Abstract
Background
Given the importance of ethnic differences in the evaluation of various aspects of symptoms in patients with Parkinson's disease (PD), we present the formal procedure for completing the traditional Chinese translation of the International and Parkinson and Movement Disorder Society/UPDRS (MDS‐UPDRS) and highlight the discrepancy in nonmotor symptoms (NMS) between patients in Eastern and Western countries.
Methods
A total of 350 native Chinese‐speaking PD patients were recruited from multiple hospitals in Eastern countries; they completed the MDS‐UPDRS. The translation process was executed and factor analysis was performed to determine the structure of the scale. Chi‐squared and t tests were used to compare frequency and severity of PD symptoms between the Chinese‐speaking and English‐speaking groups (n = 876).
Results
NMS and motor symptoms were more severe in the Western population (Part I: t (1205) = 5.36, P < 0.0001; and Part III: t (1205) = 7.64, P < 0.0001); however, the prevalence of cognitive dysfunction and impairments in activities of daily living were more frequent in the Eastern patients. The comparative fit index was 0.93 or greater, and the exploratory factor analysis revealed compatible results between the translated scale and the original version.
Conclusion
The traditional Chinese version of the MDS‐UPDRS can be designated as an official translation of the original scale, and it is now available for use. Moreover, NMS in PD constitute a major issue worldwide, and the pattern of NMS among the Chinese population is more marked in terms of cognition‐based symptoms and activities of daily living.
Keywords: Parkinson's disease, Unified Parkinson's Disease Rating Scale, nonmotor symptoms, Chinese trasnslation
Parkinson's disease (PD) is a common, progressive degenerative disease that affected 4.1 to 4.6 million people over the age of 50 years in 2005; this number is expected to double in two decades.1 PD is pathologically characterized by loss of dopaminergic neurons in the SN, and it clinically manifests as resting tremor, rigidity, bradykinesia, and postural instability. Whereas motor symptoms are the main clinical features of PD, growing evidence shows that PD patients have nonmotor symptoms (NMS).2 Most studies explore these NMS with a screening questionnaire, and these studies have shown that such NMS have a huge impact not only on caregivers, but also on the patient's quality of life.3, 4 In one Western multicenter survey, researchers found that 98.6% of patients with PD reported the presence of NMS5; furthermore, our recent study also revealed that up to 98% of PD patients in an Eastern country reported having at least one NMS,6 including nocturnal disturbance7 and neuropsychological dysfunction.8 It is believed that NMS in patients with PD is a world‐wide issue. Few studies have investigated the differences in NMS between the Western and Eastern world, and many reports have not focused on specific NMS, such as gastrointestinal (GI) symptoms. The results of these sparse investigations showed that GI symptoms are more prevalent in Asian countries9; nevertheless, some studies have shown that constipation rates in Eastern countries were not higher than in Western groups.6, 10 According to these limited findings, it is apparent that the effects of ethnicity on NMS have not yet been fully explored; as such, a comparison of the comprehensive NMS profile between different countries is needed.
A rating scale for the comprehensive measurement of both motor symptoms and NMS among patients with PD is pivotal for making decisions in clinical practice and research.2 The International Parkinson and Movement Disorder Society (MDS) sponsored a critique of the UPDRS, and although many strengths of the original scale were recognized, it was recommended that a revised scale be developed.11 This new version, the MDS‐sponsored revision of the UPDRS (MDS‐UPDRS), features a modified severity scale that captures milder manifestations of the disease, and it also offers expanded coverage of NMS and complications associated with therapy. In 2008, the reliability and validity of the MDS‐UPDRS were demonstrated.12 The MDS‐UPDRS was created to replace the original UPDRS, and it is essential that properly tested translations are made available for clinicians or patients in non‐English‐speaking countries. The MDS developed a specific protocol that could designate successful translations of non‐English‐language versions of the scale as official MDS translations.13 To date, several official MDS translations have been developed in languages including Estonian, French, German, Hungarian, Italian,14 Spanish,15 Japanese,16 Russian, and Slovak.17
In order to establish a successful, culturally unbiased translation, as well as to explore the Eastern‐Western difference in NMS, the aims of this study were 2‐fold: (1) to execute the translation process of the traditional Chinese version of the MDS‐UPDRS, so as to make the MDS‐UPDRS available for use in a Chinese‐speaking population, and (2) to investigate the differences in the NMS profiles between Chinese‐speaking and English‐speaking PD patients using the MDS‐UPDRS, which comprehensively covers both motor symptoms and NMS.
Patients and Methods
Participants
Patients were recruited from November 2013 to June 2014 by Chinese‐speaking investigators at three hospitals, including the National Taiwan University Hospital (NTUH) and the Chang Gung Memorial Hospital (CGMH) in Taiwan, as well as from the Prince of Wales Hospital in Hong Kong (PWH). Three hundred fifty patients with PD were included in this study (NTUH, 250; CGMH, 50; PWH, 50). The sample size required for the translation study was based on the need for a minimum of 5 participants per questionnaire item.18 All seven raters completed the MDS‐UPDRS Training Program and Certificate Exercise before examining the patients.19
Measurement
The following data were recorded for each subject: sociodemographics, disease duration, and scores on the traditional Chinese version of the MDS‐UPDRS. The MDS‐UPDRS is comprised of four parts12: Part I: Non‐motor Experiences of Daily Living (nM‐EDL), which has six rater‐based items and seven patient/caregiver self‐report measurements; Part II: Motor Experiences of Daily Living (motor‐EDL), featuring 13 patient‐based items; Part III: Motor Examination (MEx), which involves objective examination by the rater for 18 items (33 scores); and Part IV: Motor Complications (MCompl), with six items that cover dyskinesia and fluctuations. Scoring for each item is based on a 0 to 4 Likert‐type scale, where 0 reflects no impairment and 4 reflects maximal impairment. The total score for each part of the scale was obtained by summing the corresponding item scores.
Procedure
The MDS‐UPDRS was translated into traditional Chinese by a team of PD expert investigators in Taiwan and Hong Kong, as led by Dr. Ruey‐Meei Wu (Taiwan). The translated scale was then back‐translated into English by colleagues fluent in English and Chinese; these individuals were not involved in the original translation. The back‐translated version of the scale was reviewed by a team of experts in the United States (G.S., C.G., N.L.P., and B.T.); these individuals had been involved in the development of the original English version of the instrument. After this review, modifications to the translation were suggested to bring the two versions into alignment.
The translation was then submitted to cognitive pretesting.20 Cognitive pretesting is a qualitative approach used to assess task difficulty, determine rater and respondent ease of comprehension, and examine participant interest in and comfort when completing the scale.21 Based on the results of the initial cognitive pretesting process, other round(s) of translation, back‐translation, and cognitive pretesting may be required. Once all cognitive pretesting was completed and no additional issues were noted, the translated version of the scale was designated as an official working document that was submitted to large field testing.
Statistical Analysis
Deidentified data from the 350 subjects were uploaded to a secure website, and the data analyses were performed by the analytic team (S.L., L.W., and B.C.T.). Any participants with missing values for a given part of the survey were deleted from the analysis for that part alone; thus, the sample size could vary from section to section. Demographic and disease‐related characteristics were summarized with descriptive statistics. Patients were classified as having a specific symptom if they had a score greater than zero on a given item. Symptom severity was represented by the mean score for each part of the MDS‐UPDRS. Primary and secondary analyses were carried out with M‐plus (version 7). An unweighted least squares approach to factor estimation, which minimizes the sum of squared differences between observed and estimated correlation matrices (not counting diagonal elements), was used. This approach was chosen because of the ordered categorical nature of the data. To assist in the interpretation of the factors in the MDS‐UPDRS, we used a CF‐Varimax rotation that constrains the factors so they are uncorrelated. These methods were chosen to follow the original validation method used for the English version of the MDS‐UPDRS.
Primary Analysis
Separate confirmatory factor analyses (CFAs) were conducted for each part of the traditional Chinese version of the MDS‐UPDRS, with the data constrained to fall into the factors defined in the English‐language data. Evaluation of the CFA results was based on the comparative fit index (CFI). A successful translation was defined by CFI ≥0.90 for all parts of the scale. We also examined the mean and variance‐adjusted weighted least squares estimators to confirm model fit.
Secondary Analysis
Exploratory factor analyses (EFAs) were performed using the same factor estimation and rotation methods listed above. A scree plot test was applied to select the number of factors involved in this analysis. The subjective scree test uses a scatter plot of eigenvalues, which are plotted against their ranks based on magnitude, to extract as many factors as there are eigenvalues that fall before the last large drop in the plot.22 Once the factors were chosen, an item was retained in a factor if the factor loading for that item was 0.40 or greater.
Finally, we used the chi‐squared test to compare occurrence of symptoms and t tests to compare severity of symptoms between the Chinese‐speaking group of this study and the English‐speaking group tested previously for the English version of MDS‐UPDRS, of which were contained 876 data of English‐speaking PD patients.12 P values less than 0.05 were considered statistically significant.
Ethical Standards
All participants provided their written informed consent before enrollment, which was done in accord with the ethical standards of the Declaration of Helsinki (1964). The ethical research committees of three hospitals approved this study.
Results
Cognitive Pretesting
A total of 10 patients with PD and their examiners were interviewed using a structured interview format that is typically employed in cognitive pretesting. The raters identified no problems. One of the ten patients that were interviewed had difficulty understanding the question on cognitive impairment, 2 noted that the font size on the questionnaire was too small, and 1 had difficulty with the question pertaining to amount of time spent with dyskinesia. No other difficulties were reported by the patients. Slight modifications of the scale were recommended after this round of testing. Ten patients completed a second round of cognitive pretesting and no difficulties were identified. The modified version of the scale was approved as the official working document of the traditional Chinese MDS‐UPDRS; this document was used for psychometric testing in a larger group of patients with PD.
Large Validation Sample
Patients' demographic characteristics are shown in Table 1. The traditional Chinese data set included 350 native Chinese‐speaking PD patients who were evaluated with the MDS‐UPDRS (52.3% were male; mean age: 65.4 ± 10.4 years; mean disease duration: 8.6 ± 5.9 years).
Table 1.
Demographic characteristics of patients in Chinese‐speaking and English‐speaking groupsa
| EG | CG | |
|---|---|---|
| N | 876 | 350 |
| Sex, % male | 63.2 | 52.3 |
| Age, years | 67.5 (10.9) | 65.4 (10.4) |
| Years of disease diagnosed | 8.3 (6.7) | 8.6 (5.9) |
| H & Y stage | ||
| 1 | 63 | 57 |
| 2 | 467 | 163 |
| 3 | 174 | 80 |
| 4 | 109 | 36 |
| 5 | 53 | 11 |
Mean and standard deviation.
EG, English‐speaking group reported in 2008 by Goetz et al.12; CG, Chinese‐speaking group in this study.
Factor Analysis
Primary Analysis: CFA
CFA results for each part of the MDS‐UPDRS are displayed in Table 2. For all four parts of the traditional Chinese MDS‐UPDRS, the CFI score was 0.93 or greater, exceeding our criterion of 0.90.
Table 2.
Confirmatory factor analysis model fit
| Part I: nM‐EDL (a two‐factor model)a | |
| Traditional Chinese | CFI = 0.93, RMSEA = 0.06 (347 patients) |
| English language | CFI = 0.96, RMSEA = 0.06 (849 patients) |
| Part II: motor‐EDL (a three‐factor model) | |
| Traditional Chinese | CFI = 0.98, RMSEA = 0.09 (348 patients) |
| English language | CFI = 0.97, RMSEA = 0.09 (851 patients) |
| Part III: MEx (a seven‐factor model) | |
| Traditional Chinese | CFI = 0.95, RMSEA = 0.06 (348 patients) |
| English language | CFI = 0.95, RMSEA = 0.07 (801 patients) |
| Part IV: MCompl (a two‐factor model) | |
| Traditional Chinese | CFI = 0.99, RMSEA = 0.08 (347 patients) |
| English language | CFI = 1.00, RMSEA = 0.04 (848 patients) |
Dopamine dysregulation syndrome was not included in this analysis because it did not load on any factor in the English version.
RMSEA, root mean square error of approximation.
Secondary Analysis: EFA
The scree plots from each part of the translated MDS‐UPDRS were used to determine the number of factors in the EFA. A comparison of the traditional Chinese scree plot revealed a similar shape to that of the English‐language scree plot (Fig. 1). The number of factors for each part of the traditional Chinese MDS‐UPDRS was as follows: Part I = 2; Part II = 3; Part III = 7; and Part IV = 2.
Figure 1.
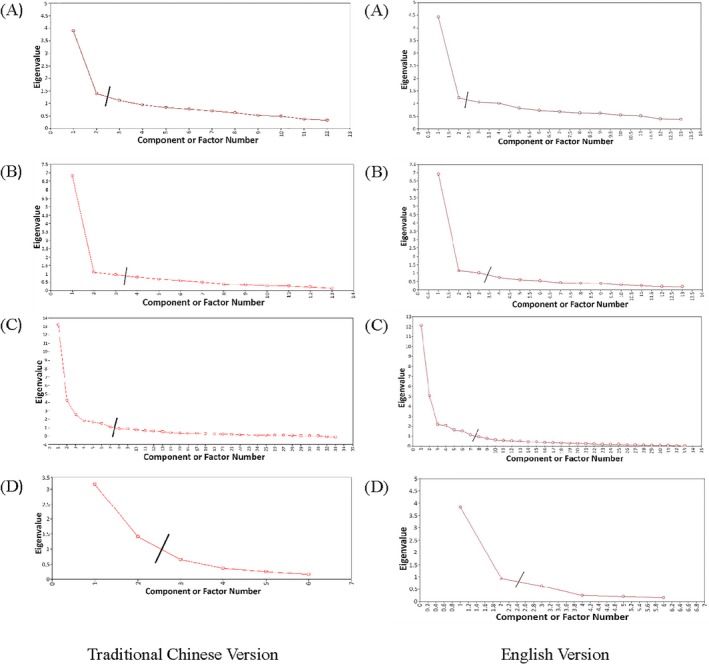
Scree plot of (A) Part I, (B) Part II, (C) Part III, and (D) Part IV.
The results of the EFA for both the English‐language and Chinese‐language versions of the scale are shown in Table 3. For Part I, and in contrast to the English version of the MDS‐UPDRS, daytime sleepiness and urinary problems did not load on any of the factors. In the traditional Chinese translation, cognitive impairment and hallucinations loaded on factor 2; in contrast, they were loaded on factor 1 in the English version. For Part II, speech did not load on any of the factors. Walking and balance, hygiene, freezing, handwriting, and “engaging inhobbies and other activities” loaded on factor 2; this was in contrast to the English version, where these elements loaded on factor 3. In Part III, 6 of the 33 items loaded on different factors on the two scales, and eight items loaded on more than one factor. Most of the items that loaded on different factors in the two versions also had cross‐loadings on multiple factors. In Part IV, time spent in the OFF state loaded on factor 2 in the Traditional Chinese version of the scale, whereas this element loaded on factor 1 in the English version.
Table 3.
Comparison of item factor loading of the English and traditional Chinese exploratory factor structures for each part of the MDS‐UPDRS
| Factor | Item | EGa | CG |
|---|---|---|---|
| Part I: nM‐EDL | |||
| Factor 1 | Percent variance | 34.0 | 32.4 |
| 1.8. Daytime sleepiness | 0.53 | b | |
| 1.7. Sleep problems | 0.35 | 0.48 | |
| 1.1. Cognitive impairment | 0.55 | xxxx | |
| 1.9. Pain and other sensations | 0.43 | 0.63 | |
| 1.2. Hallucinations and psychosis | 0.56 | xxxx | |
| 1.10. Urinary problems | 0.61 | b | |
| 1.11. Constipation problems | 0.46 | 0.57 | |
| 1.12. Lightheadedness on standing | 0.46 | 0.52 | |
| 1.13. Fatigue | 0.47 | 0.54 | |
| Factor 2 | Percent variance | 9.5 | 11.7 |
| 1.3. Depressed mood | 0.81 | 0.68 | |
| 1.4. Anxious mood | 0.68 | 0.58 | |
| 1.5. Apathy | 0.55 | 0.74 | |
| 1.1.Cognitive impairment | xxxx | 0.56 | |
| 1.2. Hallucinations and psychosis | xxxx | 0.41 | |
| Part II: motor‐EDL | |||
| Factor 1 | Percent variance | 53.1 | 52.3 |
| 2.1. Speech | 0.79 | b | |
| 2.2. Saliva and drooling | 0.45 | 0.85 | |
| 2.3. Chewing and swallowing | 0.60 | 0.52 | |
| 2.7. Handwriting | 0.46 | xxxx | |
| 2.8. Doing hobbies and other activities | 0.46 | 0.51 | |
| Factor 2 | Percent variance | 8.7 | 8.3 |
| 2.4. Eating tasks | 0.68 | 0.49 | |
| 2.10. Tremor | 0.43 | 0.70 | |
| 2.12. Walking and balance | xxxx | 0.74 | |
| 2.6. Hygiene | xxxx | 0.87 | |
| 2.13. Freezing | xxxx | 0.68 | |
| 2.7. Handwriting | xxxx | 0.87 | |
| 2.8. Doing hobbies and other activities | xxxx | 0.43 | |
| 2.5. Dressing | xxxx | 0.65c | |
| 2.9. Turning in bed | xxxx | 0.57c | |
| Factor 3 | Percent variance | 7.7 | 7.3 |
| 2.5. Dressing | 0.64 | xxxx | |
| 2.6. Hygiene | 0.65 | xxxx | |
| 2.9. Turning in bed | 0.65 | 0.46c | |
| 2.11. Getting out of bed | 0.73 | 0.56 | |
| 2.12. Walking and balance | 0.82 | xxxx | |
| 2.13. Freezing | 0.76 | xxxx | |
| Part III: MEx | |||
| Factor 1 | Percent variance | 36.7 | 40.0 |
| 3.1. Speech | 0.60 | 0.57 | |
| 3.2. Facial expression | 0.54 | 0.54 | |
| 3.9. Arising from chair | 0.80 | 0.87 | |
| 3.10. Gait | 0.87 | 0.87 | |
| 3.11. FOG | 0.83 | 0.73 | |
| 3.12. Postural stability | 0.80 | 0.80 | |
| 3.13. Posture | 0.70 | 0.63 | |
| 3.14. Global spontaneity of movement | 0.67 | 0.68 | |
| 3.7a. Toe tapping, right foot | xxxx | 0.52c | |
| 3.7b. Toe tapping, left foot | xxxx | 0.44c | |
| 3.8a. Leg agility, right leg | xxxx | 0.59c | |
| 3.8b. Leg agility, left leg | xxxx | 0.52c | |
| Factor 2 | Percent variance | 15.3 | 12.8 |
| 3.17a. Rest tremor amplitude, RUE | 0.73 | 0.82 | |
| 3.17b. Rest tremor amplitude, LUE | 0.71 | 0.75c | |
| 3.17c. Rest tremor amplitude, RLE | 0.74 | 0.76 | |
| 3.17d. Rest tremor amplitude, LLE | 0.70 | 0.81 | |
| 3.17e. Rest tremor amplitude, lip/jaw | 0.60 | 0.64 | |
| 3.18. Constancy of rest tremor | 0.88 | 0.93 | |
| Factor 3 | Percent variance | 6.6 | 7.6 |
| 3.3a. Rigidity, neck | 0.68 | 0.62 | |
| 3.3b. Rigidity, RUE | 0.73 | 0.75 | |
| 3.3c. Rigidity, LUE | 0.74 | 0.81 | |
| 3.3d. Rigidity, RLE | 0.80 | 0.57 | |
| 3.3e. Rigidity, LLE | 0.82 | 0.62 | |
| Factor 4 | Percent variance | 6.2 | 5.5 |
| 3.4a. Finger tapping, right hand | 0.67 | 0.59 | |
| 3.5a. Hand movements, right hand | 0.67 | 0.71 | |
| 3.6a. Pronation movements, right hand | 0.70 | 0.59 | |
| 3.7a. Toe tapping, right foot | xxxx | 0.42c | |
| 3.8a. Leg agility, right leg | xxxx | 0.46c | |
| Factor 5 | Percent variance | 4.9 | 5.1 |
| 3.4b. Finger tapping, left hand | 0.67 | 0.62 | |
| 3.5b. Hand movements, left hand | 0.70 | 0.63 | |
| 3.6b. Pronation movements, left hand | 0.65 | 0.60 | |
| 3.3c. Rigidity, LUE | xxxx | 0.76 | |
| 3.7b. Toe tapping, left foot | xxxx | 0.76 | |
| 3.17b. Rest tremor amplitude, LUE | xxxx | 0.43c | |
| 3.15b. Postural tremor, left hand | xxxx | 0.64 | |
| Factor 6 | Percent variance | 4.5 | 4.5 |
| 3.15a. Postural tremor, right hand | 0.66 | 0.87 | |
| 3.15b. Postural tremor, left hand | 0.71 | 0.56 | |
| 3.16a. Kinetic tremor, right hand | 0.81 | xxxx | |
| 3.16b. Kinetic tremor, left hand | 0.81 | xxxx | |
| Factor 7 | Percent variance | 3.3 | 3.3 |
| 3.7a. Toe tapping, right foot | 0.65 | xxxx | |
| 3.7b. Toe tapping, left foot | 0.62 | xxxx | |
| 3.8a. Leg agility, right leg | 0.62 | xxxx | |
| 3.8b. Leg agility, left leg | 0.60 | xxxx | |
| 3.16a. Kinetic tremor, right hand | xxxx | 0.75 | |
| 3.16b. Kinetic tremor, left hand | xxxx | 0.90 | |
| Part IV: MCompl | |||
| Factor 1 | Percent variance | 63.9 | 52.7 |
| 4.3. Time spent in the OFF state | 0.87 | xxxx | |
| 4.4. Functional impact of fluctuations | 0.84 | 0.75 | |
| 4.5. Complexity of motor fluctuations | 0.82 | 0.86 | |
| 4.6. Painful OFF state dystonia | 0.50 | 0.86 | |
| Factor 2 | Percent variance | 15.6 | 23.5 |
| 4.1. Time spent with dyskinesias | 0.71 | 0.52 | |
| 4.2. Functional impact of dyskinesias | 0.95 | 0.85 | |
| 4.3. Time spent in the OFF state | xxxx | 0.74 | |
xxxx implies the listed item did not load on the factor indicated.
Because we are using a different version of M‐Plus, the factor loadings may vary slightly from the published version.
The item did not load on any of the factors.
Item load on more than one factor with factor loading ≥0.40.
RUE, right upper extremity; LUE, left upper extremity; RLE, right lower extremity; LLE, left lower extremity.
Frequency and Group Comparisons
More than half of our patients reported the following symptoms in Part I of the MDS‐UPDRS: constipation problems (64.5%); cognitive impairment (63.6%); anxious mood (57.1%); urinary problems (55.4%); and daytime sleepiness (54.8%). In Part II, they reported difficulties with the following activities: walking and balance (82.9%); dressing (81.8%); hygiene (76.7%); getting out of bed (76.4%); handwriting (75.8%); speech (71.6%); eating tasks (69.3%); engaging in hobbies and other activities (69%); turning in bed (69%); tremor (58.8%); and saliva and drooling (52%).
Mean scores for each part of the scale in the English12 and present groups were as follows: Part I, 11.5 (standard deviation [SD] = 7.0) versus 9.2 (SD = 5. 9); Part II, 16.0 (SD = 10.0) versus 14.5 (SD = 9.6); Part III, 36.8 (SD = 18.4) versus 28.0 (SD = 16.9); and Part IV, 4.0 (SD = 4.2) versus 4.8 (SD = 4.4), respectively. Significant differences were found between the two groups in each part (Part I: t (1205) = 5.36, P < 0.0001; Part II: t (1205) = 2.37, P = 0.0179; Part III: t (1205) = 7.64, P < 0.0001; and Part IV: t (1205) = 2.94, P = 0.0034). Some differences between the two groups in terms of frequency and severity of the MDS‐UPDRS items were found (Figs. 2 and 3).
Figure 2.
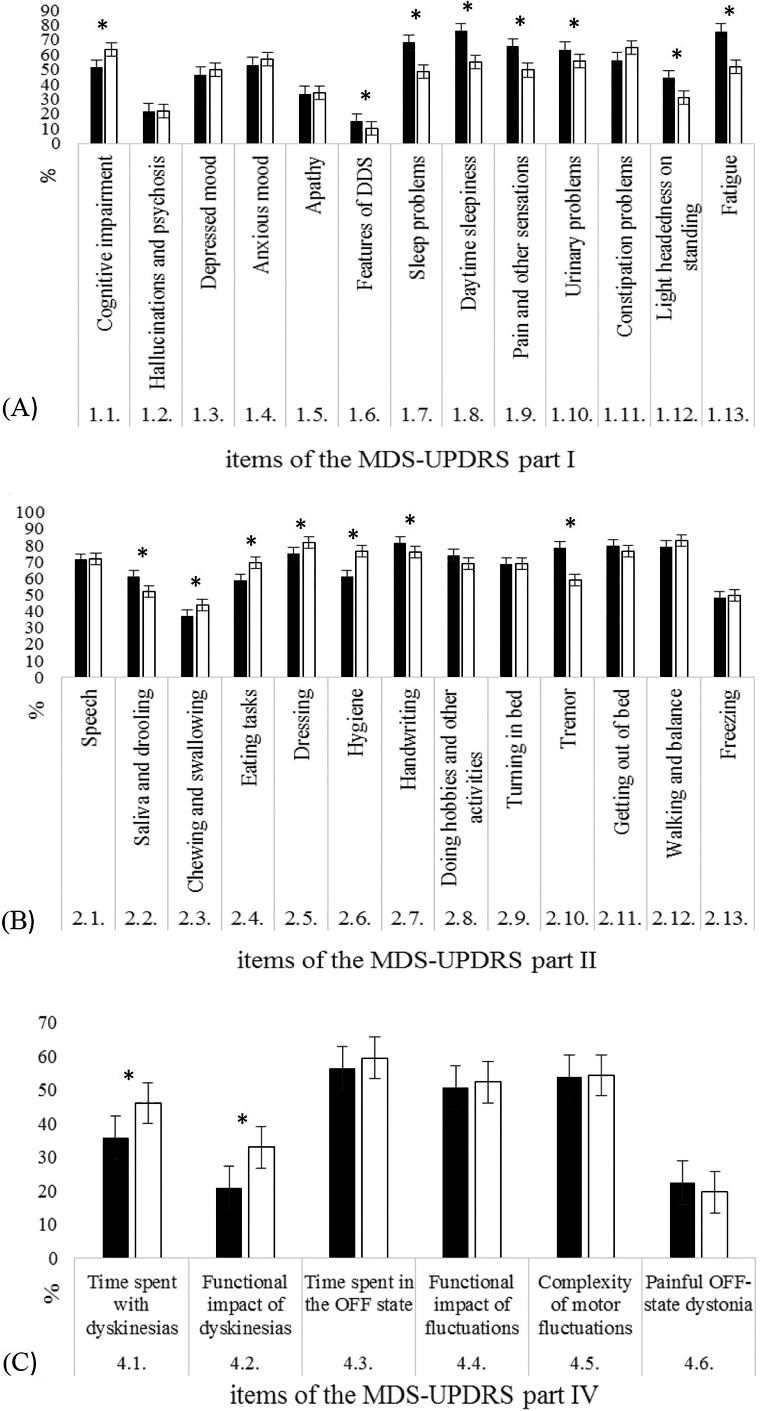
Frequency of the two study groups in (A) Part I, (B) Part II, and (C) Part IV of the MDS‐UPDRS. ■English‐speaking group; □Chinese‐speaking group. *Statistically significant difference between groups. DDS, dopamine dysregulation syndrome.
Figure 3.
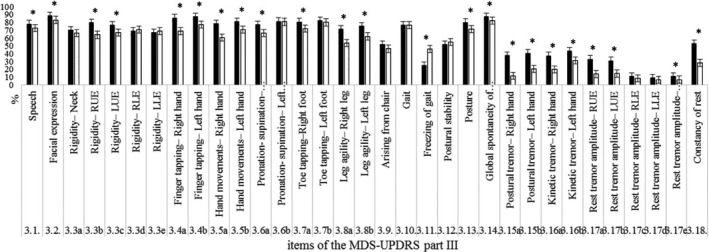
Frequency between the two groups in Part III of the MDS‐UPDRS. ■English‐speaking group; □Chinese‐speaking group. *Statistically significant difference between groups. RUE, right upper extremity; LUE, left upper extremity; RLE, right lower extremity; LLE, left lower extremity.
Discussion
This study produced a cross‐cultural adaptation of the MDS‐UPDRS; specifically, we developed an official traditional Chinese translation of this scale. The main contributions of the study are: (1) It provided a translation of the traditional Chinese version of the MDS‐UPDRS and offered the MDS‐UPDRS for use in Chinese‐speaking populations; and (2) it expanded upon our knowledge of cultural/ethnic differences in NMS, as based on the MDS‐UPDRS.
In the present study, we conducted a CFA to determine whether the factor structure for the English version of the MDS‐UPDRS12 could be confirmed in the data collected using the traditional Chinese version of the MDS‐UPDRS. Based on the criteria that were established for the “official MDS translation,” the CFI for each part (I–IV) had to be 0.90 or greater relative to the English‐language version. For all four parts of the traditional Chinese MDS‐UPDRS, the CFI—when compared with the English‐language factor structure—was 0.93 or greater for each part. Hence, the prespecified English factor structure was confirmed in our traditional Chinese data set. EFA was conducted to explore the underlying factor structure without the constraints of a prespecified factor structure; a scree plot was used to select the number of factors to retain for each part of the MDS‐UPDRS. Overall, the distribution of factors was similar across the two languages, and, in general, the EFA revealed that the results were compatible for each part of the original version.
Some variation between the Chinese‐speaking and English‐speaking populations was observed during the factor analysis. The original UPDRS was primarily criticized for its focus on the irregular placement of nonmotor elements in PD, especially the mental features captured in Part I. One interesting finding in this study is the distribution of the factor loadings in Part I (nM‐EDL). In contrast to the English‐language version, the items that focused on cognitive impairment and hallucinations in the traditional Chinese translation loaded on the same factor as the items that assessed mood and apathy. Our findings suggest that cognitive impairment and hallucinations may share a common underlying pathology with mood states. Previous pathological and clinical studies also support such a relationship.23, 24 In addition, daytime sleepiness and urinary problems did not load on any factor in Part I of the traditional Chinese version of the MDS‐UPDRS. Daytime sleepiness and urinary problems seem to be separate manifestations, given that they differ from other NMS in PD; they might also have distinct underlying mechanisms.25 Nevertheless, urinary problems often receive less recognition as an NMS of PD, and they need more attention in future studies.
Research on NMS has grown exponentially, reflecting an increasing awareness that symptoms are critical for mental health and well‐being. In our sample, we found a high prevalence of NMS measured in Part I of the MDS‐UPDRS, including constipation, cognitive impairment, anxious mood, urinary problems, daytime sleepiness, and fatigue. Similar to what was observed in Japan,16 constipation was found to be the most frequent NMS in Asian PD patients. This finding is consistent with the findings of a questionnaire‐based study, which was performed with a Malaysian PD population.9 Evidence showed that the dorsal motor nucleus of the vagus nerve, which controls the parasympathetic nerve of the GI system, is affected in PD.26 It has been suggested that alpha‐synuclein deposition within the enteric nervous system may be the earliest site of pathology in PD,27 and one nation‐wide population‐based study also indicated that severity of constipation is a precursor to PD.28 The GI system may be the point at which the pathophysiological process initiates, ultimately resulting in the clinical syndrome of PD. A comparison of two cultures indicated that prevalence of NMS (Part I) was more frequent in a Western population; however, it is worth noting that our patients demonstrated more‐frequent cognitive function deficits than those in Western countries. A previous study revealed a lower percentage of concentration difficulties among Asian patients, as well as a similar percentage of memory complaints between a Western and Eastern population.6 These inconsistent results may be related to methodology issues, because the previous study used a self‐report questionnaire, which contained closed‐ended (yes/no) questions to assess the state of the patients’ memory and concentration. The sensitivity of the questionnaire was low and self‐report bias may have influenced the results. In addition, our previous study also found that not all patients are aware of and can report their own cognitive function accurately because of the pathology involved in PD.29 Based on the findings of the current study, the crucial role played by cognitive function is evident, and the high prevalence rate of cognitive dysfunction is compatible with that found in our previous study.8 Our team conducted a comprehensive performance‐based neuropsychological assessment in 94 early‐stage PD patients and 84 healthy controls. We found that up to 46.8% of patients have mild cognitive impairment, and it was also shown that executive function was the domain most likely to be affected by a cognitive decline process in PD. In addition to the self‐report questionnaire, it is believed that the performance‐based assessment of neuropsychological abilities, which are sensitive and specific to cognitive performance, was needed and that ethnic effects on cognitive function were still controversial and needed further investigation.
For Part II of the MDS‐UPDRS (Motor Experiences of Daily Living), the two most frequently observed daily disturbances in the Asian study groups (Chinese and Japanese) were similar, with walking/balance and dressing being the most common. We suggest that medical care programs for Asians are given high priority, because they help patients with walking, buttoning, or putting on or taking off their clothes or jewelry. The strategies used to prevent falls or the use of a device to enhance a patient's walking ability or dressing skills are crucial for paramedical therapy (e.g., physical and occupational therapy) among a PD population. One systematic review indicated that moderate‐to‐strong evidence exists for task‐specific benefits of targeted physical activity training on postural stability and balance in PD.30 In general, impairments in patients’ activities of daily living are more common in our patients than in Western patients, especially with respect to chewing, eating, dressing, and performing hygiene‐related activities. This discrepancy between the two populations might be related to factors such as eating habits and food style, or lifestyle differences across different cultures. These specific activities of daily living, which pose great problems for PD patients, should be properly managed. Evidence showed that a moderate effect exists for paramedical interventions for patients with PD in physical activity training, environmental cues, and individualized programs promoting personal control and quality of life.31 Further large, pragmatic, randomized, controlled trials to investigate the effect of occupational therapy focusing on these specific items of daily living activities observed in this study are required to improve the quality of life in the Chinese‐speaking population of patients with PD.
Regarding symptom frequency, the results of the chi‐squared test indicate that motor symptoms (Part III of the MDS‐UPDRS) are generally more common in patients in the English‐language sample than among those in our group, with the exception of freezing of gait (FOG). The reason of higher scores on UPDRS part III in the English population can be seen simply as a difference in severity in the population samples. However, of note, the finding of a higher rate of FOG in the Chinese‐speaking population is compatible with the high prevalence of cognitive dysfunction observed in this study in part I of MDS‐UPDRS. It has been shown that FOG is associated with cognitive dysfunction; greater disruptions of the frontostriatal‐parietal networks are correlated with freezing severity.32 A high prevalence of FOG might be explained by the high prevalence of cognitive dysfunction in our patients.
One potential limitation of this study was the presence of referral bias; the distribution of our patients showed a predominance of patients in the early disease stages, with very few patients in stage 5. As such, our ability to extrapolate our findings to an entire PD population might be restricted. Second, there was a lack of more complete demographic data, such as years of education and patients’ medicine usage. Education is a protective factors in cognition, and use of levodopa may influence the presence of motor symptoms; these aforementioned factors were not well controlled between the two groups in this study. Further research is needed to investigate the ethnic differences in NMS among PD patients in a well‐designed, controlled study to understand the ethnic‐specific mechanism of NMS, especially cognitive function and daily self‐care activities. Last, the present study did not collect data pertaining to test‐retest reliability or concurrent validity, which are important for scale validation.
Conclusion
NMS in the Chinese PD population are common, and the pattern of NMS in a Chinese PD population is such that there is a greater predominance of symptoms related to cognition and activities of daily living when compared to Caucasian samples. Our study highlighted the important role of NMS in PD and suggested that management programs or development of equipment for NMS are needed in the future. The traditional Chinese version of the MDS‐UPDRS meets the criteria for designation as an official MDS translation of the MDS‐UPDRS; as such, it is now available for use in a Chinese‐speaking population.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
R.L.Y.: 1C, 2A, 2B, 2C, 3A, 3B
R.M.W.: 1A, 1B, 1C, 2C, 3A, 3B
A.Y.Y.C.: 1C, 3B
V.M.: 1C, 3B
Y.R.W.: 1C, 3B
B.C.T.: 2B, 2C, 3B
S.L.: 2B, 2C, 3B
L.W.: 2B, 2C, 3B
N.R.L.P.: 2A, 2B, 3B
G.T.S.: 1A, 1B, 1C, 2A, 2C, 3B
C.G.G.: 1A, 1B, 1C, 2A, 2C, 3B
Disclosures
Funding Sources and Conflicts of Interest: The program was supported by a grant from the International Parkinson and Movement Disorder Society (MDS), for the Chinese MDS‐UPDRS Translation Program for Non‐English Official versions, and the grant from the National Taiwan University Hospital (NTUH), Taipei, Taiwan (NTUH 103‐S2485) and the Ministry of Science and Technology (MOST), Taipei, Taiwan (MOST 103‐2410‐H‐006‐120‐MY2, MOST 103‐2633‐H‐006‐001, and MOST 104‐2633‐H‐006‐001). The authors report no conflicts of interests to disclose.
Financial Disclosures for previous 12 months: R.L.Y. has received a salary from National Cheng Kung University and research support through funding from the National Science Council, Taipei, Taiwan (MOST 103‐2410‐H‐006‐120‐MY2, MOST 103‐2633‐H‐006‐001, and MOST 104‐2633‐H‐006 ‐001). R.M.W. has received a salary from the National Taiwan University Hospital and a grant from National Taiwan University Hospital (100‐S1636). A.Y.Y.C. and V.M. has received a salary from the Prince of Wales Hospital. Y.R.W. has received a salary from the Chang Gung Memorial Hospital‐Linkou branch. B.C.T. has been awarded grants from the National Institutes of Health (NIH; National Institute of Neurological Disorders and Stroke, National Heart, Lung and Blood Institute, National Institute on Minority Health and Health Disparities, and National Institute of General Medical Sciences), the Pfizer Data and Safety Monitoring Committee and the NIH Data and Safety Monitoring Committees, and has received a salary from the University of Texas Health Science Center School of Public Health at Houston, Division of Biostatistics. S.L. has received grants from the NIH, MDS, and Child Health and Development Institute (CHDI) Foundation. N.R.L.P. has worked in cognitive testing, qualitative research, and program/process evaluation consulting for the University of Massachusetts (UMass) Medical School (UMMS) Lamar Soutter Library, UMass Medical School Inter‐Professional Development, The Association of Academic Health Sciences Libraries, Medical University of South Carolina (MUSC) College of Nursing and Hollings Cancer Center, and the MDS. Dr. Lapelle is a subcontractor on a variety of research and evaluation grants with principal investigators at UMMS and MUSC. G.T.S. has worked as a consultant for and held advisory board membership with honoraria with Acadia Pharmaceuticals, Adamas Pharmaceuticals, Inc., Ceregene, Inc., CHDI Management, Inc., Ingenix Pharmaceutical Services (i3 Research), Neurocrine Biosciences, Inc., and Pfizer, Inc.; has been awarded grants and research support from the NIH, the Michael J. Fox Foundation (MJFF) for Parkinson's Research, and the Dystonia Coalition, CHDI, and MDS; has received honoraria from the MDS, American Academy of Neurology, and the MJFF; and has received a salary from Rush University Medical Center (RUMC). C.G.G. is on the consulting and advisory board membership of and has received honoraria from Acadia (Deborah Wood Associates), AstraZeneca, Avanir, Boston Scientific, Clearview, Health Advances, Chelsea Pharmaceuticals (Link Medical Communications), ICON Pricespective LLC, MED‐IQ Educational Services, Neurocrine, Pfizer, Teva, and WPP Group Kantor Health LLC and directs the Rush Parkinson's Disease Research Center that receives support from the Parkinson's Disease Foundation. He directs the translation program for the MDS‐UPDRS and UDysRS and receives funds from the IPMDS for this efforts; has received grants and research support through funding from the NIH and the MJFF; has received honoraria from the University of California, University of Luxembourg, University of Rochester, and World Parkinson Coalition; has received royalties from Oxford University Press, Elsevier Publishers, Wolters Kluwer Health‐Lippincott, and Wilkins and Wilkins; and has received a salary from RUMC.
Acknowledgments
We are grateful to the participants involved in this study.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007;68:384–386. [DOI] [PubMed] [Google Scholar]
- 2. Schapira AH. The measurement and importance of non‐motor symptoms in Parkinson disease. Eur J Neurol 2015;22:2–3. [DOI] [PubMed] [Google Scholar]
- 3. Chaudhuri KR, Sauerbier A, Rojo J, et al. The burden of non‐motor symptoms in Parkinson's disease using a self‐completed non‐motor questionnaire: a simple grading system. Parkinsonism Relat Disord 2015;21:287–291. [DOI] [PubMed] [Google Scholar]
- 4. Chaudhuri KR, Martinez‐Martin P, Schapira AH, et al. International multicenter pilot study of the first comprehensive self‐completed nonmotor symptoms questionnaire for Parkinson's disease: the NMSQuest study. Mov Disord 2006;21:916–923. [DOI] [PubMed] [Google Scholar]
- 5. Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord 2009;24:1641–1649. [DOI] [PubMed] [Google Scholar]
- 6. Liu WM, Lin RJ, Yu RL, Tai CH, Lin CH, Wu RM. The impact of non‐motor symptoms on quality of life in patients with Parkinson's disease in Taiwan. Neuropsychiatr Dis Treat 2015. Nov 11;. doi: 10.2147/NDT.S88968. [Epub ahaead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu RL, Tan CH, Wu RM. The impact of nocturnal disturbances on daily quality of life in patients with Parkinson's disease. Neuropsychiatr Dis Treat 2015;11:2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu RL, Wu RM, Tai CH, Lin CH, Cheng TW, Hua MS. Neuropsychological profile in patients with early stage of Parkinson’'s disease in Taiwan. Parkinsonism Relat Disord 2012;18:1067–1072. [DOI] [PubMed] [Google Scholar]
- 9. Azmin S, Khairul Anuar AM, Tan HJ, et al. Nonmotor symptoms in a Malaysian Parkinson's disease population. Parkinsons Dis 2014;2014:472157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu Y, Guo XY, Wei QQ, et al. Non‐motor symptoms and quality of life in tremor dominant vs postural instability gait disorder Parkinson's disease patients. Acta Neurol Scand 2015. doi: 10.1111/ane.12461. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11. Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease . The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003;18:738–750. [DOI] [PubMed] [Google Scholar]
- 12. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 13. Goetz CG, Stebbins GT, Wang L, LaPelle NR, Luo S, Tilley BC. IPMDS‐Sponsored Scale Translation Program: process, format, and clinimetric testing plan for the MDS‐UPDRS and UDysRS. Mov Disord Clin Pract 2014;1:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Antonini A, Abbruzzese G, Ferini‐Strambi L, et al. Validation of the Italian version of the Movement Disorder Society‐Unified Parkinson's Disease Rating Scale. Neurol Sci 2013;34:683–687. [DOI] [PubMed] [Google Scholar]
- 15. Martinez‐Martin P, Rodriguez‐Blazquez C, Alvarez‐Sanchez M, et al. Expanded and independent validation of the Movement Disorder Society‐Unified Parkinson's Disease Rating Scale (MDS‐UPDRS). J Neurol 2013;260:228–236. [DOI] [PubMed] [Google Scholar]
- 16. Kashihara K, Kondo T, Mizuno Y, et al. Official japanese version of the Movement Disorder Society‐Unified Parkinson's Disease Rating Scale: validation against the original English version. Mov Disord Clin Pract 2014;1:200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skorvanek M, Kosutzka Z, Valkovic P, et al. Validation of Slovak version of the Movement Disorder Society‐Unified Parkinson's Disease Rating Scale. Cesk Slov Neurol N 2013;76:463–468. [Google Scholar]
- 18. Hatcher L. A Step‐by‐Step Approach to Using the SAS System for Factor Analysis and Structural Equation Modeling. Cary, NC: SAS Institute; 1994. [Google Scholar]
- 19. Goetz CG, Stebbins GT, Chmura TA, Fahn S, Poewe W, Tanner CM. Teaching program for the Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale:(MDS‐UPDRS). Mov Disord 2010;25:1190–1194. [DOI] [PubMed] [Google Scholar]
- 20. Tilley BC, LaPelle NR, Goetz CG, Stebbins GT; MDS‐UPDRS Task Force . Using cognitive pretesting in scale development for Parkinson's disease: the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) example. J Parkinsons Dis 2014;4:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fowler FJ. Improving Survey Questions: Design and Evaluation. Thousand Oaks, CA: Sage; 1995. [Google Scholar]
- 22. Gorsuch RL. Factor Analysis, 2nd ed Hillsdale, NJ: Erlbaum; 1983. [Google Scholar]
- 23. Aarsland D, Taylor JP, Weintraub D. Psychiatric issues in cognitive impairment. Mov Disord 2014;29:651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gallagher DA, Parkkinen L, O'Sullivan SS, et al. Testing an aetiological model of visual hallucinations in Parkinson's disease. Brain 2011;134:3299–3309. [DOI] [PubMed] [Google Scholar]
- 25. Höglund A, Broman JE, Pålhagen S, Fredrikson S, Hagell P. Is excessive daytime sleepiness a separate manifestation in Parkinson's disease? Acta Neurol Scand 2015;132:97–104. [DOI] [PubMed] [Google Scholar]
- 26. Del Tredici K, Rüb U, de Vos RA, Bohl JR, Braak H. Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 2002;61:413–426. [DOI] [PubMed] [Google Scholar]
- 27. Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric α‐synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease‐related brain pathology. Neurosci Lett 2006;396:67–72. [DOI] [PubMed] [Google Scholar]
- 28. Abbott RD, Ross GW, White LR, et al. Environmental, life‐style, and physical precursors of clinical Parkinson's disease: recent findings from the Honolulu‐Asia Aging Study. J Neurol 2003;250:iii30–iii39. [DOI] [PubMed] [Google Scholar]
- 29. Yu RL, Wu RM, Tai CH, Lin CH, Hua MS. Feeling‐of‐knowing in episodic memory in patients with Parkinson's disease with various motor symptoms. Mov Disord 2010;25:1034–1039. [DOI] [PubMed] [Google Scholar]
- 30. Foster ER, Bedekar M, Tickle‐Degnen L. Systematic review of the effectiveness of occupational therapy‐related interventions for people with Parkinson's disease. Am J Occup Ther 2014;68:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liddle J, Eagles R. Moderate evidence exists for occupational therapy‐related interventions for people with Parkinson's disease in physical activity training, environmental cues and individualised programmes promoting personal control and quality of life. Aust Occup Ther J 2014;61:287–288. [DOI] [PubMed] [Google Scholar]
- 32. Heremans E, Nieuwboer A, Vercruysse S. Freezing of gait in Parkinson's disease: where are we now? Curr Neurol Neurosci Rep 2013;13:350. [DOI] [PubMed] [Google Scholar]


