Abstract
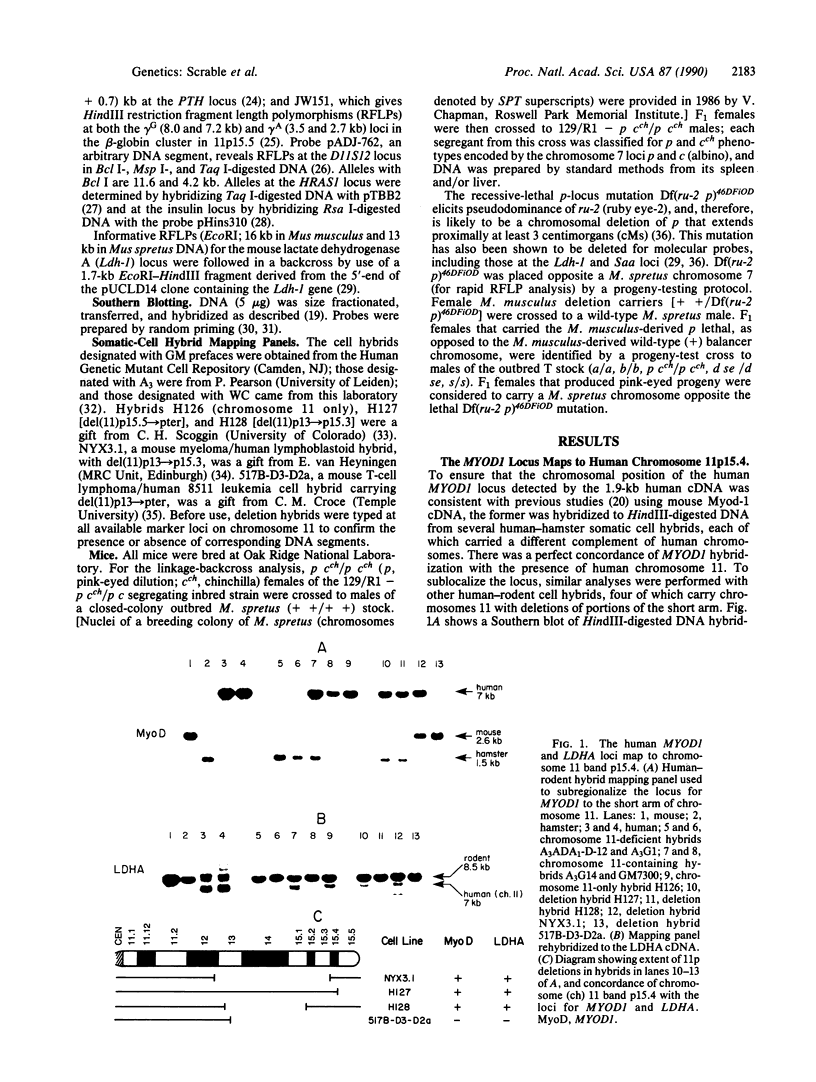
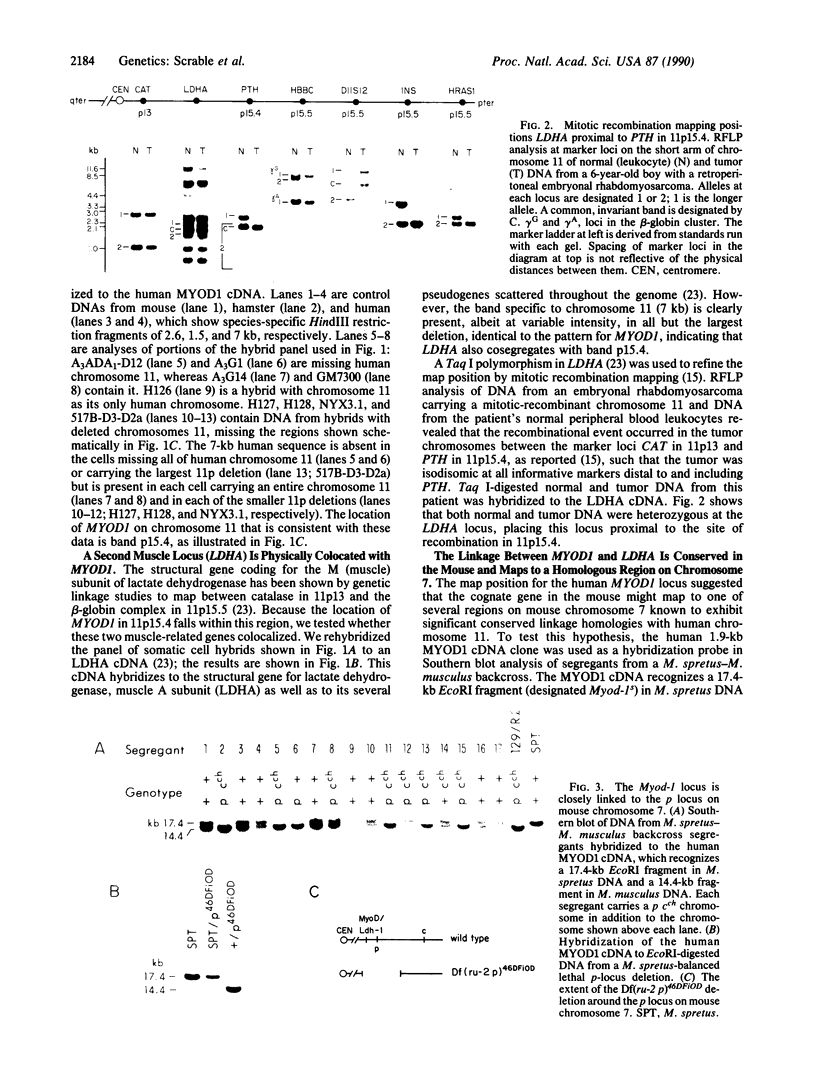
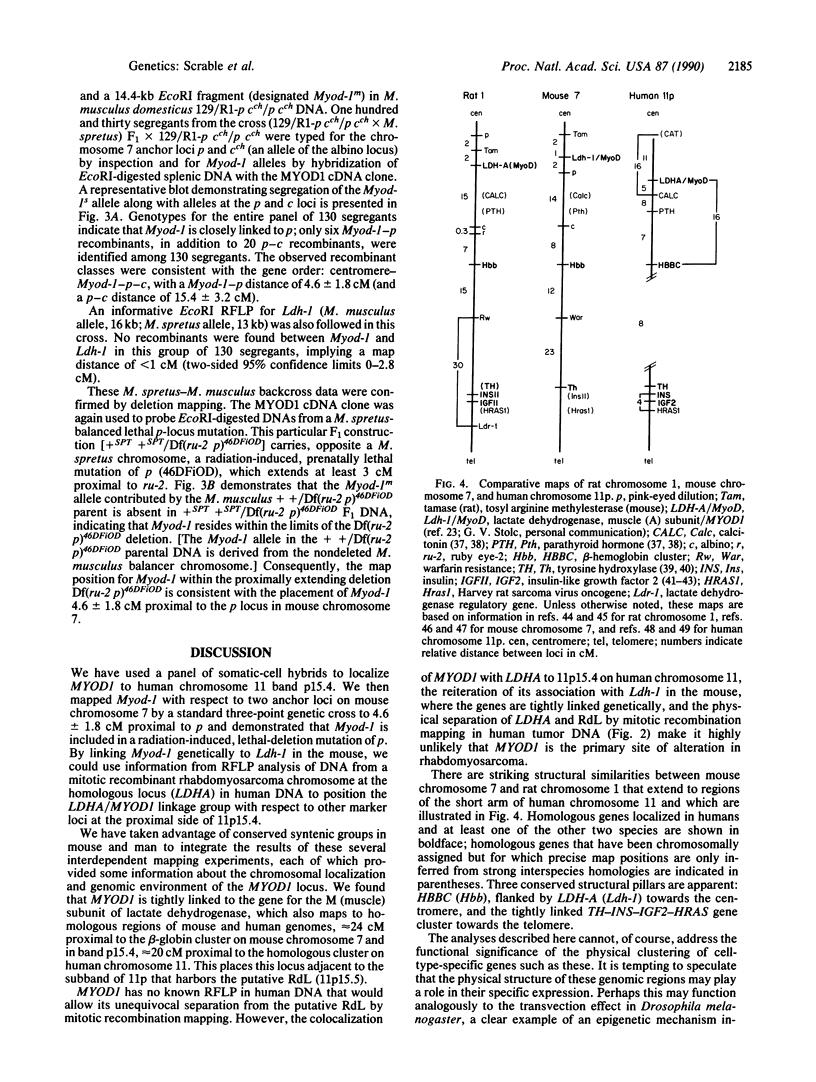
The MYOD1 locus is preferentially expressed in skeletal muscle and at higher levels in its related neoplasm, rhabdomyosarcoma. We have combined physical mapping of the human locus with meiotic and physical mapping in the mouse, together with synteny homologies between the two species, to compare the physical relationship between MYOD1 and the genetically ascertained human rhabdomyosarcoma-associated locus. We have determined that the myogenic differentiation gene is tightly linked to the structural gene for the M (muscle) subunit of lactate dehydrogenase in band p15.4 on human chromosome 11 and close to the p and Ldh-1 loci in the homologous region of mouse chromosome 7. Because the rhabdomyosarcoma locus maps to 11p15.5, MYOD1 is very unlikely to be the primary site of alteration in these tumors. Further, these analyses identify two syntenic clusters of muscle-associated genes on the short arm of human chromosome 11, one in the region of rhabdomyosarcoma locus that includes IGF2 and TH and the second the tightly linked MYOD1 and LDHA loci, which have been evolutionarily conserved in homologous regions of both the mouse and the rat genomes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonarakis S. E., Boehm C. D., Giardina P. J., Kazazian H. H., Jr Nonrandom association of polymorphic restriction sites in the beta-globin gene cluster. Proc Natl Acad Sci U S A. 1982 Jan;79(1):137–141. doi: 10.1073/pnas.79.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban G., Gilbert F., Nichols W., Meadows A. T., Shields J. Abnormalities of chromosome #13 in retinoblastomas from individuals with normal constitutional karyotypes. Cancer Genet Cytogenet. 1982 Jul;6(3):213–221. doi: 10.1016/0165-4608(82)90058-9. [DOI] [PubMed] [Google Scholar]
- Barker D., Holm T., White R. A locus on chromosome 11p with multiple restriction site polymorphisms. Am J Hum Genet. 1984 Nov;36(6):1159–1171. [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Gerhard D. S., Fong N. M., Sanchez-Pescador R., Rall L. B. Isolation of the human insulin-like growth factor genes: insulin-like growth factor II and insulin genes are contiguous. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6450–6454. doi: 10.1073/pnas.82.19.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilliant M. H., Niemann M. M., Eicher E. M. Murine tyrosine hydroxylase maps to the distal end of chromosome 7 within a region conserved in mouse and man. J Neurogenet. 1987 Aug;4(5):259–266. [PubMed] [Google Scholar]
- Butcher G. W., Clarke S., Tucker E. M. Close linkage of peripheral T-lymphocyte antigen A (PtaA) to the hemoglobin variant Hbb on linkage group I of the rat. Transplant Proc. 1979 Sep;11(3):1629–1630. [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989 May;3(5):628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- Erikson J., Williams D. L., Finan J., Nowell P. C., Croce C. M. Locus of the alpha-chain of the T-cell receptor is split by chromosome translocation in T-cell leukemias. Science. 1985 Aug 23;229(4715):784–786. doi: 10.1126/science.3875152. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher J. H., Miller Y. E., Sparkes R. S., Bateman J. B., Kimmel K. A., Carey T. E., Rodell T., Shoemaker S. A., Scoggin C. H. Wilms' tumor-aniridia association: segregation of affected chromosome in somatic cell hybrids, identification of cell surface antigen associated with deleted area, and regional mapping of c-Ha-ras-1 oncogene, insulin gene, and beta-globin gene. Somat Cell Mol Genet. 1984 Sep;10(5):455–464. doi: 10.1007/BF01534850. [DOI] [PubMed] [Google Scholar]
- Francke U. Retinoblastoma and chromosome 13. Cytogenet Cell Genet. 1976;16(1-5):131–134. doi: 10.1159/000130573. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Goldfarb M., Shimizu K., Perucho M., Wigler M. Isolation and preliminary characterization of a human transforming gene from T24 bladder carcinoma cells. Nature. 1982 Apr 1;296(5856):404–409. doi: 10.1038/296404a0. [DOI] [PubMed] [Google Scholar]
- Griffin C. A., Emanuel B. S., Hansen J. R., Cavenee W. K., Myers J. C. Human collagen genes encoding basement membrane alpha 1 (IV) and alpha 2 (IV) chains map to the distal long arm of chromosome 13. Proc Natl Acad Sci U S A. 1987 Jan;84(2):512–516. doi: 10.1073/pnas.84.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R. Chromosome error propagation and cancer. Trends Genet. 1989 Feb;5(2):42–45. doi: 10.1016/0168-9525(89)90020-6. [DOI] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Kittur S. D., Hoppener J. W., Antonarakis S. E., Daniels J. D., Meyers D. A., Maestri N. E., Jansen M., Korneluk R. G., Nelkin B. D., Kazazian H. H., Jr Linkage map of the short arm of human chromosome 11: location of the genes for catalase, calcitonin, and insulin-like growth factor II. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5064–5067. doi: 10.1073/pnas.82.15.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G. The approaching era of the tumor suppressor genes. Science. 1987 Dec 11;238(4833):1539–1545. doi: 10.1126/science.3317834. [DOI] [PubMed] [Google Scholar]
- Koufos A., Grundy P., Morgan K., Aleck K. A., Hadro T., Lampkin B. C., Kalbakji A., Cavenee W. K. Familial Wiedemann-Beckwith syndrome and a second Wilms tumor locus both map to 11p15.5. Am J Hum Genet. 1989 May;44(5):711–719. [PMC free article] [PubMed] [Google Scholar]
- LEWIS E. B. The phenomenon of position effect. Adv Genet. 1950;3:73–115. doi: 10.1016/s0065-2660(08)60083-8. [DOI] [PubMed] [Google Scholar]
- Lalley P. A., Sakaguchi A. Y., Eddy R. L., Honey N. H., Bell G. I., Shen L. P., Rutter W. J., Jacobs J. W., Heinrich G., Chin W. W. Mapping polypeptide hormone genes in the mouse: somatostatin, glucagon, calcitonin, and parathyroid hormone. Cytogenet Cell Genet. 1987;44(2-3):92–97. doi: 10.1159/000132350. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- O'Malley K. L., Rotwein P. Human tyrosine hydroxylase and insulin genes are contiguous on chromosome 11. Nucleic Acids Res. 1988 May 25;16(10):4437–4446. [PMC free article] [PubMed] [Google Scholar]
- Ping A. J., Reeve A. E., Law D. J., Young M. R., Boehnke M., Feinberg A. P. Genetic linkage of Beckwith-Wiedemann syndrome to 11p15. Am J Hum Genet. 1989 May;44(5):720–723. [PMC free article] [PubMed] [Google Scholar]
- Porteous D. J., Bickmore W., Christie S., Boyd P. A., Cranston G., Fletcher J. M., Gosden J. R., Rout D., Seawright A., Simola K. O. HRAS1-selected chromosome transfer generates markers that colocalize aniridia- and genitourinary dysplasia-associated translocation breakpoints and the Wilms tumor gene within band 11p13. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5355–5359. doi: 10.1073/pnas.84.15.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke J., Pape B., Krengel U., Langenbeck U., Cooper D. N., Breyel E., Mayer H. Restriction fragment length polymorphisms at the human parathyroid hormone gene locus. Hum Genet. 1984;67(4):428–431. doi: 10.1007/BF00291404. [DOI] [PubMed] [Google Scholar]
- Scrable H. J., Witte D. P., Lampkin B. C., Cavenee W. K. Chromosomal localization of the human rhabdomyosarcoma locus by mitotic recombination mapping. Nature. 1987 Oct 15;329(6140):645–647. doi: 10.1038/329645a0. [DOI] [PubMed] [Google Scholar]
- Scrable H., Cavenee W., Ghavimi F., Lovell M., Morgan K., Sapienza C. A model for embryonal rhabdomyosarcoma tumorigenesis that involves genome imprinting. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7480–7484. doi: 10.1073/pnas.86.19.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrable H., Witte D., Shimada H., Seemayer T., Sheng W. W., Soukup S., Koufos A., Houghton P., Lampkin B., Cavenee W. Molecular differential pathology of rhabdomyosarcoma. Genes Chromosomes Cancer. 1989 Sep;1(1):23–35. doi: 10.1002/gcc.2870010106. [DOI] [PubMed] [Google Scholar]
- Soares M. B., Schon E., Henderson A., Karathanasis S. K., Cate R., Zeitlin S., Chirgwin J., Efstratiadis A. RNA-mediated gene duplication: the rat preproinsulin I gene is a functional retroposon. Mol Cell Biol. 1985 Aug;5(8):2090–2103. doi: 10.1128/mcb.5.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares M. B., Turken A., Ishii D., Mills L., Episkopou V., Cotter S., Zeitlin S., Efstratiadis A. Rat insulin-like growth factor II gene. A single gene with two promoters expressing a multitranscript family. J Mol Biol. 1986 Dec 20;192(4):737–752. doi: 10.1016/0022-2836(86)90025-2. [DOI] [PubMed] [Google Scholar]
- Sotelo-Avila C., Gooch W. M., 3rd Neoplasms associated with the Beckwith-Wiedemann syndrome. Perspect Pediatr Pathol. 1976;3:255–272. [PubMed] [Google Scholar]
- Tapscott S. J., Davis R. L., Thayer M. J., Cheng P. F., Weintraub H., Lassar A. B. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988 Oct 21;242(4877):405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Todd S., Yoshida M. C., Fang X. E., McDonald L., Jacobs J., Heinrich G., Bell G. I., Naylor S. L., Sakaguchi A. Y. Genes for insulin I and II, parathyroid hormone, and calcitonin are on rat chromosome 1. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1175–1180. doi: 10.1016/0006-291x(85)90214-1. [DOI] [PubMed] [Google Scholar]
- Turleau C., de Grouchy J., Chavin-Colin F., Martelli H., Voyer M., Charlas R. Trisomy 11p15 and Beckwith-Wiedemann syndrome. A report of two cases. Hum Genet. 1984;67(2):219–221. doi: 10.1007/BF00273006. [DOI] [PubMed] [Google Scholar]
- Waziri M., Patil S. R., Hanson J. W., Bartley J. A. Abnormality of chromosome 11 in patients with features of Beckwith-Wiedemann syndrome. J Pediatr. 1983 Jun;102(6):873–876. doi: 10.1016/s0022-3476(83)80014-6. [DOI] [PubMed] [Google Scholar]
- White R., Leppert M., Bishop D. T., Barker D., Berkowitz J., Brown C., Callahan P., Holm T., Jerominski L. Construction of linkage maps with DNA markers for human chromosomes. Nature. 1985 Jan 10;313(5998):101–105. doi: 10.1038/313101a0. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Wu C. T., Goldberg M. L. The Drosophila zeste gene and transvection. Trends Genet. 1989 Jun;5(6):189–194. doi: 10.1016/0168-9525(89)90074-7. [DOI] [PubMed] [Google Scholar]