Abstract
Bruck syndrome (BS) is a disorder characterized by joint flexion contractures and skeletal dysplasia that shows strong clinical overlap with the brittle bone disease Osteogenesis Imperfecta (OI). BS is caused by bi-allelic mutations in either the FKBP10 or the PLOD2 gene. PLOD2 encodes the lysyl hydroxylase 2 (LH2) enzyme, which is responsible for the hydroxylation of lysine residues in fibrillar collagen telopeptides. This hydroxylation directs cross-linking of collagen fibrils in the extracellular matrix, which is necessary to provide stability and tensile integrity to the collagen fibrils. To further elucidate the function of LH2 in vertebrate skeletal development, we created a zebrafish model harboring a homozygous plod2 nonsense mutation resulting in reduced telopeptide hydroxylation and cross-linking of bone type I collagen. Adult plod2 mutants present with a shortened body axis and severe skeletal abnormalities with evidence of bone fragility and fractures. The vertebral column of plod2 mutants is short and scoliotic with compressed vertebrae that show excessive bone formation at the vertebral end plates, and increased tissue mineral density in the vertebral centra. The muscle fibers of mutant zebrafish have a reduced diameter near the horizontal myoseptum. The endomysium, a layer of connective tissue ensheathing the individual muscle fibers, is enlarged. Transmission electron microscopy of mutant vertebral bone shows type I collagen fibrils that are less organized with loss of the typical plywood-like structure. In conclusion, plod2 mutant zebrafish show molecular and tissue abnormalities in the musculoskeletal system that are concordant with clinical findings in BS patients. Therefore, the plod2 zebrafish mutant is a promising model for the elucidation of the underlying pathogenetic mechanisms leading to BS and the development of novel therapeutic avenues in this syndrome.
Keywords: BRUCK SYNDROME, OSTEOGENESIS IMPERFECTA, PLOD2, LYSYL HYDROXYLASE 2, COLLAGEN
INTRODUCTION
Bruck syndrome (BS) is a rare congenital connective tissue disorder first described a century ago(1). The hallmark of BS is a combination of joint contractures with various skeletal anomalies. The contractures are mainly flexion deformities of the large joints (knees, elbows, ankles) with pterygia, and to a lesser extent flexion deformities of the small joints (camptodactyly, adducted thumb). The skeletal anomalies include generalized osteoporosis and bone fragility resulting in fractures starting in infancy or early childhood. The vertebrae are severely malformed with platyspondyly, leading to a severely shortened trunk, dwarfism and progressive kyphoscoliosis. The extremities show curvature deformities of the long bones that are slender and have thin cortices. Macrocephaly with Wormian bones might be present, while dentinogenesis imperfecta is uncommon(2,3).
BS is a monogenic disorder caused by bi-allelic mutations in either the FKBP10 or the PLOD2 gene, resulting in respectively type I and type II BS, which are clinically indistinguishable. With regard to the skeletal anomalies and bone fragility, BS shows significant clinical overlap with Osteogenesis Imperfecta (OI). OI is usually inherited as an autosomal dominant (AD) disorder, caused by a heterozygous mutation in either COL1A1 or COL1A2, encoding the α1 and α2 chain of type I (pro)collagen, respectively(2). A small proportion of patients have an autosomal recessive form of OI due to bi-allelic mutations in genes encoding key players in the biosynthesis and processing of type I collagen, osteoblast differentiation and function or bone matrix mineralization(3).
Type I (pro)collagen belongs to the family of the fibril-forming collagens and is the major extracellular matrix (ECM) protein of bone, dentin and skin. Type I procollagen is composed of two proα1(I) chains and one proα2(I) chain that fold into a heterotrimeric triple helix. The proα chains contain a central core of uninterrupted repeats of the Gly-X-Y tripeptide, flanked by globular propeptide domains at the amino (N-) and carboxyl (C-) ends which are necessary for chain association and triple helix formation. These proα chains are synthesized at the rough endoplasmic reticulum (ER), post-translationally modified in the ER lumen and subsequently assembled into a trimeric procollagen molecule. This procollagen molecule is then transported through the trans-golgi network to the extracellular matrix (ECM). The propeptides are serially removed first by C- then N-propeptidases preceding and during the process of assembly of mature triple helical collagen molecules into fibril polymers(4). Collagen molecules become cross-linked covalently by lysyl oxidase during this process. The unique pattern of cross-linking of bone collagen is believed to be important for the normal mineralization and material strength of bone tissue.
The causal genes for BS (PLOD2, FKBP10) encode ER-residing proteins that play an important role in the biosynthesis of type I (pro)collagen. FKBP65, encoded by the FKBP10 gene, functions as a molecular chaperone that aids in the folding of type I procollagen(5). PLOD2 encodes lysyl hydroxylase 2 (LH2), which is the enzyme responsible for the hydroxylation of lysine residues in collagen telopeptides. Telopeptide hydroxylysines are essential for the hydroxyallysine pathway of cross-linking, which produces mature (permanent) lysylpyridinoline and hydroxylysylpyridinoline cross-links in extracellular collagen fibrils. A combination of these mature cross-links and labile cross-links formed from lysine aldehydes are believed to be required for the normal material properties of bone(6–9). Patients suffering from BS have reduced levels of hydroxyallysine-derived cross-links in bone type I collagen(10,11).
Despite the finding that BS is generally caused by defective type I collagen cross-linking, many questions remain to be answered regarding the pathogenesis of this disease. First, although BS and OI patients both suffer from a decrease in bone strength and an increase in bone fragility that lead to a higher susceptibility to fractures, both syndromes show a large clinical variability that is still poorly understood. Second, in BS, defects in LH2 and FKBP65 result in a phenocopy, both clinically and in terms of abnormal collagen cross-linking, while the role of FKBP65 (FKBP10) in collagen cross-linking and its relation to LH2 (PLOD2) has yet to be established. Third, the pathogenic basis of the congenital joint contractures in BS and how they are linked to defective collagen cross-linking remain unsolved. Therefore, a better understanding of the pathogenetic mechanisms in BS and OI is needed to answer these questions and to fully establish gene-phenotype relationships. Moreover, this will aid in the development of more effective therapeutics, as current therapeutic strategies for BS and OI fail to positively affect the quality of the bone.
Insights into the disease mechanisms underlying skeletal disorders such as BS and OI are strongly coupled to the tractability of model systems available to understand their pathogenesis. Growing studies suggest the small fresh water teleost D. rerio (zebrafish) may serve as a model for skeletal dysplasias (12–14). As zebrafish are aquatic animals, the weight-bearing demand on the skeleton is reduced, enabling many skeletal mutant zebrafish lines to survive into adulthood far longer than their orthologous mouse counterparts, even those that may be embryonic lethal. Furthermore, the molecular basis and the key regulators of skeletogenesis appear to be highly conserved between zebrafish and mammals (15,16). The broad availability of transgenic zebrafish lines expressing fluorescent proteins under the control of skeletal specific promoters enables in vivo visualization of cell and signaling dynamics that are difficult to resolve in mammalian systems. Finally, the amenability of zebrafish to genetic analysis has facilitated the rapid generation of mutant lines for cartilage and bone genes to understand their functional roles in skeletogenesis (13,17,18). These lines include the zebrafish mutants frilly fins, microwaved and chihuahua, that have been reported to model aspects of human OI(19,20).
Plod2-null mice do not survive beyond embryonic day 12(21). Thus, a viable animal model of BS caused by mutations in PLOD2 has yet to be identified. In this work we show that a zebrafish mutant carrying a nonsense mutation in plod2 displays molecular and tissue abnormalities in the musculoskeletal system resembling those in human patients with BS. Hence, this mutant can contribute to a better understanding of the pathogenetic mechanisms in BS caused by PLOD2 mutations, and by virtue of the unique attributes of zebrafish, accelerate the identification of new therapeutic targets.
MATERIAL AND METHODS
Animals
plod2sa1768 mutant zebrafish were generated by the zebrafish mutation project (ZMP)(22). plod2sa1768 mutant and wild-type AB fish were obtained from the Zebrafish International Resource Center (ZIRC, http://zebrafish.org). Heterozygote plod2sa1768 mutant zebrafish were incrossed to obtain homozygous plod2 mutants. Fish were housed in ZebTEC semi-closed recirculation housing systems (Techniplast, Italy) and were kept at a constant temperature (27–28°C), pH (7.5) and conductivity (500 μS) on a 14/10 light/dark cycle. Fish were fed three times a day with dry food (Gemma Micro, Skretting, UK) and brine shrimps (Ocean Nutrition, Belgium). Embryos were collected by natural spawning, staged and raised according to Kimmel and colleagues(23). All animal studies were performed in agreement with EU Directive 2010/63/EU for animals, permit Number: ECD 15/68.
Alcian Blue and Alizarin red staining
Cartilage was stained with Alcian Blue, using a modified protocol of Neuhauss et al. (24) (details in supplement) and stained specimens were analyzed under a Zeiss Axio Imager Microscope and photographed using an Axiocam MRC camera. Mineralized bone was stained with Alizarin Red using a modified protocol of Spoorendonk et al. (25) for larvae and as described by Lewis(26) for adult fish (details in supplement), and the stained specimens were examined under a Leica M165 FC Fluorescent Stereo Microscope.
Histology and Transmission Electron Microscopy (TEM)
Fish were fixed and embedded in epon following the procedures outlined by Huysseune & Sire(27) (details in supplement). All semi-thin sections were examined using a Zeiss Axio Imager Microscope and photographed using an Axiocam MRC camera. Ultrathin sections were observed in a Jeol JEM 1010 transmission electron microscope (Jeol Ltd., Tokyo, Japan). Pictures were digitized using a Ditabis system (Ditabis Ltd., Pforzheim, Germany).
μCT-scanning analysis
For μCT-based phenotyping and quantification, adult zebrafish were euthanized using 0.4% tricaine, stored frozen, and thawed immediately prior to scanning. Whole body μCT scans of 3 mutant and 3 control fish were acquired on a Scanco Viva CT 40 using the following scan parameters: 55kV, 145μA, 200ms integration time, 500 proj/180°, and 21μm voxel size. For each vertebral body, the haemal arch/ribs, centrum, and neural arch were manually segmented, and the mean tissue mineral density (TMD) and bone thickness computed. TMD was defined as previously described(28), i.e. the mineral density in only thresholded and segmented out bone was taken into account. A global test for association of groups of covariates/features with a response variable (29) was used to assess for phenotypic alterations across all vertebrae with genotype. For each fish, standard length was measured on maximal projections of scans. For whole-body μCT projections, adult specimens were fixed in 4 % paraformaldehyde for 48 hours and then transferred to a 70% ethanol solution for scanning. Specimens were scanned with the HECTOR μCT scanner at the Centre for X-ray Tomography of the Ghent University (UGCT, www.ugct.ugent.be(30,31)) using a tube voltage of 130kV, 15W target power, 1s integration time, 1801 projections, and 13.5μm voxel size. All projections were reconstructed using the Octopus-package developed at UGCT (http://www.octopusreconstruction.com(32)).
Immunoblotting
Embryos were collected at 2 dpf, chorion and yolk sac were manually removed and protein extracts were prepared from whole embryos in 1xSDS-laemmli buffer containing 5% 2-mercapto-ethanol. For adult specimens the caudal fin was amputated, tissues were collected in 1xSDS-laemmli buffer containing 5% 2-mercapto-ethanol and homogenized using a TissueLyzer (Retsch, Germany). All lysates were subjected to SDS-PAGE using a 4–12% Bis-Tris gel (Invitrogen, NuPAGE). Subsequently, proteins were transferred to a nitrocellulose membrane using the Mini Trans-Blot® Electrophoretic Transfer Cell system (Bio-rad, USA), immunolabeled with anti-LH2 primary antibody (21214-1-AP, Proteintech, USA) and horseradish peroxidase (HRP) conjugated secondary antibody (Cell Signaling Technologies, USA). Membranes were developed using the SuperSignal® West Dura Extended Duration Substrate kit (ThermoScientific, Life Technologies Europe, Belgium) and scanned with the ChemiDoc-It® 500 Imaging System (UVP, USA). Following stripping, the membrane was reprobed with α-tubulin (Sigma-Aldrich, USA) in order to check for equal loading. Intensities were quantified using Fiji software(33).
Collagen cross-linking analysis
Adult zebrafish were euthanized with 0.4% tricaine and vertebral bone was dissected manually from two plod2 mutant and two control fish, residual soft tissue was removed by incubation in Accumax solution (Sigma-Aldrich) for 1 hour. Bone was demineralized in 0.1 M HCl at 4°C, washed, and solubilized by heat denaturation in SDS-PAGE sample buffer. The method of Laemmli was used with 6% gels for the denaturant extracts. Collagen α-chains were cut from SDS-PAGE and subjected to in-gel trypsin digestion. Electrospray MS was performed on tryptic peptides using an LTQ XL ion-trap mass spectrometer (Thermo Scientific) equipped with in-line liquid chromatography using a C4 5μm capillary column (300um × 150mm; Higgins Analytical RS-15M3-W045) eluted at 4.μl min. The LC mobile phase consisted of buffer A (0.1% formic acid in MilliQ water) and buffer B (0.1% formic acid in 3:1 acetonitrile:n-propanol v/v). The LC sample stream was introduced into the mass spectrometer by electrospray ionization (ESI) with a spray voltage of 4kV. Proteome Discoverer search software (Thermo Scientific) was used for peptide identification using the NCBI protein database. Proline and lysine modifications were examined manually by scrolling or averaging the full scan over several minutes so that all of the post-translational variations of a given peptide appeared together in the full scan.
RESULTS
Zebrafish plod2 nonsense mutation resides in highly conserved catalytic domain of lh2
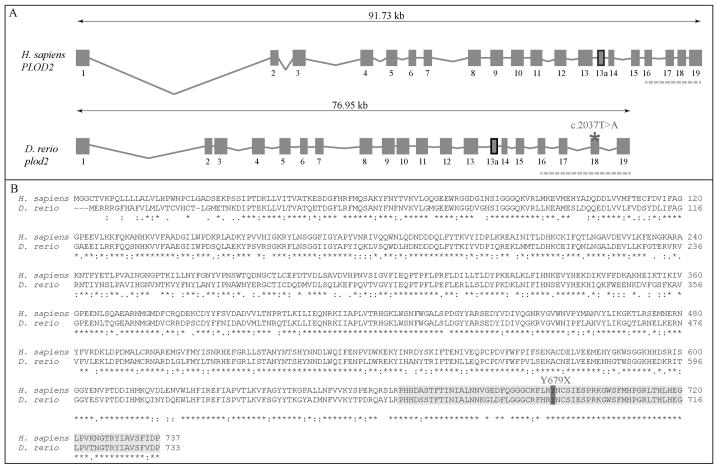
The zebrafish plod2 gene resides on chromosome 24 and spans a region of 77 kb. Its genomic structure is highly similar to that of the human PLOD2 gene as it consist of an equal number of exons (Fig. 1A). Both orthologues encode 2 differentially spliced transcripts, which differ by the presence or absence of a 63bp exon 13a. Alignment of the zebrafish and human lysyl hydroxylase 2 (lh2/LH2) protein sequence, which is encoded by plod2/PLOD2, revealed an overall 69 % amino acid (AA) similarity (Fig. 1B). The highest AA conservation (81%), can be found in the C-terminal region of the protein, encoded by the last 4 exons, and containing the catalytic domain of the protein which is vital for its lysyl hydroxylase activity, suggesting a similar enzymatic function for both orthologues. The zebrafish mutant that we report here (ZIRC, sa1768) harbors a homozygous c.2037T>A mutation in plod2, generating a premature stop codon in exon 18 at AA position 679 (p.Y679X), located centrally in the highly conserved catalytic domain.
Figure 1. Evolutionary conservation of the zebrafish and human plod2/PLOD2 gene and lh2/LH2 protein.
A. Schematic representation of the genomic structure of the H. Sapiens PLOD2 and D. Rerio plod2 genes. Boxes and lines represent exons and introns of the PLOD2/plod2 genes, respectively. Exon 13a (thick box border) is subject to differential splicing. The catalytic domain is encoded by the last four exons of the PLOD2/plod2 genes (indicated by the dashed line). The asterisk shows the position of the mutation (c.2037T>A) in the plod2sa1768 mutant zebrafish. B. Amino acid sequence alignment (with Clustal Omega) between H. Sapiens LH2 and D. Rerio lh2 (long isoform). The catalytic domain is highlighted in light grey and the mutated residue (p.Y679X) in the plod2sa1768 mutant is indicated in dark grey. Conservation of amino acid sequences are shown below the alignment: “*” means residues identical in all sequences in the alignment; “:” means conserved substitutions; “.” means semi-conserved substitutions; space means no conservation.
Reduced telopeptide lysyl hydroxylation with aberrant cross-linking of bone type I collagen in plod2 mutants
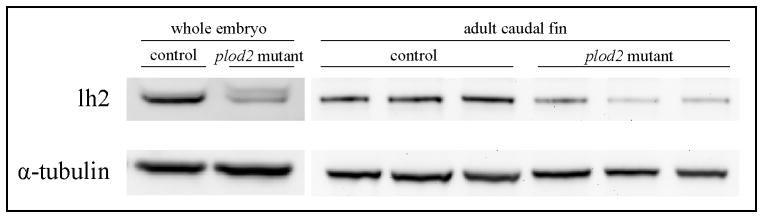
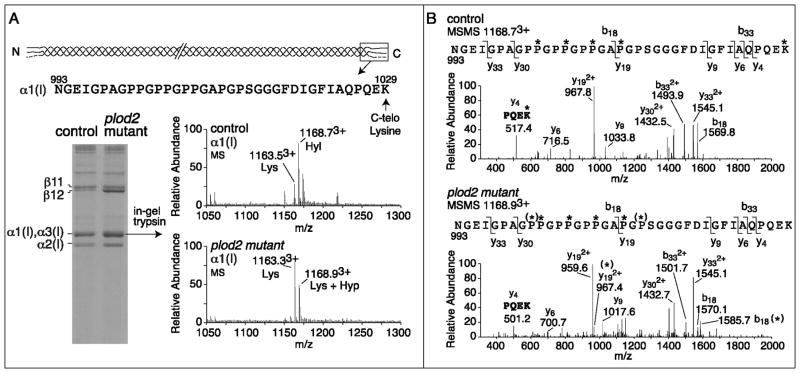
The presence of a nonsense mutation in plod2 predicts nonsense-mediated decay of mutant plod2 mRNA and consequently diminished levels of lh2 protein. In order to assess lh2 protein levels in the plod2 mutant fish, immunoblotting was performed on protein extracts prepared from whole embryos at 2 days post fertilization (dpf) and from amputated caudal fins from adult fish. In homozygous mutant embryos and adult fins, a mean lh2 reduction of resp. 60% and 65% was observed (Fig. 2). Although a residual signal is still detected upon immunoblotting in plod2 mutants, this signal could be explained by cross reaction of the antibody with the lh2 paralogues lysyl hydroxylase 1 (lh1) and lysyl hydroxylase 3 (lh3), which share the same approximate molecular weight (85 kDa) and have a significant sequence similarity with lh2. It is unlikely that this residual signal accounts for lh2 encoded by mutant plod2 mRNA that escapes NMD, as the presence of the non-sense mutation would result in a 6 kDa smaller protein which would cause a band shift upon immunoblotting. To evaluate the consequences of lh2 protein reduction on type I collagen modification in bone, we examined the hydroxylation status of the C-telopeptide lysines, which are crucial for intermolecular cross-linking in collagen fibrils of bone matrix and are conserved residues in zebrafish and human type I collagen(34). For this purpose, collagen was extracted from vertebral bone dissected from plod2 mutant and control fish and subjected to SDS-PAGE (Fig. 3A). After electrophoresis, an increase in the relative amount of extractable type I collagen was shown, consistent with a decrease in mature cross-links(10,11) in plod2 mutant bone when compared to control. Individual collagen chains were excised from the gel, digested with trypsin and lysine hydroxylation was assessed by mass spectrometry in targeted peptides. While 75% of the K1029 residues in the C-telopeptide of α1(I) were shown to be hydroxylated in samples from control fish, a complete loss of K1029 hydroxylation was evident for plod2 mutant fish (Fig. 3A–B). Since in zebrafish a paralogue of the α1(I) encoding col1a1a gene exists, namely col1a1b, which encodes the α3(I) chain, we also analyzed the equivalent tryptic peptide from α3(I) and observed similar results as for α1(I) (data not shown): The K1029 residue in the α3(I) C-telopeptide was 70 % hydroxylated in samples from control fish while in plod2 mutant fish there was a complete lack of hydroxylation at this residue. These results confirm the dysfunctionality of lh2 in plod2 mutant fish. Interestingly, the mass spectra revealed evidence of partial additional prolyl hydroxylation at residues P1001 and P1013 in plod2 mutant bone but not control. The significance and exact nature of this apparent overmodification is unclear but could reflect a suspected interplay between lysyl and prolyl hydroxylation mechanisms in the ER during synthesis(9,35).
Figure 2. Immunoblotting analysis of lh2 protein in plod2 mutants.
Analysis of lh2 expression in protein extracts prepared from a pool of whole embryos (whole embryo) at 2 dpf and from amputated caudal fins from three different plod2 mutant and three different control fish at 4 months of age (adult caudal fin). Normalization was carried out by reprobing for α-tubuline (bottom lane).
Figure 3. Cross-linking and mass spectral analysis of bone type I collagen.
Collagen was extracted from vertebral bone of 6 month old adult fish (two fish per genotype) and run on SDS-PAGE. In-gel trypsin digestion was performed on individual chains and the degree of hydroxylation of K1029 in the α1(I) C-telopeptide was analyzed by mass-spectrometry. A. SDS-PAGE of heat-denatured collagen shows a higher solubility of collagen, illustrated by an increased α and β band intensity from plod2 mutant fish, compared with control fish. The upper alpha band was shown to contain both α1(I) (65%) and α3(I) (35%) after mass-spectrometry. The sequence given at the top is of the tryptic peptide containing the α1(I) C-telopeptide obtained on mass spectrometry analysis after in-gel trypsin digestion. The lysine residue indicated (C-telo Lysine, K1029) is subject to hydroxylation by lh2. The MS spectra show the presence of both lysine (Lys) and hydroxylysine (Hyl) at K1029 in control, while in plod2 mutant fish Hyl was not detected. The molecular ions 1163.53+, 1168.73+, 1163.33+ and 1168.93+ are labeled as Lys, Hyl, Lys and Lys + Hyp respectively based on the MSMS fragmentation analysis shown in panel B. B. The fragmentation spectra (MSMS) of 1168.73+ from control α1(I) and 1168.93+ from plod2 mutant α1(I) confirms the loss of K1029 hydroxylation in plod2 mutant fish (see y4 fragment ion in plod2 mutant (501.2) and y4 (517.4) in control, K = Lys, K*= Hyl). Partial prolyl hydroxylation [illustrated by (*)] at both P1001 and P1013 was revealed by the fragmentation spectra to be the source of the additional 16 mass units in the plod2 mutant bone peptide by the MSMS spectra [see y192+ with and without (*) and b18 with and without (*)].
Plod2 mutants display severe skeletal and muscular malformations
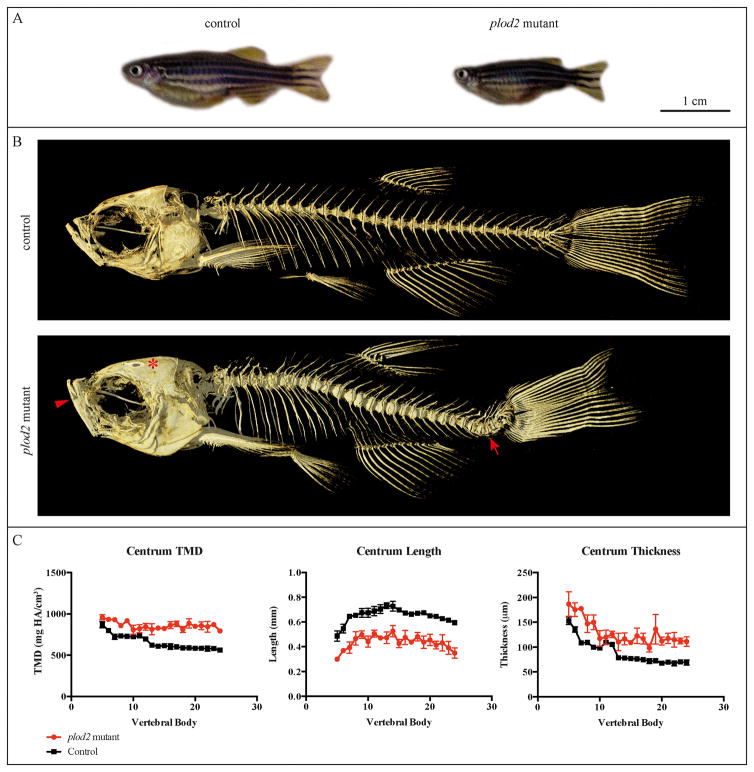
Homozygous plod2 mutant fish were generated at an expected Mendelian ratio and though there is higher mortality, most survive into adulthood. As adults, plod2 mutant fish have a shorter standard length (20.1 ± 1.2 mm, age 6 months) when compared to their wild type siblings (27.9 ± 0.7 mm, age 6 months) (p<0.001) and they display cranial malformations that are most apparent in the jaws, the opercular apparatus and the neuro-cranial roof (Fig. 4A–B). Within the axial skeleton μCT reconstructions revealed that plod2 mutant adult fish have a severely malformed vertebral column with compression of the vertebrae along the anteroposterior axis and marked kyphoscoliosis (Fig. 4B). Abundant ectopic bone formation was noted at the vertebral body centra throughout the vertebral column.
Figure 4. μCT analysis of adult fish.
A. plod2 mutant fish (4 months old) have a shorter body length compared to their wild type siblings and exhibit cranial malformation. B. μCT scans of adult plod2 mutant fish (4 months old) reveal a severe malformation of the vertebral column with compression of the vertebrae along the anteroposterior axis. The animals also suffer from kyphoscoliosis (arrow, on these lateral images only kyphosis is visible) and increased bone formation. Plod2 mutants also display malformation of the cranium, with apparent increased vertical positioning of the jaw (arrowhead) and the excess growth of the frontal and parietal bones (asterisk). C. Quantitative μCT analysis of the vertebral column in three mutant versus three control fish was performed at the age of 6 months. The x-axis represents the different vertebra (centrum) from anterior to posterior. Tissue mineral density (TMD), was significantly increased in the vertebral bodies of plod2 mutant fish (p<0.01). The Centrum length, reflecting the length of each vertebral body along the anteroposterior axis, was shown to be significantly decreased in plod2 mutant fish (p<0.01), while centrum thickness, representing the diameter of each vertebral body along the left-right axis (coronal plane) was significantly increased in plod2 mutant fish (p<0.01). Error bars indicate standard errors.
Quantitative μCT analysis revealed multi-faceted effects on vertebral morphology and mineralization. Vertebral body (centrum) tissue mineral density (TMD) and thickness were both significantly elevated in the vertebral column of plod2 mutant fish (p<0.01) (Fig. 4C). Intervertebral variability for these quantitative characters was increased in plod2 mutant fish compared to the control group. Analysis of the haemal arch, neural arch, and ribs revealed no significant effects of the plod2 mutation on bone thickness or TMD. Consistent with an overall reduction in standard length, the length of vertebral body centra was significantly reduced in plod2 mutant fish (p<0.001). The length of neural arches (p<0.05) and haemal arches/ribs (p=0.06) were also reduced, though the latter did not reach statistical significance (Fig. S1).
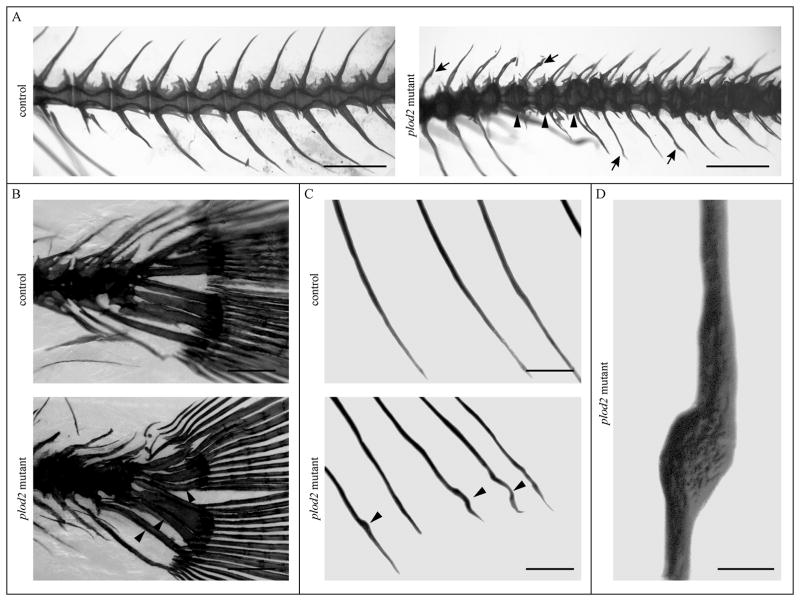
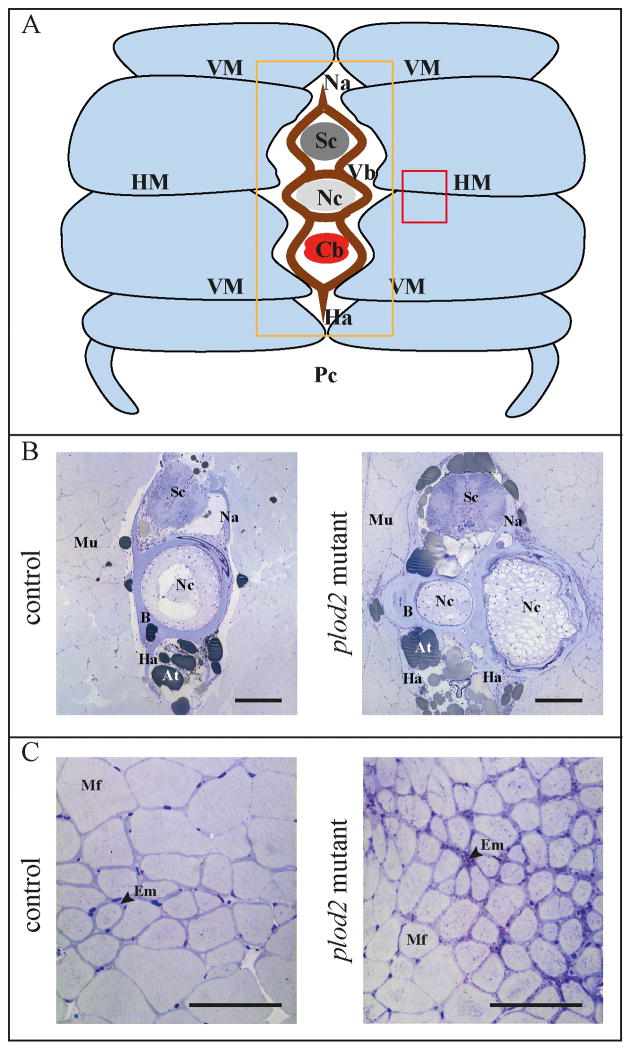
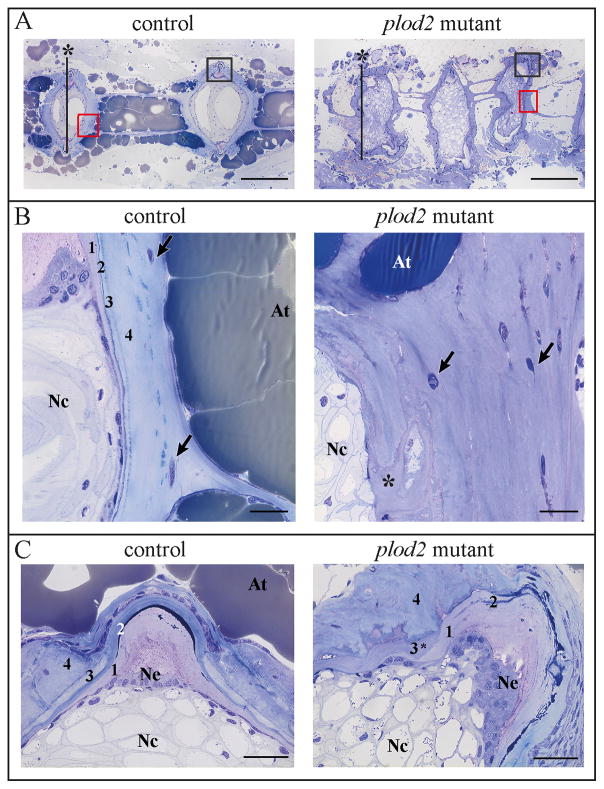
Whole-mount alizarin red staining for mineralized bone further illustrated that vertebral bodies in plod2 mutant fish are deformed through ectopic bone formation located at the vertebral endplates. Excess bone causes a loss of the typical hourglass-shape morphology of zebrafish vertebral bodies (Fig. 5A). Bowing and kinking was observed in the ribs and in the hypurals of the caudal fin endoskeleton (Fig. 5B and 5C). Bone callus formation in ribs of mutant zebrafish is indicative for fracture repair (Fig. 5D). Fractures are likely a result of increased bone fragility. Toluidine blue stained semi-thin sections (Fig. 6A–B) revealed excess bone, altering the shape of the vertebral bodies and causing the protuberances that were also observed on whole mount stains (Fig. 5A). Further, cross sections illustrated that the horizontal myoseptum, which functions as a tendon transducing force from the contracting trunk muscles towards the vertebral column(36), was also affected. The endomysium, a layer of connective tissue ensheathing the muscle fibers, was enlarged along the horizontal myoseptum of plod2 mutant fish (Fig. 6C). In addition, muscle fibers appeared smaller in diameter and were more densely packed. Sagittal sections confirmed the compressed nature of the vertebral bodies in mutant as opposed to regularly formed vertebrae in wildtype fish (Fig.7A). Excess bone was developed around the notochord and showed irregular remodeling (Fig.7B). Vertebral endplates and intervertebral space were distorted in mutant fish and lacked the orderly stratification (Fig.7C). In return, the notochord epithelium had significantly proliferated and the notochord sheath was greatly expanded in the mutant fish.
Figure 5. Alizarin Red staining for mineralized bone.
Adult fish were analyzed at 4 months of age by whole mount Alizarin Red staining. A. Most apparent defects were observed in the vertebral column, with vertebral bodies displaying an altered shape and bone protuberance occurring at the vertebral endplates (indicated at three positions by arrowheads) in plod2 mutant fish. Neural and haemal arches also display irregularities in shape (arrows) B. Bowing of the parhypurals and hypurals in the caudal fin of plod2 mutants (indicated by arrowheads). C. Bowing and kinking (arrowheads) of the ribs in mutant fish. D. Detail of a plod2 mutant rib showing callus formation upon fracture healing. Scale bars represent 1 mm in A, 200 μm in B and C and 100 μm in D.
Figure 6. Histological analysis of the vertebral column of adult fish, cross sections.
The vertebral column of 4 months old adult fish was embedded, serially sectioned and stained with toluidine blue. A. Schematic representation of the transverse profile through the trunk of an adult zebrafish. Light blue segments represent the individual muscle segments that are separated from each other by sheets of matrix, namely the horizontal and vertical myosepta (HM, VM). The orange and red square indicate the region investigated in panel B and C respectively. B. Transverse sections at a position close to the intervertebral space (location of displayed sections is also indicated in figure 7A by asterisk), showing the presence of excessive bone (B) in Plod2 mutants. This bone prominently envelops the notochord (Nc), which is bent in the coronal plane (along the left-right axis), and is consequently sectioned twice in the same plane. C. Transverse sections demonstrating a reduced muscle fiber (Mf) diameter and the presence of a thicker endomysium (Em) between the muscle fibers, at the horizontal myoseptum of plod2 mutant fish. Other abbreviations: At, adipose tissue; Cb, caudal blood vessels; Ha, haemal arch; Mu, muscle tissue; Na, neural arch; Pc, peritoneal cavity; Sc, spinal cord; Vb, vertebral body. Scale bars represent 200 μm in B and C.
Figure 7. Histological analysis of the vertebral column of adult fish, sagittal sections.
A. Sagittal sections, stained with toluidine blue, showing compressed and deformed vertebral bodies in plod2 mutants, as opposed to regularly formed vertebrae in control fish. The red and black squares indicate the regions investigated in panel B and C respectively. The vertical lines marked with an asterisk indicate the location of the transverse sections displayed in Fig 6B B. Sagittal sections demonstrating excess bone that is heavily remodeled (asterisk) in the mutant. Note that the stratification in layers, typically surrounding the notochord (1 = notochord sheath, 2 = elastin layer, 3 = lamellar bone layer, 4 = Sharpey fiber bone), is absent or severely distorted in the mutant. Osteocytes are indicated by arrows. C. Sagittal sections at the level of the intervertebral space, which is dislocated in mutants. Note the proliferated notochord epithelium (Ne), expanded notochord sheath (1), and fragmented elastin layer (2) underneath a putative lamellar bone layer (3*) and Sharpey fiber bone (4). Other abbreviations: At, adipose tissue; Nc, notochord. Scale bars represent 200 μm in A, 20 μm in B, and 30 μm in C.
The notochord sheath of plod2 mutant larvae displays structural defects
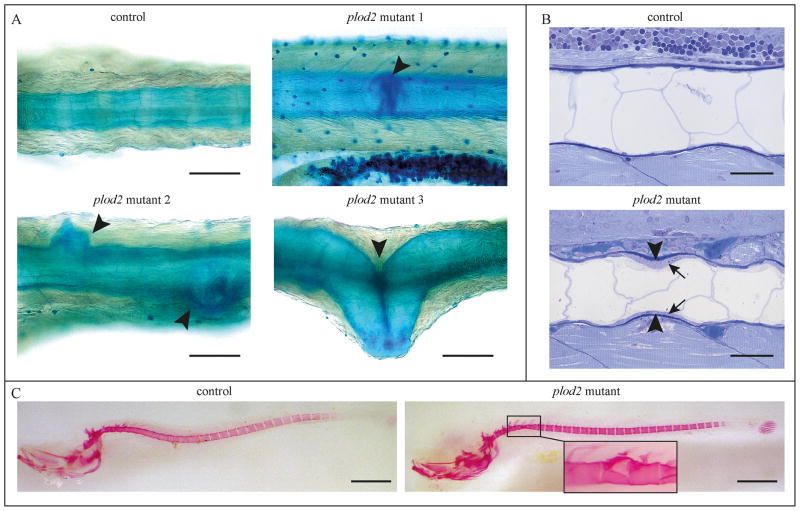
To determine at what stage the musculoskeletal abnormalities arise in the plod2 mutant fish, 8 dpf, 15 dpf and 20 dpf larvae were stained with Alcian Blue and Alizarin Red to visualize cartilage and mineralized structures. No abnormalities in the cranial cartilage could be observed at these time points (data not shown). However, from 15 dpf onwards, severe structural defects, including a disturbed vertebral centra formation, protuberance and kinking of the notochord sheath, could be seen in mutant larvae (Fig. 8A). Sagittal sections at 15 dpf revealed a wavy appearance of the notochord, confirming the phenotype observed in Alcian Blue stained whole mounts, as well as a precocious thickening of the notochordal sheath (Fig. 8B), which was also detected in the adult mutants (Fig. 7C). Alizarin Red staining in control and mutant fish at 20 dpf showed a similar pattern of segmented notochord sheath mineralization in wild type and mutant fish. However, the notochord showed the same protuberances as seen upon Alcian Blue staining (Fig. 8C). This finding was expected since Alizarin Red indicates the mineralized portion of the notochord sheath, which itself was deformed at this stage.
Figure 8. Phenotypic analysis of plod2 mutant larvae.
A. Whole mount Alcian Blue staining at 15 dpf marking the notochord sheath. Three different Plod2 mutant larvae are shown (mutant 1–3), each displaying a similar, but structurally different abnormality (arrowheads) in the notochord sheath. B. Toluidine blue stained sagittal sections of 15 dpf control fish show a smooth and rather straight notochord, while the notochord in the mutant has a wavy appearance, consistent with the phenotypes shown in panel A. Note early thickening of the notochord sheath (arrowheads), produced by a thickened notochord epithelium (arrows). C. Whole mount Alizarin Red staining for mineralized bone at 20 dpf in a control and representative plod2 mutant larva indicates no obvious abnormalities in plod2 mutants. Detail on the right shows that upon detailed examination subtle abnormalities are noted, which are similar to the structural defects observed after Alcian Blue staining. Scale bars represent 100 μm in A, 30 μm in B and 500 μm and 100 μm (detail) in C.
Type I collagen exhibits a disturbed fibrillar organization in plod2 mutant fish
In zebrafish, after initial mineralization of the notochord sheath, bone collagen is deposited by osteoblasts around the mineralized notochord sheath, especially in the vertebral body growth zone that is located at the periphery of the vertebral end plates. This collagen matures with age while developing a more pronounced banding pattern and a better organized structure, causing collagen fibril bundles to increase in density and thickness from the outer to the inner bone layer, with their orientation gradually becoming more ordered(37). To assess bone collagen fibrillar architecture in the vertebrae of plod2 mutant fish, TEM photographs were acquired in the vertebral body growth zone (Fig. 9A). In general, bone type I collagen in plod2 mutant fish displayed a lower degree of organization, which is especially evident in mature collagen adjoining the notochord. In wild type fish this collagen is present in layers exhibiting the typical plywood-like organization for bone, while in mutant fish this is not observed (Fig. 9B).
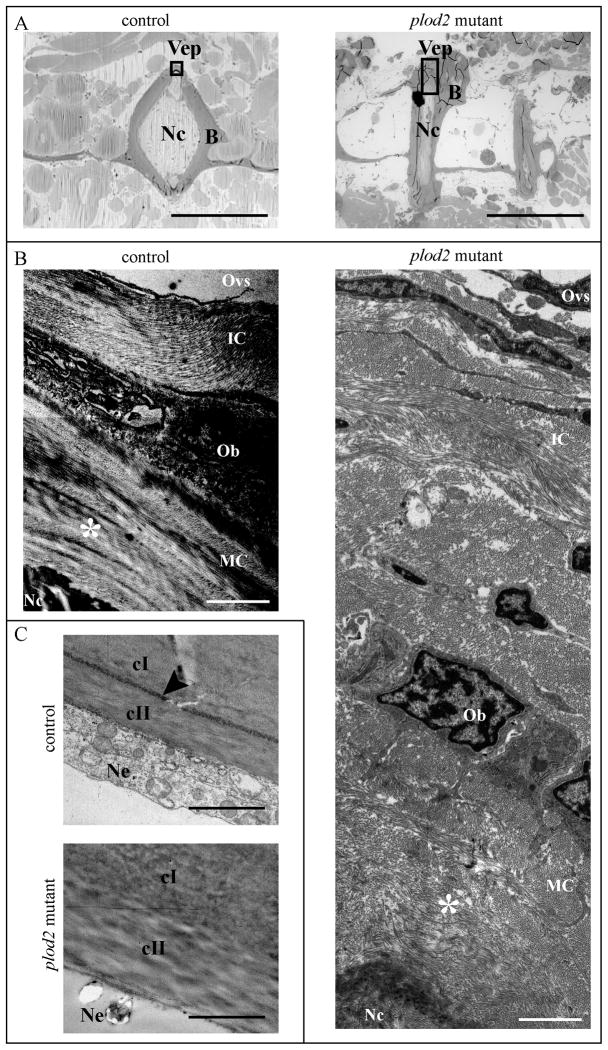
Figure 9. Analysis of bone type I collagen in adult fish using transmission electron microscopy (TEM).
A. Toluidine blue stained longitudinal sections of the vertebral column to illustrate where TEM images were acquired (black rectangles) both in control and plod2 mutant fish. B. TEM images of bone type I collagen in the vertebral end plates (Vep), which represents the growth zone of the vertebral bodies. Due to the presence of excessive periosteal bone (B) in mutant fish a larger image is shown for the plod2 mutant, compared to the control. Collagen is deposited by Osteoblasts (Ob) on the outer edges of the Vep as immature collagen (IC) and gradually matures towards the inside, adjoining the notochord (Nc), while developing a more organized structure. Hence mature collagen (MC) displays a typical plywood-like organization (indicated by asterisk), which is clearly visible in control fish but not in comparable regions (asterisk) for plod2 mutant fish. C. TEM images of the notochord sheath showing a region where the elastin layer delineating the notochord sheath (elastica externa, arrowhead), is clearly visible in control fish but interrupted in plod2 mutants. Also the collagen of the notochord sheath, which is mainly type II collagen (cII), has a disorganized appearance. Other abbreviations: cI, type I collagen; B, bone; Ne, notochord epithelium; Ovs, outer vertebral space; Vep, vertebral endplate. Scale bars represent 500 μm in A, 2 μm in B and 4 μm in C.
The notochord sheath, a cartilage-like extracellular matrix structure that surrounds the notochord, was also examined using TEM analysis. The outer portion of the sheath consists of a continuous layer of elastin (the elastica externa), which covers thick lamellar layers of mainly collagen type II. This elastica externa is covered on the outside by collagen type I rich connective tissue that surrounds the notochord(38). Interestingly, the elastica externa was of irregular thickness and fragmented in plod2 mutant fish, pointing to a disturbed integrity of the outer layer of the notochord sheath (Fig. 9C, 7C). Furthermore, the collagen fibers of the notochord sheath, adjoining the chordoblasts, also displayed a more disorganized distribution in plod2 mutant fish when compared to the control fish.
DISCUSSION
In this work we present a zebrafish model of BS, characterized by severe musculoskeletal malformations with evidence of bone fragility, and caused by a loss-of-function mutation in the plod2 gene, which encodes the lh2 protein. It has been shown that human patients with BS exhibit reduced hydroxylation of the lysine residues in bone type I collagen telopeptides (39,40), a step which is catalyzed by the LH2 enzyme. The lack of telopeptide hydroxylysines prevents pyridinoline and pyrrole formation and results in the absence of hydroxylysine aldehyde-based cross-links in bone collagen(41). Using SDS-PAGE and mass-spectrometry we showed a similar bone collagen cross-linking defect in plod2 mutant fish. While 75 % of the lysine K1029 residues in the α1(I) C-telopeptide were shown to be hydroxylated in wild type fish, a complete lack of hydroxylation of the same residue was observed in bone collagen from plod2 mutant fish, consistent with a reduction of telopeptide hydroxylysine-aldehyde cross-linking as evidenced by the increased solubility(42) of type I bone collagen in these mutants. Structurally, type I collagen fibrils were shown to exhibit a lower degree of organization with loss of the typical plywood-like structure in the vertebral bone of plod2 mutant fish when compared to control. The disturbed fibrillar architecture of collagen is most likely a consequence of the reduction of intermolecular telopeptide hydroxylysine-aldehyde cross-links within the collagen fibril, resulting in a decreased fibril stability, also affecting the supramolecular structure. In a study of human patients with recessive mutations in FKBP10 the authors also report a reduced fibrillar architecture of type I collagen in cultured patient fibroblasts(43). Moreover, RNAi-mediated knock-down of PLOD2 in cell culture was shown to negatively affect collagen fibril architecture and block parallel fiber arrangement(44). Although little is known about the relation of the ultrastructure of collagen to the mechanical integrity of mineralized bone, most likely the observed abnormalities in collagen fibril organization and ultrastructural deviations contribute to the compromised mechanical integrity of the bone in plod2 mutant fish, comparable to observations made in mouse models for OI(45,46).
At the tissue level, loss of lh2 function in zebrafish causes an early-onset and progressive phenotype that is strongly reminiscent of human BS. Human BS patients with biallelic PLOD2 mutations display a variable degree of pterygia, congenital or progressive joint contractures, (kypho)scoliosis, bone fragility with osteopenia or osteoporosis, bowing of the limbs, spontaneous fractures, including rib and vertebral fractures, and short stature (40). Many of these features are recapitulated in the plod2 mutant fish: they have a short body axis, kyphoscoliosis and compression of the vertebral column, and bowing and kinking of the ribs and the fin endoskeletal bones (hypurals). The plod2 mutants also display a reduced diameter of the muscle fibers at the horizontal myoseptum, a structure that is functionally a tendon that serves as attachment site for the trunk muscles to transduce contractile force to the vertebral column during swimming(47). An enlargement of the endomysium, a connective tissue layer ensheating the muscle fibers, was also observed. These abnormalities may reflect a similar dysfunctionality as seen in the tendons of contracted joints in human BS patients. Contractures in BS are thought to arise due to an altered cross-linking chemistry in the tendons of large joints, however the exact etiology remains unclear(9). We could demonstrate a higher TMD within the vertebral centra of plod2 mutant fish, as well as the presence of excessive periosteal bone. Although this has not been described for humans with PLOD2 mutations, hypermineralization has been reported as a feature in different forms of dominant and recessive OI and is believed to be related to the compromised bone properties in OI.
We also observed structural notochord abnormalities in both mutant larvae and adults. In mutant adult fish histological and TEM analysis revealed a loss of the integrity of the elastica externa, the elastin layer that forms the outer portion of the notochord sheath. During early larval development, this layer is important to restrict the expansion of the notochord sheath that results from the internal osmotic pressure generated by vacuoles in the notochord lumen(38). Although the connective tissue of the notochord sheath is believed to be mainly composed of collagen type II, also the presence of collagen type I has been reported for different vertebrates(48–50). As the elastica externa is intimately associated with this underlying collagen network, defective cross-linking of type I collagen in the notochord sheath could negatively affect the structure of this elastin layer. Alternatively, this defect could arise due to a more direct effect of lh2 deficiency on elastin. Although cross-linking in elastin is independent of lysine hydroxylation(7), interaction of elastin and FKBP65 has been reported(51). As a result of the disturbed integrity of the elastin layer, the ability to resist internal colloid osmotic pressure may be impaired, leading to the protuberances of the notochord and possibly scoliosis in adult fish, as scoliosis in humans is also associated with elastic fiber degeneration (52). Interestingly, fragmentation of elastic fibers was reported as a feature in the dermis of the skin of OI patients (53).
Until now only 9 different mutations in PLOD2 have been reported in patients with either OI or BS (https://oi.gene.le.ac.uk(40,41,54,55)). Five of these are missense mutations and two splice site mutations that cause an in-frame exon deletion, all affecting both LH2 isoforms. The remaining two account for insertion-deletion mutations causing a frameshift, that are either in compound heterozygous state with a missense mutation or in homozygous state but only affecting the long isoform of LH2(54). This may suggest that a complete loss of LH2 function is not likely to be viable in humans. Similarly, Lh2-null mice do not survive beyond embryonic stage(21). Conversely, most of plod2 zebrafish mutants survive into adulthood, despite the complete loss of functional lh2 protein. This could be explained by the reduced requirement for weight bearing on the zebrafish skeleton within the aquatic environment, which causes skeletal phenotypes in fish to often present as being milder and having less impact on general survival, when compared to humans or mice enabling the study of skeletal abnormalities beyond embryonal and larval stages.
Taken together, we report a plod2 zebrafish mutant with diminished type I collagen telopeptide lysine hydroxylation affecting the formation of intermolecular cross-links. This mutant displays musculoskeletal abnormalities resembling the clinical features seen in BS. Given the lack of viable animal models of BS caused by PLOD2 mutations, we believe that this mutant can serve as a useful model to help unravel the underlying pathogenetic mechanisms in BS as well as to serve as a tool for the development and evaluation of possible therapeutic interventions. Finally, in this work we further illustrate the usefulness and relevance of using zebrafish as a model for heritable bone diseases such as BS and OI.
Supplementary Material
Acknowledgments
We would like to thank Petra Vermassen and Hanna De Saffel for the excellent technical assistance. We thank Patrick Willems for the helpful discussions. This work was supported by the Ghent University Methusalem grant BOF08/01M01108 to ADP, funding from the Belgian Science Policy Office Interuniversity Attraction Poles (BELSPO-IAP) program through the project IAP P7/43-BeMGI, and grants from the NIH to DRE (AR037318 and HD070394), RYK (AR066061) and the ZIRC (RR12546). Authors’ roles: Project design: AW, PJC, SS, ADP and FM. Data collection: CG, AH, PS, DL, MD, LVH, RYK, MW and DRE. Data analysis: CG, AH, DL, MW, RYK and DRE. Data interpretation: CG, AW, EPW and AH. Drafting manuscript: CG and AW. Revising the manuscript, content and approving final version of manuscript: all authors. CG and AW take responsibility for the integrity of the data analysis.
Footnotes
Disclosures: All authors state that they have no conflict of interest
References
- 1.AB Ueber eine seltene Form von Erkrankung der Knochen und Gelenke. Dtsch Med. 1897;23:152–5. [Google Scholar]
- 2.Forlino A, Cabral WA, Barnes AM, Marini JC. New perspectives on osteogenesis imperfecta. Nature reviews Endocrinology. 2011;7(9):540–57. doi: 10.1038/nrendo.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forlino A, Marini JC. Osteogenesis imperfecta. Lancet. 2015 doi: 10.1016/S0140-6736(15)00728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinckmann J, Notbohm H, Müller PK. collagen: Primer in Structure, Processing and Assembly. Topics in Current Chemistry. 2005:247. [Google Scholar]
- 5.Alanay Y, Avaygan H, Camacho N, et al. Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2010;86(4):551–9. doi: 10.1016/j.ajhg.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eyre DR, Koob TJ, Van Ness KP. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal Biochem. 1984;137(2):380–8. doi: 10.1016/0003-2697(84)90101-5. [DOI] [PubMed] [Google Scholar]
- 7.Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–48. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- 8.Oxlund H, Barckman M, Ortoft G, Andreassen TT. Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone. 1995;17(4 Suppl):365S–71S. doi: 10.1016/8756-3282(95)00328-b. [DOI] [PubMed] [Google Scholar]
- 9.Eyre DR, Weis MA. Bone collagen: new clues to its mineralization mechanism from recessive osteogenesis imperfecta. Calcified tissue international. 2013;93(4):338–47. doi: 10.1007/s00223-013-9723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bank RA, Robins SP, Wijmenga C, et al. Defective collagen crosslinking in bone, but not in ligament or cartilage, in Bruck syndrome: indications for a bone-specific telopeptide lysyl hydroxylase on chromosome 17. Proc Natl Acad Sci U S A. 1999;96(3):1054–8. doi: 10.1073/pnas.96.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarze U, Cundy T, Pyott SM, et al. Mutations in FKBP10, which result in Bruck syndrome and recessive forms of osteogenesis imperfecta, inhibit the hydroxylation of telopeptide lysines in bone collagen. Human molecular genetics. 2013;22(1):1–17. doi: 10.1093/hmg/dds371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apschner A, Schulte-Merker S, Witten PE. Not all bones are created equal - using zebrafish and other teleost species in osteogenesis research. Methods Cell Biol. 2011;105:239–55. doi: 10.1016/B978-0-12-381320-6.00010-2. [DOI] [PubMed] [Google Scholar]
- 13.Mackay EW, Apschner A, Schulte-Merker S. A bone to pick with zebrafish. Bonekey Rep. 2013;2:445. doi: 10.1038/bonekey.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spoorendonk KM, Hammond CL, Huitema FA, Vanoevelen J, Schulte-Merker S. Zebrafish as a unique model system in bone research: the power of genetics and in vivo imaging. Applied Ichthyology. 2010;26(2) [Google Scholar]
- 15.Li N, Felber K, Elks P, Croucher P, Roehl HH. Tracking gene expression during zebrafish osteoblast differentiation. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238(2):459–66. doi: 10.1002/dvdy.21838. [DOI] [PubMed] [Google Scholar]
- 16.Witten PE, Huysseune A. A comparative view on mechanisms and functions of skeletal remodelling in teleost fish, with special emphasis on osteoclasts and their function. Biological reviews of the Cambridge Philosophical Society. 2009;84(2):315–46. doi: 10.1111/j.1469-185X.2009.00077.x. [DOI] [PubMed] [Google Scholar]
- 17.Andreeva V, Connolly MH, Stewart-Swift C, et al. Identification of adult mineralized tissue zebrafish mutants. Genesis. 2011;49(4):360–6. doi: 10.1002/dvg.20712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammond CL, Moro E. Using transgenic reporters to visualize bone and cartilage signaling during development in vivo. Front Endocrinol (Lausanne) 2012;3:91. doi: 10.3389/fendo.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asharani PV, Keupp K, Semler O, et al. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am J Hum Genet. 2012;90(4):661–74. doi: 10.1016/j.ajhg.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher S, Jagadeeswaran P, Halpern ME. Radiographic analysis of zebrafish skeletal defects. Developmental biology. 2003;264(1):64–76. doi: 10.1016/s0012-1606(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 21.Hyry M, Lantto J, Myllyharju J. Missense mutations that cause Bruck syndrome affect enzymatic activity, folding, and oligomerization of lysyl hydroxylase 2. The Journal of biological chemistry. 2009;284(45):30917–24. doi: 10.1074/jbc.M109.021238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kettleborough RN, Busch-Nentwich EM, Harvey SA, et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature. 2013;496(7446):494–7. doi: 10.1038/nature11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 24.Neuhauss SC, Solnica-Krezel L, Schier AF, et al. Mutations affecting craniofacial development in zebrafish. Development. 1996;123:357–67. doi: 10.1242/dev.123.1.357. [DOI] [PubMed] [Google Scholar]
- 25.Spoorendonk KM, Peterson-Maduro J, Renn J, et al. Retinoic acid and Cyp26b1 are critical regulators of osteogenesis in the axial skeleton. Development. 2008;135(22):3765–74. doi: 10.1242/dev.024034. [DOI] [PubMed] [Google Scholar]
- 26.Lewis LM, Lall SP, Witten PE. Morphological descriptions of the early stages of spine and vertebral development in hatchery-reared larval and juvenile Atlantic halibut (Hippoglossus hippoglossus) Aquaculture. 2004;241(1–4):47–59. [Google Scholar]
- 27.Huysseune A, Sire JY. Bone and cartilage resorption in relation to tooth development in the anterior part of the mandible in cichlid fish: a light and TEM study. Anat Rec. 1992;234(1):1–14. doi: 10.1002/ar.1092340102. [DOI] [PubMed] [Google Scholar]
- 28.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25(7):1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 29.Goeman JJ, van de Geer SA, van Houwelingen HC. Testing against a high dimensional alternative. J Roy Stat Soc B. 2006;68:477–93. [Google Scholar]
- 30.Masschaele B, Dierick M, Van Loo D, et al. HECTOR: A 240kV micro-CT setup optimized for research. J Phys Conf Ser. 2013:463. [Google Scholar]
- 31.Masschaele BC, Cnudde V, Dierick M, Jacobs P, Hoorebeke L, Vlassenbroeck J. UGCT: New X-ray radiography and tomography facility. Nuclear Instruments and Methods in Physics Research. 2007;39(23):266–9. [Google Scholar]
- 32.Vlassenbroeck J, Dierick M, Masschaelea B, VCL, VH, PJ Software tools for quantification of X-ray microtomography at the UGCT. Proceedings of the 10 th International Symposium on Radiation Physics; 2007; pp. 442–5. [Google Scholar]
- 33.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gistelinck C, Gioia R, Gagliardi A, et al. Zebrafish Collagen Type I: Molecular and Biochemical Characterization of the Major Structural Protein in Bone and Skin. Sci Rep. 2016;6:21540. doi: 10.1038/srep21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heard ME, Besio R, Weis M, et al. Sc65-Null Mice Provide Evidence for a Novel Endoplasmic Reticulum Complex Regulating Collagen Lysyl Hydroxylation. PLoS genetics. 2016;12(4):e1006002. doi: 10.1371/journal.pgen.1006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bassett DI, Currie PD. The zebrafish as a model for muscular dystrophy and congenital myopathy. Human molecular genetics. 2003;12:R265–R70. doi: 10.1093/hmg/ddg279. [DOI] [PubMed] [Google Scholar]
- 37.Ge J, Wang XM, Cui FZ. Microstructural characteristics and nanomechanical properties across the thickness of the wild-type zebrafish skeletal bone. Mat Sci Eng C-Bio S. 2006;26(4):710–5. [Google Scholar]
- 38.Grotmol S, Kryvi H, Keynes R, Krossoy C, Nordvik K, Totland GK. Stepwise enforcement of the notochord and its intersection with the myoseptum: an evolutionary path leading to development of the vertebra? J Anat. 2006;209(3):339–57. doi: 10.1111/j.1469-7580.2006.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bank RA, Robins SP, Wijmenga C, et al. Defective collagen crosslinking in bone, but not in ligament or cartilage, in Bruck syndrome: Indications for a bone-specific telopeptide lysyl hydroxylase on chromosome 17. P Natl Acad Sci USA. 1999;96(3):1054–8. doi: 10.1073/pnas.96.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ha-Vinh R, Alanay Y, Bank RA, et al. Phenotypic and molecular characterization of Bruck syndrome (osteogenesis imperfecta with contractures of the large joints) caused by a recessive mutation in PLOD2. Am J Med Genet A. 2004;131(2):115–20. doi: 10.1002/ajmg.a.30231. [DOI] [PubMed] [Google Scholar]
- 41.van der Slot AJ, Zuurmond AM, Bardoel AFJ, et al. Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. Journal of Biological Chemistry. 2003;278(42):40967–72. doi: 10.1074/jbc.M307380200. [DOI] [PubMed] [Google Scholar]
- 42.van der Slot AJ, Zuurmond AM, van den Bogaerdt AJ, et al. Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biology. 2004;23(4):251–7. doi: 10.1016/j.matbio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Barnes AM, Duncan G, Weis M, et al. Kuskokwim Syndrome, a Recessive Congenital Contracture Disorder, Extends the Phenotype of FKBP10 Mutations. Human mutation. 2013;34(9):1279–88. doi: 10.1002/humu.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sada M, Ohuchida K, Horioka K, et al. Hypoxic stellate cells of pancreatic cancer stroma regulate extracellular matrix fiber organization and cancer cell motility. Cancer Lett. 2016;372(2):210–8. doi: 10.1016/j.canlet.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 45.McBride DJ, Choe V, Shapiro JR, Brodsky B. Altered collagen structure in mouse tail tendon lacking the alpha 2(I) chain. J Mol Biol. 1997;270(2):275–84. doi: 10.1006/jmbi.1997.1106. [DOI] [PubMed] [Google Scholar]
- 46.Wallace JM, Orr BG, Marini JC, Holl MMB. Nanoscale morphology of Type I collagen is altered in the Brtl mouse model of Osteogenesis Imperfecta. J Struct Biol. 2011;173(1):146–52. doi: 10.1016/j.jsb.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gemballa S, Vogel F. Spatial arrangement of white muscle fibers and myoseptal tendons in fishes. Comp Biochem Physiol A Mol Integr Physiol. 2002;133(4):1013–37. doi: 10.1016/s1095-6433(02)00186-1. [DOI] [PubMed] [Google Scholar]
- 48.Ghanem E. Immunohistochemical localization of type I and II collagens in the involuting chick notochords in vivo and in vitro. Cell Biol Int. 1996;20(10):681–5. doi: 10.1006/cbir.1996.0090. [DOI] [PubMed] [Google Scholar]
- 49.Kenney MC, Carlson E. Ultrastructural identification of collagen and glycosaminoglycans in notochordal extracellular matrix in vivo and in vitro. Anat Rec. 1978;190(4):827–49. doi: 10.1002/ar.1091900405. [DOI] [PubMed] [Google Scholar]
- 50.Kimura S, Kamimura T. The Characterization of Lamprey Notochord Collagen with Special Reference to Its Skin Collagen. Comp Biochem Phys B. 1982;73(2):335–9. [Google Scholar]
- 51.Davis EC, Broekelmann TJ, Ozawa Y, Mecham RP. Identification of tropoelastin as a ligand for the 65-kD FK506-binding protein, FKBP65, in the secretory pathway. J Cell Biol. 1998;140(2):295–303. doi: 10.1083/jcb.140.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu J, Fairbank JC, Roberts S, Urban JP. The elastic fiber network of the anulus fibrosus of the normal and scoliotic human intervertebral disc. Spine (Phila Pa 1976) 2005;30(16):1815–20. doi: 10.1097/01.brs.0000173899.97415.5b. [DOI] [PubMed] [Google Scholar]
- 53.Balasubramanian M, Sobey GJ, Wagner BE, et al. Osteogenesis imperfecta: Ultrastructural and histological findings on examination of skin revealing novel insights into genotype-phenotype correlation. Ultrastruct Pathol. 2016;40(2):71–6. doi: 10.3109/01913123.2016.1140253. [DOI] [PubMed] [Google Scholar]
- 54.Puig-Hervas MT, Temtamy S, Aglan M, et al. Mutations in PLOD2 cause autosomal-recessive connective tissue disorders within the Bruck syndrome--osteogenesis imperfecta phenotypic spectrum. Human mutation. 2012;33(10):1444–9. doi: 10.1002/humu.22133. [DOI] [PubMed] [Google Scholar]
- 55.Zhou P, Liu Y, Lv F, et al. Novel mutations in FKBP10 and PLOD2 cause rare Bruck syndrome in Chinese patients. PLoS One. 2014;9(9):e107594. doi: 10.1371/journal.pone.0107594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.