Abstract
Rho GDP-dissociation inhibitor β (RhoGDIβ), a regulator of the Rho family of proteins, is expressed abundantly in the hematopoietic cell lineage. During apoptosis of hematopoietic cells, RhoGDIβ is cleaved by caspase-3 at Asp19 and this cleaved form (Δ19-RhoGDIβ) has been implicated in the apoptotic pathway. To clarify the role of RhoGDIβ in hematopoietic cells, the present study performed immunoblotting and immunofluorescence staining to examine the expression of RhoGDIβ and ∆19-RhoGDIβ during phorbol 12-myristate 13-acetate (PMA)-stimulated differentiation of human THP-1 monocytic cells to macrophages. During differentiation of the THP-1 cells to macrophages, the expression of RhoGDIβ remained stable; however, the expression of Δ19-RhoGDIβ increased, particularly in well-spreading, non-apoptotic cells, which differentiated into macrophages. These results suggested that Δ19-RhoGDIβ has an apoptosis-independent role in the PMA-induced differentiation of THP-1 cells to macrophages.
Keywords: caspase-3, differentiation, Rho GDP-dissociation inhibitor, THP-1, macrophages, phorbol 12-myristate 13-acetate
Introduction
Rho GDP-dissociation inhibitors (RhoGDIs) are regulators of the Rho family of proteins and function as molecular switches in numerous cellular processes, including actin cytoskeletal organization, microtubule dynamics, vesicle trafficking, cell polarity and cell cycle progression (1). A total of three RhoGDIs (RhoGDIα/RhoGDI1, RhoGDIβ/RhoGDI2/LyGDI/D4GDI and RhoGDIγ/RhoGDI3) have been identified in mammals (2). RhoGDIβ is expressed abundantly in hematopoietic cells (3,4) and is also expressed in non-hematopoietic cells, including keratinocytes, fibroblasts and amnion cells (5), non-hematopoietic tumors (6–8) and various cancer cells (9).
During apoptosis of hematopoietic cells, RhoGDIβ is cleaved by caspase-3 at Asp19 (10–16), and the cleaved form, Δ19-RhoGDIβ, translocates to the nucleus (12,15–17), suggesting the pro-apoptotic role of Δ19-RhoGDIβ. Therefore, the cleavage of RhoGDIβ by caspase-3 has been implicated in the apoptotic pathway, however, the precise role of Δ19-RhoGDIβ remains to be elucidated. Caspase-3 is known to have apoptosis-independent roles in the differentiation and proliferation of various cell types, including hematopoietic cells (18). In the differentiation of monocytes into macrophages, caspase-3 and caspase-9 are activated, and the inhibition of these caspases prevents this differentiation (19). Furthermore, phorbol 12-myristate 13-acetate (PMA)-stimulated THP-1 cells express increased levels of caspase-3 (20). However, whether RhoGDIβ is cleaved at Asp19 during the differentiation of these cells remains to be elucidated.
In the present study, to clarify the role of Δ19-RhoGDIβ in the differentiation of macrophages, the expression of RhoGDIβ and Δ19-RhoGDIβ were examined during PMA-stimulated differentiation of human THP-1 monocytic cells to macrophages. The results confirmed that RhoGDIβ was cleaved at Asp19 and it was shown that this cleaved form was expressed in non-apoptotic cells. These results suggested the apoptosis-independent role of Δ19-RhoGDIβ during the differentiation of THP-1 cells to macrophages.
Materials and methods
Cell culture and induction of differentiation
The THP-1 human myelomonocytic cell line was provided by Dr Masaharu Wano, Department of Hematology and Immunology, Kanazawa Medical University (Uchinada, Japan). The cells were cultured in RPMI 1640 medium containing 2 mM L-glutamine (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and were maintained at 37°C in a humidified atmosphere of 5% CO2 in air. The differentiation of THP-1 cells into macrophages was achieved using the method described by Daigneault et al (21). Briefly, THP-1 cells (~1.5×105/ml) were cultured with 200 nM PMA (Sigma-Aldrich; Merck Millipore) for 3 days at 37°C, the PMA-containing media was removed and the cells were incubated for a further 5 days. For counting the total cell number, the culture medium containing floating cells was removed and reserved, and the attached cells were detached using 0.25% Trypsin/0.01% EDTA in phosphate-buffered saline (PBS), following which they were suspended with the previously reserved medium containing the floating cells. The cell numbers were then counted using a hemocytometer. The relative numbers of flattened cells on the dish were observed under a phase-contrast microscope. The proportion of flattened cells was estimated as it was difficult to distinguish between flattened and unflattened cells precisely.
Antibodies
Anti-RhoGDIβ antibody (cat. no. sc-6047) raised against amino acid residues 175–194 was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). This antibody recognizes full-length and Δ19-RhoGDIβ. Anti-Δ19-RhoGDIβ antibody (clone 97A1015; cat. no. 14-6628-81) raised against the caspase-3 cleavage site of human RhoGDIβ was purchased from eBioscience, Inc. (San Diego, CA, USA). Anti-a-tubulin antibody (clone B-5-1-2; cat. no. T6074) was purchased from Sigma-Aldrich; Merck Millipore. Peroxidase-conjugated anti-mouse (cat. no; K4001) and anti-rabbit IgG antibodies (cat. no. K4002) were purchased from DakoCytomation (Glostrup, Denmark). Peroxidase-conjugated anti-goat IgG antibody (cat. no; 414351) was purchased from the Nichirei Corporation (Tokyo, Japan). Alexa Fluor 594-conjugated goat anti-mouse IgG (H+L; cat. no. A-11032) was purchased from Invitrogen; Thermo Fisher Scientific, Inc.
Immunoblotting
The cells not exposed to PMA (untreated cells) were found not to attach to the culture dish, whereas >95% of the PMA-stimulated cells attached. When the cell lysates of the PMA-stimulated cells were prepared, floating cells and cell debris were removed to avoid contamination by the dead cells. The cells were lysed using Laemmli buffer containing 4% sodium dodecyl sulfate (SDS), 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromophenol blue and 0.0125 M Tris-HCl (pH 6.8), and the protein concentrations of the lysate were measured using a Bradford Ultra kit (Novexin, Ltd., Cambridge, UK). The proteins (10 µg) were resolved by SDS-polyacrylamide gel electrophoresis and transferred onto Immobilon-P membranes (EMD Millipore, Billerica, MA, USA). The membranes were then probed with a primary antibody (sc-6047, 1:10,000 dilution; clone 97A1015, 1:10,000 dilution; clone B-5-1-2, 1:100,000 dilution) overnight at 4°C, followed by incubation with a peroxidase-conjugated secondary antibody (1:500 dilution) for 90 min at room temperature. The immunoreactive proteins were visualized using ECL Prime reagents (GE Healthcare Life Sciences, Ltd., Little Chalfont, UK). The same quantity of protein was applied in all immunoblot experiments.
Annexin V and immunofluorescence staining
The cells were grown in 35-mm culture dishes. To remove dead and apoptotic cells, the dishes were washed twice with PBS at 4°C. The cells were stained with the Annexin V-FITC Apoptosis Detection kit I (BD Biosciences, San Jose, CA, USA) according to the manufacturer's protocol, and fixed with freshly prepared 3.7% paraformaldehyde in Annexin V binding buffer containing 140 mM NaCl, 2.5 mM CaCl2 and 10 mM HEPES (pH 7.5) for 30 min at room temperature. The cells were then permeabilized with 0.5% Triton X-100 for 5 min at room temperature. Following washing with PBS, the cells were incubated with 0.5% bovine serum albumin (BSA) in PBS for 60 min at room temperature, and then incubated overnight at 4°C with anti-Δ19-RhoGDIβ antibody (clone 97A1015) diluted 1:400 in PBS containing 0.5% BSA. Following three washes with PBS, the cells were incubated for 60 min at room temperature with a secondary antibody (A-11032), diluted 1:400 in PBS containing 0.5% BSA and 0.1 µg/ml 4′,6-diamidino-2-phenylindole. Following three washes with PBS, the cells were mounted with ProLong Gold (Invitrogen; Thermo Fisher Scientific, Inc.). Images were captured using an Axiovert 200 inverted fluorescence microscope (Plan Neofluar 40X/0.75 NA objective lens) with AxioVision 4.4 software (Carl Zeiss AG, Jena, Germany). Images of the green, red and blue channels were captured using a 38HE bandpass filter (excitation, 450–490 nm; emission, 500–550 nm), a 43HE bandpass filter (excitation, 537–563 nm; emission 570–640 nm) and a 49 bandpass filter (excitation, G 365 nm; Emission, 420–470 nm), respectively.
Results
Induction of THP-1 cell differentiation into macrophages
The THP-1 cells were cultured with 200 nM PMA for 3 days, following which the PMA-containing media was removed and the cells were then incubated for a further 5 days (Fig. 1). Cell proliferation was suppressed and the proportion of attached cells increased considerably within 2 days. In addition >90% of the attached cells were flattened 2 days following treatment with PMA. The differentiation of THP-1 cells to macrophages is known to be accompanied by a loss of proliferation (22); furthermore, cell adhesion and spreading are used as functional indicators of macrophage differentiation (21,23). The results obtained indicated that the majority of THP-1 cells were induced to differentiate into macrophages.
Figure 1.
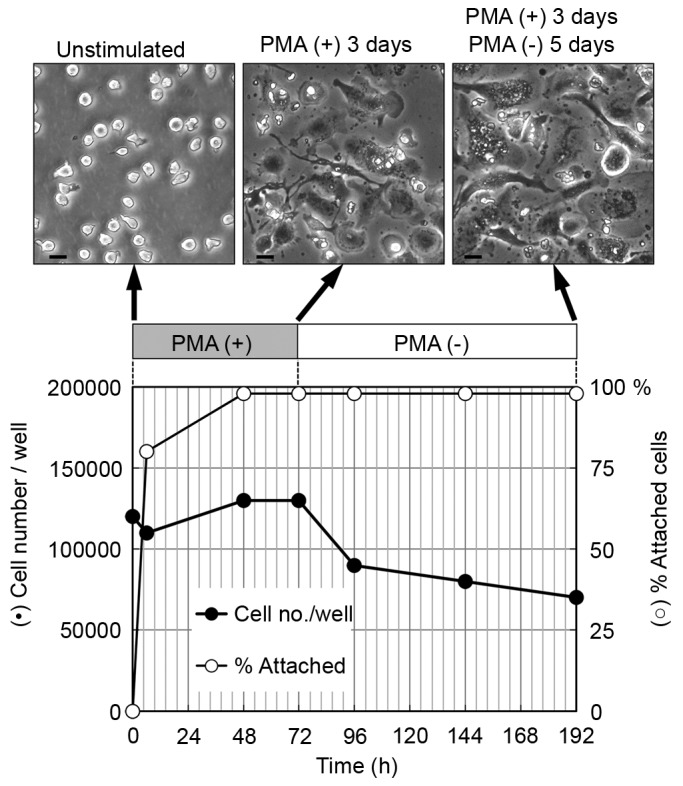
Induction of THP-1 cell differentiation into macrophages by PMA. Cells were seeded with 200 nM PMA in a 24-well plate and cultured for 3 days, following which the cells were cultured without PMA for a further 5 days. Total cell number and the proportion of attached cells were measured. Scale bar=20 µm. Similar results were obtained for three independent experiments. Representative results are shown. PMA, phorbol 12-myristate 13-acetate.
The cells cultured for 5 days following PMA removal showed a higher degree of flattened morphology, compared with the cells cultured for 3 days with PMA (Fig. 1, top panel). It has been reported that cultures rested for 5 days in PMA-free media adopt a phenotype, which more closely resembles human monocyte-derived macrophages, compared with differentiated THP-1 cells cultured for only 3 days with PMA (21). The flattened morphology observed in the present study of the cells cultured 5 days following PMA removal is likely to reflect the increased degree of differentiation of these cells.
Expression of Δ19-RhoGDI in THP-1 cells differentiated into macrophages
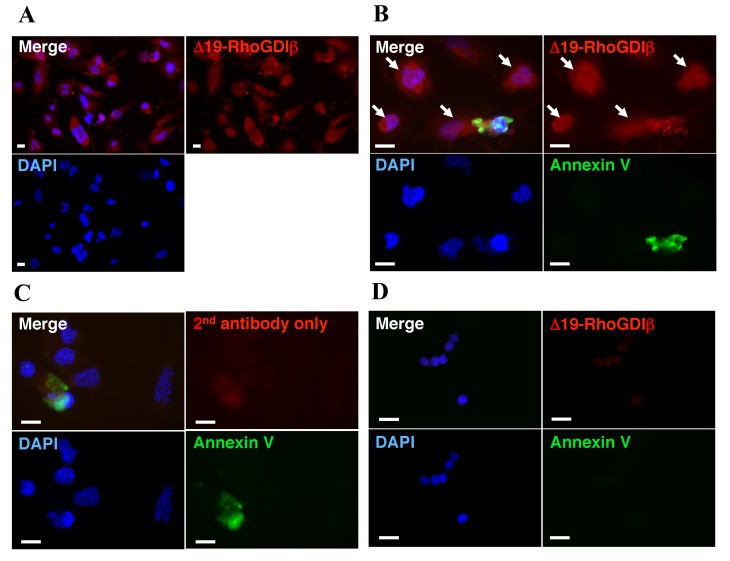
The present study used immunoblotting to examine the expression of RhoGDIβ and its cleaved form at Asp19 during the PMA-stimulated differentiation of THP-1 cells to macrophages. Following treatment with PMA, the expression of full-length RhoGDIβ remained unchanged, whereas the expression of Δ19-RhoGDIβ increased and was correlated with the differentiation of the cells into macrophages, although only a small fraction of full-length RhoGDIβ was cleaved at Asp19 (Fig. 2, top panel). The increased expression of Δ19-RhoGDIβ was confirmed using anti-Δ19-RhoGDIβ antibody (97A1015; Fig. 2, middle panel). The expression level of Δ19-RhoGDIβ was greater in cells cultured 5 days following PMA removal, compared with cells cultured for 3 days only with PMA. Therefore, the expression level of Δ19-RhoGDIβ appeared to correlate with the degree of differentiation into macrophages. To confirm the expression of Δ19-RhoGDIβ in macrophage-differentiated THP-1 cells, the differentiated cells were stained with anti-Δ19-RhoGDIβ antibody. Δ19-RhoGDIβ was detected in the macrophage-differentiated THP-1 cells (Fig. 3A).
Figure 2.
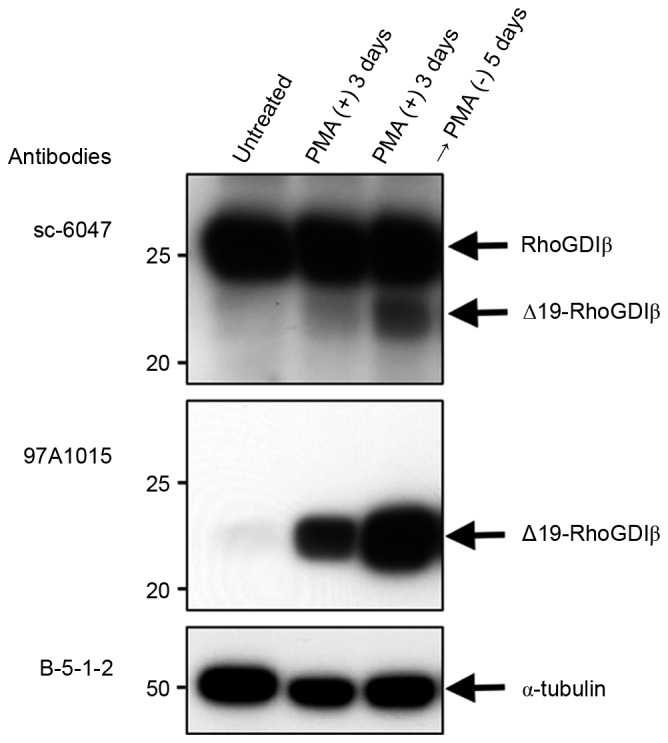
Expression of RhoGDIβ during the differentiation of THP-1 cells to macrophages. For the sampling of differentiation-induced cells, unattached dead and apoptotic cells were removed. The cell lysate was immunoblotted with an antibody (sc-6047), which recognizes full-length RhoGDIβ and Δ19-RhoGDIβ, and an antibody (97A1015), which specifically recognizes Δ19-RhoGDIβ. α-tubulin was stained with B-5-1-2 antibody as a loading control. Similar results were obtained for three independent experiments. Representative results are shown. RhoGDIβ, Rho GDP-dissociation inhibitor β.
Figure 3.
Expression of Δ19-RhoGDIβ in non-apoptotic cells during the differentiation of THP-1 cells to macrophages. For immunostaining of differentiation-induced cells unattached dead and apoptotic cells were removed. The cells were cultured with 200 nM PMA for 3 days, and then cultured without PMA for a further 5 days. (A) Differentiated cells were stained with anti-Δ19-RhoGDIβ antibody (red). (B) Differentiated cells were stained with Annexin V (green) and anti-Δ19-RhoGDIβ antibody (red). Arrows indicate Δ19-RhoGDIβ-positive non-apoptotic cells. (C) Differentiated cells stained with Annexin V (green) and only with secondary antibody (red). (D) Unstimulated cells were stained with Annexin V (green) and anti-Δ19-RhoGDIβ antibody (red). Scale bar=20 µm. Similar results were obtained for three independent experiments. Representative results are shown. RhoGDIβ, Rho GDP-dissociation inhibitor β; PMA, PMA, phorbol 12-myristate 13-acetate; DAPI, 4′,6-diamidino-2-phenylindole.
During the differentiation of THP-1 cells to macrophages, the total cell number gradually decreased (Fig. 1). As low levels of apoptotic cell death are reported to occur in macrophage-differentiated THP-1 cells in the absence of apoptotic stimuli (24), the decrease in cell number observed in the present study was likely due to apoptotic cell death. To exclude the possibility that the detected levels of Δ19-RhoGDIβ in the differentiated cells were derived from apoptotic cells, the differentiated cells attached to the culture dish were stained with Annexin V and anti-Δ19-RhoGDIβ antibody (Fig. 3B). The unattached dead and apoptotic cells were almost completely removed by washing with PBS prior to staining. The proportion of cells stained with Annexin V was <1%. The observed Annexin V-positive cells are shown in Fig. 3B and C. Δ19-RhoGDIβ was detected in the apoptotic cells and macrophage-differentiated THP-1 cells, and the majority of the expression of Δ19-RhoGDIβ was observed in the non-apoptotic cells, which differentiated into macrophages.
Discussion
During the apoptosis of hematopoietic cells, RhoGDIβ is cleaved by caspase-3 at Asp19 (10–16), and the cleaved form, Δ19-RhoGDIβ, has been suggested to have a pro-apoptotic function in K562 leukemia cells (17). In the present study, it was shown that, during the PMA-stimulated differentiation of THP-1 cells to macrophages, RhoGDIβ was also cleaved at Asp19 in non-apoptotic differentiating cells. Although it is unknown whether Δ19-RhoGDIβ is involved in the differentiation process or the differentiation phenotype, these results suggested that Δ19-RhoGDIβ is involved in cellular processes other than apoptosis, at least in THP-1 cells. Δ19-RhoGDIβ may have different roles depending on the cellular context.
RhoGDIβ is implicated in cancer progression; however, the correlation between malignancy and the expression level of RhoGDIβ is contradictory (25). It has been previously reported that RhoGDIβ may have a positive (7,26,27) and negative (28) role in cancer progression. Caspase-3 is considered to be involved in cancer susceptibility in squamous-cell carcinomas of the head and neck (29), endometrial cancer (30) and lung cancer (31), therefore, RhoGDIβ may be cleaved by casapse-3 in various cancer cells. The inconsistent correlation of RhoGDIβ with malignancy may be a reflection of the different functions of Δ19-RhoGDIβ in different types of cancer.
The function of Δ19-RhoGDIβ remains to be elucidated, and cleavage of RhoGDIβ at Asp19 is unlikely to result in the inactivation of RhoGDIβ. Δ22-RhoGDIα (32,33) and Δ20-RhoGDIα (34) have been reported to be almost fully functional for extracting GTPases from the membrane, although the inhibition of GTPase activity by Δ20-RhoGDIα is less effective, compared with that by wild-type RhoGDIα (34). The amino acid sequence and protein structure are similar between RhoGDIα and RhoGDIβ; therefore, Δ19-RhoGDIβ is expected to retain certain regulatory functions of full-length RhoGDIβ. Our previous study reported that RhoGDIs may act as a positive and negative regulator of Rho GTPases, depending on their expression level, and their affinity for guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) (35). The cleavage of RhoGDIβ at Asp19 may modify the regulatory function of RhoGDIβ towards Rho GTPases by altering the affinity of RhoGDIβ to GEFs and GAPs.
In conclusion, the present study showed that Δ19-RhoGDIβ was expressed in non-apoptotic, differentiating cells, thereby suggesting an apoptosis-independent role for this protein. The identification of this novel function of Δ19-RhoGDIβ may assist in further elucidating the regulatory system of Rho GTPases, which is involved in the regulation of numerous cellular processes.
Acknowledgements
Mr. Mamoru Fujiwara and Dr Masaaki Tatsuka (Department of Life Sciences, Life and Environmental Sciences, Prefectural University of Hiroshima, Shoubara, Japan) were supported by grants from the Prefectural University of Hiroshima Important Research Project; Interdisciplinary/Priority Research (grant nos. (S) H-26 and (S) H-27).
Glossary
Abbreviations
- BSA
bovine serum albumin
- GAP
GTPase-activating protein
- GEF
guanine nucleotide exchange factor
- PBS
phosphate-buffered saline
- PMA
phorbol 12-myristate 13-acetate
- RhoGDI
Rho GDP-dissociation inhibitor
- SDS
sodium dodecyl sulphate
References
- 1.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 2.Dovas A, Couchman JR. RhoGDI: Multiple functions in the regulation of Rho family GTPase activities. Biochem J. 2005;390:1–9. doi: 10.1042/BJ20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lelias JM, Adra CN, Wulf GM, Guillemot JC, Khagad M, Caput D, Lim B. cDNA cloning of a human mRNA preferentially expressed in hematopoietic cells and with homology to a GDP-dissociation inhibitor for the rho GTP- binding proteins. Proc Natl Acad Sci USA. 1993;90:1479–1483. doi: 10.1073/pnas.90.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherle P, Behrens T, Staudt LM. Ly-GDI, a GDP-dissociation inhibitor of the RhoA GTP-binding protein, is expressed preferentially in lymphocytes. Proc Natl Acad Sci USA. 1993;90:7568–7572. doi: 10.1073/pnas.90.16.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leffers H, Nielsen MS, Andersen AH, Honoré B, Madsen P, Vandekerckhove J, Celis JE. Identification of two human Rho GDP dissociation inhibitor proteins whose overexpression leads to disruption of the actin cytoskeleton. Exp Cell Res. 1993;209:165–174. doi: 10.1006/excr.1993.1298. [DOI] [PubMed] [Google Scholar]
- 6.Gildea JJ, Seraj MJ, Oxford G, Harding MA, Hampton GM, Moskaluk CA, Frierson HF, Conaway MR, Theodorescu D. RhoGDI2 is an invasion and metastasis suppressor gene in human cancer. Cancer Res. 2002;62:6418–6423. [PubMed] [Google Scholar]
- 7.Ota T, Maeda M, Suto S, Tatsuka M. LyGDI functions in cancer metastasis by anchoring Rho proteins to the cell membrane. Mol Carcinog. 2004;39:206–220. doi: 10.1002/mc.20006. [DOI] [PubMed] [Google Scholar]
- 8.Seraj MJ, Harding MA, Gildea JJ, Welch DR, Theodorescu D. The relationship of BRMS1 and RhoGDI2 gene expression to metastatic potential in lineage related human bladder cancer cell lines. Clin Exp Metastasis. 2000;18:519–525. doi: 10.1023/A:1011819621859. [DOI] [PubMed] [Google Scholar]
- 9.Harding MA, Theodorescu D. RhoGDI signaling provides targets for cancer therapy. Eur J Cancer. 2010;46:1525–1559. doi: 10.1016/j.ejca.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Essmann F, Wieder T, Otto A, Müller EC, Dörken B, Daniel PT. GDP dissociation inhibitor D4-GDI (Rho-GDI 2), but not the homologous rho-GDI 1, is cleaved by caspase-3 during drug-induced apoptosis. Biochem J 346 Pt. 2000;3:777–783. doi: 10.1042/bj3460777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kettritz R, Xu YX, Faass B, Klein JB, Müller EC, Otto A, Busjahn A, Luft FC, Haller H. TNF-alpha-mediated neutrophil apoptosis involves Ly-GDI, a Rho GTPase regulator. J Leukoc Biol. 2000;68:277–283. [PubMed] [Google Scholar]
- 12.Krieser RJ, Eastman A. Cleavage and nuclear translocation of the caspase 3 substrate Rho GDP-dissociation inhibitor, D4-GDI, during apoptosis. Cell Death Differ. 1999;6:412–419. doi: 10.1038/sj.cdd.4400515. [DOI] [PubMed] [Google Scholar]
- 13.Na S, Chuang TH, Cunningham A, Turi TG, Hanke JH, Bokoch GM, Danley DE. D4-GDI, a substrate of CPP32, is proteolyzed during Fas-induced apoptosis. J Biol Chem. 1996;271:11209–11213. doi: 10.1074/jbc.271.19.11209. [DOI] [PubMed] [Google Scholar]
- 14.Rickers A, Brockstedt E, Mapara MY, Otto A, Dörken B, Bommert K. Inhibition of CPP32 blocks surface IgM-mediated apoptosis and D4-GDI cleavage in human BL60 Burkitt lymphoma cells. Eur J Immunol. 1998;28:296–304. doi: 10.1002/(SICI)1521-4141(199801)28:01<296::AID-IMMU296>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Thiede B, Siejak F, Dimmler C, Rudel T. Prediction of translocation and cleavage of heterogeneous ribonuclear proteins and Rho guanine nucleotide dissociation inhibitor 2 during apoptosis by subcellular proteome analysis. Proteomics. 2002;2:996–1006. doi: 10.1002/1615-9861(200208)2:8<996::AID-PROT996>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X, Suto S, Ota T, Tatsuka M. Nuclear Translocation of Cleaved LyGDI dissociated from Rho and Rac during Trp53-dependent ionizing radiation-induced apoptosis of thymus cells in vitro. Radiat Res. 2004;162:287–295. doi: 10.1667/RR3220. [DOI] [PubMed] [Google Scholar]
- 17.Choi MR, Groot M, Drexler HC. Functional implications of caspase-mediated RhoGDI2 processing during apoptosis of HL60 and K562 leukemia cells. Apoptosis. 2007;12:2025–2035. doi: 10.1007/s10495-007-0121-5. [DOI] [PubMed] [Google Scholar]
- 18.Shalini S, Dorstyn L, Dawar S, Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22:526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sordet O, Rébè C, Plenchette S, Zermati Y, Hermine O, Vainchenker W, Garrido C, Solary E, Dubrez-Daloz L. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood. 2002;100:4446–4453. doi: 10.1182/blood-2002-06-1778. [DOI] [PubMed] [Google Scholar]
- 20.Liao HS, Matsumoto A, Itakura H, Pittman T, Kodama T, Geng YJ. De novo expression of the class-A macrophage scavenger receptor conferring resistance to apoptosis in differentiated human THP-1 monocytic cells. Cell Death Differ. 1999;6:245–255. doi: 10.1038/sj.cdd.4400485. [DOI] [PubMed] [Google Scholar]
- 21.Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The Identification of Markers of Macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 2010;5:e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwende H, Fitzke E, Ambs P, Dieter P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J Leukoc Biol. 1996;59:555–561. [PubMed] [Google Scholar]
- 23.Auwerx J. The human leukemia cell line, THP-1: A multifacetted model for the study of monocyte-macrophage differentiation. Experientia. 1991;47:22–31. doi: 10.1007/BF02041244. [DOI] [PubMed] [Google Scholar]
- 24.Vicca S, Hennequin C, Nguyen-Khoa T, Massy ZA, Descamps-Latscha B, Drueke TB, Lacour B. Caspase-dependent apoptosis in THP-1 cells exposed to oxidized low-density lipoproteins. Biochem Biophys Res Commun. 2000;273:948–954. doi: 10.1006/bbrc.2000.3017. [DOI] [PubMed] [Google Scholar]
- 25.Griner EM, Theodorescu D. The faces and friends of RhoGDI2. Cancer Metastasis Rev. 2012;31:519–528. doi: 10.1007/s10555-012-9376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang YS, Maeda M, Okamoto M, Fujii M, Fukutomi R, Hori M, Tatsuka M, Ota T. Centrosomal localization of RhoGDIβ and its relevance to mitotic processes in cancer cells. Int J Oncol. 2013;42:460–468. doi: 10.3892/ijo.2012.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ota T, Maeda M, Murakami M, Takegami T, Suto S, Tatsuka M. Activation of Rac1 by Rho-guanine nucleotide dissociation inhibitor-beta with defective isoprenyl-binding pocket. Cell Biol Int. 2007;31:92–96. doi: 10.1016/j.cellbi.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Ota T, Maeda M, Sakita-Suto S, Zhou X, Murakami M, Takegami T, Tatsuka M. RhoGDIbeta lacking the N-terminal regulatory domain suppresses metastasis by promoting anoikis in v-src-transformed cells. Clin Exp Metastasis. 2006;23:323–334. doi: 10.1007/s10585-006-9041-y. [DOI] [PubMed] [Google Scholar]
- 29.Chen K, Zhao H, Hu Z, Wang LE, Zhang W, Sturgis EM, Wei Q. CASP3 polymorphisms and risk of squamous cell carcinoma of the head and neck. Clin Cancer Res. 2008;14:6343–6349. doi: 10.1158/1078-0432.CCR-08-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu HL, Xu WH, Cai Q, Feng M, Long J, Zheng W, Xiang YB, Shu XO. Polymorphisms and haplotypes in the caspase-3, caspase-7, and caspase-8 genes and risk for endometrial cancer: A population-based, case-control study in a Chinese population. Cancer Epidemiol Biomarkers Prev. 2009;18:2114–2122. doi: 10.1158/1055-9965.EPI-09-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang JS, Kim KM, Choi JE, Cha SI, Kim CH, Lee WK, Kam S, Jung TH, Park JY. Identification of polymorphisms in the Caspase-3 gene and their association with lung cancer risk. Mol Carcinog. 2008;47:383–390. doi: 10.1002/mc.20397. [DOI] [PubMed] [Google Scholar]
- 32.Gosser YQ, Nomanbhoy TK, Aghazadeh B, Manor D, Combs C, Cerione RA, Rosen MK. C-terminal binding domain of Rho GDP-dissociation inhibitor directs N-terminal inhibitory peptide to GTPases. Nature. 1997;387:814–819. doi: 10.1038/42961. [DOI] [PubMed] [Google Scholar]
- 33.Platko JV, Leonard DA, Adra CN, Shaw RJ, Cerione RA, Lim B. A single residue can modify target-binding affinity and activity of the functional domain of the Rho-subfamily GDP dissociation inhibitors. Proc Natl Acad Sci USA. 1995;92:2974–2978. doi: 10.1073/pnas.92.7.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golovanov AP, Chuang TH, DerMardirossian C, Barsukov I, Hawkins D, Badii R, Bokoch GM, Lian LY, Roberts GC. Structure-activity relationships in flexible protein domains: Regulation of rho GTPases by RhoGDI and D4 GDI. J Mol Biol. 2001;305:121–135. doi: 10.1006/jmbi.2000.4262. [DOI] [PubMed] [Google Scholar]
- 35.Ota T, Maeda M, Okamoto M, Tatsuka M. Positive regulation of Rho GTPase activity by RhoGDIs as a result of their direct interaction with GAPs. BMC Syst Biol. 2015;9:3. doi: 10.1186/s12918-015-0143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]