Abstract
The utility of fish oil supplements (FOS) in patients who survive an acute myocardial infarction (MI) remains controversial, with randomized trials showing less benefit than observational studies would suggest. The differences in the characteristics of MI patients who use FOS in routine clinical care are unknown, but may help explain this discrepancy. We utilized data from a 24-site registry study in which extensive information was available on 4340 MI patients at admission and 1, 6, and 12 months post discharge. After excluding those using FOS at admission (n=651), who died before the 1-month follow-up visit (n=63), and those with missing data at 1 month (n=1228), 2398 remained. Of them 377 (16%) started FOS within 1 month of their MI. We analyzed 53 patient characteristics associated with FOS use. We observed differences (p<0.001) in 20 demographic, socio-economic, treatment, disease severity and health status domains. FOS users were more likely than non-users to be white, married, financially secure, highly educated, and eating fish. They also had a higher ejection fraction at discharge, were more likely to have had in-hospital percutaneous coronary interventions and were more likely to have participated in cardiac rehabilitation programs. FOS users were less likely to have a history of diabetes, alcohol abuse, stroke, MI and angina. In conclusion, post-MI patients who initiate FOS within 1 month of discharge in routine clinical practice differ substantially from those who do not. These differences are strongly associated with a better post-MI prognosis and may illuminate several sources of unmeasured confounding in observational studies.
Keywords: fish oil, nutritional supplements, omega-3 fatty acids, myocardial infarction, confounding, human
1. Introduction
The use of fish oil supplements (FOS) for the prevention of coronary heart disease (CHD) events is controversial, with observational studies [1–4] and earlier randomized controlled trials (RCTs) [5, 6] demonstrating benefit, but with recent RCTs in patients in the post-myocardial infarction (MI) setting showed no significant survival advantage with FOS [7, 8]. One potential explanation for this discrepancy is that, in observational studies, those subjects that use FOS, or regularly eat fish, are significantly different (i.e., healthier) than those that do not. While statistical adjustments are often performed in observational studies to control for such confounding, all of the relevant differences in patient characteristics, comorbidities, socio-economic status, concurrent treatments, etc. may not have been measured. It is therefore possible that it is not omega-3 fatty acid consumption, per sethat provides cardioprotection but a complex milieu of healthier behaviors for which omega-3 user serves as a marker for these healthcare characteristics. On the other hand, a substantial evidence base (including RCTs [9], biomarker-based prospective cohort studies [3, 4], experimental studies with intermediate endpoints[10–12], animal experiments [13], transgenic studies [14, 15], etc.) suggests a beneficial role of omega-3 fatty acids in CHD risk reduction [16–18], and thus the improved outcomes associated with increased omega-3 fatty acid consumption in observational studies could, in fact, be due to increased in vivo levels of these nutrients.
Given that RCTs are the gold standard for proof of causal relations (especially for pharmaceuticals), and observational studies are better at defining the real-world effectiveness, reconciling the differences between these two study types on the efficacy of FOS is important [19]. A deeper understanding of potential differences between FOS users and non-users can help inform what data elements need to be collected in observational studies to minimize confounding. To date, little attention has been paid to defining the differences in patients and their treatments between users and non-users of FOS.
To address this gap in knowledge, and lay the foundation for future comparative effectiveness studies, we conducted a detailed comparison of numerous patient and treatment characteristics associated with FOS use after MI. We hypothesized that FOS users would be healthier, more financially secure and more aggressive in the use of secondary prevention, such as cardiac rehabilitation, than non-users. This study could help clarify potential confounders in observational studies of FOS and by identify important patient characteristics for adjustment for in future analyses.
2. Methods and Materials
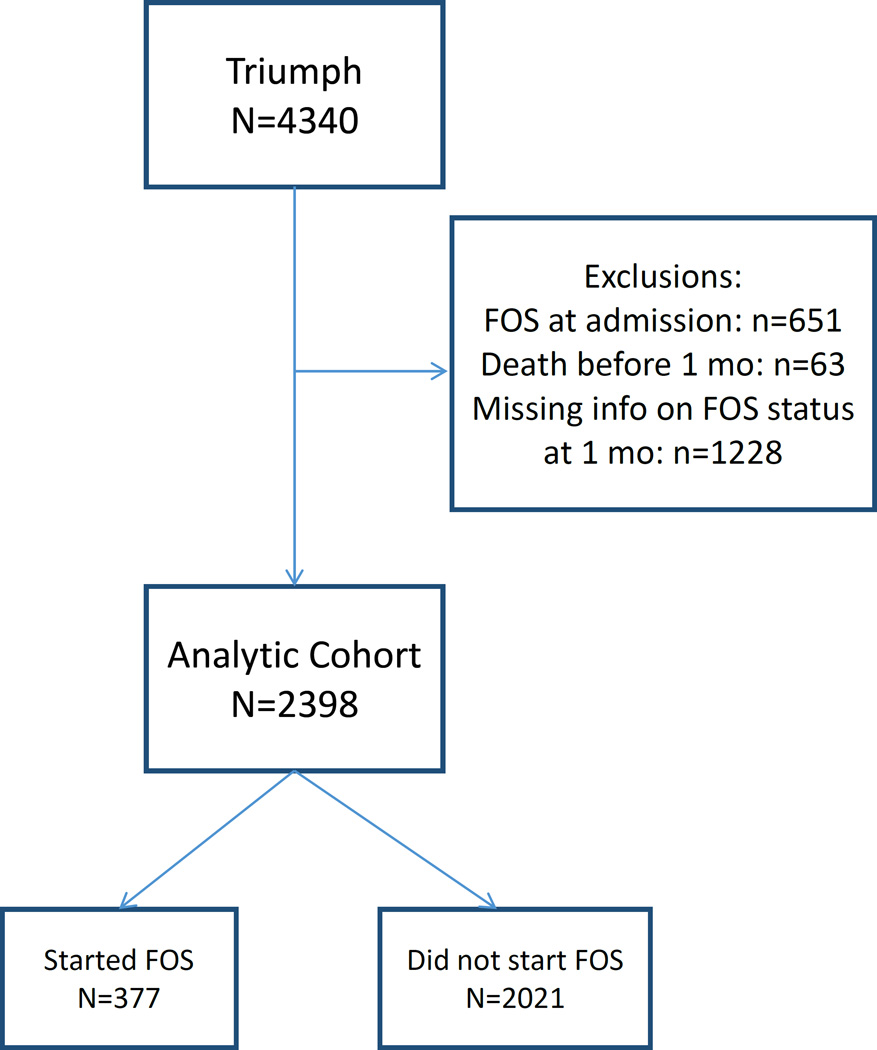
The Translational Research Investigating Underlying disparities in recovery from acute Myocardial infarction Patient Health status (TRIUMPH) study is a prospective MI registry of 24-centers across the United States that is unique in the breadth of patient characteristics it assessed. Enrollment occurred between April 2005 and December 2008. Among 4340 MI patients enrolled in TRIUMPH, charts were abstracted for medical history and processes of inpatient care. These data were supplemented with a detailed baseline interview that explicitly asked patients whether or not they used FOS at the time of their MI. Centralized follow-up interviews at 1 (6 and 12) months sought to quantify patients’ post-discharge care and outcomes, with a focus on their health status (symptoms, function, and quality of life) as well as FOS use [20]. In order to focus on the immediate initiation of FOS after MI, we compared the characteristics of those patients who started FOS between discharge and the 1-month follow up interview. Accordingly, we excluded those who were taking FOS at the time of their MI (n=651), who died within 1 month (n=63), and who could not be contacted for the 1 month interview and hence were missing information on FOS status (n=1228). This resulted in a sample of 2398 patients with evaluable data (Figure 1). The 1-month time frame was selected because the benefits of omega-3 treatment appear to be greatest when started soon after an MI [2, 5].
Figure 1.
Study Flow Chart. (FOS, Fish Oil Supplementation)
2.1 Statistical Analysis
The primary analysis was to compare the differences in patient and treatment characteristics for users and non-users. This was performed using descriptive statistics (means, standard deviations and frequencies), t-tests and linear trend tests for continuous variables, and Chi-squares or Mantel-Haenszel trend tests for categorical variables. Differences in patient variables with a p-value <0.001 were considered statistically significant. All statistical analyses were performed using SAS Version 9.3 (SAS Institute, Cary, NC).
3. Results
Among the 2398 post-MI patients included in this study, 377 patients (16%) initiated FOS within 1 month of discharge (Figure 1). Patient characteristics of those who did and did not initiate FOS (n=2021) in that time frame are shown in Tables 1–3. Among the demographic and socioeconomic factors (Table 1), FOS users were more likely to be married, white, and they tended to be about 1.5 years younger. They had better socio-economic status, were more likely to be highly educated, employed full-time and to have money left over at the end of the month. They also tended to be better insured. For the disease severity and risk factors/health status factors (Table 2), those who initiated FOS use after discharge were more likely to have had an ST-elevation MI. Their Global Registry of Acute Coronary Events (GRACE) score [21] was lower (indicating a better predicted prognosis), and they had higher left ventricular ejection fractions. The Canadian Cardiology Society Class did not differ between groups. Serum high density lipoprotein cholesterol was lower and triglycerides were higher in the FOS users. There were marginally significant differences in systolic and diastolic blood pressure (both higher in the FOS users), and creatinine was slightly lower. FOS users also had higher scores on the physical component of the Short Form12v2 and were more likely to report eating omega-3 fatty acid-rich fish. Notable similarities between the groups included low density lipoprotein cholesterol levels, depression scores, body mass index, heart rate and smoking habits. Finally, treatment status and medical history differences are shown in Table 3. FOS users were much more likely to have had in-hospital percutaneous coronary intervention and to have participated in cardiac rehab programs post discharge. Overall compliance with prescribed medications was not different between groups. In terms of medical histories, the FOS users were less likely to have had angina, a prior MI, a stroke, diabetes and chronic kidney disease. They were also fewer patients who admitted to alcohol abuse. Marginal differences (p<0.02) were seen for being on a statin at discharge or on dialysis on arrival; having a history of hypertension, peripheral vascular disease, coronary artery bypass graft surgery, cocaine use; and the use of oxygen at home.
Table 1.
Differences in demographic and socioeconomic variables between post-myocardial infarction patients who did or did not start taking fish oil supplements within 1 month of discharge
| Reported fish oil use at 1 month post | P-Value | Missing | ||
|---|---|---|---|---|
| Yes n = 377 |
No n = 2021 |
|||
| Demographic | ||||
| Age (yrs) | 58.0 ± 11.2 | 59.5 ± 12.3 | 0.034 | |
| Marital status | < 0.001 | 0.2% | ||
| Married | 236 (62.9%) | 1046 (51.8%) | ||
| Divorced | 61 (16.3%) | 351 (17.4%) | ||
| Separated | 15 (4.0%) | 91 (4.5%) | ||
| Widowed | 21 (5.6%) | 254 (12.6%) | ||
| Single | 39 (10.4%) | 231 (11.4%) | ||
| Male Sex | 272 (72.1%) | 1303 (64.5%) | 0.004 | |
| Race | < 0.001 | 0.3% | ||
| White/Caucasian | 316 (83.8%) | 1348 (67.0%) | ||
| Black/African-American | 49 (13.0%) | 511 (25.4%) | ||
| Other | 12 (3.2%) | 154 (7.7%) | ||
| Socioeconomic | ||||
| Work for pay | < 0.001 | 1.0% | ||
| No, 1 don't currently work for pay | 142 (38.2%) | 1066 (53.2%) | ||
| Yes, work full time | 197 (53.0%) | 754 (37.7%) | ||
| Yes, work part-time | 33 (8.9%) | 182 (9.1%) | ||
| Has insurance | 323 (85.7%) | 1613 (79.8%) | 0.008 | |
| Finances at the End of the Month | < 0.001 | |||
| Some money left over | 200 (53.1%) | 859 (42.5%) | ||
| Just enough to make ends meet | 130 (34.5%) | 737 (36.5%) | ||
| Not enough to make ends meet | 47 (12.5%) | 425 (21.0%) | ||
| Education | < 0.001 | 0.5% | ||
| High school education | 330 (88.0%) | 1570 (78.1%) | ||
| Some college/vocational school | 140 (37.3%) | 571 (28.4%) | ||
| Graduated from college | 53 (14.1%) | 255 (12.7%) | ||
| Post-graduate degree | 34 (9.1%) | 155 (7.7%) | ||
Table 3.
Differences in treatment and medical history variables between post-myocardial infarction patients who did or did not start taking fish oil supplements within 1 month of discharge
| Reported fish oil use at 1 month post | P-Value | Missing | ||
|---|---|---|---|---|
| Yes n = 377 |
No n = 2021 |
|||
| Treatment | 3.1% | |||
| Statin at Discharge | 348 (92.3%) | 1776 (87.9%) | 0.013 | |
| In-Hospital Percutaneous Coronary Intervention | 305 (80.9%) | 1309 (64.8%) | < 0.001 | |
| Participated in Cardiac Rehabilitation Program | 189 (52.4%) | 477 (24.3%) | < 0.001 | |
| Missing | 16 | 61 | ||
| Compliance with Prescriptions in the Past Month | 11.1% | |||
| All of the time (100%) | 272 (85.8%) | 1618 (89.4%) | ||
| Nearly all of the time (90%) | 39 (12.3%) | 155 (8.6%) | ||
| Most of the time (75%) | 3 (0.9%) | 23 (1.3%) | 0.216 | |
| About half the time (50%) | 3 (0.9%) | 7 (0.4%) | ||
| Less than half the time (<50%) | 0 (0.0%) | 6 (0.3%) | ||
| Missing | 60 | 212 | ||
| Medical History | ||||
| Family history of Coronary Artery Disease | 285 (77.0%) | 1454 (72.5%) | 0.072 | |
| Atrial Fibrillation | 10 (2.7%) | 95 (4.7%) | 0.074 | |
| Chronic Heart Failure | 18 (4.8%) | 156 (7.7%) | 0.043 | |
| Dyslipidemia | 195 (51.7%) | 971 (48.0%) | 0.19 | |
| Hypertension | 228 (60.5%) | 1350 (66.8%) | 0.018 | |
| Peripheral Vascular Disease | 11 (2.9%) | 109 (5.4%) | 0.043 | |
| Angina | 30 (8.0%) | 318 (15.7%) | < 0.001 | |
| Myocardial Infarction | 40 (10.6%) | 428 (21.2%) | < 0.001 | |
| Percutaneous Coronary Intervention | 54 (14.3%) | 372 (18.4%) | 0.057 | |
| Coronary Artery Bypass Graft | 24 (6.4%) | 225 (11.1%) | 0.005 | |
| Inplantable Cardioversion Defibrillator | 4 (1.1%) | 31 (1.5%) | 0.482 | |
| Stroke | 4 (1.1%) | 109 (5.4%) | < 0.001 | |
| Transient Ischemic Attack | 6 (1.6%) | 52 (2.6%) | 0.255 | |
| Alcohol Abuse | 16 (4.2%) | 221 (10.9%) | < 0.001 | |
| Cancer | 29 (7.7%) | 152 (7.5%) | 0.908 | |
| Depression RequringTreatment | 23 (6.1%) | 157 (7.8%) | 0.259 | |
| Diabetes | 78 (20.7%) | 608 (30.1%) | < 0.001 | |
| Cocaine Use | 10 (2.7%) | 112 (5.5%) | 0.019 | |
| Other Illlicit Drug Use | 8 (2.1%) | 74 (3.7%) | 0.131 | |
| Chronic Kidney Disease | 7 (1.9%) | 144 (7.1%) | < 0.001 | |
| Dialysis on Arrival | 1 (0.3%) | 32 (1.6%) | 0.044 | |
| Chronic Lung Disease | 21 (5.6%) | 162 (8.0%) | 0.101 | |
| Home Oxygen | 0 (0.0%) | 31 (1.5%) | 0.016 | |
| Sleep Apnea | 10 (2.7%) | 54 (2.7%) | 0.983 | |
Table 2.
Differences in disease severity and risk factors/health status variables between post-myocardial infarction patients who did or did not start taking fish oil supplements within 1 month of discharge
| Reported fish oil use at 1 month | P-Value | Missing | ||
|---|---|---|---|---|
| Yes n = 377 |
No n = 2021 |
|||
| Disease Severity | ||||
| ST-segment elevation myocardial infarction | 229 (60.7%) | 859 (42.5%) | < 0.001 | |
| GRACE 6m Mortality Risk Score | 93.9 ± 25.7 | 101.4 ± 29.7 | < 0.001 | |
| Ejection Fraction (%) | 51.5 ± 11.1 | 48.6 ± 13.1 | < 0.001 | 14.0% |
| Canadian Cardiology Society Class | 0.79 | |||
| 0 No angina with any level of activity | 206 (54.6%) | 1180 (58.4%) | ||
| I Ordinary physical activity does not cause angina | 70 (18.6%) | 282 (14.0%) | ||
| II Slight limitation of ordinary activity | 58 (15.4%) | 310(15.3%) | ||
| III Marked limitation of ordinary physical activity | 29 (7.7%) | 170 (8.4%) | ||
| IV Inability to carry on any physical activity without discomfort | 14 (3.7%) | 79 (3.9%) | ||
| Risk Factors/Health Status | ||||
| Serum lipids (mmol/L)* | ||||
| HDL-Cholesterol | 1.02 ± 0.33 | 1.10 ± 0.37 | < 0.001 | 7.0% |
| LDL-Cholesterol | 2.74 ± 1.01 | 2.70 ± 1.07 | 0.511 | 9.5% |
| Total Cholesterol | 4.69 ± 1.44 | 4.53 ± 1.09 | 0.032 | 6.4% |
| Triglycerides | 2.13 ± 3.10 | 1.65 ± 1.90 | < 0.001 | 7.0% |
| Patient Health Questionnaire-9 Depression Score | 5.0 ± 4.9 | 5.1 ± 5.4 | 0.663 | 6.3% |
| Body Mass Index (kg/m2) | 30.1 ± 6.3 | 29.5 ± 6.4 | 0.098 | 4.7% |
| Heart Rate (beats per minute) | 80.4 ± 21.4 | 82.9 ± 22.8 | 0.054 | 0.4% |
| Systolic Blood Pressure (mm Hg) | 146.5 ± 30.6 | 142.4 ± 30.6 | 0.017 | 0.5% |
| Diastolic Blood Pressure (mm Hg) | 85.3 ± 18.1 | 82.8 ± 19.2 | 0.022 | 0.6% |
| Creatinine (mg/dL) | 1.1 ± 0.5 | 1.2 ± 1.0 | 0.021 | 0.2% |
| Short form-12v2 Mental Component Score | 51.0 ± 10.1 | 49.9 ± 11.5 | 0.075 | 4.5% |
| Short form-12v2 Physical Component Score | 44.9 ± 11.5 | 42.4 ± 12.3 | < 0.001 | 4.5% |
| How active during leisure time | 0.001 | 0.8% | ||
| Mainly sedentary | 128 (34.3%) | 923 (46.0%) | ||
| Mild to moderate exercise | 228 (61.2%) | 1005 (50.1%) | ||
| Strenuous exercise | 17 (4.6%) | 77 (3.8%) | ||
| Smoking Status | 0.425 | 0.8% | ||
| Current (<30d) | 140 (37.9%) | 826(41.1%) | ||
| Former (>30d) | 128 (34.7%) | 643 (32.0%) | ||
| Never (or <100 total) | 101 (27.4%) | 540 (26.9%) | ||
| Frequency of eating tuna/non-fried fish | < 0.001 | 2.6% | ||
| Less than once a month | 86 (23.1%) | 635 (32.4%) | ||
| 1–3 times a month | 100 (26.9%) | 612 (31.2%) | ||
| 1–2 times a week | 139 (37.4%) | 588 (30.0%) | ||
| 3–4 times a week | 40 (10.8%) | 113 (5.8%) | ||
| 5 or more times a week | 7 (1.9%) | 14 (0.7%) | ||
| Frequency of eating fast food | < 0.001 | 1.2% | ||
| Less than once a month | 96 (25.7%) | 750 (37.6%) | ||
| 1–3 times a month | 104 (27.9%) | 556 (27.9%) | ||
| 1–2 times a week | 93 (24.9%) | 390(19.6%) | ||
| 3–4 times a week | 53 (14.2%) | 172 (8.6%) | ||
| 5 or more times a week | 27 (7.2%) | 126 (6.3%) | ||
Most recent chart value within the last year
4. Discussion
As hypothesized, we found numerous differences between the patients who did and did not start FOS after their MI, most of which would be associated with a better post-MI prognosis. Among these would be better socioeconomic status and histories of less cardiovascular and metabolic diseases among the FOS users, more participation in cardiac rehabilitation programs, and more common consumption of oily fish. Lower GRACE scores and better left ventricular ejection fractions are also associated with better prognoses. Demographically, users were likely to be white, married, and to be more secure financially. All of these factors (and likely others associated with these that were not assessed) would argue that patients who start FOS post MI are simply “healthier” or “better off” than those who do not. Some of the group differences were not so clearly in favor of the FOS users, however. The users were more likely to have an in-hospital revascularization procedure and to have suffered ST-elevation MIs, both of which might suggest more severe disease. They were more likely to be men, who at least in their late 50s, are more likely to suffer cardiovascular events than women (which, if men believed themselves to be at greater risk, might explain increased usage of FOS presuming them to be helpful.) Although blood pressure tended to be lower in users, so were serum high density lipoprotein cholesterol levels while triglyceride levels were somewhat higher. To this last point, elevated triglycerides is one of the most common indications for taking omega-3 fatty acids, thus some patients may have become users in hopes of lowering their triglyceride levels.
In light of the discrepancies between RCTs (where baseline differences are minimized by randomization) and observational studies with FOS regarding prognosis after acute MI, the goal of this study was to carefully define the differences between patients who did and did not start taking FOS within 1 month of their MI. We focused upon early FOS use, within a 1-month window, because previous reports suggested that post-MI patients who started FOS within 1 month had better cardiovascular and mortality outcomes than patients who waited longer or never started FOS [2]. Moreover, early treatment with omega-3 fatty acids was associated with reduced risk for sudden death and total mortality in the GISSI-Prevenzione Study [5] (but not in a later study [7]). Clarifying whether FOS use is a marker for other patient characteristics associated with a favorable prognosis is essential in designing and interpreting future observational research studies focused on the effectiveness of FOS use.
Previous reports of the relations between omega-3 fatty acid intakes or biomarker levels and disease have included data on baseline differences in patient characteristics across the span of omega-3 exposures. A sampling of studies examining the relations between omega-3 fatty acid exposure and CHD that also presented data on patient characteristics as a function of exposure is enlightening (Table 4). Common factors associated with higher fish intakes/omega-3 levels are intakes of fruits, vegetables, and alcohol, and physical activity and education. Higher red blood cell omega-3 levels were associated with more favorable socio-economic status [22] and lower intakes of fast food [23]. Inverse associations are frequently reported for the intake of trans and saturated fats and smoking [23–26]. These factors, in and of themselves, could render individuals with higher omega-3 intakes/levels at reduced risk for CHD.
Table 4.
Variables associated with omega-3 fatty acid intake and/or blood levels in prior studies
| First author | N-3 Fatty Acid Exposure |
Variables | |
|---|---|---|---|
| Directly | Inversely | ||
| Present Study | Initiation offish oil supplementation within 1 month post-myocardial infarction | Married, male sex, education, health insurance, full-time employment, an income meeting needs, ST-elevation myocardial infarction, GRACE score[21], left ventricular ejection fraction, serum triglycerides, physical fitness, in-hospital angioplasty, non-fried fish consumption, and participation in cardiac rehabilitation | White race; high density lipoprotein cholesterol; a history of myocardial infarction, coronary artery bypass graft, angina, stroke, diabetes, chronic kidney disease, and alcohol abuse |
| Bell [26] | EPA+DHA intake | Male sex; education; physical activity; intake of fruits, vegetables, energy, and alcohol. | Hormone replacement therapy; intake of saturated and trans fats |
| Miyagawa [38] | Long chain n-3 fatty acid intake | Age; rural residence; intake of sodium | Intake of energy, fat, n-6 polyunsaturated fatty acids |
| Engeset [39] | Fish consumption | Age; BMI; physical activity; smoking [men]; intake of energy, fruits, vegetables, alcohol (men), meat | University degree |
| Mozaffarian [3] | Plasma phospholipid EPA | Education; alcohol, fish, fruit and vegetable intake | Meat consumption, age, male sex |
| Plasma phospholipid DHA | Education; fish, fruit and vegetable intake | Meat consumption, white race, smoking | |
| Otto [24] | Plasma phospholipid DHA+DPA+EPA | Age; education; physical activity; lipid medication use; intake of fruits, vegetables, carbohydrates, protein | Smoking; BMI; intake of saturated and trans fats |
| Nishizaki [40] | Serum EPA/AA ratio | Age; male sex; # of CHD risk factors; use of anti-platelet agents and calcium channel blockers | Diabetes |
| Wennberg [25] | Fish intake (< vs. > 3 servings/wk.) | Age; physical activity; education; intake of fruits, vegetables, and wine | Smoking (men) |
| Salisbury [23] | Erythrocyte EPA+DHA | Age; education; financial security; and non-fried fish intake | White race; smoking; fast food intake; history of coronary artery bypass surgery, congestive heart failure |
Abbreviations: CHD, coronary heart disease; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic acid; AA, arachidonic acid.
The conflicting conclusions from observational studies (that have commonly observed morbidity and mortality benefits associated with higher omega-3 fatty acid intakes/levels [2–4, 27]) and RCTs (which recently have not [9]) may have several explanations. On the one hand, the RCTs may have enrolled particularly compliant patients with higher than expected background dietary intakes of omega-3 fatty acids. Other factors could be modern concurrent drug therapy (e.g., omega-3 fatty acids may be more effective in patients not on statins vs those being treated [28]), low doses of omega-3 fatty acid supplements (which are unlikely to have achieved a therapeutic omega-3 index [29–31]), the potentially poor absorption of ethyl esters used in most RCTs [32], short durations of treatment, use of combined endpoints, and inclusion of older patients with pre-existing disease [33–35]. [Two major RCTs are currently underway using four-fold higher doses of omega-3 fatty acids; these may help address some of these weaknesses: REDUCE-IT (NCT01492361) and STRENGTH (NCT02104817)]. On the other hand, the observational studies may have failed to measure the myriad of potential confounders detected here, such that their statistical attempts to control for confounding was incomplete. (A sample of the covariates included in past studies is provided in Table 4).
Our findings should be interpreted in the context of several potential limitations. First, it is likely that, despite our extensive abstraction of clinical details and our detailed patient interviews, there were still other differences that were not captured in this investigation. Another important limitation was the lack of any information (including FOS status) between discharge and 1 month for patients who died or who could not be contacted for the 1-month follow-up interview. We also had only limited dietary information (e.g. fish consumption), which may have identified other eating habits that differed between the groups. Variations in diet can have a marked effect on risk for death in a population with CHD [36, 37]. Nevertheless, these limitations are offset by strengths including a broad range of patient variables, the use of data from 24 clinical centers, and the inclusion of all post-MI patients.
In conclusions, our findings document the many health-related differences between patients who initiate FOS post MI and those who do not. Several prior observational studies did not control for the depth and breadth of patient characteristics measured here, and this omission may, at least in part, explain the differences between their findings and those of RCTs. Future observational studies should collect and adjust for all relevant variables potentially related to the outcomes under study.
Acknowledgments
Funding Sources
The TRIUMPH study was supported by grants from the National Heart, Lung, and Blood Institute Specialized Center of Clinically Oriented Research in Cardiac Dysfunction and Disease (grant no. P50 HL077113) and Cardiovascular Outcomes, Kansas City, MO.
ABBREVIATIONS
- CHD
coronary heart disease
- FOS
fish oil supplements
- GRACE
Global Registry of Acute Coronary Events
- MI
myocardial infarction
- RCT
randomized controlled trial
- TRIUMPH
Translational Research Investigating Underlying disparities in recovery from acute Myocardial infarction Patient Health status
Footnotes
Disclosures
WSH: Ownership interest in OmegaQuant Analytics, LLC; employment by Health Diagnostic Laboratory, Inc.; Scientific advisory boards for Aker Biomarine Antarctica, the Seafood Nutrition Partnership, and the Global Organization for EPA and DHA. All other authors have no relationships to disclose.
References
- 1.Zheng J, Huang T, Yu Y, Hu X, Yang B, Li D. Fish consumption and CHD mortality: an updated meta-analysis of seventeen cohort studies. Pub Health Nutr. 2012;15:725–737. doi: 10.1017/S1368980011002254. [DOI] [PubMed] [Google Scholar]
- 2.Poole CD, Halcox JP, Jenkins-Jones S, Carr ES, Schifflers MG, Ray KK, et al. Omega-3 Fatty acids and mortality outcome in patients with and without type 2 diabetes after myocardial infarction: a retrospective, matched-cohort study. Clin Therap. 2013;35:40–51. doi: 10.1016/j.clinthera.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Lemaitre RN, King IB, Song X, Huang H, Sacks FM, et al. Plasma phospholipid long-chain omega-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann Intern Med. 2013;158:515–525. doi: 10.7326/0003-4819-158-7-201304020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160:398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 5.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 6.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 7.Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 8.Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. doi: 10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 10.Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hyperten. 2014;27:885–896. doi: 10.1093/ajh/hpu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xin W, Wei W, Li X. Effects of fish oil supplementation on cardiac function in chronic heart failure: a meta-analysis of randomised controlled trials. Heart. 2012;98:1620–1625. doi: 10.1136/heartjnl-2012-302119. [DOI] [PubMed] [Google Scholar]
- 12.Xin W, Wei W, Li X. Effect of fish oil supplementation on fasting vascular endothelial function in humans: a meta-analysis of randomized controlled trials. PloS one. 2012;7:e46028. doi: 10.1371/journal.pone.0046028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiner BH, Ockene IS, Levine PH, Cuenoud HF, Fisher M, Johnson BF, et al. Inhibition of atherosclerosis by cod-liver oil in a hyperlipidemic swine model. The New England journal of medicine. 1986;315:841–846. doi: 10.1056/NEJM198610023151401. [DOI] [PubMed] [Google Scholar]
- 14.Kang JX. Reduction of heart rate by omega-3 fatty acids and the potential underlying mechanisms. Frontiers in physiology. 2012;3:416. doi: 10.3389/fphys.2012.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo C, Ren H, Wan JB, Yao X, Zhang X, He C, et al. Enriched endogenous omega-3 fatty acids in mice protect against global ischemia injury. Journal of lipid research. 2014;55:1288–1297. doi: 10.1194/jlr.M046466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 17.De Caterina R. n-3 fatty acids in cardiovascular disease. The New England journal of medicine. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 18.Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet. 2010;376:540–550. doi: 10.1016/S0140-6736(10)60445-X. [DOI] [PubMed] [Google Scholar]
- 19.Nallamothu BK, Hayward RA, Bates ER. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008;118:1294–1303. doi: 10.1161/CIRCULATIONAHA.107.703579. [DOI] [PubMed] [Google Scholar]
- 20.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status (TRIUMPH): Design and Rationale of a Prospective Multicenter Registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de WF, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 22.Cohen BE, Garg SK, Ali S, Harris WS, Whooley MA. Red blood cell docosahexaenoic acid and eicosapentaenoic acid concentrations are positively associated with socioeconomic status in patients with established coronary artery disease: data from the heart and soul study. The Journal of nutrition. 2008;138:1135–1140. doi: 10.1093/jn/138.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salisbury AC, Amin AP, Harris WS, Chan PS, Gosch KL, Rich MW, et al. Predictors of omega-3 index in patients with acute myocardial infarction. Mayo Clin Proc. 2011;86:626–632. doi: 10.4065/mcp.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Oliveira Otto MC, Wu JH, Baylin A, Vaidya D, Rich SS, Tsai MY, et al. Circulating and Dietary Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. Journal of the American Heart Association. 2013;2:e000506. doi: 10.1161/JAHA.113.000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wennberg M, Tornevi A, Johansson I, Hornell A, Norberg M, Bergdahl IA. Diet and lifestyle factors associated with fish consumption in men and women: a study of whether gender differences can result in gender-specific confounding. Nutrition journal. 2012;11:101. doi: 10.1186/1475-2891-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell GA, Kantor ED, Lampe JW, Kristal AR, Heckbert SR, White E. Intake of long-chain omega-3 fatty acids from diet and supplements in relation to mortality. American journal of epidemiology. 2014;179:710–720. doi: 10.1093/aje/kwt326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benedetto U, Melina G, di Bartolomeo R, Angeloni E, Sansone D, Falaschi G, et al. n-3 Polyunsaturated Fatty Acids After Coronary Artery Bypass Grafting. Ann Thorac Surg. 2011;91:1169–1175. doi: 10.1016/j.athoracsur.2010.11.068. [DOI] [PubMed] [Google Scholar]
- 28.Eussen SR, Geleijnse JM, Giltay EJ, Rompelberg CJ, Klungel OH, Kromhout D. Effects of n-3 fatty acids on major cardiovascular events in statin users and non-users with a history of myocardial infarction. Eur Heart J. 2012;33:1582–1588. doi: 10.1093/eurheartj/ehr499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Stasi D, Bernasconi R, Marchioli R, Marfisi RM, Rossi G, Tognoni G, et al. Early modifications of fatty acid composition in plasma phospholipids, platelets and mononucleates of healthy volunteers after low doses of n-3 polyunsaturated fatty acids. Eur J Clin Pharmacol. 2004;60:183–190. doi: 10.1007/s00228-004-0758-8. [DOI] [PubMed] [Google Scholar]
- 30.Flock MR, Skulas-Ray AC, Harris WS, Etherton TD, Fleming JA, Kris-Etherton PM. Determinants of Erythrocyte Omega-3 Fatty Acid Content in Response to Fish Oil Supplementation: A Dose-Response Randomized Controlled Trial. Journal of the American Heart Association. 2013;2:e000513. doi: 10.1161/JAHA.113.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris WS, von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 32.Maki KC, Orloff DG, Nicholls SJ, Dunbar RL, Roth EM, Curcio D, et al. A highly bioavailable omega-3 free fatty acid formulation improves the cardiovascular risk profile in high-risk, statin-treated patients with residual hypertriglyceridemia (the ESPRIT trial) Clinical therapeutics. 2013;35:1400–1411. e1–e3. doi: 10.1016/j.clinthera.2013.07.420. [DOI] [PubMed] [Google Scholar]
- 33.Harris WS. Are n-3 fatty acids still cardioprotective? Current opinion in clinical nutrition and metabolic care. 2013;16:141–149. doi: 10.1097/MCO.0b013e32835bf380. [DOI] [PubMed] [Google Scholar]
- 34.Deckelbaum RJ, Calder PC. Different outcomes for omega-3 heart trials: why? Current opinion in clinical nutrition and metabolic care. 2012;15:97–98. doi: 10.1097/MCO.0b013e32834ec9e5. [DOI] [PubMed] [Google Scholar]
- 35.James MJ, Sullivan TR, Metcalf RG, Cleland LG. Pitfalls in the use of randomised controlled trials for fish oil studies with cardiac patients. The British journal of nutrition. 2014;112:812–820. doi: 10.1017/S0007114514001408. [DOI] [PubMed] [Google Scholar]
- 36.Barzi F, Woodward M, Marfisi RM, Tavazzi L, Valagussa F, Marchioli R. Mediterranean diet and all-causes mortality after myocardial infarction: results from the GISSI-Prevenzione trial. European journal of clinical nutrition. 2003;57:604–611. doi: 10.1038/sj.ejcn.1601575. [DOI] [PubMed] [Google Scholar]
- 37.de Lorgeril M, Salen P, Martin JL, Renaud S, Monjaud I, Mamelle N, et al. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 38.Miyagawa N, Miura K, Okuda N, Kadowaki T, Takashima N, Nagasawa SY, et al. Long-chain n-3 polyunsaturated fatty acids intake and cardiovascular disease mortality risk in japanese: a 24-year follow-up of NIPPON DATA80. Atherosclerosis. 2014;232:384–389. doi: 10.1016/j.atherosclerosis.2013.11.073. [DOI] [PubMed] [Google Scholar]
- 39.Engeset D, Braaten T, Teucher B, Kuhn T, Bueno-de-Mesquita HB, Leenders M, et al. Fish consumption and mortality in the European Prospective Investigation into Cancer and Nutrition cohort. European journal of epidemiology. 2014 doi: 10.1007/s10654-014-9966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishizaki Y, Shimada K, Tani S, Ogawa T, Ando J, Takahashi M, et al. Significance of imbalance in the ratio of serum n-3 to n-6 polyunsaturated fatty acids in patients with acute coronary syndrome. Am J Cardiol. 2014;113:441–445. doi: 10.1016/j.amjcard.2013.10.011. [DOI] [PubMed] [Google Scholar]