Abstract
Background
Frailty is associated with immune activation and inflammation in the elderly general population, but whether this is true in the younger HIV-infected (HIV+) population is not known.
Methods
We analyzed 24 serologic biomarkers of monocyte, T cell, or B cell activation in HIV− (n=207) and HIV+ (n=714; 75% virologically suppressed) men who have sex with men in the Multicenter AIDS Cohort Study and were classified as frail or nonfrail according to expression or non-expression of the frailty phenotype at two consecutive study visits.
Results
After correction for multiple comparisons and adjustment for age, race, study site, and education, frailty in HIV+ men was significantly (p<0.002) associated with higher levels of sCD14, sIL2Rα, sTNF-R2, IL-6, and TNF-α; the association with higher levels of C-reactive protein (CRP) approached significance (p=.003). After further adjustment for BMI, smoking, and co-morbidities, only the association with CRP was significant at p<0.002, with levels approximately 50% higher in frail compared to nonfrail men. These conclusions were not altered by restricting the analysis to HIV+ men who were virologically suppressed. Among HIV− men, none of these markers differed significantly by frailty.
Conclusion
These data suggest that frailty in virologically suppressed HIV+ men was associated with immune activation beyond that due to treated HIV infection. The inflammatory markers associated with frailty were primarily products of activated monocytes/macrophages. Much, but not all, activation was accounted for by harmful behaviors and comorbidities. However, CRP, which is regulated by IL-6, was elevated in HIV+ frail men independent of these factors.
Keywords: Frailty, cytokines, chemokines, cohort study, HIV, C-reactive protein
Introduction
With the advent of highly active antiretroviral therapy (HAART), people with HIV infection are surviving to older ages1. Whether the aging process is affected by treated HIV infection is controversial. HIV infection and aging in general have been associated with immune activation and inflammation2, and the profound immune activation observed in untreated HIV infection is not completely resolved by HAART3. This residual immune activation in treated HIV infection could contribute to aging and other chronic diseases.
Frailty, an aging-related syndrome characterized by vulnerability to stressors and predictive of adverse health outcomes4, is of particular interest for several reasons. First, it is common in aging populations. Second, it often occurs in the absence of any specific identifiable disease5;6. Third, it is thought to represent the simultaneous failure of multiple homeostatic systems and possibly of a higher order whole-body homeostasis that transcends any particular organ system7–9. Fourth, although its etiology is unknown, frailty has been associated with activation of immune effector mechanisms that are pro-inflammatory and/or catabolic10. For example, many studies have found that in the general population higher circulating levels of markers of inflammation, such as interleukin-6 (IL-6), connote a higher risk of death11, disability12 and frailty10;13–15.
In HIV-infected (HIV+) people receiving HAART, the links between aging, frailty, and inflammation are less established than they are in the general population. We6;16 and others17–19 (reviewed in20) have documented that frailty occurs more frequently in HIV+ than similar HIV− populations. Moreover, frailty often occurs at an earlier age in HIV+ populations6;16, at least partly because of a higher burden of co-morbidities6, and predicts mortality21–23. The contribution of residual inflammation in treated HIV+ people to the development of frailty is unknown. Piggott et al23 found that an inflammatory index derived from serum levels of IL-6 and sTNFR1 predicted frailty, but Kooij et al18 did not find an association between frailty and other serologic markers of inflammation. We used data from the Multicenter AIDS Cohort Study (MACS) to address the hypothesis that inflammation is associated with frailty in HIV+ men.
Methods
Study design
The MACS, a longitudinal cohort study of HIV infection in men who have sex with men, recruited a total of 6972 HIV+ and HIV− men in 1984–5, 1987–90, and 2001–3. Recruitment and study procedures have been published24. Briefly, participants are followed semiannually with standardized questionnaires, brief physical examinations, and laboratory testing including HIV serological testing on HIV− men and plasma HIV RNA measurement on HIV+ men. Plasma, serum, and peripheral blood mononuclear cells (PBMC) are stored at each study visit. Waist circumference was measured by standardized methods as described25.
The frailty phenotype (FP) described by Fried et al5, has been measured at all study visits since 20076; briefly, this phenotype requires expression of 3 of 5 characteristics: slow walking speed, low grip strength, exhaustion, low physical activity, and weight loss. Time to walk 4 meters was measured twice, and the faster value was used. Grip strength with the dominant hand was measured 3 times, and the average value was used. For these two criteria, having a value below the 20th percentile of the HIV− population was taken as expression of the criterion. The other three criteria were assessed by questionnaire6. Because the expression of the FP can vary extensively between semiannual assessments6, we defined frailty as expression of the FP at 2 consecutive study visits within one year of each other, and non-frailty as similar non-expression of the FP, with no previous expression of the FP at any visit. The index visit was defined as the first of the two visits used to assess frailty. For individuals who had more than one visit pair with frailty or non-frailty, only the first pair was used.
The study population was selected from a previous MACS substudy of the long-term effects of HIV infection and HAART on biomarkers of inflammation and immune activation26. That study assessed 24 such biomarkers longitudinally in 1885 MACS participants in four partially overlapping populations: 1) all incident cases of HIV infection whose infection dates were known to within ±0.75 years (n =541), 2) all HAART initiators (n =1348), 3) all HIV+ men with >15 years without AIDS with no antiretroviral therapy (long-term nonprogressors; n=57), and 4) a representative sample of 250 men who remained HIV−; this included all HIV− men with hepatitis C (n=35), to best address the effect of HCV infection on the biomarkers. Thus, that study, and hence the present study, contained primarily HIV+ men, particularly all those on treatment when FP assessments were initiated in the study. The men included in the present analysis were those who had biomarker measurements at the frailty assessment or within two years before it. If biomarkers at the index visit were missing, prior measurements closest to that visit were used.
Laboratory testing
All measurements were performed on stored serum, as described26,27. At Johns Hopkins, serum cytokines (IL-1β, -2, -6, -8,-10,-12p70; GM-CSF, IFN-γ, and TNF-α) were determined using the Human ProInflammatory 9-plex Ultra-Sensitive Kit (Meso-Scale Discovery (MSD), Gaithersburg, MD), and serum chemokines (CCL2/MCP-1, CCL4/MIP-1β, CCL11/eotaxin, 13/MCP-4, CCL17/TARC, CXCL8/IL-8 and CXCL10/IP-10) using the Human Chemokine 7-plex Ultra-Sensitive Kit (MSD), per manufacturer’s instructions with the Sector 2400 analyzer (MSD). The lower limit of quantification (LLOQ) for each analyte was defined as the lowest concentration on the standard curve which yielded a) a measured concentration within 25% of the nominal value, and b) a coefficient of variation (%CV) less than 25%28. The lower limit of detection (LLOD) was defined as the lowest concentration on the standard curve that was >2.5 standard deviations above the blank. 10% of samples were run in duplicate. Using these duplicates, CVs were determined for analytes that were above the LLOQ in ≥ 80% of samples (all but IL-1β, IL-2, IFN-γ, and GM-CSF). These ranged from 6.8 to 18.8% in HIV− men, and 11.8 to 32.0% in HIV+ men. Testing was done on one lot of multiplex kits.
Serum levels of soluble (s) CD14, sCD27, sgp130, sIL-6 receptor (sIL-6R), sIL-2 receptor-α (sCD25), and sTNF receptor-2 (sTNFR2), plus a cytokine known as B cell activating factor or B Lymphocyte Stimulator (BAFF/BLyS), and the chemokine CXCL13 (B-lymphocyte Chemoattractant/B Cell-attracting Chemokine 1; BLC/BCA1), were measured in one multiplexed assay panel (Human Biomarker Custom Premix Kit A), at the University of California, Los Angeles, using custom-manufactured multiplexed (Luminex platform) assay kits (Fluorokine MAP, R & D Systems, Minneapolis, MN) per manufacturer’s directions, and a Bio-Plex 200 Luminex instrument and Bio-Plex analysis software (Bio-Rad, Hercules, CA). Coefficients of variation ranged from 10.2 to 29.6%. Again, testing was done on one lot of assay kits.
C-reactive protein (CRP) was measured at Quest Diagnostics by a high-sensitivity nephelometric assay with an LLOD of 0.2 mg/L and a CV of 7.6% (Dade Behring, Newark, DE). Prothrombin time was also measured at Quest.
Statistical analysis
Differences between the frail and nonfrail groups in demographic, behavioral and clinical characteristics were assessed by Chi-square and Wilcoxon rank sum statistics for discrete and continuous variables, respectively. Date of birth, race, and highest education attained were obtained by self-report at study entry. All other data were from the index visit if available; otherwise data from the closest visit within the preceding 2 years were used. Covariates included body mass index (BMI=weight(kg)/height(cm)2), current smoking, hepatitis C virus (HCV) infection (defined by detectable serum HCV RNA), and presence of depressive symptoms, defined as a score ≥16 on the Center for Epidemiology Studies-Depression questionnaire (CES-D), since we have found that HCV infection and depressive symptoms were associated with expression of the frailty phenotype6. Kidney disease was defined as either an estimated glomerular filtration rate (MDRD eGFR) < 60 mL/min/1.73m2 body surface area or the presence of proteinuria ≥200 mg/g creatinine. Liver disease was defined as serum liver enzymes (ALT or AST) > 150 IU/L. Other chronic illnesses (hypertension, diabetes, dyslipidemia) were considered confirmed if present at two or more visits including at the time of the measured biomarker. Hypertension was defined as either an average systolic blood pressure ≥ 140 mm Hg, an average diastolic blood pressure ≥ 90 mm Hg, or the use of medication for hypertension. Diabetes was defined as a fasting glucose ≥ 126 mg/dl or the use of medication for diabetes; and dyslipidemia was defined as any of the following: fasting total cholesterol ≥200 mg/dL, low-density lipoprotein (LDL) ≥130 mg/dL, high-density lipoprotein (HDL) <40 mg/dL, triglycerides ≥150 mg/dL, or use of lipid-lowering medications with self-reported previous clinical diagnosis. The 1993 CDC definition of AIDS29 was used, except that a CD4 T-cell count <200/ul by itself was not considered AIDS.
Two multivariable regression approaches were used to evaluate associations between frailty and biomarkers. First, generalized gamma (GG) regression was used with left censoring to account for values below the limit of detection. GG regression was chosen since many of the biomarker levels were not normally distributed by either raw or log-transformed values. GG distributions are defined by three parameters – location (β), scale (σ) and shape (κ)30; we assessed effects on the location parameter only. Relative percentiles were calculated comparing each HIV/frailty group to the HIV− nonfrail men as the reference group. Second, for markers with > 25% undetectable values, we report the odds ratio for detectability from logistic regression models. In each approach, we fit two models: 1) minimally adjusting for age, race, center, and education; and 2) adjusting also for BMI, smoking, HCV, depressive symptoms, hypertension, diabetes, dyslipidemia, kidney disease, liver disease, and cancer. For analyzing the relationship between biomarkers and nadir CD4 cell count (among HIV+ men), nadir CD4 cell count was dichotomized as ≤ 200 or > 200 cells/μL(reference group). Age-adjusted median and interquartile range of biomarkers were obtained by using quantile regressions at 25th, 50th, and 75th percentiles including age (centered at 50 years) as an independent variable. Using a Bonferroni correction for multiple testing, p < 0.002 was considered statistically significant; 0.002 < p-values < 0.05 were considered marginally significant. There were insufficient HIV− men to formally test for differential effects of HIV status on the relationships between inflammatory markers and frailty. Analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
Results
Of the 1141 MACS participants who had biomarkers measured within two years of a frailty assessment, 1072 had the required 2 visits with FP assessments (+ or −) within one year (Figure 1). This included 809 HAART initiators, 33 long-term nonprogressors, and 230 HIV− men. The two FP determinations were consistent (i.e., +/+ (frail) or −/− (nonfrail)) for 921 men, who were included in the present analysis; 109 (15.3%) of the HIV+ men were frail, as were 26 (12.6%) of the HIV− men. As shown in Table 1, frail men (both HIV+ and HIV−) were older than nonfrail men and more likely to be from Baltimore, had less education, and more reported current smoking and had HCV infection, depressive symptoms, diabetes, hypertension, and kidney disease. Among HIV+ men, >90% of both frail and non-frail men were receiving HAART, 75% or more for at least 7 years, and >75% had undetectable plasma HIV RNA levels; frail HIV+ men had lower nadir and current CD4 cell counts and were more likely to have had an AIDS-defining illness. Frail and nonfrail HIV+ men had similar durations of untreated HIV infection, ART histories, timing of biomarker measurement relative to frailty assessment, waist circumferences, and proportions with both CD4 cell count >500 and CD4/CD8 ratio >1. CD4/CD8 ratio was slightly higher in the nonfrail HIV+ men.
Figure 1.
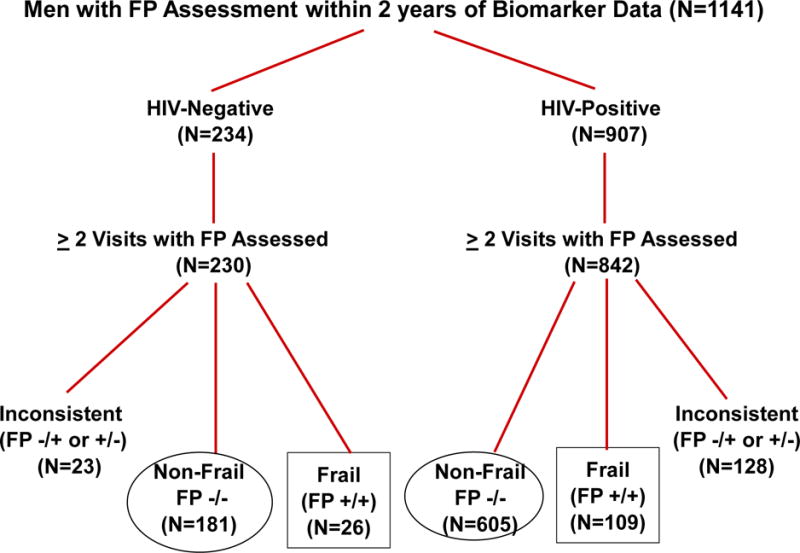
Selection of study population. + and − signs refer to expression and non-expression, respectively, of the frailty phenotype (FP) at consecutive MACS study visits.
Table 1.
Characteristics of study population.
| Characteristics | HIV− | HIV+ | |||||
|---|---|---|---|---|---|---|---|
| Nonfrail | Frail | P1 | Nonfrail | Frail | P2 | P3 | |
| N | 181 | 26 | 605 | 109 | |||
| Age (years), Mean (SD) | 52.3 (10.3) | 55.1 (8.9) | 0.398 | 49.9 (8.7) | 53.9 (7.8) | <0.001 | 0.989 |
| Ethnicity | 0.061 | 0.641 | 0.018 | ||||
| White | 102 (56.4) | 10 (38.5) | 331 (54.7) | 63 (57.8) | |||
| Black | 60 (33.1) | 15 (57.7) | 167 (27.6) | 31 (28.4) | |||
| Hispanic/Other | 19 (10.5) | 1 (3.8) | 107 (17.7) | 15 (13.8) | |||
| Site | 0.18 | 0.073 | 0.103 | ||||
| Baltimore | 34 (18.8) | 9 (34.6) | 135 (22.3) | 36 (33.0) | |||
| Chicago | 49 (27.1) | 5 (19.2) | 166 (27.4) | 21 (19.3) | |||
| Pittsburgh | 63 (34.8) | 10 (38.5) | 123 (20.3) | 23 (21.1) | |||
| Los Angeles | 35 (19.3) | 2 (7.7) | 181 (29.9) | 29 (26.6) | |||
| College education or above | 96 (53) | 5 (19.2) | 0.001 | 283 (46.8) | 36 (33.0) | 0.009 | 0.236 |
| BMI, Mean (SD) | 27.3 (5) | 27.7 (5) | 0.735 | 25.8 (4.2) | 25.1 (5.0) | 0.067 | 0.025 |
| Smoking | <0.001 | 0.006 | 0.109 | ||||
| Current | 41 (22.7) | 18 (69.2) | 177 (29.3) | 49 (45.0) | |||
| Former | 96 (53) | 5 (19.2) | 266 (44) | 38 (34.9) | |||
| HCV-infection | 24 (13.3) | 11 (42.3) | <0.001 | 45 (7.4) | 25 (22.9) | <0.001 | 0.052 |
| Depressive Symptoms | 36 (19.9) | 9 (34.6) | 0.124 | 110 (18.2) | 54 (49.5) | <0.001 | 0.195 |
| Hypertension | 84 (46.4) | 19 (73.1) | 0.012 | 246 (40.7) | 58 (53.2) | 0.016 | 0.08 |
| Diabetes | 21 (11.6) | 4 (15.4) | 0.529 | 59 (9.8) | 18 (16.5) | 0.044 | 1 |
| Dyslipidemia | 130 (71.8) | 16 (61.5) | 0.357 | 509 (84.1) | 89 (81.7) | 0.572 | 0.036 |
| Kidney disease | 9 (5) | 7 (26.9) | 0.001 | 107 (17.7) | 51 (46.8) | <0.001 | 0.08 |
| Liver disease | 1 (0.6) | 0 (0) | 1 | 9 (1.5) | 1 (0.9) | 1 | 1 |
| Cancer | 1 (0.6) | 0 (0) | 1 | 6 (1) | 4 (3.7) | 0.052 | 1 |
| Waist circumference (cm), Median (IQR) | 96 (88,106) | 101 (89,108) | 0.409 | 93 (85,99) | 94 (83,102) | 0.996 | 0.051 |
| Biomarkers measured at the index visit | 43 (23.8) | 2 (7.7) | 0.076 | 173 (28.6) | 23 (21.1) | 0.129 | 0.161 |
| Nadir CD4 cell count (/μL), Median (IQR) | 252 (149,346) | 201 (88,307) | 0.003 | ||||
| Current CD4 cell count (/μL), Median (IQR) | 572 (419,748) | 520 (352,704) | 0.037 | ||||
| Receiving HAART | 576 (95.2) | 102 (93.6) | 0.475 | ||||
| Undetectable HIV RNA (<50 copies/ml) | 466 (77) | 82 (75.2) | 0.712 | ||||
| HIV RNA among detectable (log10 copies/mL), Median (IQR)_ | 3.7 (2.5, 4.5) | 3.2 (2.1, 4.6) | 0.81 | ||||
| History of AIDS | 67 (11.1) | 29 (26.6) | <0.001 | ||||
| Receiving HAART | 576 (95.2) | 102 (93.6) | 0.475 | ||||
| Years received HAART, Median (IQR) | 10 (7,12) | 11 (8,13) | 0.007 | ||||
| Duration of untreated HIV-infection (years), Median (IQR) | 2 (0,6.5) | 2.4 (0,7.2) | 0.891 | ||||
| Experienced ART before the index visit | 579 (95.7) | 104 (95.4) | 0.802 | ||||
| Calendar year of HIV-infection diagnosis, Median (IQR) | 2001 (1985,2002) | 1992 (1984,2002) | 0.409 | ||||
| Calendar year of clinical AIDS diagnosis, Median (IQR) | 1997 (1995,2002) | 1995 (1994,2002) | 0.423 | ||||
| CD4/CD8 ratio, Median (IQR) | 0.7 (0.5,1) | 0.6 (0.4,0.9) | 0.074 | ||||
| CD4/CD8 ratio>1 and CD4>500 for ≥5 years | 40 (6.6) | 5 (4.6) | 0.525 | ||||
Data are numbers and percentages of observations unless otherwise noted.
Comparing HIV−/Nonfrail vs. HIV−/Frail
Comparing HIV+/Nonfrail vs. HIV+/Frail
Comparing HIV−/Frail vs. HIV+/Frail
HIV+ and HIV− frail men did not differ significantly in the FP criteria that were met in order to be classified as FP+ at a given visit, although HIV+ men were slightly more likely to have met the criteria of weight loss and slow walking speed, and slightly less likely the criterion of low grip strength (Supplemental Table 1).
The 151 HIV+ and HIV− men who were excluded from the analysis because of inconsistent expression of the FP at consecutive visits (i.e., −/+ or +/−) tended to have demographic characteristics intermediate to those of non-frail and frail men of the same HIV status (Supplemental Table 2).
The biomarker measurements were from the index visit for 26% of men and from a visit preceding the index visit for 74% of men (81% of frail men, and 73% of non-frail men). Distributions of biomarker values, stratified by HIV and frailty status, are shown in Supplemental Figure S1 for the overall study population, and Supplemental Figure S2 for the four HIV and frailty subgroups. In view of the age difference between frail men and non-frail men (Table 1), age-adjusted biomarker values are summarized in Table 2. Among HIV− men, the frail had higher age-adjusted medians than the nonfrail for 15 of 24 biomarkers; these differences were marginally significant (.002 < p <.05) for BAFF, sCD27, and sIL2Rα before any adjustment other than age (Table 2, left columns). In minimally adjusted models (including age, race, center, and education), the frail HIV− had lower MCP-4 values that were marginally significant (RP =0.84, p=.043). After adjustment for HCV infection and smoking, this difference persisted but was no longer significant (RP= 0.86; p=.063). After further adjusting for other co-morbidities, none of the differences between frail and nonfrail HIV− men was significant (Figure 2, left bars).
Table 2.
Summary Statistics (median, IQR) of Biomarkers by HIV and Frailty Status.
| HIV− | HIV+ | P+ | |||
|---|---|---|---|---|---|
| Biomarker | Nonfrail | Frail | Nonfrail | Frail | |
| N | 181 | 26 | 605 | 109 | |
| BAFF/BLyS |
1947 (1709, 2241) |
2033 (1856, 2355) |
2075++ (1798, 2498) |
2411++ (1934, 3058) |
0.044 |
| BLC/BCA-1 | 296 (247, 349) |
301 (230, 351) |
285 (230, 345) |
294 (252, 373) |
0.861 |
| sCD14** | 2004 (1777, 2388) |
1956 (1724, 2391) |
2477 (2042, 2953) |
2663 (2159, 3214) |
0.001 |
| sCD27 |
8757 (7161, 11370) |
9608 (8587, 12612) |
10530++ (8601, 14062) |
13587++ (9072, 18898) |
<0.001 |
| sgp130** | 252 (223, 285) |
260 (233, 304) |
266 (235, 298) |
274 (240, 339) |
0.416 |
| sIL-6R** | 47.8 (39.9, 58.6) |
45.5 (35.9, 52.4) |
48.0 (39.0, 57.8) |
47.8 (38.7, 58.4) |
0.951 |
| sIL-2Ra |
1331 (1081, 1666) |
1522 (1117, 1899) |
1383++ (1121, 1815) |
1751++ (1259, 2298) |
0.399 |
| sTNF-R2 | 2258 (1828, 2774) |
2510 (2196, 3333) |
2500++ (2021, 3249) |
3213++ (2483, 4395) |
0.058 |
| GM-CSF | 0.6 (0.4, 1.7) |
0.9 (0.3, 2.2) |
0.5 (0.2, 1.1) |
0.6 (0.2, 1.2) |
0.766 |
| IFN-ᵞ | 0.8 (0.4, 1.3) |
1.2 (0.4, 1.3) |
0.9 (0.6, 1.6) |
1 (0.7, 1.8) |
0.503 |
| IL-10 | 3.1 (2, 7.1) |
3.1 (1.7, 5.2) |
2.7 (1.7, 4.6) |
3 (1.9, 4.9) |
0.982 |
| IL-12p70 | 2.4 (1.2, 5.9) |
2.3 (0.8, 3.2) |
1.7 (0.8, 3.4) |
1.7 (1.1, 3.2) |
0.927 |
| IL-1β | 0.3 (0.2, 0.7) |
0.3 (0.1, 1) |
0.3 (0.1, 0.5) |
0.3 (0.1, 0.6) |
0.507 |
| IL-2 | 0.6 (0.3, 1.2) |
0.6 (0.3, 2.8) |
0.5 (0.3, 0.9) |
0.6 (0.3, 1) |
0.619 |
| IL-6 | 1 (0.8, 1.7) |
1.3 (0.8, 2.3) |
1 (0.7, 1.6) |
1.3 (0.8, 2.1) |
0.764 |
| IL-8*** | 15 (10, 47) |
18 (11, 92) |
14 (10, 25) |
16 (11, 28) |
0.469 |
| TNF-α | 8 (7, 11) |
9 (7, 13) |
9 (7, 13) |
11 (8, 15) |
0.028 |
| IP-10 | 147 (108, 215) |
121 (75, 578) |
244 (161, 407) |
271 (191, 418) |
0.005 |
| MCP-4 | 827 (638, 1068) |
853 (444, 1045) |
875 (657, 1125) |
888 (624, 1190) |
0.438 |
| Eotaxin | 1765 (1193, 2490) |
1474 (970, 1733) |
1900 (1357, 2598) |
1723 (1165, 2507) |
0.171 |
| MCP-1 |
531 (376, 682) |
407 (295, 672) |
554 (420, 704) |
523 385, 689) |
0.006 |
| MIP-1B | 146 (99, 212) |
181 (95, 232) |
132 (94, 181) |
123 (83, 203) |
0.452 |
| TARC | 572 (379, 843) |
621 (319, 1195) |
517 (321, 819) |
632 (363, 1005) |
0.901 |
| CRP†† | 1 (0.6, 2.6) |
1.1 (0.4, 3.0) |
1.3 (0.6, 3.2) |
2.3 (0.8, 4.3) |
0.004 |
Data in pg/ml except as noted.
bold = p<0.05 by frailty status within HIV status (by non-parametric Wilcoxon test).
bold, italic, underline
= p<0.002 by frailty status within HIV status
sCD14, sGP130, sIL-6R in ng/ml.
CRP in mg/L.
IL-8 was measured in both proinflammatory and chemokine panels, with equivalent results. Results from proinflammatory panel are shown.
P value comparing HIV− frail to HIV+ frail men.
Figure 2.
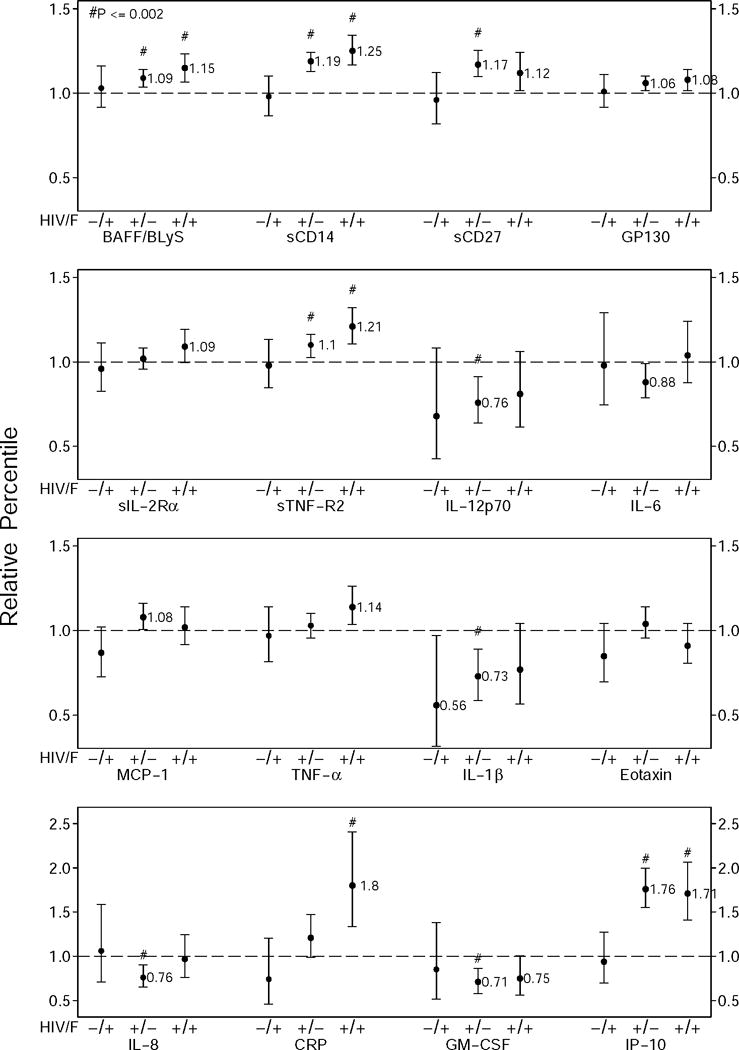
Relative percentile estimates for the HIV− frail group, the HIV+ nonfrail group, and the HIV+ frail group (left, middle, and right ●, respectively), and 95% confidence limits of the estimates (error bars), for exposure groups relative to the nonfrail HIV− group, obtained by generalized gamma regression models for each biomarker, adjusted for age, race, study sites, and education, as well as BMI, current smoking, depressive symptoms, HCV infection, hypertension, diabetes, dyslipidemia, kidney disease, liver disease, and cancer. Significant differences (p≤0.002) are indicated by #. Marginally significant differences (0.002<p<0.05) are those for which the 95% confidence intervals do not include 1.0. Relative percentile estimates are given for differences that were significant or marginally significant. The biomarkers that had ≥1 significant (p<0.05) comparison in any analysis are shown,
Among the HIV+ men, the frail had higher age-adjusted medians than the nonfrail for 18 of the 24 biomarkers (Table 2, right columns). In unadjusted analyses, these differences were marginally significant for the three markers that were marginally significant for HIV− men (BAFF, sIL2Rα, sCD27) and also for sCD14, sgp130, sTNFR2, IL-6, IL-8, TNF-α, TARC, and CRP. Of these markers, BAFF, sCD27, sIL2Rα, and sTNFR2 remained significant (p<.002) after adjustment for multiple comparisons, and the higher levels of sCD14 (p=0.003), TNF-α (p=0.003), and IL-6 (p=0.009) approached significance after this adjustment. All of these markers except sIL-2Rα, IL-6, and TNF-α differed significantly between HIV− and HIV+ nonfrail groups after full adjustment, as did IP-10 (Table 2; Figure 2, middle bars). Most markers were higher in HIV+ frail men than in HIV+ non-frail men (Figure 2, right bars and middle bars, respectively). These results were essentially unchanged when analyses were restricted to those who were virologically suppressed (Supplemental Table 2).
The adjusted results comparing biomarker levels according to frailty status in the HIV+ men are shown in Figure 3. After adjustment for demographics (Figure 3, left bars), sCD14, sIL2Rα, sTNFR2, IL-6, and TNF-α were significantly higher in frail men (p < 0.002). CRP levels were also higher, and this difference was nearly significant (p= 0.003). However, after further adjustment for co-morbidities only the difference in CRP was significant at the p<0.002 level (Figure 3, right bars). Higher levels of IL-6, TNF-α, and sTNFR2 in the frail men persisted, but with marginal significance (0.002 < p < 0.05). One marker, eotaxin, was marginally significantly lower in the frail men. These results were not affected by excluding long-term nonprogressors (n=33) or men with HCV infection (35 HIV−, 70 HIV+) from the analyses, except that in the latter case, the difference of TNF-α by frailty in the HIV+ was no longer marginally significant although its relative distribution did not change (data not shown). In fully adjusted models of HIV+ men, most biomarkers did not differ significantly by nadir CD4 cell count (≤ 200 vs >200 cells/μL); however, IL-6 concentrations were about 6% lower in frail men with low nadirs (p<.001) and 17% higher in non-frail men with low nadirs (p=.006), and TARC was 51% higher in frail men with low nadirs (p<.001). Prothrombin times did not differ significantly by either HIV status or frailty status (not shown).
Figure 3.
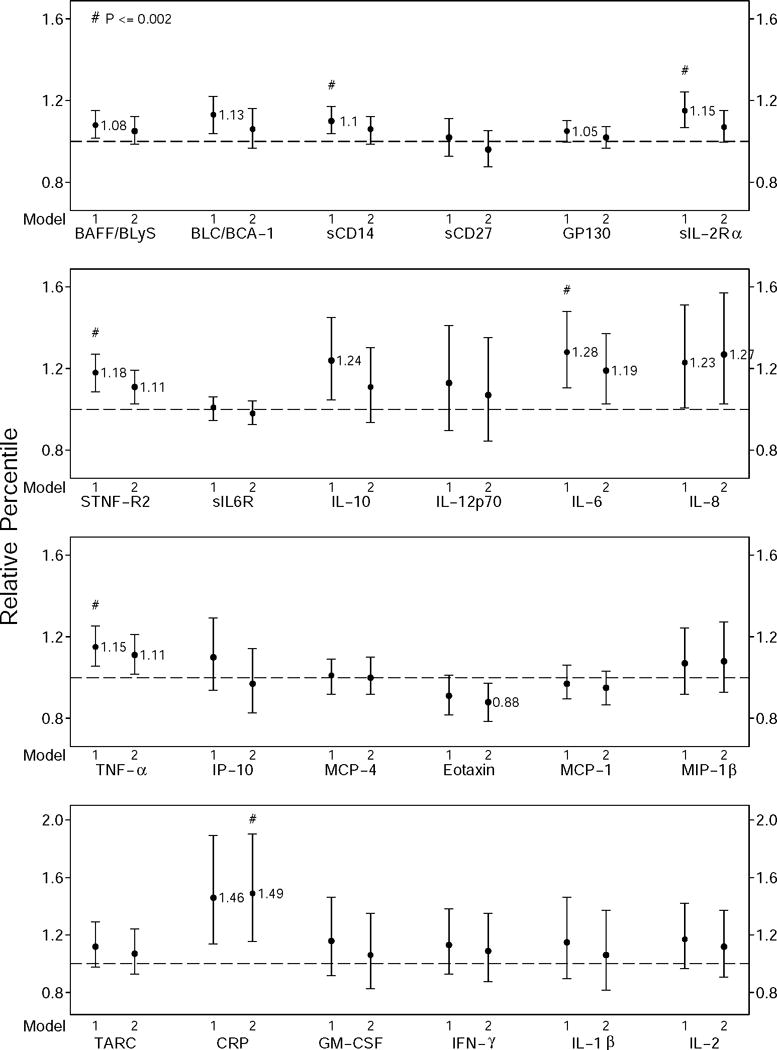
Relative percentile estimates (●) and 95% confidence limits (error bars) of the estimates for the frail HIV+ men compared to nonfrail HIV+ men. Data were generated using generalized gamma regression models for each biomarker adjusted for age, race, study sites, and education (left bars with ●, model 1), as well as further adjusted for BMI, current smoking, presence of depressive symptoms, HCV infection, hypertension, diabetes, dyslipidemia, kidney disease, liver disease, and cancer (right bars with ●, model 2). Relative percentile differences between the frail and nonfrail HIV+ men that were significantly different at p ≤0.002 are indicated by #; those that were significantly different at 0.002 ≤p ≤0.05 are those for which the 95% confidence intervals do not include 1.0.
Among markers with >25% undetectable samples, frail HIV− men were less likely than the nonfrail HIV− men to have detectable IL-1β but this association was only of marginal statistical significance (odds ratio (OR) = 0.40, p = 0.039). This difference persisted in the fully adjusted model (OR = 0.36, p = 0.027). Detectability of other markers did not differ significantly by frailty in either HIV− or HIV+ men.
To compare levels of inflammatory markers in frail HIV− and frail HIV+ men, we used only data from virologically suppressed HIV+ men to exclude the effect of HIV viremia. sCD14, BAFF, IP-10, and CRP were higher in the frail HIV+ men (Supplemental Table 3) but with only marginal significance. They were also higher in the HIV+ nonfrail men than in the HIV− nonfrail men.
Discussion
In this study, the longitudinal data of the MACS allowed us to define frailty based on confirmed expression of the frailty phenotype. This likely resulted in a more conservative and robust classification of frailty and nonfrailty than in previous studies, which have relied on single assessments. We found that compared to HIV+ nonfrail men and after adjustment for multiple comparisons, age, race, study site, and education, HIV+ frail men had significantly (p<0.002) higher serum levels of sCD14, sIL2Rα, sTNF-R2, IL-6, and TNF-α; and higher levels of C-reactive protein (CRP) that were nearly significant (p=.003). After further adjustment for BMI, smoking, and co-morbidities, only CRP was significant at p<0.002, with levels approximately 50% higher in the frail men than in the nonfrail men. All of these markers except sIL2Rα are products of activated monocyte/macrophages, and therefore their elevation suggests greater activation of these cells in the frail HIV+ men. In the general population, serum IL-6 increases with age31, predicts disability12 and mortality11, and is associated with frailty10. CRP, whose production is regulated by IL-6, has the same associations. Thus, the present results are consistent with data from the elderly general population linking frailty to monocyte/macrophage activation.
The present results are also consistent with recent studies in HIV+ populations. Erlandson et al found that low levels of physical functioning, including frailty, were associated with higher levels of CD8 T cell activation (expression of CD38 and HLA-DR) and serum IL-6 in HIV+ people32. Piggott et al23 found that an inflammatory index derived from serum levels of IL-6 and sTNFR1 was significantly associated with the Fried frailty phenotype and with mortality in a large study of HIV+ and HIV− injection drug users. However, Kooij et al18, in a predominantly male Dutch cohort, found that incorporation of inflammatory markers (CRP, d-dimer, sCD163, sCD14) into analytic models did not attenuate the observed association of HIV infection with expression of a modified Fried frailty phenotype. This inconsistency may be explained by the more advanced level of HIV disease in their cohort (e.g., 69.5% had had AIDS) compared to the present study and that of Piggott et al. Overall, the present findings, based on a large cohort, a robust definition of frailty and non-frailty, and quality-assured analysis of a panel of selected inflammatory markers, substantially strengthen the association of frailty with higher inflammation in men with treated HIV infection.
The difference in CRP was of the greatest magnitude, and was the only difference whose significance persisted after adjustment for demographics and co-morbidities which may be involved in the causal pathway for frailty. Most of the significant differences in inflammatory markers between frail and non-frail HIV+ men in the present study became non-significant after adjustment for harmful behaviors and co-morbidities that are common in treated HIV+ men. This finding suggests that these factors contribute to frailty6 through inflammatory pathways, and that they account to some extent for the greater inflammation in the HIV+ men with frailty compared to those who were nonfrail. However, the fact that these factors did not account for all of the inflammation associated with frailty is consistent with earlier studies5,6. Thus, the present study adds to our understanding of inflammation and frailty in people with treated HIV infection, but there is more to be learned about the inflammation that is still independently associated with frailty.
The HIV− men in the present study exhibited fewer significant differences between frail and nonfrail men than did the HIV+ men. This may be related to the smaller number of HIV− men studied, one limitation of using existing biomarker data. Unlike the treated HIV+ participants, for whom the biomarkers were measured in all participants, assessment was done on only a sample of HIV− men. Most of the variables that differed significantly by frailty in the HIV+ men had similar trends in the HIV− men. However, CRP, which was almost 50% higher in frail HIV+ men than in non-frail HIV+ men, was essentially identical in frail and nonfrail HIV− men. This discrepancy may be due at least in part to our deliberate inclusion of all HCV-infected HIV− men in the parent biomarker study; this infection can lower CRP levels33 and was much more common in the HIV− frail men (42%) than in the HIV− nonfrail men (13%). Taken together, the present data suggest that inflammation is associated with frailty in HIV+ men as it is in elderly HIV− populations, but that the mechanisms of this association may differ between HIV− and HIV+ people in the age range studied here.
In this study, serum IL-6 levels were similar in the HIV+ nonfrail men and the HIV− men (whether frail or nonfrail). Previous studies have generally found higher IL-6 levels in HIV+ than HIV− people, even after HAART3. The reason for this discrepancy is unknown but could be related to the fact that previous analyses of IL-6 by HIV status did not take frailty into account. Alternatively, the HIV− men in this study may have had higher than expected IL-6 levels for unknown reasons.
In the present study, the frail HIV+ men had higher levels of sIL2Rα (sCD25) than the nonfrail HIV+ men, providing some evidence of greater T-cell activation. The role of T cell activation in HIV− populations who develop frailty has not been well-defined10.
This study examined the relationship between inflammation and frailty in the context of HIV infection. We carefully selected biomarkers that are considered as reflective of inflammation for inclusion in this study to test our hypotheses. Although this study could be considered exploratory in a sense, because our hypotheses were not restricted to a specific inflammatory marker(s), the study was not without such hypotheses. The proinflammatory cytokine and chemokine panels used for electrochemiluminescence were selected because they covered markers of monocyte activation, T cell activation, and recruitment of monocytes and T cells to sites of inflammation. The B cell cytokine and soluble receptor panel used for the Luminex-based study was custom-designed by the MACS to include markers of specific interest. While some results were not hypothesis-driven, most of the data obtained were directly pertinent to the overall hypothesis of the study, and the fact that clusters of functionally related biomarkers often differed in parallel between frail and non-frail men supports the value of this experimental approach. The likelihood of falsely identifying significant correlations with frailty because of large numbers of markers tested was minimized by using the Bonferroni correction, which is considered conservative34.
The study had some other limitations. First, the temporal relationship between inflammation and onset of frailty could not be determined from this cross-sectional analysis nested in the cohort. Although we have reported that the biomarkers analyzed here were very stable in virologically suppressed HIV+ men after the first year of HAART35, and the participants in this study had been receiving HAART for many years, there could still be some variability in the biomarker trajectory that may be due to frailty. Second, the frailty phenotype used has not been clinically validated in HIV+ men, as it has in HIV− people4. However, this frailty phenotype predicted mortality in a cohort of injection drug users, especially those who were HIV+21, and in the MACS a phenotype similar to the frailty phenotype, termed the frailty-related phenotype, independently predicted decreased AIDS-free survival and overall survival after initiation of HAART36. These data suggest that frailty will predict adverse health outcomes in HIV+ people as it does in the HIV− elderly. Finally, CMV infection has been associated with a higher risk of frailty in the general population in some studies37–41, but we did not have serologic data on CMV infection of the present cohort and so could not assess the relation of this infection to frailty.
Generalizability of the present findings is affected by the fact that the study population represents men who have sex with men, recruited before 2003. This limits generalizability to women and perhaps to men who acquired HIV by other behaviors. The cohort also may not reflect people who have acquired HIV more recently. Because the study population included all HIV+ men in the MACS cohort under antiretroviral treatment and a random sample of the HIV− men other than those with HCV infection, it was not a convenience sample but rather should reflect the underlying cohort. As men who have sex with men still comprise the group with the greatest risk of HIV in the United States, and particularly those aging with HIV, these results on frailty are highly relevant to older HIV-infected persons. In this study, frail men with HIV generally exhibited similar components of the frailty phenotype as frail HIV− men, suggesting that the findings also may have pertinence for the general population of frail people. Other aspects of HIV infection, such as time without antiretroviral treatment, nadir CD4 cell count, and duration of antiretroviral therapy, were similar between frail and nonfrail men with HIV, supporting the interpretation that the differences in inflammation between the two groups were not due to different stages of HIV infection or different levels of viral suppression, although the frail men were more likely to have had a history of clinically-defined AIDS. Also, as mentioned, the relatively small size of the HIV− population in the present study may have limited power to find differences between frail and nonfrail HIV− men.
The mechanisms for the association of immune activation with aging are unknown42. Postulated explanations include a) production of macrophage-activating cytokines by innate and adaptive immune responses against ongoing infections;43;44 b) production of such cytokines by senescent cells;45 immune-activating effects of circulating cell-free DNA46 or microbial components; or c) deficient inhibition of T cell activation47. In the latter connection, number and function of T-regulatory cells, which can suppress immune activation, were not reduced in frail relative to non-frail MACS participants, whether HIV+ or HIV−48. In the present study, markers that were significantly elevated in frail vs nonfrail HIV+ men after adjustment for multiple comparisons (i.e., p<.002) and for age, race, study site, and education, included the proinflammatory cytokines IL-6 and TNF-α, the TNF receptor superfamily members sCD27 and sTNFR2, and the scavenger receptor sCD14, all suggesting monocyte/macrophage activation. CRP was significantly elevated in the frail vs nonfrail HIV+ men after all adjustments including behaviors and comorbidities; since CRP production is regulated by IL-6, produced by monocytes among other cells, this again suggests activation of these cells.
The increased inflammation that exists among virologically suppressed HIV+ persons, and its association with frailty, provide support for monitoring people living with HIV for age-related illnesses. Little is known about trajectories of CRP preceding the onset of frailty, or how the higher levels observed here in frail HIV+ men developed over time. Given the well-established relation between inflammation and aging in the general population, this information may inform mechanisms of aging in general, especially when independent comorbidities can be taken into account. Monocyte activation represents a prime target for such studies.
Supplementary Material
Acknowledgments
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI35042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Jay Bream, Todd Brown, Adrian Dobs, W. David Hardy, Michelle Estrella, Lisette Johnson-Hill, Sean Leng, Anne Monroe, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), John P. Phair, Sheila Badri, Dana Gabuzda, Frank J. Palella, Jr., Sudhir Penugonda, Susheel Reddy, Matthew Stephens, Linda Teplin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels and Otoniel Martínez-Maza (joint-PIs), Aaron Aronow, Peter Anton, Robert Bolan, Elizabeth Breen, Anthony Butch, Shehnaz Hussain, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (Co-PI), James T. Becker, Phalguni Gupta, Kenneth Ho, Susan Koletar, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D’Souza (PI), Alison Abraham, Keri Althoff, Priya Duggal, Sabina Haberlen, Eithne Keelaghan, Alvaro Munoz, Derek Ng, Eric C. Seaberg, Sol Su, Pamela Surkan, Nikolas Wada. Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The MACS website is located at http://aidscohortstudy.org/.
Funding
National Institutes of Health U01-Al35042; U01-Al35039; U01-Al35040; U01-Al35041; UM1-Al35043
National Center for Advancing Translational Sciences: UL1-TR001079
Reference List
- 1.Wada N, Jacobson LP, Cohen M, French AL, Phair J, Munoz A. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol. 2013;177:116–125. doi: 10.1093/aje/kws321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regidor DL, Detels R, Breen EC, et al. Effect of highly active antiretroviral therapy on biomarkers of B-lymphocyte activation and inflammation. AIDS. 2011;25:303–314. doi: 10.1097/QAD.0b013e32834273ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 5.Fried L, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. Journal of Gerontology: Medical Sciences J Gerontol Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 6.Althoff KN, Jacobson LP, Cranston RD, et al. Age, comorbidities and AIDS predict the frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci. 2014;69A:189–198. doi: 10.1093/gerona/glt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64:1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao X, Li H, Leng SX. Inflammation and immune system alterations in frailty. Clin Geriatr Med. 2011;27:79–87. doi: 10.1016/j.cger.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 12.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 13.Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133:456–466. doi: 10.1016/j.mad.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 14.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Leng SX, Tian X, Matteini A, et al. IL-6-independent association of elevated serum neopterin levels with prevalent frailty in community-dwelling older adults. Age Ageing. 2011;40:475–481. doi: 10.1093/ageing/afr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desquilbet L, Jacobson L, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62:1279–1286. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 17.Rees HC, Ianas V, McCracken P, et al. Measuring frailty in HIV-infected individuals. Identification of frail patients is the first step to amelioration and reversal of frailty. J Vis Exp. 2013 doi: 10.3791/50537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kooij KW, Wit FW, Schouten J, et al. HIV infection is independently associated with frailty in middle-aged HIV type 1-infected individuals compared with similar but uninfected controls. AIDS. 2016;30:241–250. doi: 10.1097/QAD.0000000000000910. [DOI] [PubMed] [Google Scholar]
- 19.Greene M, Covinsky KE, Valcour V, et al. Geriatric Syndromes in Older HIV-Infected Adults. J Acquir Immune Defic Syndr. 2015;69:161–167. doi: 10.1097/QAI.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brothers TD, Kirkland S, Guaraldi G, et al. Frailty in people aging with human immunodeficiency virus (HIV) infection. J Infect Dis. 2014;210:1170–1179. doi: 10.1093/infdis/jiu258. [DOI] [PubMed] [Google Scholar]
- 21.Piggott DA, Muzaale AD, Mehta SH, et al. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS ONE. 2013;8:e54910. doi: 10.1371/journal.pone.0054910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akgun KM, Tate JP, Crothers K, et al. An adapted frailty-related phenotype and the VACS index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. J Acquir Immune Defic Syndr. 2014;67:397–404. doi: 10.1097/QAI.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piggott DA, Varadhan R, Mehta SH, et al. Frailty, Inflammation, and Mortality Among Persons Aging With HIV Infection and Injection Drug Use. J Gerontol A Biol Sci Med Sci. 2015;70:1542–1547. doi: 10.1093/gerona/glv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 25.Brown T, Wang Z, Chu H, et al. Longitudinal anthropometric changes in HIV-infected and HIV-uninfected men. J Acquir Immune Defic Syndr. 2006;43:356–362. doi: 10.1097/01.qai.0000243052.73321.8e. [DOI] [PubMed] [Google Scholar]
- 26.Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29:463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKay HS, Margolick JB, Martinez-Maza O, et al. Multiplex assay reliability and long-term intra-individual variation of serologic inflammatory biomarkers. Cytokine. doi: 10.1016/j.cyto.2016.09.018. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chowdhury F, Williams A, Johnson P. Validation and comparison of two multiplex technologies, Luminex and Mesoscale Discovery, for human cytokine profiling. J Immunol Methods. 2009;340:55–64. doi: 10.1016/j.jim.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 29.CDC. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 30.Cox C, Chu H, Schneider MF, Munoz A. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med. 2007;26:4352–4357. doi: 10.1002/sim.2836. [DOI] [PubMed] [Google Scholar]
- 31.Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41:176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 32.Erlandson KM, Allshouse AA, Jankowski CM, et al. Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Infect Dis. 2013;208:249–259. doi: 10.1093/infdis/jit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah S, Ma Y, Scherzer R, et al. Association of HIV, hepatitis C virus and liver fibrosis severity with interleukin-6 and C-reactive protein levels. AIDS. 2015;29:1325–1333. doi: 10.1097/QAD.0000000000000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of Royal statistical Society. 1995;57:289–300. [Google Scholar]
- 35.Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2014;20:463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desquilbet L, Jacobson L, Fried LP, et al. A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men. J Gerontol A Biol Sci Med Sci. 2011;66A:1030–1038. doi: 10.1093/gerona/glr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haeseker MB, Pijpers E, Dukers-Muijrers NH, et al. Association of cytomegalovirus and other pathogens with frailty and diabetes mellitus, but not with cardiovascular disease and mortality in psycho-geriatric patients; a prospective cohort study. Immun Ageing. 2013;10:30. doi: 10.1186/1742-4933-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matheï C, Vaes B, Wallemacq P, Degryse J. Associations between cytomegalovirus infection and functional impairment and frailty in the BELFRAIL Cohort. J Am Geriatr Soc. 2011;59:2201–2208. doi: 10.1111/j.1532-5415.2011.03719.x. [DOI] [PubMed] [Google Scholar]
- 39.Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 40.Vallejo AN, Hamel DL, Jr, Mueller RG, et al. NK-like T cells and plasma cytokines, but not anti-viral serology, define immune fingerprints of resilience and mild disability in exceptional aging. PLoS ONE. 2011;6:e26558. doi: 10.1371/journal.pone.0026558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang GC, Kao WH, Murakami P, et al. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol. 2010;171:1144–1152. doi: 10.1093/aje/kwq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michaud M, Balardy L, Moulis G, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14:877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Ferrando-Martinez S, Ruiz-Mateos E, Casazza JP, et al. IFNgamma-TNFalpha-IL2-MIP1alpha-CD107a+PRF1+ CD8 pp65-Specific T-Cell Response Is Independently Associated With Time to Death in Elderly Humans. J Gerontol A Biol Sci Med Sci. 2014 doi: 10.1093/gerona/glu171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Compte N, Zouaoui BK, Vanhaeverbeek M, et al. Frailty in old age is associated with decreased interleukin-12/23 production in response to toll-like receptor ligation. PLoS ONE. 2013;8:e65325. doi: 10.1371/journal.pone.0065325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tchkonia T, Zhu Y, van DJ, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jylhävä J, Nevalainen T, Marttila S, Jylha M, Hervonen A, Hurme M. Characterization of the role of distinct plasma cell-free DNA species in age-associated inflammation and frailty. Aging Cell. 2013;12:388–397. doi: 10.1111/acel.12058. [DOI] [PubMed] [Google Scholar]
- 47.Ryan SO, Johnson JL, Cobb BA. Neutrophils confer T cell resistance to myeloid-derived suppressor cell-mediated suppression to promote chronic inflammation. J Immunol. 2013;190:5037–5047. doi: 10.4049/jimmunol.1203404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang W, Nilles TL, Johnson JR, Margolick JB. Regulatory T cells, frailty, and immune activation in men who have sex with men in the Multicenter AIDS Cohort Study. J Gerontol A Biol Sci Med Sci. 2015;70:1533–1541. doi: 10.1093/gerona/glv132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


