Abstract
Modification of p53 expression levels and its principle apoptosis and cell cycle regulatory partners, mouse double minute 2 homolog (MDM-2) and p21, has been previously reported in various types of cancer. In the current study, the expression of Δ133p53 isoforms was investigated in lung carcinomas with respect to the expression of the aforementioned genes. The expression of p53 full-length transcript and Δ133p53 isoforms α, β and γ transcripts, MDM-2 and p21 transcripts were determined by reverse transcription-quantitative polymerase chain reaction, in total RNA isolated from 17 lung carcinoma specimens and 17 corresponding adjacent non-cancerous tissues. RNA expression analysis was performed according to the Pfaffl equation and Rest tool using β-actin as a reference gene. Detection of the above proteins was additionally performed by western blotting. Significant overexpression of the Δ133p53 mRNAs was observed in cancerous as compared with adjacent non-cancerous tissues (3.94-fold), whereas full-length p53 and MDM-2 expression exhibited a smaller, however significant, increase. The expression of the p21 transcript was significantly reduced in cancerous specimens. Δ133p53 and p21 expression levels varied in parallel, however were not significantly correlated. p53 full-length protein expression observed by western blot analysis strongly varied from the Δ133p53 isoforms, however MDM-2 protein isoforms were not detectable and p21 protein was more abundant in non-cancerous tissues. In conclusion, Δ133p53 mRNA levels is suggested as a potentially useful marker of malignancy in lung cancer. The absence of Δ133p53 protein in lung carcinomas, which overexpress Δ133p53 transcripts, may indicate the role of the latter in post-transcriptional regulation through RNA interference in the cell cycle and apoptosis.
Keywords: Δ133p53 isoforms, MDM-2, p21, lung cancer
Introduction
p53 is a key regulator of growth arrest, senescence and apoptosis in response to a wide array of cell damage events (1). Rapid induction of high p53 protein levels under different stress conditions prevents inappropriate propagation of cells carrying potentially mutagenic, damaged DNA (2). The central role of p53 in the cell stress response lends significance to the existence of at least nine different p53 isoforms arising from differential splicing and promoter usage (3). These isoforms are expressed in normal tissue in a tissue-dependent manner and their differentiated expression in human cancer suggests that they may be involved in tumor development or progression (4).
The Δ133p53 isoform(s) of tumor suppressor p53 are transactivated by p53 in response to stress (5). The Δ133p53 isoforms are lacking the first 133 amino acids and the expression of their mRNA is derived from an alternative internal promoter located in intron 4 of p53. Lack of the p53 N-terminus is responsible for a dominant negative inhibition of normal apoptotic function of the p53 protein. Therefore, the excessive expression of these isoforms may serve a critical role and interfere with normal p53 function. The Δ133p53 isoforms are implicated in controlling cellular senescence and elevated levels of these isoforms have been observed in a variety of tumors. Elevated expression of Δ133p53 isoform(s) has been observed in breast cancer and in renal cell carcinoma, whereas in colon cancer, progression from colon adenoma to carcinoma is accompanied by an increase of Δ133p53 mRNA (6). In addition to its multiple functions, p53 has been additionally identified to suppress metastasis and inhibit angiogenesis, a process strongly contributing to tumor development (7). Bernard et al (8) established that angiogenesis and growth of glioblastoma U87 tumors are inhibited upon depletion of the Δ133p53α isoform and that this isoform induces pro-angiogenic gene expression and represses anti-angiogenic gene expression. Furthermore, previous analysis of germline p53 mutations in breast cancer by Kouidou et al (9) demonstrated that the Li-Fraumeni and Li-Fraumeni-like syndromes are closely associated with the loss of the initiation codon 133 of the Δ133p53 isoforms, thus indicating that these isoforms serve a regulatory role and are associated with carcinogenesis.
In the current study, the expression levels of transcripts leading to the expression of Δ133p53 isoforms were identified in lung cancer. In addition, due to the fact that p53 serves a pivotal role within the cell and is subjected to a tight and orchestrated control, creating a network of positive and negative regulations (10), the association of Δ133p53 mRNA expression with respect to that of mouse double minute 2 homolog (MDM-2) and p21, two of its principle regulatory partners, was investigated. MDM-2 has been identified as the principal cellular antagonist of p53 by limiting p53 tumor suppressor activities (11). Through binding to the N-terminal transactivation domain, MDM-2 is involved in p53 degradation in the cytoplasm (12), thus suppressing its activity. MDM-2, an E3 ubiquitin ligase of the RING-finger family, is involved in p53 regulation. The RING domain permits the direct binding of ubiquitin enzymes resulting in mono- or poly-ubiquitination of p53 (13). p21 is a notable effector of p53 and is a general inhibitor of cyclin-dependent kinases, functioning to negatively regulate the cell cycle. The expression of p21 is upregulated by the p53 response to DNA damage, leading to cell-cycle arrest at the G1 checkpoint (14). Similar to other types of human cancer, approximately 50% of lung cancer types exhibit mutations in p53 (15), however the relative expression of the Δ133p53 isoforms remains to be fully elucidated in association with this disease.
Materials and methods
Tissue specimens
Surgical specimens, primary tumor samples and corresponding non-malignant tissues, were obtained from 17 patients admitted to Theageneion Anticancer Hospital (Thessaloniki, Greece) immediately after the excision of non-small cell lung carcinoma during resection surgery (Table I). Thirteen of the patients were male (mean age, 61.27 years) and three female (mean age, 58 years). Macroscopically non-cancerous lung tissue was obtained from a distal site at the excision limit from the same individual. Samples were immediately snap-frozen and stored in liquid nitrogen prior to long-term storage at −80°C. This study was approved by the ethics committee of Theageneion Anticancer Hospital. The human lung adenocarcinoma epithelial cell line (A549) and the human lung fibroblast cell line (MRC-5) were also analysed. The cell lines were kindly provided by Dr George Geromichalos (Theageneion Anticancer Hospital).
Table I.
Epidemiological characteristics of samples used in the experiments of the current study, documentation of the histopathology analysis of each patient, the age, gender and the smoking habits (packs per years) are presented.
| Sample no. | Histopathology | Age | Gender | Smoking (packs/year) |
|---|---|---|---|---|
| 1 | Squamous | 44 | Μ | 60 |
| 6 | NSCLC | N/A | F | 70 |
| 9 | NSCLC | N/A | M | Non-smoker |
| 11 | NSCLC | 73 | M | 55 |
| 12 | Adenocarcinoma | 42 | M | 63 |
| 13 | Squamous | N/A | M | 40 |
| 20 | Adenocarcinoma | 47 | M | 60 |
| 27 | NSCLC | N/A | N/A | N/A |
| 38 | Adenocarcinoma | 75 | M | Non-smoker |
| 39 | NSCLC | 58 | Μ | 30 |
| 40 | NSCLC | 66 | F | Non-smoker |
| 41 | Adenocarcinoma | 67 | M | 150 |
| 42 | NSCLC | 65 | M | 140 |
| 43 | NSCLC | 66 | M | 200 |
| 44 | NSCLC | 60 | M | 60 |
| 45 | NSCLC | 77 | Μ | 60 |
| 46 | Adenocarcinoma | 48 | F | 70 |
N/A, not available; NSCLC, non-small cell lung cancer.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assays for detection of p53, full-length and Δ133p53, MDM-2 and p21 mRNA and statistical analysis of the results
Total RNA was extracted with the SV Total RNA Isolation System (Promega Corporation, Madison, WI, USA) treated with DNase (Promega Corporation), both integrity and purity were confirmed via spectrophotometry and agarose gel electrophoresis. cDNA was synthesized using oligodT and Superscript II Reverse Transcriptase (Promega Corporation). cDNA (2 µg) were then used for the PCR reaction. For quantification of p53, full-length and Δ133p53 isoforms, MDM-2 and p21 transcripts, RT-qPCR was performed using 125 ng RNA with 2X Power SYBR-Green PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer's instructions and performed on Applied Biosystems StepOnePlus Real Time PCR System (Thermo Fisher Scientific, Inc.). The cycling conditions were as follows: 95°C for 10 min, then 40 cycles of 95°C for 30 sec, 56°C for 30 sec, 72°C for 30 sec, followed by melting curve analysis up to 95°C. The primer sequences used were as follows: Full-length p53, P53EX3S 5′-TCCATGGGACTGACTTTCTGC-3′ and P53EX4AS 5′-CTGGCATTCTGGGAGCTTCA-3′; Δ133p53 isoforms, P534b 5′-TAGACGCCAACTCTCTCTAG-3′ and P53RE5 5′-TTGGCAAAACATCTTGTTGAGGGC-3′; MDM-2, MDM2S 5′-CTGAAATTTCCTTAGCTGAC-3′ and MDM2AS 5′-TTCAGGAAGCCAATTCTCAC-3′; p21, P21S 5′-TGGACCTGTCACTGTCTTGT-3′ and P21AS 5′-TCCTGTGGGCGGATTAG-3′ and β-actin: B-ACTINS 5′-CGTCTTCCCCTCCATCGTG-3′ and B-ACTINAS 5′-CTTCTGACCCATGCCCACCA-3′. The primers were designed in our laboratory with the exception of primers P21S and P21AS, which were designed as described previously (16). All primers were synthesized by Invitrogen (Thermo Fisher Scientific, Inc.) The molecular weight of PCR products was verified by agarose gel electrophoresis.
In order to quantify and compare the amplification products, Cq data corresponding to the target genes were normalised relative to those of an internal housekeeping gene, β-actin. Each data point was obtained twice. Quantitative values were estimated from the quantification PCR cycle number (Cq) at which the increase in signal associated with an exponential growth for PCR product starts to be detected. The relative mRNA levels in each sample were normalised to its β-actin mRNA content. The relative expression level of the target gene was analysed by the 2−ΔΔCq method (17). For each sample, the difference in Cq values for the gene of interest and the endogenous control was calculated (ΔCq) (17). In addition, the REST© tool (relative expression software, version 2009; Qiagen GmbH, Hilden, Germany) which compares two groups (cancerous vs. non-cancerous tissue) with up to 16 data points in each group for reference, and up to four target genes (18).
Protein extraction
The surgical specimens were homogenized into a fine powder using a mortar and pestle in liquid nitrogen and maintained at −80°C. For the total protein extraction 100 mg of homogenized tissue was resuspended in 300 µl lysis buffer (50 mM Tris/HCl pH 8, 150 mM NaCl, 0.02% (w/v) sodium azide, 0.1% (w/v) SDS, 0.5% (w/v) sodium deoxycholate, 0.4 mM EDTA, 10 mM NaF, 0.75 mM PMSF, 1% v/v protease inhibitor cocktail EDTA-free). The cells were then lysed by performing 5 freeze-thaw cycles in liquid nitrogen and 37°C water bath. Subsequent to incubation for 4.5 h at 4°C under rotation, the suspension was centrifuged at 15,000 × g for 25 min at 4°C. The supernatant was maintained at −80°C until later use.
Semi-dry western blotting analysis
Following electrophoresis, the proteins were transferred onto nitrocellulose membranes (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) in the presence of blotting buffer (12.5 mM H3BO3, 12.5 mM Tris/HCl pH 8.5, 0.02% w/v SDS) by applying 64 mA for 15 min. The membranes were blocked for 1 h in 5% non-fat dry milk in phosphate-buffered saline with 0.1% Tween-20 at room temperature. Incubation with the primary antibodies was performed overnight at 4°C, followed by incubation with the secondary antibody conjugated to alkaline phosphatase for 1 h at room temperature. The signals in the immunoblots were detected indirectly by staining with NBT/BCIP (Biotium, Fremont, CA, USA). The mouse anti-p53 antibody (Pab 240; sc-99) raised against amino acids 156–214 of p53 of human origin and the rabbit anti-p21 (C-19, sc-397) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) and used at a working dilution of 1:500. The rabbit anti-MDM-2 antibody (S166; AP1253e) was purchased from Abgent (San Diego, CA, USA) and used at a 1:1,000 working dilution. The secondary anti-rabbit (7054F) and anti-mouse (7056) IgG-alkaline phosphatase antibodies (1:4,000 working dilution) were purchased from Cell Signalling Technology, Inc. (Danvers, MA, USA). The image capturing was performed with the Molecular Imager® Gel Doc™ XR+ System with Image Lab™ Software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Due to the fact that staining of the β-actin protein was inadequate, Coomassie Blue Staining was used as a loading control for the western blot analysis (19). Variation of β-actin steady-state expression levels in the lung may be responsible for the limited use of this protein as a control (19).
Results
Expression of p53, full-length and Δ133p53, MDM-2 and p21 transcripts, using RT-qPCR
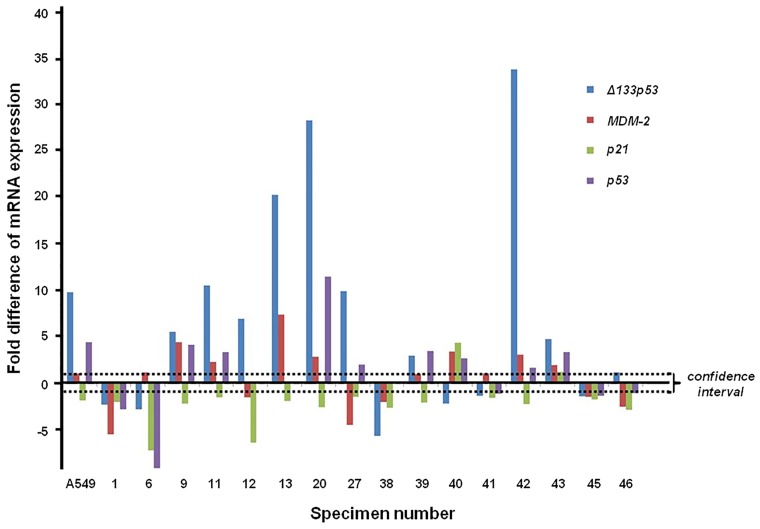
Using specific primers and RT-qPCR, the full-length p53 transcript, Δ133p53 (for Δ133p53α, Δ133p53β and Δ133p53γ a common set of primers was used), in addition to MDM-2 and p21 transcripts were amplified. In each case, the data were verified by replicate tests. The normalized average for each sample relative to the actin control was calculated using the Pfaffl equation (Fig. 1). Fig. 1 illustrates the wide variation of the relative expression of p53, full length and Δ133p53, MDM-2 and p21 transcripts in the majority of samples. However, the most considerable variation was observed in Δ133p53 transcript expression (3.94-fold increase), which according to the Pfaffl criteria (>1.5) was statistically significant.
Figure 1.
mRNA expression of p53, full-length and Δ133p53, MDM-2 and p21 relative to β-actin (reference gene) for each individual lung carcinoma and corresponding adjacent non-cancerous specimens. The Cq values were analyzed by the 2−ΔΔCq method using Pfaffl analysis. ▪▪▪, 95% confidence interval limit. MDM-2, mouse double minute 2 homolog.
Analysis of the relative expression of p53, full-length and Δ133p53, MDM-2 and p21 transcripts in cancerous vs. non-cancerous tissue
In order to quantitfy the relative alterations in expression of the genes studied, REST© analysis was used. The results presented in Table IIA demonstrate significant overexpression of the Δ133p53 transcripts in cancerous vs. non-cancerous tissue, in addition to a smaller overexpression of p53 and MDM-2 in the above tissues. On the contrary, p21 was observed to undergo a small reduction of expression in the cancerous tissue relative to the non-cancerous tissues. The same conclusions were drawn for the relative expression of p53, full-length, Δ133p53 and the MDM-2 transcripts in the cancerous A549 cell line compared with the non-cancerous MRC-5 cell line (Table IIB). p21 was significantly underexpressed in the cancerous A549 cell line. Thus, Δ133p53 appears to be the most overexpressed of the above transcripts in the cancerous tissues.
Table II.
Expression of full-length p53, Δ133p53, MDM-2 and p21 transcripts in the different tissues and cell lines.
| A, Cancerous tissues relative to non-cancerous tissues for the carcinoma specimens | |||||
|---|---|---|---|---|---|
| Gene | Type | Reaction efficiency | Expression | P(H1) | Result |
| β-actin | REF | 1.0 | 0.872 | 0.845 | |
| p53 | TRG | 1.0 | 1.379 | 0.787 | |
| Δ133p53 | TRG | 1.0 | 3.436 | 0.012 | Up |
| MDM-2 | TRG | 1.0 | 1.232 | 0.756 | |
| p21 | TRG | 1.0 | 0.509 | 0.470 | |
| B, The adenocarcinoma epithelial cell line A549 and the human lung fibroblast cell line MRC-5 | |||||
| Gene | Type | Reaction efficiency | Expression | P(H1) | Result |
| β-actin | REF | 1.0 | 0.521 | 0.509 | |
| p53 | TRG | 1.0 | 2.346 | 0.000 | Up |
| Δ133p53 | TRG | 1.0 | 5.169 | 0.000 | Up |
| MDM-2 | TRG | 1.0 | 0.570 | 0.509 | |
| p21 | TRG | 1.0 | 0.287 | 0.000 | Down |
Values were normalized relative to the β-actin using the Rest statistical analytical tool. REF, reference gene; TRG, target gene; MDM-2, mouse double minute 2 homolog.
Comparison of MDM-2 and p21 expression relative to p53 full-length and to Δ133p53
In order to acquire a more sensitive marker indicative of the alterations associated with cancer, the relative expression differences of MDM-2 and p21 vs. Δ133p53 were investigated in cancerous tissues compared with non-cancerous tissues. The results presented in Table III indicate that the relative expression of p21 vs. Δ133p53 exhibited a marginally stronger, however not significant, variation (P=0.052). Furthermore, investigation of the MDM-2, p21 and Δ133p53 expression vs. full-length p53 expression in cancerous and non-cancerous tissue (results not shown) identified smaller differences of the above transcripts with respect to both MDM-2 and p21 expression.
Table III.
Comparison of Δ133p53 expression vs. MDM-2 and p21 in cancerous vs. non-cancerous tissue using the Rest statistical analytical tool.
| Gene | Type | Reaction Efficiency | Expression | P(H1) |
|---|---|---|---|---|
| Δ133p53 | REF | 1.0 | 1.000 | |
| MDM-2 | TRG | 1.0 | 0.384 | 0.208 |
| p21 | TRG | 1.0 | 0.157 | 0.052 |
MDM-2, mouse double minute 2 homolog; REF, reference gene; TRG, target gene.
Comparison of p53, full-length and Δ133p53, MDM-2 and p21 expression relative to smoking
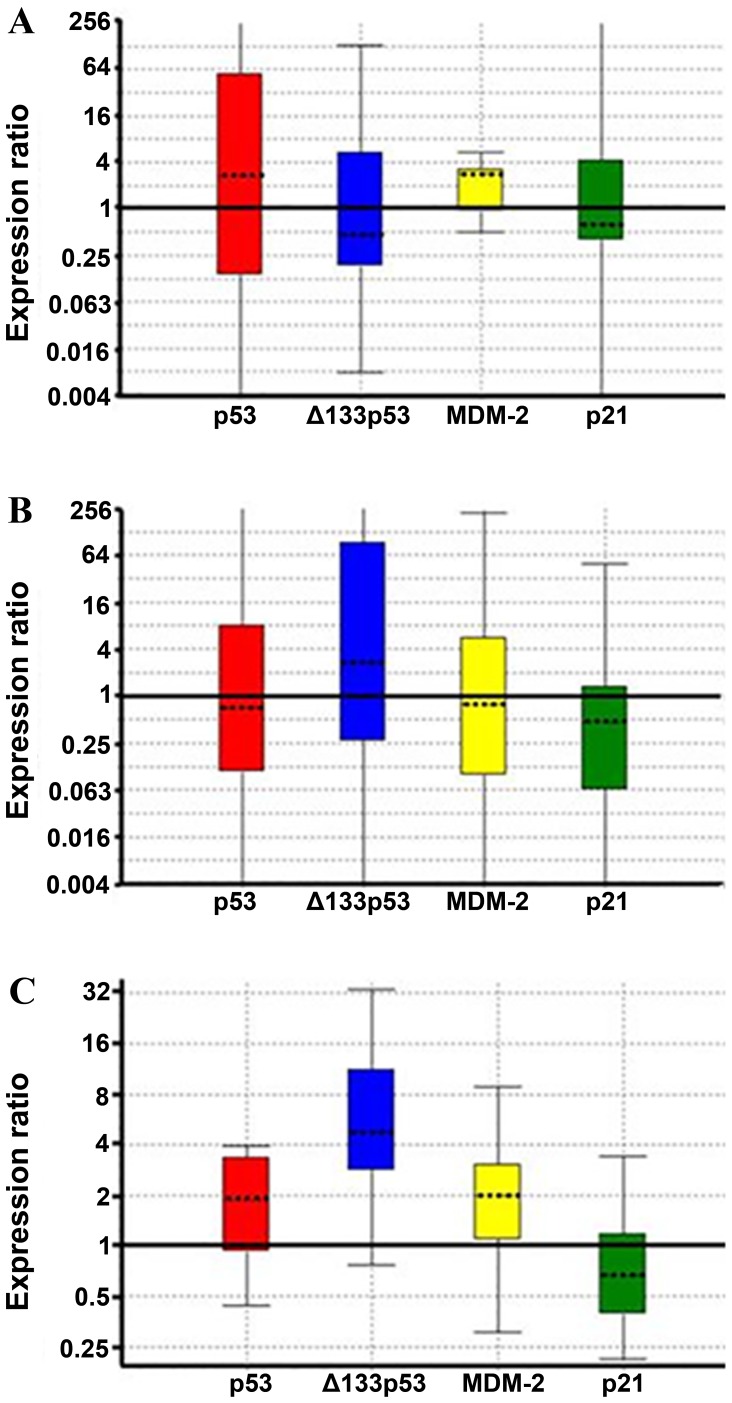
Due to the fact that cigarette consumption (packs/year) may be another parameter affecting the expression of these genes, the transcript differences with respect to smoking were additionally measured. Of the patients, three were heavy smokers (140–200 packs/year), while, three other patients had never smoked and the remaining 10 patients were intermediate smokers who consumed a smaller number of packs/year (40–70 packs/year). The analysis conducted for cancerous vs. non-cancerous tissues relative to smoking (Fig. 2) indicated that there were several consistent differences in gene expression. One difference was the absence of Δ133p53 overexpression in cancerous vs. non-cancerous tissue among non-smokers (Fig. 2A), detectable overexpression among intermediate smokers (Fig. 2B) and strong overexpression in heavy smokers (Fig. 2C). On the contrary, p53 and MDM-2 differences were similar in all groups in cancerous and non-cancerous tissues and thus not differentiated with respect to smoking. p21 expression did not vary considerably between cancerous and non-cancerous tissue with respect to smoking. In the small number of these cases, the above results were consistent although not statistically significant.
Figure 2.
Expression of p53, full-length and Δ133p53, MDM-2 and p21 transcripts in cancerous vs. non-cancerous tissue in (A) non-smokers (B) intermediate smokers (40–70 packs/year) and (C) heavy smokers (140–200 packs/year) using the Rest statistical analytical tool. ▬, difference limit for the relative expression levels. MDM-2, mouse double minute 2 homolog.
Western blotting analysis of p53, full-length and Δ133p53 isoforms, MDM-2 and p21. Comparison to mRNA expression
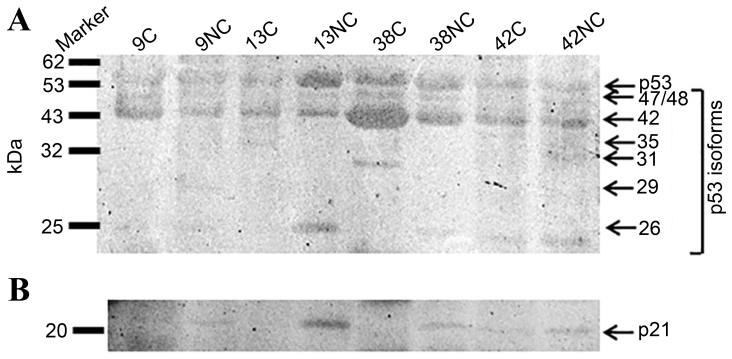
p53 protein expression and the presence of its different isoforms, in addition to MDM-2 and p21 proteins were then analyzed in representative samples with varying levels of Δ133p53 transcript expression: High (samples 13 and 42); intermediate level (sample 9); and low (sample 38). For this purpose the Pab-240 anti-p53 antibody was used, specific for the common exons 5–6 in the DNA binding region of p53, amino acids 156–214, which does not differentiate between α, β and γ variants. Using this antibody, almost all p53 isoforms were detected at differing in addition to the Δ40p53 β and γ isoforms (42 kDa) formed major intensity products (Fig. 3). Detectable truncated isoform Δ133p53α (35 kDa) was observed in sample 13C and Δ133p53β and γ (29 kDa) were weakly detected in sample 9NC. The C-terminus truncated p53β and p53γ were detectable in almost all samples. The relative abundance of the smaller isoforms could not be correlated with the corresponding transcript abundance (Fig. 1). Thus, in samples 9 and 42, overexpressing the Δ133 transcripts, corresponding isoforms were not observed.
Figure 3.
Western blot analysis of (A) p53 and (B) p21 proteins in C and NC specimens. The samples represent varying levels of Δ133p53 transcript expression: High (samples 13 and 42); low (sample 38) and intermediate (sample 9). Molecular weight markers are displayed on the left, whereas the predicted isoforms and corresponding molecular weights (kDa) are displayed on the right. Positive samples are indicated with arrows corresponding to their molecular weights in A. p53 was present at 53 kDa and its isoforms with variable molecular weights, and p21 is presented in B at 21 kDa. C, carcinoma; NC, adjacent normal lung specimens.
In the majority of cases, p21 protein was more abundant in adjacent normal lung tissue (Fig. 3B) compared with cancerous samples, and in accordance with the corresponding RT-qPCR results. Western blotting analysis did not provide conclusive evidence for MDM-2 protein isoforms (results not shown).
Discussion
Expression of full-length p53 mRNA, its isoforms and their principle co-regulators, apoptosis effector MDM-2 and cell cycle regulator p21, serve a critical role in the process of lung carcinogenesis. In the current study, it was reported, to the best of our knowledge, for the first time, that in addition to the parallel MDM-2 and p53 expression variation in cancerous compared with adjacent non-cancerous lung tissue, Δ133p53 isoforms are significantly overexpressed in cancerous lung tissue. On the contrary, p21 is significantly under-expressed in cancerous lung tissue samples. The observed inverse association of Δ133p53 isoforms and p21 expression was not statistically significant.
Although the Δ133p53/p53 mRNA was most abundant in the cancerous samples, the Δ133p53 protein isoforms were not detectable in samples overexpressing the mRNA of these isoforms. By contrast, p21 protein expression was correlated with p21 mRNA expression, in the cancerous and non-cancerous lung tissues used in the present study. The differences observed by western blotting analysis demonstrated inconsistencies between mRNA and protein production, which are observed primarily in cancerous tissues and also verified in previous studies in other types of cancer (20,21). It is suggested that the overexpression of Δ133p53 isoforms may exert its effect through p53 transcript interference. Transcript interference appears to be a common mechanism by which mRNAs lacking their 5′ sequences act (22,23). It has been previously reported that Δ133p53 isoforms differentially modulate p53 target gene expression to antagonize p53 apoptotic activity at the physiological level in zebra fish and that the knockdown of Δ133p53 enhances p53-mediated apoptosis under stress conditions (24). A recent study did however demonstrate that the Δ133p53 isoforms are also negatively involved in DNA damage repair and inhibition of cell senescence (25). The observations of the current study demonstrate that the Δ133p53 transcript overexpression may additionally have a negative effect on DNA damage repair in lung cancer where mutations are very frequent. This condition paralleled by the p21 transcript reduction is suggested to strongly affect carcinogenesis in the lung.
Numerous studies have identified the impact of different isoforms on cancer biology (26–28). Alternatively spliced tissue factor (TF) isoform expression is differentially modulated on a post-transcriptional level via regulatory factors including serine/arginine-rich (SR) proteins, SR protein kinases and microRNAs. These isoforms participate in a variety of physiological and pathophysiological functions, including thrombogenicity, angiogenesis, cell signalling, tumor cell proliferation and metastasis (26). Specifically, Goldin-Lang et al (27) observed that upregulation of human full length tissue factor and, particularly, an alternatively spliced human tissue factor in pulmonary adenomatosis, suggested raised risk of thrombosis and tumor progression, thereby indicating poor prognosis in these patients. In addition, in A549 human lung cancer cells, modulation of TF isoform expression, which is controlled by alternative splicing, modulates the pro-angiogenic potential of these cells under hypoxic conditions (28).
In conclusion, the present study verifies the potential value of Δ133p53 isoform(s) overexpression as a marker for lung malignancy, and contributes to our understanding of its role in cell cycle regulation. This information is vital for elucidating lung carcinogenesis and investigating its drug sensitivity.
Acknowledgements
The current study was supported by the National Strategic Reference Framework Central Actions for PhD studies (grant no. 84643; 2007–2013) and the Programme for Development, European Social Fund, Education and Lifelong Learning, Heraclitus II.
References
- 1.Herce DH, Deng W, Helma J, Leonhardt H, Cardoso MC. Visualization and targeted disruption of protein interactions in living cells. Nat Commun. 2013;4:2660. doi: 10.1038/ncomms3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vousden KH, Lu X. Live or let die: The cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 3.Davidson RW, Kari C, Ren Q, Daroczi B, Dicker PA, Rodeck U. Differential regulation of p53 function by the N-terminal ΔNp53 and Δ113p53 isoforms in zebrafish embryos. BMC Dev Biol. 2010;10:102. doi: 10.1186/1471-213X-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song W, Huo SW, Lü JJ, Liu Z, Fang XL, Jin XB, Yuan MZ. Expression of p53 isoforms in renal cell carcinoma. Clim Med J (Engl) 2009;122:921–926. [PubMed] [Google Scholar]
- 5.Khoury MP, Bourdon C. p53 Isoforms. An intracellular microprocessor? Genes Cancer. 2011;2:453–465. doi: 10.1177/1947601911408893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wel J, Zaika E, Zaika A. p53 Family: Role of protein isoforms in human cancer. J Nucleic Acids. 2012;2012:687359. doi: 10.1155/2012/687359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teodoro JG, Evans SK, Green MR. Inhibition of tumour angiogenesis by p53: A new role for the guardian of the genome. J Mol Med (Berl) 2007;85:1175–1186. doi: 10.1007/s00109-007-0221-2. [DOI] [PubMed] [Google Scholar]
- 8.Bernard H, Garmy-Susini B, Ainaoui N, Van Den Berghe L, Peurichard A, Javerzat S, Bikfalvi A, Lane DP, Bourdon JC, Prats AC. The p53 isoform, Δ133p53α, stimulates angiogenesis and tumour progression. Oncogene. 2013;32:2150–2160. doi: 10.1038/onc.2012.242. [DOI] [PubMed] [Google Scholar]
- 9.Kouidou S, Malousi A, Kyventidis A, Fragou A, Maglaveras N. G:C>A:T mutations and potential epigenetic regulation of p53 in breast cancer. Breast Cancer Res Treat. 2007;106:351–360. doi: 10.1007/s10549-007-9514-y. [DOI] [PubMed] [Google Scholar]
- 10.Pekova S, Cmejla R, Smolej L, Kozak T, Spacek M, Prucha M. Identification of a novel, transactivation-defective splicing variant of p53 gene in patients with chronic lymphocytic leukemia. Leuk Res. 2008;32:395–400. doi: 10.1016/j.leukres.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Ute M, Petrenko O. The MDM2-p53 interaction. Mol Cancer Res. 2003;1:1001–1008. [PubMed] [Google Scholar]
- 12.Freedman DA, Lavine AJ. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol Cell Biol. 1998;18:7288–7293. doi: 10.1128/MCB.18.12.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itahana K, Mao H, Jin A, Itahana Y, Clegg H, Lindström MS, Bhat KP, Godfrey VL, Evan GI, Zhang Y. Targeted inactivation of Mdm2 Ring finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation. Cancer Cell. 2007;12:355–366. doi: 10.1016/j.ccr.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 15.Guimaraes DP, Hainaut P. TP53: A key gene in human cancer. Biochimie. 2002;84:83–93. doi: 10.1016/S0300-9084(01)01356-6. [DOI] [PubMed] [Google Scholar]
- 16.Sun P, Qiu Y, Zhang Z, Wan J, Wang T, Jin X, Lan Q, Rothman N, Xia ZL. Association of genetic polymorphisms, mRNA expression of p53 and p21 with chronic benzene poisoning in a Chinese occupational population. Cancer Epidemiol Biomarkers Prev. 2009;18:1821–1828. doi: 10.1158/1055-9965.EPI-09-0140. [DOI] [PubMed] [Google Scholar]
- 17.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 18.Pfaffl MW, Graham WH, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucl Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welinder C, Ekblad L. Coomassie staining as loading control in Western blot analysis. J Proteome Res. 2011;10:1416–1419. doi: 10.1021/pr1011476. [DOI] [PubMed] [Google Scholar]
- 20.Hofstetter G, Berger A, Schuster E, Wolf A, Hager G, Vergote I, Cadron I, Sehouli J, Braicu EI, Mahner S, et al. Δ133p53 is an independent prognostic marker in p53 mutant advanced serous ovarian cancer. Br J Cancer. 2011;105:1593–709. doi: 10.1038/bjc.2011.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore CH, Jordan BL, Bray ES, Baker L, Quinlan RP, Purdie CA, Thompson AM, Bourdon JC, Fuller-Pace FV. The RNA helicase p68 modulates expression and function of the Δ133 isoform(s) of p53, and is inversely associated with Δ133p53 expression in breast cancer. Oncogene. 2010;29:6475–6484. doi: 10.1038/onc.2010.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewing AS, Rueli RH, Robles MJ, Nguyen-Wu ED, Zeyda T, Berry MJ, Bellinger FP. Expression and regulation of mouse selenoprotein P transcript variants differing in non-coding RNA. RNA Biol. 2012;9:1361–1369. doi: 10.4161/rna.22290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keene JD. RNA regulons: Coordination of post-transcriptional events. Nat Rev Gene. 2007;8:533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Ng SM, Chang C, Zhang Z, Bourdon JC, Lane DP, Peng J. p53 isoform D113p53 is a p53 target gene that antagonizes p53 apoptotic activity via BclxL activation in zebrafish. Genes Dev. 2009;23:278–290. doi: 10.1101/gad.1761609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong L, Gong H, Pan X, Chang C, Ou Z, Ye S, Yin L, Yang L, Tao T, Zhang Z, et al. p53 isoform Δ113p53/Δ133p53 promotes DNA double-strand break repair to protect cell from death and senescence in response to DNA damage. Cell Res. 2015;25:351–369. doi: 10.1038/cr.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leppert U, Eisenreich A. The role of tissue factor isoforms in cancer biology. Int J Cancer. 2015;137:497–503. doi: 10.1002/ijc.28959. [DOI] [PubMed] [Google Scholar]
- 27.Goldin-Lang P, Tran QV, Fichtner I, Eisenreich A, Antoniak S, Schulze K, Coupland SE, Poller W, Schultheiss HP, Rauch U. Tissue factor expression pattern in human non-small cell lung cancer tissues indicate increased blood thrombogenicity and tumour metastasis. Oncol Rep. 2008;20:123–128. [PubMed] [Google Scholar]
- 28.Eisenreich A, Zakrzewicz A, Huber K, Thierbach H, Pepke W, Goldin-Lang P, Schultheiss HP, Pries A, Rauch U. Regulation of pro-angiogenic tissue factor expression in hypoxia-induced human lung cancer cells. Oncol Rep. 2013;30:462–470. doi: 10.3892/or.2013.2413. [DOI] [PubMed] [Google Scholar]