Abstract
Apoptosis and DNA oxidative damage serve significant roles in the pathogenesis of steroid-induced femoral head necrosis. Vitamin E demonstrates anti-apoptotic and anti-oxidant properties. Therefore, the present study investigated the effects of vitamin E on osteocyte apoptosis and DNA oxidative damage in bone marrow hemopoietic cells at an early stage of steroid-induced femoral head osteonecrosis. Japanese white rabbits were randomly divided into three groups (steroid, vitamin E-treated, and control groups), each comprising 12 rabbits. Those in the steroid group (group S) were initially injected twice with an intravenous dose of 100 µg/kg Escherichia coli endotoxin, with a 24 h interval between the two injections, and then with an intramuscular dose of 20 mg/kg methylprednisolone, three times at intervals of 24 h in order to establish a rabbit model of osteonecrosis. The vitamin E treated group (group E) received the same treatment as group S, and were administered 0.6 g/kg/d vitamin E daily from the beginning of modeling. The control group (group C) was injected with normal saline at the equivalent dosage and times as the aforementioned two groups. Two time points, weeks 4 and 6 following the completion of modeling, were selected. Osteonecrosis was verified by histopathology with hematoxylin-eosin staining. The apoptosis rate of osteonecrosis was analyzed by terminal deoxynucleotidyl transferase dUTP nick end labeling assay. The apoptosis expression levels of caspase-3 and B-cell lymphoma 2 (Bcl-2), and DNA oxidative damage of bone marrow hematopoietic cells were analyzed by immunohistochemistry. At weeks 4 and 6 following the completion of modeling, the vacant bone lacunae rates of group E were 15.87±1.97 and 25.09±2.67%, respectively, lower than the results of 20.02±2.21 and 27.79±1.39% for group S; and the osteocyte apoptosis indexes of group E were 20.99±2.95 and 33.93±1.62%, respectively, lower than the results of 26.46±3.37 and 39.90±3.74% from group S. In addition, the Bcl-2 expression at week 4 in the femoral head tissues of group E was higher compared with group S; and the proportion of Bcl-2-positive cells of group E was 9.81±1.01%, higher compared with group S at 8.26±1.13%. The caspase-3 staining data at week 4 in femoral head tissues demonstrated that in the 12 femoral heads of group S, four were negative (32%) and eight were positive (68%); in group E, five were negative (45%) and seven were positive (55%); and in group C, 11 were negative (95%) and one was positive (5%). In addition, the DNA oxidative damage rate at week 4 in the bone marrow hemopoietic cells of group E was (7.24±1.44%), lower compared with group S (11.80±1.26%), and higher compared with group C (5.75±1.47%). Vitamin E is effective in intervening in apoptosis through decreasing caspase-3 expression and upregulating Bcl-2 expression, and by alleviating DNA oxidative damage in bone marrow hemopoietic cells at the early stage of steroid-induced femoral head necrosis in rabbit models.
Keywords: glucocorticoid, femoral head necrosis, vitamin E, early stage, apoptosis
Introduction
Glucocorticoid has been widely used for treating connective tissue diseases, including systemic lupus erythematosus, nephrotic syndrome and complications following organ transplantation (1–3). However, glucocorticoid treatment always induces femoral head necrosis, which, if not properly treated, may cause femoral head collapse and osteoarthritis that consequently requires artificial joint replacement (4,5). At present, the incidence of steroid-induced necrosis of the femoral head (SINFH) exceeds trauma-induced femoral head necrosis (6). However, the pathogenesis of SINFH remains controversial, and the various theories explaining SINFH pathogenesis are predominantly classified into the osteoporosis theory, the lipid metabolism disorders theory, the vasculopathy theory, the intraosseous hypertension theory and the glucocorticoid cytotoxicity theory (7–12).
Accumulating evidence indicates that steroids can cause osteocyte, osteoblast and chondrocyte apoptosis, which is the cytological basis for femoral head necrosis (13). In addition, oxidative stress also serves an important role in SINFH pathogenesis (14). When cells are stimulated by the external environment, reactive oxygen species (ROS) increase, which alters the balance between the oxidative system and anti-oxidization system of the body, and consequently causes oxidative stress (15). Furthermore, oxidative stress can cause DNA peroxidation, which results in DNA oxidative damage (16). It has been reported that steroids can increase ROS and induce oxidative stress (14).
Vitamin E is an important anti-oxidant substance and can regulate oxidative stress and control the generation of free radicals (17). It has been reported that vitamin E can markedly reduce the incidence of SINFH in rabbits and may be used for the prevention of SINFH (18). However, the underlying mechanism of the effect of vitamin E in SINFH has, to the best of our knowledge, never been thoroughly investigated. Therefore, the present study aimed to investigate the effects of vitamin E on osteocyte apoptosis and DNA oxidative damage in bone marrow hemopoietic cells at the early stage of SINFH.
Materials and methods
Animals, grouping and treatment
Healthy Japanese white rabbits at 4 months old (weight, 2.5±0.5 kg) were selected, fed with a standard diet and raised separately. All the animals were supplied by the Test Animal Center of the Inner Mongolia Medical University (Huhhot, China) and the entire experimental procedure was approved by the Ethics Committee of the Inner Mongolia Medical University. The animals were randomly divided into three groups. Based on the modeling method of Yamamoto et al (19), the Steroid group (group S, n=12) was treated by injecting twice with Escherichia coli (E. coli) endotoxin (100 µg/kg; The National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China) through a marginal ear vein, with a 24 h-interval between the two injections. On the occasion of the second injection with E. coli endotoxin, methylprednisolone (20 mg/kg; Pfizer, Puurs, Belgium) was injected into the right gluteus of the rabbits three times in total, and at intervals of 24 h. The vitamin E treated group (group E, n=12) was treated the same way as the group S, and was fed daily with vitamin E (0.6 g/kg/d; Zhejiang Medicine Co., Ltd., Xinchang Pharmaceutical Factory, Xinchang, China) from the beginning of the experiment. The control group (group C, n=12) was treated with the equivalent volume of normal saline at the same time as the aforementioned two groups. All the experimental animals were administered penicillin by intramuscular injection, 800,000 units per rabbit, twice a week, to prevent infection.
Tissue treatment
Animals in all groups were sacrificed with an intravenous injection dose of 3% sodium pentobarbital (30 mg/kg; Beijing Propbs Biotechnology Co., Ltd., Beijing, China). The animals were euthanized at two time points: Weeks 4 and 6. Left femoral heads were fixed in 5% glutaraldehyde for 1 week, and decalcified for 2 h with 5% nitric acid. The femoral head weight-bearing area was opened along the coronal plane, and 1.0×1.5×1.0 mm3 tissue samples were obtained from the subchondral area, embedded in paraffin wax and then cut along the coronal plane into 4 µm thick sections. Hematoxylin and eosin (H&E) staining was performed and vacant bone lacunae were counted to establish femoral head necrosis. Right femoral heads were fixed in 10% formaldehyde for 1 week and decalcified for 45 days with 15% EDTA. Then the appearance and color of the bone specimens were noted and the degree of decalcification was established physically.
Histopathology
The pathological change of osteocyte necrosis was always indicated by the percentage of vacant bone lacunae. More specifically, five visual fields were selected and 50 bone lacunae were randomly selected from each to count vacant bone lacunae in each field via optical microscopy, at magnification, ×200. These observations were used to estimate the percentage of vacant bone lacunae in the bone lacunae, that is, the vacant bone lacunae rate. The vacant bone lacunae rate in the femoral head subchondral area of a normal grown-up rabbit ranged between 8 and 12%, a higher vacant bone lacunae rate in the femoral head subchondral area in comparable sections indicated osteonecrosis (20).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay apoptosis detection
A TUNEL cell apoptosis test kit was used to test osteocyte apoptosis. TUNEL staining was performed using an in situ cell-death detection kit AP (Roche Diagnostics GmbH, Mannheim, Germany) in accordance with the manufacturer's protocol. Particles stained yellow in a cell nucleus indicated an apoptotic cell. A total of 10 visual fields were randomly selected from each section and imaged via light microscopy, magnification, ×200, and 50 osteocytes were counted in each field to calculate apoptotic cell index (number of apoptotic cells/total number of cells in the visual field).
Immunohistochemistry
A caspase-3 colorimetric kit (Wuhan Boster Biological Technology Ltd., Wuhan, China) and B-cell lymphoma 2 (Bcl-2) immunohistochemistry kit (Wuhan Boster Biological Technology Ltd.) were used. For the Bcl-2 assay, the femoral head tissues of each group at week 4 were sectioned, and streptavidin-peroxidase immunohistochemical staining was performed to observe Bcl-2 expression under a light microscope. Positive Bcl-2 expression appeared as intracytoplasmic yellow particles. A known positively stained section was used as a positive control, and PBS instead of primary antibodies was used as a negative control. A total of 10 visual fields were randomly selected under a light microscope at magnification, ×200, to calculate the Bcl-2-positive cell percentage. For caspase-3 analysis, femoral head tissues of each group at week 4 were sectioned, and observed with a light microscope. A known positively stained section was used as a positive control, and PBS instead of primary antibodies was used as a negative control. Particles stained a tan color in the cytoplasm indicated a positive result and the scoring method of Cakir et al (21) was used as follows: i) Cytoplasm staining degree; 1, weak staining, stained light yellow or only a very small number of yellow or brown cells; 3, strong staining, the majority of cells are stained yellow or tan; and, 2, medium staining, a staining degree between 1 and 2; and ii) Percentage of stained cells in the cells counted; 0, <1%; 1, 1–5%; 2, 6–50%; 3, 51–75%; and 4, >75%. Finally, the total score of each section was calculated as follows: Total score=staining degree score × stained cell percentage. A total score of 36 indicated strongly positive (+++), 3–4 medium positive (++), l-2 weakly positive (+) and 0 negative (−) staining.
Assessment of DNA oxidative damage
Accumulation of ROS can cause DNA oxidative damage and 8-hydroxy-2′-deoxyguanosine (8-OHdG) is recognized as a marker for DNA oxidative damage. The monoclonal antibody N45.1 specifically binds with 8-OHdG. Sections were placed into H2O2 with a volume fraction of 0.3% for 30 min incubation at 37°C, digested with 0.1% trypsin for 15 min, and then reacted with the monoclonal antibody N45.1 (catalog no. ab48508; 1:20; Abcam, Cambridge, UK). The sections were incubated for 2 h at 37°C, then 3,3′-diaminobenzidine was used for color development for 5 min, and finally H&E staining was performed. Marrow bone hematopoietic cells included hematopoietic stem cells, myeloid stem cells, lymphoid stem cells and their differentiated cells. Five visual fields were randomly selected to count marrow bone hematopoietic cells with DNA oxidative damage, 50 marrow bone hematopoietic cells were counted in each field to calculate the percentage of count marrow bone hematopoietic cells with DNA oxidative damage in total marrow bone hematopoietic cells, that is, the DNA oxidative damage rate of marrow bone hematopoietic cells.
Statistical analysis
All the data were in the form of mean ± standard deviation, and SPSS software version 13.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. The statistical test of vacant bone lacunae rate and osteocyte apoptosis index was carried out by the variance analysis method for completely randomized design. Pairwise comparison of several specimens' averages was carried out by least significant difference. Correlation analysis was carried out using Person correlation analysis and linear regression analysis methods. P<0.05 was considered to indicate a statistically significant difference. An independent sample t-test was carried out for comparing all Bcl-2 test data, and P<0.01 was considered to indicate a statistically significant difference. For caspase-3 test results, Fisher's exact probability in a 2×2 table was adopted for pairwise specimen rate difference test. A t-test was carried to the resulting quantitative data, or t-test in case of heterogeneity of variance. P<0.05 was considered to indicate a statistically significant difference.
Results
Histopathological changes
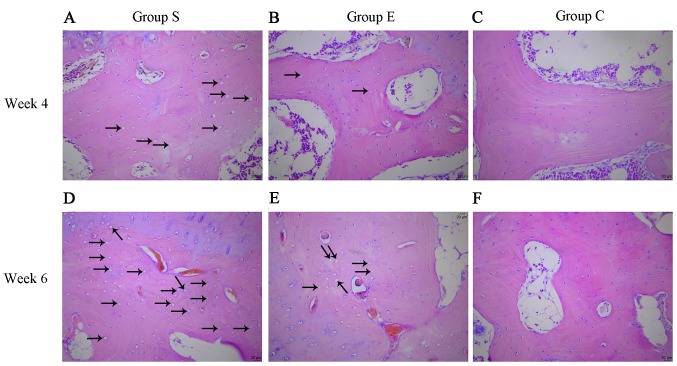
At week 4, group S femoral head pathological sections exhibited typical osteonecrosis; bone trabeculae were fewer and thinner, became broken, fragmented and structurally disordered, intraosseous adipose cells became enlarged, the arrangement of the osteoblasts became irregular and vacant bone lacunae increased (Fig. 1A). In group E the bone trabeculae in the subchondral area of femoral head were fewer and thinner, a number bone lacunae became empty and empty bone lacunae were distributed locally, abundant hematopoietic cells existed in the medullary space and there was an increase in the numbers of mast adipose cells (Fig. 1B). In group C the bone trabeculae remained intact, well organized, dense and complete, and the osteocytes had big and central cell nuclei (Fig. 1C).
Figure 1.
Histological appearance of the femoral heads of rabbits in the three groups at weeks 4 and 6 (hematoxylin-eosin staining, original magnification, ×200). (A) Group S, (B) group E and (C) group C at week 4. (D) Group S, (E) group E and (F) group C at week 6. The black arrows indicate empty osteocytic lacunae in the bone trabeculae. It was evident that there were more empty osteocytic lacunae in group S. Group S, steroid group; Group E, vitamin E-treated group; Group C, control group.
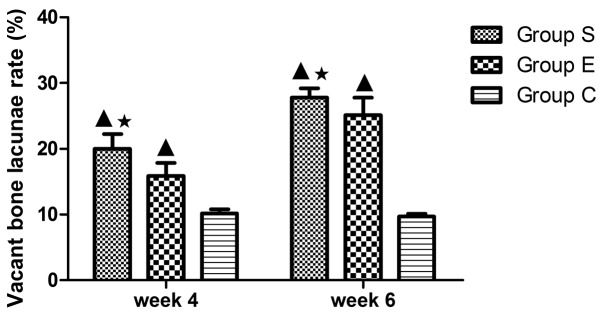
At week 6, group S sections demonstrated osteocyte necrosis, including karyopyknosis, karyolysis and karyorrhexis; more bone lacunae vacuoles occurred, intramedullary vessels were compressed, vascular lumens became narrow and fat embolus and thrombus were observed (Fig. 1D). In group E, bone trabeculae in the femoral head subchondral area were thin and fragile, hematopoietic cells in the medullary space became scarcer, the number of mast adipose cells increased and the number of soft bone lacunae vacuoles increased (Fig. 1E). In the group C sections, bone trabeculae remained intact, well organized, dense and complete, and the osteocytes had big and central cell nuclei (Fig. 1F). In group S, the rate of vacant bone lacunae demonstrated significant differences compared with group C at weeks 4 and 6 (P<0.01); compared with the group E, there was a statistical significance at weeks 4 and 6 (P<0.05). The average vacant bone lacunae rates of each group at weeks 4 and 6 are presented in Fig. 2.
Figure 2.
Rate of empty lacunae in the three groups at weeks 4 and 6. In group S, the rate of vacant bone lacunae demonstrated significant differences compared with group C at weeks 4 and 6 (P<0.01); compared with the group E, there was a statistical significance at weeks 4 and 6 (P<0.05). Values are expressed as mean ± standard deviation (n=8). ▲P<0.01 vs. group C; *P<0.05 vs. group E. Group S, steroid group; group E, vitamin E-treated group; group C, control group.
TUNEL apoptosis
Osteocyte apoptosis was examined in each group at weeks 4 and 6 following modeling (Fig. 3). As presented in Fig. 3, apoptotic osteocytes (cells with yellow particles in the bone lacunae were apoptotic) were mainly in group S (Fig. 3A and D) and a large number of apoptotic osteocytes were observed in the bone trabeculae; in group E (Fig. 3B and E), apoptotic osteocytes were fewer compared with group S; and a small number of apoptotic osteocytes were identified in group C (Fig. 3C and F). The apoptosis index of osteocytes in each group at weeks 4 and 6 are presented in Fig. 4. These findings indicated that treatment with vitamin E inhibited steroid-induced osteocyte apoptosis.
Figure 3.
Photomicrograph of TUNEL reaction demonstrating the presence of apoptotic cells in the femoral heads of rabbits at weeks 4 and 6 (original magnification, ×200). (A) Group S, (B) group E and (C) group C at week 4. (D) Group S, (E) group E and (F) group C at week 6. Cells with brown nuclei (black arrows) are TUNEL-positive cells. It was clear that there were more TUNEL-positive cells in group S compared with group E and C. TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; group S, steroid group; group E, vitamin-E treated group; group C, control group.
Figure 4.
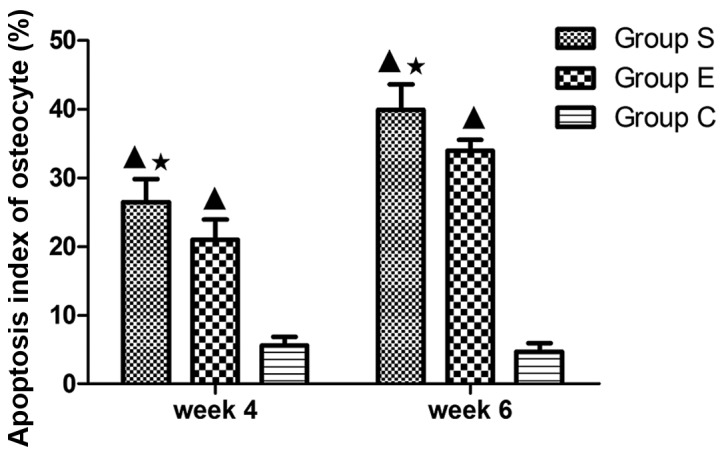
Levels of apoptotic cells in the three groups at weeks 4 and 6. In group S, the apoptosis index of osteocyte demonstrated significant differences compared with group C at weeks 4 and 6 (P<0.01); compared with the group E, there was a statistical significance at weeks 4 and 6 (P<0.05). Values are expressed as mean ± standard deviation (n=8). ▲P<0.01 vs. group C; *P<0.05 vs. group E. Group S, steroid group; group E, vitamin E-treated group; group C, control group.
Expression of caspase-3 and Bcl-2
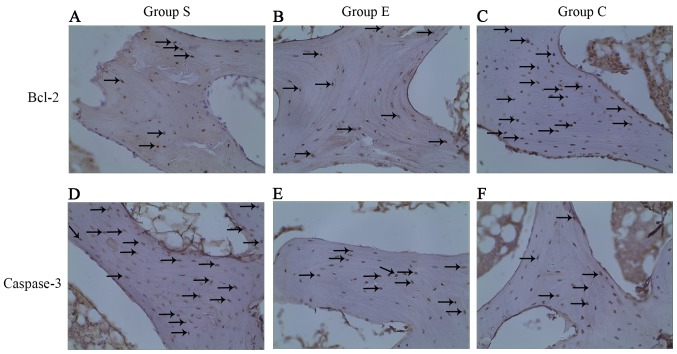
The expression of two apoptosis-associated proteins, Bcl-2 and caspase-3, was examined in each group at week 4 of the model (Fig. 5) and the proportion of Bcl-2 positive cells was counted (Fig. 6). Bcl-2 is an inhibitor of cell apoptosis. In group S, numerous cells demonstrated positive expression of Bcl-2 (Fig. 5A), the proportion of positive cells was 8.26±1.13% (Fig. 6). In group E, there were more cells expressing Bcl-2 (Fig. 5B); the proportion of positive cells was 9.81±1.01% (Fig. 6). In group C, cells expressing Bcl-2 were the majority (Fig. 5C); the proportion of positive cells was 12.60±1.03% (Fig. 6). Subsequently, the expression of caspase-3 was examined in each group at week 4 of the model. As presented in Table I, in the 12 femoral heads (bilateral femoral heads, 6×2) of group S, four were negative (32%), and eight were positive (68%), of which two were weakly positive (18%), five were medium positive (38%), and one was strongly positive (12%; Fig. 5D). In the 12 femoral heads (bilateral femoral heads: 6×2) of group E, five were negative (45%), and seven were positive (55%), of which three were weakly positive (18%), three were medium positive (27%), and one was strongly positive (10%; Fig. 5E). In the 12 femoral heads (bilateral femoral heads: 6×2) of group C, 11 were negative (95%), and one was weakly positive (5%); none was medium or strongly positive (Fig. 5F). Accordingly, the data from the present study suggest that vitamin E suppresses the steroid-induced downregulation of anti-apoptotic Bcl-2 in addition to the upregulation of pro-apoptotic caspase-3 in osteocytes, and thus inhibits steroid-induced osteocyte apoptosis.
Figure 5.
Expression of Bcl-2 and caspase-3 with immunohistochemical staining in femoral heads of rabbits at week 4 after modeling (original magnification, ×200). Bcl-2 staining was performed on (A) group S, (B) group E and (C) group C, and caspase-3 staining was also performed on (D) group S, (E) group E and (F) group C The positive staining is brown (black arrows), and the staining in group S was less intense compared with groups E and C. The positive staining is brown (black arrows) and the staining in the group S was more intense compared with groups E and C. Bcl-2, B-cell lymphoma 2; group S, steroid group; group E, vitamin E-treated group; group C, control group.
Figure 6.
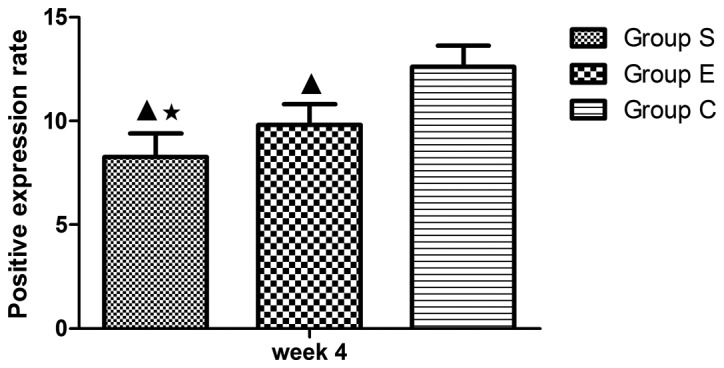
Average staining area of Bcl-2 in the three groups at week 4. In group S, the proportion of positive cells demonstrated significant differences compared with group C at week 4 (P<0.01); compared with the group E, there was a statistical significance at week 4 (P<0.05). Values are expressed as mean ± standard deviation (n=8). ▲P<0.01 vs. group C; *P<0.05 vs. group E. Bcl-2, B-cell lymphoma 2; group S, steroid group; group E, vitamin E-treated group; group C, control group.
Table I.
Positive expression of caspase-3 at week 4.
| Number of samples | ||||
|---|---|---|---|---|
| Group | − (%) | + (%) | ++ (%) | +++ (%) |
| Group S | 4 (32) | 2 (18) | 5 (38) | 1 (12) |
| Group E | 5 (45) | 3 (18) | 3 (27) | 1 (10) |
| Group C | 11 (95) | 1 (5) | 0 (0) | 0 (0) |
Group S, steroid group; Group E, vitamin E-treated group; Group C, control group; ‘-’, negative; ‘+’, weakly positive; ‘++’, medium positive; ‘+++’, strongly positive.
Assessment of DNA oxidative damage in marrow bone hematopoietic cells
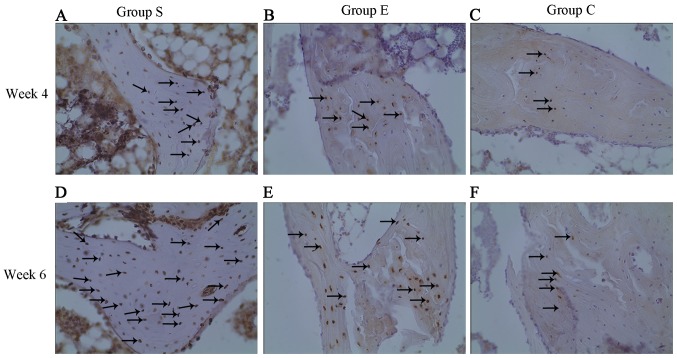
The DNA oxidative damage of bone marrow hemopoietic cells was investigated in groups S (Fig. 7A), E (Fig. 7B) and C (Fig. 7C) at week 4 following the modeling. As presented in Fig. 8, the DNA oxidative damage rate of marrow bone hematopoietic cells of group E was lower (7.24±1.44%) compared with group S (11.80±1.26%), while higher compared with group C (5.75±1.47%). These data suggested an inhibitory effect of vitamin E on the DNA oxidative damage in bone marrow hemopoietic cells at an early stage of SINFH.
Figure 7.
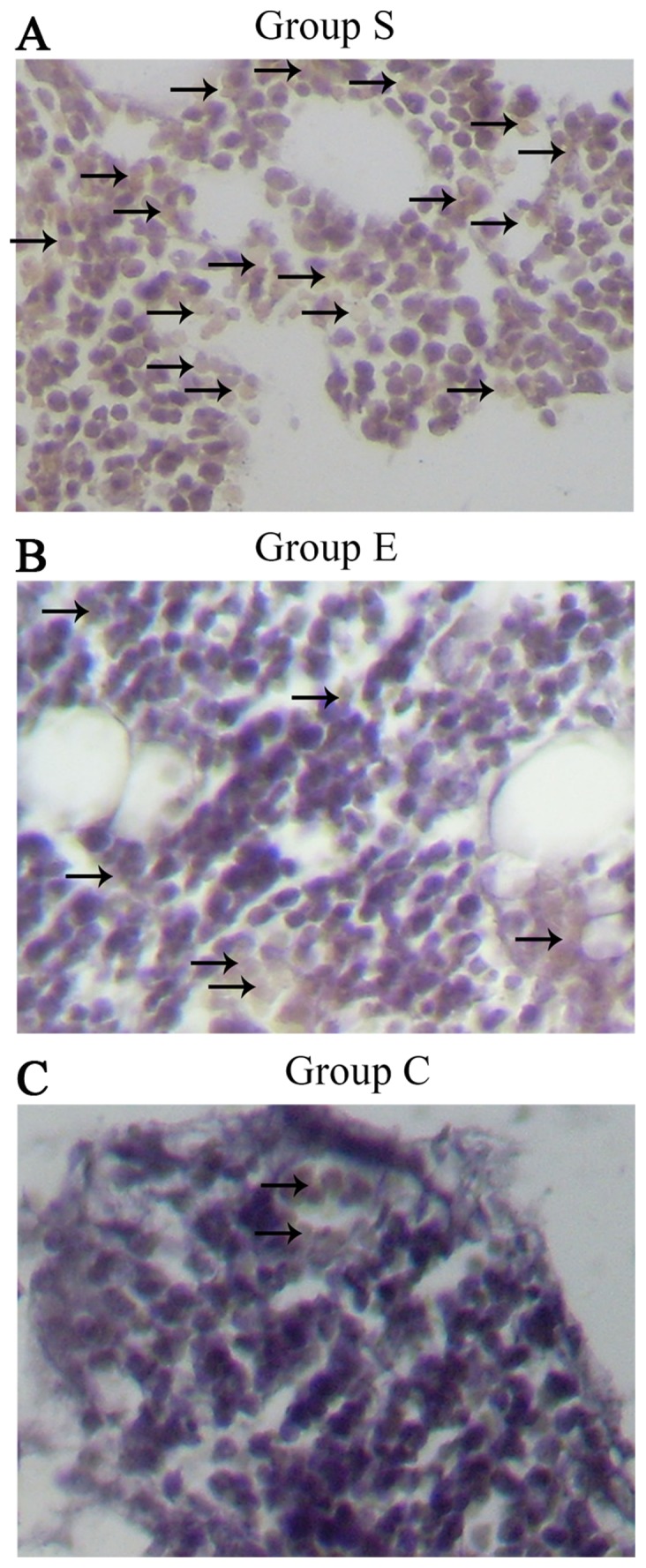
Immunohistochemical staining of 8-hydroxy-2′-deoxyguanosine assay for DNA oxidative damage of marrow bone hematopoietic cells in femoral heads of rabbits in groups (A) S, (B) E an (C) C at week 4 after modeling (original magnification, ×200). The black arrows represent positive hematopoietic cells. It was evident that there were more positive hematopoietic cells in group S. Group S, steroid group; group E, vitamin E-treated group; group C, control group.
Figure 8.
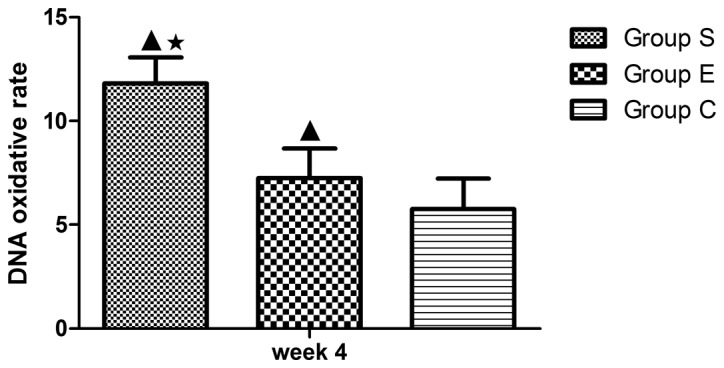
DNA oxidative damage rate of marrow bone hematopoietic cells in the three groups at week 4. In group S, the DNA oxidative damage rate of marrow bone hematopoietic cells demonstrated significant differences compared with group C at week 4 (P<0.01); compared with the group E, there was a statistical significance at week 4 (P<0.05). Values are expressed as mean ± standard deviation (n=8). ▲P<0.01 vs. group C; *P<0.05 vs. group E. Group S, steroid group; Group E, vitamin E-treated group; Group C, control group.
Discussion
Oxidative stress is caused by ROS accumulation or the dysfunction of ROS clearance and/or oxidative damage repair capability. ROS can activate the JNK pathway in osteoblasts, inactivate the ERK-l/2 and Akt pathways, and induce osteoblast apoptosis (22). Reduced glutathione (GSH) is necessary for maintaining the oxidative balance in the body, while the oxidative stress inducer buthionine sulfoximine can cause femoral head necrosis by inhibiting GSH synthesis (23), thus indicating that steroids can increase ROS. In addition, ROS can cause protein structure mutation or biological deactivation, DNA strand breakage, DNA locus mutation, DNA double-strand aberration, and finally cause oxidative damage (24). In the present study, it was demonstrated that the DNA oxidative damage of marrow bone hematopoietic cells following exposure to steroids was markedly greater compared with the control group.
A previous study (25) indicated that anti-oxidants can effectively eliminate free radicals or maintain the dynamic equilibrium of free radicals, and thus contribute to maintaining normal cell metabolism and preventing DNA damage. Vitamin E is a powerful free radical scavenger, interacting with active free radicals and changing lipid peroxide into hydroxyl lipid (26). Furthermore, vitamin E can inhibit oxidative enzymes and activate the anti-oxidase system (27). Therefore, administration of vitamin E can facilitate anti-oxidant capacity and protect DNA from free radical attack. A previous study (18) identified that vitamin E may inhibit the occurrence of SINFH in rabbits. However, the underlying mechanism remains to be elucidated. In the present study, the DNA oxidative damage in marrow bone hematopoietic cells following treatment with vitamin E was significantly decreased compared with the model group, suggesting that vitamin E may suppress the oxidative stress reaction and alleviate steroid-induced bone injury via the inhibition of DNA oxidative damage in marrow bone hematopoietic cells.
Glucocorticoid is recognized as an inducer of cell apoptosis (28). Long-term exposure to glucocorticoid or exposure to high levels of glucocorticoid directly leads to the apoptosis of osteoblasts, osteocytes and osteoclasts (29). In addition, Weinstein et al (30) suggested that glucocorticoid can also cause femoral head necrosis. Kogianni et al (31) demonstrated that cell apoptosis occurred in steroid-induced femoral head necrosis from the perspective of Fas cell surface death receptor/CD95. Kabata et al (32) demonstrated that in SINFH, a large number of osteoblasts and osteoclasts become apoptotic, and, as apoptosis continues, osteoporosis is caused, the mechanical bearing structure of the bone is damaged, the femoral heads in the weight-bearing area become flattened and collapse, and ultimately femoral head necrosis is caused. The present study indicated that the number of apoptotic cells was directly proportional to the duration of exposure to steroids. In addition, steroid treatment was associated with the downregulation of Bcl-2, an inhibitor of cell apoptosis (33).
The core mechanism of cell apoptosis is the activation of the caspase family, which is an important molecule group for regulating cell apoptosis, and serves a crucial executor role in cell apoptosis (34). The caspase family can directly split cell structure, prevent DNA repair and interfere with DNA and RNA assembly by degrading nuclear lamina and the connection between apoptotic and surrounding cells; furthermore, caspases can break the nuclear structure and generate apoptotic bodies (35). For example, activated caspase-3 affects DNA replication and transcription, and damages DNA repair (36). In addition, caspase-3 and Bcl-2 control cell apoptosis separately without mutual interference. In normal osteocytes, Bcl-2 inhibits apoptosis by preventing apoptosis signaling (37). The present study demonstrated that Bcl-2 expression in the osteocytes of group S was lower compared with group C. In animal models with SINFH, treatment with steroids reduced the expression of Bcl-2 and increased the expression of caspase-3, and thus the apoptosis of osteocytes is upregulated, which consequently causes femoral head necrosis.
A previous study suggested that vitamin E has the potential to be used as an anti-oxidant for inhibition of thioacetamide-induced caspase activation and hepatic cell apoptosis (38). Vitamin E also suppresses pulmonary epithelial cell apoptosis via decreasing tumor necrosis factor and myeloperoxidase (39). When used jointly with vitamin C, vitamin E increases the Bcl-2 level in mitochondria and decreases the level of Bcl-2-like protein 4, suppresses the release of cytochrome C, activates caspase-9 and caspase-3, and thus, inhibits myocardial cell apoptosis (40). In the present study, it is suggested that vitamin E may inhibit steroid-induced osteocyte apoptosis and DNA oxidative damage in bone marrow hemopoietic cells, potentially via upregulation of Bcl-2 and downregulation of caspase-3. Therefore, vitamin E may be used for the prevention of steroid-induced bone damage.
Acknowledgements
This study was supported by The Inner Mongolia Autonomous Region Natural Funded Projects (grant no. 2015MS0895).
References
- 1.Tanaka Y, Yoshikawa N, Hattori S, Sasaki S, Ando T, Ikesa M, Honda M. Japanese Study Group for Renal Disease in Children: Combination therapy with steroids and mizoribine in juvenile SLE: A randomized controlled trial. Pediatr Nephrol. 2010;25:877–882. doi: 10.1007/s00467-009-1411-7. [DOI] [PubMed] [Google Scholar]
- 2.Hodson EM, Alexander SI. Evaluation and management of steroid-sensitive nephrotic syndrome. Curr Opin Pediatr. 2008;20:145–150. doi: 10.1097/MOP.0b013e3282f4307a. [DOI] [PubMed] [Google Scholar]
- 3.Tönshoff B, Höcker B, Weber LT. Steroid withdrawal in pediatric and adult renal transplant recipients. Pediatr Nephrol. 2005;20:409–417. doi: 10.1007/s00467-004-1765-9. [DOI] [PubMed] [Google Scholar]
- 4.Simon JP, Berger P, Bellemans J. Total hip arthroplasty in patients less than 40 years old with avascular necrosis of the femoral head. A 5 to 19-year follow-up study. Acta Orthop Belg. 2011;77:53–60. [PubMed] [Google Scholar]
- 5.Motomura G, Yamamoto T, Yamaguchi R, Ikemura S, Nakashima Y, Mawatari T, Iwamoto Y. Morphological analysis of collapsed regions in osteonecrosis of the femoral head. J Bone Joint Surg Br. 2011;93:184–187. doi: 10.1302/0301-620X.93B225476. [DOI] [PubMed] [Google Scholar]
- 6.Nagasawa K, Tada Y, Koarada S, Tsukamoto H, Horiuchi T, Yoshizawa S, Murai K, Ueda A, Haruta Y, Ohta A. Prevention of steroid-induced osteonecrosis of femoral head in systemic lupus erythematosus by anti-coagulant. Lupus. 2006;15:354–357. doi: 10.1191/0961203306lu2311oa. [DOI] [PubMed] [Google Scholar]
- 7.Yin L, Li YB, Wang YS. Dexamethasone-induced adipogenesis in primary marrow stromal cell cultures: Mechanism of steroid-induced osteonecrosis. Chin Med J (Engl) 2006;119:581–588. [PubMed] [Google Scholar]
- 8.Drescher W, Schlieper G, Floege J, Eitner F. Steroid-related osteonecrosis-an update. Nephrol Dial Transplant. 2011;26:2728–2731. doi: 10.1093/ndt/gfr212. [DOI] [PubMed] [Google Scholar]
- 9.Takano-Murakami R, Tokunaga K, Kondo N, Ito T, Kitahara H, Ito M, Endo N. Glucocorticoid inhibits bone regeneration after osteonecrosis of the femoral head in aged female rats. Tohoku J Exp Med. 2009;217:51–58. doi: 10.1620/tjem.217.51. [DOI] [PubMed] [Google Scholar]
- 10.Siler U, Rousselle P, Müller CA, Klein G. Laminin gamma2 chain as a stromal cell marker of the human bone marrow microenvironment. Br J Haematol. 2002;119:212–220. doi: 10.1046/j.1365-2141.2002.03800.x. [DOI] [PubMed] [Google Scholar]
- 11.Miyanishi K, Yamamoto T, Irisa T, Yamashita A, Jingushi S, Noguchi Y, Iwamoto Y. Bone marrow fat cell enlargement and a rise in intraosseous pressure in steroid-treated rabbits with osteonecrosis. Bone. 2002;30:185–190. doi: 10.1016/S8756-3282(01)00663-9. [DOI] [PubMed] [Google Scholar]
- 12.Liu YF, Chen WM, Lin YF, Yang RC, Lin MW, Li LH, Chang YH, Jou YS, Lin PY, Su JS, et al. Type II collagen gene variants and inherited osteonecrosis of the femoral head. N Engl J Med. 2005;352:2294–2301. doi: 10.1056/NEJMoa042480. [DOI] [PubMed] [Google Scholar]
- 13.Zalavras C, Shah S, Birnbaum MJ, Frenkel B. Role of apoptosis in glucocorticoid-induced osteoporosis and osteonecrosis. Crit Rev Eukaryot Gene Expr. 2003;13:221–235. doi: 10.1615/CritRevEukaryotGeneExpr.v13.i24.140. [DOI] [PubMed] [Google Scholar]
- 14.Ichiseki T, Kaneuji A, Katsuda S, Ueda Y, Sugimori T, Matsumoto T. DNA oxidation injury in bone early after steroid administration is involved in the pathogenesis of steroid-induced osteonecrosis. Rheumatology (Oxford) 2005;44:456–460. doi: 10.1093/rheumatology/keh518. [DOI] [PubMed] [Google Scholar]
- 15.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jørgensen A. Oxidatively generated DNA/RNA damage in psychological stress states. Dan Med J. 2013;60:B4685. [PubMed] [Google Scholar]
- 17.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuribayashi M, Fujioka M, Takahashi KA, Arai Y, Ishida M, Goto T, Kubo T. Vitamin E prevents steroid-induced osteonecrosis in rabbits. Acta Orthop. 2010;81:154–160. doi: 10.3109/17453671003628772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto T, Miyanishi K, Motomura G, Nishida K, Iwamoto Y, Sueishi K. Animal models for steroid-induced osteonecrosis. Clin Calcium. 2007;17:879–886. (In Japanese) [PubMed] [Google Scholar]
- 20.Conradie MM, De Wet H, Kotze DD, Burrin JM, Hough FS, Hulley PA. Vanadate prevents glucocorticoid-induced apoptosis of osteoblasts in vitro and osteocytes in vivo. J Endocrinol. 2007;195:229–240. doi: 10.1677/JOE-07-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cakir E, Yilmaz A, Demirag F, Oguztuzun S, Sahin S, Yazici UE, Aydin M. Prognostic significance of micropapillary pattern in lung adenocarcinoma and expression of apoptosis-related markers: Caspase-3, bcl-2 and p53. APMIS. 2011;119:574–580. doi: 10.1111/j.1600-0463.2011.02778.x. [DOI] [PubMed] [Google Scholar]
- 22.Lu PZ, Lai CY, Chan WH. Caffeine induces cell death via activation of apoptotic signal and inactivation of survival signal in human osteoblasts. Int J Mol Sci. 2008;9:698–718. doi: 10.3390/ijms9050698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichiseki T, Ueda Y, Katsuda S, Kitamura K, Kaneuji A, Matsumoto T. Oxidative stress by glutathione depletion induces osteonecrosis in rats. Rheumatology (Oxford) 2006;45:287–290. doi: 10.1093/rheumatology/kei149. [DOI] [PubMed] [Google Scholar]
- 24.Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical-induced damage to DNA: Mechanisms and measurement. Free Radic Biol Med. 2002;32:1102–1115. doi: 10.1016/S0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- 26.Constantinou C, Neophytou CM, Vraka P, Hyatt JA, Papas KA, Constantinou AI. Induction of DNA damage and caspase-independent programmed cell death by vitamin E. Nutr Cancer. 2012;64:136–152. doi: 10.1080/01635581.2012.630167. [DOI] [PubMed] [Google Scholar]
- 27.Itoh H, Ohkuwa T, Yamazaki Y, Shimoda T, Wakayama A, Tamura S, Yamamoto T, Sato Y, Miyamura M. Vitamin E supplementation attenuates leakage of enzymes following 6 successive days of running training. Int J Sports Med. 2000;21:369–374. doi: 10.1055/s-2000-3777. [DOI] [PubMed] [Google Scholar]
- 28.Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci. 2002;966:73–81. doi: 10.1111/j.1749-6632.2002.tb04204.x. [DOI] [PubMed] [Google Scholar]
- 29.Sato M, Sugano N, Ohzono K, Nomura S, Kitamura Y, Tsukamoto Y, Ogawa S. Apoptosis and expression of stress protein (ORP150, HO1) during development of ischaemic osteonecrosis in the rat. J Bone Joint Surg Br. 2001;83:751–759. doi: 10.1302/0301-620X.83B5.10801. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85:2907–2912. doi: 10.1210/jc.85.8.2907. [DOI] [PubMed] [Google Scholar]
- 31.Kogianni G, Mann V, Ebetino F, Nuttall M, Nijweide P, Simpson H, Noble B. Fas/CD95 is associated with glucocorticoid-induced osteocyte apoptosis. Life Sci. 2004;75:2879–2895. doi: 10.1016/j.lfs.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 32.Kabata T, Kubo T, Matsumoto T, Nishino M, Tomita K, Katsuda S, Horii T, Uto N, Kitajima I. Apoptotic cell death in steroid induced osteonecrosis: An experimental study in rabbits. J Rheumatol. 2000;27:2166–2171. [PubMed] [Google Scholar]
- 33.Haÿ E, Lemonnier J, Fromigué O, Marie PJ. Bone morphogenetic protein-2 promotes osteoblast apoptosis through a Smad-independent, protein kinase C-dependent signaling pathway. J Biol Chem. 2001;276:29028–29036. doi: 10.1074/jbc.M011265200. [DOI] [PubMed] [Google Scholar]
- 34.Fiandalo MV, Kyprianou N. Caspase control: Protagonists of cancer cell apoptosis. Exp Oncol. 2012;34:165–175. [PMC free article] [PubMed] [Google Scholar]
- 35.Poreba M, Strózyk A, Salvesen GS, Drag M. Caspase Substrates and Inhibitors. Cold Spring Harb Perspect Biol. 2013;5:a008680. doi: 10.1101/cshperspect.a008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Végran F, Boidot R, Solary E, Lizard-Nacol S. A short caspase-3 isoform inhibits chemotherapy-induced apoptosis by blocking apoptosome assembly. PLoS One. 2011;6:e29058. doi: 10.1371/journal.pone.0029058. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Byrd JC, Shinn C, Waselenko JK, Fuchs EJ, Lehman TA, Nguyen PL, Flinn IW, Diehl LF, Sausville E, Grever MR. Flavopiridol induces apoptosis in chronic lymphocytic leukemia cells via activation of caspase-3 without evidence of bcl-2 modulation or dependence on functional p53. Blood. 1998;92:3804–3816. [PubMed] [Google Scholar]
- 38.Sun F, Hayami S, Ogiri Y, Haruna S, Tanaka K, Yamada Y, Tokumaru S, Kojo S. Evaluation of oxidative stress based on lipid hydroperoxide, vitamin C and vitamin E during apoptosis and necrosis caused by thioacetamide in rat liver. Biochim Biophys Acta. 2000;1500:181–185. doi: 10.1016/S0925-4439(99)00100-3. [DOI] [PubMed] [Google Scholar]
- 39.Demiralay R, Gürsan N, Erdem H. The effects of erdosteine, N-acetylcysteine, and vitamin E on nicotine-induced apoptosis of pulmonary cells. Toxicology. 2006;219:197–207. doi: 10.1016/j.tox.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 40.Qin F, Yan C, Patel R, Liu W, Dong E. Vitamins C and E attenuate apoptosis, beta-adrenergic receptor desensitization, and sarcoplasmic reticular Ca2+ ATPase downregulation after myocardial infarction. Free Radic Biol Med. 2006;40:1827–1842. doi: 10.1016/j.freeradbiomed.2006.01.019. [DOI] [PubMed] [Google Scholar]