Abstract
The study of ankylosing spondylitis (AS) has made significant progress over the last decade. Genome-wide association studies have identified and further substantiated the role of susceptibility genes outside the major histocompatibility complex locus. However, human leukocyte antigen (HLA)-B27 has been suggested to be important in the pathogenesis of AS, contributing to ~20.1% of AS heritability. The current review will present the classical and non-classical forms of HLA-B27, as well as their pathogenic roles, and further discuss the hypotheses regarding the potential pathogenesis of AS. In addition, the association between the pathogenic role of HLA-B27 and inflammatory indexes, including the interleukin-23/−17 axis will be investigated to provide novel insights into the pathogenesis of AS. The aim of the present review is to provide an update of the current research into the pathogenesis of AS, and provide a comprehensive description of the pathogenic role of HLA-B27 in AS.
Keywords: ankylosing spondylitis, human leukocyte antigen-B27, endoplasmic reticulum aminopeptidase 1, interleukin-23/−17 axis, pathogenesis
1. Introduction
Ankylosing spondylitis (AS), which is the most frequently occurring form of Spondyloarthritis (SpA), is a chronic immune-mediated inflammatory disease characterized by inflammation that predominantly affects the axial skeleton. Peripheral arthritis and enthesopathy have been reported to be present in a large group of AS patients (1). In addition, specific organ involvement including anterior uveitis, psoriasis and chronic inflammatory bowel disease, may simultaneously occur in AS, accompanied by an increased risk of cardiovascular or pulmonary complications (1,2). Chronic inflammation in the attachment of tendons, ligaments and joint capsules to bone leads to alterations in joint architecture, with new bone formations and joint fusions (3). The unique structural alterations in syndesmophyte formation and ankylosis of the vertebrae are the primary causes of early severe disability in patients with AS during disease progression. However, the pathogenesis and contributing etiological factors of AS remain to be elucidated.
Genetic studies have contributed the most significant information to our understanding of AS. Familial aggregation studies have revealed that heritability contributes a substantial proportion of AS susceptibility (4). A strong genetic predisposition was confirmed by the discovery of a remarkably high association between AS and the human leukocyte antigen (HLA)-B27 in 1973 (5). A proportion of AS cases that do not involve HLA-B27 have been reported, however, it is still regarded as one of the most important factors for the development of AS with a high association (>100), and is present in up to 90% of patients in the majority of ethnic groups that present with the disease (6). Genetic studies have concluded that HLA-B27 in the major histocompatibility complex (MHC) locus contributes to ~20.1% of AS heritability, with 4.3% associated with loci other than HLA-B (7). It has a high degree of genetic polymorphism, with up to 105 known subtypes, termed HLA-B*27:01 to HLA-B*27:106, which are encoded by 132 alleles. The most common subtypes associated with AS are HLA-B*27:05 (Caucasians), HLA-B*27:04 (Chinese), and HLA-B*27:02 (Mediterranean) (8). However, two subtypes, HLA-B*27:06 and HLA-B*27:09, do not appear to present any association with the disease (9). In addition, genome-wide association studies and those involving Immunochip arrays, have further substantiated that the development of AS is determined, to a large extent, by genes located outside of the MHC locus. These involve loci of the interleukin (IL)-23/IL-17 axis, including IL23R, IL12B, tyrosine kinase 2, signal transducer and activator of transcription 3, IL6R, IL27, as well as several aminopeptidase genes, including endoplasmic reticulum aminopeptidase (ERAP) 1 and 2, leucyl and cystinyl aminopeptidase (LNPEP) and aminopeptidase puromycin sensitive (NPEPPS) genes (7,10). It has previously been suggested that a strong association exists between ERAP1 and AS that is restricted to HLA-B27-positive patients (11,12). Previous studies suggest that the development of AS may be associated with antigen processing and presentation (11,12).
2. Classical and non-classical forms of HLA-B27
MHC Class I molecules are important for the initiation and propagation of immune responses (13,14). The classical heterotrimeric MHC class I molecule is composed of three non-covalently bound individual polypeptides: A highly polymorphic heavy chain (HC), β2-microglobulin (β2m) light chain and an oligopeptide, typically 8 to 10 residues in length (13,14). Assembly of a stable MHC molecule in the endoplasmic reticulum (ER) is a necessary step prior to export to the cell surface. Following synthesis and glycosylation, free HCs are initially stabilized by chaperones (calreticulin and tapasin) until a conformation suitable to bind β2m and a peptide with suitable length is achieved (15). Nascent MHC class I molecules typically bind antigen peptides and transport them to the cell surface for presentation to the T-cell receptors (TCR) on T lymphocytes (15).
In the absence of β2m, HCs will misfold and ER-associated degradation may occur in the ER. However, HLA-B27 appears to exhibit a tendency to misfold and a predilection for forming dimers or multimers (16). HLA-B27 possesses three unpaired cysteine (C) residues at positions 67, 308 and 325, and four conserved cysteine residues at positions 101, 164, 203 and 259 (17). Three distinct forms of dimeric MHC-I structures have been described previously, including cell surface HLA-B27 homodimers, intracellular and the exosomal fully-folded MHC-I dimers (18). The intracellular dimers are termed as ER-resident and endosomal dimers, the latter of which may be expressed on the cell surface. Previous studies have proposed that the cysteine at position 67 (C67) is involved in intracellular and cell surface dimer formation (15,19). However, the structurally conserved C101 and C164 residues have additionally been demonstrated to participate in the formation of a pool of intracellular dimers (17).
In addition to the widely-accepted cell surface HLA-B27 homodimers, ER-resident dimers and endosomal dimers, the present review introduces the exosomal fully-folded MHC-I dimers. Exosomes are small vesicles of different sizes that are formed by the inward budding of endosomes to generate multivesicular bodies (MVBs). A proportion of these MVBs will fuse with the plasma membrane, releasing the internal vesicles to the extracellular environment (20). An extensive variety of cell types have been demonstrated to release exosomes and various MHC-I dimers have been revealed to be expressed on the surface of these vesicles (20). The exosome-associated HLA-B27 dimers are fully-folded, and the cysteine residues at position 325 in the cytoplasmic tail domain participate in the formation of these structures (21). In addition, a novel population of mixed-allele dimers comprising HLA-A2 and HLA-B27 has been described in exosomes. Further details of the various forms of HLA-B27 are described in Table I.
Table I.
Different forms of HLA-B27 and their pathogenic roles in ankylosing spondylitis.
| First author, year | HLA-B27 form | Location | Structural feature | Formation | Condition | Pathogenic role/receptor | Refs. |
|---|---|---|---|---|---|---|---|
| Chen, 2013; | Classical | ER | Expressed at the cell | Assembly of a stable | Three non-covalently | TCR, KIR3DL1, | 23 |
| Allen, 2004; | HLA-B27 | surface as heterotrimeric | HLA-B27 molecule in the ER | bound individual | LILRB1, | 35 | |
| Allen, 2001; | peptide-MHC complexes | is necessary. Following synthesis | polypeptides are all | LILRB2, | 37 | ||
| Giles, 2012; | with β2m and peptide | and glycosylation, free HCs | required: a highly | LILRA1 | 40 | ||
| Shaw, 2014 | are initially stabilized by | polymorphic HC, β2m | 57 | ||||
| chaperones (calreticulin and tapasin) | light, chain and an | ||||||
| until a conformation suitable to | oligopeptide, typically | ||||||
| bind β2m and a peptide is achieved | of 8 to 10 residues | ||||||
| Kollenberger, 2007; | Cell surface | Endosome | Formed by two covalently | Recycling of fully-folded | Acidic environment of | KIR3DL1, | 36 |
| Allen, 2001; | HLA-B27 | bonded β2m-dissociated | HLA-B27 cell surface molecules | the endosome and | KIR3DL2, | 37 | |
| Giles, 2012; | homodimers | HCs | via the endocytic pathway, the | the low affinity binding | LILRB2, | 40 | |
| Campbell, 2012; | β2m-dissociated HCs form covalent | of β2m and peptides | LILRA1 | 43 | |||
| Shaw, 2014 | homodimers by cysteine residue at | with HC | 57 | ||||
| C67 in the α1 domain, and are | |||||||
| re-express at the cell surface | |||||||
| Lenart, 2012; | ER HLA-B27 | ER | The two β2m-dissociated, | Form via C67-C67, C101-C101 or | HLA-B27 exhibited an enhanced | UPR | 17 |
| Colbert, 2009; | homodimers | partially unfolded HCs form | C164-C164 disulfide bonds. | tendency to misfold and was | 48 | ||
| Turner, 2005 | covalent homodimers, but | susceptible to aggregation | 49 | ||||
| do not transit out of the ER | |||||||
| Raposo, 2013; | Redox-induced | Exosomes/ | Fully-folded β2m- | Critically depend on C325 in the | Lower levels of glutathione inside | Intercellular | 20 |
| Lynch, 2009; | HLA-B27 | Apoptosing | associated HLA-B27 | cytoplasmic tail (or with C339 in | exosomes creating a more oxidizing | communication | 21 |
| Campbell, 2012; | dimers | cells | dimers that are detected | HLA-A alleles) | environment | 43 | |
| Shaw, 2014 | on exosomes | 57 | |||||
| Luthra-Guptasarma, | HLA-B27 | ER | Misfolded monomeric | Residues 169–181 (identical to a | β2m-free, peptide-free HCs support | UPR/recognized | 58 |
| 2004 | with peptide | HLA-B27 with the | known HLA-B27 ligand) loop | a helix-coil transition facilitating | by receptors | ||
| binding cleft | molecule's own peptide | around and occupy the molecules | rotation of backbone angles around | ||||
| occupied | binding cleft occupied | own peptide-binding cleft | amino acid 167/168 | ||||
| Dakwar, 2008; | HLA-B27 that | ER | Misfolded HLA-B27 | Misfolding occurs in the ER | B pocket in the peptide- | UPR | 26 |
| Bowness, 2011; | have not yet | monomer that folds | prior to β2m association and | binding groove conferred a | 39 | ||
| Rajagopalan, 2012 | folded properly | improperly | peptide optimization | slow folding phenotype and | 46 | ||
| a tendency to misfold |
HLA-B27, human leucocyte antigen B27; ER, endoplasmic reticulum; MHC, major histocompatibility complex; HC, heavy chain; β2m, β2-microglobulin; TCR, T-cell receptors; KIR3DL1/KIR3DL2, killer cell immunoglobulin-like receptor, three domains, long cytoplasmic tail 1/2; LILRA1, leukocyte immunoglobulin-like receptor, subfamily A (with TM domain), member 1; LILRB1/LILRB2, leukocyte immunoglobulin-like receptor, subfamily B (with TM and ITIM domains), member 1/2; UPR, unfolded protein response.
3. Role of HLA-B27 in the pathogenesis of AS
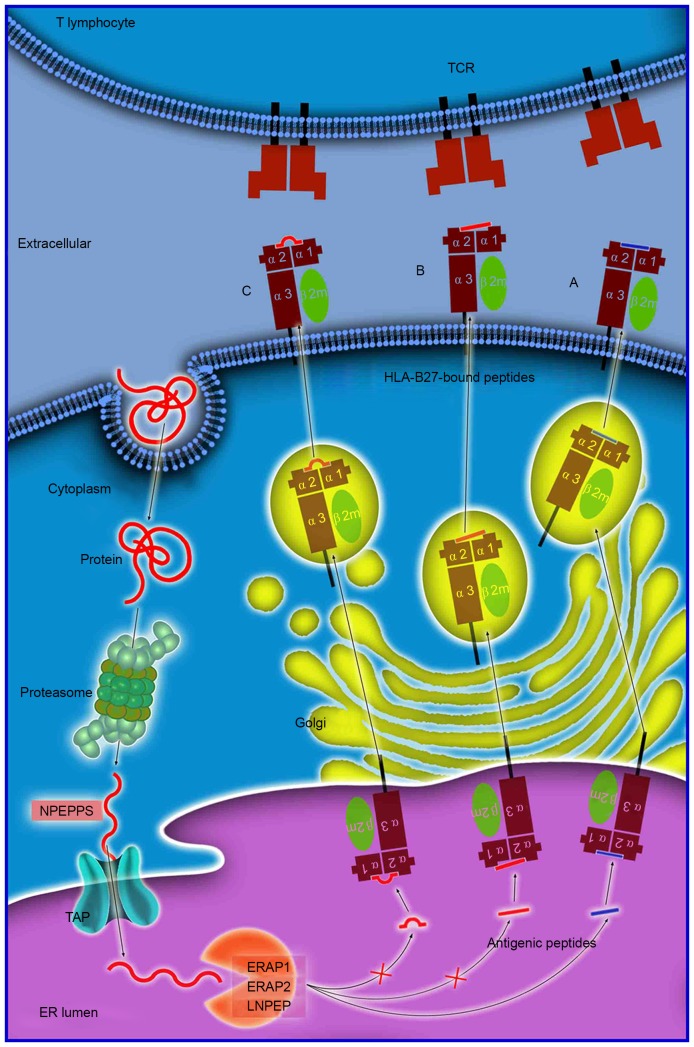
The mechanism of antigen processing and presentation is presented in Fig. 1. The majority of proteins are initially degraded in the cytoplasm by the multi-unit proteasome complex, typically generating peptide fragments of up to 25 amino acids in length. These antigen peptides and their N-terminal extended precursors are subsequently transported into the ER by a transporter associated with antigen processing that preferentially transports peptides 8–16 residues in length. Transporter, ATP-binding cassette subfamily B member (TAP) is an adenosine triphospate-driven transporter welladapted for the transfer of the precursor peptides that are continuously generated by the proteasome or other cytosolic proteases (22). Subsequently, longer peptides will be further cleaved to the length required for antigen presentation by ERAP1 residing in the ER. ERAP1 efficiently cleaves the precursors to oligopeptides 8 or 9 residues in length, which is optimal for binding to HLA-B27. These peptide-MHC complexes will subsequently enter the Golgi apparatus for the generation of mature epitopes (23). However, previous genetic studies have identified an association between various aminopeptidase genes (NPEPPS, LNPEP and ERAP2) and AS (7). The NPEPPS protein localizes to the cytoplasm and has previously been demonstrated to be involved in processing proteasome-derived peptides prior to their transport to the ER. LNPEP and ERAP2 are members of the ER aminopeptidase family and have substantial sequence homology to ERAP1 (7,24).
Figure 1.
Antigen processing and presentation of peptides of various sizes. Antigen processing and presentation is a sequenced process. Numerous proteins are initially degraded into peptide fragments of up to 25 amino acids in length by the multi-unit proteasome complex followed by NPEPPS. TAP preferentially transports antigen peptides of 8–16 residues into the ER. N-terminal extended precursors will be further cleaved by ERAP1/ERAP2/LNPEP into oligopeptides of 8 or 9 residues, which is the optimal length for binding to HLA-B27. The peptides subsequently (A) enter the Golgi apparatus for generation of mature epitopes. However, various longer peptides may bind to HLA-B27, where they reside in the peptide groove of the HLA-B27 with (B) a protruding C-terminus, or (C) a bulge in the center. These HLA-B27-bound peptides may be highly immunogenic and may stimulate an extremely biased T-cell response repertoire. NPEPPS, aminopeptidase puromycin sensitive; TAP, transporter, ATP-binding cassette subfamily B member; HLA, human leukocyte antigen; ER, endoplasmic reticulum; ERAP1/2, endoplasmic reticulum aminopeptidase 1/2; LNPEP, leucyl cystinyl aminopeptidase; TCR, T-cell receptor.
Structurally unique peptide-MHC complexes
The process of antigen peptide processing and presentation to receptors on immune cells may occur via a variety of pathways. The ‘arthritogenic’ peptide hypothesis suggests that the HLA-B27-specific autoimmune response may be directly initiated for structurally unique peptide-MHC complexes, depending on the amino-acid composition of the antigen peptides (6). The theory of ‘molecular mimicry’ suggests that a cross-reactive peptide derived from an infecting bacterial pathogen stimulates T cells, which subsequently respond to an HLA-B27 associated ‘self-peptide’, or to peptides derived from HLA-B27 directly (25). Numerous self and foreign antigen peptides have previously been investigated and sequenced, however, there is no conclusive evidence demonstrating that any of these peptides are indeed cross-reactive or self-peptides (26). Furthermore, Taurog et al (27) demonstrated that disease manifestations arose in HLA-B27/Huβ2m-transgenic rats in the absence of functional cluster of differentiation (CD)8+ T cells. Generating a model of AS disease pathogenesis, whereby HLA-B27 presents a peptide to CD8+ T cells, presents a significant challenge.
The length of antigen peptides
The length of antigen peptides is an important consideration in the process of antigen presenting. Extended peptides which are generated via aberrant peptide processing may bind HLA-B27, and have been demonstrated to reside in the peptide groove of MHC class I molecules, with either a protruding C-terminus (28), or a middle bulge (Fig. 1) (29). These HLA-B27-bound peptides may be highly immunogenic and may stimulate an extremely biased TCR repertoire. When they are presented to the TCR on T cells, a HLA-B27-specific, T-cell-mediated impaired peptide presentation is initiated, which leads to differences in immunodominance hierarchies for the presentation of a variety of aberrant epitopes, which is important in the pathogenesis of AS (23).
It was previously demonstrated that three antigens of the Epstein-Barr virus with overlapping sequences of different lengths, including the 9-mer (56LPQGQLTAY64), 11-mer (54EPLPQGQLTAY64) and 13-mer (52LPEPLPQGQLTAY64) peptides, all bound efficiently to HLA-B*35:01. By contrast, the cytotoxic T cell (CTL) response in individuals expressing HLA-B*35:01 was directed exclusively toward the 11-mer peptide (30). Conversely, individuals with HLA-B*35:03 demonstrated no significant CTL response to these peptides; however, individuals expressing HLA-B*35:08 exhibited a CTL response targeted to the 13-mer peptide (30). It was therefore hypothesized that the CTL response to infecting pathogens may target antigen peptides of >9 amino acids in length. It is therefore formally possible that HLA-B27 may exhibit a similar role in the pathogenesis of AS, by targeting unusually long peptides with subtype specificity.
For numerous variants of ERAP1, when the function of peptide processing is suppressed or disordered, the modification of peptide fragments may be deregulated and lead to an increase in the number of extended peptides or abnormal peptides. It has previously been demonstrated that mice lacking the ERAP1 enzyme exhibit disrupted presentation of peptide-MHC-I molecules, which leads to a marked shift in the hierarchy of immunodominance (31). This is followed by a 100-fold increase in the immune response to various ERAP1-sensitive viral peptides, a reduced or absent immune response to those that are ERAP1-dependent, and an unaltered response to ERAP1-independent peptides (31). Furthermore, ERAP-deficient cells present numerous unstable and structurally unique peptide-MHC complexes, which may elicit potent CD8+ T cell and B cell responses (32). An additional factor that may influence structural features of antigen peptides is TAP, as it serves a role in altering antigen peptide selection and transportation, which is the basis for further processing (33). The increase in the frequency of longer peptides for dysfunctional ERAP1 and TAP may increase the antigen peptide precursors and the frequency of downstream abnormalities, thus resulting in a greater incidence of AS. Therefore, it may be feasible to ameliorate CTL-mediated autoimmune assaults by altering epitope generation via the administration of selective proteasome inhibitors (34).
Cell surface HLA-B27 dimers and their receptors
As the low binding affinity of β2m and peptides with HLA-B27 HCs by hydrogen bonding, as well as HCs may form covalent homodimers via the α1 domain of C67, the ‘cell surface HLA-B27 homodimers’ hypothesis proposes that formation of disulphide bonds between the cysteine residue at C67 in the peptide binding groove of two separate HC molecules generates homodimers without the participation of β2m (23). It has been previously demonstrated that HLA-B27 homodimers may bind to immunoreceptors expressed on natural killer (NK) cells, myelomonotic cells or lymphocytes [killer cell immunoglobulin-like receptors (KIR) and leucocyte immunoglobulin-like receptors (LILR)], and therefore may be important in the pathogenesis of autoimmune disorders (37). A previous study revealed that patients with SpA exhibit an increased number of NK and CD4+ T cells (38). These cells express the killer cell immunoglobulin-like receptor, three Ig domains and long cytoplasmic tail 2 (KIR3DL2) receptor, which recognizes cell surface HLA-B27 homodimers (38,39). Interestingly, LILRB2 and KIR3DL2 bind to HLA-B27 dimers with a stronger affinity than HLA-B27, as well as additional conventional HLA-class I heterotrimers, and the binding of KIR3DL2 has been demonstrated to promote the survival and differentiation of inflammatory leukocytes in SpA (40,41). Enhanced proliferation and survival of KIR3DL2+ CD4+ T cells and increased IL-17 production have been observed in AS patients upon stimulation with antigen presenting cells that express HLA-B27 homodimers (38). In addition, the majority of IL-17-producing KIR3DL2+ CD4+ T cells were reported to produce tumor necrosis factor (TNF)-α, and were enriched with the production of interferon (IFN)-γ, when compared with KIR3DL2-Th17 cells. KIR3DL2-expressing CD4+ T cells account for the majority of peripheral blood CD4+ T cell IL-23 receptor expression, and produce increased IL-17 in the presence of IL-23 (39). In addition to KIR3DL2, the interaction between these cell surface HLA-B27 dimers and their receptors require further investigation.
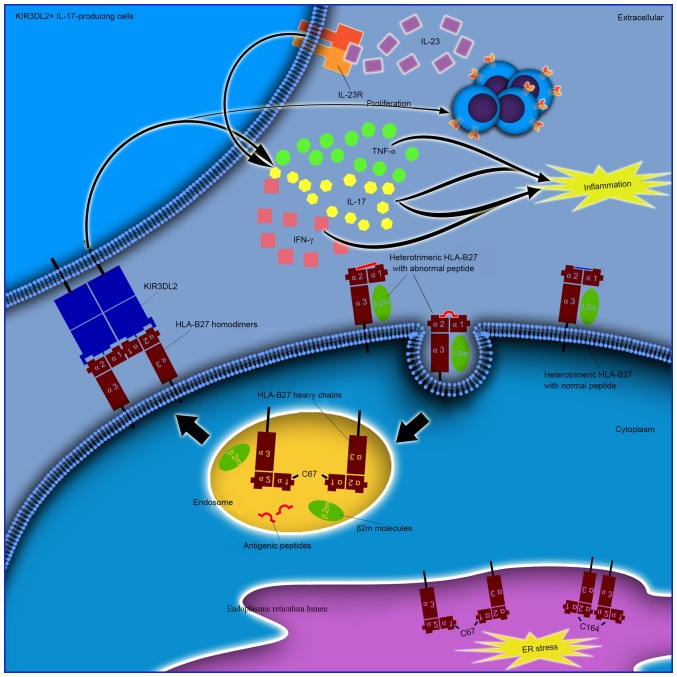
ER-resident and cell surface dimers are not associated
The cysteine at position 67 is known to be involved in cell surface and intracellular dimer formation (42). In addition, the structurally conserved cysteines at positions 101 and 164 have been demonstrated to be involved in the formation of ER-resident dimers (17). ER-resident dimers form via C67-C67 or C164-C164 disulfide bonds, however, they do not transit out of the ER (43). Cell surface dimers form following the recycling of fully-folded HLA-B27 cell surface molecules via the endocytic pathway, prior to re-expression as dimers mediated by C67-C67 interactions (25). It is important to note that intracellular dimers and cell surface dimers may represent two different mechanisms or hypotheses (Fig. 2). One potential mechanism may be that the structurally unique peptide-MHC complexes are unstable, which may lead to dissociation of heterotrimeric HLA-B27 from the cell surface and the formation of cell surface homodimers. Furthermore, the formation of these dimers from different HLA-B27 subtypes may contribute to the differential association of these alleles with AS. It has been reported that HLA-B*27:05, which is strongly associated with AS, forms a greater number of HLA-B27 dimers for KIR3DL2 compared with HLA-B*27:09 which is not associated with AS (33). Increased proportions of peripheral blood NK and CD4+ T cells that express KIR3DL2 have been demonstrated to be present in AS patients with HLA-B*27:05+, compared with healthy HLA-B*27:05+, HLA-B*27:09+ or HLA-B27- controls (44). By contrast, it is possible that HLA-B27 homodimers may affect prior to their expression on the cell surface (45). An additional subtype of MHC-I molecules, HLA-G, has been demonstrated to form homodimers in endosomes with a fully-folded β2m-associated form (21). This non-classical HLA-G subtype is considered to be the ligand for KIR2DL4 (CD158d). Unlike the other KIRs that are expressed on the surface of NK cells, KIR2DL4 resides in endosomes (46). It remains to be verified whether additional potential receptors are present in endosomes that recognize endosomal HLA-B27 dimers, and whether they demonstrate pathogenic roles in AS.
Figure 2.
Different pathogenic roles of ER-resident and cell surface HLA-B27 dimers. ER resident dimers may result in ER stress as a cellular response and lead to activation of the unfolded protein response. Cell surface dimers are reported to form following the recycling of fully-folded HLA-B27 cell surface molecules via the endocytic pathway, and re-express as dimers for presentation to immunoreceptors, including KIR and LILR. Enhanced proliferation and survival of KIR3DL2+ CD4+ T cells and increased IL-17 production in patients with AS following stimulation with antigen presenting cells expressing HLA-B27 homodimers has been previously demonstrated. The majority of these cells have been reported to produce TNF-α and IFN-γ. IL-17 has been demonstrated to synergize with TNF-α or IFN-γ to promote the release of inflammatory mediators and influence bone metabolism, thus demonstrating its important role in the pathogenesis of AS. ER, endoplasmic reticulum; HLA, human leukocyte antigen; IFN-γ, interferon-γ; IL-17, interleukin-17; KIR, killer cell immunoglobulin-like receptor; KIR3DL2, killer cell immunoglobulin-like receptor three domains long cytoplasmic tail 2; LILR, leucocyte immunoglobulin-like receptor; TNF-α, tumor necrosis factor-α; AS, ankylosing spondylitis.
Misfolded forms with or without the unfolded protein response (UPR)
The majority of the disease-associated forms of HLA-B27 molecules (HLA-B*27:05, HLA-B*27:04 and HLA-B*27:02) demonstrate a reduced rate of folding when compared with HLA-B*27:06 and HLA-B*27:09 and the majority of other MHC-I molecules, which are not associated with AS (38,47). The increase in duration for the disease-associated HLA-B27 molecules to assemble appears to subsequently lead to the accumulation of misfolded HLA-B27 molecules in the ER; a proportion of which may be in the form of dimers. The accumulation of misfolded or unfolded proteins may perturb ER function and result in ER stress and activation of the UPR, in an attempt to rescue or dispose of the burden of misfolded proteins (48,49). Furthermore, IL-23p19 (the unique subunit of the active IL-23 cytokine) was revealed to be synergistically upregulated by lipopolysaccharide (LPS) in macrophages undergoing a UPR in an HLA-B27 transgenic rat model of spondyloarthropathy (50). Furthermore, IL-23p19 was increased in the colon of these rats, which was concurrent with the development of intestinal inflammation. IL-17 exhibited robust upregulation in a similar temporal pattern, with an expansion of IL-17-expressing CD4+ T cells (50). However, increased production of IL-23 in response to LPS without induction of significant UPR has been reported in AS macrophages (51). Ciccia et al (52) reported that HLA-B27 misfolding occurs in the gut of patients with AS, and is accompanied by activation of autophagy rather than a UPR. However, Neerinckx et al (53) failed to demonstrate any significant increase in the expression of synovium autophagy-associated genes by reverse transcription-polymerase chain reaction, and no significant overexpression of IL-23p19 was observed when compared with disease and healthy controls. IL-23p19 has been previously demonstrated, to be overexpressed in the inflamed tissues of patients with AS (such as the gut and zygapophysial joints) as determined by immunohistochemical analysis (54,55). IL-23p19 may exhibit a tissue-specific role in the gut and/or in the lymph nodes, by priming specific subsets of IL-23-responsive proinflammatory cells. A previous study investigated the hypothesis that ERAP1-mediated HLA-B27 misfolding increases ER stress and induces an IL-23-dependent, pro-inflammatory immune response (56). It was demonstrated that disease-associated polymorphisms in the ERAP1 and HLA-B27 genes do not alter ER-stress levels in AS (56). Therefore, it remains to be elucidated whether ER-resident misfolded HLA-B27 molecules are associated with the UPR and exhibit pathogenic roles in AS.
Exosomal fully-folded MHC I dimers
The redox-induced dimers in exosomes are fully-folded and are independent of the cysteine residues at positions 67 and 308. However these dimers are critically dependent on cysteine 325 in the cytoplasmic tail (21). It has been suggested that they are redox-induced due to the relative absence of the reducing agent glutathione in exosomes, which contrasts with the low millimolar levels normally observed in the cell cytoplasm (43). Considering that exosomes will be released as extracellular vesicles, they may represent an important mode of intercellular communication (20,57). Therefore, the exosomal fully-folded MHC I dimers may transfer signals to the resident cells in entheses to induce inflammation, which may lead to alterations in joint architecture and new bone formation.
Additional HLA-B27 hypotheses
β2m-free, peptide-free HCs support a helix-coil transition facilitating rotation of backbone angles around amino acid 167/168, thus leading to the residues 169–181 (identical to a known HLA-B27 ligand) to loop around and occupy the molecule's own peptide binding cleft. This ‘auto-display’, that occurs within or between HLA-B27 molecules, may induce an autoimmune disease and be important in the pathogenesis of AS (58).
Upon dissociation of the HLA-B27 dimers, β2m may accumulate and become trapped in the synovia, where they may bind to collagen and form amyloid deposits or interact with synovial fibroblasts, thereby inducing the synthesis and secretion of proteins involved in tissue destruction, finally resulting in AS (59). This is termed the ‘β2m deposition’ hypothesis. It was further hypothesized that β2m expression levels may be associated with AS pathogenesis, as spondylitis was successfully induced in a novel HLA-B27/β2m transgenic rat model expressing increased levels of β2m (60). This lead to the proposed ‘β2m over-expression’ hypothesis (60).
4. Discussion
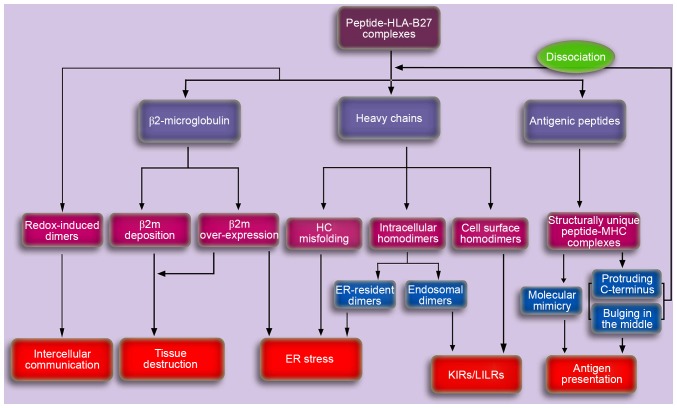
According to the results of previous studies discussed in the present review, it is possible that the onset of AS may result from aberrant peptide presentation (11,12), misfolded HLA-B27 molecules (16), HLA-B27 dimers (17,19) or β2m accumulation and deposition (6,23) (Fig. 3). Previous studies have demonstrated the involvement of the IL-23/IL-17 axis in the pathogenesis of AS (61,62). However, aberrant recognition and cytokine dysregulation may not be two independent procedures. Aberrant recognition by specific immunoreceptors may occur in upstream pathways, which may subsequently contribute to downstream cytokine dysregulation, particularly in the IL-23/IL-17 axis.
Figure 3.
A schematic depicting the potential pathogenesis of AS caused by HLA-B27. Aberrant processing and presentation of structurally unique peptides were initially proposed to explain the potential pathogenesis of AS. Cell surface HLA-B27 dimers may be recognized by various immunoreceptors and may be important in the pathogenesis of autoimmune disorders. Accumulation of proteins in the ER, including ER-resident dimers, misfolded HCs and β2m may result in the ER stress response, thereby activating the unfolded protein response, which is associated with cytokine dysregulation. In addition, the accumulating β2m in synovia for dissociation and/or overexpression, may induce the synthesis and secretion of proteins involved in tissue destruction, thus leading to AS. The exosomal fully-folded HLA-B27 dimers may be important in the pathogenesis of AS via intercellular communication. AS, ankylosing spondylitis; HLA, human leukocyte antigen; HC, heavy chain; KIR, killer cell immunoglobulin-like receptor; LILR, leucocyte immunoglobulin-like receptor; ER, endoplasmic reticulum; MHC, major histocompatibility complex; β2m, β2microglobulin.
Currently, three major mechanistic hypotheses exist to describe the association between HLA-B27 and AS. Firstly, aberrant peptide processing and presentation may be involved in the pathogenesis of AS due to the interaction between HLA-B27 and ERAP1 (11,12). However, the molecular mechanisms underlying this process remain to be fully elucidated. Secondly, misfolded HLA-B27 molecules in the ER may trigger ER stress and provoke the UPR (16). It has been previously demonstrated that this is followed by the subsequent upregulation of various cytokines, particularly IL-23 and IL-17, accompanied by the development of immune dysregulation. However, macrophages from AS patients exhibited greater IL-23 production in response to LPS, and no significant UPR induction was observed. The induction of the UPR is dependent on the magnitude and duration of ER stress, as well as the type of cells that are affected. Further studies are required to determine whether cells process misfolded monomers, intracellular HLA-B27 homodimers or β2m in the ER differently, and whether they may be associated with the UPR and further cytokine dysregulation. In addition, further studies are required to reassess the cellular source of IL-17 in the primary target tissues of AS, including γδ T cells, mast cells, neutrophils or innate lymphoid cells that have been implicated in previous studies (3). Furthermore, cell surface HLA-B27 dimers may be important in AS pathogenesis, due to their role in binding to receptors on immune cells (17,19). The recognition of HLA-B27 dimers by KIR3DL2 is reportedly associated with KIR3DL2+ IL-17-producing CD4+ T cells, IL-23 receptor expression and the production of IL-17, TNF-α and IFN-γ. Future studies investigating the intrinsic association between the pathogenic role of HLA-B27 and the IL-23/IL-17 axis may provide novel insights into understanding the molecular mechanisms involved in the development and progression of AS.
Acknowledgements
The present review was supported by grant-in-aid for scientific research from the National Natural Science Foundation of China (grant no. 81171686) and the Natural Science Foundation of Shanghai (grant no. 14140903802).
References
- 1.Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011;377:2127–2137. doi: 10.1016/S0140-6736(11)60071-8. [DOI] [PubMed] [Google Scholar]
- 2.Cho H, Kim T, Kim TH, Lee S, Lee KH. Spinal mobility, vertebral squaring, pulmonary function, pain, fatigue, and quality of life in patients with ankylosing spondylitis. Ann Rehabil Med. 2013;37:675–682. doi: 10.5535/arm.2013.37.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–1390. doi: 10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- 4.Végvári A, Szabó Z, Szántó S, Glant TT, Mikecz K, Szekanecz Z. The genetic background of ankylosing spondylitis. Joint Bone Spine. 2009;76:623–628. doi: 10.1016/j.jbspin.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DC, Sturrock RD. Ankylosing spondylitis and HL-A 27. Lancet. 1973;1:904–907. doi: 10.1016/S0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- 6.Chatzikyriakidou A, Voulgari PV, Drosos AA. What is the role of HLA-B27 in spondyloarthropathies? Autoimmun Rev. 2011;10:464–468. doi: 10.1016/j.autrev.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 7.International Genetics of Ankylosing Spondylitis Consortium (IGAS) Cortes A, Hadler J, Pointon JP, Robinson PC, Karaderi T, Leo P, Cremin K, Pryce K, Harris J, et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet. 2013;45:730–738. doi: 10.1038/ng.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheehan NJ. HLA-B27: What's new? Rheumatology (Oxford) 2010;49:621–631. doi: 10.1093/rheumatology/kep450. [DOI] [PubMed] [Google Scholar]
- 9.Khan MA. Polymorphism of HLA-B27: 105 subtypes currently known. Curr Rheumatol Rep. 2013;15:362. doi: 10.1007/s11926-013-0362-y. [DOI] [PubMed] [Google Scholar]
- 10.Brown MA. Progress in the genetics of ankylosing spondylitis. Brief Funct Genomics. 2011;10:249–257. doi: 10.1093/bfgp/elr023. [DOI] [PubMed] [Google Scholar]
- 11.Warde N. Spondyloarthropathies: HLA-B27 and ERAP1 contribute to ankylosing spondylitis via aberrant peptide processing and presentation. Nat Rev Rheumatol. 2011;7:498. doi: 10.1038/nrrheum.2011.112. [DOI] [PubMed] [Google Scholar]
- 12.Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, Kochan G, Oppermann U, Dilthey A, Pirinen M, Stone MA, et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet. 2011;43:761–767. doi: 10.1038/ng.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen TT, Chang SC, Evnouchidou I, York IA, Zikos C, Rock KL, Goldberg AL, Stratikos E, Stern LJ. Structural basis for antigenic peptide precursor processing by the endoplasmic reticulum aminopeptidase ERAP1. Nat Struct Mol Biol. 2011;18:604–613. doi: 10.1038/nsmb.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yewdell JW. DRiPs solidify: Progress in understanding endogenous MHC class I antigen processing. Trends Immunol. 2011;32:548–558. doi: 10.1016/j.it.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madden DR. The three-dimensional structure of peptide-MHC complexes. Annu Rev Immunol. 1995;13:587–622. doi: 10.1146/annurev.iy.13.040195.003103. [DOI] [PubMed] [Google Scholar]
- 16.Colbert RA, Tran TM, Layh-Schmitt G. HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol. 2014;57:44–51. doi: 10.1016/j.molimm.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenart I, Guiliano DB, Burn G, Campbell EC, Morley KD, Fussell H, Powis SJ, Antoniou AN. The MHC Class I heavy chain structurally conserved cysteines 101 and 164 participate in HLA-B27 dimer formation. Antioxid Redox Signal. 2012;16:33–43. doi: 10.1089/ars.2010.3693. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez-Navarro C, López de Castro JA. ERAP1 structure, function and pathogenetic role in ankylosing spondylitis and other MHC-associated diseases. Mol Immunol. 2014;57:12–21. doi: 10.1016/j.molimm.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Colbert RA. The immunobiology of HLA-B27: Variations on a theme. Curr Mol Med. 2004;4:21–30. doi: 10.2174/1566524043479293. [DOI] [PubMed] [Google Scholar]
- 20.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch S, Santos SG, Campbell EC, Nimmo AM, Botting C, Prescott A, Antoniou AN, Powis SJ. Novel MHC class I structures on exosomes. J Immunol. 2009;183:1884–1891. doi: 10.4049/jimmunol.0900798. [DOI] [PubMed] [Google Scholar]
- 22.Lorente E, Infantes S, Abia D, Barnea E, Beer I, García R, Lasala F, Jiménez M, Mir C, Morreale A, et al. A viral, transporter associated with antigen processing (TAP)-independent, high affinity ligand with alternative interactions endogenously presented by the nonclassical human leukocyte antigen E class I molecule. J Biol Chem. 2012;287:34895–34903. doi: 10.1074/jbc.M111.314856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen B, Li D, Xu W. Association of ankylosing spondylitis with HLA-B27 and ERAP1: Pathogenic role of antigenic peptide. Med Hypotheses. 2013;80:36–38. doi: 10.1016/j.mehy.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Lévy F, Burri L, Morel S, Peitrequin AL, Lévy N, Bachi A, Hellman U, Van den Eynde BJ, Servis C. The final N-terminal trimming of a subaminoterminal proline-containing HLA class I-restricted antigenic peptide in the cytosol is mediated by two peptidases. J Immunol. 2002;169:4161–4171. doi: 10.4049/jimmunol.169.8.4161. [DOI] [PubMed] [Google Scholar]
- 25.Antoniou AN, Lenart I, Guiliano DB. Pathogenicity of misfolded and dimeric HLA-B27 molecules. Int J Rheumatol. 2011;2011:486856. doi: 10.1155/2011/486856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dakwar E, Reddy J, Vale FL, Uribe JS. A review of the pathogenesis of ankylosing spondylitis. Neurosurg Focus. 2008;24:E2. doi: 10.3171/FOC/2008/24/1/E2. [DOI] [PubMed] [Google Scholar]
- 27.Taurog JD, Dorris ML, Satumtira N, Tran TM, Sharma R, Dressel R, van den Brandt J, Reichardt HM. Spondylarthritis in HLA-B27/human beta2-microglobulin-transgenic rats is not prevented by lack of CD8. Arthritis Rheum. 2009;60:1977–1984. doi: 10.1002/art.24599. [DOI] [PubMed] [Google Scholar]
- 28.Collins EJ, Garboczi DN, Wiley DC. Three-dimensional structure of a peptide extending from one end of a class I MHC binding site. Nature. 1994;371:626–629. doi: 10.1038/371626a0. [DOI] [PubMed] [Google Scholar]
- 29.Probst-Kepper M, Hecht HJ, Herrmann H, Janke V, Ocklenburg F, Klempnauer J, van den Eynde BJ, Weiss S. Conformational restraints and flexibility of 14-meric peptides in complex with HLA-B*3501. J Immunol. 2004;173:5610–5616. doi: 10.4049/jimmunol.173.9.5610. [DOI] [PubMed] [Google Scholar]
- 30.Green KJ, Miles JJ, Tellam J, van Zuylen WJ, Connolly G, Burrows SR. Potent T cell response to a class I-binding 13-mer viral epitope and the influence of HLA micropolymorphism in controlling epitope length. Eur J Immunol. 2004;34:2510–2519. doi: 10.1002/eji.200425193. [DOI] [PubMed] [Google Scholar]
- 31.York IA, Brehm MA, Zendzian S, Towne CF, Rock KL. Endoplasmic reticulum aminopeptidase 1 (ERAP1) trims MHC class I-present edpeptides in vivo and plays an important role in immunodominance. Proc Natl Acad Sci USA. 2006;103:9202–9207. doi: 10.1073/pnas.0603095103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammer GE, Gonzalez F, James E, Nolla H, Shastri N. In the absence of aminopeptidase ERAAP, MHC class I molecules present many unstable and highly immunogenic peptides. Nat Immunol. 2007;8:101–108. doi: 10.1038/ni1409. [DOI] [PubMed] [Google Scholar]
- 33.Lorente E, García R, Mir C, Barriga A, Lemonnier FA, Ramos M, López D. Role of metalloproteases in vaccinia virus epitope processing for transporter associated with antigen processing (TAP)-independent human leukocyte antigen (HLA)-B7 class I antigen presentation. J Biol Chem. 2012;287:9990–10000. doi: 10.1074/jbc.M111.314856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarz K, De Giuli R, Schmidtke G, Kostka S, van den Broek M, Kim KB, Crews CM, Kraft R, Groettrup M. The selective proteasome inhibitors lactacystin and epoxomicin can be used to either up- or down-regulate antigen presentation at nontoxic doses. J Immunol. 2000;164:6147–6157. doi: 10.4049/jimmunol.164.12.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen RL, Trowsdale J. Recognition of classical and heavy chain forms of HLA-B27 by leukocyte receptors. Curr Mol Med. 2004;4:59–65. doi: 10.2174/1566524043479329. [DOI] [PubMed] [Google Scholar]
- 36.Kollnberger S, Chan A, Sun MY, Chen LY, Wright C, di Gleria K, McMichael A, Bowness P. Interaction of HLA-B27 homodimers with KIR3DL1 and KIR3DL2, unlike HLA-B27 heterotrimers, is independent of the sequence of bound peptide. Eur J Immunol. 2007;37:1313–1322. doi: 10.1002/eji.200635997. [DOI] [PubMed] [Google Scholar]
- 37.Allen RL, Raine T, Haude A, Trowsdale J, Wilson MJ. Leukocyte receptor complex-encoded immunomodulatory receptors show differing specificity for alternative HLA-B27 structures. J Immunol. 2001;167:5543–5547. doi: 10.4049/jimmunol.167.10.5543. [DOI] [PubMed] [Google Scholar]
- 38.Chan AT, Kollnberger SD, Wedderburn LR, Bowness P. Expansion and enhanced survival of natural killer cells expressing the killer immunoglobulin-like receptor KIR3DL2 in spondylarthritis. Arthritis Rheum. 2005;52:3586–3595. doi: 10.1002/art.21395. [DOI] [PubMed] [Google Scholar]
- 39.Bowness P, Ridley A, Shaw J, Chan AT, Wong-Baeza I, Fleming M, Cummings F, McMichael A, Kollnberger S. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol. 2011;186:2672–2680. doi: 10.4049/jimmunol.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giles J, Shaw J, Piper C, Wong-Baeza I, McHugh K, Ridley A, Li D, Lenart I, Antoniou AN, DiGleria K, et al. HLA-B27 homodimers and free H chains are stronger ligands for leukocyte Ig-like receptor B2 than classical HLA class I. J Immunol. 2012;188:6184–6193. doi: 10.4049/jimmunol.1102711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong-Baeza I, Ridley A, Shaw J, Hatano H, Rysnik O, McHugh K, Piper C, Brackenbridge S, Fernandes R, Chan A, et al. KIR3DL2 binds to HLA-B27 dimers and free H chains more strongly than other HLA class I and promotes the expansion of T cells in ankylosing spondylitis. J Immunol. 2013;190:3216–3224. doi: 10.4049/jimmunol.1202926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dangoria NS, DeLay ML, Kingsbury DJ, Mear JP, Uchanska-Ziegler B, Ziegler A, Colbert RA. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J Biol Chem. 2002;277:23459–23468. doi: 10.1074/jbc.M110336200. [DOI] [PubMed] [Google Scholar]
- 43.Campbell EC, Antoniou AN, Powis SJ. The multi-faceted nature of HLA class I dimer molecules. Immunology. 2012;136:380–384. doi: 10.1111/j.1365-2567.2012.03593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cauli A, Shaw J, Giles J, Hatano H, Rysnik O, Payeli S, McHugh K, Dessole G, Porru G, Desogus E, et al. The arthritis-associated HLA-B*27:05 allele forms more cell surface B27 dimer and free heavy chain ligands for KIR3DL2 than HLA-B*27:09. Rheumatology (Oxford) 2013;52:1952–1962. doi: 10.1093/rheumatology/ket219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuśnierczyk P, Majorczyk E. Pas de quatre: An interaction of HLA-B*27:05 and KIR3DL2 homodimers in spondyloarthropathies. Rheumatology (Oxford) 2013;52:1931–1912. doi: 10.1093/rheumatology/ket268. [DOI] [PubMed] [Google Scholar]
- 46.Rajagopalan S, Long EO. KIR2DL4 (CD158d): An activation receptor for HLA-G. Front Immunol. 2012;3:258. doi: 10.3389/fimmu.2012.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antoniou AN, Ford S, Taurog JD, Butcher GW, Powis SJ. Formation of HLA-B27 homodimers and their relationship to assembly kinetics. J Biol Chem. 2004;279:8895–8902. doi: 10.1074/jbc.M311757200. [DOI] [PubMed] [Google Scholar]
- 48.Colbert RA, DeLay ML, Layh-Schmitt G, Sowders DP. HLA-B27 misfolding and spondyloarthropathies. Prion. 2009;3:15–26. doi: 10.4161/pri.3.1.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turner MJ, Sowders DP, DeLay ML, Mohapatra R, Bai S, Smith JA, Brandewie JR, Taurog JD, Colbert RA. HLA-B27 misfolding in transgenic rats is associated with activation of the unfolded protein response. J Immunol. 2005;175:2438–2348. doi: 10.4049/jimmunol.175.4.2438. [DOI] [PubMed] [Google Scholar]
- 50.DeLay ML, Turner MJ, Klenk EI, Smith JA, Sowders DP, Colbert RA. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009;60:2633–2643. doi: 10.1002/art.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng L, Lindstrom MJ, Smith JA. Ankylosing spondylitis macrophage production of higher levels of interleukin-23 in response to lipopolysaccharide without induction of a significant unfolded protein response. Arthritis Rheum. 2011;63:3807–3817. doi: 10.1002/art.30593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciccia F, Accardo-Palumbo A, Rizzo A, Guggino G, Raimondo S, Giardina A, Cannizzaro A, Colbert RA, Alessandro R, Triolo G. Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation. Ann Rheum Dis. 2014;73:1566–1574. doi: 10.1136/annrheumdis-2012-202925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neerinckx B, Carter S, Lories R. IL-23 expression and activation of autophagy in synovium and PBMCs of HLA-B27 positive patients with ankylosing spondylitis. Response to: ‘Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation’ by Ciccia. Ann Rheum Dis. 2014;73:e68. doi: 10.1136/annrheumdis-2014-206277. [DOI] [PubMed] [Google Scholar]
- 54.Ciccia F, Bombardieri M, Principato A, Giardina A, Tripodo C, Porcasi R, Peralta S, Franco V, Giardina E, Craxi A, et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 2009;60:955–965. doi: 10.1002/art.24389. [DOI] [PubMed] [Google Scholar]
- 55.Appel H, Maier R, Bleil J, Hempfing A, Loddenkemper C, Schlichting U, Syrbe U, Sieper J. In situ analysis of interleukin-23- and interleukin-12-positive cells in the spine of patients with ankylosing spondylitis. Arthritis Rheum. 2013;65:1522–1529. doi: 10.1002/art.37937. [DOI] [PubMed] [Google Scholar]
- 56.Kenna TJ, Lau MC, Keith P, Ciccia F, Costello ME, Bradbury L, Low PL, Agrawal N, Triolo G, Alessandro R, et al. Disease-associated polymorphisms in ERAP1 do not alter endoplasmic reticulum stress in patients with ankylosing spondylitis. Genes Immun. 2015;16:35–42. doi: 10.1038/gene.2014.62. [DOI] [PubMed] [Google Scholar]
- 57.Shaw J, Hatano H, Kollnberger S. The biochemistry and immunology of non-canonical forms of HLA-B27. Mol Immunol. 2014;57:52–58. doi: 10.1016/j.molimm.2013.05.243. [DOI] [PubMed] [Google Scholar]
- 58.Luthra-Guptasarma M, Singh B. HLA-B27 lacking associated beta2-microglobulin rearranges to auto-display or cross-display residues 169–181: A novel molecular mechanism for spondyloarthropathies. FEBS Lett. 2004;575:1–8. doi: 10.1016/j.febslet.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 59.Uchanska-Ziegler B, Ziegler A. Ankylosing spondylitis: A beta2m-deposition disease? Trends Immunol. 2003;24:73–76. doi: 10.1016/S1471-4906(03)00078-4. [DOI] [PubMed] [Google Scholar]
- 60.Tran TM, Dorris ML, Satumtira N, Richardson JA, Hammer RE, Shang J, Taurog JD. Additional human beta2-microglobulin curbs HLA-B27 misfolding and promotes arthritis and spondylitis without colitis in male HLA-B27-transgenic rats. Arthritis Rheum. 2006;54:1317–1327. doi: 10.1002/art.21740. [DOI] [PubMed] [Google Scholar]
- 61.Yeremenko N, Paramarta JE, Baeten D. The interleukin-23/interleukin-17 immune axis as a promising new target in the treatment of spondyloarthritis. Curr Opin Rheumatol. 2014;26:361–370. doi: 10.1097/BOR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 62.Jethwa H, Bowness P. The interleukin (IL)-23/IL-17 axis in ankylosing spondylitis: New advances and potentials for treatment. Clin Exp Immunol. 2016;183:30–36. doi: 10.1111/cei.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]