed by surgery, intratumoral bleeding and thrombus formations were not observed. This indicates that in such cases the tumorous nature of embolus might be expected. Unfortunately, we did not have information on the cardiac tumor at the time of the neurological emergency.
Liebeskind et al. reported that HMCAS was notably linked with red blood cell (RBC)-dominant thrombi, but infrequent with fibrin rich thrombi.19) In our case, the pathology of the retrieved emboli contained less RBCs despite of HMCAS at the onset. Apparently, HMCAS can be found in patients with tumorous embolism showing acute cerebral ischemia without atrial fibrillation. Therefore, mechanical endovascular thrombectomy should be considered in addition for establishing complete recanalization in these cases.
In the only two embolic stroke cases with cardiac myxoma that were treated by mechanical endovascular thrombectomy, stent retriever (Solitaire FR Device, Trevo) was used.13) These authors speculated on the usefulness of the Merci Retriever for hard thrombus such as cardiac myxoma. To our knowledge, there is no report, which addresses the treatment for tumorous emboli from cardiac myxoma by Merci Retriever. In ineffective rt-PA treatment embolic cases, mechanical thrombectomy could be under consideration to obtain rapid recanalization.
Conclusion
We treated a cardiac myxoma embolus case successfully with Merci Retriever. This case showed that mechanical thrombectomy may be an option in rt-PA ineffective elastic hard tumorous emboli.
Footnotes
Conflicts of Interest Disclosure
There are no financial supports and industry affiliations. The authors have registered COI Disclosure Statement Forms for The Japan Neurosurgical Society.
References
- 1). McManus B, Lee CH. Primary tumors of the heart, in Libby P. (ed): Braunwald’s Heart Disease, ed 8 Philadelphia, Saunders, 2008, pp 1817– 1827 [Google Scholar]
- 2). Wold LE, Lie JT: Cardiac myxomas: a clinicopathologic profile. Am J Pathol 101: 219– 240, 1980. [PMC free article] [PubMed] [Google Scholar]
- 3). Ibrahim M, Iliescu C, Safi HJ, Buja ML, McPherson DD, Fuentes F: Biatrial myxoma and cerebral ischemia successfully treated with intravenous thrombolytic therapy and surgical resection. Tex Heart Inst J 35: 193– 195, 2008. [PMC free article] [PubMed] [Google Scholar]
- 4). Yamanome T, Yoshida K, Miura K, Ogawa A: [Superselective fibrinolysis for a middle cerebral artery embolism caused by a left atrial myxoma: case report]. No Shinkei Geka 28: 653– 658, 2000. (Japanese) [PubMed] [Google Scholar]
- 5). Gassanov N, Nia AM, Dahlem KM, Ederer S, Wedemeyer I, Caglayan E, Erdmann E, Er F: Local thrombolysis for successful treatment of acute stroke in an adolescent with cardiac myxoma. Scientific World Journal 11: 891– 893, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Abe M, Kohama A, Takeda T, Ishikawa A, Yamada Y, Kawase Y, Ishii Y, Bessho R, Oaki Y, Haruta S, Ohashi T: Effective intravenous thrombolytic therapy in a patient with cerebral infarction associated with left atrial myxoma. Intern Med 50: 2401– 2405, 2011. [DOI] [PubMed] [Google Scholar]
- 7). Kohno N, Kawakami Y, Hamada C, Toyoda G, Bokura H, Yamaguchi S: Cerebral embolism associated with left atrial myxoma that was treated with thrombolytic therapy. Case Rep Neurol 4: 38– 42, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Nagy CD, Levy M, Mulhearn TJ, 4th, Shapland M, Sun H, Yuh DD, Cheung D, Chandra-Strobos N: Safe and effective intravenous thrombolysis for acute ischemic stroke caused by left atrial myxoma. J Stroke Cerebrovasc Dis 18: 398– 402, 2009. [DOI] [PubMed] [Google Scholar]
- 9). Ong CT, Chang RY: Intravenous thrombolysis of occlusion in the middle cerebral and retinal arteries from presumed ventricular myxoma. Stroke Res Treat 2011: 735057, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Nishimura H, Nakajima T, Ukita T, Tsuji M, Miyake H, Ohmura T, Tachibana H: A case of acute cerebral infarction associated with left atrial myxoma treated by intravenous tissue plasminogen activator. Jpn J Stroke 32: 156– 162, 2010. [Google Scholar]
- 11). Sun MC, Tai HC, Lee CH: Intravenous thrombolysis for embolic stroke due to cardiac myxoma. Case Rep Neurol 3: 21– 26, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Chong JY, Vraniak P, Etienne M, Sherman D, Elkind MS: Intravenous thrombolytic treatment of acute ischemic stroke associated with left atrial myxoma: a case report. J Stroke Cerebrovasc Dis 14: 39– 41, 2005. [DOI] [PubMed] [Google Scholar]
- 13). Garcia-Ptacek S, Matias-Guiu JA, Valencia-Sánchez C, Gil A, Bernal-Becerra I, De las Heras-Revilla V, Serna-Candel C: Mechanical endovascular treatment of acute stroke due to cardiac myxoma. J Neurointerv Surg 6: e1, 2014. [DOI] [PubMed] [Google Scholar]
- 14). Barber PA, Demchuk AM, Zhang J, Buchan AM: Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 355: 1670– 1674, 2000. [DOI] [PubMed] [Google Scholar]
- 15). Hirai T, Sasaki M, Maeda M, Ida M, Katsuragawa S, Sakoh M, Takano K, Arai S, Hirano T, Kai Y, Kakeda S, Murakami R, Ikeda R, Fukuoka H, Sasao A, Yamashita Y, Acute Stroke Imaging Standardization Group-Japan (ASIST-Japan) Investigators : Diffusion-weighted imaging in ischemic stroke: effect of display method on observers’ diagnostic performance. Acad Radiol 16: 305– 312, 2009. [DOI] [PubMed] [Google Scholar]
- 16). Higashida RT, Furian AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, Sacks D, Technology Assessment Committee of the American Society of Interventional and Therapeutic Neuroradiology; Technology Assessment Committee of the Society of Interventional Radiology : Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 34: e109– e137, 2003. [DOI] [PubMed] [Google Scholar]
- 17). Sugawara T, Takahashi A, So K, Yoshimoto T, Suzuki J, Suzuki Y, Horiuchi T: [A case of cerebral embolism caused by atrial myxoma—superselective fibrinolytic therapy]. No Shinkei Geka 15: 1321– 1326, 1987. (Japanese) [PubMed] [Google Scholar]
- 18). Bekavac I, Hanna JP, Wallace RC, Powers J, Ratliff NB, Furlan AJ: Intra-arterial thrombolysis of embolic proximal middle cerebral artery occlusion from presumed atrial myxoma. Neurology 49: 618– 620, 1997. [DOI] [PubMed] [Google Scholar]
- 19). Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, Zheng DD, Abolian AM, Kim D, Ali LK, Shah SH, Towfighi A, Ovbiagele B, Kidwell CS, Tateshima S, Jahan R, Duckwiler GR, Viñuela F, Salamon N, Villablanca JP, Vinters HV, Marder VJ, Saver JL: CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 42: 1237– 1243, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]