Abstract
Dual-energy X-ray absorptiometry (DXA) provides a noninvasive way to determine lean tissue mass (LTM), fat mass (FM), bone mineral content (BMC), and bone mineral density (BMD) in humans and small mammals. Live channel catfish (n=74, 78g – 1200 g) were anesthetized and scanned in both a lateral position and a dorsa-ventral position. Six individual fish (300g – 600g) were scanned five times each to determine precision by the coefficient of variation. Precision was good for LTM (0.75–1.06%) and for BMC and BMD (2–2.6%). Precision for FM was not good (27–34%), which was due to the very low FM (0–1g) recorded by the DXA. However, using the predicted values, FM precision improved to 5–5.5%. DXA values for LTM, FM, and BMC were significantly different from chemical analysis (P< 0.001). DXA overestimated LTM and underestimated FM and BMC. However, all three compartments were strongly correlated with carcass values (P <0.0001). Using the prediction equations and the jackknife procedure, predicted values of LTM, FM, and BMC were not significantly different from the carcass values (P >0.05). DXA may also be a valuable tool for evaluating body condition longitudinally in commercial or in threatened or endangered fish species, where non-invasive procedures would be invaluable.
Introduction
The goal of a producer is to obtain a quality, marketable-sized fish in the shortest amount of time for the lowest cost. The ability to reach this goal is accomplished primarily through feed formulation and feed management and their inherent costs. Feeds can differ greatly in ingredient composition and macronutrient content. Different ratios of macronutrients greatly influence body composition in different fish species including hybrid striped bass (Nematipour et al. 1992), tilapia (El-Sayed and Garling 1988), channel catfish fingerlings (Garling and Wilson 1977), and adult channel catfish (Garling and Wilson 1977). With protein being a costly ingredient, primary energy sources such as carbohydrates and lipids constitute a large percentage of feeds. Consequently, decreasing the amount of protein in a diet can reduce costs associated with feed production; however, increased lipid and carbohydrate levels can lead to increased fat in the intraperitoneal cavity, fillet, and liver of the fish (Craig and Helfrich 2002). Increases in fat content in the fillet can not only decrease the amount of the fillet being processed but it can also affect the quality of the fillet itself. Fat deposition has been shown to result in an off-flavor in the taste of the fish as well as lead to poor storage quality (Tucker and van der Ploeg 1999; Bosworth and Wolters 2001).
Evaluation of the biochemical composition of fish generally requires destructive sampling. This assessment process is very time consuming and destructive sampling also results in the loss of potential marketable product. The ability to determine the composition of fish noninvasively could provide for longitudinal studies of individual organisms while modifying other factors such as feed content or stocking density. Knowledge of those factors that influence body composition and, as a consequence, fillet quality can have substantial economic benefit in animal husbandry technologies.
Dual-energy X-ray absorptiometry (DXA) operates by scanning the subject with two low-dose X-rays of differing energy levels. The amount of energy absorbed by the tissues is detected and used to differentiate between bone and soft tissue, including fat and lean tissue composition (Nagy, 2001). DXA provides measures of fat mass (FM), lean tissue mass (LTM), bone mineral content (BMC), and bone mineral density (BMD). Although designed for humans, DXA has been used to accurately determine body composition of smaller mammals such as mice, rats, and lemmings (Nagy and Clair 2000; Nagy et al. 2001; Hunter and Nagy 2002) and reptiles such as snakes (Secor and Nagy 2003). DXA could be a valuable predictor of the body composition of fish, although to date only one study has evaluated its use (Wood 2004). The ability of the producer to be able to predict or control the quality of the fish would be an important tool in fisheries and aquaculture. The purpose of this experiment is to validate DXA in predicting the body composition of channel catfish.
Materials and Methods
A mixed-sex population of channel catfish (n=74) ranging in size from 78 to 1200g were reared in 0.4-hectare ponds at Gadsden State Community College, Alabama. Fish were either fed Cargill Catfish Food (32% protein) ad libitum or held off feed to provide variation in the nutritional status of the fish. Catfish were harvested using seines and single-line sampling.
Live catfish were transported to UAB in chilled water and anesthetized using tricaine methanesulfonate (MS222). Each fish was then weighed and scanned using a GE Lunar, Madison, WI, Prodigy DXA scanner (small animal software v. 3.60.031). Each fish was scanned twice, once laterally and once dorsa-ventrally to determine if position influenced the scans. To determine precision, six catfish (300 – 600g) were scanned five times each (with repositioning between scans) in both the lateral and dorsa-ventral (d-v) positions. The DXA scans gave values of BMC (g), FM (g) and lean mass (g), which were compared to the chemical values of ash, fat and lean mass.
After scanning, the fish were euthanized by an overdose of MS222 and dissected to determine sex. Intraperitoneal fat (IP fat), ovaries (if female), and fillets were removed from each fish and weighed. The remaining frame (carcass minus IP fat, ovaries, and fillets) was also weighed. All samples were placed in a convection oven to dry at 60 C until constant mass was achieved (<1% change in 24 h) to determine water content. IP fat, ovaries, and fillets typically dried in 3–4 d, whereas frames generally dried in 7–9 d. The difference in weight before drying versus after drying was assumed to be water loss and was calculated to be the water content of the fish. Ovaries and fillets were ground to a powder using a mortar and pestle. Frames were minced into small pieces with scissors. The dry samples were then placed in cellulose thimbles in the Soxhlet apparatus overnight with circulating petroleum ether to determine fat content (Dobush et al. 1985). Frames were divided into triplicate subsamples for fat determination. Samples were weighed before and after the Soxhlet procedure. The difference in weights was determined to be fat and calculated as a percentage of the whole tissue/component. Ash was determined by burning the fat-free dry samples in a muffle furnace overnight at 600 C. Based on preliminary studies, ash from the IP fat was determined to be <1% and levels were considered to be negligible. Ash content was calculated by multiplying the percent ash by the fat-free dry tissue. Ash is a measure of inorganic mineral content and as the majority of inorganic mineral is found in bone it is used as a measure of BMC. LTM was determined as fat-free mass minus ash content plus water content. Each individual component was then added together to calculate the total water, fat, lean, and ash content of each catfish.
The effect of scanning position was analyzed by paired t tests, and the DXA data were compared to chemical analysis determinations by paired t tests and regression analyses. Residual plots were calculated to show any bias in the measurements. Prediction equations were generated using backward elimination with BMC, FM, and LTM as variables. Variables were omitted from the equations if P>0.1. Testing prediction equations usually involves applying them to a separate independent group in a cross validation. However, as there was no cross-validation group in this study, the equations were tested using the jackknife procedure (Sokal and Rohlf 1995), in which the data for each fish were removed in turn and equations developed on the data minus that fish. The data for this fish were then placed into these equations to obtain predicted values. These predicted values were then compared with chemical carcass analyses using paired t tests. Graphs are shown for the lateral scan only as the data were very similar for the lateral and d-v positions. All statistical analyses were performed using SAS (Version 9.1; SAS Institute, Cary, NC). Data were considered significant when P≤ 0.05.
Results
Precision
Precision (as indicated by the coefficient of variation, CV) was assessed for both scanning positions and the data are shown in Table 1. There was a trend for the precision for lean tissue mass to be better for the lateral scan than the d-v scan (P =0.087), however both precisions were very good (1.06% d-v vs. 0.75% lateral). Scanning position did not affect FM precision (P =0.383), but precision was not good (−42 vs. 109%). This was due mainly to the data from one fish whose precision was −418% for the d-v and 518% for the lateral scan. This large CV was due to the FM measured by DXA being around 0–1g with a range of 6 and 10g respectively. This range for FM was not unlike that of the other fish, however, when combined with the very low mean FM, it resulted in a very large CV. When this point was removed the precision for FM was improved to 34 and 27% for the d-v and lateral scans respectively. There were no significant differences in the precision of BMD (P =0.561) or BMC (P =0.780) between the two positions. Precision was 2–2.6% for both bone parameters.
TABLE 1.
Precision of the dual-energy X-ray absorptiometry.a
| Dorsa-ventral CV (%) |
Lateral CV (%) |
P-value | |
|---|---|---|---|
| Bone mineral density (g/cm2) | 2.27 ± 0.43 | 2.11 ± 0.33 | 0.561 |
| Bone mineral content (g) | 2.45 ± 0.24 | 2.59 ± 0.48 | 0.780 |
| Area (cm2) | 2.39 ± 0.27 | 2.16 ± 0.14 | 0.455 |
| Total tissue (g) | 0.44 ± 0.04 | 0.34 ± 0.05 | 0.203 |
| Lean tissue mass (g) | 1.06 ± 0.08 | 0.75 ± 0.17 | 0.087 |
| Fat mass (g) | 41.63 ± 76.27 | 108.75 ± 82.41 | 0.383 |
| Fat mass (g)* | 33.70 ± 14.57 | 26.82 ± 10.84 | 0.270 |
CV – coefficient of variation; Fat mass (g)* = CV for fat mass after removing the fish with a mean fat mass of close to zero.
Data presented are mean coefficients of variation of 5 repeated scans on six fish.
Accuracy
The mean values for the carcass parameters and the DXA results for both scanning positions are shown in Table 2. DXA significantly overestimated lean mass (P <0.0001) by more than 69g (17%) for both positions. The two DXA positions were significantly different from each other (P =0.0011) with the lateral lean mass being lower than the d-v lean mass by more than 2g. Both scanning positions were significantly related to carcass lean (P <0.0001, dorsa-ventral r2=0.99, lateral r2=0.99) (Fig. 1A). There was a significant bias in the overestimation of carcass lean (P <0.0001) (Fig. 1B). The greater the lean mass, the larger the overestimation.
TABLE 2.
| Carcass analysis | Dorsa-ventral DXA | Lateral DXA | |
|---|---|---|---|
| Lean mass (g) | 402.17 ± 24.08a | 474.31 ± 28.42b | 471.96 ± 28.35c |
| Fat mass (g) | 40.04 ± 2.54a | 6.96 ± 1.98b | 10.62 ± 2.03c |
| Bone mineral content/ash (g) | 28.01 ± 1.85a | 10.66 ± 0.65b | 10.45 ± 0.63c |
DXA = dual-energy X-ray absorptiometry
Data are presented as means ± sem
Different letters represent values that are significantly different from each other (P<0.01).
FIGURE 1.
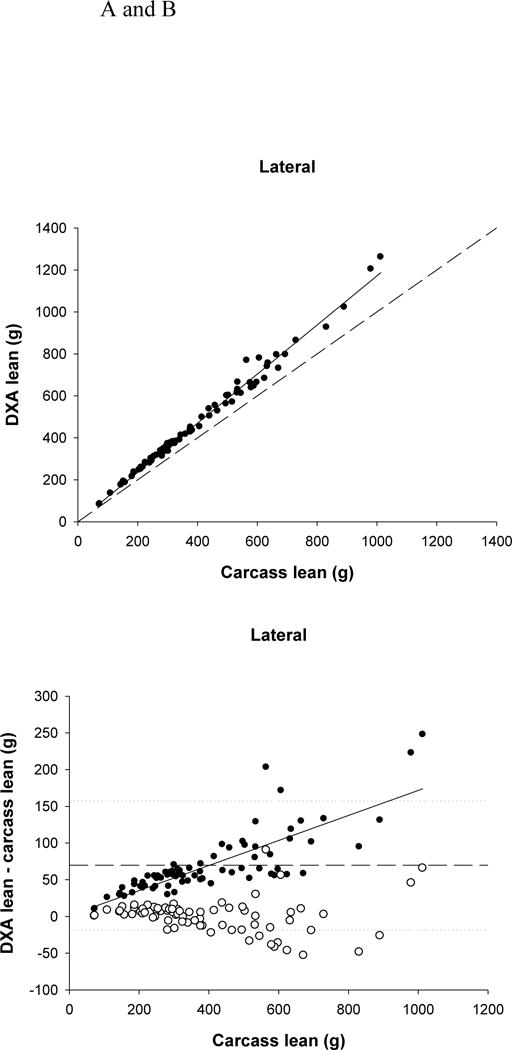
(A) Relationship between carcass lean and dual-energy X-ray absorptiometry (DXA) lean tissue mass (LTM) (lateral position, r2=0.989, P<0.0001). The dashed line represents the line of identity, and the solid line is the regression line.
(B) Residual plot of carcass lean and the difference between carcass and DXA LTM (for the lateral position; filled circles). The solid line represents the regression line (P<0.0001) indicating a significant bias in the relationship. The dashed line represents the mean difference between DXA and carcass lean, with the dotted lines representing ± 2 SDs from the mean. The open circles represent the difference between the predicted lean (using the equations) and carcass lean.
DXA significantly underestimated FM (P <0.0001) by more than 29g (73.5%) for both positions, and the lateral FM was significantly higher than the d-v FM (P <0.0001) by about 3.7g. Both DXA positions were significantly related to carcass fat (P <0.0001, d-v r2=0.50, lateral r2=0.64) (Fig. 2A). There was a significant bias in the underestimation of carcass fat (P <0.0001), with the underestimation being greater with larger amounts of fat (Fig. 2B).
FIGURE 2.
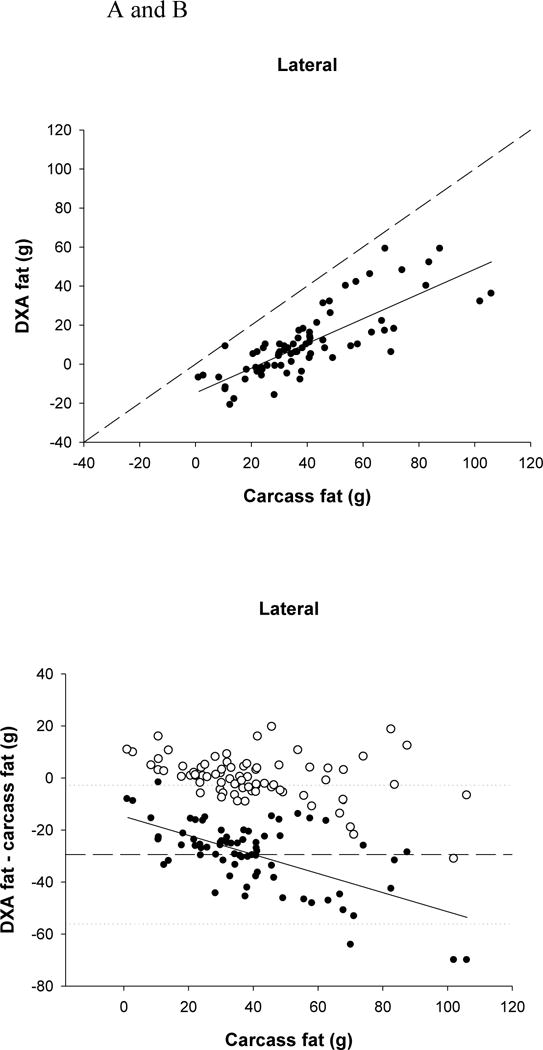
(A) Relationship between carcass fat and dual-energy X-ray absorptiometry (DXA) fat (lateral position r2=0.638, P<0.0001). The dashed line represents the line of identity, and the solid line is the regression line.
(B) Residual plot of carcass fat and the difference between carcass and DXA fat (for the lateral position; filled circles). The solid line represents the regression line (P<0.0001) indicating a significant bias in the relationship. The dashed line represents the mean difference between DXA and carcass fat, with the dotted lines representing ± 2 SDs from the mean. The open circles represent the difference between the predicted fat (using the equations) and carcass fat.
DXA significantly underestimated ash for both scanning positions (P <0.0001) by approximately 17.5g (62%). In addition, the lateral scan BMC value was significantly lower than d-v BMC (P =0.002), but only by 0.2g. However, both DXA values of BMC were significantly related to carcass ash (P <0.0001, d-v r2=0.83, lateral r2=0.83) (Fig. 3A). There was a significant bias in the underestimation of ash (P <0.0001) (Fig. 3B). The higher the ash content, the greater the underestimation by DXA.
FIGURE 3.
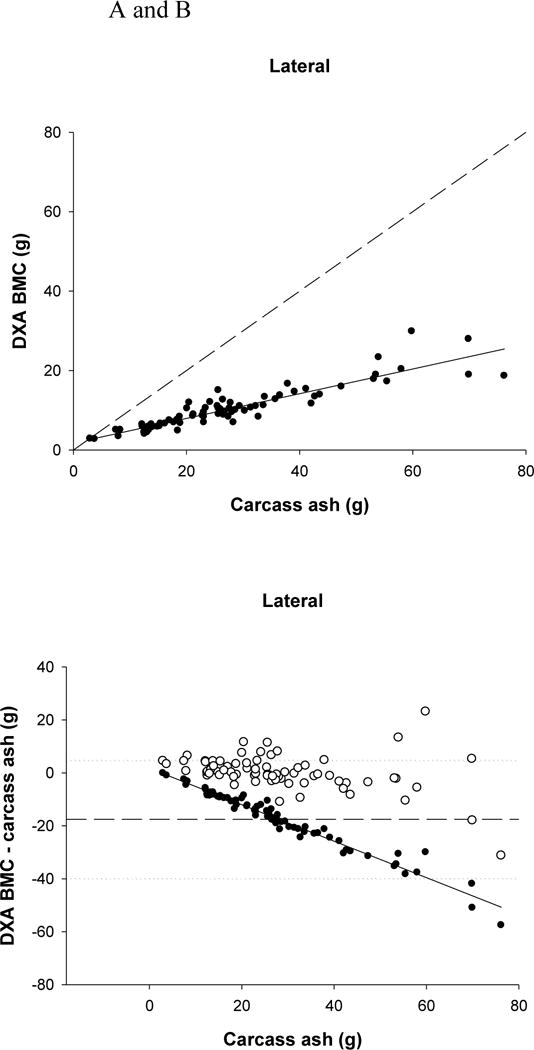
(A) Relationship between carcass ash and dual-energy X-ray absorptiometry (DXA) bone mineral content (BMC) (lateral position r2=0.831, P<0.0001). The dashed line represents the line of identity, and the solid line is the regression line.
(B) Residual plot of carcass ash and the difference between carcass ash and DXA BMC (for the lateral position; filled circles). The solid line represents the regression line (P<0.0001) indicating a significant bias in the relationship. The dashed line represents the mean difference between DXA and carcass values, with the dotted lines representing ± 2 SDs from the mean. The open circles represent the difference between the predicted BMC (using the equations) and carcass ash.
Prediction equations were developed using backward elimination regression analyses with all DXA parameters entered at the start of the model (Table 3). Both carcass ash and carcass fat were best predicted using equations containing both DXA BMC and DXA fat, whereas carcass lean was best predicted by an equation using only DXA lean. The predicted values from the equations in Table 3 were compared against the carcass values using the jackknife procedure. Predicted lean mass was not significantly different from carcass lean for either the d-v (P =0.946; 0.19g/0.05%) or the lateral (P =0.959; 0.14g/0.03%) positions. Predicted FM was not significantly different from carcass fat for either the d-v (P =0.947; 0.07g/0.17%) or the lateral position (P =0.924; 0.09g/0.22%). Predicted BMC was not significantly different than carcass ash for either the d-v (P =0.642; 0.4g/1.43%) or the lateral (P =0.952; 0.05g/0.18%) positions. In addition, all predicted values were highly significantly related to carcass values (P <0.001), with intercepts that were not significantly different than zero (P >0.05) and slopes that were not significantly than 1 (P >0.05).
TABLE 3.
Prediction equations for DXA-predicted body components.
| Position | Prediction equationsa | Model r2 | P value |
|---|---|---|---|
| Dorsa-ventral | Carcass Lean (g) = 0.842(DXA LTM) + 2.72 | 0.99 | < 0.001 |
| Lateral | Carcass Lean (g) = 0.845(DXA LTM) + 3.50 | 0.99 | < 0.001 |
| Dorsa-ventral | Carcass Fat (g) = 0.513(DXA fat) + 2.64(DXA BMC) + 8.35 | 0.86 | < 0.001 |
| Lateral | Carcass Fat (g) = 0.574(DXA fat) + 2.38(DXA BMC) + 9.11 | 0.87 | < 0.001 |
| Dorsa-ventral | Carcass Ash (g) = 2.433(DXA BMC) + 0.112 (DXA Fat) +1.29 | 0.84 | < 0.001 |
| Lateral | Carcass Ash (g) = 2.441(DXA BMC) + 0.126 (DXA Fat) +1.17 | 0.84 | < 0.001 |
BMC = bone mineral content; DXA = dual-energy X-ray absorptiometry; LTM = lean tissue mass,
Backward elimination regression techniques were used. All models started with DXA BMC, DXA LTM, and DXA fat as independent variables. Variables were removed from the model if P> 0.1.
Discussion
The ability to assess body composition in live fish is an important development in fish culture management. In addition, carcass quality can be rapidly estimated in an entire fish or within fillets. This study demonstrates that DXA can be used to accurately determine LTM, FM and ash in channel catfish. By identifying factors that can promote lean tissue growth and prevent excessive fat production, strategies can be developed to provide maximal growth and minimize costs for the fish producer, as well as provide a quality product for the consumer.
There were no significant differences in the precision of the scans between the lateral and d-v positions. However, there was a trend for greater precision for lean mass when scanned in the lateral position (P =0.087). In addition, scanning in the lateral position gave values of FM that were closer to the chemical carcass value for fat, and had a greater r2 value than the d-v position albeit very small. Lean mass from the lateral scan was also closer to the carcass value, and the r2 values for both BMC and lean mass were higher for the lateral scan, although the differences were not as great as for FM. Both positions underestimated fat and bone and overestimated lean mass, however given the choice of positions, the data from this study suggest that the lateral position should be used. The significance of this finding is that most fishes, due to anatomical constraints could not be easily scanned from the top (too laterally compressed) and thus the lateral position would be the only option.
The precision (CV) of the DXA was very good for LTM (around 1.0%) and BMC (around 2.5%), but was fairly large for FM (around 30%). This large CV for FM was due, at least in part, to the low mean value for fat from DXA (close to zero), which meant that any variation in this value led to a very high CV (calculated as the SD divided by the mean). Using the prediction equations obtained from the accuracy analysis to adjust the precision data did not affect the precision of lean mass or bone much, but it did improve the CV for fat from 30% down to 5–5.5%. This indicates that the poor precision of the unadjusted fat values was mainly due to the very low values of fat artificially elevating the CV. These precision values of 1–2% for lean and bone mass were similar to those observed for lemmings (Hunter and Nagy 2002), voles (Stevenson and van Tets 2008), water snakes (Secor and Nagy 2003), small birds (Korine et al. 2004), chickens (Swennen et al. 2004) and mice (Nagy and Clair 2000). The precision for FM in catfish (5.5%) was better than the 9.2% observed in water snakes (Secor and Nagy 2003), and 7% in voles (Stevenson and van Tets 2008), similar to chickens (6%; Swennen et al. 2004), small birds (4%; Korine et al. 2004), and lemmings (4%; Hunter and Nagy 2002), but not as good as the DXA fat precision for mice (2.2%; Nagy and Clair 2000). Although there has been one previous validation of DXA in fish (Wood 2004), the study only looked at accuracy and not precision in hybrid striped bass.
DXA underestimated the amount of ash when compared to chemical analysis. This is probably due, at least in part, to the DXA only quantifying the minerals associated with bone, whereas the carcass value for ash includes not only the minerals from bone but also minerals that are present throughout the body in all cells. In addition, some of the catfish may have had food in their gut, which may not have been detected by DXA, but would have been measured by chemical analysis. Therefore, ash is probably an overestimation of BMC. To examine this in the current study, data were divided into the fed and feed-deprived groups and the overestimation determined for the groups separately. The feed-deprived fish had an average overestimation of 14g compared with 21g for the fed fish. This suggests that gut fill in this study may have contributed to approximately a third of the overestimation. While the entire discrepancy cannot be completely resolved, it may help to fast the fish for 24 h to ensure that their guts are cleared of contents prior to DXA scanning. In addition, Nagy and Clair (2000) found that 1.17% of non bone tissue (fat and lean) in mice was composed of ash. There is no figure for what this may be in fish, however using this value (from mice) would account for about 5.2g of the 17.5g discrepancy in bone. So while nonbone ash does contribute to the discrepancy it does not explain it completely. Other DXA validation studies have shown significant underestimations of ash by 45% in chickens (Swennen et al. 2004), 24% in lemmings (Hunter and Nagy 2002), and cats and dogs (Speakman et al. 2001). The underestimation of 17.5g in this study is very similar to the 14–28g (41–75%) underestimation in adult hybrid striped bass (Wood 2004). By using the prediction equations (and the jackknife procedure), the discrepancy between DXA and chemical analysis in this study improved from 62% to less than 1.5%, which was not significantly different than the carcass value.
DXA was also shown to underestimate FM, as was found in the validation of hybrid striped bass (Wood 2004). This underestimation varied between 74 and 90g (62–74%) depending on machine and scanning settings in bass compared with 73.5% in catfish. Using the jackknife procedure, the model improved for the detection of FM (r2 = 0.86), and comparing the predicted values to the carcass values resulted in no significant difference, indicating that total fish fat can be accurately predicted using DXA analysis. The predicted values were only 0.2% different than chemical values, which were much improved from the raw values of 73.5%. There has been wide variability in the accuracy of DXA to measure FM, ranging from 0.6% in small birds (Korine et al. 2004), 2% in cats and dogs (Speakman et al. 2001), 4% in lemmings (Hunter and Nagy 2002), 6% and 15% in chickens (Mitchell et al. 1997; Swennen et al. 2004), to 14% in water snakes (Secor and Nagy 2003) and 109% in mice (Nagy and Clair 2000). These average differences can be very deceiving by masking larger individual differences. For example, although the discrepancy was only 2% in cats and dogs (Speakman et al. 2001), the range was much higher, from an underestimation of 21% to an overestimation of 32%. Similarly the range in chickens was from −45% to +84%, even though the average was 15% (Mitchell et al. 1997). Importantly the discrepancy in this study was eliminated when the predicted values were used.
Lean mass was overestimated by DXA in this study by 17%. This is similar to the validations in hybrid striped bass (7–25%; adding water and protein; Wood 2004), and chickens (15%; Swennen et al. 2004), but greater than in lemmings (8%; Hunter and Nagy 2002), mice (3%; Nagy and Clair 2000), cats and dogs (2.6%; Speakman et al. 2001), water snakes (2%; Secor and Nagy 2003) and small birds (0.01%; Korine et al. 2004). After using the jackknife procedure in this study to get the predicted values, the r2 values remained very high (0.98) and importantly the slope of the relationship fit the line of identity more closely. In addition, the predicted values for lean mass were not significantly different from the carcass values. Using the predicted values improved the discrepancy between DXA and chemical values from 17 to 0.05%. The errors in both the catfish data (this study) and chickens (Swennen et al. 2004) were almost eliminated after using the predicted values.
In the validation of juvenile hybrid striped bass (Wood 2004), calculating the predicted values from validations of two DXA machines, did not eliminate the discrepancies between the DXA and chemical values. For the PIXI, predicted fat and ash values were 28 and 50% different from chemical values, and for the high resolution pencil beam DXA the differences were 16 and 35% respectively. The higher discrepancy in the PIXI values was likely due to the scanning area being too small to image the entire fish; however, this was not applicable to the pencil beam DXA.
From the residual plots (Figs. 1–3B) it appeared that there was higher variation with the larger fish compared with the smaller fish even in the predicted values, especially for lean mass and ash. This would suggest that DXA may be better at predicting body composition in small fish. However, when the data were replotted as the difference as a percentage, rather than in grams, this pattern disappears. There are no longer greater differences between DXA and chemical values with larger fish. In fact, the relationship may be reversed in fat and ash, with the very smallest fish having a large percentage difference. Therefore, proportionately there is no difference in the variation.
Though DXA was not originally designed for nonmammalian animals, it has proved to be comparable to other non-invasive techniques previously validated for fish. Brown et al. (1993) evaluated total body electrical conductivity (TOBEC) on hybrid sunshine bass. Overall, TOBEC proved useful when measuring lean mass (r2 = 0.99), fat (r2 = 0.93), and ash (r2 = 0.86). For fat analysis, TOBEC predicted a higher value than observed (0.5g or 13%) and a lower value for ash content (0.45g or 17.4%). Fischer et al. (1996) showed that TOBEC could be used to determine lean mass (r2 = 0.99) and nonpolar lipids (r2 = 0.81) in bluegills, Lepomis macrochirus, with a difference of 0.3% in the measurement of nonpolar lipids. This was similar to the 0.2% difference for DXA fat in this study. Barziza and Gatlin (2000) had similar findings with largemouth bass (lean r2 = 0.99, lipid content r2 = 0.87, and ash r2 = 0.87), however body weight and length proved to be just as good or better at predicting body composition than the TOBEC reading. Using weight and length results in differences of 1.8% for lean mass, and more than 22% for both ash and FM. Validating TOBEC for channel catfish, Jaramillo et al. (1994) calculated values for lean, lipid content, and ash as r2 = 0.99, 0.90, and 0.86 respectively. Using the prediction equations, Jaramillo et al. (1994) found that TOBEC-predicted ash was 0.09g or 3.3% lower than observed; predicted lean was 0.28g or 0.4% higher than observed; and predicted lipid was 0.28g or 3.6% lower than observed. These differences for the predicted TOBEC values were all higher than the DXA-predicted values in this study. Although all three studies were successful in accurately predicting the body composition of the fish, the authors stress the importance of placement of the fish in the apparatus (Fischer et al. 1996) as well as the temperature of the subject and the temperature of the surrounding environment (Fischer et al. 1996; Barziza and Gatlin 2000). Brown et al. (1993) reported that care must be taken by the individual operator of the equipment used to determine body composition, as the results were greatly affected by slight variations in the procedure. They suggested more research was necessary to evaluate poikilotherms before TOBEC can be used as a reliable indicator.
Bosworth and Wolters (2001) evaluated carcass composition in channel catfish, Ictalurus punctatus using bioelectrical impedance analysis (BIA). R2 values ranged from 0.41 for fillet moisture to 0.62 for fillet fat, 0.75 for carcass fat, and 0.65 for carcass moisture. These results showed that there was a linear correlation between resistance and carcass and fillet fat (albeit not a strong correlation). The mean accuracy was shown to be 0.51 and 0.98% for carcass and fillet fat, and 0.55 and 1.49% for carcass and fillet moisture. These researchers concluded that BIA has the potential to be a reliable predictor of body composition, but further validation needs to be completed.
The use of ultrasound imagery to determine meat yield in channel catfish was assessed by Bosworth et al. (2001), using three variable models. The calculated r2 values were carcass (0.38), whole fillet (0.31), and shank fillet (0.31) for males and carcass (0.54), whole fillet (0.48), and shank fillet (0.56) for females. This study showed that ultrasound measurements were moderately correlated to meat yield in catfish; however, the authors stress the need for improved accuracy.
In this study, body weight was a significant predictor of fat, lean and ash content (P’s<0.001), with r2 values of 0.81, 0.99 and 0.78 respectively. These values were similar to those from the DXA values, with DXA explaining approximately 4% more of the variation in fat and ash mass than body weight, however there was no difference for lean mass, as both explained almost all of the variation (99%).
DXA can be used to predict body composition for channel catfish, Ictalurus punctatus. DXA provides a fast, reliable, and noninvasive measurement of body composition. Individual scans take only a few minutes (3–5) and can be accomplished while the fish is under general anesthesia, providing for longitudinal studies for fish under experimental trials. Data from this study showed that the overall position of the fish on the scanning bed did not significantly affect the results. One potential disadvantage of the GE Prodigy DXA, other than the cost, is its lower weight limit of approximately 250g. In this study, there were several fish (n=12) that weighed less than 250g. To determine whether the inclusion of these small fish affected the relationships, data were reanalyzed excluding these 12 fish. Rather than improving the relationships, removal of these fish resulted in a drop in the r2 values of approximately 0.05 for fat and BMC and of 0.005 for lean mass. Therefore, the inclusion of these fish actually improved the relationships between DXA and chemical carcass measures. However, this may be simply due to the extension of the range of values over which the prediction equations were developed and caution should be used if the majority of the fish are below the 250g limit. Also, because DXA employs only two X-ray beams, it can only distinguish between two types of tissue at one time. Therefore, when DXA scans an area containing both bone and soft tissue, it is unable to differentiate the soft tissue into fat or lean and must estimate based on the surrounding tissue. This can lead to errors in the estimation of fat and lean mass close to bone. An additional disadvantage of DXA is that currently it is not ‘field’ compatible, as it requires relative sophisticated equipment and a trained operator. However, the accuracy and precision of DXA is at least comparable to BIA and TOBEC, and is actually superior to TOBEC in some studies when used to measure body composition in fish. Consequently, DXA could be an important tool for studies investigating factors that influence body composition in scientific trials before management strategies can be adopted by producers. Once validated, DXA could be a valuable tool for evaluating fish in traditional freshwater or saltwater fisheries, or in evaluating threatened or endangered species.
Acknowledgments
This work was supported by the UAB Animal Models Core (P30DK056336 and P30DK079626) including the Aquatic Animal Research Subcore, of the Nutrition and Obesity Research Center at UAB. We also appreciate the Department of Biology at UAB for ongoing support.
Literature Cited
- Barziza DF, Gatlin DM., III An evaluation of total body electrical conductivity to estimate body composition of largemouth bass Micropterus salmoides. Aquatic Living Resources. 2000;13:439–447. [Google Scholar]
- Bosworth BG, Wolters WR. Evaluation of bioelectric impedance to predict carcass yield, carcass composition, and fillet composition in farm-raised catfish. Journal of the World Aquaculture Society. 2001;32(1):72–78. [Google Scholar]
- Bosworth BG, Holland M, Brazil BL. Evaluation of ultrasound imagery and body shape to predict carcass and fillet yield in farm-raised catfish. Journal of Animal Science. 2001;79:1483–1490. doi: 10.2527/2001.7961483x. [DOI] [PubMed] [Google Scholar]
- Brown ML, Gatlin DM, III, Murphy BR. Non-destructive measurement of sunshine bass, Morone chrysops (Rafinesque) × Morone saxatilis (Walbaum), body composition using electrical conductivity. Aquaculture and Fisheries Management. 1993;24:585–592. [Google Scholar]
- Craig S, Helfrich LA. Understanding fish nutrition, feeds, and feeding. Virginia Cooperative Extension. 2002:420–256. [Google Scholar]
- Dobush GR, Ankey CD, Krementz DG. The effect of apparatus, extraction time, and solvent type on lipid extractions of snow geese. Canadian Journal of Zoology. 1985;63:1917–1920. [Google Scholar]
- El-Sayed AM, Garling DL. Carbohydrate-to-lipid ratios in diets for Tilapia zilli fingerlings. Aquaculture. 1988;73:157–163. [Google Scholar]
- Fischer RU, Congdon JD, Brock M. Total body electrical conductivity (TOBEC): a tool to estimate lean mass and nonpolar lipids of an aquatic organism? Copeia. 1996;2:459–462. [Google Scholar]
- Garling DL, Wilson RP. Effects of dietary carbohydrate-lipid ratios on growth and body composition of fingerling channel catfish. The Progressive Fish-Culturist. 1977;39(1):43–47. [Google Scholar]
- Hunter HL, Nagy TR. Body composition in a seasonal model of obesity longitudinal measures and validation of DXA. Obesity Research. 2002;10(11):1180–1187. doi: 10.1038/oby.2002.160. [DOI] [PubMed] [Google Scholar]
- Jaramillo J, Jr, Bai SC, Murphy BR, Gatlin DM., III Application of electrical conductivity for non-destructive measurement of channel catfish, Ictalurus punctatus, body composition. Aquatic Living Resources. 1994;7:87–91. [Google Scholar]
- Korine C, Daniel S, van Tets IG, Yosef R, Pinshow B. Measuring fat mass in small birds by dual-energy X-ray absorptiometry. Physiological and Biochemical Zoology. 2004;77(3):522–529. doi: 10.1086/383507. [DOI] [PubMed] [Google Scholar]
- Mitchell AD, Rosebrough RW, Conway JM. Body composition analysis of chickens by dual energy X-ray absorptiometry. Poultry Science. 1997;76:1746–1752. doi: 10.1093/ps/76.12.1746. [DOI] [PubMed] [Google Scholar]
- Nagy TR. The use of dual-energy X-ray absorptiometry for the measurement of body composition. In: Speakman JR, editor. Body composition analysis of animals: a handbook of non-destructive methods. Cambridge University Press; Cambridge, UK: 2001. pp. 211–229. [Google Scholar]
- Nagy TR, Clair AL. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obesity Research. 2000;8(5):392–398. doi: 10.1038/oby.2000.47. [DOI] [PubMed] [Google Scholar]
- Nagy TR, Prince CW, Li J. Validation of peripheral dual-energy X-ray absorptiometry for the measurement of bone mineral in intact and excised long bones of rats. Journal of Bone and Mineral Research. 2001;16(9):1682–1687. doi: 10.1359/jbmr.2001.16.9.1682. [DOI] [PubMed] [Google Scholar]
- Nematipour GR, Brown ML, Gatlin DM., III Effects of dietary carbohydrate: lipid ratio on growth and body composition of hybrid striped bass. Journal of World Aquaculture Society. 1992;23(2):128–132. [Google Scholar]
- Secor SM, Nagy TR. Non-invasive measure of body composition of snakes using dual-energy X-ray absorptiometry. Comparative Biochemistry and Physiology A: Molecular & Integrative Physiology. 2003;136(2):379–389. doi: 10.1016/s1095-6433(03)00176-4. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf RJ. Biometry. W.H. Freeman and Company; New York, New York, USA: 1995. [Google Scholar]
- Speakman JR, Booles D, Butterwick R. Validation of dual energy X-ray absorptiometry (DXA) by comparison with chemical analysis of dogs and cats. International Journal of Obesity. 2001;25:439–447. doi: 10.1038/sj.ijo.0801544. [DOI] [PubMed] [Google Scholar]
- Stevenson KT, van Tets IG. Dual-energy X-ray absorptiometry (DXA) can accurately and nondestructively measure the body composition of small free-living rodents. Physiological and Biochemical Zoology. 2008;81(3):373–382. doi: 10.1086/587096. [DOI] [PubMed] [Google Scholar]
- Swennen Q, Janssens GPJ, Geers R, Decuypere E, Buyse J. Validation of dual-energy X-ray absorptiometry for determining in vivo body composition of chickens. Poultry Science. 2004;83:1348–1357. doi: 10.1093/ps/83.8.1348. [DOI] [PubMed] [Google Scholar]
- Tucker CS, van der Ploeg M. Managing off-flavor problems in pond-raised catfish. 1999. (SRAC Publication No. 186). [Google Scholar]
- Wood SE. Master’s thesis. University of Maryland; 2004. The effectiveness of dual energy X-ray absorptiometry to non-invasively determine body composition of hybrid striped bass. Accessed April 18, 2012 at http://hdl.handle.net/1903/1476. [Google Scholar]


