Abstract
Preterm infants are susceptible to neonatal inflammatory/infective diseases requiring drug therapy. The present study hypothesized that mRNA expression in the blood may be modulated by signaling pathways during treatment. The current study aimed to explore changes in global gene expression in the blood from preterm infants with the objective of identifying patterns or pathways of potential relevance to drug therapy. The infants involved were selected based on maternal criteria indicating increased risk for therapeutic intervention. Global mRNA expression was measured in 107 longitudinal whole blood samples using Affymetrix Human-Genome-U133 Plus 2.0-arrays; samples were obtained from 20 preterm infants. Unsupervised clustering revealed a distinct homogeneous gene expression pattern in 13 samples derived from seven infants undergoing continuous oxygen therapy. At these sampling times, all but one of the seven infants exhibited severe drops in peripheral capillary saturation levels below 60%. The infants were reoxygenated with 100% inspired oxygen concentration. The other samples (n=94) represented the infants from the cohort at time points when they did not undergo continuous oxygen therapy. Comparing these two sets of samples identified a distinct gene expression pattern of 5,986 significantly differentially expressed genes, of which 5,167 genes exhibited reduced expression levels during transient hypoxia. This expression pattern was reversed when the infants became stable, i.e., when they were not continuously oxygenated and had no events of hypoxia. To identify signaling pathways involved in gene regulation, the Database for Annotation, Visualization and Integrated Discovery online tool was used. Mitogen-activated protein kinases, which are normally induced by oxidative stress, exhibited reduced gene expression during hypoxia. In addition, nuclear factor erythroid 2-related factor 2-antioxidant response element target genes involved in oxidative stress protection were also expressed at lower levels, suggesting reduced transcription of this pathway. The findings of the present study suggest that oxidative stress-dependent signaling is reduced during hypoxia. Understanding the molecular response in preterm infants during continuous oxygenation may aid in refining therapeutic strategies for oxygen therapy.
Keywords: gene expression profile, preterm infants, transcription, molecular pattern, signaling pathway
Introduction
Preterm infants are vulnerable to xenobiotics and morbidity due to the presence of an immature immune system, reduced antioxidant capacity, and oxidative stress (1,2). The respiratory function of immature lungs is incomplete, for which traditionally oxygen has been given as a therapeutic agent (3). Controversies regarding the most appropriate inspired oxygen concentrations (FiO2) in resuscitation and in the treatment of preterm infants have been extensively discussed in the literature (1,4–9). However, the most suitable FiO2 levels and target range of oxygen saturation remain unknown (4,10,11).
Peripheral capillary saturation level (SpO2) is used for monitoring postnatal stabilization (12). The medicinal indication for administering oxygen is to avoid hypoxia and to ensure reperfusion after hypoxemia following apneic spells and drops in SpO2. Therefore, oxygen has become an integral part of therapeutic respiratory support worldwide, and is the most commonly used drug in neonatal intensive care units (13). Adverse consequences and toxicity from administering oxygen are associated with excessive oxidative stress and reactive oxygen species (ROS) generation. Despite this understanding, high levels of FiO2 have long been used in the treatment of preterm infants (1,3,7). Morbidities associated with excessive oxidative stress have been widely described in the literature, including bronchopulmonary dysplasia (BPD), patent ductus arteriosus (PDA), periventricular leukomalacia (PVL), intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC) and retinopathy of prematurity (ROP) (7,14–17).
At present, detailed molecular characterizations and understanding of gene-regulatory mechanisms during treatment in preterm infants are lacking, but could provide an entry point for developing therapies and/or avoiding adverse effects. Differentially expressed genes in peripheral blood may exhibit individual responses or expression patterns describing biological responses or effects from therapeutic interventions (18). High-throughput arrays provide the potential to investigate a large number of genes and signaling pathways in a small number of human blood samples (19,20), whilst allowing flexibility in sample comparison (18). Recently, data from genome-wide expression studies in preterm infants have emerged (21,22).
Therapeutic intervention is often initiated in preterm infants exposed to maternal inflammatory/infective processes. Therefore, the present study aimed to explore global gene expression patterns to identify signaling pathways of potential significance to therapeutics. Genome-wide mRNA expression was determined using the NuGEN Ovation RNA-Amplification system on whole blood from preterm infants in the presence of fetal globins. A distinct transcription pattern was identified in infants that received continuous oxygen therapy during hypoxia. In addition, it was identified that this pattern was reversed when continuous oxygen therapy was terminated. The transcription profile included low expression levels of mitogen-activated protein kinases (MAPK) genes and target genes of the nuclear factor erythroid 2-related factor 2-antioxidant response element (Nrf2-ARE) pathway, indicating reduced oxidative stress-dependent signaling during hypoxia. The identified profile may allow a better understanding of the basic mechanisms involved in gene regulatory networks during continuous oxygen therapy and transient hypoxia in preterm infants. Furthermore, this profile may potentially serve as a biomarker to improve treatment strategies for oxygen therapy in preterm infants.
Materials and methods
Study population
The cohort included 20 preterm infants (<33 weeks at birth) from a level III NICU at Oslo University Hospital, Rikshospitalet (Oslo, Norway), which admits infants from regional, multiregional and national populations. The study was conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent to participate was provided by the parents prior to enrollment. All the methods used in the study were performed in accordance with the guidelines and regulations according to the approval from the Regional Committees for Medical and Health Research Ethics in Norway (‘REK-South’) and the Institutional Review Board at Oslo University Hospital, Rikshospitalet; project nο. 11690.
Whole blood samples were longitudinally obtained from the cohort throughout the neonatal and postnatal period (Table I). The samples were collected in conjunction with the morning routine laboratory samples as requested by the consultant physicians. Therefore, the number of samples from each infant varied. The infants were selected based on maternal criteria indicating risk for neonatal inflammatory/infective processes, such as preterm labor, including antenatal steroid treatment to improve fetal lung development, clinical chorioamnionitis, preterm premature rupture of membranes (p-PROM) >24 h involving systemic antibiotic treatment prior to delivery, preeclampsia, or hemolysis, elevated liver enzyme levels and low platelet count (HELLP) (Table II). The gestational age (GA) was assessed by obstetric history, and confirmed by the consultant physician and during the newborn examination. Infants with congenital malformations, metabolic and neurologic diseases, and chromosomal anomalies were excluded from the study.
Table I.
Distribution of all samples (n=107) according to age and gender of the infants.
| Samples | |||
|---|---|---|---|
| Age of infants after birth | Male (n=33) | Female (n=74) | Total (n=107) |
| Days after birth | |||
| 1–15 | 6 | 27 | 33 |
| 16–30 | 8 | 21 | 29 |
| 31–45 | 4 | 11 | 15 |
| 46–60 | 5 | 9 | 14 |
| 61–73 | 4 | 4 | 8 |
| 74–226 | 6 | 2 | 8 |
| PMA, weeks after birth | |||
| 23.9–26.9 | 0 | 5 | 5 |
| 27.0–29.9 | 9 | 12 | 21 |
| 30.0–32.9 | 6 | 23 | 29 |
| 33.0–36.9 | 5 | 26 | 31 |
| 37.0–41.9 | 10 | 6 | 16 |
| 42.0–61.6 | 3 | 2 | 5 |
PMA, postmenstrual age. PMA=gestational age + chronological age (weeks).
Table II.
Characteristics of the cohort (n=20) and the maternal criteria, including infants with hypoxia (n=7) and without hypoxia (n=13).
| Characteristic | All infants (n=20) | Infants; transient hypoxia (n=7) | Infants; without hypoxia (n=13) |
| Gestational age (week)a | 27.6 (23.7–36.9) | 27.9 (25.2–33.0) | 29.3 (23.7–36.9) |
| Gender | |||
| Male | 7 | 2 | 5 |
| Female | 13 | 5 | 8 |
| Birth weight (g)a | 1,200 (560–3,000) | 1,088 (585–1,980) | 1,205 (560–3,000) |
| Delivery | |||
| Vaginal | 4 | 1 | 3 |
| Caesarean | 16 | 6 | 10 |
| Maternal criteria | |||
| Clinical chorioamnionitis | 7 | 4 | 3 |
| Prenatal antibiotics | 7 | 4 | 3 |
| Prenatal steroids (doses) | 14 | 6 | 8 |
| 0 | 6 | 1 | 5 |
| 1 | 11 | 5 | 6 |
| 2 | 3 | 1 | 2 |
| Tractocile™ | 9 | 4 | 5 |
| Preeclampsia | 9 | 3 | 6 |
| HELLP | 5 | 2 | 3 |
| Curosurf (doses) | |||
| 1 | 5 | 2 | 3 |
| 2 | 3 | 1 | 2 |
| Steroid treatment | 5 | 3 | 2 |
| Antibiotics (courses) | |||
| 0 | 5 | 1 | 4 |
| 1 | 7 | 3 | 4 |
| 2 | 2 | 0 | 2 |
| 3 | 3 | 1 | 2 |
| 4 | 2 | 1 | 1 |
| 5 | 1 | 1 | 0 |
| Outcome morbidities | |||
| PVL | 3 | 3 | 0 |
| IVH grade 1 | 1 | 1 | 0 |
| IVH grade 2 | 5 | 3 | 2 |
| PDA | 4 | 4 | 0 |
| BPD | 4 | 4 | 0 |
| NEC | 2 | 1 | 1 |
| ROP | 2 | 1 | 1 |
| Death | 1 | 0 | 1 |
median (min-max). PVL, periventricular leukomalacia; IVH, intraventricular hemorrhage; PDA, patent ductus arteriosus; BPD, bronchopulmonary dysplasia; NEC, necrotizing enterocolitis; ROP, retinopathy of prematurity.
Treatment strategies of the cohort
Oxygen therapy, antibiotics, and supportive treatment were prescribed according to standard procedures in the present neonatal intensive care unit (NICU). All infants on respiratory support; nasal CPAP or ventilator and/or when oxygen was administered, received 2.5 mg/kg/day caffeine citrate maintenance dose and 15 mg/kg/day antioxidant Vitamin E once daily. Vitamin E was administered as an ‘anti-oxidant’ drug to prevent oxygenation from reactive oxygen species. Caffeine was administered to stimulate the central respiratory center and prevent from apneas. The maintenance dose prescribed for preterm infants in the NICU was lower than the standard recommendation dose in order to prevent the occurrence of tachycardia; a known side effect. These products were produced at the hospital pharmacy at Rikshospitalet. Antibiotics were administered on indication of sepsis, as prescribed by the consultant physicians. The diagnoses of sepsis was confirmed as respiratory failure and distress, tachypnea, apnea, lethargy, temperature instability and hypotension, in addition to confirmation from laboratory findings. These findings included a positive blood culture that confirmed growth of a microorganism, according to standard procedures, and maternal antibiotic-treated chorioamnionitis or p-PROM >24 h.
Treatment of infants with continuous oxygen
A caffeine citrate maintenance dose of 2.5 mg/kg/day and Vitamin E 15 mg/kg/day (as previously described) were prescribed once daily in conjunction with the administered continuous oxygen therapy for a minimum of 21 consecutive days. Infants with sepsis were prescribed antibiotics according to standard regimens in the NICU regarding bacterial growth.
Data collection
Diagnostic information, including the cause of prematurity, was obtained from individual records, intensive care charts, and from daily reports by the physicians and nurses.
Blood collection
A previous study identified that a sufficient amount of RNA may be isolated from peripheral blood (18). Whole blood was chosen for study due to the presence of cells involved in progression of infection, regardless of gestation (18). Blood sampling was conducted in a non-stressful manner following analgesia with oral dextrose (25%). Samples were collected in BD Microtainer K2E tubes (BD Biosciences, Franklin Lakes, NJ, USA) containing EDTA as an anticoagulant. A total of 143 samples were obtained, of which 36 samples were excluded; 22 had poor RNA yield and quality, and 14 could not be processed due to insufficient RNA binding buffer. The remaining 107 samples were derived from 7 males and 13 females (Table I). A total of 62/107 samples were obtained during the first four living weeks.
Blood processing and RNA preparation
A 0.3–0.5 ml aliquot of peripheral whole blood was obtained by heel stick, according to standard procedure, at clinical site while the infant was in the incubator or bed. A 2.0 ml microfuge tube (Ambion; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 1.3 ml RNA-stabilization solution, RNAlater (Ambion; Thermo Fisher Scientific, Inc.) was used for this purpose. The samples were mixed thoroughly to stabilize RNA, and stored at room temperature for 36–48 h, in order to allow the stabilization solution to penetrate the cells. According to the RiboPure-protocol (Ambion; Thermo Fisher Scientific, Inc.) (23), the tubes were stored at −20°C until RNA extraction. These steps were performed by one person to ensure uniformity of sample collection and processing. For blood preparation, the RiboPure-Blood kit (23) and GLOBINclear-Human kit (Ambion; Thermo Fisher Scientific, Inc.) (24) were used, according to the manufacturer's protocols. Following isolation of total RNA, all samples were stored at −80°C and shipped overnight on dry ice to Uppsala Array Platform at Uppsala University (Uppsala, Sweden) for further processing and analysis.
The NuGEN Ovation RNA-Amplification system (NuGEN Technologies, Inc., San Carlos, CA, USA) (24) for application on Affymetrix arrays (Affymetrix, Inc., Santa Clara, CA, USA) was used for RNA labeling, according to the manufacturer's protocol. The methods use of the GLOBINclear-Human kit eliminated the effects of all α- and β-globin RNA (from red and white blood cells) in two steps via hybridization technology and magnetic separation (23). The removal of globin is essential for the expression of genes. An abundance of globin may mask gene expression. The initial microarray samples identified that the gene expression was masked in the present study using standard methods. The remaining globin identified in the samples was most likely fetal globin. mRNA was then amplified in the presence of fetal globin, and any possible α- and β-globin leftover from the initial step, using the NuGEN Ovation RNA-Amplification system (25); the remaining globin did not affect gene expression.
Microarray analysis
A total of 107 samples were processed for global mRNA gene expression and analyzed using Human Genome U133-Plus 2.0 arrays from Affymetrix, Inc. The RNA concentrations were measured with a NanoDrop-1000 spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE, USA) and the quality was evaluated prior to labeling using the 2100 Bioanalyzer system (Agilent Technologies, Inc., Santa Clara, CA, USA). From each sample, 100 ng total RNA was used to prepare biotinylated fragmented cRNA, according to the NuGEN Ovation WB Reagent User Guide (version, 05.01.07; NuGEN Technologies, Inc.) and FL-Ovation cDNA Biotin Module V2 User Guide (version, 05.13.08; NuGEN Technologies, Inc.) (25). Purification of cDNA was conducted according to manufacturer's protocol (FL-Ovation cDNA Biotin Module V2 User Guide (version, 05.13.08; NuGEN Technologies, Inc.) using the DNA Clean & Concentrator-25 (Zymo Research Corporation, Irvine, CA, USA). The Human Genome U133-Plus 2.0 arrays (Affymetrix, Inc.) were hybridized for 16 h in a 45°C incubator while being rotated at 60 rpm according to the manufacturer's protocol (The Human Genome U133-Plus 2.0 arrays; Affymetrix, Inc.), thereafter the arrays were washed and stained with Affymetrix Fluidics Station 450, and finally scanned using the GeneChip Scanner 3000 7G (Affymetrix, Inc.). All samples passed the quality control steps.
Statistical analysis
The gene expression data were analyzed in the freely available statistical computing language R (http://www.r-project.org) version number R 2.10.0 using packages available from the Bioconductor project (www.bioconductor.org). The raw data were normalized using the robust multiarray average method (26), which was first suggested by Li and Wong (27). To interpret the complex data and the role of the genes, the distinct expression pattern was observed, and the clinical and therapeutic characteristics of the infants at each sampling time were described.
Differences in gene expression levels between the two identified groups of infants were analyzed by applying an empirical Bayes moderated t-test (28) using the Limma package (29). The P-values were adjusted using the method of Benjamini and Hochberg to address the problem of multiple testing (30). Significant differences in gene expression levels were defined at P<0.05 (adjusted) and at >2-fold differences. To further characterize the 5,986 differentially expressed genes during hypoxia, k-means clustering was investigated to identify whether some of these genes could have a more similar gene expression pattern than others. The freely available software, Genesis (31), was used for k-means clustering and initial median centering of the genes (data not shown). The default setting of 10 clusters was chosen.
Two large clusters of genes were identified. One group of 1,876 genes had the smallest next nearest neighbor variance. This group of genes was chosen for further analysis. These genes were expressed at lower levels during hypoxia. Subsequently, the genes from this group were analyzed using the online tool Database for Annotation, Visualization and Integrated Discovery (DAVID) (32,33). DAVID is a high-throughput and integrated data-mining environment used to analyze gene lists derived from high-throughput genomic experiments. The aim is to systematically map the genes to the associated biological annotations (e.g., Gene Ontology terms) and to statistically highlight the most overrepresented (enriched) biological annotation. DAVID includes the molecular pathway databases Kyoto Encyclopedia of Genes and Genomes (KEGG) and Biocarta that were also used for the analysis (32,33). The strategy for enrichment analysis is to take the user's gene list, and then iteratively test the enrichment of each annotation term one-by-one. The enrichment P-value calculation, i.e., the number of genes within the list that hit a given biology class as compared to pure random chance, can be performed with the aid of well-known statistical methods, including Fisher's exact test and the hypergeometric distribution. The association between morbidities and the observed gene expression pattern was determined using the two-tailed Fisher's exact test.
Results
To identify gene expression profiles that may correlate with therapeutic intervention, the present study conducted research on 20 preterm infants ranging between 23.7–36.9 weeks of gestation at birth, following high-risk pregnancies. A total of 107 samples were eligible for further processing. All infants were monitored due to respiratory distress, with the exception of one healthy baby that was delivered by acute caesarean operation due to HELLP at GA 36.9 weeks.
A distinct gene expression pattern
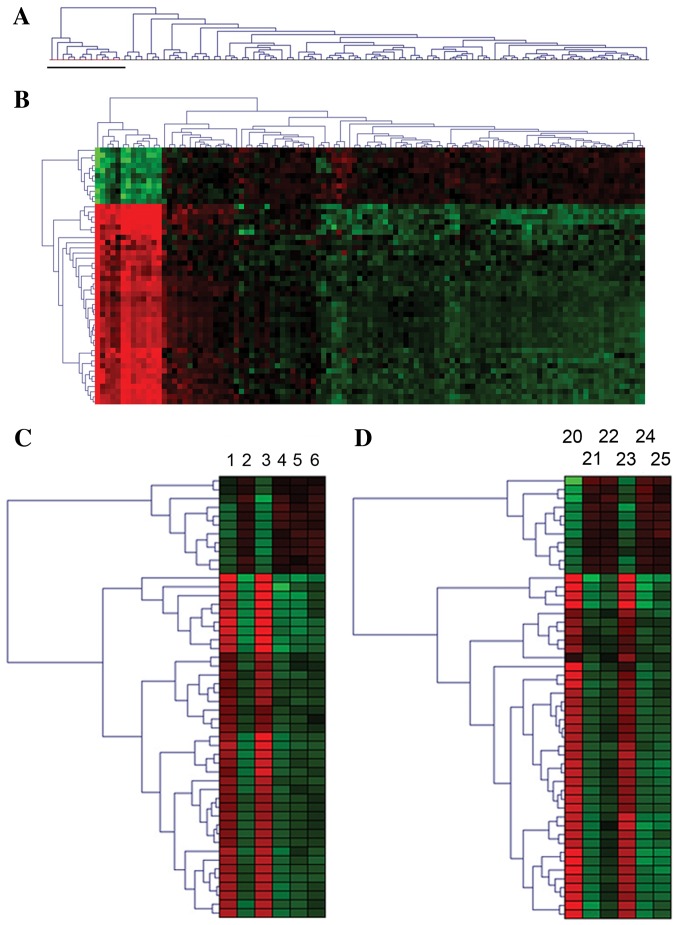
Gene expression levels were analyzed from total RNA using NuGEN Ovation amplification and Affymetrix Human-Genome-U133 Plus 2.0-arrays. Unsupervised clustering of the data identified a homogenous expression pattern in 13 samples that differed distinctly from the other samples (Fig. 1A). These 13 samples were derived from seven infants that underwent continuous oxygen therapy, and coincided with severe drops in SpO2 below 60%; also known as transient hypoxia. The other 13 infants in the cohort did not receive continuous oxygen therapy and exhibited no hypoxic events (Table II).
Figure 1.
A distinct and reversible gene expression pattern was identified in preterm infants undergoing continuous oxygen therapy during transient hypoxia. (A) Dendrogram of unsupervised clustering of all expression data identified a homogenous expression pattern in 13 blood samples, as indicated by a black bar, from seven preterm infants on long-term continuous oxygen therapy exhibiting hypoxia. (B) Hierarchical cluster analyses of the 50 most differentially expressed genes (P<0.05) at the time of hypoxia when continuous oxygen therapy was administered compared to when the infants did not receive continuous oxygen and had no events of hypoxia. The red area represents highly upregulated genes and the green area represents downregulated genes. The darker shades represent genes with smaller changes in gene expression. (C) Example 1 illustrates the reversible nature of the transcription profile: Transient gene expression profile of differentially expressed genes in a preterm infant born at 25.2 weeks gestation during transient hypoxia and reoxygenation with 100% FiO2 on the 17th and 23rd day after birth, as represented by the sample ID nos. 1 and 3. At the other time points, the infant was stable without hypoxia and continuous oxygen therapy. (D) Example 2 illustrates the reversible nature of the transcription profile: Transient gene expression profile of differentially expressed genes in a preterm infant born at 28.1 weeks gestation during transient hypoxia and reoxygenation with 100% FiO2 on the 5 and 25th day after birth, as represented by the sample ID nos. 20 and 23. At the other time points, the infant was stable without hypoxia and continuous oxygen therapy.
Further characteristics of the seven infants at each sampling time are listed in Table III. Detailed analysis of the clinical and therapeutic features revealed that these seven infants received continuous oxygen all h of the day for a minimum of 21 days. Six of these seven infants received FiO2 >80% continuously and exhibited transient hypoxia followed by reoxygenation with 100% FiO2. Nursing interventions, in terms of multiple tactile stimuli of the extremities were performed to induce pain, and subsequent initiation of respiration together with 100% oxygen, in order to resume regular respiration (Table III). The infant that did not present with transient hypoxia received 30% FiO2 continuously, and was described as extremely fatigued.
Table III.
Characteristics of the seven preterm infants exhibiting the unique gene expression pattern at each sampling time (n=13), corresponding with hypoxia.
| Infanta (n=7) | Corresponding IU condition | GA (weeks) | BW (g) | Sample ID. no.b (n=13) | Current weight (g) | PMA (weeks) | Living days | NM | Transient hypoxia | Extra stimulid |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chorio-amnionitis, | 25.2 | 585 | 1 | 807 | 27.9 | 17 | PDA, PVL, IVH | + | + |
| p-PROM | 3 | 810 | 28.4 | 23 | PVL | + | + | |||
| 2 | Chorio-amnionitis, | 25.2 | 586 | 7 | 690 | 27.9 | 17 | PDA, NEC, | + | + |
| p-PROM | PVL, IVH | |||||||||
| 3 | Chorio-amnionitis, | 27.6 | 1,000 | 12 | 1,030 | 30.4 | 19 | PVL | + | + |
| p-PROM | 14 | 1,125 | 31.2 | 24 | PVL | + | + | |||
| 15 | 1,195 | 31.6 | 28 | PVL, ROP | + | + | ||||
| 4 | Preeclampsia, | 28.1 | 1,354 | 20 | 1,200 | 28.6 | 5 | IVH | + | + |
| HELLP | 23 | 1,617 | 31.5 | 25 | IVH | + | + | |||
| 5 | Preeclampsia, | 26.5 | 805 | 29 | 834 | 28.3 | 12 | PDA | + | + |
| HELLP | ||||||||||
| 6 | Preeclampsia, | 33.0 | 1,980 | 83 | 1,759 | 34.5 | 7 | − | c | + |
| HELLP | ||||||||||
| 7 | Chorio-amnionitis, | 29.6 | 1,305 | 98 | 2,227 | 38.2 | 59 | PDA, BPD, IVH gr 1 | + | + |
| p-PROM | 99 | 2,270 | 38.3 | 60 | PDA, BPD, IVH gr 1 | + | + | |||
| 100 | 2,404 | 39.1 | 65 | PDA, BPD, IVH gr 1 | + | + |
Each infant is presented together with its corresponding intrauterine inflammatory conditions, GA at birth, BW, current weight and age (PMA and days since birth) at each sampling time, in addition to the associated morbidities diagnosed prior to sample collection.
All infants presented with respiratory distress and prolonged respiratory difficulties during treatment with continuous oxygen; FiO2 >80–100%, with the exception of one infant (infant no. 6; sample ID no. 83 who received 30% FiO2.
Each infant is listed with their sample ID numbers.
Tachycardia >200 beats/min.
Following severe SpO2-drops, powerful and multiple tactile stimuli of the extremities were performed by the nurses to induce pain and initiation of respiration together with 100% oxygen, in order to resume regular respiration. BPD, bronchopulmonary dysplasia; BW, birth weight; GA, gestational age; HELLP, hemolysis, elevated liver enzyme levels and low platelet count; IVH gr 1, intraventricular hemorrhage, grade 1; IU, intrauterine; NEC, necrotizing enterocolitis; NM, neonatal morbidity; p-PROM, preterm premature rupture of membranes; PDA, patent ductus arteriosus; PVL, periventricular leukomalacia; PMA, postmenstrual age (GA + chronological age, weeks); ROP, retinopathy of prematurity; SpO2, peripheral capillary saturation level. Drops in SpO2 below 60% led to reoxygenation with 100% FiO2 in addition to powerful and multiple tactile stimuli of the extremities to resume regular respiration.
The remaining samples (94/107) represented all infants of the cohort, including the group of seven infants (32/94 samples), since none of the infants received continuous oxygen therapy at these sampling times and no hypoxic events were detected. A total of 62/94 samples were from the 13 infants that did not exhibit the expression pattern. These infants exhibited smaller fluctuations in SpO2 and drops in SpO2 between 80 and 85%; however, the drops were more frequent compared to when the infants presented with transient hypoxia. Furthermore, these infants were able to auto-adjust to SpO2-levels between 88 and 92%. In part, this may be attributed to normal physiological adaptation processes.
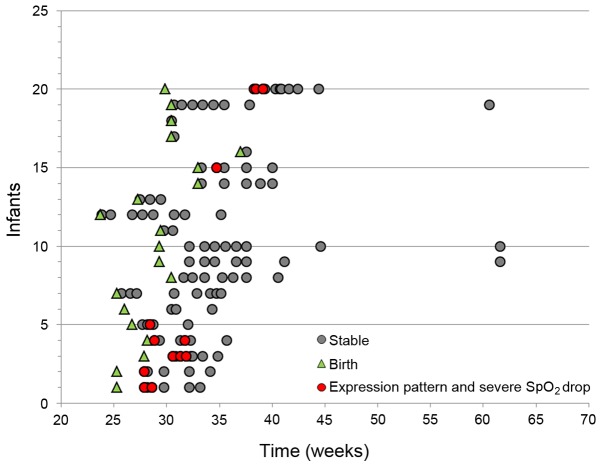
To identify differentially expressed genes during hypoxia, the corresponding 13 samples were compared with the 94 samples. The result provided a distinct expression pattern that contained 5,986 significantly differentially expressed genes following adjustment for multiple testing. A total of 5,167 genes were expressed at lower levels and 819 genes at higher levels (data not shown). The 50 most significantly differentially expressed genes are presented in Fig. 1B. The expression pattern appeared transient as it was reversed when continuous oxygen therapy was terminated and the infants had no events of hypoxia, as illustrated by two patients in Fig. 1C and D. The sampling times for all samples, (n=107) according to postmenstrual age (PMA), are presented together with the time of hypoxia (PMA, 26–39 weeks; Fig. 2).
Figure 2.
Sampling times for all samples (n=107) according to age of the infants. Each infant is indicated on the y-axis (n=20). All samples (n=107; the red and grey dots) are indicated on the x-axis according to the postmenstrual age of the infants. For each infant, the GA at birth is marked by green triangles. Samples representing time points when the infants received continuous oxygen therapy and transient hypoxia are indicated by red dots (n=13). Sampling times when the infants were not continuously oxygenated and had no events of transient hypoxia (stable) are indicated by grey dots (n=94). GA, gestational age; postmenstrual age of infants=GA+chronological age (weeks).
The characteristics of the two groups of infants at birth (GA, birth weight, gender, antibiotics and prenatal steroids) and their neonatal morbidities are outlined in Table IV. Six of the seven infants exhibiting the transcription pattern were administered systemic antibiotic treatment due to proven infection and sepsis (Table IV). Four of these infants were treated with systemic antibiotics prior to the events of transient hypoxia. The infant that was not treated with systemic antibiotics was the infant previously described as fatigued. Conversely, eight of the 13 infants that did not exhibit the expression pattern received treatment for sepsis with systemic antibiotics.
Table IV.
Characteristics of two groups of infants (hypoxia, n=7; without hypoxia, n=13) at birth and their neonatal morbidities.
| Characteristics | ||||||
|---|---|---|---|---|---|---|
| Preterm infants | GA (weeks) | BW (g) | Gender | Antibiotics administered | Neonatal morbidities | Prenatal steroids administered |
| Infants with hypoxia (n=7) | ||||||
| 1 | 25.2 | 585 | Male | + | BPD, PDA, PVL, IVH | + |
| 2 | 25.2 | 586 | Female | + | BPD, PDA, NEC, PVL, IVH | + |
| 3 | 27.6 | 1,000 | Female | + | PVL, ROP | + |
| 4 | 28.1 | 1,354 | Female | + | BPD, IVH | + |
| 5 | 26.5 | 805 | Female | + | PDA | + |
| 6 | 33.0 | 1,980 | Female | − | − | − |
| 7 | 29.6 | 1,305 | Male | + | PDA, BPD, IVH | + |
| Infants with no hypoxia (n=13) | ||||||
| 1 | 26.0 | 1,225 | Male | + | IVH | + |
| 2 | 25.2 | 913 | Female | + | − | − |
| 3 | 30.3 | 1,365 | Female | + | − | − |
| 4 | 29.2 | 1,204 | Female | + | − | + |
| 5 | 29.2 | 588 | Male | + | − | + |
| 6 | 29.3 | 1,036 | Male | + | − | − |
| 7 | 23.5 | 560 | Female | + | IVH, NEC, ROP | + |
| 8 | 27.2 | 754 | Male | + | − | + |
| 9 | 33.0 | 2,110 | Female | − | − | − |
| 10 | 36.6 | 3,000 | Male | − | − | − |
| 11 | 30.3 | 1,195 | Female | − | − | + |
| 12 | 30.3 | 1,340 | Female | − | − | + |
| 13 | 30.3 | 1,374 | Female | − | − | + |
BPD, bronchopulmonary dysplasia according to the clinicians' final diagnosis; BW, birth weight; GA, gestational age; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity.
Biological functions of the differentially expressed genes
The present study aimed to identify altered biological processes in cells during transient hypoxia and to identify the potential environmental stimuli that brought about the changes in differentially expressed genes. When a large proportion of genes are differentially expressed, it is intrinsically difficult to identify processes or pathways by overrepresentation. Therefore, it was hypothesized that some of the significantly differentially expressed genes may represent primary pathways that altered the expression of genes, whereas other genes may be part of secondary transcriptional programs involved in regulatory networks or cascades, modulated by genetic and environmental factors. Therefore, the present study explored whether there were groups of genes bearing a more similar gene expression pattern than others, by performing a k-means clustering test with 10 gene groups (data not shown). The result identified one group, which contained 1,876 genes that were expressed at lower levels during transient hypoxia. In addition, the genes within this group were more tightly co-regulated compared with genes in the other groups (data not shown).
To identify any potential enrichment of molecular pathway annotations, the 1,876 genes were analyzed using the online tool, DAVID, as well as the molecular pathway databases Kyoto Encyclopedia of Genes and Genomes (KEGG) and Biocarta (32,33). Whilst analyzing these genes with KEGG, a number of signaling pathway annotations that were significantly enriched were identified. These pathways have been identified in various cellular systems (Table V). Many of these enriched pathways involved MAPK signaling as a common theme, including the Toll-like receptor (TLR) signaling pathway (Table V). Therefore, the present study examined the proportion of genes in each pathway that were in common with the generic MAPK pathway (Table V). The proportions were between 16 and 50% (data not shown), which represents large overlaps that may have led to the identification of some broader pathways (Table V). Several of these have no clinical relevance to this cohort, exemplified by the colorectal cancer or oocyte maturation pathway. It is well understood that MAPK signaling is involved in various biological systems.
Table V.
Enrichment of signaling pathways annotations was identified using the Kyoto Encyclopedia of Genes and Genomes pathway database.
| Name of pathway | Enrichment score | P-value |
|---|---|---|
| Toll-like receptor signaling | 3.0 | 0.000064 |
| Pancreatic cancer | 3.3 | 0.0002 |
| Pathways in cancer | 1.8 | 0.00044 |
| NOD-like receptor signaling | 3.3 | 0.00099 |
| Colorectal cancer | 2.8 | 0.001 |
| Insulin signaling | 2.3 | 0.0011 |
| ErbB signaling | 2.7 | 0.0012 |
| Acute myeloid leukemia | 3.1 | 0.0023 |
| Progesterone-mediated oocyte | 2.5 | 0.0078 |
| maturation | ||
| T cell receptor signaling | 2.2 | 0.018 |
| Neurotrophin signaling | 2.1 | 0.017 |
| Endocytosis | 1.8 | 0.020 |
| Prostate cancer | 2.3 | 0.023 |
| B cell receptor signaling | 2.4 | 0.024 |
| Chronic myeloid leukemia | 2.4 | 0.024 |
| MAPK signaling | 1.6 | 0.025 |
NOD, nucleotide-binding oligomerization domain; MAPK, mitogen-activated protein kinase.
Since the pathways with the lowest P-values (Table V) were heavily dependent on MAPK genes, the results were interpreted conservatively as an overrepresentation of genes associated with MAPK signaling. Therefore, among the significantly differentially expressed genes during transient hypoxia, it may be suggested that MAPK signaling is the most relevant pathway for further investigation. Specific information regarding the pathways and biological processes involved during transient hypoxia was determined using the Biocarta pathway database (Table VI) where further details on the significantly differentially expressed genes were identified. One of the top candidate pathways in the Biocarta analysis involved oxidative stress-induced expression via the Nrf2-ARE pathway (Table VI), although the P-values were high (Table VI).
Table VI.
Enrichment of signaling pathway annotations identified by Biocarta.
| Name of pathway | Enrichment score | P-value |
|---|---|---|
| EGF signaling | 3.0 | 0.61 |
| Inhibition of cellular proliferation | 3.2 | 0.60 |
| by Gleevec | ||
| Oxidative stress induced expression | 3.2 | 0.60 |
| via Nrf2 | ||
| PDGF signaling | 2.8 | 0.59 |
| Keratinocyte differentiation | 2.2 | 0.70 |
| IGF-1 signaling | 2.9 | 0.76 |
Using the group of 1,876 genes obtained by k-means clustering analysis, overrepresentation of pathways was identified using the Biocarta database through the online tool, database for annotation, visualization and integrated discovery. EGF, epidermal growth factor; Nrf2, nuclear factor erythroid 2-related factor 2; PDGF, platelet-derived growth factor; IGF-1, insulin-like growth factor-1.
The Nrf2-ARE target genes are a group of genes transcriptionally activated by MAPKs in response to oxidative stress (34). Originally, genes involved in the Nrf2-ARE pathway were identified in nerve cells (35). The transcription factor Nrf2 is involved in the transcription program to protect against oxidative stress using the ARE response element. Therefore, the additional finding of the Nrf2-ARE pathway, and the evidence that the Nrf2-ARE target genes are transcriptionally activated by MAPKs in response to oxidative stress, was the rationale to further investigate the Nrf2-ARE pathway.
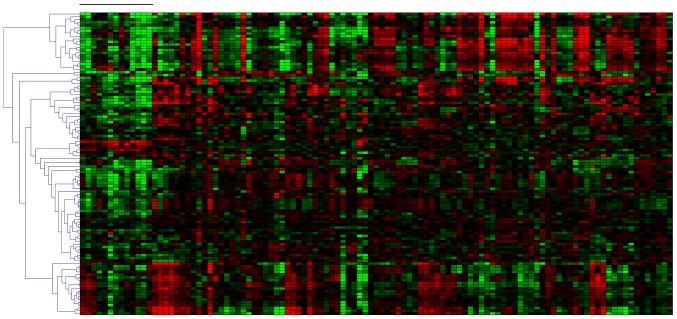
To determine the mRNA expression of Nrf2-ARE target genes, the expression levels of the Nrf2-ARE genes, as defined by Johnson et al in 2008 (35), were investigated. A total of 109 probe sets on the DNA microarray corresponded with Nrf2-ARE genes. Out of these probe sets, 69 were included in the set of significantly differentially expressed genes previously identified (P<0.05), which represented the majority of classical Nrf2-ARE target genes that were expressed at lower levels during transient hypoxia (Fig. 3). This result provided evidence indicating that the transcription of these antioxidant genes was reduced during transient hypoxia.
Figure 3.
Expression of Nrf2-ARE target genes analyzed using 109 probe sets within a DNA microarray. Gene expression levels from 109 probe sets corresponding with the nuclear factor erythroid 2-related factor 2-antioxidant response element target genes are presented for all 107 samples. The 13 leftmost samples, as indicated by the black bar, represent transient hypoxia. Note a predominantly lower expression at these sampling times. A total of 69 probe sets are differentially expressed between the two groups of infants with a P<0.05. The red area represents highly upregulated genes and the green area represents downregulated genes.
Since the differentially expressed genes in the distinct expression pattern were identified during hypoxia, the hypoxia-inducible factor (HIF)-1α target genes were also examined, since they are known to be induced by hypoxic conditions (36). The HIF-1α target genes were not overexpressed (Table VII), suggesting that the HIF-1α pathway was not active in infants during transient hypoxia.
Table VII.
HIF-1α target expression within the gene profile.
| Probe set ID | Gene title | Gene symbol | Log FC | Adj. P-value |
|---|---|---|---|---|
| 210512_s_at | vascular endotdelial growtd factor A | VEGFA | −1.10 | 2.11E-04 |
| 210513_s_at | vascular endotdelial growtd factor A | VEGFA | 0.35 | 5.65E-03 |
| 212171_x_at | vascular endotdelial growtd factor A | VEGFA | −0.41 | 4.99E-02 |
| 232809_s_at | Fms-related tyrosine kinase 1 (vascular endotdelial growtd factor receptor) | FLT1 | −0.12 | 5.04E-01 |
| 202718_at | insulin-like growtd factor binding protein 2, 36 kDa | IGFBP2 | −1.02 | 2.80E-07 |
| 202934_at | hexokinase 2 | HK2 | 0.10 | 5.16E-01 |
| 200697_at | hexokinase 1 | HK1 | −0.63 | 4.94E-05 |
| 238996_x_at | aldolase A, fructose-bisphosphate | ALDOA | −0.31 | 2.65E-02 |
| 202022_at | aldolase C, fructose-bisphosphate | ALDOC | −0.74 | 1.39E-08 |
| 200966_x_at | aldolase A, fructose-bisphosphate | ALDOA | −0.22 | 6.12E-02 |
| 224151_s_at | adenylate kinase 3 | AK3 | −1.25 | 4.36E-11 |
| 224655_at | adenylate kinase 3 | AK3 | −0.44 | 2.69E-04 |
| 202912_at | adrenomedullin | ADM | −0.47 | 6.80E-02 |
| 228143_at | ceruloplasmin (ferroxidase), similar to ceruloplasmin precursor (ferroxidase) | CP///LOC100132553 | 0.82 | 2.92E-10 |
| 218995_s_at | endotdelin 1 | EDN1 | 1.50 | 6.93E-20 |
| 207257_at | erytdropoietin | EPO | 0.16 | 8.20E-02 |
| M33197_5_at | glyceraldehyde-3-phosphate dehydrogenase | GAPDH | 0.00 | 9.97E-01 |
| 203665_at | heme oxygenase (decycling) 1 | HMOX1 | −1.03 | 3.88E-06 |
| 200650_s_at | lactate dehydrogenase A | LDHA | 0.13 | 1.76E-01 |
| 201102_s_at | phosphofructokinase, liver | PFKL | −0.84 | 1.32E-10 |
| 227068_at | phosphoglycerate kinase 1 | PGK1 | −0.36 | 1.50E-02 |
| 202627_s_at | serpin peptidase inhibitor, clade E (plasminogen activator inhibitor type 1), member 1 | SERPINE1 | −0.19 | 4.45E-01 |
| 202628_s_at | serpin peptidase inhibitor, clade E (plasminogen activator inhibitor type 1), member 1 | SERPINE1 | −0.16 | 4.92E-01 |
| 222125_s_at | hypoxia-inducible factor prolyl 4-hydroxylase | PH-4 | −1.36 | 7.56E-18 |
| 201251_at | pyruvate kinase, muscle | PKM2 | −0.20 | 7.00E-02 |
| 240686_x_at | transferrin receptor (p90, CD71) | TFRC | −0.28 | 1.62E-02 |
| 214063_s_at | transferrin | TF | −0.83 | 5.62E-02 |
| 209747_at | transforming growtd factor, β 3 | TGFB3 | −0.53 | 1.21E-04 |
Expression of HIF-1α target genes in samples from preterm infants undergoing long-term continuous oxygen tderapy at tde time of hypoxia vs. samples from stable preterm infants witdout hypoxia and continuous oxygen tderapy. HIF-1α, hypoxia-inducible factor-1α; FC, fold-change.
Since excessive oxidative stress is associated with oxygen therapy and oxidative radical disease in neonatology, a potential link between continuous oxygenation, hypoxic events and the incidence of morbidities, including PVL/IVH, BPD, PDA, NEC and ROP, was examined. The cohort consisted of 35 patient samples representing infants diagnosed with PVL or IVH. A total of 11 of these samples exhibited the distinct expression pattern. In addition, 72 samples were examined from infants who were never diagnosed with PVL or IVH. Two of these samples had the expression pattern. The Fisher's exact test provided a P-value of 0.000062. The same test was performed for the other morbidities, as described in Table IV. Significant P-values were identified for PDA and BPD, P=0.014 and 0.003 respectively. For ROP and NEC, the P-values were not significant, P=0.41 and 1.00, respectively (data not shown).
Discussion
Preterm infants exposed to maternal inflammatory/infective processes often require therapeutic intervention. By exploring global gene expression in blood from preterm infants during treatment, novel avenues in therapeutic management at the point of care may be identified. In the present study, blood cells were analyzed by NuGEN amplification and DNA microarrays. A unique transcription pattern during transient hypoxia in infants undergoing continuous long-term oxygen therapy was identified. The majority of differentially expressed genes were downregulated. During hypoxia, the infants exhibited reduced oxidative stress-dependent signaling and the antioxidant Nrf2-ARE target genes were not activated. This transcription pattern was reversed when infants became stable without hypoxia and continuous oxygen therapy.
Biological processes associated with the transcription profile
The distinct gene expression pattern was exclusively observed during transient hypoxia in infants that were receiving continuous oxygen therapy. The uniformity of the homogeneous expression pattern in blood may suggest similar regulatory mechanisms causing the differentially expressed genes. The majority of classical Nrf2-ARE target genes exhibited a reduced MAPK-dependent Nrf2-ARE antioxidant transcriptional response during transient hypoxia. Overexpression of MAPKs may be associated with responses to high FiO2 levels or activity at the transcription level (37–39). Hyperoxia in the newborn period is associated with excessive ROS production and oxidative stress (7), resulting in effects on gene expression and cellular responses (40). Excessive ROS are suggested to be associated with the chemical processes that modulate signaling pathways and activate members of the MAPK family (37,41). Continuous oxygen therapy or reoxygenation during transient hypoxia may have induced an antioxidant Nrf2-ARE response. Nevertheless, there is no direct information clarifying whether the varying responses observed have any favorable or unfavorable consequences for the infants due to the transient nature of the response.
TLR activation is known to be involved in systemic inflammation and neuroinflammation. However, TLR signaling (Table V) possesses multifunctional roles in various biological systems, including hematopoiesis, infective and non-infective pathogenesis, cardiovascular and cerebrovascular diseases, and autoimmune, vascular function and dermatologic diseases, and cancer, and is therefore hard to interpret (42,43). The findings of the signaling pathways, should be interpreted with caution, as various pathways were identified that may not be relevant to this cohort, due to large overlaps among them. However, all the gene groups demonstrated an overrepresentation of differentially expressed MAPK genes (data not shown).
Notably, the MAPK signaling pathway is suggested to be involved in various mechanisms in cell physiology, including apoptosis, cellular stress, hyperoxic cell death, ischemia/reperfusion and ROS-induced cell death (37,38,44,45), all of which are involved in oxygen-related injury (17). The Fisher's exact test provided an association between the occurrence of the following diagnoses: PVL/IVH, PDA and BPD, and the infants with the unique expression pattern during hypoxia (Tables II and III). The morbidities could possibly have led to alterations in the mRNA expression of inflammatory and immunological markers. However, the occurrence of these morbidities did not coincide at the direct time of the transcription profile. The diagnoses were made between 2 days and 1 month prior to the obtained samples as illustrated by the occurrence of PVL/IVH and PDA. At present, the biomarkers for PVL and IVH are protein markers released from the site of injury (46). The results of the present study suggested that there could be an association between the presence of PVL/IVH, PDA, and BPD, and this transcription profile; however, the P-values should be interpreted with caution due to co-variation and dependencies between samples. Ultimately, the identification of the MAPK-dependent pathways did not clearly point towards inflammation as expected from PVL/IVH.
Gender has been identified to be relevant with regards to antioxidant capacity; female infants have been demonstrated to have increased antioxidant activity and reduced oxidative stress compared to males (47). In the current study, the number of female infants was somewhat higher among the infants exhibiting the expression pattern (5/7 infants) compared with the other group of infants (8/13). Accordingly, the number of samples also differed. The proportion of those with the expression pattern was 5/13 samples from males and 8/13 from females (Fisher's exact test P-value=1.00). The proportion of samples without the expression pattern was 28/94 from males and 66/94 from females (Fisher's exact test P=0.53). Therefore, there is no evidence with regards to any association between gender and the exhibited gene expression pattern in the present study.
A possible explanation for the differential gene expression pattern could be altered composition of blood cells in the samples. A previous study identified specific gene expression patterns for various types of blood cells (48). The expression of cell-type specific genes for B cells, T cells and granulocytes, which have previously been described by Palmer et al (48), was examined from both groups of infants. There was a small deviation towards a lower log fold-change during transient hypoxia vs. no hypoxia for T cell-, B cell- and granulocyte-specific genes (median, −0.8). However, the observed differences in cell-type specific genes were relatively small and followed the general downregulatory expression pattern. Therefore, differences in cell type composition cannot explain the exhibited pattern.
All infants in this cohort were at risk for inflammatory/infective processes due to intrauterine conditions. However, only seven of the 20 infants exhibited the distinct transcription pattern, of which one infant exhibited the profile as late as 59–65 days after birth. Furthermore, four of these infants finished treatment for sepsis with systemic antibiotics prior to detection of the exhibited profile. These findings, including the indication of the reversible nature of the profile, may suggest that intrauterine inflammation and sepsis are not directly implicated in the transcriptional response of the distinct expression pattern.
A distinct gene expression pattern suggests similar causes
Notably, the aforementioned unique gene expression pattern was homogeneous (Fig. 1A). However, little is understood regarding the biological functions of a large number of the genes identified as significantly differentially expressed. The similarities between the morbidities of the infants (Table IV), known to be accompanied by oxidative stress and excessive ROS (7,16,17,49,50), may suggest a common effect culminating in the same gene expression response. Furthermore, excessive ROS is associated with oxygen dependence (51), which in part could explain the infants' requirement for continuous long-term oxygen therapy.
The 20 infants selected for this cohort were all at risk for therapeutic intervention due to maternal conditions. Intrauterine inflammation may cause fetal inflammatory responses with increased risk for respiratory failure regardless of gestation (52). The maternal conditions (p-PROM, chorioamnionitis and aggravation of preeclampsia, HELLP) are associated with increased products from oxidative stress, and are known to influence fetal growth and signaling pathways (52). One of these conditions, HELLP, has been recognized as a ‘putative marker for fetal hypoxia’ (53). However, the common feature for all seven infants exhibiting the expression pattern was continuous oxygen therapy (Table III). The other common feature of these infants was the events of hypoxia, likewise the gene expression pattern was reversed when the infants no longer received continuous oxygen therapy (Fig. 1C and D). The mechanism underlying the shift in gene expression is unknown; however, it may be explained by MAPK-regulated transcription (37,45).
There was an exception in this cohort relating to one infant that was operated on for NEC. The maternal condition was clinical chorioamnionitis and treatment with prenatal steroids. Unlike the other infants that received continuous oxygen, the administration of oxygen to this patient differed. The FiO2 level varied largely and oxygen was given in an on-and-off manner. In contrast to continuous oxygen administration, oxygen was discontinued and re-introduced after some h. This infant did not have transient hypoxia. This observation, although related to one infant only, may suggest that factors other than high FiO2 levels may induce similar effects on gene activity.
Gene expression may be affected by numerous factors. To limit genetic contributions to the variation of gene expression, infants with chromosomal and congenital abnormalities were excluded, as were those with metabolic, neurologic or known genetic diseases. Additional covariates that may potentially influence the expression of genes were also assessed, including different origins of the onset of labor, body weight, GA and days after birth. Despite differences in prematurity, BW and GA, intrauterine inflammation was common for all infants of the cohort. The BW and GA ranged between 560–3,000 g and 23.7–36.9 weeks, respectively (Table II), whereas in infants exhibiting the transcription pattern body weight was between 585–1,980 g and current body weight ranged between 690–2,404 g (Table III). The PMA by these time points ranged between 27.9–39.1 weeks (Table III).
Additional variables that potentially may influence the distinct transcription pattern were investigated with regards to blood transfusion, steroid treatment, and antibiotics, that were given on the day before sample collection. None of the infants received a blood transfusion 24 h prior to collection of a sample exhibiting the distinct transcription pattern. Furthermore, four samples did not present the transcription pattern even though the infants received a blood transfusion on the day prior to sample collection (Fisher's exact test P=1.00). Similarly, for one sample exhibiting the pattern, the infant received steroid treatment 24 h prior to sample collection, whereas nine samples from three infants did not exhibit the pattern even though steroids were administered to the infants on the previous day (Fisher's exact test P=1.00). In addition, at 10 of the 13 sampling times in which the pattern was exhibited, sepsis was not present and systemic antibiotics were not administered on the day prior to collection of the samples, whereas for 3/13 samples the infants had sepsis and antibiotics were administered on the same day. Sepsis and antibiotics coincided with 16 samples not exhibiting the expression pattern (Fisher's exact test P=0.70). Therefore, the presence of the differential gene expression did not appear to correlate with blood transfusions, steroid treatment, nor sepsis or the administration of antibiotics.
Although the majority of samples (10/13) exhibiting the distinct transcription pattern were obtained from the neonatal period between 5 and 28 days after birth, there was a wide age-related variability of the infants, ranging between 5 and 65 days after birth (Table III) and between 25.2 and 33.0 weeks gestation (Table III). The majority of these samples (8/13) were obtained between 12–28 days after birth, whereas the remaining samples (5/13) were from days 5 and 7, and days 59, 60 and 65 after birth (Table III). Therefore, alterations in the transcription pattern in blood were not limited to a particular time window of postnatal development. The preterm infant that exhibited the profile later after birth (59–65 days after birth) received a total of five courses of systemic antibiotics, due to culture-positive sepsis. Potentially, previous episodes of sepsis and antibiotics may have weakened the antioxidant capacity that eventually could have contributed to the exhibited expression pattern at this postnatal age. Although the infants of this cohort were at risk for developing systemic inflammation, no specific inflammatory genes were identified. Therefore, the present study suggested that sepsis and intrauterine inflammation were not directly implicated in the significantly differentially gene expression exhibited within the distinct transcription pattern.
A molecular marker in blood during transient hypoxia
Biomarkers identified by the use of high-throughput technologies may indicate a biological state relevant for clinical practice or therapeutic intervention (54). The elicited response coincided at transient hypoxia during continuous oxygen therapy. These events of hypoxia appear to represent a form of transient disorder of respiratory control, which is associated with impaired oxidative stress-dependent signaling in the blood. There is an ongoing debate regarding safe oxygen saturation targeting to avoid hypoxia and hyperoxia, and how to identify the most appropriate FiO2 levels considering the age of the infant (10,55).
In the present study, premature infants born prior to 33 weeks gestation exhibited the transcription profile even as late as 65 days after birth, which potentially may reflect a therapeutic group of infants for particular monitoring during continuous oxygenation. The network of genes and signaling pathways within the transcription pattern were exhibited across various gestations at birth, different postnatal development and days after birth. In line with a previous study, the present findings indicated the potential relevance of applying gene expression profiles in blood to serve as biomarkers for molecular diagnostic analysis, and possibly facilitating an individualized approach to safe oxygen therapy in preterm infants during neonatal and postnatal life (54).
In conclusion, the present genome-wide expression study provided information regarding transcriptional processes, and may improve understanding of the molecular responses in blood throughout early life in preterm infants. To the best of our knowledge, these findings have previously not been described. The significantly differentially expressed genes exhibited a unique expression pattern solely in infants that received continuous long-term oxygen therapy during hypoxia compared with infants that did not receive this type of therapeutic intervention. Most differentially expressed genes were expressed at lower levels. The profile detected low expression levels of MAPK genes and target genes of the Nrf2-ARE pathway, thus indicating reduced oxidative stress-dependent signaling in the blood. The antioxidant genes were not activated during transient hypoxia. The high FiO2 level or reoxygenation with 100% FiO2 may have induced an antioxidant Nrf2-ARE response. The distinct profile was transient in nature, since it was reversed when the infants were no longer continuously oxygenated. However, the study design does not allow us to conclusively determine the cause of the differentially expressed genes within the MAPK signaling pathways or the cause of the dynamic process behind the shift in gene expression. It may be hypothesized that the identified transcription profile will improve understanding regarding the basic mechanisms involved in gene regulatory networks during oxygen therapy and transient hypoxia in preterm infants. Future investigations should aim to further clarify the mechanisms underlying the altered gene expression, as well as the individual susceptibility to the influences of small molecules administered, such as oxygen.
Acknowledgments
The present study was supported by grants from Anne Margrethe Bakke's fund, and the Neonatal Fund (both intramural funds at Oslo University Hospital, Rikshospitalet, Division of Pediatrics); University of Oslo's Research Initiating program and Small Research Grant; The Anders Jahre Fund for the Advancement of Science; The Medinnova Research Fund; The Sigval and Nanki Bergesen Foundation; The Renee and Bredo Grimsgaard Foundation; Leif Richard Erichsen and Maren Hertzberg Erichsen's Fund for Norwegian Medicine; Gertrude and Jack Nelson's Fund; and Joh. H. Andresen's Medical Fund.
The authors wish to gratefully acknowledge the contribution of the parents and their children in this study. The authors would like to thank Prof. Thor Willy Ruud Hansen (University of Oslo), for his kind help to obtain funding for the study, and for reading an early version of the manuscript. The highly skillful technical assistance of Mrs. Maria Rydåker, Uppsala Array Platform was greatly acknowledged. The authors also thank the NICU, Oslo University Hospital, Rikshospitalet and the Department of Medical Biochemistry for support. Particularly, the authors thank the involved nurses for writing reports of high quality, which became helpful for retrospective analysis of the accurate medical information.
Data availability
The data are publically available at GEO (accession no. GSE 70461).
References
- 1.Vento M, Escobar J, Cernada M, Escrig R, Aguar M. The use and misuse of oxygen during the neonatal period. Clin Perinatol. 2012;39:165–176. doi: 10.1016/j.clp.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Baba LM, McGrath JM. Oxygen free radicals: Effects in the newborn period. Adv Neonatal Care. 2008;8:256–264. doi: 10.1097/01.ANC.0000338015.25911.8a. [DOI] [PubMed] [Google Scholar]
- 3.Vento M, Saugstad OD. Oxygen as a therapeutic agent in neonatology: A comprehensive approach. Semin Fetal Neonatal Med. 2010;15:185. doi: 10.1016/j.siny.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Vento M. Oxygen supplementation in the neonatal period: Changing the paradigm. Neonatology. 2014;105:323–331. doi: 10.1159/000360646. [DOI] [PubMed] [Google Scholar]
- 5.Cernada M, Cubells E, Torres-Cuevas I, Kuligowski J, Escobar J, Aguar M, Escrig R, Vento M. Oxygen in the delivery room. Early Hum Dev. 2013;89(Suppl 1):S11–S13. doi: 10.1016/S0378-3782(13)70004-5. [DOI] [PubMed] [Google Scholar]
- 6.Finer N, Saugstad O, Vento M, Barrington K, Davis P, Duara S, Leone T, Lui K, Martin R, Morley C, et al. Use of oxygen for resuscitation of the extremely low birth weight infant. Pediatrics. 2010;125:389–391. doi: 10.1542/peds.2009-1247. [DOI] [PubMed] [Google Scholar]
- 7.Gitto E, Pellegrino S, D'Arrigo S, Barberi I, Reiter RJ. Oxidative stress in resuscitation and in ventilation of newborns. Eur Respir J. 2009;34:1461–1469. doi: 10.1183/09031936.00032809. [DOI] [PubMed] [Google Scholar]
- 8.Escrig R, Arruza L, Izquierdo I, Villar G, Sáenz P, Gimeno A, Moro M, Vento M. Achievement of targeted saturation values in extremely low gestational age neonates resuscitated with low or high oxygen concentrations: A prospective, randomized trial. Pediatrics. 2008;121:875–881. doi: 10.1542/peds.2007-1984. [DOI] [PubMed] [Google Scholar]
- 9.Saugstad OD. Oxygen for newborns: How much is too much? J Perinatol. 2005;25(Suppl 2):S45–S50. doi: 10.1038/sj.jp.7211321. [DOI] [PubMed] [Google Scholar]
- 10.Torres-Cuevas I, Cernada M, Nuñez A, Escobar J, Kuligowski J, Chafer-Pericas J, Vento M. Oxygen supplementation to stabilize preterm infants in the fetal to neonatal transition: No satisfactory answer. Front Pediatr. 2016;4:29. doi: 10.3389/fped.2016.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vento M. Tailoring oxygen needs of extremely low birth weight infants in the delivery room. Neonatology. 2011;99:342–348. doi: 10.1159/000326626. [DOI] [PubMed] [Google Scholar]
- 12.Vento M, Cubells E, Escobar JJ, Escrig R, Aguar M, Brugada M, Cernada M, Saénz P, Izquierdo I. Oxygen saturation after birth in preterm infants treated with continuous positive airway pressure and air: Assessment of gender differences and comparison with a published nomogram. Arch Dis Child Fetal Neonatal Ed. 2013;98:F228–F232. doi: 10.1136/archdischild-2012-302369. [DOI] [PubMed] [Google Scholar]
- 13.Aversa S, Marseglia L, Manti S, D'Angelo G, Cuppari C, David A, Chirico G, Gitto E. Ventilation strategies for preventing oxidative stress-induced injury in preterm infants with respiratory disease: An update. Paediatr Respir Rev. 2016;17:71–79. doi: 10.1016/j.prrv.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Maltepe E, Saugstad OD. Oxygen in health and disease: Regulation of oxygen homeostasis-clinical implications. Pediatr Res. 2009;65:261–268. doi: 10.1203/PDR.0b013e31818fc83f. [DOI] [PubMed] [Google Scholar]
- 15.Saugstad OD. Oxygen and retinopathy of prematurity. J Perinatol. 2006;26(Suppl 1):S46–S50. S60–S64. doi: 10.1038/sj.jp.7211475. [DOI] [PubMed] [Google Scholar]
- 16.Saugstad OD. Oxidative stress in the newborn-a 30-year perspective. Biol Neonate. 2005;88:228–236. doi: 10.1159/000087586. [DOI] [PubMed] [Google Scholar]
- 17.Saugstad OD. Update on oxygen radical disease in neonatology. Curr Opin Obstet Gynecol. 2001;13:147–153. doi: 10.1097/00001703-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Whitney AR, Diehn M, Popper SJ, Alizadeh AA, Boldrick JC, Relman DA, Brown PO. Individuality and variation in gene expression patterns in human blood. Proc Natl Acad Sci USA. 2003;100:1896–3901. doi: 10.1073/pnas.252784499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mardinoglu A, Nielsen J. Systems medicine and metabolic modelling. J Intern Med. 2012;271:142–154. doi: 10.1111/j.1365-2796.2011.02493.x. [DOI] [PubMed] [Google Scholar]
- 20.Nayak RR, Kearns M, Spielman RS, Cheung VG. Coexpression network based on natural variation in human gene expression reveals gene interactions and functions. Genome Res. 2009;19:1953–1962. doi: 10.1101/gr.097600.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cernada M, Serna E, Bauerl C, Collado MC, Pérez-Martínez G, Vento M. Genome-wide expression profiles in very low birth weight infants with neonatal sepsis. Pediatrics. 2014;133:e1203–e1211. doi: 10.1542/peds.2013-2552. [DOI] [PubMed] [Google Scholar]
- 22.Pietrzyk JJ, Kwinta P, Bik-Multanowski M, Madetko-Talowska A, Jagła M, Tomasik T, Mitkowska Z, Wollen EJ, Nygård S, Saugstad OD. New insight into the pathogenesis of retinopathy of prematurity: Assessment of whole-genome expression. Pediatr Res. 2013;73:476–483. doi: 10.1038/pr.2012.195. [DOI] [PubMed] [Google Scholar]
- 23.Protocol: RiboPure-Blood Kit (Catalog no. 1928) Instruction Manual. 2010 [Google Scholar]
- 24.Protocol: GLOBINclear-Human (Catalog no. 1980) Instruction Manual. 2010 [Google Scholar]
- 25.Protocol: NuGEN®Ovation RNA-Amplification system, corp-author. Ovation® RNA Amplification System V2 (Catalog no. 3100), Ovation® WB Reagent (Catalog no. 1300), FL-Ovation cDNA Biotin Module V2 (Catalog no. 4200) 2010 [Google Scholar]
- 26.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 27.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: Expression index computation and outlier detection. Proc Natl Acad Sci USA. 2001;98:31–36. doi: 10.1073/pnas.98.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smyth GK. Linear Models and Empirical Bayes Methods for Assessing Differential Expression in Microarray Experiments. http://www.bepress.com/sagmb/vol3/iss1/art3. Statistical Applications in Genetics and Molecular Biology. 2004;3 doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 29.Smyth GK. Bioinformatics and Computational Biology Solutions using R and Bioconductor. Springer; New York: 2005. [DOI] [Google Scholar]
- 30.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the royal statistical society series B (Methodological) 1995;57:289–300. [Google Scholar]
- 31.Sturn A, Quackenbush J, Trajanoski Z. Genesis: Cluster analysis of microarray data. Bioinformatics. 2002;18:207–208. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- 32.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 33.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths towards the comprehenisve functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 35.Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC. The Nrf-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci. 2008;1147:61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semenza GL. Hypoxia-inducible factor 1: Control of oxygen homeostasis in health and disease. Pediatr Res. 2001;49:614–617. doi: 10.1203/00006450-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Saugstad OD, Sejersted Y, Solberg R, Wollen EJ, Bjørås M. Oxygenation of the newborn: A molecular approach. Neonatology. 2012;101:315–325. doi: 10.1159/000337345. [DOI] [PubMed] [Google Scholar]
- 38.Jennifer LM, Nikki JH. Cellular response to oxidative stress: Signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 39.Griendling KK, Sorescu D, Lassègue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.ATV.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 40.Wright CJ, Dennery PA. Manipulation of gene expression by oxygen: A primer from bedside to bench. Pediatr Res. 2009;66:3–10. doi: 10.1203/PDR.0b013e3181a2c184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85:753–766. doi: 10.1161/01.RES.85.8.753. [DOI] [PubMed] [Google Scholar]
- 42.Goulopoulou S, McCarthy CG, Webb RC. Toll-like receptors in the vascular system: Sensing the dangers within. Pharmacol Rev. 2016;68:142–167. doi: 10.1124/pr.114.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atkinson TJ. Toll-like receptors, transduction-effector pathways and disease diversity: Evidence of an immunobiological paradigm explaining all human illness? Int Rev Immunol. 2008;27:255–281. doi: 10.1080/08830180801959072. [DOI] [PubMed] [Google Scholar]
- 44.Cowan KJ, Storey KB. Mitogen-activated protein kinases: New signaling pathways functioning in cellular responses to environmental stress. J Exp Biol. 2003;206:1107–1115. doi: 10.1242/jeb.00220. [DOI] [PubMed] [Google Scholar]
- 45.Hazzalin CA, Mahadevan LC. MAPK-regulated transcription: A continuously variable gene switch? Nat Rev Mol Cell Biol. 2002;3:30–40. doi: 10.1038/nrm715. [DOI] [PubMed] [Google Scholar]
- 46.Andrikopoulou M, Almalki A, Farzin A, Cordeiro CN, Johnston MV, Burd I. Perinatal biomarkers in prematurity: Early identification of neurologic injury. Int J Dev Neurosci. 2014;36:25–31. doi: 10.1016/j.ijdevneu.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vento M, Aguar M, Escobar J, Arduini A, Escrig R, Brugada M, Izquierdo I, Asensi MA, Sastre J, Saenz P, Gimeno A. Antenatal steroids and antioxidant enzyme activity in preterm infants: Influence of gender and timing. Antioxid Redox Signal. 2009;11:2945–2955. doi: 10.1089/ars.2009.2671. [DOI] [PubMed] [Google Scholar]
- 48.Palmer C, Dieh M, Alizadeh AA, Brown PO. Cell-type specific gene expression profiles of leukocytes in human peripheral blood. BMC Genomics. 2006;7 doi: 10.1186/1471-2164-7-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gitto E, Reiter RJ, Karbownik M, Xian-Tan D, Barberi I. Respiratory distress syndrome in the newborn: Role of oxidative stress. Intensive Care Med. 2001;27:1116–1123. doi: 10.1007/s001340100977. [DOI] [PubMed] [Google Scholar]
- 50.Gitto E, Reiter RJ, Karbownik M, Tan DX, Gitto P, Barberi S, Barberi I. Causes of oxidative stress in the pre- and perinatal period. Biol Neonate. 2002;81:146–157. doi: 10.1159/000051527. [DOI] [PubMed] [Google Scholar]
- 51.Sehgal A, Saili A, Gupta RP, Bajaj P. Free oxygen radicals and immune profile in newborns with lung diseases. J Trop Pediatr. 2000;46:335–337. doi: 10.1093/tropej/46.6.335. [DOI] [PubMed] [Google Scholar]
- 52.Vedovato S, Zanardo V. Chorioamnionitis and inflammatory disease in the premature newborn infant. Minerva Pediatr. 2010;62(3 Suppl 1):S155–S156. (In Italian) [PubMed] [Google Scholar]
- 53.Ment LR, Adán U, Lin A, Kwon SH, Choi M, Hallman M, Lifton RP, Zhang H, Bauer CR. Gene Targets for IVH Study Group: Gene-environment interactions in severe intraventricular hemorrhage of preterm neonates. Pediatr Res. 2014;75:241–250. doi: 10.1038/pr.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mussap M, Noto A, Cibecchini F, Fanos V. The importance of biomarkers in neonatology. Semin Fetal Neonatal Med. 2013;18:56–64. doi: 10.1016/j.siny.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Sola A, Golombek SG, Bueno MT Montes, Lemus-Varela L, Zuluaga C, Domínguez F, Baquero H, Sarmiento AEY, Natta D, Perez JMR, et al. Safe oxygen saturation targeting and monitoring in preterm infants: Can we avoid hypoxia and hyperoxia? Acta Paediatr. 2014;13:1009–1018. doi: 10.1111/apa.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]