Abstract
Gorham’s disease is a rare disorder of unknown etiology and variable clinical presentation and is characterized by the proliferation of lymphatic vessels associated with massive regional osteolysis. Although 10 cases involving the skull and cervical spine have been reported in the literature, little is available concerning the surgical treatment of either atlantoaxial dislocation or basilar impression. Most cases have experienced universally unsuccessful treatment with bone grafts, which have led to dissolution. This case report describes the clinical course, and radiotherapeutic, medical, and surgical treatment for Gorham’s disease with basilar impression and massive osteolysis of the skull and upper cervical spine. The case of a 27-year-old man with progressive massive osteolysis of the skull and cervical spine is reported. Multiple surgical treatments to decompress the spinal cord and stabilize the skull and upper cervical spine with autologous fibular grafts were performed in order to prevent the progression of atlantoaxial dislocation and basilar impression. Pathologically, radiotherapy failed to show any effect. The efficacy of antiresorptive therapy with bisphosphonates could not be confirmed either clinically or radiologically. Although solid bone fusion was not obtained, the patient has achieved a satisfactory functional outcome and remains completely active after repeated surgeries. Surgical treatment is extremely difficult in cases of Gorham’s disease involving the skull and upper cervical spine. Fibular bone grafts seem to show resistance to erosion to osteolytic tissue.
Keywords: Gorham’s disease, basilar impression, massive osteolysis, radiotherapy, surgical treatment
Introduction
Gorham’s disease is a rare disorder of unknown etiology, which is characterized by massive osteolysis of a portion of the bone involved. Idiopathic osteolysis was first reported in 1838 by Jackson, who reported the case of “a boneless arm”, describing a 12-year-old boy with advanced osteolysis of the humerus.1) In 1955, Gorham and Stout tabulated 24 previously reported cases and concluded that the massive osteolysis in those cases was associated with angiomatosis of the blood or lymphatic vessels.2) This condition has since become known as Gorham’s disease.
To date, more than 200 cases of Gorham’s disease have been reported in the literature; however, only 10 of these cases involved the skull and cervical spine (Table 1).3–11) Only two cases exhibited kyphosis or subluxation of the cervical spine due to massive osteolysis, and one of them was associated with Chiari’s malformation with basilar impression.5,10)
Table 1.
Previously reported cases with involvement of both skull and cervical spine
| First author, year | Age | Sex | Affected bone(s) | Clinical signs | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Ellis et al. (1971)4) | 61 | F | Mandible, maxilla, sphenoid, palate, & cervical vertebrae | Lower jaw being eaten up & speech disorder | Biopsy | Progression of disease; death from transection of spinal cord at C4 |
| Hoffman et al. (1980)7) | 7 | F | Petrous, sphenoid, & C1–2 | Cerebrospinal fluid rhinorrhea, meningitis, torticollis, & headache | RT | AD; remineralization |
| Kurczynski et al. (1981)9) | 14 | F | Left orbit, zygoma, mandible, sphenoid, & C1 | Pressure pain of occiput & enophthalmia | RT | AD; remineralization |
| Heffez et al. (1983)6) | 13 | M | Mandible, hyoid, zygoma, occiput, sphenoid, maxilla, C1, & C4–6 | Loose mandibular molar & depression on left side of face | RT | AD; remineralization |
| Dunbar et al. (1993)3) | 40 | M | Occiput & C1–2 | Neck pain | RT | AD |
| - | - | Occiput & C1–2 | Neck stiffness | RT | AD | |
| Khosrovi et al. (1997)8) | 62 | M | Occiput & C1–2 | Headache & pneumocephalus | RT | AD; remineralization |
| Mark et al. (1997)11) | 6 | M | Skull base & C1–3 | Neck pain & lymphedema | RT & HV | AD; remineralization |
| Girn et al. (2006)5) | 2 | F | Skull base & cervical spine | Lump in temporal region, head-ache, deafness, paraplegia, & Arnold-Chiari malformation | Pamidronate, laminectomy, & RT | Progression of disease; death from chylothorax |
| Lekovic et al. (2006)10 | 10 | M | Clivus, petrous, & C1–2 | Painful neck, torticollis, dysphagia, & quadriparesis | Posterior occiput-C4 fusion, transoral odontoidectomy, HV, & RT | Unstable spine; HV required |
-: unknown, RT: radiotherapy, HV: halo vest, AD: arrest disease.
The present case report describes Gorham’s disease in a 27-year-old man who developed both atlantoaxial dislocation and basilar impression due to massive osteolysis of the skull and upper cervical spine.
Case Report
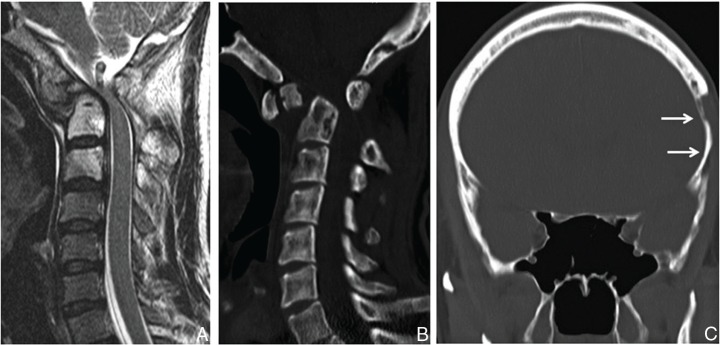
A 27-year-old man presented with bilateral upper-limb hypoesthesia and nagging neck pain of 2 months’ duration. There was no history of trauma. Shortly thereafter, he rapidly developed disturbances in gait and deglutition. Neurological examination revealed muscular weakness in the right upper limb and hypoesthesia of the extremities. Deep tendon reflexes were hyperactive and equal bilaterally in both upper and lower limbs. Bowel and bladder function was normal. Radiographs of the cervical spine revealed atlantoaxial dislocation due to osteolysis. Magnetic resonance imaging (MRI) scans revealed severe compression at the level of atlantoaxial dislocation and high intensity in the C2 vertebral body (Fig. 1A). Reconstructed computed tomography (CT) scanning of the skull and cervical spine revealed thinning of the whole skull and a pathological fracture of the odontoid process (Fig. 1B, C).
Fig. 1.
Preoperative sagittal T2-weighted MRI revealing compression at the level of atlantoaxial dislocation (A). Sagittal (B) and coronal (C) reconstructed CT images showingpathological fracture of the odontoid process and massive osteolytic lesion in the temporal bone (solid arrows). CT: computed tomography, MRI: magnetic resonance imaging.
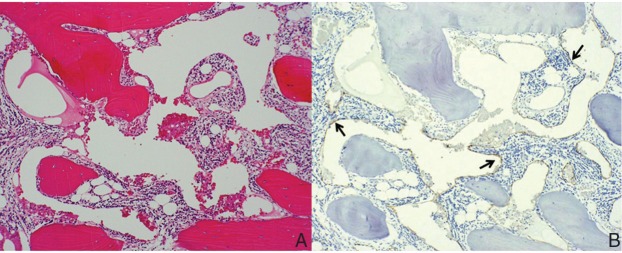
Emergency laminectomy of levels C1 to C2 with suboccipital decompression was performed in order to decompress the spinal cord, and a halo brace was used. During surgery, it was noted that the bone quality of the basilar bone and upper cervical spine appeared highly soft and spongy. The patient immediately experienced a significant neurological improvement, regaining normal muscular strength in the extremities and recovering the ability of deglutition. Four months after surgery, he continued to require a halo brace, which was removed due to a part of the skull pin penetrating the fragile skull bone. Histological examination of the C2 spinous process showed evidence of inflammatory granulation tissue and thin-walled vascular channels, with a positive result for lymphatic endothelial markers in immunohistochemical examination. The appearance was consistent with lymphangiomatosis (Fig. 2). A diagnosis of Gorham’s disease was made, based on the clinical, radiographic, and pathological findings.
Fig. 2.
Photomicrograph of hematoxylin and eosin staining of the resected specimen revealing both bone lamella and irregular fibrous tissue, and in part, granulation tissue with neutrophils, plasma cells and lymphocytes in the marrow cavity. Original magnification ×25 (A). Immunohistochemical staining for D2-40 revealing positive endothelial cells in many dilated, thin-walled and vascular channels, as shown in light brown (solid arrows). Original magnification ×25 (B).
Radiotherapy with a dose of 38 Gy in 19 fractions was administered to the occipital bone and C1–2 in an attempt to arrest the disease progression and improve the quality and density of the bone. Although the patient was well, radiographs revealed no evidence of either disease progression or remodeling of the affected bone. Six months after the radiotherapy, an open biopsy was performed to assess the effects which were not detected in the pathological examination (Fig. 3). Medical treatment by oral administration of alendronate sodium hydrate (35 mg/week) and an intravenous drip of zoledronic acid (4 mg/month) was started. The clinical course remained stable for 4.2 years after posterior cervical decompression by way of a sternal occiput mandibular immobilization (SOMI)-type brace; however, a lateral radiogram of the cervical spine showed gradual invagination of the foramen magnum and upper cervical spine to the base of the skull, namely basilar impression.
Fig. 3.
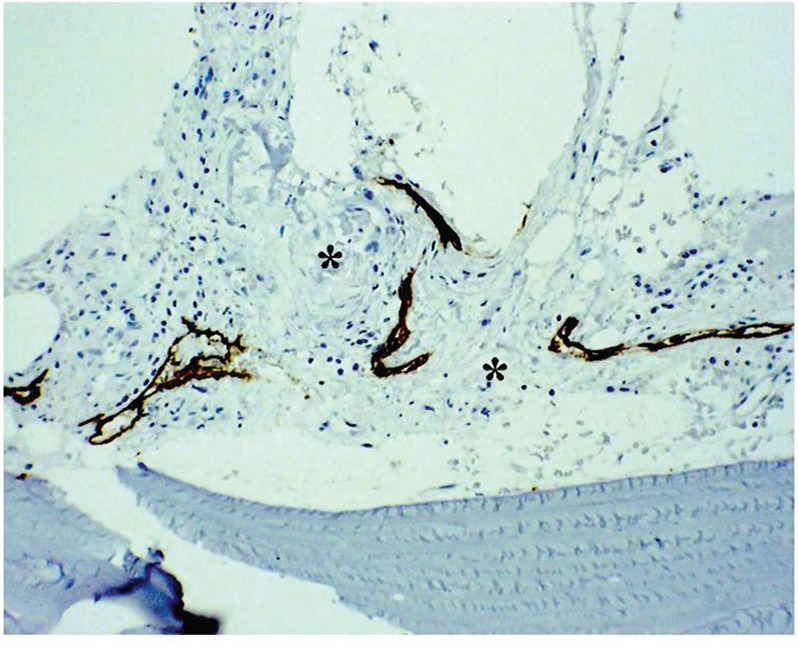
Repeated D2-40 immunohistochemical examination of the C2 spinous process revealing absent bone in part replaced by fibrous tissue (asterisk). Original magnification ×50.
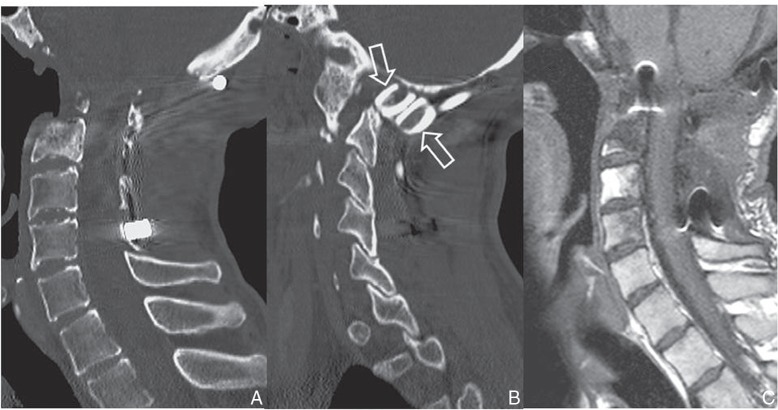
He then presented with facial hypoesthesia, deglutition disorder, and spastic gait. MRI revealed spinal cord and medulla oblongata compression at the level of the basilar impression. Although posterior suboccipital decompression was performed, a CT scan of the head and cervical spine revealed the progression and severity of basilar impression due to loss of atlantoaxial supporting structures, with the tip of the odontoid process far above McGregor’s line (Fig. 4).12) Posterior occiput-C5 stabilization with the use of sublaminar/ occipital cables (ultra-high molecular weight polyethylene tapes, Nesplon; Alfresa, Inc., Osaka), contoured rods and autologous bone was performed. Although he showed improvement in neurological function, there was no radiological evidence of bone fusion. Three months later, transoral decompression surgery, which involved odontoidectomy and C2–3 vertebrectomy, was performed (Fig. 5).
Fig. 4.
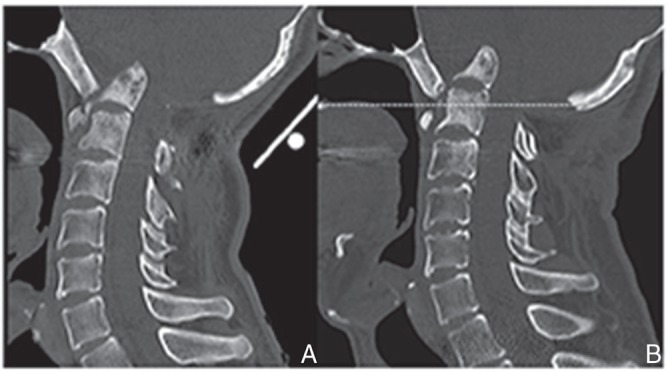
Two years after first surgery, reconstructed sagittal CT image revealing the progress of basilar impression with osteolysis of the anterior arch of C1 and isolated fragment of the odontoid process (A). At 4.2-year follow-up, CT image revealing the tip of the odontoid process more than 30 mm above McGregor’s line (dotted line) (B). CT: computed tomography.
Fig. 5.
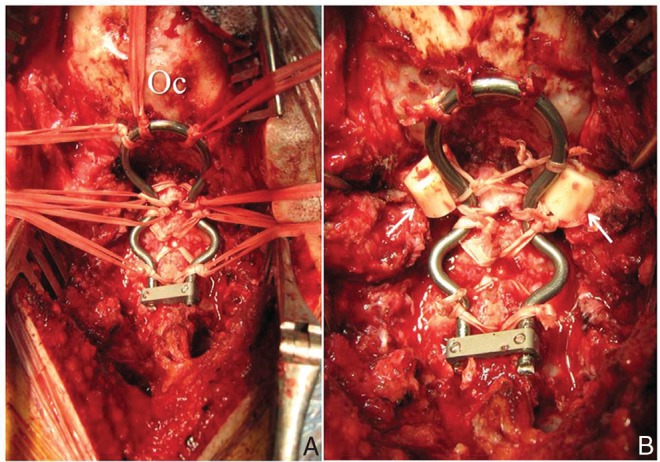
Intraoperative photograph showing posterior occiput-C5 stabilization with the use of sublaminar/occipital cables and contoured rods (A). Autologous fibular grafts (solid arrows) are integrally connected with the cables for fixation between occipital bone and upper cervical spine (B). Oc: occipital bone.
At 1 year after that surgery, he developed incisional surgical site methicillin-resistant Staphylococcus aureus infection (SSI) and rhinolalia due to mucosal defect in the epipharynx. SSI was successfully treated by repeated debridement, and the untreated rhinolalia was gradually improved by conservation. At the 2-year follow-up evaluation after the final surgery, the spine remained stable radiologically, although solid bone fusion had not been obtained (Fig. 6). At the latest follow-up, 7 years from the first visit, he was neurologically asymptomatic with the use of a cervical collar.
Fig. 6.
Two years after the reconstruction surgery, a mid-sagittal CT image of the upper cervical spine revealing odontoidectomy and C2–3 vertebrectomy defect (A). Parasagittal section showing autologous fibular grafts (open arrows), without resorption or bone fusion (B). Sagittal T1-weighted MRI showing sufficient decompression of the medulla oblongata and spinal cord (C). CT: computed tomography, MRI: magnetic resonance imaging.
Discussion
A diagnosis of Gorham’s disease is based on a combination of clinical, radiographic, and histological examinations. Metastasis, infection, and aseptic necrosis must be excluded by biopsy. The following eight criteria were suggested by Heffez et al.,6) to distinguish Gorham’s disease from other diseases involving bone destruction: (1) a positive biopsy for angiomatous tissue; (2) absence of cellular atypia; (3) minimal or no osteoblastic response, and absence of dystrophic calcification; (4) evidence of local progressive osseous resorption; (5) nonexpansile, nonulcerative lesions; (6) absence of visceral involvement; (7) an osteolytic radiographic pattern; and (8) negative hereditary, metabolic, neoplastic, immunological, or infectious etiology.
Gorham’s disease can involve either sex and any age group, although most cases are discovered before the age of 40 years. Neither connected familial nor hereditary predisposition seems to be a factor in its incidence. Although the frequently affected bones are the clavicle, scapula, humerus, ribs, and pelvis, any bone in the body can be affected.13) The patient is often asymptomatic because the bone erosion itself is painless; however, a pathological fracture often leads to its discovery. Spontaneous arrest of this disease without treatment has been reported,14) but the common course is progression to deformity and disability. Spinal dislocation and chest involvement, which often lead to chylothorax, are the most frequent causes of death associated with this disease.5)
No standardized treatment for Gorham’s disease has yet been established, due to the poor understanding of its etiology. Radiotherapeutic, medical, and surgical treatments for this disease have been selected, alone or in combination, but the results have been far from satisfactory. Radiotherapy has proven to be successful in many cases,3,6–9,11) and some authors have reported the regrowth of bone.8,9),11) However, others have stated that radiotherapy could not halt the progression of the disease.5,10) In the present case, radiotherapy showed little effect on the pathology.
Some authors have reported the ability of bisphosphonates and/or interferon to stabilize bone destruction.15–18) Silva reported that early potent antiresorptive therapy with bisphosphonates may prevent progressive local osteolysis.19) On the other hand, pamidronate combined with radiotherapy and surgical treatment has not been of any benefit.5) In the present case, bisphosphonates were administered for 6 years; however, their effect could not be confirmed either clinically or radiologically. There was no clear progression of the osteolysis; however, the patient had worsened neurological symptoms because of the deterioration of the basilar impression over time.
Surgical treatment has been proposed as the method of choice; however, standard bone-grafting methods have yielded poor results in the majority of cases, and many authors have reported a high incidence of resorption.20,21) Turra et al. reported that the reconstruction of a pathological fracture of the humerus in a 19-year-old male was successful using an autologous fibular strut graft, and the cortical bone showed greater resistance than the cancellous bone to erosion to osteolytic tissue.22)
Surgical results for the skull and cervical spine have been discouraging clinically.5,10) In cases with spinal involvement that have been treated surgically, both instability and dislocation due to massive bone destruction are frequent findings and must be managed without delay; treatment based on spinal cord decompression and column stabilization are problematic because of the technical difficulty, resorption of the graft, and other factors.23,24) In the present case, occipitocervical fusion was extremely difficult because of thinning in the occiput, poor bone quality, and the absence of a suitable site for the bone graft and resulted in pseudoarthrosis. However, the fibular grafts have not been resorbed, and so the anterior or vertical atlantoaxial subluxation have not been aggravated for 2 years.
Gorham’s disease with skull involvement is difficult to treat with the spinal instrumentation used for rigid fixation. The combination of repeated surgery, administration of bisphosphonates and application of a cervical collar has allowed the present subject to remain active for 7 years from the first visit. This case highlights the need for careful follow-up because solid bone fusion has not been obtained.
Footnotes
Conflicts of Interest Disclosure
No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1). Jackson JBS: A boneless arm. Boston Med Surg J 18: 368– 369, 1838. [Google Scholar]
- 2). GORHAM LW, STOUT AP: Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am 37-A: 985– 1004, 1955. [PubMed] [Google Scholar]
- 3). Dunbar SF, Rosenberg A, Mankin H, Rosenthal D, Suit HD: Gorham’s massive osteolysis: the role of radiation therapy and a review of the literature. Int J Radiat Oncol Biol Phys 26: 491– 497, 1993. [DOI] [PubMed] [Google Scholar]
- 4). Ellis DJ, Adams TO: Massive osteolysis: report of case. J Oral Surg 29: 659– 663, 1971. [PubMed] [Google Scholar]
- 5). Girn HR, Towns G, Chumas P, Holland P, Chakrabarty A: Gorham’s disease of skull base and cervical spine—confusing picture in a two year old. Acta Neurochir (Wien) 148: 909– 913; discussion 913, 2006. [DOI] [PubMed] [Google Scholar]
- 6). Heffez L, Doku HC, Carter BL, Feeney JE: Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol 55: 331– 343, 1983. [DOI] [PubMed] [Google Scholar]
- 7). Hoffman HJ, Harwood-Nash DC, Morley TP, Rewcastle NB: University of Toronto Neurosurgical Rounds no. 1. Massive osteolysis in association with multiple cerebrospinal fluid fistulae. Can J Neurol Sci 7: 39– 44, 1980. [PubMed] [Google Scholar]
- 8). Khosrovi H, Ortiz O, Kaufman HH, Schochet SS, Reddy GN, Simmons D: Massive osteolysis of the skull and upper cervical spine. Case report and review of the literature. J Neurosurg 87: 773– 780, 1997. [DOI] [PubMed] [Google Scholar]
- 9). Kurczynski E, Horwitz SJ: Response of lymphangiectasis to radiotherapy. Cancer 48: 255– 256, 1981. [DOI] [PubMed] [Google Scholar]
- 10). Lekovic GP, Mariwalla NR, Horn EM, Chang S, Rekate HL, Theodore N: Skeletal dysplasia involving the subaxial cervical spine. Report of two cases and review of the literature. Neurosurg Focus 20: E8, 2006. [DOI] [PubMed] [Google Scholar]
- 11). Mawk JR, Obukhov SK, Nichols WD, Wynne TD, Odell JM, Urman SM: Successful conservative management of Gorham disease of the skull base and cervical spine. Childs Nerv Syst 13: 622– 625, 1997. [DOI] [PubMed] [Google Scholar]
- 12). McGreger M: The significance of certain measurements of the skull in the diagnosis of basilar impression. Br J Radiol 21: 171– 181, 1948. [DOI] [PubMed] [Google Scholar]
- 13). Patel DV: Gorham’s disease or massive osteolysis. Clin Med Res 3: 65– 74, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Campbell J, Almond HG, Johnson R: Massive osteolysis of the humerus with spontaneous recovery. J Bone Joint Surg Br 57: 238– 240, 1975. [PubMed] [Google Scholar]
- 15). Hagberg H, Lamgerg K, Aström G: Alpha-2b interferon and oral clodronate for Gorham’s disease. Lancet 350: 1822– 1823, 1997. [DOI] [PubMed] [Google Scholar]
- 16). Hammer F, Kenn W, Wesselmann U, Hofbauer LC, Delling G, Allolio B, Arlt W: Gorham-Stout disease—stabilization during bisphosphonate treatment. J Bone Miner Res 20: 350– 353, 2005. [DOI] [PubMed] [Google Scholar]
- 17). Mignogna MD, Fedele S, Lo Russo L, Ciccarelli R: Treatment of Gorham’s disease with zoledronic acid. Oral Oncol 41: 747– 750, 2005. [DOI] [PubMed] [Google Scholar]
- 18). Shimizu T, Sato K, Yoshida T, Takahashi A, Yanagawa T, Wada N, Sohmiya M, Shirakura K, Watanabe H: A case report of Gorham-Stout syndrome remission. J Orthop Sci 17: 199– 204, 2012. [DOI] [PubMed] [Google Scholar]
- 19). Silva S: Gorham-Stout disease affecting both hands: stabilisation during biphosphonate treatment. Hand (N Y) 6: 85– 89, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Woodward HR, Chan DPK, Lee J: Massive osteolysis of the cervical spine. A case report of bone graft failure. Spine (Phila Pa 1976) 6: 545– 549, 1981. [DOI] [PubMed] [Google Scholar]
- 21). Cannon SR: Massive osteolysis. A review of seven cases. J Bone Joint Surg Br 68: 24– 28, 1986. [DOI] [PubMed] [Google Scholar]
- 22). Turra S, Gigante C, Scapinelli R: A 20-year follow-up study of a case of surgically treated massive osteolysis. Clin Orthop Relat Res 297– 302, 1990. [PubMed] [Google Scholar]
- 23). Chong Ng L, Sell P: Gorham disease of the cervical spine-a case report and review of the literature. Spine 28: E355– 358, 2003. [DOI] [PubMed] [Google Scholar]
- 24). Foult H, Goupille P, Aesch B, Valat JP, Burdin P, Jan M: Massive osteolysis of the cervical spine. A case report. Spine 20: 1636– 1639, 1995. [DOI] [PubMed] [Google Scholar]