Abstract
Exposure to estrous ewe urine stimulates investigation and mounting activity in sexually active but not sexually inactive rams. It was hypothesized sexual indifference may result from an inability to detect olfactory cues or an interruption of the pathway from detection of the olfactory stimulus to the motor response. Sexually active (n=4) and inactive (n=3) rams were exposed to urine from estrous ewes. An additional group of sexually active rams (n=3) were exposed to urine from ovariectomized ewes. Rams were exsanguinated following 1 h of exposure to stimulus. Neural activity was determined in tissues of interest by the presence of fos and fos-related proteins detected by immunohistochemistry procedures. Sexually active rams exposed to urine from ovariectomized ewes had more (P ≤ 0.05) fos-positive cells in the olfactory bulb, but fewer (P = 0.03) fos-positive cells in the cortical amygdala compared to sexually active rams exposed to urine from estrous ewes. Sexually inactive rams had similar (P ≥ 0.13) numbers of fos positive neurons in the olfactory bulb and medial amygdala but fewer (P ≤ 0.04) in the central amygdala, bed nucleus of the stria terminalis and the medial preoptic area compared to sexually active rams exposed to urine from estrous ewes. Sexual inactivity was not associated with decreased hypothalamic function since fos activity was similar (P ≥ 0.14) among groups in the suprachiasmatic and ventral medial nucleus. Sexual inactivity is not likely due to an impaired ability to detect or process olfactory stimuli by the main olfactory bulb and medial-cortical amygdala. Sexually inactive rams may have reduced attentiveness to sexual stimuli and/or decreased responsiveness of regions in the brain which regulate reproductive behaviors.
Keywords: Rams, Sexual Behavior, olfactory pathway, c-fos
INTRODUCTION
Rams use olfaction to identify ewes in estrus, and display a preference for urine from ewes during the peri-estrual period (Blissitt et al., 1994a). Olfactory sexual cues facilitate sexual interest in rams and shorten the interval to mounting and ejaculation (Maina and Katz, 1999). However, regardless of the sexual stimuli, approximately 20% of rams are either slow to initiate sexual activity or fail to mount sexually-receptive females (Alexander et al., 1999).
Both the main and accessory olfactory organs detect chemosensory cues. Although the vomeronasal organ is not required for sexually experienced rams to discriminate among estrous and non-estrous females, a functional vomeronasal organ does enhance the expression of reproductive behaviors (Blissitt et al., 1990). As opposed to rodents (Coquelin et al., 1984; Meredith, 1986; Keverne, 1999), the main olfactory bulb identifies sexually relevant odors in rams and is required for the expression of sexual behavior (Blissitt et al., 1990). Olfactory sexual cues are likely processed directly through the amygdala (Martel and Baum, 2009) influencing the bed nucleus of the stria terminalis (BNST) and preoptic area of the hypothalamus (Petrulis, 2013).
Immediate early gene expression is used as a marker of neural activity as a first and transient indicator of gene expression following sensory stimulation in the central nervous system (Halpern and Martinez-Marcos, 2003). Expression of fos and fos-related proteins is used to evaluate neural activity following exposure to a broad range of stimuli (Bialy and Kaczmarek, 1996). Basal levels of c-fos expression are low in the brain and increase precipitously in brain regions known to be important for male sexual behaviors following sexual stimulation (Bialy and Kaczmarek, 1996).
This study was designed to determine if sexual disinterest was a result of an inability to detect sexually relevant olfactory cues, or whether the processing of those cues is compromised in sexually inactive rams.
MATERIALS AND METHODS
All animal procedures were approved by the University of Wyoming animal care and use committee.
Rams
Mature Western white-faced (Rambouillet × Columbia) commercial rams (Ovis aries, 2–3 yrs of age) from the University of Wyoming flock were sequentially evaluated for sexual interest and preference. Rams were maintained as a cohort in a 8.5 × 25.6 m pen fed a forage-based diet with water provided ad libitum. Ovariectomized ewes used as stimulus animals were kept in a distant pen prior to behavior testing. To determine sexual interest, rams were subjected to a serving capacity test. For the serving capacity test, rams were penned with two or three ewes in estrus for 20 min in an enclosed pen (3.7 m × 4.0 m). Rams not exhibiting mounting behavior were fitted with a marking harness and exposed overnight in their home pen to their pen mates (n = 12 ram cohorts) or to ewes (n = 6) in estrus. To insure high performing rams had preferential interest in estrous females, and sexually inactive rams did not have latent sexual interest in males, rams were subjected to sexual preference test. Sexual preference tests were conducted by exposing rams to ewes in estrus and cohort rams restrained in a four-way stanchion (Perkins et al., 1992) giving the subject ram free and equal access to all stimulus animals. For classification, rams were tested each breeding season as lambs between 6–10 months of age, yearlings between 18–22 months, and at 2+ years. For each breeding season, each ram was tested in 3 serving capacity tests on three consecutive days followed one week later by 3 sexual preference tests. For previous and unrelated experiments, two sexually inactive rams were castrated following puberty (8 mo) and initial behavior evaluation. Serum concentrations of testosterone were maintained by four Synovex H implants (200 mg testosterone and 20 mg estradiol/implant; Pfizer Animal Health, New York, NY, USA) into their ears (2/ear) one month prior to behavior testing during each breeding season.
Rams were classified based on expression of sexual behaviors during serving capacity and sexual preference tests (Alexander et al., 1999). Investigatory behaviors exhibited were recorded and include numbers of ano-genital investigatory sniffs (nosing of the perineum), flehmen (upper lip curl), fore-leg kicks (foreleg is extended and then flexed in short choppy motions), vocalizations (low-pitched, guttural), and rear nudges (ram positions behind the ewe and contacts the rear of the ewe with his shoulder—often followed by a fore-leg kick)(Banks, 1964). Consummatory behaviors recorded include mount attempts (brief, incomplete mounting of the ewe from behind), mounts (full mount of the ewe without intromission), and ejaculations (mounting with intromission and ejaculatory thrusting) (Banks, 1964). Investigatory and consummatory behaviors were combined for analysis. Sexually active rams exclusively mounted females during all behavior evaluations, and achieved ≥ 6 ejaculation during serving capacity tests. Rams failing to exhibit sexual interest, having a long latency (>10 min) to mount or achieved ≤ 3 ejaculations in serving capacity tests, and exhibited no interest or preference for males during preference tests were considered sexually inactive.
Ewes
Urine collected from non-treated ovariectomized ewes (n= 2) was used as the control stimulus. Ovariectomized ewes (n = 2) were induced into estrus by exposure to progesterone for 14 d with an Eazi-Breed CIDR® for sheep (Pfizer Animal Health, New York, NY, USA). Following CIDR removal, ewes were treated with estradiol (50μg) on 2 consecutive days prior to urine collection. Ewes were placed in metabolism crates on the day of CIDR removal for urine collection. Fresh urine was collected on the day of ram exposure and maintained at 37°C.
Exposure to Olfactory Cues
The final behavior testing occurred 1 month prior to exsanguination during the late breeding season. All rams were 2–3 years of age at the time of odor exposure which occurred within 3 d of each other during the month of November. To decrease stress on the day of exposure, rams were isolated from ewes and placed in indoor treatment pens two days prior to exposure and provided with ad libitum access to grass hay and water. Rams were individually penned to prevent sexual contact but allowed visual and olfactory contact with cohort rams. Ten milliliters of ewe urine from either estrous or ovariectomized ewes was placed on a stack of 3″×3″ cotton pads inside of a facemask. Rams were exposed to the olfactory stimuli for one hour.
Blood and Tissue Collection
Blood was collected from the jugular vein of each ram prior to and following olfactory exposure. Immediately following exposure, rams were anesthetized with intravenous injection of 1.5mL Ketamine/Xylazine mixture (1 part Xylazine to 10 parts Ketamine) then exsanguinated. Heads were removed and perfused via the carotid artery with 500mL of 0.9% saline with 75,000 units of heparin followed by 3L of 4% paraformaldehyde in 0.1M Sorensen’s phosphate buffer. Olfactory bulbs and brains were removed from the cranium and the intact brain was postfixed overnight in a 4% paraformaldehyde solution.
Following the post-fix period, tissues were dissected using surface landmarks. The amygdala was dissected on the rostral/caudal plane at the level of the optic chiasm (Glass et al., 1984) (Alexander et al., 2001). The preoptic area of the hypothalamus was identified anterior of the optic chiasm, posterior to the anterior commissure, and lateral to the third ventricle (Glass et al., 1984) (Alexander et al., 2001). The ventromedial hypothalamus was dissected caudal to the preoptic area and lateral to the third ventricle with the fornix located in the anterior portion (Alexander et al., 2001). Fixed brain tissue was cryoprotected in a 20% sucrose fixative solution at 4°C and then rapidly frozen on dry ice and stored at −80°C until sectioned.
Testosterone Radioimmunoassay Procedure
Blood samples were allowed to clot overnight at 4°C then centrifuged at 2,000 × g for 20 min. Serum was separated and stored at −20°C until analysis. Serum concentrations of testosterone were analyzed by solid-phase commercially available RIA (Diagnostic Products Corporation, Los Angeles, CA, USA). Standards were prepared by serial dilutions of a stock solution in charcoal-stripped wether serum. Standard or sample were dispensed (0.1mL) in duplicate into antibody-coated tubes and incubated with radiolabeled testosterone for 4h at room temperature. Samples were analyzed in a single assay with an intra-assay coefficient of variation of 10%.
Immunohistochemistry Staining Procedure
Fos and fos related proteins (FRP) were quantified in fixed frozen tissues cut into 40μm coronal sections on a cryostat and stored in cryoprotectant (50% 0.05M sodium phosphate buffer, 30% Ethylene Glycol, 20% Glycerol) at −20°C. Every third section for a total of 24 sections was collected for each tissue. Tissue integrity of the main olfactory bulb was problematic making mounting of consecutive sections difficult; however, 24 total intact sections were stained. Immunohistochemistry was carried out on free-floating sections that were washed overnight in 0.1 M phosphate buffer with 0.9% saline and 0.4% TritonX-100 (PBSTx) to remove cryoprotectant. After inactivation of endogenous peroxidases with 1% H2O2 and further PBS washings, the sections were incubated for 2 h in PBS containing 4% normal goat serum diluted in PBTX. Sections were then incubated at 4°C in affinity-purified rabbit polyclonal antibody (FRP, Ab-2, PC38, Calbiochem, San Diego, CA, USA) diluted 1:20,000 in PBSTx. Following incubation, sections were washed and placed in a solution of PBTX + 4% NDS with biotinylated goat anti-rabbit (dilution 1:200) for 1 h. The sections were subsequently washed and incubated for 1 h in avidin-biotin-horseradish peroxidase (HRP) complex (dilution 1:100 in PBS, Vector Laboratories, Burlingame, CA, USA). HRP was visualized using 3,3′diaminobenzidine as the chromagen and reacted for 10 min. Sections were mounted on Superfrost Plus microslides (VWR International, LLC., Radnor, PA, USA) and dried on a drying rack overnight. Mounted sections were dehydrated in ascending ethanol solutions, cleared with xylene, and coverslipped with Permount (Fisher Scientific, Fair Lawn, NJ, USA). Nonspecific binding was determined in tissue incubated in absence of anti-fos primary antibody.
Analysis of Immunohistochemistry Staining
Sections were viewed using an Olympus Vannox microscope equipped with a digital camera. Images were not altered with the exception of adjusting contrast as necessary. For each region of interest (Fig. 1), 9–12 sections were analyzed using the current version of Image J software (USA National Institute of Health) for fos immunoreactive positive cells. A grey scale threshold established the parameters of the fos-positive nuclei, and size exclusion parameters were adjusted by slide based on the size of the positive nuclei. The program subsequently quantified positive cells within a region of interest. Fos-positive cells on the border of the region of interest were excluded from quantification. Fos-positive cells were verified and artifacts removed by visual inspection without knowledge of ram group.
Figure 1.
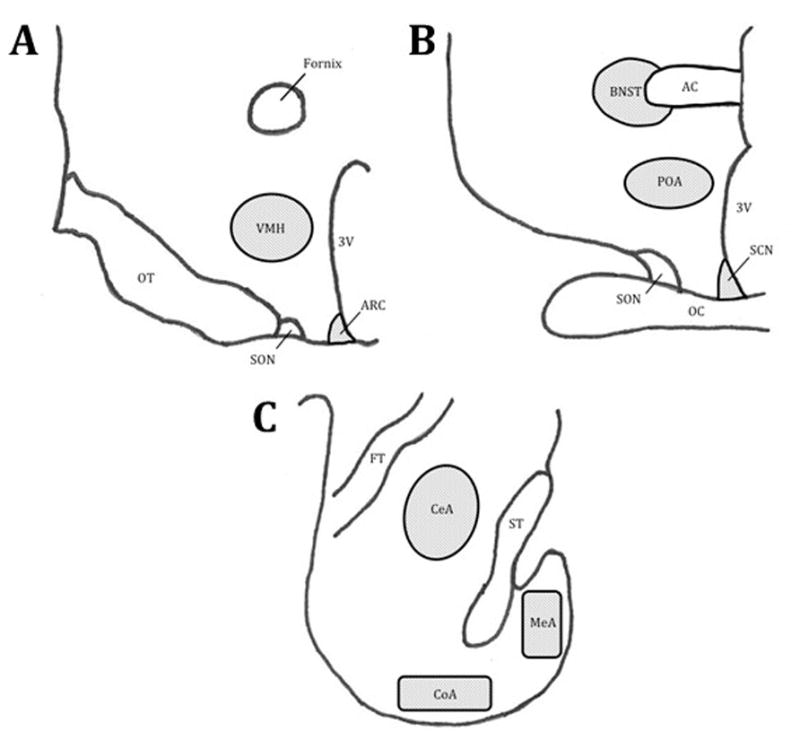
Nuclei and tissue regions analyzed: Panel A, Ventral medial hypothalamus (VMH); Panel B, Bed Nucleus of the Stria Terminalis (BNST), medial Preoptic Area of the Hypothalamus (POA), and suprachiasmatic nuclei (SCN); Panel C, Central Amygdala (CeA), Medial Amygdala (MeA), and Cortical Amygdala (CoA). Other identified regions: optic tract (OT), optic chiasm (OC), arcuate nucleus of the hypothalamus (ARC), supraoptic nucleus of the hypothalamus (SON), third ventricle (3V), anterior commissure (AC), fiber tract (FT), and stria terminalis (ST).
Density of FRP-positive neurons was defined as total number of Fos-IR positive cells divided by the area of the region of interest. Cell counts were transformed to average number/mm2 by dividing the count by area.
Statistics
Cell counts from each region of interest were averaged for statistical analysis. GLM procedure (one-way ANOVA for unbalanced sample size) in SAS (Ver 9.3, SAS Institute, Cary, NC, USA) was used to analyze the data, means ± SEM are reported. To determine effect of stimulus on serum concentration of testosterone and neural expression of fos and fos related peptides, preplanned comparisons of stimulus exposure (estrous vs ovariectomized ewe urine) in sexually active rams was conducted. Effect of ram classification (sexually active vs inactive) was determined by comparing sexually active to inactive rams with both groups exposed to urine from ewes in estrus.
RESULTS
Behavior Testing
Total investigatory and consummatory behaviors exhibited during the final two serving capacity tests and behaviors exhibited towards rams during the final two sexual preference tests are reported in Table 1. As expected, rams classified as sexually active exhibited the greatest numbers of investigatory (35.5 ± 9.7) and consummatory behaviors (21.4 ± 18.4) towards ewes in estrous. Sexually inactive rams exhibited few investigatory behaviors towards ewes in estrous (4 ± 5.2) and only one sexually inactive achieved intromission.
Table 1.
Total number of investigatory and consummatory behaviors exhibited by sexually active (A) and sexually inactive (I) rams in the final two behavior tests.
| Serving Capacity Test | Behavior Toward Rams | ||||
|---|---|---|---|---|---|
|
| |||||
| Ram | Group | Investa | Consumb | Investa | Consumb |
| 8507 | A | 33 | 17 | 4 | 0 |
| 8011 | A | 25 | 11 | 3 | 0 |
| 8027 | A | 39 | 5 | 2 | 0 |
| 8045 | A | 48 | 31 | 1 | 0 |
| 8114 | A | 31 | 15 | 1 | 0 |
| 8576 | A | 46 | 63 | 0 | 0 |
| 8587 | A | 41 | 18 | 3 | 0 |
| 85136 | A | 21 | 11 | 2 | 0 |
| 7044 | I | 1 | 0 | 5 | 0 |
| 7111 | I | 1 | 0 | 0 | 0 |
| 8507 | I | 10 | 1 | 4 | 0 |
Invest = Investigatory behaviors expressed and include ano-genital sniffs, flehmen, foreleg kick and vocalizations
Consum = Consummatory behaviors expressed and include attempted mounts, mounts, and mounts with intromission or ejaculation
Testosterone
Basal concentrations of serum testosterone (1.1 ± 0.2 ng/mL) did not differ (F (2, 7) = 1.1, P = 0.4) among sexually active and inactive rams prior to urine exposure. Serum concentrations of testosterone in sexually active rams did not change (F (1, 5) = 0.8, P = 0.4) following exposure to urine from estrous or ovariectomized ewes (−0.1 ± 0.1 vs 0.3 ± 0.5 ng/mL, respectively). Due to testosterone-replacement in two of three sexually inactive rams, change in serum concentrations of testosterone could not be evaluated in sexually inactive rams.
Fos Immunostaining
Absence of anti-fos antibody resulted in no staining (Fig. 2). Sexually active rams exposed to urine from ovariectomized ewes had greater numbers of FRP-positive neurons in all layers of the olfactory bulb including the granular (F (1, 5) = 19.4, P = 0.007) and mitral and tufted layer (F (1, 5) = 6.61, P = 0.05) compared to sexually active rams exposed to urine from estrous ewes (Fig. 3). Differences were not noted (F (1, 5) = 0.2, P > 0.7) among sexually active and inactive rams exposed to urine from ewes in estrus.
Figure 2.
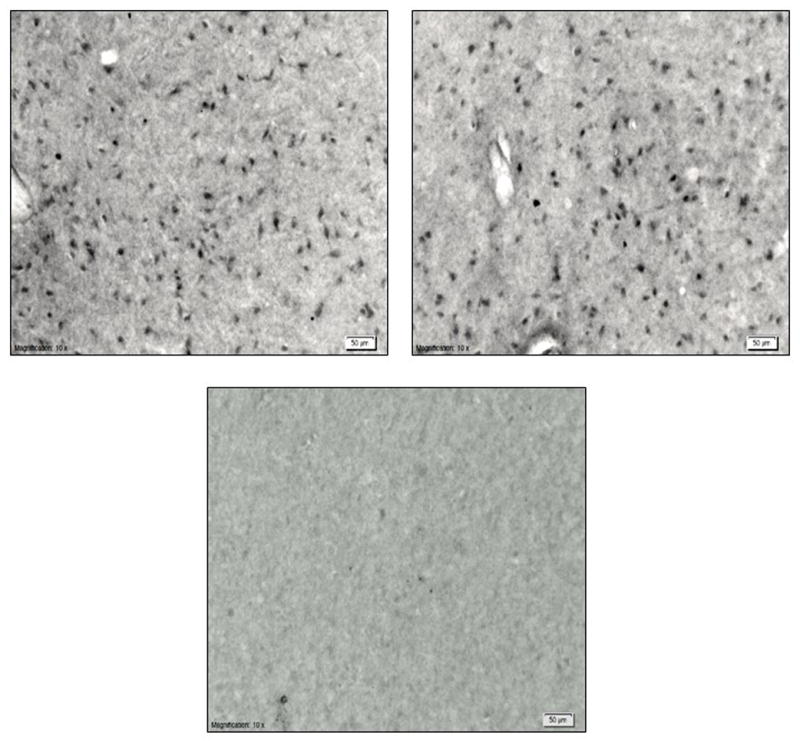
Preoptic area of the hypothalamus stained for c-fos and fos-related proteins from treatment groups: (clockwise) sexually active rams exposed to estrous ewe urine, sexually active rams exposed to ovariectomized ewe urine and sexually inactive rams exposed to urine from estrous ewes.
Figure 3.
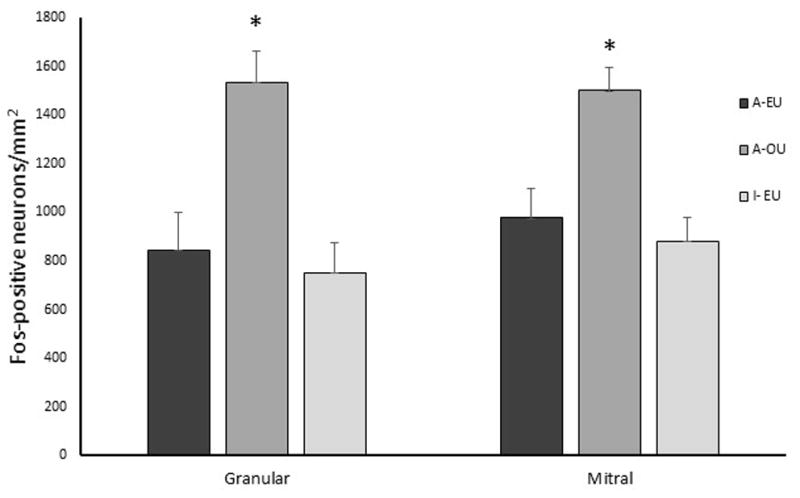
Average fos and fos-related proteins positive neurons in the granular and mitral and tufted layer of the olfactory bulb per mm2. Sexually active (A) or inactive (I) rams were exposed to urine from ewes in estrus (EU) or ovariectomized ewes (OU). *Denotes difference compared to sexually active rams exposed to urine from ewes in estrus (A-EU) (P < 0.05).
Olfactory stimulation of urine from estrous ewes evoked a greater response in the cortical (F (1, 5) = 9.0, P = 0.03) but not the medial (F (1, 5) = 3.3, P = 0.13) or central (F (1, 5) = 5.3, P = 0.07) nuclei of the amygdala when compared to sexually active rams exposed to urine from ovariectomized ewes (Fig. 4).
Figure 4.
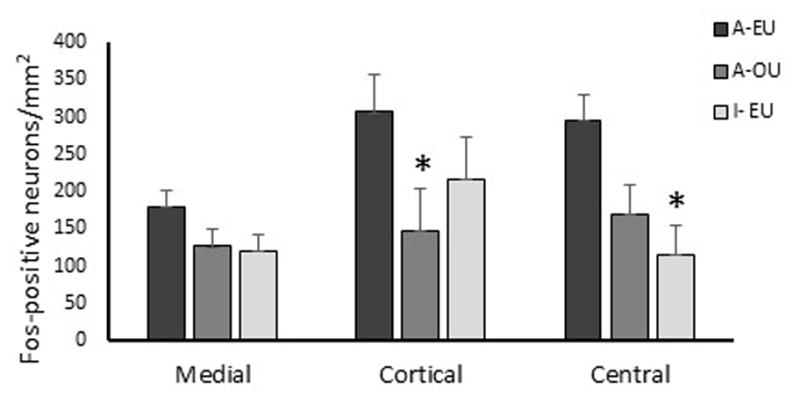
Average fos and fos-related proteins positive neurons in the medial, cortical, and central amygdala per mm2. Sexually active (A) or inactive (I) rams were exposed to urine from ewes in estrus (EU) or ovariectomized ewes (OU). *Denotes difference compared to sexually active rams exposed to urine from ewes in estrus (A-EU) (P < 0.05).
Sexually inactive rams had fewer (F (1, 5) = 11.3, P = 0.02) numbers of fos positive neurons in the central amygdala when exposed to urine from estrous ewes than sexually active rams exposed to similar stimuli, but differences in the medial amygdala were not noted (F (1, 5) = 3.3, P ≥ 0.13). Sexually inactive rams had fewer (F (1, 5) = 7.6, P = 0.04) numbers of fos positive neurons in the BNST than sexually active rams following exposure to estrous ewe urine (Fig. 5). Hypothalamic responsiveness as measured by numbers of fos-positive neurons was similar among rams in the ventral medial (F (1, 5) = 3.1, P ≥ 0.14) and suprachiasmatic (F (1, 5) = 0.3, P ≥ 0.60) nuclei (Fig. 5). Numbers of fos-positive neurons in the medial preoptic area (mPOA), however, were fewer (F (1, 5) = 34.7, P = 0.002) for sexually inactive compared to sexually active rams exposed to urine from estrous ewes (Fig. 5). Differences in fos activity in the mPOA were not noted among sexually active rams exposed to urine from ovariectomized vs estrous ewes (Fig. 5).
Figure 5.
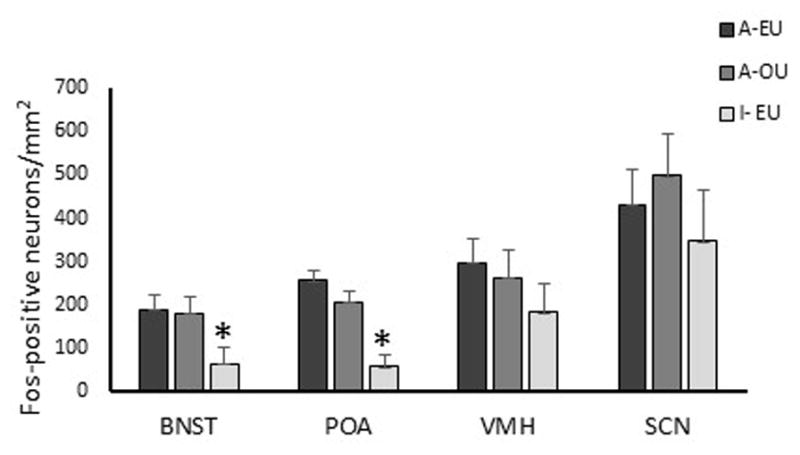
Average fos and fos-related proteins positive neurons in the bed nucleus of the stria terminalis (BNST), preoptic area of the hypothalamus (POA), ventromedial hypothalamus (VMH), and the suprachiasmatic nucleus (SCN) per mm2. Sexually active (A) or inactive (I) rams were exposed to urine from ewes in estrus (EU) or ovariectomized ewes (OU). *Denotes difference compared to sexually active rams exposed to urine from ewes in estrus (A-EU) (P < 0.05).
DISCUSSION
Sexually active rams placed among randomly cycling females quickly identify ewes is estrus. Although ewes do exhibit limited proceptive behavior such as seeking and affiliation (Lindsay, 1974), rams primarily use olfactory cues to discriminate the estrus state of ewes (Blissitt et al., 1994). Exposure of rams to ewes in estrus increases serum concentrations of LH and testosterone (Ungerfeld and Silva, 2004), but olfactory cues alone are not sufficient to elicit this increase(Gonzalez et al., 1991a). Similarly, in the present study, sexually active rams exposed to urine from estrous ewes did not experience a rise in testosterone in response to olfactory stimuli. Testosterone may influence fos activity independent of stimulus (De Lorme and Sisk, 2013); therefore, it is important to note serum concentrations of testosterone were similar among groups prior to the presentation of olfactory stimulus and did not rise in response to stimulus.
Urine from ovariectomized ewes elicited a greater fos response in the main olfactory bulb in sexually active rams than did urine from estrus ewes. It is possible the chemical composition of ovariectomized ewe urine is sufficiently more complex than urine from ewes in estrus to elicit this increased response. Regardless of urine complexity, sexually experienced rams clearly differentiate among ewes. Ewes will characteristically void urine when approached by a ram if they are not in estrus (Bland and Jubilan, 1987). In response to main olfactory stimuli (Hart, 1987), rams sniff and sample voided urine and typically follow with a flehmen response. The flehmen response facilitates transport of the urine to the vomeronasal organ (Ladewig and Hart, 1980). Although it is not clear how rams learn to associate particular urine composition to a willing vs unwilling mate, classic conditioning may be involved. Classic conditioning response was demonstrated when a foot shock was paired with the presentation of an odor, rats had increased fos activity in the main as well as accessory olfactory bulb when compared to presentation of odor alone (Funk and Amir, 2000). Differences in fos activity, however, were not apparent among high and low sexually performing rams exposed to urine from estrous ewes. Thus, it appears rams with low libido have an equal ability to detect potentially sexually evocative stimuli. It should be noted, however, the urine stimulus in the present study primarily provided volatile cues, and non-volatile cues from urine saturating the facial mask would have been limited. Because of the study design, potential differences in the ability to detect non-volatile sexual cues cannot be disregarded.
Initial recognition and discrimination of a ewe’s reproductive status likely occurs at the level of the olfactory bulb (Blissitt et al., 1994b). However, the motor response to that stimuli requires further processing of the stimulus. Olfactory information through the main and accessory bulb is transmitted to the amygdala (Brennan and Keverne, 1997). In sheep, the cortical amygdala receives input from both the main and accessory olfactory bulbs while the medial amygdala receives input only from the accessory bulb (Meurisse et al., 2009). Although fos activity in the medial amygdala was similar among sexually evocative and non-evocative stimuli, fos activity was decreased in the cortical amygdala when rams were exposed to urine from ovariectomized ewes compared to exposure to a putative sexual stimuli. It is unexpected that fos activity in the medial amygdala was not different among rams exposed to urine from ovariectomized or estrous ewes especially since flehmen occurs most often toward non-estrous females and connections with the accessory olfactory bulb are primarily to the medial amygdala (Meurisse et al., 2009). In absence of a flehmen response, however, minimal fluid is transported into the vomeronasal organ (Hart, 1987). In the present study the face mask likely prohibited flehmen, and may account for the lack of differentiation in the medial amygdala. Lesions to the cortical amygdala in Syrian hamsters did not influence odor preference or discrimination, but did influence behavior patterns associated with sexual satiety (Maras and Petrulis, 2008). Neural activity in the cortical amygdala may facilitate the appropriate behavioral response to olfactory stimuli (i.e. mounting vs feeding). A dense interconnection of these two amygdala nuclei (Meurisse et al., 2009) may also refine olfactory signals. Similar to fos activity in the olfactory bulb, rams characterized as sexually inactive had similar fos activity in the medial and cortical amygdala as sexually active rams when exposed to a putative sexual stimuli. Similar fos activity was also noted in rams allowed to interact with ewes in estrus (Borja and Fabre-Nys, 2012). Our study along with Borja and Fabre-Nys (2012) clearly indicate that medial and cortical amygdala function is not responsible for the lack of sexual interest in rams with low libido.
Fos activity in the central amygdala was lower in sexually inactive rams compared to sexually active rams following exposure to urine from ewes in estrus. The central nuclei initiates a state of arousal towards non-specific stimuli (Gallagher et al., 1990). Sexually active rams may be more aware and vigilant than sexually inactive rams and that vigilance facilitates increased sexual activity. In a production setting, sexually active rams seem to investigate their environment more and track to the feed source more often without consuming feed (Uthlaut et al., 2011). In the present study, similar fos activity in the central amygdala of sexually active rams exposed to either putative sexually evocative or non-evocative stimuli lends credence to this hypothesis.
In sheep, both the medial as well as the cortical amygdala have reciprocal connections to the BNST and the mPOA (Meurisse et al., 2009). Although differences in fos expression in the medial and cortical amygdala was not noted, sexually inactive rams had fewer numbers of fos positive neurons in the BNST and mPOA when compared to sexually active rams exposed to urine from ewes in estrus. This contrasts with (Borja and Fabre-Nys, 2012), who noted less fos expression in the POA but not BNST of low libido rams with full access to ewes in estrus. Differences in stimuli exposure may explain the differences. The POA is known to be essential for the expression of male reproductive behaviors—especially mounting and intromission (Hart and Leedy, 1985). Lower fos expression in the mPOA may be explained in the Borja and Fabre-Nys (2012) experiment by the reduced mounting activity in their low libido rams. However, in conjunction with present results, it seems plausible that inherent low activity in the mPOA may be at least partially responsible for the lack of sexual activity in low sexually performing rams. Similar levels of fos expression among sexually active rams exposed to putative sexually evocative and non-evocative stimuli suggests sexually active rams require more sensory input to elicit a sexual response. This is supported by evidence that olfactory stimulus alone does not cause an increase in serum concentrations of testosterone (Alexander et al., 1999) (Gonzalez et al., 1991b), present study.
Aberrant hypothalamic function could be cause for decreased sexual interest in rams as has been shown in males of many species with large hypothalamic lesions (for review: (Hart and Leedy, 1985). However, it does not appear sexually inactive rams have a global impairment/reduction in hypothalamic function—clearly low sexually performing rams have normal serum concentrations of testosterone (Alexander et al., 1999), grow similarly and are selected as flock sires (Uthlaut et al., 2011), and also fos expression in the suprachiasmatic and ventral medial hypothalamus is similar among all rams.
CONCLUSION
Sexual inactivity is not likely due to an impaired ability to detect or process olfactory stimuli from by the main olfactory bulb or medial and cortical amygdala, but may be caused by a reduced vigilance to sexual stimuli and/or decreased responsiveness of the BNST and mPOA. This study advances our knowledge of mechanisms which regulate reproductive behavior. A thorough understanding of such basic mechanisms is a prerequisite to the development of reliable methods to regulate behavior.
Highlights.
Sexually active and inactive rams have equal olfactory response to sexual stimuli
Sexual olfactory cue increase neural activity in the cortical amygdala of active rams
Sexual inactivity was associated with decreased neural activity in the BNST and POA
Acknowledgments
Funded in part by 3RO1RR014270-10S1 NIH/DHHS (CER, PI) and USDA-NRI 2007-55618-18176 (BMA, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Alexander BM, Rose JD, Stellflug JN, Fitzgerald JA, Moss GE. Fos-like immunoreactivity in brain regions of domestic rams following exposure to rams or ewes. Phys Behav. 2001;73:75–80. doi: 10.1016/s0031-9384(01)00441-3. [DOI] [PubMed] [Google Scholar]
- Alexander BM, Stellflug JN, Rose JD, Fitzgerald JA, Moss GE. Behavior and endocrine changes in high-performing, low-performing, and male-oriented domestic rams following exposure to rams and ewes in estrus when copulation is precluded. J Anim Sci. 1999;77:1869–1874. doi: 10.2527/1999.7771869x. [DOI] [PubMed] [Google Scholar]
- Banks EM. Some axpects of sexual behavior in domestic sheep, ovis aries. Behaviour. 1964;23:249–279. [Google Scholar]
- Bialy M, Kaczmarek L. c-Fos expression as a tool to search for the neurobiological base of the sexual behaviour of males. Acta Neurobiol Exp (Wars) 1996;56:567–577. doi: 10.55782/ane-1996-1162. [DOI] [PubMed] [Google Scholar]
- Bland KP, Jubilan BM. Correlation of Flehmen by Male Sheep with Female Behavior and Estrus. Anim Behav. 1987;35:735–738. [Google Scholar]
- Blissitt MJ, Bland KP, Cottrell DF. Olfactory and Vomeronasal Chemoreception and the Discrimination of Estrous and Non-Estrous Ewe Urine Odors by the Ram. Appl Anim Behav Sci. 1990;27:325–335. [Google Scholar]
- Blissitt MJ, Bland KP, Cottrell DF. Detection of Estrous-Related Odor in Ewe Urine by Rams. J Reprod Fertil. 1994a;101:189–191. doi: 10.1530/jrf.0.1010189. [DOI] [PubMed] [Google Scholar]
- Blissitt MJ, Bland KP, Cottrell DF. Detection of Estrous-Related Odor in Ewe Urine by Rams. J Reprod Fertil. 1994b;101:189–191. doi: 10.1530/jrf.0.1010189. [DOI] [PubMed] [Google Scholar]
- Borja F, Fabre-Nys C. Brain structures involved in the sexual behaviour of Ile de France rams with different sexual preferences and levels of sexual activity. Behav Brain Res. 2012;226:411–419. doi: 10.1016/j.bbr.2011.09.037. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Prog Neurobiol. 1997;51:457–481. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Coquelin A, Clancy AN, Macrides F, Noble EP, Gorski RA. Pheromonally induced release of luteinizing hormone in male mice: involvement of the vomeronasal system. J Neurosci. 1984;4:2230–2236. doi: 10.1523/JNEUROSCI.04-09-02230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorme KC, Sisk CL. Pubertal testosterone programs context-appropriate agonistic behavior and associated neural activation patterns in male Syrian hamsters. Phys Behav. 2013;112–113:1–7. doi: 10.1016/j.physbeh.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Amir S. Enhanced fos expression within the primary olfactory and limbic pathways induced by an aversive conditioned odor stimulus. Neuroscience. 2000;98:403–406. doi: 10.1016/s0306-4522(00)00217-7. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. J Neurosci. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Amann RP, Nett TM. Effects of season and sex on the distribution of cytosolic estrogen receptors within the brain and the anterior pituitary gland of sheep. Biol Reprod. 1984;30:894–902. doi: 10.1095/biolreprod30.4.894. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Levy F, Orgeur P, Poindron P, Signoret JP. Female effect in sheep. II Role of volatile substances from the sexually receptive female; implication of the sense of smell. Reprod Nutr Dev. 1991a;31:103–109. doi: 10.1051/rnd:19910110. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Levy F, Orgeur P, Poindron P, Signoret JP. Female effect in sheep. II Role of volatile substances from the sexually receptive female; implication of the sense of smell. Reprod Nutr Dev. 1991b;31:103–109. doi: 10.1051/rnd:19910110. [DOI] [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Hart B, Leedy M. Neurological Bases of Male Sexual Behavior. In: Adler N, Pfaff D, Goy R, editors. Reproduction. Springer; US: 1985. pp. 373–422. [Google Scholar]
- Hart BL. Roles of the olfactory and vomeronasal systems in behavior. Vet Clin N Am-Food A. 1987;3:463–475. doi: 10.1016/s0749-0720(15)31163-4. [DOI] [PubMed] [Google Scholar]
- Keverne EB. The vomeronasal organ. Science. 1999;286:716–720. doi: 10.1126/science.286.5440.716. [DOI] [PubMed] [Google Scholar]
- Ladewig J, Hart BL. Flehmen and vomeronasal organ function in male goats. Phys Behav. 1980;24:1067–1071. doi: 10.1016/0031-9384(80)90049-9. [DOI] [PubMed] [Google Scholar]
- Lindsay DRaFIC. Ram seeking activity associated with oestrous behavior in ewes. Anim Behav. 1974;20:452–456. doi: 10.1016/s0003-3472(72)80008-3. [DOI] [PubMed] [Google Scholar]
- Maina D, Katz LS. Scent of a Ewe: transmission of a social cue by conspecifics affects sexual performance in male sheep. Biol Reprod. 1999;60:1373–1377. doi: 10.1095/biolreprod60.6.1373. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. The posteromedial cortical amygdala regulates copulatory behavior, but not sexual odor preference, in the male Syrian hamster (Mesocricetus auratus) Neuroscience. 2008;156:425–435. doi: 10.1016/j.neuroscience.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. A centrifugal pathway to the mouse accessory olfactory bulb from the medial amygdala conveys gender-specific volatile pheromonal signals. European Journal of Neuroscience. 2009;29:368–376. doi: 10.1111/j.1460-9568.2008.06564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal organ removal before sexual experience impairs male hamster mating behavior. Physiol Behav. 1986;36:737–743. doi: 10.1016/0031-9384(86)90362-8. [DOI] [PubMed] [Google Scholar]
- Meurisse M, Chaillou E, Levy F. Afferent and efferent connections of the cortical and medial nuclei of the amygdala in sheep. J Chem Neuroanat. 2009;37:87–97. doi: 10.1016/j.jchemneu.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Perkins A, Fitzgerald JA, Price EO. Luteinizing hormone and testosterone response of sexually active and inactive rams. J Anim Sci. 1992;70:2086–2093. doi: 10.2527/1992.7072086x. [DOI] [PubMed] [Google Scholar]
- Petrulis A. Chemosignals and hormones in the neural control of mammalian sexual behavior. Front Neuroendocrin. 2013;34:255–267. doi: 10.1016/j.yfrne.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Ungerfeld R, Silva L. Ewe effect: endocrine and testicular changes in experienced adult and inexperienced young Corriedale rams used for the ram effect. Anim Reprod Sci. 2004;80:251–259. doi: 10.1016/j.anireprosci.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Uthlaut VA, Moss GE, Stobart RH, Larson BA, Alexander BM. Sexual performance and production traits in white-faced yearling rams. Small Ruminant Res. 2011;100:63–66. [Google Scholar]


