Abstract
Due to their unique characteristics such as multifold change of volume in response to minute change in the environment, resemblance of soft biological tissues, ability to operate in wet environments, and chemical tailorability, stimuli responsive gels represent a versatile and very promising class of materials for sensors, muscle-type actuators, biomedical applications, and autonomous intelligent structures. Success of these materials in practical applications largely depends on their ability to fulfill application-specific mechanical requirements. This article provides an overview of recent application-driven development of covalent polymer gels with special emphasis on the relevant mechanical factors and properties. A short account of mechanisms of gel swelling and mechanical characteristics of importance to stimuli-responsive gels is presented. The review highlights major barriers for wider application of these materials and discusses latest advances and potential future directions toward overcoming these barriers, including interpenetrating networks, homogeneous networks, nanocomposites, and nanofilamentary gels.
Keywords: hydrogels, stimuli-responsive gels, mechanical properties, swelling, sensors, actuators
Graphical Abstract

1. Introduction
Covalent polymer gels are crosslinked polymer networks swollen by a solvent. They consist mostly of liquid, but behave like a solid due to three-dimensional crosslinked macromolecular network. Their properties can be varied in a broad range because a wide variety of polymers can form 3D gel networks, while density and structure of the cross-links allow additional degree of control. Large inner volume of gels, accessible for storage, is used in many applications such as superabsorbents, drug delivery carriers, and batteries. The gels are particularly attractive for biomedical applications, such as tissue engineering, owing to their resemblance of soft biological tissues and their operation in wet environments. In fundamental research, gels proved to be useful for understanding polymer thermodynamics and rubber elasticity.
Gels are known to change their volume in response to alteration of the environmental parameters. The change in volume results from the absorption or release of the fluid and may reach hundreds and even thousands percent. Often it is accompanied by considerable swelling force. Gels that demonstrate substantial (and often abrupt) volume change in response to small environmental change and gels that are selective to a specific stimulus are called stimuli-responsive or responsive gels (SRGs).
One of the first experimental observations of reversible and profound volume change in gels was reported by Kuhn and Katchalsky who also noted the resemblance of such gels to muscles [1–3]. Almost at the same time, Flory and Rehner developed a theory of swelling of polymer networks [4–6] that was found to be capable of explaining gel response to stimuli.
The development of responsive gels accelerated in the last quarter of the twentieth century. Tanaka demonstrated abrupt gel volume change caused by small change of environmental conditions, which was named volume phase transition [7]. He created gels responsive to different stimuli, such as solvent composition [7], temperature [7], metal ions [8], electric field [9], and light [10]. Tanaka also advanced the theory of equilibrium swelling of responsive gels and developed a theory of gel swelling kinetics [11–14]. By the end of the twentieth century, responsive gels became an important class of functional materials. The number of applications of SRGs, as well as the amount of research in this field, is continually increasing. Several applications have already been commercialized.
To ensure further success of SRGs in a broad range of practical application, it is becoming critical to better understand and control their mechanical properties. This report describes the current state in the assessment and control of properties of gels relevant to their mechanical behavior, discusses challenges and their proposed or potential solutions, and identifies and reviews new directions.
The scope of this report is limited to covalent stimuli-responsive polymer gels. Other relevant materials, including self-assembled gels and stimuli-responsive and shape-memory materials that are not gel-based are not covered. Nevertheless, it still would be impossible to include every important result and publication in a single review. The preference was given to recent publications. Additional information can be found in the relevant previously published reviews [15–25] as well as in a significant number of more specialized papers and reviews that are referenced below in the following sections.
2. Underlying Mechanisms of Gel Swelling (Classification and Theoretical Fundamentals)
Gel properties, including gel volume, are mostly influenced by interaction of macromolecules that constitute the gel network with mobile molecules in the solution (although other interaction may also be present). According to Flory-Rehner model [4,5,26], the free energy of the gel can be considered to be a sum of contributions from mixing of the polymer network with the solvent, elasticity of the network, and interactions between mobile charges and charges immobilized on the network electrical charges (Fig. 1). The gel reaches its equilibrium volume when a balance of the forces is reached, i.e. the osmotic pressure inside and outside the gel equilibrates. Osmotic pressure of the solvent in the gel is a derivative of the free energy and therefore also includes three components:. Πtotal = Πmix + Πelast + Πcharge
Fig. 1.
Schematic representation of the thermodynamics of gel equilibrium. The total free energy of the gel (Gtotal) is considered to be a sum of the energy of mixing (Gmixing), the elasticity energy of network (Gelasticity), and the energy associated with electrical charges (Gcharge). The mixing can be described by Flory-Huggins theory. The elasticity can be described by a model of rubber elasticity. The contribution of the electrical charges arises from electrostatic repulsion and attraction and osmotic pressure produced by Donnan equilibrium.
Changes in the environment disturb the balance and cause mass transfer until a new equilibrium is reached. Often, the response of the gel is caused by the stimuli-induced change in the miscibility of the polymer network and the solvent. The most studied example of such gels are the hydrogels composed of poly(N-isopropylacrylamide) (PNIPAM). This polymer has low critical solution temperature (LCST) around 33°C in water. Above that temperature, PNIPAM-based network’s miscibility with water rapidly decreases and hydrogel shrinks loosing almost all of its water [27,28].
The elasticity of the gel arises from the forces generated by thermal motion of the network chains constrained by the crosslinks. According to classical theories of polymer network elasticity [29], the elastic forces are proportional to the number of effective chains per unit volume and therefore proportional to the crosslinking density. Stimuli that cause dissociation of the crosslinks reduce the elastic component of the gel osmotic pressure and increase the swelling of gels. Other stimuli may cause formation of additional cross-links, for example, as a result of formation of complexes involving two functional groups attached to the polymer network, and therefore trigger gel shrinkage.
Ionic and polyelectrolyte gels contain charged groups immobilized on the polymer network. The network of such gel is usually not electrically neutral by itself. As a result, mobile ions must distribute unequally between the gel interior and the outside solution to maintain electroneutrality [30]. The unequal distribution of the mobile ions, which is called Donnan equilibrium [31], produces osmotic pressure that must be counterbalanced by other forces. Donnan equilibrium results in additional swelling that augments the swelling of the neutral network. This additional swelling is most pronounced in solutions with low ionic strength. Change of the solution ionic strength or change of the network charge cause substantial change of the gel volume. The most studied gels of this class are polyacrylic acid-based pH-sensitive hydrogels. Electrical charges can also contribute to the swelling through their electrostatic repulsion or attraction. However, the electrical charges are often effectively screened by mobile ions or the solvent, and therefore the contribution of electrostatic repulsion/attraction between the charges may be negligible even when the Donnan osmotic pressure is quite high. Nevertheless, in highly charged polyelectrolytes, electrostatic interactions are significant and accompanied by other phenomena such as counterion condensation and chain stiffening [30].
SRGs can be classified by the mechanisms of response i.e. by the part of their osmotic pressure that is most affected by stimuli. Thus, SRGs may be divided in three groups: gels responsive in consequence of change of free energy of mixing, gels responsive due to change of the network elasticity, and gels responsive through ion interactions. It is important to remember, however, that the division of the free energy of gels into these three parts is somewhat artificial. Many molecular interactions can be included in one part or another. For example, effects of hydrogen bonding are usually associated with the mixing part although they can be considered as crosslinks and thus included into the elastic part. The electrostatic repulsion of polyelectrolyte segments often thought to be a part of the charge interactions, even though for linear polyelectrolytes, the electrostatic repulsion is normally included in the free energy of mixing. Also, many stimuli affect more than one part. For example, dissociation of acid groups gives rise to the “charge interactions” part, but it also may increase the hydrophilicity of the gel network, i.e. alter the “mixing” part.
The transition of the gel from a state that is no longer in equilibrium with the environment to a new equilibrium state, i.e. the kinetics of swelling, can be described by Tanaka-Fillmore theory [11]. According to the theory, which is based on the linear model of elasticity of the gel network, the characteristic time of the transition for spherical gel is directly proportional to the square of the dimension of the gel and inversely proportional to the collective diffusion coefficient D, which is defined as a ratio of the longitudinal bulk modulus of the network to the “coefficient of friction” between the network and the solvent f:
where r(t) is the current radius of gel, r0 is the initial radius, req is the equilibrium radius, τ is the characteristic time, and K and μ are bulk and shear moduli of the gel network.
The prediction of the Tanaka-Fillmore theory that the response time is proportional to the square of the gel’s smallest spatial dimension has been extended to other gel shapes and confirmed experimentally [32]. Yet many aspects of swelling kinetics remain poorly understood. The swelling kinetics of SRGs is often complicated by concurrent diffusion of several types of mobile solutes coupled with chemical reactions. These processes occur simultaneously with the swelling, proceed with different rates, and influence each other. Therefore, in some cases the gel may approach the equilibrium very slowly [33,34], or “overshoot” the balance point [35,36], as shown in Fig. 2.
Fig. 2.

Complexity of gel response kinetics is demonstrated by this example of gel response overshoot. In response to addition of 1 mM glucose, a poly(acrylamide) gel functionalized with fluorophenylboronic acid groups shrinks past the equilibrium point and then re-swells. In the subsequent test (Exp 2) the gel reaches the equilibrium faster and demonstrates smaller overshoot that in the initial test (Exp 1). The gel volume change is monitored by the shift of the diffraction of a photonic crystal embedded in the gel. Reprinted with permission from Ref. [35]. Copyright 2009 American Chemical Society.
3. Mechanical Factors of Importance to Stimuli-Responsive Gels
The thermodynamical forces mentioned above are directly related to mechanical properties important for applications of gels. Among the main mechanical parameters of regular gels, we consider swelling ratio, swelling time (or swelling rate), and mechanical robustness. SRGs are additionally characterized by such mechanical parameters as amplitude of response to stimuli, response time, and the amount of work that the gel is able to produce during its response to stimuli.
The swelling ratio, which represents the amount of fluid that the gel is able to hold, is the most characteristic property of gels. Most often the swelling ratio is reported for free swelling, i.e. when the gel is immersed in the fluid and not constrained in any way. Fig. 3 shows examples of dry and swollen gels [37]. The swelling ratio Q is usually measured as the mass of absorbed fluid mfluid per unit mass of dry gel network mdry:
although other definitions exist in the literature. For example, the swelling ratio may also be reported as volume percent of fluid in the gel. Notably, a constrained gel or a gel under load is not able to hold as much fluid as a free gel. Therefore it is critical to take into account any forces or constrains that are applied to the gel during characterization of its swelling ratio. It is also important to consider forces and constraints that may be applied to gels in applications.
Fig. 3.

(A) Dried and (B) swollen in tetrahydrofuran lipophilic polyelectrolyte gel demonstrates a typical magnitude of swelling ratio in polymer gels. Reprinted from Ref. [37] by permission from Macmillan Publishers Ltd, copyright 2007.
Amplitude of response is a parameter specific to stimuli-responsive behavior of the gel. It is most often reported as the ratio of the volumes of the gel with and without the stimulus. The amplitude of response can be deducted from swelling ratios of gels with and without the stimulus, but not from the single swelling ratio in the presence of the stimulus, because the gel also swells in the absence of the stimuli. Quite often in applications, the response of the gel is important in one dimension only. Moreover, the gel may be constrained or may swell anisotropically. In such cases it is more appropriate to measure the amplitude of response separately for specific dimensions. A constrained gel, being unable to change a dimension, will respond to stimuli by generating force. In this case, the amplitude of response can be measured as stress in the constrained direction or pressure generated by the gel [38].
The amount of mechanical work that the gel is able to produce is important for applications where the gel is employed as an actuator. The work is proportional to both generated force and displacement, and therefore may be also regarded as a measure of the amplitude of response.
Obviously, mechanical robustness is required for many applications. The gel must be strong enough so that its functioning is not compromised and that it is not damaged during processing, handling, service, or storage. It is important to keep in mind that during mechanical deformation a gel may behave either as a “closed system,” for which the volume fraction of solvent remains constant during deformation, or, if immersed in fluid, as a semi-open system that may absorb or give out the fluid [39]. Even in air, a highly swollen gel may expel the fluid in response to a deformation. Depending on these conditions, the results of mechanical testing require different interpretation.
Gels ability to resist deformation is typically characterized by two moduli. Bulk modulus determines the ability of the gel to maintain its volume against external pressure, while shear modulus quantifies the ability of the gel to maintain its shape. The ability to withstand the mechanical load without failure is characterized by the ultimate stress at break, and is usually determined in both compression and tensile tests. Also the maximum strain achievable without gel breakage is important for some applications.
The major structural factor contributing to all those parameters is the number of polymer chains per unit volume, which is proportional to the number of cross-links per volume. It is well known that gel robustness decreases when the gel swells because the numbers of polymer chains per volume decreases. In a recent work, Johnson et al. [40] demonstrated reduction in ultimate tensile strength from 300 to 60 kPa, accompanied by reduction in elongation at break from 150% to 30%, between swollen and unswollen states of a hydrogel. Therefore, the gels need to be characterized in dry state and/or at one or several degrees of swelling, depending on the application.
The moduli and the stress at break usually increase with the increase of crosslinking degree (Fig. 5). Five-fold increase of the crosslinking density may result in an increase in Young’s modulus by an order of magnitude [40]. At the same time, the swelling ratio and the amplitude of volume response typically decrease with the increase of crosslinking degree. This demonstrates that a compromise between the swelling ratio or response amplitude and the mechanical properties may have to be achieved depending on the application requirements.
Fig. 5.
Tensile stress vs strain dependence of Ca2+-cross-linked alginate hydrogels at Ca2+ concentration 0.24 M (1), 0.30 M (2), and 0.60 M (3). Both moduli and stress at break increase with the increase of crosslinking degree. Reprinted with permission from Ref. [41]. Copyright 2003 American Chemical Society.
Some other mechanical parameters can be important for applications. In addition to the already mentioned factors, cyclic repeatability and fatigue durability are essential, because the useful lifetime of many gel-based devices depends on the reversibility of the gel volume change.
The most important parameter for many applications is the speed with which the gel changes its volume. It is a crucial limiting factor for gels, because their volume response is a diffusion process. The volume change of the gel is accompanied by movement of the fluid through the polymer network, as well as diffusion-limited equilibration of chemical potentials of all mobile species present in the fluid. The time needed for dry gel to reach its swelling ratio after immersion in a fluid is called swelling time. Similarly, the time required to reach equilibrium after a change in environment is called response time.
Because one of the main aims of the development of responsive gels is the improvement of the speed of response, it is very important to use a standardized characteristic of this parameter that would allow comparison of gels with various structures. In spite of this, there is currently no well-defined measurement for the response time or swelling time that would suit multiple applications. In superabsorbent polymers industry, Vortex method and Voigt-based model are widely used [42], which appears to be the only examples of standardized measurement of gel swelling speed. Those methods, however, are hardly applicable for other applications, other responsive gel systems, and fundamental research.
Ideally, one would define swelling time as the time needed to reach equilibrium swelling. But gel equilibrium is reached asymptotically and thus would require an infinite time. Often, the response time is considered to be the time after which no additional change of the gel state can be observed during arbitrary long enough time interval by the method used to monitor swelling. Such method is obviously controversial. More reproducible results may be attained if the response time defined as the time needed to reach a certain degree of transition. In general however, the profile of swelling vs. time may differ significantly from one gel system to another thus requiring analysis of the complete time profile of swelling in order to compare the speed of response of the gel systems.
Because the speed of response strongly depends on the gel shape and dimensions, it is important to consider these factors when comparing results obtained on different systems. It is also important to keep in mind that the response time may depend on environmental parameters. For example, characteristic time of swelling of collagen-based materials is pH-dependent [34]. Ionic strength affects the response time of many pH responsive gels [43], while temperature affects the kinetics of almost every process including response of SRG-based sensors [35,44].
4. Overview of Gel-Based Materials by Application
The research in the field of responsive gels is often driven by specific applications. Responsive gels are finding applications in very diverse areas with different requirements. Some applications utilize gels unique ability to hold large amount of fluid, others employ them as actuators or sensors, and some use both their sensing and actuating capabilities at the same time. Different applications require gels to possess different combination of mechanical properties. This section overviews recent application-driven developments of gels and discusses relevant application-specific mechanical factors and needs.
4.1. Superabsorbents
Many applications of gels are built on their unique swelling properties. Superabsorbents, i.e. materials capable to spontaneously take in fluid in the amount of 20 times and more of their dry weight while retaining the shape, are the most straightforward example of such applications. In fact, it is the first successful application of hydrogel swelling capacity. Commercial production of hydrogel-based superabsorbents began in 1978 [42]. Hydrogels in the role of superabsorbent materials are currently widely employed in hygienic products. They also find applications in agriculture for enhancing water retention, improving soil properties, increasing fertilizer and pesticide efficiency, and mitigating contamination of the environment [45,46]. In civil engineering they are used for water blocking in optical cables [47] and for improving properties of cement-based materials [48]. The recent development in superabsorbent materials was reviewed by Zohuriaan-Mehr [42].
Swelling ratio and swelling time are the most important parameters of superabsorbents. Nevertheless, the practical use of hydrogels as superabsorbent revealed the importance of other mechanical properties of gels. Even in hygienic applications, the hydrogel particle must have sufficient integrity to retain its shape and resist the flow and fusion with the neighboring items [42]. Weak superabsorbent materials release moisture upon compression or get squeezed through seams or textile pores. Given that modulus of elasticity and strength of the superabsorbent gels in swollen state is typically low [39], special attention is required to ensure that the mechanical properties of superabsorbent materials meet the application requirements. When the importance of the mechanical properties was recognized, the priorities in diaper manufacturing, a characteristic application of superabsorbents, shifted from maximizing free swelling ratio of superabsorbents to optimizing absorbency under load (ability to absorb fluid while under static load) [49] and to improving gel stability and ability to maintain the absorption capacity against shearing. Additionally, modern superabsorbent materials are required to possess desired rate of absorption, minimal soluble content, and sufficient durability and stability in swelling environments and during storage [42].
Higher absorbency under load and better robustness are typically achieved by increasing crosslinking density, at the expense of free swelling capacity and the speed of absorption. Although the speed of absorption can be increased by creating smaller superabsorbent particles, it usually makes handling them more difficult and does not recover the loss in swelling capacity. Therefore, research of alternative approaches to improve mechanical properties of superabsorbents without compromising their swelling speed and capacity continues. For example, particles with higher crosslinking degree on surface that in the middle showed faster swelling rate and better mechanical properties than the uniformly crosslinked particles with same swelling ratio from the same materials [50]. Further improvement is expected from reinforced superabsorbents described in Section 5.1.
Superabsorbents are commonly based on polyelectrolytes. The global market of superabsorbents is currently dominated by copolymers of acrylic acid. As alternatives, systems based on hydrolyzed polyacrylonitrile, poly(isobutylene-co-disodium maleate), neutral polymers such as poly(acrylamide) (PAAm) and poly(ethylene glycol) (PEG), and natural polymers such as polysaccharides (cellulose and starch) and polypeptides (fish and soy proteins, collagen) are considered.
Although swelling in response to contact with moisture may be to some extent considered as stimuli-responsive behavior, regular superabsorbents are not usually classified as stimuli-responsive hydrogels; nevertheless they are very relevant. Materials used as superabsorbents often constitute a base of systems employed in other gels applications with stimuli-responsive capabilities. Use of gels as superabsorbents demonstrated the demands for mechanical properties that are likely to arise in other applications of gels. Meanwhile, stimuli-responsive materials were recently proposed as recyclable, multiple-use superabsorbents. As an example, Mudiyanselage and Neckers prepared azobenzene-based photochromic superabsorbent polymeric system that expel 80% of absorbed water upon irradiation at 350 nm [51].
4.2. Oil absorbents
By analogy with aqueous superabsorbents, gels can also be used as oil sorbents [52]. Oil sorbents are utilized to control oil spills on water surface and on the ground. Conventionally, polypropylene fiber or non-woven fabrics, polyurethane foam sheets, and melt blown polyesters are employed for this purpose [53,54]. However, gel-forming systems are now considered as promising high-performance oil absorbents and several examples of such systems have been reported. Thus, crosslinked cinnamoyloxyethyl methacrylate/isooctyl acrylate copolymers demonstrated 12 g/g toluene absorbency and 6 g/g crude oil absorbency with swelling time 3–5 h [55]. Docosanyl acrylate/cinnamoyloxy ethyl methacrylate demonstrate 35 g/g oil absorbency after 10 h swelling time [56]. Crosslinking polymerization of 4-tert-butylstyrene/ethylene–propylene–diene terpolymer/styrene–butadiene blend produced absorbent capable of 60 g/g oil absorbency upon ~ 5 days swelling time [57]. Graft terpolymer 4-tert-butylstyrene/ethylene–propylene–diene terpolymer/divynilbenzene attained, depending on synthesis routine, either 84 g/g maximum oil absorbency with reaching 60% of this value in 20 h, or 56 g/g maximum oil absorbency reaching 60% of this value in 10 min [58]. Regular copolymer poly(octadecene-alt-maleic anhydride) crosslinked by ethylene glycol reached 60 g/g oil absorbency within 30 min swelling time [59]. Atta and Arndt performed a detailed parametric study of copolymers of 1-octene and isodecyl acrylate and obtained an absorbent with both high oil absorbency 60 g/g and high Young modulus in the swollen state (0.4 MPa), although with a long swelling time (4–50 h) [60].
Similarly to the aqueous superabsorbent, the applications of gels as oil sorbents require optimization of swelling ratio, swelling time, and mechanical strength. Among unresolved issues remains the question of the most appropriate form/shape/format of the oil sorbents. Bulk form results in slow absorption while particulate form is harder to collect. Systems with open pores may combine faster absorption with ease of collecting provided such systems have mechanical strength in the swollen state sufficient for handling.
The most critical weaknesses of current oil sorbents are their bulkiness, high material cost, and environmental and economic problems with their disposal, as the used absorbent needs to be incinerated or buried after single use [61]. Multiple-use oil absorbents based on SRGs would alleviate these shortcomings. Additionally, stimuli-responsive oil absorbents may offer a way for oil recovery. Development of stimuli-responsive oil absorbents has been recently initiated. For instance, Kulawardana and Neckers synthesized light-sensitive oil sorbent comprising isodecyl acrylate, lauryl acrylate, tert-butylstyrene, and photoresponsive azobenzene groups. Absorbency of gasoline diluted with toluene was 12 g/g within 50 min swelling time. Upon irradiation with 350 nm light, 94% of absorbed diluted gasoline was expelled in 60 min while the nonirradiated gels needed more than 1 day to desorb the oil [62].
In summary, gels and SRGs are still in the early stages of development for oil absorption applications. They demonstrated high oil absorbency but, as in the case of superabsorbents, in order to be successful in this application, their other properties need to be investigated and improved, most notably absorbency rate and mechanical strength.
4.3. Catalysts support media
Several factors make gels very attractive as catalyst supports. First, the catalysts are easier to recycle if they are immobilized in hydrogel. Second, reactions that commonly do not proceed well enough in aqueous media may be possible in a hydrogel environment because reactants can be activated through interactions with the hydrogel. Finally, reactants can be concentrated within the hydrogel because of affinity between the reactants and the hydrogel network [63]. Approaches for entrapping man-made catalysts and enzymes in a gel have been investigated and developed since the early Bernfeld and Wan report [64] on physical immobilization of enzymes into a cross-linked synthetic acrylamide gel.
SRGs possess several advantages over regular gels as catalyst support and reaction media [65]. Responsive behavior of hydrogels simplifies recycling of the catalyst. Reversible deswelling allows further concentrating of the reactants. Control of catalyst activity and availability opens perspectives of intelligent regulation of the reaction rate. Future development in this field may lead, for example, to an antioxidant medicine, where catalytic efficiency can be controlled according to the need of the human body.
The current development of SRGs as catalyst support media has been recently reviewed by Diaz Diaz and co-authors [66]. Here we summarize just a few achievements. A thermoresponsive hydrogel of poly(glycidyl methacrylate-co-NIPAM) with gold nanoparticles was shown to act as a recyclable catalyst [67]. At a temperature below the LCST, it was a homogeneous and efficient catalyst, whereas at a temperature above the LCST, it became heterogeneous and its catalytic activity greatly decreased. A thermally recyclable (via swelling/deswelling) catalyst was attained by immobilizing the enzyme thermolysin on copolymer of NIPAM and N-acryloxysuccinimide [68]. A thermoresponsive and pH-responsive, chelating, and superabsorbent hydrogel of poly(NIPAM)-co-poly[2-methacrylic acid 3-(bis-carboxymethylamino)-2-hydroxypropyl ester] was synthesized and proposed as both a reaction medium and a recyclable Pd catalyst support for Suzuki, Heck, and other organic reactions [69]. Same polymer system with gold nanoparticles was demonstrated also to concentrate reactants within the hydrogel matrix through the reversible deswelling [70].
For applications as a catalyst support media, the gel must possess the adequate characteristics as a reaction media, i.e. required hydrophilicity and lipophilicity and activation of reactants. These applications also require that the gel efficiently immobilizes the catalyst and provides adequate mechanical robustness for handling, while allowing an easy access of reactive compounds to the catalyst.
4.4. Sensors
Gel ability to change volume in response to a specific stimulus is utilized in many sensing designs that are currently under development. A variety of gels with various chemical structures have been proposed, which can collectively achieve response to a broad range of stimuli (Table 1).
Table 1.
Characteristics of some SRG-based sensor prototypes
| Detecte d signal |
material | Sensing mechanism | shape | Respon se time |
Signal transducing technology |
Notes | Ref. |
|---|---|---|---|---|---|---|---|
| Ionic strength | Poly(AAm-co-APTAC-co-AMPS) | Mobile ions interrupt interactions between hydrogel-bound ions, reducing effective cross-linking | Film 10 μm | 30 s | Hologram | Response to ionic strength is near independent of pH | [71] |
| pH | P4VP | Donnan equilibrium (protonation of pyridyl groups) | Film ~100 nm | QCM | [72] | ||
| pH | Poly(MAAc-co-poly(ethylene glycol) dimethacrylate) | Donnan equilibrium (ionization of MAAc) | Film 2.2 μm | 10–15 min | Microcantilever | Sensitivity up to 5×10−5 pH unit | [73,74] |
| pH | Poly(AAm-co-DMAEM) | Donnan equilibrium (ionization of DMAEM) | Film 15 μm | ~ 15 min | Microcantilever | [75] | |
| pH | Poly(AAm-co-DMAEM) | Donnan equilibrium (ionization of DMAEM) | Film 400 μm | 10–30 min | Pressure sensor | Pressure up to 180 kPa | [76] |
| pH | Poly(AAm-co-DMAEM) | Donnan equilibrium (ionization of DMAEM) | Film 10 μm | 10–30 min | Pressure sensor | Pressure up to 200 kPa | [77] |
| pH | PVA/PAAc mixture | Donnan equilibrium (ionization of AAc) | Film 0.4–1 μm | 500–800 ms | QCM | [78] | |
| pH | Poly(iOA-co-AAc) | Donnan equilibrium (ionization of AAc) | Film 1–4 μm | 14–27 min | Magnetoelastic sensors | [79] | |
| pH | PVA/PAAc mixture | Donnan equilibrium (ionization of AAc) | Film 250 μm | 15–200 min | Bending plate | Response time depended on pH | [80] |
| pH | Copolymers of HEMA with MAAc, TFMPA, DMAEM, or VI | Donnan equilibrium | Film 10 μm | 250–2000 s | Hologram | Response time depended on buffer concentration | [43] |
| pH and ionic strength | Poly(AAm-co-DMAPAAm) | Donnan equilibrium (ionization of DMAPAAm) | half-sphere 60 μm radius | 8 s for ionic strength and 90–130 s for pH | Optical fiber interferometric technique | [81] | |
| pH, Ca2+, and Cu2+ | Polyvinylbenzyl chloride with dicarboxylate groups | Dissociation of dicarboxylate groups or formation of complexes between dicarboxylate groups and metal ions | Gel micro particles in gel support film | 12–30 min | Optical transmittance | pH range: 5–8; Ca2+:0–0.01 M; Cu2+: 0–0.001 M | [82] |
| K+ | Poly(HEMA) with pendant 18-crown-6 groups | Donnan equilibrium (charged complex with 18-crown-6) | Film 10 μm | 30 s | Hologram | Selective to K+ over Na+ | [83] |
| Pb2+ | Poly(AAm-co-4-acrylamidobenzo-18-crown-6) | Donnan equilibrium (charged complex with benzo-18- crown-6) | Film | PCCA | Also sensitive to K+ | [84,85] | |
| Pb2+ | Poly(AAm-co-4-acrylamidobenzo-18-crown-6) | Donnan equilibrium (charged complex with benzo-18- crown-6) | Film 15 μm | Microcantilever | [86] | ||
| Metal ions | PAAm with pendant 8-hydroxyquinoline groups | Complexation-induced additional cross-links | Film | PCCA | Sensitive to Cu2+, Co2+, Ni2+, Zn2+ | [87] | |
| Metal ions | Poly(HEMA-co-carboxymethyl-(2-methyl- acryloyl)-amino acetic acid) | Network ionization and crosslinking due to complexation between ions and chelating groups | Film 10 μm | 30 s | Hologram | Sensitive to Ni2+, Zn2+, Co2+, Ca2+, Mg2+; also sensitive to pH and ionic strength | [88] |
| CrO42− | Poly(AAm-co-APTAC) | Neutralization of network due to complex between trimethylammonium group and CrO42−. Also possible cause is exchange of a highly hydrated anion(Cl−), for a less hydrated CrO4−2 | Film 15 μm | Microcantilever | In addition to proposed mechanism, volume change may be caused by shrinkage due to additional cross-links | [89] | |
| CO2 | PMAAc grafted with DETA | Donnan equilibrium (ionization MAAc (anion) and DETA (cation)) | Micro particles | 50 min | Pressure sensor | Sensitivity over wide pH range; 400 kPa can be generated | [38] |
| urea | Poly(HEMA-co-DMAEM) with immobilized urease | Urease hydrolyses urea to ammonium bicarbonate; pH change shifts Donnan equilibrium | Film | 50–500 s | Hologram | Cross-sensitivity to pH | [90] |
| Organo phosphorus compounds | PAAm with pendant organophosphorus hydrolase and 3-aminophenol | Hydrolysis of organophosphorus compounds leads to pH change detected by pH sensitive gel | Film | PCCA | Cross-sensitivity to pH | [91,92] | |
| Organo phosphorus compounds | Poly(HEMA-co-MAAc) with immobilized organophosphorus hydrolase | Hydrolysis of organophosphorus compounds leads to pH change detected by pH sensitive gel | Film | 10–30 min | Magnetoelastic sensor | Detection limit as low as 10−7 M; cross-sensitivity to pH | [93] |
| Glucose | Hydrolyzed PAAm with immobilized GOx | GOx hydrolyzes glucose; pH change shifts Donnan equilibrium | film | PCCA | AAc is produced by hydrolysis | [84] | |
| Glucose | Poly(iOA-co-AAc) for pH sensing and GOx and catalase for glucose hydrolysis | GOx hydrolyzes glucose; pH change shifts Donnan equilibrium; catalase removes H2O2 and recovers oxygen | Two-layered film | 20–100 min | Magnetoelastic sensors | Cross-sensitivity to pH and ionic strength | [94] |
| Glucose | Poly(glucosyloxyethylmethacrylate) with pendant concanavalin A (ConA) | Free glucose breaks complex between immobilized glucose groups and ConA reducing the number of cross-links | Bulk 1 mm thick | Few hours | [95] | ||
| Glucose | PAAm with pendant phenylboronic or fluorophenylboronic acid groups | Donnan equilibrium (complexation-induced network ionization) | Film 10 μm | Hologram | Selectivity to glucose over lactose was investigated | [96] | |
| Glucose | PAAm with pendant phenylboronic acid and dimethylaminopropyl groups | Complexation-induced additional cross-links | Film 10 μm | Hologram | Selectivity to glucose over lactose, galactose, fructose, etc. | [97] | |
| Glucose | Copolymer of 2-acrylamidophenylboronic acid with PEG, APTAC, and [2-(acryloyloxy)ethyl]trimethyl ammonium chloride | Complexation-induced additional cross-links | Film | 10 min (50%) | Hologram | Little pH dependence; little sensitivity to lactose | [98] |
| Glucose | PAAm with pendant phenylboronic acid groups | Glucose binding shifts pKa of boronic acid groups and changes network ionization | Film | PCCA | [99] | ||
| Glucose | PAAm and poly(AAm-co-n- hexyl acrylate) with pendant fluorophenylboronic acid groups | Complexation-induced additional cross-links | Film | 10–60 min | PCCA | Demonstrated dependence of response speed on temperature | [35,44] |
| Glucose | 3-aminophenylboronic acid-functionalized poly(NIPAm-co-AAc) | Glucose binding shifts pKa of boronic acid groups and changes network ionization | Film 0.2–15 μm | 30–120 min | Fabry–Pérot interferometer | [100] | |
| Glucose | PMMA modified with diethylenetriamine, butylamine, and 3-aminophenylboronic acid | Complex of glucose with pendant groups acts as additional cross-links | Bulk ~8×7×1 mm | 40 min | High glucose selectivity over fructose and galactose. | [101] | |
| Glucose | Imprinted copolymer of HEMA and MAAc | Imprinted sites | Film ~1mm | 60 min | [102] | ||
| Penicillin G | Poly(HEMA-co-MAAc) with immobilized penicillinase | Penicillinase hydrolyses penicillin to penicilloic acid resulting in shrinking of pH sensitive gel | Film | 50–500 s | Hologram | [90] | |
| Penicillin G | Poly(iOA-co-AAc) with immobilized penicillinase | Penicillinase hydrolyses penicillin to penicilloic acid resulting in shrinking of pH sensitive gel | Film | 40 min | Magnetoelastic sensor | Cross-sensitivity to pH and ionic strength | [103] |
| L-lactate | Poly(AAm-co-4-acrylamidophenyl boronate) | Ionization of network as a result of complexation between boronic acid and L-lactate | Film | 200–500 s | Hologram | [104] | |
| Nucleotides (ATP/AMP) | Poly(APTAC-co-3-(acrylamido)phenylboronic acid) | Complexation that results in change in network ionization | Film | QCM | [105] | ||
| Nucleotides (AMP/GMP/CMP/UMP)) and saccharides | Imprinted poly(AAm-co-3-(acrylamido)phenylboronic acid) | Imprinted sites | Film | 300 s | QCM; ion-sensitive field-effect transistors | Imprinting enhances selectivity and sensitivity | [106] |
| herbicides | Herbicide-imprinted poly(AAm-co-MAAc) | Imprinted sites; unexplained water uptake due to complex formation | Film 10 μm | 120 s | QCM; ion-sensitive field-effect transistors | [107] | |
| Molecular weight of polyelectrolytes | Charged PNIPAm-based gel film coated with gold layer of various thickness | Thickness and average pore size of gold layer determine what polyelectrolytes can penetrate into microgel | Film | Array of Fabry–Pérot interferometers | [108] | ||
| AFP (tumor-specific marker) | Interpenetrating network of AFP-imprinted lectin (ConA) and polyclonal AFP-antibody grafted AAm gel | Reversible cross-link formation through lectin–glycoprotein–antibody complexes | Bulk | Designed to detect human hepatocellular carcinoma | [109] |
Temperature sensors are in high demand for industrial applications and to identify sites of infection, inflammation, or other pathology in health care. Many polymer systems were therefore investigated for applications in gel-based temperature sensors. Most of these systems contain polymers that exhibit LCST, i.e. shrink when heated, or upper critical solution temperature (UCST), i.e. swell when heated. Among the polymers with LCST, in addition to classical poly(NIPAM)-based hydrogels, several polymers such as N,N-Diethylacrylamide, proline methyl ester, and poly(N-vinylcaprolactam) were used in sensing devices. PAAc/PAAm blends and PAAc/PEG blends are examples of systems that exhibit UCST.
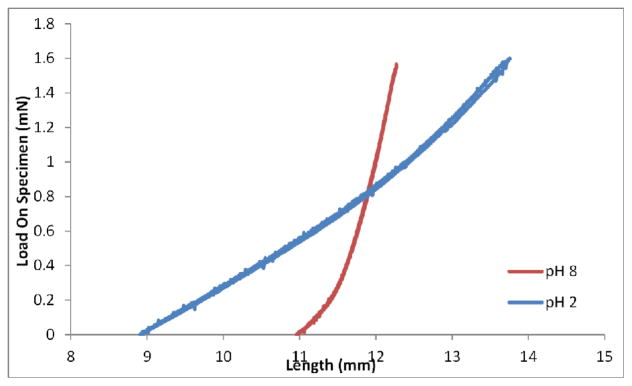
Several hydrogel-based sensors were designed to measure pH, an important parameter of aqueous solutions. Usually, such sensors utilize the pH dependence of the ionization degree of weak polyelectrolytes. The ionization degree determines the charge of the network and therefore the amount of the contribution of charge interactions to the osmotic pressure of the hydrogel. Hydrogels based on negatively charged groups, such as AAc, etc. increase their volume in response to increase of pH. Hydrogels that swell in response to decrease of pH are based on polycations, such as DMAEM. SRG-based sensors have been developed for detection of other cations, such as K+, Na+, Pb2+, and anions such as CrO42−.
Several sensing motifs have been proposed for detecting the concentration of glucose, a measurement important for controlling blood glucose level in the care of diabetes mellitus. The most common approach utilizes glucose oxidase (GOx). In this approach, glucose inside the gel is enzymatically converted to gluconic acid which lowers local pH and in turn triggers volume change of a pH-sensitive gel [110]. One shortcoming of this approach is that enzymatic reaction consumes oxygen and produces hydrogen peroxide, which inhibits the GOx activity. This shortcoming can be partially overcome by incorporation of catalase in addition to GOx. Catalase recovers a fraction of consumed oxygen and removes hydrogen peroxide [94,111]. Another shortcoming is the sensor cross-sensitivity to pH and usually also to the solution ionic strength. An alternative approach, with low sensitivity to pH or the ionic strength, is based on complexation of glucose and concanavalin A (ConA). In this approach, the portion of SRG cross-links is made by complexation between glucose groups and ConA that both are covalently attached to the gel network. In the presence of free glucose, some complexes between immobilized glucose and ConA break in favor of formation of complexes between free glucose and ConA, thus reducing the number of cross-links and causing the gel to swell [95,112,113].
Fully synthetic glucose sensors are more attractive for commercial use. The majority of such sensors are currently based on phenylboronic acid. In these sensors, glucose binds to phenylboronic acid leading to change in the dissociation constant of the acid. Thus, at pH close to pKa of the phenylboronic acid groups, the charge of a gel network with pendent phenylboronic acid groups will depend on the concentration of glucose. Unfortunately, pKa of phenylboronic acids is usually outside of the physiologically relevant pH range. Additionally, SRGs that are built on the charge interactions are sensitive to ionic strength and may lose their sensitivity at physiological ionic strength [99]. Therefore, significant effort was devoted to the development of better glucose sensors based on boronic acid. The most noticeable advancement was achieved in gels that utilize the mechanism of reversible cross-links, which can be independent of the ionic strength [35,44,96–98,114]. In such gels, a molecule of glucose forms a complex with two phenylboronic acid groups, causing gel shrinkage. pKa of phenylboronic acid groups can be lowered by using fluorinated derivatives of the acid [35,44,114] or the pH dependence of the complex formation can be eliminated by adding an acrylamide group to the ortho position of the phenylboronic acid group [98]. Another completely synthetic glucose-sensitive gel was obtained from PMMA modified with diethylenetriamine, butylamine, and 3-aminophenylboronic acid. The gel showed high selectivity to glucose over fructose and galactose [101].
Hydrogel-based sensors for other biologically relevant compounds are also being developed. The approach based on immobilization of enzymes was utilized in sensors detecting urea and penicillin [90,103]. Phenylboronic acid binding was used in sensors to nucleotides, saccharides, and L-lactate [104,105]. Several sensing motifs utilize approach of reversible crosslinking, where free analyte compete with pendent groups for binding positions within protein sites. When free analyte replaces pendent groups in complexes, the network loses effective crosslinks. When the analyte decreases in concentration within the bulk phase, the protein binds again with the pendent groups closing the network structure. Similarly, systems have been designed that have a specific antigen and corresponding antibody grafted to a semi-interpenetrating network, which swells in response to binding of the antigen due to a loss of effective crosslinks (Fig. 6) [115,116].
Fig. 6.
Scheme of response mechanism of an antigen-sensitive hydrogel. Free antigen (analyte) replaces immobilized antigen in antigen-antibody complex, reducing the number of cross-links in the gel and thus causing gel swelling. Reproduced with permission from Ref. [116]. Copyright 1999 American Chemical Society
Recently, molecular imprinting, a promising technique of artificially generated molecular recognition, has been extended to stimuli-responsive hydrogels. Molecular imprinting involves forming complexes between the template molecules and functional monomers, oligomers, or polymers prior the polymerization or crosslinking. The polymerization and crosslinking occurs in the presence of the complex. The template is then removed after the synthesis, producing a matrix with specific recognition elements for the template molecules (Fig. 7) [117]. The potential of molecular imprinted sensing hydrogels was demonstrated by Miyata et al. in a tumor marker-responsive hydrogel that can be used to detect human hepatocellular carcinoma [109]. Molecular imprinting was also used to create hydrogels sensitive to glucose [102,118,119], various nucleotides [106], herbicides [107], proteins [120], etc. More detailed account of the evolving field of molecular imprinting can be found in several recent reviews [102,117,121–127].
Fig. 7.
Scheme of the imprinting process of atrazine in a hydrogel film. The polymerization and crosslinking occurs in the presence of atrazine. After the synthesis, atrazine is removed, producing a matrix with specific recognition elements for the imprinted molecules. Reproduced from Ref. [107] with permission of The Royal Society of Chemistry.
Several methods are used to convert the stimuli-responsive changes in hydrogels to evaluable output signals. One group of methods works on optical principles. These methods monitor optical transparency [82,128], refractive index [128,129], or fluorescence intensity [130]. Other optical methods measure directly the volume of miniature hydrogels. A simple device may consist of an optical fiber coated on the end with a drop of SRG (Fig. 8A) [81,131,132]. Alternatively, the SRG can be coupled to a reflector so that the reflector is moved in response to the change of the hydrogel volume. The position of the reflector can be determined from light interference (Fig. 8B) [22,100,133,134] or from the intensity of light in a system of two optical fibers (Fig. 8C) [135].
Figure 8.
Reflective optical methods for detecting gel volume change in sensors. (A) Drop of gel at the end of optical fiber; gel volume change alters interference of reflected light in the optical fiber. (B) Gel film attached to reflective surface and covered with reflective metal islands; change in thickness of the film is determined from light interference. (C) Gel moving reflector; position of the reflector determines the amount of light delivered from one optical fiber to another. 1 – gel; 2 – optical fiber; 3 – reflector; 4 – flexible diaphragm.
Photonic crystals are another approach in optical detection of hydrogel volume. Asher et al. incorporated colloidal crystalline arrays into various SRGs [84]. Such arrays diffract the light giving rise to an intense color. Volume change of the gel modifies the lattice spacing of the colloidal array and shifts the wavelength of the maximum intensity of the diffracted light (Fig. 9). Same effect can be attained by means of inverse opal structures, which are produced by dissolving the material of the colloidal crystalline array after the fabrication of the hydrogel [136–138]. Alternatively, a hologram fabricated inside the hydrogel can be utilized to monitor the volume of the SRG by the diffraction wavelength or color [43,71,88,90,96,104]. Plasmon resonance techniques were also shown to be a feasible, although somewhat complicated, method to measure the thickness of nanosized films [139].
Fig. 9.
Detection of gel volume by diffraction maximum of embedded photonic crystal (colloidal crystalline array). When illuminated by white light, (A) shrunken gel diffracts light with shorter wavelength than (B) swollen gel. (C) Diffraction maximum is detected by spectrometer. Swelling of the gel causes red-shift of the diffraction maximum while shrinking causes blue-shift. Reproduced from Ref. [91] with permission of Springer.
Change of electric conductivity [140–142] and capacity [143] associated with the volume change of hydrogels was also used in sensing devices. Other sensors employed quartz crystal microbalance technique (Fig. 10) [78]. These sensors were built on the ability to measure very precisely the resonance frequency of piezoelectric quartz crystal resonator. The resonance frequency depends on the elastic properties and the mass of the vibrating body; these parameters in turn depend on the swelling state. Similarly, detection of mechanical resonance frequency was used in magnetoelastic sensors with remote query capabilities [79,103,144]. Finally, several approaches were developed that measure force or deformation of an elastic body generated by the constrained hydrogel. Such devices utilize, among other approaches, bending plates and other pressure sensors [38,76,77,80,143,145,145,146] or microcantilevers [73–75,89,147] (Fig. 11).
Fig. 10.
(A) Scheme of quartz crystal microbalance sensor. (B) Frequency response of poly(4-vinylpyridine)-coated quartz crystals to pH change. (B) is reprinted with permission from Ref. [72] Copyright 2005 American Chemical Society
Fig. 11.
Sensing devices based on force or deformation of an elastic body generated by SRG. (A) Pressure sensing approach: stimuli-dependent force generate by confined gel is registered by pressure sensor [38], (B) Capacitive transducer approach: stimuli-triggered expansion of gel changes distance between capacitor plates that is being captured by measuring the capacitance. Reproduced from Ref. [143] with permission of Springer. (C) Microcantilever approach: expansion of a gel layer causes deflection of cantilever that is determined by deflection of laser beam. Reproduced from Ref. [74] with permission of Springer.
Sensing applications impose specific requirement on SRG. A list of important sensor specifications includes such characteristics as sensitivity, stability, accuracy, speed of response, hysteresis, stimulus range, resolution, and selectivity [148]. Although not always obvious, these characteristics in most gel-based sensors are, in fact, determined by gel mechanical properties. The response of the gel usually consists of generation of force, change of volume or shape, or other mechanical processes.
The time of response is one of the critical parameters of gel-based sensors. Most sensors are currently comparatively slow. The time of response depends, among other factors, on the dimensions of the gel. Therefore, the sensing devices based on gels have a tendency for miniaturization. Those devices utilize either a thin film of hydrogel or small particles. Miniaturization, however, complicates the reading of the response. Additionally, small particles are hard to immobilize and the film-based sensors often suffer from delamination from the surface [149]. Future improvement of the response time is expected with the methods presented in Section 5.2.
Stimulus range, sensitivity, and resolution are other major characteristics that attract significant attention in the research on gel-based sensors. Those parameters have a direct relation to the response ratio of gels which is influenced by the structure of SRG. Other characteristics, such as accuracy, hysteresis, and stability of gel sensors are also beginning to receive attention. A very important parameter which is often overlooked by researchers is selectivity. This parameter defines the ability of the sensor to respond primarily to one stimulus in the presence of other stimuli. For SRG, selectivity issue is especially critical because the volume of the gel usually depends on several environmental parameters. For instance, many temperature sensitive hydrogel systems are also sensitive to pH; response of pH-sensitive hydrogels often depends on ionic strength; and PAAc-based and other pH sensors may also be responsive to metal ions.
Several recent scientific reports addressed this issue and demonstrated the extent of the selectivity problem, as well as methods for improving sensitivity. González et al. investigated selectivity and cross-sensitivity of poly(HEMA-co-carboxymethyl-(2-methyl-acryloyl)-amino acetic acid) hydrogel to various metal ions, pH, and ionic strength [88]. Selectivity of other metal sensors has also been investigated [83,84,87]. A significant effort was devoted to understand the requirements for selectivity of glucose sensors and to improve the existing designs [35,94,96–99,101]. Temperature, pH, ionic strength, other carbohydrates, and lactose were identified as the parameters that can cause interference in detecting glucose. Glucose itself, on the other hand, was found to be the major possible interference for detection of L-lactate in blood with the holographic sensor based on phenylboronic acid. In the last case, reducing the concentration of the ligand in the hydrogel, although decreased the sensitivity toward L-lactate, eliminated the interference from glucose [104]. Analyte specificity and responsiveness can be achieved with molecular imprinting strategies [117,150,151]. Molecular imprinting was shown to improve selectivity for glucose in one sensor design [118,119] and selectivity to metal ions in another [152]. Watanabe et al. [153] produced norephedrine- and adrenaline-imprinted stimuli-responsive gel with molecular recognition achieved by molecular imprinting. Both gels were sensitive to the analyte used for imprinting and insensitive to the other. Arnold et al. proposed another technique to improve selectivity. Their hydrogel glucose sensor was encapsulated within a bipolar ion exchange membrane impermeable for ions, but freely permeable for glucose [141]. This way the hydrogel response to glucose can be measured without the interference of ions. Finally, an array of sensors, each with selectivity to a particular parameter, can be employed to determine simultaneously the parameter of interest and all interfering factors [154].
In conclusion, SRGs are a versatile platform for development of sensors for a broad range of analytes. SRG-based sensors are especially attractive for medical applications and for development of inexpensive sensors. To avoid slow response time, gels with small dimensions can be utilized. Most often the gels are used in a form of thin films. Reading of the volumetric changes of such gels is a significant undertaking; several approaches were proposed. The majority of these approaches employ optical methods. Additional research is needed to establish and optimize SRG sensor selectivity, accuracy, and stability. Further development of theory of gel swelling and understanding of their response mechanisms is required to gain wide acceptance and utilization of this sensing platform.
4.5. Actuators
Application of gels as actuators was historically considered promising because gel properties resemble properties of muscles. Thanks to this resemblance, such systems became known as “artificial muscles”. They are particularly attractive for making biologically inspired devices and robots that mimic the movements of humans, animals, and insects. Several gel actuators systems were proposed (see Table 2) and a number of proof-of-concept devices that utilize gel actuators have been reported. Thus, Kurauchi et al. reported a robotic hand and a “swimming” device, both based on PVA-PAAc hydrogel actuated by electric field [155]. Osada and Gong fabricated similar “swimming” device based on poly[2-(acrylamido)-2-methylpropanesulfonic acid] hydrogel. The device showed eel-like motion in a surfactant solution at the 10 V alternating voltage [156]. An actuator based on poly(acrylonitrile) (PAN) gel fibers build by students from Virginia Tech participated in the first arm-wrestling match between an EAP actuated robotic arm and a human (AMERAH) organized by Bar-Cohen [157]. In the second AMERAH, the modified version of the actuator was the strongest artificial arm [158].
Table 2.
Characteristics of some SRG applications in actuators and microfluidics.
| Material | Type of control | Shape | Application | Notes | Ref. |
|---|---|---|---|---|---|
| PEDOT, PSS, PAAm, PAAc | Electroactive | Bulk | Macroscopic actuator | Compressive fracture stress of 1.2 MPa and a fracture strain of 90%. | [178] |
| Layers of PAAm and PAAc gels | Electroactive | Multilayered | Macroscopic actuator | When AAc layer expels water, AAm layer takes water, and vice versa. Thickness changes by 10–15% in response to 3 V. | [165] |
| Poly(AAc-co-vinylsulfonic acid) | Electroactive | Bulk | Macroscopic actuator | Contraction by 15–40% in response to 4.5 V in ~60 min. | [179] |
| P(Aac-co-Aam) blended with conductive polypyrrole/carbon black composite | Electroactive | 400×400×2500 μm | Bending actuator | Bending up to 30 degree. 5 s response time. | [171] |
| Saponified PAN | Electroactive | Yarns of roughly 2000 fibrils of 7 μm diameter | Artificial muscle | Response time: 10–15 min. Linear response ratio: 2 times. Contracted: 1 MPa modulus, 5 MPa strength, 2.4 elongation. Elongated: 4 MPa modulus, 1.5 MPa strength, 0.4 elongation. | [180] |
| PNIPAM | Temperature | Microparticles (~500 μm) and photopatterned microactuator (250×250×50 μm) | Control valve for microfluidics | Explored optimal particle size and optimal particles volume/chamber volume ratio. 0.3–10 s response time. Up to 840 kPa pressure resistance. | [181] |
| PNIPAM | Temperature | Photopatterned microactuator (2000×200×100 μm) | Control valve for microfluidics | 3–5 s response time. 1400 kPa pressure resistance in closed state. 300 kPa back pressure in open state at flow velocity 5 cm/s. | [182] |
| PNIPAM | Temperature | Photopatterned microactuator | Control valve for microfluidics | Applied in polymerase chain reaction device. 5 s response time. Pressure resistance 200 kPa. | [174] |
| P(NIPAM-co-NEAAm) | Temperature | Photopatterned microactuator (5000×100×40 μm) | Control valve for microfluidics | Copolymers with NEAAm enabled higher switch temperature than PNIPAM along. 1–4 s response time. Pressure resistance more than 15 MPa in closed state. | [183] |
| P(NIPAM-co-sodium acrylate) | Temperature | Microactuator | Control valve for microfluidics | 5–10 min response time. | [184] |
| PNIPAAm | Temperature | Photopatterned microactuator (5000×100×100 μm) | Control valve for microfluidics | 4–6 s response time. 9 MPa pressure resistance. | [185] |
| PNIPAAm | Temperature | Microgels | Control valve for microfluidics | Pressure resistance up to 9 MPa; opening time as low as 0.3 s; 2 s closing time. | [186] |
| PNIPAAm | Temperature and alcohol concentration | Microgels | Electronically adjustable automatic control of concentration of alcohols | Pressure resistance up to 600 kPa. Flow rate changes more than 30 times. | [187] |
| PNIPAAm | Temperature | Microactuator | Control valve for microchannel concentrator | 50–240 s valve switching time. | [188] |
| PNIPAAm | Temperature | Photopatterned microactuator | Diffusion micropump | 0.54 μl/min in peristaltic mode and 2.8 μl/min in pulsating mode at 0.43 kPa pumping pressure. 1.28 kPa maximum pumping pressure. | [189] |
| PNIPAAm | Temperature | Packed microgels | Displacement micropump | Higher performance can be achieved by increasing actuator thickness and pump chamber volume. 4.5 μl/min at 0.5 kPa and 0.2 μl/min at 7.5 kPa. | [189] |
| Composite of poly(NIPAAm-co-AAm) and light absorbing particles | Light | Microvalves | Microfluidic control of flow by light of specific wavelength | Gold colloid nanocomposite collapses in response to green light. Gold nanoshells composite collapses in response to near-IR light | [190] |
| PNIPAM functionalized with spirobenzopyran | Light | Microvalves | Microfluidic control of flow by light | 18–30 s to open; more than 1 h to close. | [191] |
| PAN | pH and electroactive | Fibers | Macroscopic actuator | 2.2 times change in length. 0.1 MPa contraction stress. | [192] |
| Poly(HEMA-co-AAc) | pH | Microvalve ~200 μm | “Self-regulated” pH control valve in microfluidic | Several minutes response time. | [193] |
| Poly(HEMA-co-AAc) or poly(HEMA-co-DMAEM) | pH | Microvalves | Switching between channels | 8–12 s response time. More than 300 kPa differential pressure. | [194,195] |
| poly(4-hydroxybutylacrylate–co-AAc) | pH | Microsphere | Microfluidic valve | Flow rate changes 10 times. Valve opens/closes in 4–8 min. | [196] |
| Poly(HEMA-co-AAc) | pH | Cylindrical microactuators | Microvalve and micropump | 35 kPa pressure of micropump. 1.5 MPa pressure resistance of microvalve. 2 μl/min flow rate by the pump along. For a fast bolus release the micropump is used in combination with the valve. The valve is opened after full swelling of the pump actuator, releasing the content of the pump chamber within a few seconds at an average flow rate of 540 μl/min. | [197] |
| Poly (AAm-co-3-methacrylamidophenylboronic acid) | Glucose | 70 μm thick film | Microfluidics; artificial pancreas. | More than 5.4 kPa pressure resistance. 17–18 min response time. 1.5 linear response ratio for 0–100 mM glucose shift. | [198] |
| Poly(NIPAM-co-AMPS-co-Ru(bpy)3) | Cyclic BZ reaction | 0.5 mm thick film | Self-oscilating | Self-waking on ratchet surface. ~100 s per cycle. Walking speed 170 μm/min. | [199] |
| Poly(NIPAM-co-AMPS-co-Ru(bpy)3) | Cyclic BZ reaction | 0.5 mm thick free-standing membrane | Self-oscilating | Cyclic pendulum motion. ~300 s per cycle. | [200] |
| Poly(NIPAM-co-AMPS-co-Ru(bpy)3) | Cyclic BZ reaction | Film | Transport of particles on surface | 1–4 mm/min particle speed. | [201] |
More recently, Lee et al. demonstrated pH-sensitive hydrogels mimicking the shape and motion of octopus and sperm [159]. Richter and Paschew fabricated a tactile display consisting of 4,225 PNIPAM actuators at a pitch of 580 um (Fig. 12) [160]. Each actuator was individually controlled by light-induced temperature field. Within several seconds, the display generated palpable information based on various height and softness of the actuators. Such display would allow advanced virtual reality devices and improved communication for visually impaired persons with electronic media. Additional examples of gel actuators can be found in several specialized reviews [161–164].
Fig. 12.

Image of a dolphin displayed by SRG-based tactile display. The length of the image from mouth to tail is 14.5 mm. Reproduced with permission from [160]. Copyright 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
However, several well developed actuator technologies already exist. In order to be compatible with other actuation technologies, gels need to match or outperform other platforms in a combination of parameters. One set of important factors is related to the “amount” of actuation that an actuator can produce. These factors include actuation strain, actuation stress, and work that the gel can produce in a single stroke. Gels perform quite well in regard to some of these factors (Fig. 13), offering one of the highest actuation strains among all devices and above average work per stroke; actuation stress, however, is relatively low. Other gel advantages include high resilience and ability to handle fragile objects. Some of the properties of gels, however, are rather unconventional for current common designs of machines and devices. The gels are wet and, on fundamental level, controlled chemically. For many applications, the requirement of keeping the gel wet represents a hurdle. It makes gels, however, a natural candidate for applications in aquatic environment or biological fluids. Solutions are being developed to circumvent the gel need to stay immersed in liquid. Liu and Calvert, for instance, suggested a “sandwiched” structure that contains a responsive gel layer and another gel layer used to store the fluid (Fig. 14) [165].
Fig. 13.
Actuation stress versus actuation strain for various actuators. Performance of SRGs usually falls into the shaded box. SRGs produce high actuating strain but improvement of actuating stress is needed. Data for non-gel-based actuators is taken from Ref. [166].
Fig. 14.
Schematic of a “sandwiched” structure of hydrogel actuator capable of working without external fluid [165]. Applying electric potential between electrodes produces flow of hydrogen ions from the positive electrode, which causes the polyacrylic acid layer to shrink and force water into the polyacrylamide. The shrinkage of polyacrylic acid layer (which is stiffer than PAAm layer) causes the whole stack to contract in the directions parallel to the interface between the gels.
Control of actuation with environmental stimuli such as temperature, pH, or light is also relatively uncommon in current actuator designs. For modern technologies, electrically driven actuators are usually more suitable. Electroactive ionic gels that can be controlled electrically are being currently investigated and developed [167–172].
The most important shortcoming of gel actuators, however, is their response rate, which diminishes such important characteristics as maximum frequency of strokes (Fig. 15) and power. Bulk macroscopic gels are unacceptably slow for most actuators applications. Therefore methods of improvement of response rate (discussed in Section 5.2) are highly relevant for gel actuators.
Fig. 15.
Work per unit volume in a single stroke versus maximum frequency of strokes for various actuators. Common performance of macroscopic SRGs is shown by a shaded block. While SRGs demonstrate generous work per unit volume in a single stroke, their frequency of strokes is limited by their slow response rate. Increases in response rate and frequency of stroke are critically needed. Data for non-gel-based actuators is taken from Ref. [166].
Nonetheless, gel actuators possess other unique properties, such as no power requirements, and no moving parts. Therefore, they are ideally suited for specialized applications, such as autonomous medical pumps for long-term drug release [173,174] and autonomous valves for power-free field irrigation [175–177].
4.6. Microfluidics and valves
If the response time is improved, gels offer significant benefits for many specific applications. An illustrative example is an application of gels in microfluidics. This application is being developed in the recent two decades. The application of SRGs in microfluidics has been reviewed in Refs [18,202,203].
SRG-based actuators are a good fit for the application in microfluidics. They are simple, have minimal amount of moving parts, and do not require power. Conventional microactuators (e.g. using electromagnetic, electrostatic, or thermopneumatic effects) require external power for operation and relatively complex assembly [194]. Another important aspect of SRG-based valves is their tolerance of the dust. Dust is a major source of malfunction in conventional lab-on-a-chip systems. Dust particles get caught in the actuator moving parts made from rigid materials, such as silicon or glass, compromising the actuator function. Because hydrogels are soft and resilient, the hydrogel actuator will shape itself around the dust particle. Hydrogels are also able to handle soft objects and substrates, such as cells and embryos, which would normally get damaged by the contact with rigid actuator parts.
Hydrogel actuators are an excellent option for microfluidic control valves. They can be activated by temperature, light, or flow composition (see Table 2). Although hydrogel is a soft material, hydrogel-based valves are remarkably pressure-resistant [204]; a hydrogel valve was able to operate at back pressure as high as 18 MPa [183]. Hydrogel actuators are also a viable option for micropumps [205]. Such micropumps can control lower flow rates, have lower dead volume, and are less expensive than conventional micropumps [189,206].
Importantly, microfluidic applications can benefit from using SRGs as sensor and actuator simultaneously. Same body acting as sensor and as actuator represents the smallest possible close-loop control system. Very few other materials can be used in such applications. With SRGs employed as both sensor and actuator, simple, autonomous controlling devices that require no power can be achieved. Several examples of such microfluidics devices have been reported to date. In one example [194], a self-regulated ‘flow sorter’ was demonstrated. This device (Fig. 16) consists of a ‘T’ channel in which the entrance to each branch is gated with a hydrogel structure of different chemical composition. The hydrogel for one branch expands at high pH and contracts at low pH, while the hydrogel for the other branch exhibits inverse behavior (that is, contracts at high pH and expands at low pH). The device routes the fluid down one of two paths based on the pH of the input. In a certain pH range (5.7–6.8), both hydrogel valves swell to seal the channel. Thus, hydrogels performs the sensing, actuating and regulating functions normally performed by discrete components. In another example, SRG was utilized to achieve a self-regulating chemostatic device that is able to maintain pH in output flow by mixing two input flows in a necessary proportion [193].
Fig. 16.
A device that directs (‘sorts’) a fluid stream on the basis of its pH. The hydrogel gating the right branch (circles) expands at high pH and contracts at low pH. The hydrogel gating the left branch (squares) behaves in the opposite manner (expands at low pH and contracts at high pH). At pH 7.8, the left branch is open and the right branch is closed. At a pH 4.7, the right branch is open and the left branch is closed. Both gates are closed at pH around 6.7. Scale bars are 300 um. fD is fractional change in diameter. Reprinted from [194] by permission from Macmillan Publishers Ltd., copyright 2000.
Notably, a dry gel will swell and automatically stop the flow when reached by liquid. If the gel is stimuli-responsive, the same valve can be opened later, for example, by heating. Valves based on such effect were proposed for use in point-of-care lab-on-a-chip devices for flow control, sample and reagent metering and distribution, and sealing of a reaction reactor [174]. Most recently, another peculiar phenomenon was observed during capillary filling of microtubes coated with a dry hydrogel film on their inner walls [207]. The filling velocity for water was three orders of magnitude smaller when compared to uncoated microtubes. Moreover, on macroscopic scale the filling velocity was constant throughout the filling process, whereas in uncoated microtubes the meniscus position initially changes proportionally to the square of time followed by a constant-velocity stage and finally the Lucas–Washburn behavior where the position is proportional to the square root of time [208]. On microscopic scale, the meniscus positon in hydrogel-coated microtubes undergos “stick-and-slip” motion that is caused by dynamically changing geometry and mechanical properties of the swelling hydrogel coating. Practically, this phenomenon may offer better temporal control in future lab-on-a-chip and MEMS devices.
Microfluidic applications demonstrate significant importance of mechanical properties of gels. Depending on application, such parameters as response time, amplitude of response, sensitivity, and pressure resistance can be important. However, similarly to other applications, optimization of one parameter is often attained at the expense of another. In a characteristic example, attempts to reduce closing and opening time of PNIPAM-based crosslinked network microvalves resulted in lower mechanical stability and lower leakage pressure resistance and vice versa [185].
4.7. Smart membranes, controllable lenses, and autonomous oscillators
With the help of stimuli-responsive polymers, functionality similar to control valves in microfluidic applications can be realized on macroscale in stimuli-responsive membranes and filters. Reports on responsive gel membranes date back to 1970s [209,210]. Such membranes were proposed for a range of application from externally controllable filters to functional valves in implantable drug delivery devices. The controllable filters could be adjusted by means of temperature or light. Temperature-controlled membranes for gel permeation chromatography would allow separation in a continuous way by varying the membrane swelling at appropriate time [211]. Membranes sensitive to water contaminants may be used to manage operation of water purification systems or in autonomous safety devices in water distribution system. Several examples of responsive membranes been reported recently, which can be controlled by such stimuli, as pH [163,212–214], glucose concentration [110,215], and temperature [216–218]. They have been reviewed in [211,219–222].
In another application, SRGs, due to their optical clarity, were employed as microlenses with controllable optical power [223–228].
Progress in understanding of SRGs and oscillating chemical reactions created possibility of coupling of a cyclic stimulus, which is produced by the chemical oscillator, with the response of gels [229–231]. One example of oscillating chemical reactions is the Belousov–Zhabotinsky (BZ) reaction, which generates autonomous oscillations in the redox potential. Employing this reaction, Murase et al. fabricated gels by copolymerizing N-isopropylacrylamide and 2-acrylamido-2′-methylpropanesulfonic acid (AMPS) with ruthenium tris(2,2′-bipyridine) (Ru(bpy)3) as the catalyst for the BZ reaction. The gels exhibited autonomous peristaltic motion and were used for directed particle transport [201]. Ryan et al. coupled SRG with another self-oscillation reaction, Landolt pH-oscillator, and demonstrated cyclic change of both gel size and generated force [232]. Dhanarajan et al. utilized pH-responsive hydrogel to build an autonomous chemomechanical oscillator driven by glucose [233].
Although such systems are only in initial development stages, they have shown a potential for several new applications such as a conveyer to transport soft materials, a formation process for ordered structures of micro- and/or nanomaterials, and a self-cleaning surface [199,201,231]. Further development may lead to a new design of artificial heart and other implantable devices and soft motors. Especially attractive is the possibility to realize such behavior in nanogels [234,235], which promises new concept of soft nanomotors.
4.8. Gene and drug delivery
Not only gels are able to hold fluid inside, the inner environment of swollen gel is thermodynamically different from the outside. This difference can be utilized for several purposes. Thus, gels ability to hold and slowly release various compounds is utilized in agriculture for controlled release of pesticides and nutrients [46,236]. More importantly, hydrogels are applied for sustained drug delivery. When an active compound has a short-term physiological effect, narrow therapeutic window, or low stability, sustained release system can extend exposure after a single administration thus significantly reducing the dosage and the number of times the drug must be administered.
One type of hydrogel based drug delivery devices, called superporous hydrogels, is developed for gastric retention applications, i.e. prolonging retention of drugs in stomach or intestine [237–241]. In this application, a compressed hydrogel filled with drug is swallowed. The hydrogel rapidly swells when reaches the stomach and becomes large enough to be physically entrapped in the stomach and tough enough to persist against stomach medium and contractions. For fast swelling, superporous hydrogels, with pore sizes in the range of 100 μm and larger, are utilized. However, because of their porous structure and high absorbency, the porous hydrogels often suffer from poor mechanical strength. Therefore, considerable attention was devoted to the improvement of the mechanical properties of such superporous hydrogel systems [242]. For instance, Demirtas et al. modified polyacrylamide-based hydrogel with hydroxyapatite. The compressive modulus for such composite was increased to 6.59 MPa as opposed to 0.63 MPa for the non-composite hydrogel, although swelling ratio decreased to 14 g/g from 54 g/g [243]. Other approaches leading to increase of mechanical strength of gels are discussed further in Section 5.1.
Applications of SRGs in delivery and release of drugs and other biologically relevant agents are being developed with the promise to help cure currently incurable disease, simplify healthcare, and improve patients’ quality of life [244]. Both implantable devices (such as insulin pumps) and pharmaceutical systems (such as nanogels) are being developed. The breadth of the research in this area is demonstrated by numerous published reviews [23,25,245–260].
The hydrogel-based delivery systems are capable of attaining both time-controlled and stimuli-induced modulated delivery of agents. Temperature, electricity, light, magnetic field, and ultrasound were employed to remotely control drug release from hydrogel materials [25,258–260]. Hydrogels also have potential to enable integrated feedback-controlled drug delivery systems, which would self-regulate depending on therapeutic needs. Furthermore, they can be used to selectively deliver therapeutic agents to diseased tissues by utilizing certain cues that are specific to the targeted site [250]. Site-specific delivery minimizes many undesired effects that result from inefficient transport across biological barriers, non-preferred distribution of drugs in tissues, and off-target or systemic toxicity. Site-specific delivery is also important in gene delivery, because the genetic material is required to reach, undamaged, its particular target, such as a specific organ in the human body or a specific organelle inside the cell [250].
As discussed above, SRGs can be made responsive to many physiologically relevant stimuli. Temperature-controlled gels are promising for fever-triggered drug delivery and for anti-tumor treatment; in the latter a drug is released into solid tumors thus limiting systemic exposure [261]. pH-sensitive gels are capable of delivering drugs to various parts of human gastrointestinal tract which maintain specific pH [262–264] or to cellular components, such as cytoplasm, endosomes, lysosomes, and mitochondria, which also maintain their own characteristic pH values [265]. Additionally, pH of some pathological sites, such as infection, inflammation, and cancer tumors, is lower than that of normal tissues [264–267]. Other cues utilized in hydrogel-based drug delivery devices are glucose level [268–271], antigens [115,272], etc.
Nanogels, i.e. swollen crosslinked polymer nanoparticles attract high interest in drug delivery research [255,273,274]. They can be utilized for improved solubilization of otherwise insoluble compounds, protection of the compounds from chemical and enzymatic degradation, controlled release of compounds triggered by external stimuli, and diagnostics. Small size of nanogel particles enables their intravenous injection or inhalation.
It is worth noticing that most current drug delivery systems used in pills and injections are designed for a single use. Therefore, not only SRGs, but also regular gels as well as degradable, self-assembled, and other “non-reversibly responsive” polymer systems are utilized. The drug delivery mechanisms of such systems may involve diffusion-controlled release, biodegradation, stimuli-induced disintegration, or slow dissolution. However, reversibility of stimuli-responsive nanogels has potential advantage of feedback-controlled drug release [275]. Another benefit of reversible nature of gel response was demonstrated by recently reported “multi-use” nanoparticles. One such particle with reversible response can deliver required amount of drug to several targeted cells [276].
4.9. Tissue engineering
Because of high water content, tissue-like elasticity, biocompatibility, and low mechanical and frictional irritation, hydrogels have been considered in tissue engineering (TE) for matrices and scaffolds [25,277–285]. As TE scaffolds, they must provide balanced cell penetrability, support cell attachment, proliferation, and differentiation, and allow movement of oxygen, nutrients, and cell products. Small molecules such as oxygen and nutrients readily diffuse throughout the gels, enabling ample supply to growing tissues [286], while diffusion of macromolecules such as proteins is only restricted by the density of cross-links [287]. This advantage makes hydrogels especially well suited to applications in highly metabolic tissues.
Migration of cells through the homogeneous hydrogel, on the other hand, is severely restricted. To overcome this restriction, ideal scaffold should resemble extracellular matrix, which possess a three-dimensional well-defined microstructure with an interconnected pore network [288]. Therefore, pores are often introduced in hydrogel-based scaffold by various methods such as solvent casting-particulate leaching or supercritical processing. Alternatively, cells are encapsulated into gels during preparation of the gels [289–292]. In the latter case, non-porous hydrogels can be employed for protection of encapsulated cells while maintaining good transport of nutrients and cell products [293,294]
Mechanical properties of TE scaffolds are attracting increasing interest [283–285,290,295,296]. Artificial tissues must provide appropriate flexibility and elasticity and adequate mechanical strength [297], suitable for reconstructing or replacing lost or damaged tissues and organs [298]. Moreover, cells are able to sense mechanical properties of their environment, which affect their proliferation, differentiation, geometry and adhesion [285,295,299–301]. Hydrogels with sufficiently high modulus and mechanical strength are generally hard to prepare [289,302]. Although increase in the crosslinking density leads to gels with higher mechanical properties, it also decreases swelling capabilities, mesh sizes, and diffusion coefficients [290]. Introducing porosity causes additional detrimental effects on the mechanical properties of gels. Accordingly, applications of hydrogel scaffolds are currently mostly limited to soft tissues, where no load bearing capacity of the polymer support is needed [286]. In order to construct complex tissues and organs, improvement of mechanical properties of hydrogel scaffolds is desired [290,298]. Nevertheless, hydrogels remains one of the most promising materials for TE. Recently, hydrogels have been developed for regeneration of cartilage [298]. Even more promising is application of in-vivo forming injectable hydrogel scaffolds [303].
TE matrices can serve not only as three-dimensional support systems but also as delivery systems of cytokines, growth factors, genetic material, and other tissue remodeling-inducing substances [25,286–288,293,304]. The control over the regenerative potential of TE scaffolds has been improved dramatically in recent years by using drug releasing scaffolds or incorporating drug delivery devices into TE scaffolds [286,288,305]. Although signaling molecules can be integrated within scaffolds by simply dispersing them in the matrix, extended (more than few days) diffusion-controlled release of growth factors through hydrogel is not easily achieved in such a way. Long-term release kinetics can be achieved by immobilization of agents with covalent bonding and subsequent degradation-controlled release of agents [306]. Alternatively, separate release systems prepared on the basis of recent advances in drug delivery can be incorporated into hydrogel matrix [287], so that drug release function does not affect swelling and degradation of the hydrogel scaffold.
Further enhancement of such scaffolds is possible through enabling control of release with specific triggers. Such release can be closed-loop controlled, meaning that the release rate is self-regulated by the system in response to feedback information without any external intervention, or open-loop controlled, i.e. monitored and activated externally [288]. Externally-regulated, on-demand release may be controlled by temperature, magnetism, light, ultrasound, electric field or irradiation [248,288]. On-demand responsive matrices may also be based on enzymatically-triggered release of growth factors, that can be realized by introducing enzyme-cleavable linkers for attaching bioactive agent to the matrix [307].
Controllable micromechanical stimulation of target cells [293] in SRG-based scaffolds can be exploited in engineering tissues such as cardiac or vascular tissues. The potential of hydrogels in such applications was demonstrated by Pelah, who developed an approach to reversibly deform cell shape using a thermoresponsive polymeric actuator [308]. SRGs have the potential to mechanically influence the fate of stem cells in responsive scaffolds, however more research is needed in this promising area.
In brief, hydrogels are attractive materials for tissue engineering. Their mechanical strength and stiffness are essential requirement and often need to be improved, especially in porous gels. SRGs can enable regulated release of cytokines, growth factors, genetic material, and other tissue-inducing substances and can allow controllable micromechanical stimulation of cells. Precision cyclic response and durability would be critically important for the latter systems.
5. Challenges and Opportunities
SRGs have certain characteristics (chemical control, wet environment) that make them inconvenient in current mainstream designs of machines and devices. At the same time, their attractive and unique features (diversity of possible mechanisms of control, biocompatibility, ability to handle fragile substrates, muscle-like movement, high actuation strain, ability to operate autonomously, etc.) allow them to occupy an important niche in the current designs. Small-size gels were successfully applied in such applications as sensors, microfluidics, tactile displays, membranes with controlled permeability, adaptive microlenses, and drug delivery systems. SRGs possess a particular advantage for autonomous applications, in which the gel can act as sensor and actuator simultaneously.
Because SRs are materials that convert chemical energy into the mechanical action, many of the performance factors of SRGs originate from the mechanical properties of the gels. Better understanding of the relationship between structure and mechanical properties is critical for further improvement of mechanical performance of SRGs needed for majority of their applications. Based on the above analysis of the development of SRGs, poor mechanical robustness and slow response rate can be identified as two gels parameters that often limit their applications [309], especially when macroscopic displacements are required.
5.1. Mechanical robustness
The gels are inherently weak because they contain large amount of fluid that does not contribute to their mechanical resistance. Additional weakness of gels is caused by underlying spatial inhomogeneity during polymerization [309]. Due to the inhomogeneity, most of the stress arisen from gel deformation is localized in the shortest polymer chains, which soon break splitting the network into several pieces. The mechanical weakness is detrimental for many practical applications of gels [178,310]. Therefore, improvements of gel elastic modulus, strength, and toughness are needed. It is important, however, to preserve other properties of the SRG such as amplitude and rate of response. Thus, traditional methods of improving gels strength, such as increasing cross-linking degree, which decrease swelling and reduce response ratio, have limited applicability.
Gel strength can be improved through optimization of structure of the polymer network. This can be achieved using grafted networks, interpenetrating networks, homogeneous networks, slide-ring gels, and so called nanocomposite gels. Results obtained on grafted networks were quite surprising. According to classic theory of elasticity of polymer networks, dangling chains and uncrosslinked polymer, although contribute to the swelling, do not contribute to mechanical properties.[311] However, grafting of polymer networks with polysaccharides led to improvement of shear modulus and stress at break [312].
Interpenetrating networks often exhibit improved mechanical properties. For instance, introducing interpenetrating networks in superporous hydrogels produced remarkably elastic superabsorbent hydrogels [242,313–315]. Most noticeable effect of interpenetrating networks was observed in so called double networks. To obtain a double network, one of the networks comprised less dense but relatively highly crosslinked polyelectrolyte [316], while the second component was much denser but more loosely crosslinked network [317] or a linear polymer [316]. The strength and toughness of the double network greatly exceeded those of both of its components. For instance, in compression test, a double network of poly(2-acrylamido-2-methylpropanesulfonic acid) (PAMPS) and PAAm sustained stress of 17 MPa and fracture strain 92%, while corresponding single-network gels broke at 0.4–0.8 MPa and 41–84%. In tensile test, the double network broke at 0.68 MPa stress and 75% strain, while PAMPS breaks at 0.05 MPa stress and 6% strain, respectively (Fig. 17) [317]. Mechanically strong double network hydrogels were obtained from natural polymers, e.g. bacterial cellulose and gelatin [318], although it is not yet clear if such structures can be stimuli-responsive.
Fig. 17.
Comparison of behavior of a double network with corresponding single networks under uniaxial compression. (A) Compression of PAMPS single network. (B) Compression of PAMPS/PAAm double network. (C) Stress-strain curves. The strength and toughness of the double network greatly exceeded those of both of its components. Reproduced with permission from [317]. Copyright 2003 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
The problem of the network inhomogeneity can be alleviated by advanced polymerization techniques capable of producing almost perfectly homogeneous networks. Among such techniques are tetra-PEG gels [309] and click-reactions [309]. The obtained gels indeed demonstrated improved mechanical properties. Unfortunately, expensive precursors and tedious synthetic procedures are significant limiting factors of these techniques. An interesting approach to minimize the loss of the gel strength caused by the network inhomogeneity was presented by Okumura and Ito [319], who produced a hydrogel by crosslinking α-cyclodextrins (α-CDs) threaded on PEG chains and trapped by capping the chains with bulky ends (Fig. 18). The resulted gel was called slide-ring gel [309]. Such gel exhibited higher stress and strain than regular gels and showed reversible strains up to 100% [319]. Additionally, it demonstrated some improvement of response time and significant improvement in swelling ratio. For instance, a polyrotaxane gel absorbed water up to 400 times its dry weight. [319]
Fig. 18.
Conceptual models of a chemical gel and a slide-ring gel during tensile deformation. (A) Chemical gel is broken down gradually because the heterogeneous distribution of cross-links localizes the stress of deformation. (B) In slide-ring gel, the tension in polymer chains can equalize cooperatively and avoid localization of the stress. Reprinted from Ref. [320] by permission from Macmillan Publishers Ltd, copyright 2007.
Another approach to lessen the effect of the gel inhomogeneity was based on nanocomposite gels [321]. Phillipova et al. [322] introduced self-assembled poly(sodium p-phenylenesulfonate) rods into PAAm network. Although reinforcement of gels usually leads to loss of absorbency, the polyionic rods not only reinforced the gel but also increased the swelling ratio. Unfortunately, the uncrosslinked polymer was leaching from the gel, which is detrimental for most applications. Another type of nanocomposite gels can be obtained by incorporating clay particles. The clay sheets can act as highly multifunctional (connecting large number of chains together) reversible crosslinks for the polymer (Fig. 19) [323]. The clay nanocomposite gels demonstrated tensile strength 0.04–1 MPa and modulus 0.002–0.4 MPa depending on concentration of clay particles, while elongation at break reached 1000% (Fig. 20). The elongation of the gels was 98% reversible while the swelling was approx. 1000 wt.% [323–325]. Responsive PNIPAM-based clay nanocomposite gels were demonstrated. At low concentrations of clay, the nanocomposite demonstrated higher swelling ratios and faster swelling and deswelling than regular PNIPAM hydrogel, possibly because of higher mobility of the chains [323,326]. However, at high concentrations of clay, the swelling ratios decreased and the nanocomposite behaved like a hydrophilic polymer without exhibiting any thermosensitive transition [324,326]. The improvement of the mechanical properties likely resulted from the high crosslinking functionality of the nanoparticles, more uniform distribution of the cross-links, reduction of short chains between cross-links because an active chain has to extend between two different nanoparticles. These principles have been recently implemented to obtain good mechanical performance using silica nanoparticles [327], acetylated allylic starch nanospheres [328], and macromolecular microspheres [329–331].
Fig. 19.

(A) Schematic representation of structure of a clay nanocomposite gel consisting of uniformly dispersed exfoliated inorganic clay sheets and flexible polymer chains. (B) Representation of elongated structure of the gel. Clay nanoparticles act as multifunctional cross-links. Polymer chains have some ability to slide “through” the clay nanoparticles somewhat similar to slide-ring gels. Such gels contain less frozen structural inhomogeneities than common gels and therefore can withstand high levels of elongation. Reproduced with permission from [323]. Copyright 2002 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Fig. 20.
Example of free (top) and stretched by hands (bottom) clay nanocomposite gel. Nanocomposite gels can withstand high levels of elongation. Reproduced with permission from [323]. Copyright 2002 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Another interesting approach for improving mechanical properties is anisotropic unidomain lamellar structure with unidirectional alignment. This system consisted of uniaxial stratified bilayer structure of a polymerizable surfactant dodecyl glyceryl itaconate (PDGI) stacked inside polyacrylamide (PAAm) matrix [310,332]. Physical association of macromolecules in PDGI domains serve as reversible bonds that rupture upon loading and recover back on unloading, thus enhancing toughness of chemically cross-linked gel and allowing self-recovery. The structure showed significant improvement of elastic modulus, strain-to-failure, ultimate strength, toughness, and crack resistance compared to regular PAAm gel [310].
One more type of extremely stretchable and tough hydrogels was produced by mixing and crosslinking two types of polymers: ionically crosslinked alginate and covalently crosslinked polyacrylamide [333]. The hydrogels could be stretched beyond 20 times their initial length, and demonstrated fracture energies of approx. 9,000 J m−2 (Fig. 22). The gel toughness arose from the synergy of two mechanisms: crack bridging by the network of covalent crosslinks and hysteresis by unzipping the network of ionic crosslinks. Moreover, the gels could recover after unloading, because the network of covalent crosslinks preserved the memory of the initial state, and ionic crosslinks healed via re-zipping.
Fig. 22.
Crack resistance of hybrid polyacrylamide/polyalginate gel. The alginate/acrylamide ratio was 1:8. (A) A strip of the undeformed gel glued to two rigid clamps. (B) The gel was stretched to 21 times its initial length. (C) A strip of the gel with a notch produced by a razor blade; the gel is stretched 1.15 times to make the notch visible. (D) The gel containing the notch was stretched to 17 times its initial length. Reprinted by permission from Macmillan Publishers Ltd from Ref. [333], copyright 2012.
In conclusion, several approaches capable of significant improvement of the mechanical robustness of gels were recently proposed. The data on the applicability of those approaches to SRGs is scarce at the moment. Most current successful techniques of improving the mechanical properties of gels involve complex synthesis of the gel network. Additional investigations are needed in order to determine the efficiency of these new approaches for SRGs and practical applications. Further development of techniques capable of improving mechanical robustness and durability of gels is critically needed. Sufficient mechanical robustness of gels would critically enhance existing and open up entirely new applications.
5.2. Response time
Most applications of SRGs would benefit from faster rate of gel response. As discussed, slow response is typical for macroscopic SRGs because gel volume change is a mass diffusion process. The volume change may slow down even further due to several factors, such as the “skin” effect [309,334–336]. This effect is triggered by the formation of a “skin”, i.e. a surface layer with high polymer content, during initial abrupt deswelling of a gel. The following deswelling of the inner part of the material slows down because of restricted diffusion of the solvent through the “skin”. “Skin” effect is observed, for instance, for PNIPAM hydrogels. It is worth noticing that the reswelling of regular PNIPAM hydrogels is even slower, because it is difficult for water to break down the hydrophobic clusters of the isopropyl groups in the collapsed hydrogel matrix [336].
Because the response rate is inversely proportional to the square of the smallest spatial dimension of the gel, miniaturization of active gel parts can be used to improve the response rate. Application of SRGs in microfluidics is an example of such approach. In general, designs that lead to miniaturization of gel dimensions will decrease the response rate. Beebe et al. [194] demonstrated how such approach can lead to further improvement of gels actuators in microfluidic devices. Instead of one larger hydrogel component, they fabricated three thinner components and attained an order of magnitude improvement in opening/closing time of a valve (Fig. 23).
Fig. 23.
(A) A schematic diagram of a microfluidic device with hydrogel jackets around the posts. (B) The top view of an actual device. (C) When the hydrogel jackets expanded, the side channel was blocked. (D) The contracted hydrogel jackets allow fluid to flow down the side channel. (E) Fractional change in diameter (fD) vs time after stimulation of the hydrogel jacket design (circles) and an alternative design that uses a single larger cylindrical structure in the same size channel (squares). Using thinner gel components allowed an order of magnitude improvement in operating time. Scale bars are 300 um. Reprinted by permission from Macmillan Publishers Ltd from Ref. [194], copyright 2000.
Micro- and especially nanogels are another example of fast SRGs. Their response rate is sufficient for many applications. Thus, temperature-sensitive nanogels based on PNIPAM responded to a temperature jump within microseconds [337,338]. Unfortunately, nanogels have their own disadvantages that are detrimental for many potential applications that could benefit from ultrafast response: they are difficult to handle and immobilize, measurement of their volume requires complicated methods, and most importantly, zero-dimensional nanoparticulate morphology prevents using these gels for actuation at the macroscale.
An alternative to gel nanoparticles is thin gel films [339–341]. Films are easier to handle or immobilize. They are widely used in sensors, albeit the reading of the film thickness requires elaborate designs. Applications of gel films as actuators, however, are limited because the films produce either weak force (such as in microcantilever bending), or small displacement. Additionally, mechanical stress can cause delamination of the film from the substrate [82,149,342].
Instead of reducing external dimensions of gels, porous gels were employed to increase the response rate. Porous gels can be obtained by performing gel network synthesis in the presence of a salt, sucrose, PEG, or another solvent [336,343–346]. Synthesis of the network during foaming or bubble generation also resulted in porous gels [241,347]. Among other methods used to create porous gels are polymerization in the presence of a surfactant [348,349], synthesis of PNIPAM hydrogel at a temperature above LCST [350], or supercritical processing and other freezing techniques [351,352]. Additionally, porous structures were produced by using sacrificial templates [353]. Most notably, this method allows producing highly organized interconnecting inverse opal porous structure [136]. The swelling speed of highly porous gel is less dependent of the external gel size. However, porous constructs are poorly suited for many practical applications, as the hydrogels lose their mechanical strength, toughness, and optical transparency [309].
The improvement of gels response rate was also achieved through tweaking the molecular structure of the gels. Thus, grafted gel networks showed improvement of rate of deswelling of PNIPAM [354,355] although the reswelling rate was not significantly improved [336]. Block-copolymer structure was used to create nanostructured gels with hydrophilic channels [356]. The channels facilitated the movement of the solvent in the gel. Similar channels were observed in gels with grafted structure [357] and interpenetrating networks [358]. Despite the significant progress in controlling the molecular structure of gels, the obtained improvement in the speed of the response of the gels still cannot meet the demands of the majority of applications. Therefore decreasing the characteristic length of gel components in gel-based devices remains the main option for increasing the response rate. Designing at micro- and nanoscale, which was demonstrated in microfluidics and nanogels, is most promicing way to accomplish significant impact on the gel response speed. Of crucial importance, when reducing the characteristic length of the gel, is not to compromise (as in case of most porous materials) the mechanical properties of the gel.
5.3. Anisotropy of response
Many of gel applications would benefit from their anisotropic mechanical response. However, conventional non-constrained gels swell isotropically. Isotropic mechanical properties of swollen gels also follow from theoretical models. Gaussian network models predict that even though polymers cross-linked in oriented state swell anisotropically, they behave isotropically once swollen [359]. Therefore, anisotropy of response of stimuli-responsive gels is hard to achieve. On the other hand, biological materials show numerous examples of anisotropic response. Two examples relevant to SRGs are forisomes and spasmonemes [360]. Achieving anisotropic behavior in SRG is desirable for strategies to mimic biological materials or biologically important processes [361]. Anisotropic gels can be also beneficial for other applications, such as sensors and actuators [362].
Anisotropic deformation of gels may result from directional or gradient stimuli field such as electric or magnetic field. [15] Thus, electro-responsive polyelectrolyte hydrogels deform under an electric field as charged ions are directed towards the anode or cathode side of the gel [363]. Also, shape distortion of magnetic composites gels [364–367] occurs when external magnetic field is applied or removed. The aroused anisotropy manifests itself in both direction dependent elastic modulus as well as direction-dependent swelling [368]. Nevertheless, in these cases the stimuli field (not the gel) is the cause of the anisotropy and such approaches are quite limiting. Moreover, magnetic and electric fields are mainly artificially created with the necessity of additional equipment.
Quite recently, anisotropy has been achieved in composite hydrogel materials with anisotropic structure. For instance, a sheet with a series of photo-patterned strips of high- and low-swelling PNIPAM-based hydrogels rolled upon swelling around the axis perpendicular to the interfaces between the strips [361]. However, when the strip width fell below a critical size proportional to the film thickness, the patterned sheets remained flat, undergoing greater expansion along the direction normal to the interfaces than in the parallel direction.
Another example is PEG–PNIPAM hydrogel with dual-network microstructure comprised of unidirectional channeled porous PEG scaffold as the primary network and PNIPAM polymer inside the pores as the secondary network [362]. The material responded to a temperature increase from 20°C to 45°C by shrinking 50% in the directions perpendicular to the channels but showed almost no change in size in the direction parallel to the channels (Fig. 24).
Fig. 24.
Photographs of cylindrical samples of temperature-sensitive hydrogels at 20°C (left) and 45°C (right): (A) PEG–PNIPAM hydrogel with an anisotropic aligned porous PEG scaffold, (B) PEG–PNIPAM hydrogel with an isotropic porous PEG scaffold, and (C) pure PNIPAM hydrogel without a PEG scaffold. (D) Comparison of the axial shrinkage (h1/h0; h0: original height, h1: height after shrinkage) and radial shrinkage (d1/d0; d0: original diameter, d1: diameter after shrinkage) between the three samples. Anisotropic PEG–PNIPAM hydrogel showed anisotropic response to temperature while the other two hydrogels responded isotropically. Reproduced from Ref. [362] with permission of The Royal Society of Chemistry.
Uniaxial layered composites can also have anisotropic response. A notable example is PNIPAM-based hydrogel with embedded cofacially oriented electrolyte titanate(IV) nanosheets [369]. PNIPAM hydrogel exhibited temperature-dependent internal electrostatic permittivity, which reversibly modulated the electrostatic repulsion of the nanosheets in the hydrogel matrix. Therefore, in a few seconds after heating, the hydrogel expanded in one direction and shrinked in the others. The actuation occurred without substantial water uptake or release and therefore could be realized in non-aqueous media. It will be interesting to see how such system would behave on longer timescale when the hydrogel is able to reach its equilibrium volume. One more example of layered composites is anisotropic uniaxial stratified lamellar bilayer structures contained PDGI. In such structures, hydrogel layers are constrained by PDGI layers. In addition to virtually one-dimensional swelling of PDGI/PAAm system in water [332], PDGI/PAAm–PAAc system also demonstrated anisotropic response to pH and temperature [370].
The abovementioned endeavors demonstrate that anisotropic response is a new emerging direction in the development of SRGs. Anisotropic SRGs show great promise for applications. However, only a few such systems have been demonstrated to date and further development is needed before their applications become possible.
6. Nanofilamentary Gels: Prospective Next Generation Stimuli-Responsive Gel Platform
Currently, nanoparticulate gels (0D nanogels) and gel films (2D nanogels) are the two main methods of obtaining fast SRGs. Recent challenges and advances in the development of responsive polymer nanostructures in colloidal and film forms were described in an excellent review [341]. However, assembling either gel films or gel nanoparticles into macrostructures that preserve fast response of nanogels and produce significant force or displacement needed for use in macroscopic actuators or autonomous systems has proven to be problematic. Responsive gels in continuous nanofibrous form (1D nanogels) can theoretically alleviate these problems. Nanofibrous gels are expected to have fast response due to nano-sized fiber diameter. At the same time, infinite nanofilament length and possibilities of assembling them into controlled 2D and 3D macrostructures would enable macroscopic shape change and offer anisotropic swelling or force generating capability.
The fibrous form can provide several advantages over the powder form in applications. For example, in superabsorbents, the powders have two significant limitations: difficult handling and instability in and on a substrate [371]. Continuous fibrous materials are not affected by these limitations. Fiber structure would provide greater fluid capacity [45] along with excellent wicking capability and liquid distribution [50]. Nanofiber gels can be applied to vertical surface with no substrate and would not require fluff in superabsorbent pads.
Attractive opportunities for 1D nanogels emerge from recent advances in electrospinning [372,373]. Electrospinning is a top-down nanomanufacturing technique that uses high voltage electric field to eject charged jets from polymer solutions or melts. The jets stretch and solidify while travelling towards a collector, producing continuous fibers with diameters ranging from single nanometers to a few microns. The top-down electrospinning process, compared to synthetic bottom-up or self-assembly methods, offers simplicity, relatively low cost, and high robustness, while at the same time alleviating the health concerns associated with discontinuous nanomaterials. The properly optimized nanofiber samples are very uniform and do not require expensive purification. Electrospinning is very flexible and has been used to manufacture nanofibers from a wide range of natural and synthetic polymers. Polymer chains in the nanofibers can be cross-linked after electrospinning to achieve nanofilamentary gels [374]. Such gels would combine ultrafine diameter with infinite length, thus allowing bridging nano- and macroscales. Electrospinning process offers unique potential for cost-effective electromechanical control of gel nanofilament size, shape, molecular orientation, and other properties, as well as control of nanofilament placement for integrated single-step manufacturing of two- and three-dimensional nanofilamentary SRG assemblies.
Electrospun nanofibers possess high polymer chain orientation resulting in improved mechanical properties. Recently, ultrafine electrospun nanofibers demonstrated simultaneous improvements in strength, modulus, and toughness [375,376]. The mechanical properties of individual nanofibers can translate into three-dimensional nanofilamentary networks [377]. These improvements are also expected to translate into cross-linked gel nanofilaments and nanofilamentary assemblies. Ultrafine nanofilamentary gels would be superstrong and tough and also have ultrafast response rate.
Recently obtained results showed that nanofilamentary gels produced from electrospun nanofibers showed anisotropy of response [374,378]. Even more possibilities to control macroscopic mechanical anisotropy arise from the recently developed advanced electrospinning techniques. Alignment and deposition methods producing highly ordered nanofiber assemblies [379] and hierarchically ordered nanofibers [380–382] can provide an unprecedented degree of control of architecture and properties of nanofilamentary materials. For instance, actuators that capitalize on anisotropic response of nanoscale fibers are possible. This feature is of interest for applications that may benefit from asymmetric mechanical transformation, such as artificial muscles [383]. In addition, electrospun nanogels can be combined into hierarchical nanostructured materials architectured to optimize the response by using combinations of nanofilaments with various response mechanisms. In sensing applications, such materials can be compensated for non-specific response, for example to ionic strength or solvent quality. Autonomous devices with no need for power source, in which 1D nanogels simultaneously perform duties of sensing and actuation, will be possible for use for implantable health control, drug release, decentralized distributed sensing, personal protection, smart textiles, and emergency shut-offs in water distribution systems, machinery, factory equipment, and space exploration.
Electrospun materials are already used in tissue engineering and drug delivery [373,384–386]. Such materials provide adequate mechanical strength and high porosity with high spatial interconnectivity, which greatly resemble the natural extracellular matrix [387]. The advantages of electrospun materials are, therefore, complimentary to the advantages of gels. Electrospun gels can combine advantages of both materials, providing unique opportunities for tissue engineering. One can envision advanced implantable scaffolds with elaborate externally controlled and/or autonomous drug delivery functions, implantables with capabilities of artificial muscles, and scaffolds that deliver to the seeded cells mechanical cues at the micro- and nanoscales.
Examples of electrospun gel materials have begun to emerge. Electrospun absorbent materials with absorbance depending on pH or temperature were reported [388,389]. Electrospun mats that changed their dimensions were also shown.[378] However, measurement of response of single nanofiber gel and investigation of response mechanisms and mechanical properties of gels in nanofilamentary form is yet to be performed. Some interesting results were recently observed in our group [374].
We have observed the change of thickness in response to pH of single gel filaments produced by cross-linking of PVA/PAAc (Fig. 25). The thickness of filaments that had diameters more than 1 μm was measured by optical microscopy. Measurement of thickness of thinner nanofilaments was performed by wet AFM.
Fig. 25.
Microscopic images of dry (a) and swollen in alkaline (b) and acidic (c) solutions PVA/PAAc filaments. The filaments were obtained by electrospinning of mixed PVA/PAAc solution, followed by thermal treatment at 150°C for 10 min. Average diameter of filaments in dry state was 1.5 μm, in alkaline solution – 4.5 μm, and in acidic solution – 1.7 μm. The diameter of filaments changes approximately 3 fold in response to the change of pH.
In addition, we observed regular buckling of aligned filaments as a result of axial swelling in alkali solution (Fig. 26), which relate to formation of regular folding patterns in pH-responsive gel films on rigid substrates [390]. Further analysis of such buckling can provide critical insights into mechanics of pattern formation and axial deformability of the novel gels that can lead to new methods of measuring axial swelling and serve as a means for unique reversible purely mechanical-based nanopatterning [390].
Fig. 26.
Optical image of PVA/PAAc geleous fibers in (A) neutral and (B) alkali solutions. In response to change of pH, the fibers elongated axially by 19% in less than 5 seconds after submission in alkali solution. The buckled fibers were approximated by sinusoidals to calculate the length.
For measurement of axial extension and mechanical properties of nanofibrous gels, a special set-up and testing procedures were developed [374]. Fig. 27 demonstrates achievable accuracies of time and length measurements and the ultrafast potential of nanofibrous gels, which are expected to exhibit even faster response at smaller diameters. Using our set-up, we measured linear responsive swelling of aligned gel nanofilaments and performed tensile tests on nanofilamentary gels in controlled wet environment (Fig. 28).
Fig. 27.
(a) Demonstration of ultrafast response of 1D nanogels. On 3 min from the start of the experiment, the sample was immersed in distilled water. On 63 min, acid was added. On 90 min, alkali was added. (b) is a close-up of the sample’s response to stepwise change of pH in (a) demonstrating nearly instant response (vertical jumps).
Fig. 28.
Length vs. load dependences of the same PVA/PAAc nanofibrous gel bundle at pH 8 and pH 2 demonstrate unusual cross-over.
Very intriguing is the anisotropy of pH response of PVA/PAAc nanofibrous gels. The length of the nanofilaments increased approx. 20% in response to change of pH from 2 to 8 (Fig. 26 and 28), while the diameter of the nanofilaments increased 150% (Fig. 25). Such high anisotropy of response in unconstrained SRG that apparently had no anisotropic composite structure is unexpected. Also interesting are unusual stimuli-dependent mechanical behavior of these gels. As shown on Fig. 28, load vs. length curves of PVA-PAAc nanofilamentary gels at different pH cross over. Such behavior was not reported for other SRGs nor predicted by theories of gel swelling. Note that the axial response of PVA-PAAc nanofilamentary gels can reverse at high tensile load.
These results indicate that nanofilamentary gels based on electrospun nanofibers can possess fast response, mechanical integrity, and response anisotropy. The dimensional changes of these dual nano-macroscale materials can be registered at the macroscale (Fig. 27–28). Even for unoptimized nanofibrous gels, dimensional changes as high as 2.5 fold can be achieved (Fig. 25) within seconds. Further development of nanofilamentary gels may resolve problems described in Sections 5.1–5.3 and therefore are of substantial interest. The results also demonstrate the need for detailed investigation of mechanical characteristics of gel swelling in general, in order to achieve sufficient understanding of phenomena observed on nanofilamentary gels, which would allow wide application of these novel responsive gels.
7. Conclusions
Summary
Stimuli responsive gels represent a versatile and rapidly developing class of stimuli-responsive materials. Unique properties of SRGs drove expansion of polymer gel applications from the field of absorbents into several new diverse directions. Simplicity, minimal amount of moving parts, and possibility of autonomous function, as well as other features, such as optical clarity, muscle-like behavior, and ability to handle fragile objects, created interest in applying SRGs as actuators. Significant response to small environmental changes and possibility to tailor the response to desired stimulus resulted in applications of SRGs in sensing and drug delivery. Possibility of combining functions of sensing and actuation in a single body make SRGs suitable for autonomous logic switches and self-regulating devices. SRGs also find applications in catalysis, tissue engineering, smart membranes, multiple-use superabsorbents, and oil recovery. In some applications, gels are employed to perform intricate functions, which can be characterized as “intelligent”. Examples of such function are sensing in non-equilibrium mode, sophisticated release profiles of drugs, and cyclic actuation.
Enormous variety of monomers, functional groups, and molecular recognitions schemes were used in SRGs during the development of SRG applications. Importance of mechanical aspects of SRG behavior for applications is being increasingly recognized. Advanced network structures (interpenetrating, homogeneous, slide-ring, hybrid, nanocomposite, etc.) emerged in attempts to enhance response speed and mechanical robustness of gels. However, despite significant progress in these directions, speed of response and robustness remain the most crucial issues of gel-based materials.
Outlook
To facilitate improvement of mechanical factors of gels for applications and to achieve level of understanding of SRG properties and dynamic behavior required for practical use, further systematic theoretical and experimental evaluation of SRGs is needed. Better understanding of mechanisms of response and better models of SRGs will help to achieve higher selectivity and sensitivity for sensing applications and to optimize important for practical applications engineering parameters such as work per stroke, work per cycle, power, generated force, repeatability, fatigue, stability, resolution, and processability.
Because properties of SRGs are highly correlated and improvement of one parameter often leads to degradation of another (for example, absorbency or response time can be improved at the expenses of robustness), it is becoming increasingly difficult to compare the performance of different network structures, response mechanisms, and designs. Standardized experimental characterization protocols are highly desirable to enable comparison of different SRG-based materials.
By reducing characteristic dimension of gel material to sub-micrometer scale, the response speed suitable for many applications can be attained, but this response speed is difficult to translate to macroscale applications. Materials that can combine the speed of nanogels with macroscopic shape change and load caring capabilities are highly desirable. One type of materials that promises such capabilities is nanofilamentary gels based on continuous nanofibers. Such hierarchical macroscopic gels with controlled 3D nanofilamentary architecture can possess fast response, mechanical integrity, and response anisotropy. Further systematic studies are needed to realize this potential.
Supplementary Material
Fig. 4.
A constrained poly(methacrylic acid) hydrogel responded to pH change by generating pressure. Reprinted from Ref. [38], copyright 2003, with permission from Elsevier.
Fig. 21.
Crack resistance of PDGI/PAAm gel with anisotropic unidomain lamellar structure. (A) The gel was cut to have an initial sharp notch. (B,C,D) The gel is stretched perpendicular to the notch directions to a strain more than 3. A blunting occurs in front of the crack tip and it suppresses the stress concentration. (E) After release, the gel returns back to a small residual strain. (F) After several minutes, the gel recovers to its original dimensions. (G) In regular PAAm gel, crack propagates easily in contrast to the PDGI/PAAm gel. The red line in every image shows the initial crack distance. Reprinted with permission from Ref. [310]. Copyright 2011 American Chemical Society.
Acknowledgments
This research was supported in part by the grants from ONR (N000141410663), NSF (DMR-1310534, CMMI-1463636), and NIH (1R01HL125736-01). The authors thank Kazi Jahan and Matthew Shea for conducting nanofilamentary experiments.
Abbreviations
- 4VP
4-vinylpyridine
- AAc
acrylic acid
- AAm
acrylamide
- AFP
α-fetoprotein (tumor-specific marker glycoprotein)
- AMERAH
arm-wrestling match between an EAP actuated robotic arm and a human
- AMP
adenosine 5′-monophosphate
- AMPS
2-acrylamido-2′-methylpropanesulfonic acid
- APTAC
(3-acrylamidopropyl)trimethylammonium chloride
- ATP
adenosine triphosphate
- BZ
Belousov–Zhabotinsky (reaction)
- CMP
cytidine 5′-monophosphate
- ConA
concanavalin A
- DETA
diethylene triamide
- DMAEM
2-dimethylamino ethyl methacrylate
- DMAPAAm
N-(3-dimethylamino propyl) acrylamide
- EAP
electroactive polymer
- GMP
guanosine 5′-monophosphate
- GOx
glucose oxidase
- HEMA
2-hydroxyethyl methacrylate
- iOA
iso-octyl acrylate
- LCST
low critical solution temperature
- MAAc
methacrylic acid
- NEAAm
N-ethylacrylamide
- NIPAM
N-isopropylacrylamide
- P4VP
poly(4-vinylpyridine)
- PAAc
poly(acrylic acid)
- PAAm
poly(acrylamide)
- PAMPS
poly(2-Acrylamido-2-methylpropanesulfonic acid)
- PAN
poly(acrylonitrile)
- PCCA
polymerized colloidal crystalline arrays
- PDGI
poly(dodecyl glyceryl itaconate)
- PEDOT
poly(3,4-ethylenedioxythiophene)
- PEG
poly(ethylene glycol)
- PMAAc
poly(methacrylic acid)
- PMMA
poly(methyl methacrylate)
- PNIPAM
poly(N-isopropylacrylamide)
- PSS
poly(styrenesulfonate)
- PVA
poly(vinyl alcohol)
- QCM
quartz crystal microbalance
- Ru(bpy)3
ruthenium tris(2,2′-bipyridine)
- SRG
stimuli-responsive gel
- TE
tissue engineering
- TFMPA
trifluoromethyl propenoic acid
- UCST
upper critical solution temperature
- UMP
uridine 5′-monophosphate
- VI
vinyl imidazole
- α-CD
α-cyclodextrin
Biographies
 Alexander Goponenko received his M.S. in Materials Science from Lomonosov Moscow State University (1997) and Ph.D. in Macromolecular Chemistry from Karpov Institute of Physical Chemistry (2000). After working as a postdoc at University of Pittsburgh (2001–2004) and at University of Nebraska Medical Center (2005–2006), he has been employed as a Research Assistant Professor in the Department of Mechanical and Materials Engineering at University of Nebraska–Lincoln. His research interests include polymer gels and nanostructured materials.
Alexander Goponenko received his M.S. in Materials Science from Lomonosov Moscow State University (1997) and Ph.D. in Macromolecular Chemistry from Karpov Institute of Physical Chemistry (2000). After working as a postdoc at University of Pittsburgh (2001–2004) and at University of Nebraska Medical Center (2005–2006), he has been employed as a Research Assistant Professor in the Department of Mechanical and Materials Engineering at University of Nebraska–Lincoln. His research interests include polymer gels and nanostructured materials.
 Yuris Dzenis is a McBroom Professor of Engineering at UNL. He has earned his PhD in Aerospace and Mechanical Engineering from the University of Texas-Arlington, PhD in Physics and Mechanics of Polymers from Latvian Academy of Sciences, and MS in Physics (Electrodynamics of Continua) from Latvian University. His research interests are in design, manufacturing, modeling, and characterization of advanced nanomaterials and composites. He has introduced and developed several nanomanufacturing and hybrid manufacturing technologies to produce advanced nanomaterials. He has pioneered development of cost-effective delamination resistant structural composites with nanoreinforced interfaces. Most recent advances include the discovery and explanation of simultaneously high strength and toughness of ultrafine continuous polymer nanofibers.
Yuris Dzenis is a McBroom Professor of Engineering at UNL. He has earned his PhD in Aerospace and Mechanical Engineering from the University of Texas-Arlington, PhD in Physics and Mechanics of Polymers from Latvian Academy of Sciences, and MS in Physics (Electrodynamics of Continua) from Latvian University. His research interests are in design, manufacturing, modeling, and characterization of advanced nanomaterials and composites. He has introduced and developed several nanomanufacturing and hybrid manufacturing technologies to produce advanced nanomaterials. He has pioneered development of cost-effective delamination resistant structural composites with nanoreinforced interfaces. Most recent advances include the discovery and explanation of simultaneously high strength and toughness of ultrafine continuous polymer nanofibers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuhn W. Reversible extension and contraction with change in ionization of a network of multivalent filamentous molecules. Experientia. 1949;5:318–9. doi: 10.1007/BF02172635. [DOI] [PubMed] [Google Scholar]
- 2.Katchalsky A. Rapid swelling and deswelling of reversible gels of polymeric acids by ionization. Experientia. 1949;5:319–20. doi: 10.1007/BF02172636. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn W, Hargitay B, Katchalsky A, Eisenberg H. Reversible Dilation and Contraction by Changing the State of Ionization of High-Polymer Acid Networks. Nature. 1950;165:514–6. [Google Scholar]
- 4.Flory PJ, Rehner J, John Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling J Chem Phys. 1943;11:521–6. [Google Scholar]
- 5.Flory PJ, Rehner JJ. Statistical Mechanics of Cross-Linked Polymer Networks I. Rubberlike Elasticity. J Chem Phys. 1943;11:512–20. [Google Scholar]
- 6.Flory PJ. Principles of polymer chemistry. Itaka. NY: Cornell University Press; 1953. [Google Scholar]
- 7.Tanaka T. Collapse of Gels and the Critical Endpoint. Phys Rev Lett. 1978;40:820–3. [Google Scholar]
- 8.Tanaka T, Wang C, Pande V, Grosberg AY, English A, Masamune S, Gold H, Levy R, King K. Polymer gels that can recognize and recover molecules. Faraday Discuss. 1995;101:201–6. [Google Scholar]
- 9.Tanaka T, Nishio I, Sun S, Ueno-Nishio S. Collapse of Gels in an Electric Field. Science. 1982;218:467–9. doi: 10.1126/science.218.4571.467. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki A, Tanaka T. Phase transition in polymer gels induced by visible light. Nature. 1990;346:345–7. [Google Scholar]
- 11.Tanaka T, Fillmore DJ. Kinetics of swelling of gels. J Chem Phys. 1979;70:1214–8. [Google Scholar]
- 12.Ricka J, Tanaka T. Swelling of ionic gels: quantitative performance of the Donnan theory. Macromolecules. 1984;17:2916–21. [Google Scholar]
- 13.Ricka J, Tanaka T. Phase transition in ionic gels induced by copper complexation. Macromolecules. 1985;18:83–5. [Google Scholar]
- 14.Li Y, Tanaka T. Kinetics of swelling and shrinking of gels. J Chem Phys. 1990;92:1365. [Google Scholar]
- 15.Roy D, Cambre JN, Sumerlin BS. Future perspectives and recent advances in stimuli-responsive materials. Progress in Polymer Science. 2010;35:278–301. [Google Scholar]
- 16.Messing R, Schmidt AM. Perspectives for the mechanical manipulation of hybrid hydrogels. Polymer Chemistry. 2011;2:18–32. [Google Scholar]
- 17.Peteu SF, Oancea F, Sicuia OA, Constantinescu F, Dinu S. Responsive Polymers for Crop Protection. Polymers. 2010;2:229–51. [Google Scholar]
- 18.van der Linden HJ, Herber S, Olthuis W, Bergveld P. Stimulus-sensitive hydrogels and their applications in chemical (micro) analysis. Analyst. 2003;128:325–31. doi: 10.1039/b210140h. [DOI] [PubMed] [Google Scholar]
- 19.Schneider H, Kato K, Strongin RM. Chemomechanical Polymers as Sensors and Actuators for Biological and Medicinal Applications. Sensors. 2007;7:1578–611. doi: 10.3390/s7081578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorne JB, Vine GJ, Snowden MJ. Microgel applications and commercial considerations. Colloid Polym Sci. 2011;289:625–46. [Google Scholar]
- 21.Zhang QM, Serpe MJ. Responsive Polymers as Sensors, Muscles, and Self-Healing Materials. In: Boulatov R, editor. Polymer Mechanochemistry. Heidelberg: Springer International Publishing; 2015. pp. 377–424. [DOI] [PubMed] [Google Scholar]
- 22.Islam MR, Lu Z, Li X, Sarker AK, Hu L, Choi P, Li X, Hakobyan N, Serpe MJ. Responsive polymers for analytical applications: A review. Anal Chim Acta. 2013;789:17–32. doi: 10.1016/j.aca.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 23.de Las Heras Alarcon C, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chem Soc Rev. 2005;34:276–85. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- 24.Galaev I. ‘Smart’ polymers and what they could do in biotechnology and medicine. Trends Biotechnol. 1999;17:335–40. doi: 10.1016/s0167-7799(99)01345-1. [DOI] [PubMed] [Google Scholar]
- 25.Kirschner CM, Anseth KS. Hydrogels in Healthcare: From Static to Dynamic Material Microenvironments. Acta Mater. 2013;61:931–44. doi: 10.1016/j.actamat.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flory PJ. Thermodynamics of Heterogeneous Polymers and Their Solutions. J Chem Phys. 1944;12:425. [Google Scholar]
- 27.Katsumoto Y, Tanaka T, Sato H, Ozaki Y. Conformational Change of Poly(N-isopropylacrylamide) during the Coil-Globule Transition Investigated by Attenuated Total Reflection/Infrared Spectroscopy and Density Functional Theory Calculation†. J Phys Chem A. 2002;106:3429–35. doi: 10.1021/jp0124903. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed Z, Gooding EA, Pimenov KV, Wang L, Asher SA. UV Resonance Raman Determination of Molecular Mechanism of Poly(N-isopropylacrylamide) Volume Phase Transition. J Phys Chem B. 2009;113:4248–56. doi: 10.1021/jp810685g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erman B, Mark JE. Structures and properties of rubberlike networks. New York: Oxford University Press; 1997. [Google Scholar]
- 30.Khokhlov A, Starodubtzev S, Vasilevskaya V. Conformational transitions in polymer gels: Theory and experiment. In: Dušek K, editor. Responsive Gels: Volume Transitions I. Berlin/Heidelberg: Springer; 1993. pp. 123–71. [Google Scholar]
- 31.Donnan FG. The Theory of Membrane Equilibria. Chem Rev. 1924;1:73–90. [Google Scholar]
- 32.English AE, Tanaka T, Edelman ER. Equilibrium and non-equilibrium phase transitions in copolymer polyelectrolyte hydrogels. J Chem Phys. 1997;107:1645–54. [Google Scholar]
- 33.Grimshaw PE, Nussbaum JH, Grodzinsky AJ, Yarmush ML. Kinetics of electrically and chemically induced swelling in polyelectrolyte gels. J Chem Phys. 1990;93:4462–72. [Google Scholar]
- 34.Nussbaum JH, Grodzinsky AJ. Proton diffusion reaction in a protein polyelectrolyte membrane and the kinetics of electromechanical forces. J Membr Sci. 1981;8:193–219. [Google Scholar]
- 35.Ward Muscatello MM, Stunja LE, Asher SA. Polymerized Crystalline Colloidal Array Sensing of High Glucose Concentrations. Anal Chem (Washington, DC, U S ) 2009;81:4978–86. doi: 10.1021/ac900006x. [DOI] [PubMed] [Google Scholar]
- 36.Murayama H, Imran AB, Nagano S, Seki T, Kidowaki M, Ito K, Takeoka Y. Chromic Slide-Ring Gel Based on Reflection from Photonic Bandgap. Macromolecules. 2008;41:1808–14. [Google Scholar]
- 37.Ono T, Sugimoto T, Shinkai S, Sada K. Lipophilic polyelectrolyte gels as super-absorbent polymers for nonpolar organic solvents. Nat Mater. 2007;6:429–33. doi: 10.1038/nmat1904. [DOI] [PubMed] [Google Scholar]
- 38.Herber S, Olthuis W, Bergveld P. A swelling hydrogel-based PCO2 sensor. Sensors Actuators B: Chem. 2003;91:378–82. [Google Scholar]
- 39.Burak E. Mechanical Behavior of Swollen Networks. In: Buchholz FL, Peppas NA, editors. Superabsorbent Polymers. Washington: American Chemical Society; 1994. pp. 50–63. [Google Scholar]
- 40.Johnson BD, Beebe DJ, Crone WC. Effects of swelling on the mechanical properties of a pH-sensitive hydrogel for use in microfluidic devices. Materials Science and Engineering: C. 2004;24:575–81. [Google Scholar]
- 41.Kong HJ, Wong E, Mooney DJ. Independent Control of Rigidity and Toughness of Polymeric Hydrogels. Macromolecules. 2003;36:4582–8. [Google Scholar]
- 42.Zohuriaan-Mehr MJ, Kabiri K. Superabsorbent polymer materials: a review. Iranian Polymer Journal. 2008;17:451–77. [Google Scholar]
- 43.Marshall AJ, Blyth J, Davidson CAB, Lowe CR. pH-Sensitive Holographic Sensors. Anal Chem. 2003;75:4423–31. doi: 10.1021/ac020730k. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Moshe M, Alexeev VL, Asher SA. Fast Responsive Crystalline Colloidal Array Photonic Crystal Glucose Sensors. Anal Chem. 2006;78:5149–57. doi: 10.1021/ac060643i. [DOI] [PubMed] [Google Scholar]
- 45.Zohuriaan-Mehr M, Omidian H, Doroudiani S, Kabiri K. Advances in non-hygienic applications of superabsorbent hydrogel materials. Journal of Materials Science. 2010;45:5711–35. [Google Scholar]
- 46.Puoci F, Iemma F, Spizzirri UG, Cirillo G, Curcio M, Picci N. Polymer in Agriculture: a Review. American Journal of Agricultural and Biological Sciences. 2008;3:299–314. [Google Scholar]
- 47.Kazuo H, Fumihiro A. Water-Blocking, Optical-Fiber Cable System Employing Water-Absorbent Materials. In: Buchholz FL, Peppas NA, editors. Superabsorbent Polymers. Washington: American Chemical Society; 1994. pp. 128–40. [Google Scholar]
- 48.Chung DDL. Use of polymers for cement-based structural materials. Journal of Materials Science. 2004;39:2973–8. [Google Scholar]
- 49.Fusayoshi M. Trends in the Development of Superabsorbent Polymers for Diapers. In: Buchholz FL, Peppas NA, editors. Superabsorbent Polymers. Washington: American Chemical Society; 1994. pp. 88–98. [Google Scholar]
- 50.Herbert N. Characterization of a New Superabsorbent Polymer Generation. In: Buchholz FL, Peppas NA, editors. Superabsorbent Polymers. Washington: American Chemical Society; 1994. pp. 99–111. [Google Scholar]
- 51.Mudiyanselage TK, Neckers DC. Photochromic superabsorbent polymers. Soft Matter. 2008;4:768–74. doi: 10.1039/b712642p. [DOI] [PubMed] [Google Scholar]
- 52.Adebajo MO, Frost RL, Kloprogge JT, Carmody O, Kokot S. Porous Materials for Oil Spill Cleanup: A Review of Synthesis and Absorbing Properties. Journal of Porous Materials. 2003;10:159–70. [Google Scholar]
- 53.Jang J, Kim B. Studies of crosslinked styrene-alkyl acrylate copolymers for oil absorbency application. I. Synthesis and characterization. J Appl Polym Sci. 2000;77:903–13. [Google Scholar]
- 54.Jang J, Kim B. Studies of crosslinked styrene-alkyl acrylate copolymers for oil absorbency application. II. Effects of polymerization conditions on oil absorbency. J Appl Polym Sci. 2000;77:914–20. [Google Scholar]
- 55.Atta AM, El-Ghazawy RA, Farag RK, El-Kafrawy AF, Abdel-Azim AA. Crosslinked cinnamoyloxyethyl methacrylate and isooctyl acrylate copolymers as oil sorbers. Polym Int. 2005;54:1088–96. [Google Scholar]
- 56.Farag RK, El-Saeed SM. Synthesis and characterization of oil sorbers based on docosanyl acrylate and methacrylates copolymers. J Appl Polym Sci. 2008;109:3704–13. [Google Scholar]
- 57.Zhou MH, Cho W. Oil absorbents based on styrene-butadiene rubber. J Appl Polym Sci. 2003;89:1818–24. [Google Scholar]
- 58.Zhou MH, Cho W. Synthesis and properties of high oil-absorbent 4-tert-butylstyrene-EPDM-divinylbenzene graft terpolymer. J Appl Polym Sci. 2002;85:2119–29. [Google Scholar]
- 59.Atta AM, El-Hamouly SH, AlSabagh AM, Gabr MM. Crosslinked poly(octadecene-alt-maleic anhydride) copolymers as crude oil sorbers. J Appl Polym Sci. 2007;105:2113–20. [Google Scholar]
- 60.Atta AM, Arndt K. Swelling and network parameters of high oil-absorptive network based on 1-octene and isodecyl acrylate copolymers. J Appl Polym Sci. 2005;97:80–91. [Google Scholar]
- 61.Wei QF, Mather RR, Fotheringham AF. Oil removal from used sorbents using a biosurfactant. Bioresour Technol. 2005;96:331–4. doi: 10.1016/j.biortech.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Kulawardana EU, Neckers DC. Photoresponsive oil sorbers. Journal of Polymer Science Part A: Polymer Chemistry. 2010;48:55–62. [Google Scholar]
- 63.Wang G, Kuroda K, Enoki T, Grosberg A, Masamune S, Oya T, Takeoka Y, Tanaka T. Gel catalysts that switch on and off. Proceedings of the National Academy of Sciences. 2000;97:9861–4. doi: 10.1073/pnas.180192597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bernfeld P, Wan J. Antigens and Enzymes Made Insoluble by Entrapping Them into Lattices of Synthetic Polymers. Science. 1963;142:678–9. doi: 10.1126/science.142.3593.678. [DOI] [PubMed] [Google Scholar]
- 65.Bergbreiter DE, Case BL, Liu Y, Caraway JW. Poly(N-isopropylacrylamide) Soluble Polymer Supports in Catalysis and Synthesis. Macromolecules. 1998;31:6053–62. [Google Scholar]
- 66.Diaz Diaz D, Kuehbeck D, Koopmans RJ. Stimuli-responsive gels as reaction vessels and reusable catalysts. Chem Soc Rev. 2011;40:427–48. doi: 10.1039/c005401c. [DOI] [PubMed] [Google Scholar]
- 67.Jiang X, Xiong D, An Y, Zheng P, Zhang W, Shi L. Thermoresponsive hydrogel of poly(glycidyl methacrylate-co-N-isopropylacrylamide) as a nanoreactor of gold nanoparticles. J Polym Sci, Part A: Polym Chem. 2007;45:2812–9. [Google Scholar]
- 68.Liu F, Tao G, Zhuo R. Synthesis of Thermal Phase Separating Reactive Polymers and Their Applications in Immobilized Enzymes. Polym J. 1993;25:561–7. [Google Scholar]
- 69.Wang Y, Zhang J, Zhang W, Zhang M. Pd-Catalyzed C-C Cross-Coupling Reactions within a Thermoresponsive and pH-Responsive and Chelating Polymeric Hydrogel. J Org Chem. 2009;74:1923–31. doi: 10.1021/jo802427k. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Yan R, Zhang J, Zhang W. Synthesis of efficient and reusable catalyst of size-controlled Au nanoparticles within a porous, chelating and intelligent hydrogel for aerobic alcohol oxidation. Journal of Molecular Catalysis A: Chemical. 2010;317:81–8. [Google Scholar]
- 71.Marshall AJ, Young DS, Kabilan S, Hussain A, Blyth J, Lowe CR. Holographic sensors for the determination of ionic strength. Anal Chim Acta. 2004;527:13–20. [Google Scholar]
- 72.Harnish B, Robinson JT, Pei Z, Ramström O, Yan M. UV-Cross-Linked Poly(vinylpyridine) Thin Films as Reversibly Responsive Surfaces. Chemistry of Materials. 2005;17:4092–6. [Google Scholar]
- 73.Bashir R, Hilt JZ, Elibol O, Gupta A, Peppas NA. Micromechanical cantilever as an ultrasensitive pH microsensor. Appl Phys Lett. 2002;81:3091–3. [Google Scholar]
- 74.Hilt JZ, Gupta AK, Bashir R, Peppas NA. Ultrasensitive Biomems Sensors Based on Microcantilevers Patterned with Environmentally Responsive Hydrogels. Biomedical Microdevices. 2003;5:177–84. [Google Scholar]
- 75.Zhang Y, Ji H, Snow D, Sterling R, Brown GM. A pH Sensor Based on a Microcantilever Coated with Intelligent Hydrogel. Instrum Sci Technol. 2004;32:361. [Google Scholar]
- 76.Han IS, Han M, Kim J, Lew S, Lee YJ, Horkay F, Magda JJ. Constant-Volume Hydrogel Osmometer: A New Device Concept for Miniature Biosensors. Biomacromolecules. 2002;3:1271–5. doi: 10.1021/bm0255894. [DOI] [PubMed] [Google Scholar]
- 77.Herber S, Eijkel J, Olthuis W, Bergveld P, van den Berg A. Study of chemically induced pressure generation of hydrogels under isochoric conditions using a microfabricated device. J Chem Phys. 2004;121:2746–51. doi: 10.1063/1.1773153. [DOI] [PubMed] [Google Scholar]
- 78.Richter A, Bund A, Keller M, Arndt K. Characterization of a microgravimetric sensor based on pH sensitive hydrogels. Sensors Actuators B: Chem. 2004;99:579–85. [Google Scholar]
- 79.Cai QY, Grimes CA. A remote query magnetoelastic pH sensor. Sensors Actuators B: Chem. 2000;71:112–7. doi: 10.1016/s0925-4005(00)00599-2. [DOI] [PubMed] [Google Scholar]
- 80.Gerlach G, Guenther M, Suchaneck G, Sorber J, Arndt K, Richter A. Application of sensitive hydrogels in chemical and pH sensors. Macromolecular Symposia. 2004;210:403–10. [Google Scholar]
- 81.Tierney S, Hjelme DR, Stokke BT. Determination of swelling of responsive gels with nanometer resolution. Fiber-optic based platform for hydrogels as signal transducers. Anal Chem. 2008;80:5086–93. doi: 10.1021/ac800292k. [DOI] [PubMed] [Google Scholar]
- 82.Shakhsher ZM, Odeh I, Jabr S, Rudolf Seitz W. An Optical Chemical Sensor Based on Swellable Dicarboxylate Functionalized Polymer Microspheres for pH Copper and Calcium Determination. Microchimica Acta. 2004;144:147–53. [Google Scholar]
- 83.Mayes AG, Blyth J, Millington RB, Lowe CR. Metal Ion-Sensitive Holographic Sensors. Anal Chem. 2002;74:3649–57. doi: 10.1021/ac020131d. [DOI] [PubMed] [Google Scholar]
- 84.Holtz JH, Asher SA. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature. 1997;389:829–32. doi: 10.1038/39834. [DOI] [PubMed] [Google Scholar]
- 85.Reese CE, Asher SA. Photonic crystal optrode sensor for detection of Pb2+ in high ionic strength environments. Anal Chem. 2003;75:3915–8. doi: 10.1021/ac034276b. [DOI] [PubMed] [Google Scholar]
- 86.Liu K, Ji H. Detection of Pb2+ Using a Hydrogel Swelling Microcantilever Sensor. Analytical Sciences. 2004;20:9–11. doi: 10.2116/analsci.20.9. [DOI] [PubMed] [Google Scholar]
- 87.Asher SA, Sharma AC, Goponenko AV, Ward MM. Photonic Crystal Aqueous Metal Cation Sensing Materials. Anal Chem. 2003;75:1676–83. doi: 10.1021/ac026328n. [DOI] [PubMed] [Google Scholar]
- 88.Madrigal González B, Christie G, Davidson CAB, Blyth J, Lowe CR. Divalent metal ion-sensitive holographic sensors. Anal Chim Acta. 2005;528:219–28. [Google Scholar]
- 89.Zhang Y, Ji H, Brown GM, Thundat T. Detection of CrO42- Using a Hydrogel Swelling Microcantilever Sensor. Anal Chem. 2003;75:4773–7. doi: 10.1021/ac0343026. [DOI] [PubMed] [Google Scholar]
- 90.Marshall AJ, Young DS, Blyth J, Kabilan S, Lowe CR. Metabolite-Sensitive Holographic Biosensors. Anal Chem. 2004;76:1518–23. doi: 10.1021/ac030357w. [DOI] [PubMed] [Google Scholar]
- 91.Walker JP, Kimble KW, Asher SA. Photonic crystal sensor for organophosphate nerve agents utilizing the organophosphorus hydrolase enzyme. Analytical & Bioanalytical Chemistry. 2007;389:2115–24. doi: 10.1007/s00216-007-1599-y. [DOI] [PubMed] [Google Scholar]
- 92.Walker JP, Asher SA. Acetylcholinesterase-Based Organophosphate Nerve Agent Sensing Photonic Crystal. Anal Chem. 2005;77:1596–600. doi: 10.1021/ac048562e. [DOI] [PubMed] [Google Scholar]
- 93.Zourob M, Ong KG, Zeng K, Mouffouk F, Grimes CA. A wireless magnetoelastic biosensor for the direct detection of organophosphorus pesticides. Analyst. 2007;132:338–43. doi: 10.1039/b616035b. [DOI] [PubMed] [Google Scholar]
- 94.Cai Q, Zeng K, Ruan C, Desai TA, Grimes CA. A Wireless, Remote Query Glucose Biosensor Based on a pH-Sensitive Polymer. Anal Chem. 2004;76:4038–43. doi: 10.1021/ac0498516. [DOI] [PubMed] [Google Scholar]
- 95.Miyata T, Jikihara A, Nakamae K, Hoffman AS. Preparation of reversibly glucose-responsive hydrogels by covalent immobilization of lectin in polymer networks having pendant glucose. Journal of Biomaterials Science, Polymer Edition. 2004;15:1085–98. doi: 10.1163/1568562041753061. [DOI] [PubMed] [Google Scholar]
- 96.Kabilan S, Marshall AJ, Sartain FK, Lee M, Hussain A, Yang X, Blyth J, Karangu N, James K, Zeng J, Smith D, Domschke A, Lowe CR. Holographic glucose sensors. Biosensors and Bioelectronics. 2005;20:1602–10. doi: 10.1016/j.bios.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 97.Dean KES, Horgan AM, Marshall AJ, Kabilan S, Pritchard J. Selective holographic detection of glucose using tertiary amines. Chem Commun. 2006:3507–9. doi: 10.1039/b605778k. [DOI] [PubMed] [Google Scholar]
- 98.Yang X, Pan X, Blyth J, Lowe CR. Towards the real-time monitoring of glucose in tear fluid: Holographic glucose sensors with reduced interference from lactate and pH. Biosensors and Bioelectronics. 2008;23:899–905. doi: 10.1016/j.bios.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 99.Asher SA, Alexeev VL, Goponenko AV, Sharma AC, Lednev IK, Wilcox CS, Finegold DN. Photonic crystal carbohydrate sensors: Low ionic strength sugar sensing. J Am Chem Soc. 2003;125:3322–9. doi: 10.1021/ja021037h. [DOI] [PubMed] [Google Scholar]
- 100.Sorrell CD, Serpe MJ. Glucose sensitive poly (N-isopropylacrylamide) microgel based etalons. Analytical and Bioanalytical Chemistry. 2012;402:2385–93. doi: 10.1007/s00216-012-5736-x. [DOI] [PubMed] [Google Scholar]
- 101.Samoei GK, Wang W, Escobedo JO, Xu X, Schneider H, Cook RL, Strongin RM. A Chemomechanical Polymer that Functions in Blood Plasma with High Glucose Selectivity. Angewandte Chemie International Edition. 2006;45:5319–22. doi: 10.1002/anie.200601398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bodugoz H, Güven O, Peppas NA. Glucose recognition capabilities of hydroxyethyl methacrylate-based hydrogels containing poly(ethylene glycol) chains. J Appl Polym Sci. 2007;103:432–41. [Google Scholar]
- 103.Gao X, Zhen R, Zhang Y, Grimes CA. Detecting Penicillin in Milk with a Wireless Magnetoelastic Biosensor. Sensor Letters. 2009;7:6–10. [Google Scholar]
- 104.Sartain FK, Yang X, Lowe CR. Holographic Lactate Sensor. Anal Chem. 2006;78:5664–70. doi: 10.1021/ac060416g. [DOI] [PubMed] [Google Scholar]
- 105.Kanekiyo Y, Sano M, Iguchi R, Shinkai S. Novel nucleotide-responsive hydrogels designed from copolymers of boronic acid and cationic units and their applications as a QCM resonator system to nucleotide sensing. Journal of Polymer Science Part A: Polymer Chemistry. 2000;38:1302–10. [Google Scholar]
- 106.Sallacan N, Zayats M, Bourenko T, Kharitonov AB, Willner I. Imprinting of Nucleotide and Monosaccharide Recognition Sites in Acrylamidephenylboronic Acid-Acrylamide Copolymer Membranes Associated with Electronic Transducers. Anal Chem. 2002;74:702–12. doi: 10.1021/ac0109873. [DOI] [PubMed] [Google Scholar]
- 107.Pogorelova SP, Bourenko T, Kharitonov AB, Willner I. Selective sensing of triazine herbicides in imprinted membranes using ion-sensitive field-effect transistors and microgravimetric quartz crystal microbalance measurements. Analyst. 2002;127:1484–91. doi: 10.1039/b204491a. [DOI] [PubMed] [Google Scholar]
- 108.Islam MR, Serpe MJ. Poly (N-isopropylacrylamide) microgel-based etalons and etalon arrays for determining the molecular weight of polymers in solution. APL Materials. 2013;1:052108. [Google Scholar]
- 109.Miyata T, Jige M, Nakaminami T, Uragami T. Tumor marker-responsive behavior of gels prepared by biomolecular imprinting. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1190–3. doi: 10.1073/pnas.0506786103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Albin G, Horbett TA, Ratner BD. Glucose sensitive membranes for controlled delivery of insulin: Insulin transport studies. J Controlled Release. 1985;2:153–64. [Google Scholar]
- 111.Traitel T, Cohen Y, Kost J. Characterization of glucose-sensitive insulin release systems in simulated in vivo conditions. Biomaterials. 2000;21:1679–87. doi: 10.1016/s0142-9612(00)00050-8. [DOI] [PubMed] [Google Scholar]
- 112.Kokufata E, Zhang Y, Tanaka T. Saccharide-sensitive phase transition of a lectin-loaded gel. Nature. 1991;351:302–4. [Google Scholar]
- 113.Miyata T, Jikihara A, Nakamae K, Hoffman AS. Preparation of poly(2-glucosyloxyethyl methacrylate)-concanavalin A complex hydrogel and its glucose-sensitivity. Macromolecular Chemistry and Physics. 1996;197:1135–46. [Google Scholar]
- 114.Alexeev VL, Sharma AC, Goponenko AV, Das S, Lednev IK, Wilcox CS, Finegold DN, Asher SA. High ionic strength glucose-sensing photonic crystal. Anal Chem. 2003;75:2316–23. doi: 10.1021/ac030021m. [DOI] [PubMed] [Google Scholar]
- 115.Miyata T, Asami N, Uragami T. A reversibly antigen-responsive hydrogel. Nature. 1999;399:766–9. doi: 10.1038/21619. [DOI] [PubMed] [Google Scholar]
- 116.Miyata T, Asami N, Uragami T. Preparation of an Antigen-Sensitive Hydrogel Using Antigen-Antibody Bindings. Macromolecules. 1999;32:2082–4. [Google Scholar]
- 117.Byrne ME, Park K, Peppas NA. Molecular imprinting within hydrogels. Adv Drug Deliv Rev. 2002;54:149–61. doi: 10.1016/s0169-409x(01)00246-0. [DOI] [PubMed] [Google Scholar]
- 118.Wizeman JW, Kofinas P. Molecularly imprinted polymer hydrogels displaying isomerically resolved glucose binding. Biomaterials. 2001;22:1485–91. doi: 10.1016/s0142-9612(00)00303-3. [DOI] [PubMed] [Google Scholar]
- 119.Byrne ME, Oral E, Zachary Hilt J, Peppas NA. Networks for recognition of biomolecules: molecular imprinting and micropatterning poly(ethylene glycol)- Containing films. Polym Adv Technol. 2002;13:798–816. [Google Scholar]
- 120.Adrus N, Ulbricht M. Molecularly imprinted stimuli-responsive hydrogels for protein recognition. Polymer. 2012;53:4359–4366. [Google Scholar]
- 121.Takeuchi T, Haginaka J. Separation and sensing based on molecular recognition using molecularly imprinted polymers. Journal of Chromatography B: Biomedical Sciences and Applications. 1999;728:1–20. doi: 10.1016/s0378-4347(99)00057-2. [DOI] [PubMed] [Google Scholar]
- 122.Whitcombe MJ, Vulfson EN. Imprinted Polymers. Adv Mater. 2001;13:467–78. [Google Scholar]
- 123.Takeuchi T, Matsui J. Molecular imprinting: An approach to “tailor-made” synthetic polymers with biomimetic functions. Acta Polymerica. 1996;47:471–80. [Google Scholar]
- 124.Mosbach K, Haupt K. Some new developments and challenges in non-covalent molecular imprinting technology. Journal of Molecular Recognition. 1998;11:62–8. doi: 10.1002/(SICI)1099-1352(199812)11:1/6<62::AID-JMR391>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 125.Wulff G. Molecular Imprinting in Cross-Linked Materials with the Aid of Molecular Templates? A Way towards. Artificial Antibodies Angewandte Chemie International Edition in English. 1995;34:1812–32. [Google Scholar]
- 126.Sellergren B. Noncovalent molecular imprinting: antibody-like molecular recognition in polymeric network materials. TrAC Trends in Analytical Chemistry. 1997;16:310–20. [Google Scholar]
- 127.Asanuma H, Hishiya T, Komiyama M. Tailor-Made Receptors by Molecular Imprinting. Adv Mater. 2000;12:1019–30. [Google Scholar]
- 128.Rooney M, TV, Rudolf Seitz W. An optically sensitive membrane for pH based on swellable polymer microspheres in a hydrogel. Anal Commun. 1999;36:267–70. [Google Scholar]
- 129.Dübendorfer J, Kunz RE, Jobst G, Moser I, Urban G. Integrated optical pH sensor using replicated chirped grating coupler sensor chips. Sensors Actuators B: Chem. 1998;50:210–9. doi: 10.1364/ao.37.001890. [DOI] [PubMed] [Google Scholar]
- 130.McCurley MF. An optical biosensor using a fluorescent, swelling sensing element. Biosensors and Bioelectronics. 1994;9:527–33. [Google Scholar]
- 131.Shakhsher Z, Seitz WR, Legg KD. Single Fiber-Optic pH Sensor Based on Changes in Reflection Accompanying Polymer Swelling. Anal Chem. 1994;66:1731–5. doi: 10.1021/ac00082a021. [DOI] [PubMed] [Google Scholar]
- 132.Zhang L, Langmuir ME, Bai M, Rudolf Seitz W. A sensor for pH based on an optical reflective device coupled to the swelling of an aminated polystyrene membrane. Talanta. 1997;44:1691–8. doi: 10.1016/s0039-9140(97)00080-5. [DOI] [PubMed] [Google Scholar]
- 133.Schalkhammer T, Lobmaier C, Pittner F, Leitner A, Brunner H, Aussenegg FR. The use of metal-island-coated pH-sensitive swelling polymers for biosensor applications. Sensors and Actuators B: Chemical. 1995;24:166–72. [Google Scholar]
- 134.Aussenegg FR, Brunner H, Leitner A, Lobmaier C, Schalkhammer T, Pittner F. The metal island coated swelling polymer over mirror system (MICSPOMS): a new principle for measuring ionic strength. Sensors Actuators B: Chem. 1995;29:204–9. [Google Scholar]
- 135.Rudolf Seitz W. New directions in fiber optic chemical sensors: Sensors based on polymer swelling. J Mol Struct. 1993;292:105–13. [Google Scholar]
- 136.Takeoka Y, Watanabe M. Polymer Gels that Memorize Structures of Mesoscopically Sized Templates. Dynamic and Optical Nature of Periodic Ordered Mesoporous Chemical Gels. Langmuir. 2002;18:5977–80. [Google Scholar]
- 137.Lee Y, Pruzinsky SA, Braun PV. Glucose-Sensitive Inverse Opal Hydrogels: Analysis of Optical Diffraction Response. Langmuir. 2004;20:3096–106. [PubMed] [Google Scholar]
- 138.Wang JY, Cao Y, Feng Y, Yin F, Gao JP. Multiresponsive Inverse-Opal Hydrogels. Adv Mater. 2007;19:3865–71. [Google Scholar]
- 139.Kuckling D, Harmon ME, Frank CW. Photo-Cross-Linkable PNIPAAm Copolymers. 1. Synthesis and Characterization of Constrained Temperature-Responsive Hydrogel Layers. Macromolecules. 2002;35:6377–83. [Google Scholar]
- 140.Sheppard NF, Jr, Lesho MJ, McNally P, Shaun Francomacaro A. Microfabricated conductimetric pH sensor. Sensors Actuators B: Chem. 1995;28:95–102. [Google Scholar]
- 141.Arnold FH, Zheng W, Michaels AS. A membrane-moderated, conductimetric sensor for the detection and measurement of specific organic solutes in aqueous solutions. J Membr Sci. 2000;167:227–39. [Google Scholar]
- 142.Kikuchi A, Suzuki K, Okabayashi O, Hoshino H, Kataoka K, Sakurai Y, Okano T. Glucose-Sensing Electrode Coated with Polymer Complex Gel Containing Phenylboronic Acid. Anal Chem. 1996;68:823–8. doi: 10.1021/ac950748d. [DOI] [PubMed] [Google Scholar]
- 143.Strong ZA, Wang AW, McConaghy CF. Hydrogel-Actuated Capacitive Transducer for Wireless Biosensors. Biomedical Microdevices. 2002;4:97–103. [Google Scholar]
- 144.Grimes CA, Roy SC, Rani S, Cai Q. Theory, Instrumentation and Applications of Magnetoelastic Resonance Sensors: A Review. Sensors. 2011;11:2809–44. doi: 10.3390/s110302809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thong Trinh Q, Gerlach G, Sorber J, Arndt K. Hydrogel-based piezoresistive pH sensors: Design, simulation and output characteristics. Sensors Actuators B: Chem. 2006;117:17–26. [Google Scholar]
- 146.Gerlach G, Guenther M, Sorber J, Suchaneck G, Arndt K, Richter A. Chemical and pH sensors based on the swelling behavior of hydrogels. Sensors Actuators B: Chem. 2005;111–112:555–61. [Google Scholar]
- 147.Carrascosa LG, Moreno M, Álvarez M, Lechuga LM. Nanomechanical biosensors: a new sensing tool. TrAC Trends in Analytical Chemistry. 2006;25:196–206. [Google Scholar]
- 148.Fraden J. Handbook of Modern Sensors: Physics, Designs, and Applications. New York: Springer; 2010. [Google Scholar]
- 149.Richter A, Paschew G, Klatt S, Lienig J, Arndt K, Adler HP. Review on Hydrogel-based pH Sensors and Microsensors. Sensors. 2008;8:561–81. doi: 10.3390/s8010561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Oya T, Enoki T, Grosberg AY, Masamune S, Sakiyama T, Takeoka Y, Tanaka K, Wang G, Yilmaz Y, Feld MS, Dasari R, Tanaka T. Reversible Molecular Adsorption Based on Multiple-Point Interaction by Shrinkable Gels. Science. 1999;286:1543–5. doi: 10.1126/science.286.5444.1543. [DOI] [PubMed] [Google Scholar]
- 151.Alvarez-Lorenzo C, Hiratani H, Tanaka K, Stancil K, Grosberg AY, Tanaka T. Simultaneous Multiple-Point Adsorption of Aluminum Ions and Charged Molecules by a Polyampholyte Thermosensitive Gel:3 Controlling Frustrations in a Heteropolymer Gel. Langmuir. 2001;17:3616–22. [Google Scholar]
- 152.Schneider H, Tianjun L, Lomadze N. Dimension Changes in a Chemomechanical Polymer Containing Ethylenediamine and Alkyl Functions as Selective Recognition Units. European Journal of Organic Chemistry. 2006;2006:677–92. [Google Scholar]
- 153.Watanabe M, Akahoshi T, Tabata Y, Nakayama D. Molecular Specific Swelling Change of Hydrogels in Accordance with the Concentration of Guest Molecules. J Am Chem Soc. 1998;120:5577–8. [Google Scholar]
- 154.Cai QY, Grimes Ca. A salt-independent pH sensor. Sensors Actuators B: Chem. 2001;79:144–9. [Google Scholar]
- 155.Kurauchi T, Shiga T, Hirose Y, Okada A. Deformation Behaviors of Polymer Gels in Electric Field. In: DeRossi D, Kajiwara K, Osada Y, Yamauchi A, editors. Polymer Gels: Fundamentals and Biomedical Applications. New York: Plenum Press; 1991. pp. 237–46. [Google Scholar]
- 156.Osada Y, Gong J. Soft and Wet Materials: Polymer Gels. Adv Mater. 1998;10:827–37. [Google Scholar]
- 157.Pransky J. First armwrestling match between an EAP actuated robotic arm and a human. Industrial Robot: An International Journal. 2005:32. [Google Scholar]
- 158.Bar-Cohen Y. Artificial muscles based on electroactive polymers as an enabling tool in biomimetics. Proc Inst Mech Eng Part C. 2007;221:1149–56. [Google Scholar]
- 159.Kwon GH, Park JY, Kim JY, Frisk ML, Beebe DJ, Lee S. Biomimetic Soft Multifunctional Miniature Aquabots. Small. 2008;4:2148–53. doi: 10.1002/smll.200800315. [DOI] [PubMed] [Google Scholar]
- 160.Richter A, Paschew G. Optoelectrothermic Control of Highly Integrated Polymer-Based MEMS Applied in an Artificial Skin. Adv Mater. 2009;21:979–83. [Google Scholar]
- 161.Maeda S, Hara Y, Yoshida R, Hashimoto S. Active polymer gel actuators. Int J Mol Sci. 2010;11:52–66. doi: 10.3390/ijms11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Geryak R, Tsukruk VV. Reconfigurable and actuating structures from soft materials. Soft Matter. 2014;10:1246–1263. doi: 10.1039/c3sm51768c. [DOI] [PubMed] [Google Scholar]
- 163.Tokarev I, Minko S. Multiresponsive, Hierarchically Structured Membranes: New, Challenging, Biomimetic Materials for Biosensors, Controlled Release, Biochemical Gates, and Nanoreactors. Adv Mater. 2009;21:241–7. [Google Scholar]
- 164.Ionov L. Hydrogel-based actuators: possibilities and limitations. Materials Today. 2014;17:494–503. [Google Scholar]
- 165.Liu Z, Calvert P. Multilayer Hydrogels as Muscle-Like Actuators. Adv Mater. 2000;12:288–91. [Google Scholar]
- 166.Huber JE, Fleck NA, Ashby MF. The selection of mechanical actuators based on performance indices. Proceedings of the Royal Society of London Series A: Mathematical, Physical and Engineering Sciences. 1997;453:2185–205. [Google Scholar]
- 167.Yoshioka Y, Calvert P. Epoxy-based electroactive polymer gels. Experimental Mechanics. 2002;42:404–8. [Google Scholar]
- 168.Kim SJ, Yoon SG, Lee YM, Kim SI. Electrical sensitive behavior of poly(vinyl alcohol)/poly (diallyldimethylammonium chloride) IPN hydrogel. Sensors Actuators B: Chem. 2003;88:286–91. [Google Scholar]
- 169.Kurkuri Mahaveer D, et al. Electroactive behavior of poly(acrylic acid) grafted poly(vinyl alcohol) samples, their synthesis using a Ce (IV) glucose redox system and their characterization. Smart Mater Struct. 2006;15:417. [Google Scholar]
- 170.Samui AB, et al. Electroactive polymer gels based on epoxy resin. Smart Mater Struct. 2007;16:237. [Google Scholar]
- 171.Moschou EA, Peteu SF, Bachas LG, Madou MJ, Daunert S. Artificial Muscle Material with Fast Electroactuation under Neutral pH Conditions. Chemistry of Materials. 2004;16:2499–502. [Google Scholar]
- 172.Siddhanta SK, Gangopadhyay R. Conducting polymer gel: formation of a novel semi-IPN from polyaniline and crosslinked poly(2-acrylamido-2-methyl propanesulphonicacid) Polymer. 2005;46:2993–3000. [Google Scholar]
- 173.Richter A, Klenke C, Arndt K. Adjustable low dynamic pumps based on hydrogels. Macromolecular Symposia. 2004;210:377–84. [Google Scholar]
- 174.Wang J, Chen Z, Mauk M, Hong K, Li M, Yang S, Bau H. Self-Actuated, Thermo-Responsive Hydrogel Valves for Lab on a Chip. Biomedical Microdevices. 2005;7:313–22. doi: 10.1007/s10544-005-6073-z. [DOI] [PubMed] [Google Scholar]
- 175.Brown ME. Moisture responsive device and method. WO/2008/068496 2008;GB2007/004680. WIPO Patent Application.
- 176.Graham NB, Szmidt RAK, Kirkwood RC. Plant growth media comprising cross-linked hydrogel particles. 5,382,270 1991;613913. Us.
- 177.Ornstein L. Method of controlling the relative humidity in a soil environment and apparatus for accomplishing same. 4,182,357 1980;05/882,789. Us.
- 178.Dai T, Qing X, Shen C, Wang J, Lu Y. High-strength multifunctional conducting polymer hydrogels. Advanced Materials Research. 2010;123–125:117–20. [Google Scholar]
- 179.Kim SJ, Kim HI, Park SJ, Kim IY, Lee SH, Lee TS, Kim SI. Behavior in electric fields of smart hydrogels with potential application as bio-inspired actuators. Smart Mater Struct. 2005;14:511–4. [Google Scholar]
- 180.Schreyer HB, Gebhart N, Kim KJ, Shahinpoor M. Electrical Activation of Artificial Muscles Containing Polyacrylonitrile Gel Fibers. Biomacromolecules. 2000;1:642–7. doi: 10.1021/bm005557l. [DOI] [PubMed] [Google Scholar]
- 181.Richter A, Kuckling D, Howitz S, Gehring Thomas, Arndt K. Electronically controllable microvalves based on smart hydrogels: magnitudes and potential applications. Journal of Microelectromechanical Systems. 2003;12:748–53. [Google Scholar]
- 182.Yu C, Mutlu S, Selvaganapathy P, Mastrangelo CH, Svec F, Fréchet JMJ. Flow Control Valves for Analytical Microfluidic Chips without Mechanical Parts Based on Thermally Responsive Monolithic Polymers. Anal Chem. 2003;75:1958–61. doi: 10.1021/ac026455j. [DOI] [PubMed] [Google Scholar]
- 183.Luo Q, Mutlu S, Gianchandani YB, Svec F, Fréchet JMJ. Monolithic valves for microfluidic chips based on thermoresponsive polymer gels. Electrophoresis. 2003;24:3694–702. doi: 10.1002/elps.200305577. [DOI] [PubMed] [Google Scholar]
- 184.Harmon ME, Tang M, Frank CW. A microfluidic actuator based on thermoresponsive hydrogels. Polymer. 2003;44:4547–56. [Google Scholar]
- 185.Chen G, Svec F, Knapp DR. Light-actuated high pressure-resisting microvalve for on-chip flow control based on thermo-responsive nanostructured polymer. Lab Chip. 2008;8:1198–204. doi: 10.1039/b803293a. [DOI] [PubMed] [Google Scholar]
- 186.Richter A, Howitz S, Kuckling D, Arndt K. Influence of volume phase transition phenomena on the behavior of hydrogel-based valves. Sensors Actuators B: Chem. 2004;99:451–8. [Google Scholar]
- 187.Richter A, Türke A, Pich A. Controlled Double-Sensitivity of Microgels Applied to Electronically Adjustable Chemostats. Adv Mater. 2007;19:1109–12. [Google Scholar]
- 188.Zhang Y, Kato S, Anazawa T. A microchannel concentrator controlled by integral thermoresponsive valves. Sensors Actuators B: Chem. 2008;129:481–6. [Google Scholar]
- 189.Richter A, Klatt S, Paschew G, Klenke C. Micropumps operated by swelling and shrinking of temperature-sensitive hydrogels. Lab Chip. 2009;9:613–8. doi: 10.1039/b810256b. [DOI] [PubMed] [Google Scholar]
- 190.Sershen SR, Mensing GA, Ng M, Halas NJ, Beebe DJ, West JL. Independent Optical Control of Microfluidic Valves Formed from Optomechanically Responsive Nanocomposite Hydrogels. Adv Mater. 2005;17:1366–8. doi: 10.1002/adma.200401239. [DOI] [PubMed] [Google Scholar]
- 191.Sugiura S, Sumaru K, Ohi K, Hiroki K, Takagi T, Kanamori T. Photoresponsive polymer gel microvalves controlled by local light irradiation. Sensors and Actuators A: Physical. 2007;140:176–84. [Google Scholar]
- 192.Choe K, Kim KJ. Polyacrylonitrile linear actuators: Chemomechanical and electro-chemomechanical properties. Sensors and Actuators, A: Physical. 2006;126:165–72. [Google Scholar]
- 193.Eddington DT, Liu RH, Moore JS, Beebe DJ. An organic self-regulating microfluidic system. Lab Chip. 2001;1:96–9. doi: 10.1039/b108078d. [DOI] [PubMed] [Google Scholar]
- 194.Beebe DJ, Moore JS, Bauer JM, Yu Q, Liu RH, Devadoss C, Jo B. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature. 2000;404:588–90. doi: 10.1038/35007047. [DOI] [PubMed] [Google Scholar]
- 195.Liu RH, Yu Qing, Beebe DJ. Fabrication and characterization of hydrogel-based microvalves. Microelectromechanical Systems, Journal Of. 2002;11:45–53. [Google Scholar]
- 196.Park Ji Young, et al. A polymeric microfluidic valve employing a pH-responsive hydrogel microsphere as an actuating source. J Micromech Microengineering. 2006;16:656. [Google Scholar]
- 197.Eddington DT, Beebe DJ. A valved responsive hydrogel microdispensing device with integrated pressure source. Microelectromechanical Systems, Journal Of. 2004;13:586–93. [Google Scholar]
- 198.Baldi A, Gu Yuandong, Loftness PE, Siegel RA, Ziaie B. A hydrogel-actuated environmentally sensitive microvalve for active flow control. Journal of Microelectromechanical Systems. 2003;12:613–21. [Google Scholar]
- 199.Takeoka Y, Watanabe M, Yoshida R. Self-Sustaining Peristaltic Motion on the Surface of a Porous Gel. J Am Chem Soc. 2003;125:13320–1. doi: 10.1021/ja036904c. [DOI] [PubMed] [Google Scholar]
- 200.Maeda S, Hara Y, Yoshida R, Hashimoto S. Control of the Dynamic Motion of a Gel Actuator Driven by the Belousov-Zhabotinsky Reaction. Macromolecular Rapid Communications. 2008;29:401–5. [Google Scholar]
- 201.Murase Y, Maeda S, Hashimoto S, Yoshida R. Design of a Mass Transport Surface Utilizing Peristaltic Motion of a Self-Oscillating Gel. Langmuir. 2009;25:483–9. doi: 10.1021/la8029006. [DOI] [PubMed] [Google Scholar]
- 202.Eddington DT, Beebe DJ. Flow control with hydrogels. Adv Drug Deliv Rev. 2004;56:199–210. doi: 10.1016/j.addr.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 203.Dong L, Jiang H. Autonomous microfluidics with stimuli-responsive hydrogels. Soft Matter. 2007;3:1223–30. doi: 10.1039/b706563a. [DOI] [PubMed] [Google Scholar]
- 204.Richter A. Hydrogels for Actuators. In: Gerlach G, Arndt K, editors. Hydrogel Sensors and Actuators. Berlin: Springer; 2010. pp. 221–48. [Google Scholar]
- 205.Kobayashi K, Suzuki H. A sampling mechanism employing the phase transition of a gel and its application to a micro analysis system imitating a mosquito. Sensors and Actuators B: Chemical. 2001;80:1–8. [Google Scholar]
- 206.Nguyen N, Huang X, Chuan TK. MEMS-Micropumps: A Review. J Fluids Eng. 2002;124:384–92. [Google Scholar]
- 207.Silva JE, Geryak R, Loney DA, Kottke PA, Naik RR, Tsukruk VV, Fedorov AG. Stick-slip water penetration into capillaries coated with swelling hydrogel. Soft Matter. 2015;11:5933–9. doi: 10.1039/c5sm00660k. [DOI] [PubMed] [Google Scholar]
- 208.Stange M, Dreyer ME, Rath HJ. Capillary driven flow in circular cylindrical tubes. Phys Fluids. 2003;15:2587–601. [Google Scholar]
- 209.Vacík J, Kopeček J. Specific resistances of hydrophilic membranes containing ionogenic groups. J Appl Polym Sci. 1975;19:3029–44. [Google Scholar]
- 210.Kopeček J, Vacík J, Lím D. Permeability of membranes containing ionogenic groups. J Polym Sci A-1 Polym Chem. 1971;9:2801–15. [Google Scholar]
- 211.Feil H, Bae YH, Feijen J, Kim SW. Molecular separation by thermosensitive hydrogel membranes. J Membr Sci. 1991;64:283–94. [Google Scholar]
- 212.Mika AM, Childs RF, Dickson JM. Salt separation and hydrodynamic permeability of a porous membrane filled with pH-sensitive gel. J Membr Sci. 2002;206:19–30. [Google Scholar]
- 213.Tokarev I, Orlov M, Minko S. Responsive Polyelectrolyte Gel Membranes. Adv Mater. 2006;18:2458–60. [Google Scholar]
- 214.Iwata H, Matsuda T. Preparation and properties of novel environment-sensitive membranes prepared by graft polymerization onto a porous membrane. J Membr Sci. 1988;38:185–99. [Google Scholar]
- 215.Ishihara K, Kobayashi M, Ishimaru N, Shinohara I. Glucose Induced Permeation Control of Insulin through a Complex Membrane Consisting of Immobilized Glucose Oxidase and a Poly(amine) Polym J. 1984;16:625–31. [Google Scholar]
- 216.Li SK, D’Emanuele A. On-off transport through a thermoresponsive hydrogel composite membrane. J Controlled Release. 2001;75:55–67. doi: 10.1016/s0168-3659(01)00365-0. [DOI] [PubMed] [Google Scholar]
- 217.Yoshida M, Asano M, Safranj A, Omichi H, Spohr R, Vetter J, Katakai R. Novel Thin Film with Cylindrical Nanopores That Open and Close Depending on Temperature: First Successful Synthesis. Macromolecules. 1996;29:8987–9. [Google Scholar]
- 218.Chu L, Li Y, Zhu J, Chen W. Negatively Thermoresponsive Membranes with Functional Gates Driven by Zipper-Type Hydrogen-Bonding Interactions. Angewandte Chemie International Edition. 2005;44:2124–7. doi: 10.1002/anie.200462687. [DOI] [PubMed] [Google Scholar]
- 219.Kikuchi A, Okano T. Intelligent thermoresponsive polymeric stationary phases for aqueous chromatography of biological compounds. Progress in Polymer Science. 2002;27:1165–93. [Google Scholar]
- 220.Yakushiji T, Sakai K, Kikuchi A, Aoyagi T, Sakurai Y, Okano T. Effects of Cross-Linked Structure on Temperature-Responsive Hydrophobic Interaction of Poly(N-isopropylacrylamide) Hydrogel-Modified Surfaces with Steroids. Anal Chem. 1999;71:1125–30. [Google Scholar]
- 221.Kim J, Park K. Smart hydrogels for bioseparation. Bioseparation. 1998;7:177–84. doi: 10.1023/a:1008050124949. [DOI] [PubMed] [Google Scholar]
- 222.Gopishetty V, Roiter Y, Tokarev I, Minko S. Multiresponsive Biopolyelectrolyte Membrane. Adv Mater. 2008;20:4588–93. [Google Scholar]
- 223.Dong L, Agarwal AK, Beebe DJ, Jiang H. Adaptive liquid microlenses activated by stimuli-responsive hydrogels. Nature. 2006;442:551–4. doi: 10.1038/nature05024. [DOI] [PubMed] [Google Scholar]
- 224.Holmes DP, Crosby AJ. Snapping Surfaces. Adv Mater. 2007;19:3589–93. [Google Scholar]
- 225.Kim J, Serpe MJ, Lyon LA. Photoswitchable Microlens Arrays. Angewandte Chemie International Edition. 2005;44:1333–6. doi: 10.1002/anie.200461538. [DOI] [PubMed] [Google Scholar]
- 226.Kim J, Nayak S, Lyon LA. Bioresponsive Hydrogel Microlenses. J Am Chem Soc. 2005;127:9588–92. doi: 10.1021/ja0519076. [DOI] [PubMed] [Google Scholar]
- 227.Kim J, Serpe MJ, Lyon LA. Hydrogel Microparticles as Dynamically Tunable Microlenses. J Am Chem Soc. 2004;126:9512–3. doi: 10.1021/ja047274x. [DOI] [PubMed] [Google Scholar]
- 228.Ehrick JD, Stokes S, Bachas-Daunert S, Moschou EA, Deo SK, Bachas LG, Daunert S. Chemically Tunable Lensing of Stimuli-Responsive Hydrogel Microdomes. Adv Mater. 2007;19:4024–7. [Google Scholar]
- 229.Yoshida R, Ichijo H, Hakuta T, Yamaguchi T. Self-oscillating swelling and deswelling of polymer gels. Macromolecular Rapid Communications. 1995;16:305–10. [Google Scholar]
- 230.Crook CJ, Smith A, Jones RAL, Ryan AJ. Chemically induced oscillations in a pH-responsive hydrogel. Phys Chem Chem Phys. 2002;4:1367–9. [Google Scholar]
- 231.Shinohara S, Seki T, Sakai T, Yoshida R, Takeoka Y. Chemical and optical control of peristaltic actuator based on self-oscillating porous gel. Chem Commun. 2008:4735–7. doi: 10.1039/b808427k. [DOI] [PubMed] [Google Scholar]
- 232.Ryan AJ, Crook CJ, Howse JR, Topham P, Jones RAL, Geoghegan M, Parnell AJ, Ruiz-Perez L, Martin SJ, Cadby A, Menelle A, Webster JRP, Gleeson AJ, Bras W. Responsive brushes and gels as components of soft nanotechnology. Faraday Discuss. 2005;128:55–74. doi: 10.1039/b405700g. [DOI] [PubMed] [Google Scholar]
- 233.Dhanarajan AP, Misra GP, Siegel RA. Autonomous Chemomechanical Oscillations in a Hydrogel/Enzyme System Driven by Glucose. The Journal of Physical Chemistry A. 2002;106:8835–8. [Google Scholar]
- 234.Varga I, Szalai I, Mészaros R, Gilányi T. Pulsating pH-Responsive Nanogels. The Journal of Physical Chemistry B. 2006;110:20297–301. doi: 10.1021/jp064282m. [DOI] [PubMed] [Google Scholar]
- 235.Hara Y, Yoshida R. Control of Oscillating Behavior for the Self-Oscillating Polymer with pH-Control Site. Langmuir. 2005;21:9773–6. doi: 10.1021/la052070v. [DOI] [PubMed] [Google Scholar]
- 236.Rudzinski WE, Chipuk T, Dave AM, Kumbar SG, Aminabhavi TM. pH-sensitive acrylic-based copolymeric hydrogels for the controlled release of a pesticide and a micronutrient. J Appl Polym Sci. 2003;87:394–403. [Google Scholar]
- 237.Polnok A, Verhoef JC, Borchard G, Sarisuta N, Junginger HE. In vitro evaluation of intestinal absorption of desmopressin using drug-delivery systems based on superporous hydrogels. Int J Pharm. 2004;269:303–10. doi: 10.1016/j.ijpharm.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 238.Chen J, Park K. Synthesis and characterization of superporous hydrogel composites. J Controlled Release. 2000;65:73–82. doi: 10.1016/s0168-3659(99)00238-2. [DOI] [PubMed] [Google Scholar]
- 239.Dorkoosh FA, Verhoef JC, Verheijden JHM, Rafiee-Tehrani M, Borchard G, Junginger HE. Peroral Absorption of Octreotide in Pigs Formulated in Delivery Systems on the Basis of Superporous Hydrogel Polymers. Pharmaceutical Research. 2002;19:1532–6. doi: 10.1023/a:1020416918624. [DOI] [PubMed] [Google Scholar]
- 240.Dorkoosh FA, Verhoef JC, Borchard G, Rafiee-Tehrani M, Verheijden JHM, Junginger HE. Intestinal absorption of human insulin in pigs using delivery systems based on superporous hydrogel polymers. Int J Pharm. 2002;247:47–55. doi: 10.1016/s0378-5173(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 241.Chen J, Park H, Park K. Synthesis of superporous hydrogels: Hydrogels with fast swelling and superabsorbent properties. J Biomed Mater Res. 1999;44:53–62. doi: 10.1002/(sici)1097-4636(199901)44:1<53::aid-jbm6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 242.Omidian H, Rocca JG, Park K. Advances in superporous hydrogels. J Controlled Release. 2005;102:3–12. doi: 10.1016/j.jconrel.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 243.Tolga Demirtaş T, Karakeçili A, Gümüşderelioğlu M. Hydroxyapatite containing superporous hydrogel composites: synthesis and in-vitro characterization. Journal of Materials Science: Materials in Medicine. 2008;19:729–35. doi: 10.1007/s10856-007-3008-7. [DOI] [PubMed] [Google Scholar]
- 244.Bayer CL, Peppas NA. Advances in recognitive, conductive and responsive delivery systems. J Controlled Release. 2008;132:216–21. doi: 10.1016/j.jconrel.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 245.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53:321–39. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 246.Gupta P, Vermani K, Garg S. Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov Today. 2002;7:569–79. doi: 10.1016/s1359-6446(02)02255-9. [DOI] [PubMed] [Google Scholar]
- 247.Peppas N. Hydrogels in pharmaceutical formulations. European Journal of Pharmaceutics and Biopharmaceutics. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 248.Kost J, Langer R. Responsive polymeric delivery systems. Adv Drug Deliv Rev. 2001;46:125–48. doi: 10.1016/s0169-409x(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 249.Chaterji S, Kwon IK, Park K. Smart polymeric gels: Redefining the limits of biomedical devices. Progress in Polymer Science. 2007;32:1083–122. doi: 10.1016/j.progpolymsci.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Grainger SJ, El-Sayed MEH. Stimuli-sensitive particles for drug delivery. In: Jabbari E, Khademhosseini A, editors. Biologically-Responsive Hybrid Biomaterials: A Reference for Material Scientists and Bioengineers. New Jersey: World Scientific Publishing Company; 2010. pp. 171–90. [Google Scholar]
- 251.Bajpai AK, Shukla SK, Bhanu S, Kankane S. Responsive polymers in controlled drug delivery. Progress in Polymer Science. 2008;33:1088–118. [Google Scholar]
- 252.De Geest BG, Sanders NN, Sukhorukov GB, Demeester J, De Smedt SC. Release mechanisms for polyelectrolyte capsules. Chem Soc Rev. 2007;36:636–49. doi: 10.1039/b600460c. [DOI] [PubMed] [Google Scholar]
- 253.Kikuchi A, Okano T. Pulsatile drug release control using hydrogels. Adv Drug Deliv Rev. 2002;54:53–77. doi: 10.1016/s0169-409x(01)00243-5. [DOI] [PubMed] [Google Scholar]
- 254.Peppas N, Hilt J, Khademhosseini A, Langer R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv Mater. 2006;18:1345–60. [Google Scholar]
- 255.Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev. 2008;60:1638–49. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 256.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 257.Mano JF. Stimuli-Responsive Polymeric Systems for Biomedical Applications. Advanced Engineering Materials. 2008;10:515–27. [Google Scholar]
- 258.Timko BP, Whitehead K, Gao W, Kohane DS, Farokhzad O, Anderson D, Langer R. Advances in Drug Delivery. Annual Review of Materials Research. 2011;41:1–20. [Google Scholar]
- 259.Sirsi SR, Borden MA. State-of-the-art materials for ultrasound-triggered drug delivery. Adv Drug Deliv Rev. 2014;72:3–14. doi: 10.1016/j.addr.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sershen S, West J. Implantable, polymeric systems for modulated drug delivery. Adv Drug Deliv Rev. 2002;54:1225–35. doi: 10.1016/s0169-409x(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 261.Chilkoti A, Dreher MR, Meyer DE, Raucher D. Targeted drug delivery by thermally responsive polymers. Adv Drug Deliv Rev. 2002;54:613–30. doi: 10.1016/s0169-409x(02)00041-8. [DOI] [PubMed] [Google Scholar]
- 262.Nakamura K, Murray RJ, Joseph JI, Peppas NA, Morishita M, Lowman AM. Oral insulin delivery using P(MAA-g-EG) hydrogels: effects of network morphology on insulin delivery characteristics. J Controlled Release. 2004;95:589–99. doi: 10.1016/j.jconrel.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 263.Risbud MV, Hardikar AA, Bhat SV, Bhonde RR. pH-sensitive freeze-dried chitosan–polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J Controlled Release. 2000;68:23–30. doi: 10.1016/s0168-3659(00)00208-x. [DOI] [PubMed] [Google Scholar]
- 264.Zhang H, Mardyani S, Chan WCW, Kumacheva E. Design of Biocompatible Chitosan Microgels for Targeted pH-Mediated Intracellular Release of Cancer Therapeutics. Biomacromolecules. 2006;7:1568–72. doi: 10.1021/bm050912z. [DOI] [PubMed] [Google Scholar]
- 265.Oishi M, Hayashi H, Iijima M, Nagasaki Y. Endosomal release and intracellular delivery of anticancer drugs using pH-sensitive PEGylated nanogels. Journal of Materials Chemistry. 2007;17:3720–5. [Google Scholar]
- 266.Na K, Bae YH. Self-Assembled Hydrogel Nanoparticles Responsive to Tumor Extracellular pH from Pullulan Derivative/Sulfonamide Conjugate: Characterization, Aggregation, and Adriamycin Release in Vitro. Pharm Res. 2002;19:681–8. doi: 10.1023/a:1015370532543. [DOI] [PubMed] [Google Scholar]
- 267.Na K, Lee ES, Bae YH. Self-Organized Nanogels Responding to Tumor Extracellular pH: pH-Dependent Drug Release and in Vitro Cytotoxicity against MCF-7 Cells. Bioconjug Chem. 2007;18:1568–74. doi: 10.1021/bc070052e. [DOI] [PubMed] [Google Scholar]
- 268.Ravaine V, Ancla C, Catargi B. Chemically controlled closed-loop insulin delivery. J Controlled Release. 2008;132:2–11. doi: 10.1016/j.jconrel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 269.Shiino D, Murata Y, Kubo A, Kim YJ, Kataoka K, Koyama Y, Kikuchi A, Yokoyama M, Sakurai Y, Okano T. Amine containing phenylboronic acid gel for glucose-responsive insulin release under physiological pH. J Controlled Release. 1995;37:269–76. [Google Scholar]
- 270.Kang SI, Bae YH. A sulfonamide based glucose-responsive hydrogel with covalently immobilized glucose oxidase and catalase. J Controlled Release. 2003;86:115–21. doi: 10.1016/s0168-3659(02)00409-1. [DOI] [PubMed] [Google Scholar]
- 271.Chu L, Li Y, Zhu J, Wang H, Liang Y. Control of pore size and permeability of a glucose-responsive gating membrane for insulin delivery. J Controlled Release. 2004;97:43–53. doi: 10.1016/j.jconrel.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 272.Tang M, Zhang R, Bowyer A, Eisenthal R, Hubble J. NAD-sensitive hydrogel for the release of macromolecules. Biotechnol Bioeng. 2004;87:791–6. doi: 10.1002/bit.20210. [DOI] [PubMed] [Google Scholar]
- 273.Vinogradov SV, Bronich TK, Kabanov AV. Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv Drug Deliv Rev. 2002;54:135–47. doi: 10.1016/s0169-409x(01)00245-9. [DOI] [PubMed] [Google Scholar]
- 274.Raemdonck K, Demeester J, Smedt SD. Advanced nanogel engineering for drug delivery. Soft Matter. 2009;5:707–15. [Google Scholar]
- 275.Hoare T, Pelton R. Charge-Switching, Amphoteric Glucose-Responsive Microgels with Physiological Swelling Activity. Biomacromolecules. 2008;9:733–40. doi: 10.1021/bm701203r. [DOI] [PubMed] [Google Scholar]
- 276.Lee ES, Kim D, Youn YS, Oh KT, Bae YH. A Virus-Mimetic Nanogel Vehicle. Angewandte Chemie International Edition. 2008;47:2418–21. doi: 10.1002/anie.200704121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.von Recum H, Okano T, Wan Kim S. Growth factor release from thermally reversible tissue culture substrates. J Controlled Release. 1998;55:121–30. doi: 10.1016/s0168-3659(98)00042-x. [DOI] [PubMed] [Google Scholar]
- 278.Stile RA, Burghardt WR, Healy KE. Synthesis and Characterization of Injectable Poly(N-isopropylacrylamide)-Based Hydrogels That Support Tissue Formation in Vitro. Macromolecules. 1999;32:7370–9. [Google Scholar]
- 279.Lee KY, Mooney DJ. Hydrogels for Tissue Engineering. Chem Rev. 2001;101:1869–80. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 280.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proceedings of the National Academy of Sciences. 2003;100:5413–8. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Khan F, Tare RS, Oreffo ROC, Bradley M. Versatile Biocompatible Polymer Hydrogels: Scaffolds for Cell Growth. Angewandte Chemie International Edition. 2009;48:978–82. doi: 10.1002/anie.200804096. [DOI] [PubMed] [Google Scholar]
- 282.Van Vlierberghe S, Dubruel P, Schacht E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules. 2011;12:1387–408. doi: 10.1021/bm200083n. [DOI] [PubMed] [Google Scholar]
- 283.Brandl F, Sommer F, Goepferich A. Rational design of hydrogels for tissue engineering: Impact of physical factors on cell behavior. Biomaterials. 2007;28:134–46. doi: 10.1016/j.biomaterials.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 284.Kamata H, Akagi Y, Kayasuga-Kariya Y, Chung U, Sakai T. “Nonswellable” Hydrogel Without Mechanical Hysteresis. Science. 2014;343:873–5. doi: 10.1126/science.1247811. [DOI] [PubMed] [Google Scholar]
- 285.Huang G, Wang L, Wang S, Han Y, Wu J, Zhang Q, Xu F, Lu TJ. Engineering three-dimensional cell mechanical microenvironment with hydrogels. Biofabrication. 2012;4:042001. doi: 10.1088/1758-5082/4/4/042001. [DOI] [PubMed] [Google Scholar]
- 286.Tessmar JK, Göpferich AM. Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:274–91. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 287.Tayalia P, Mooney DJ. Controlled Growth Factor Delivery for Tissue Engineering. Adv Mater. 2009;21:3269–85. doi: 10.1002/adma.200900241. [DOI] [PubMed] [Google Scholar]
- 288.Biondi M, Ungaro F, Quaglia F, Netti PA. Controlled drug delivery in tissue engineering. Adv Drug Deliv Rev. 2008;60:229–42. doi: 10.1016/j.addr.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 289.Huang G, Zhang X, Xiao Z, Zhang Q, Zhou J, Xu F, Lu TJ. Cell-encapsulating microfluidic hydrogels with enhanced mechanical stability. Soft Matter. 2012;8:10687. [Google Scholar]
- 290.Nicodemus GD, Bryant SJ. Cell Encapsulation in Biodegradable Hydrogels for Tissue Engineering Applications. Tissue Engineering Part B: Reviews. 2008;14:149–65. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Jen AC, Wake MC, Mikos AG. Review: Hydrogels for cell immobilization. Biotechnol Bioeng. 1996;50:357–64. doi: 10.1002/(SICI)1097-0290(19960520)50:4<357::AID-BIT2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 292.Nuttelman CR, Rice MA, Rydholm AE, Salinas CN, Shah DN, Anseth KS. Macromolecular Monomers for the Synthesis of Hydrogel Niches and Their Application in Cell Encapsulation and Tissue Engineering. Prog Polym Sci. 2008;33:167–79. doi: 10.1016/j.progpolymsci.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Baroli B. Hydrogels for tissue engineering and delivery of tissue-inducing substances. J Pharm Sci. 2007;96:2197–223. doi: 10.1002/jps.20873. [DOI] [PubMed] [Google Scholar]
- 294.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–14. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 295.Kaklamani G, Cheneler D, Grover LM, Adams MJ, Bowen J. Mechanical properties of alginate hydrogels manufactured using external gelation. J Mech Behav Biomed Mater. 2014;36:135–42. doi: 10.1016/j.jmbbm.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 296.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–51. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 297.Nuttelman CR, Henry SM, Anseth KS. Synthesis and characterization of photocrosslinkable, degradable poly(vinyl alcohol)-based tissue engineering scaffolds. Biomaterials. 2002;23:3617–26. doi: 10.1016/s0142-9612(02)00093-5. [DOI] [PubMed] [Google Scholar]
- 298.Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials. 2011;32:8771–82. doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Liu WF, Chen CS. Engineering biomaterials to control cell function. Materials Today. 2005;8:28–35. [Google Scholar]
- 300.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 301.Discher DE. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science. 2005;310:1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 302.Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues — state of the art and future perspectives. Journal of Biomaterials Science, Polymer Edition. 2001;12:107–24. doi: 10.1163/156856201744489. [DOI] [PubMed] [Google Scholar]
- 303.Bhattarai N, Ramay HR, Gunn J, Matsen FA, Zhang M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J Controlled Release. 2005;103:609–24. doi: 10.1016/j.jconrel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 304.Lee K, Ea Silva, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. Journal of the Royal Society Interface. 2011;8:153–70. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Chen RR, Mooney DJ. Polymeric Growth Factor Delivery Strategies for Tissue Engineering. Pharmaceutical Research. 2003;20:1103–12. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- 306.Sokolsky-Papkov M, Agashi K, Olaye A, Shakesheff K, Domb AJ. Polymer carriers for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:187–206. doi: 10.1016/j.addr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 307.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotech. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 308.Pelah A, Seemann R, Jovin TM. Reversible Cell Deformation by a Polymeric Actuator. J Am Chem Soc. 2007;129:468–9. doi: 10.1021/ja067171+. [DOI] [PubMed] [Google Scholar]
- 309.Imran AB, Seki T, Takeoka Y. Recent advances in hydrogels in terms of fast stimuli responsiveness and superior mechanical performance. Polym J. 2010;42:839–51. [Google Scholar]
- 310.Haque MA, Kurokawa T, Kamita G, Gong JP. Lamellar bilayers as reversible sacrificial bonds to toughen hydrogel: Hysteresis, self-recovery, fatigue resistance, and crack blunting. Macromolecules. 2011;44:8916–24. [Google Scholar]
- 311.Bulhholtz FL. Preparation and structure of polyacrylates. In: Brannon-Peppas L, Harland RS, editors. Absorbent Polymer Technology. New York: Elsevier; 1990. pp. 23–44. [Google Scholar]
- 312.Chen L, Dong J, Ding Y, Han W. Environmental responses of poly(N-isopropylacrylamide-co-glycidyl methacrylate derivatized dextran) hydrogels. J Appl Polym Sci. 2005;96:2435–9. [Google Scholar]
- 313.Qiu Y, Park K. Superporous IPN hydrogels having enhanced mechanical properties. AAPS PharmSciTech. 2003;4:406–12. doi: 10.1208/pt040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Yang S, Park K, Rocca JG. Semi-interpenetrating Polymer Network Superporous Hydrogels Based on Poly(3-Sulfopropyl Acrylate, Potassium Salt) and Poly(Vinyl Alcohol): Synthesis and Characterization. Journal of Bioactive and Compatible Polymers. 2004;19:81–100. [Google Scholar]
- 315.Kim D, Park K. Swelling and mechanical properties of superporous hydrogels of poly(acrylamide-co-acrylic acid)/polyethylenimine interpenetrating polymer networks. Polymer. 2004;45:189–96. doi: 10.1163/156856204322793575. [DOI] [PubMed] [Google Scholar]
- 316.Tsukeshiba H, Huang M, Na Y, Kurokawa T, Kuwabara R, Tanaka Y, Furukawa H, Osada Y, Gong JP. Effect of Polymer Entanglement on the Toughening of Double Network Hydrogels. The Journal of Physical Chemistry B. 2005;109:16304–9. doi: 10.1021/jp052419n. [DOI] [PubMed] [Google Scholar]
- 317.Gong JP, Katsuyama Y, Kurokawa T, Osada Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv Mater. 2003;15:1155–8. [Google Scholar]
- 318.Nakayama A, Kakugo A, Gong JP, Osada Y, Takai M, Erata T, Kawano S. High Mechanical Strength Double-Network Hydrogel with Bacterial Cellulose. Advanced Functional Materials. 2004;14:1124–8. [Google Scholar]
- 319.Okumura Y, Ito K. The Polyrotaxane Gel: A Topological Gel by Figure-of-Eight Cross-links. Adv Mater. 2001;13:485–7. [Google Scholar]
- 320.Ito K. Novel Cross-Linking Concept of Polymer Network: Synthesis, Structure, and Properties of Slide-Ring Gels with Freely Movable Junctions. Polym J. 2007;39:489–99. [Google Scholar]
- 321.Schexnailder P, Schmidt G. Nanocomposite polymer hydrogels. Colloid Polym Sci. 2009;287:1–11. [Google Scholar]
- 322.Philippova OE, Zaroslov YD, Khokhlov AR, Wegner G. Reinforced superabsorbent polyacrylamide hydrogels. Macromolecular Symposia. 2003;200:45–54. [Google Scholar]
- 323.Haraguchi K, Takehisa T. Nanocomposite Hydrogels: A Unique Organic-Inorganic Network Structure with Extraordinary Mechanical, Optical, and Swelling/De-swelling Properties. Adv Mater. 2002;14:1120–4. [Google Scholar]
- 324.Haraguchi K, Li H. Control of the Coil-to-Globule Transition and Ultrahigh Mechanical Properties of PNIPA in Nanocomposite Hydrogels. Angewandte Chemie International Edition. 2005;44:6500–4. doi: 10.1002/anie.200502004. [DOI] [PubMed] [Google Scholar]
- 325.Haraguchi K, Farnworth R, Ohbayashi A, Takehisa T. Compositional Effects on Mechanical Properties of Nanocomposite Hydrogels Composed of Poly(N,N-dimethylacrylamide) and Clay. Macromolecules. 2003;36:5732–41. [Google Scholar]
- 326.Haraguchi K, Takehisa T, Fan S. Effects of Clay Content on the Properties of Nanocomposite Hydrogels Composed of Poly(N-isopropylacrylamide) and Clay. Macromolecules. 2002;35:10162–71. [Google Scholar]
- 327.Saric M, Dietsch H, Schurtenberger P. In situ polymerisation as a route towards transparent nanocomposites: Time-resolved light and neutron scattering experiments. Colloids Surf Physicochem Eng Aspects. 2006;291:110–6. [Google Scholar]
- 328.Tan Y, Xu K, Wang P, Li W, Sun S, Dong L. High mechanical strength and rapid response rate of poly(N-isopropyl acrylamide) hydrogel crosslinked by starch-based nanospheres. Soft Matter. 2010;6:1467. [Google Scholar]
- 329.Huang T, Xu HG, Jiao KX, Zhu LP, Brown HR, Wang HL. A Novel Hydrogel with High Mechanical Strength: A Macromolecular Microsphere Composite Hydrogel. Adv Mater. 2007;19:1622–6. [Google Scholar]
- 330.Cai T, Wang G, Thompson S, Marquez M, Hu Z. Photonic Hydrogels with Poly(ethylene glycol) Derivative Colloidal Spheres as Building Blocks. Macromolecules. 2008;41:9508–12. [Google Scholar]
- 331.Morimoto N, Ohki T, Kurita K, Akiyoshi K. Thermo-Responsive Hydrogels with Nanodomains: Rapid Shrinking of a Nanogel-Crosslinking Hydrogel of Poly(N-isopropyl acrylamide) Macromolecular Rapid Communications. 2008;29:672–6. [Google Scholar]
- 332.Haque MA, Kamita G, Kurokawa T, Tsujii K, Gong JP. Unidirectional alignment of lamellar bilayer in hydrogel: One-dimensional swelling, anisotropic modulus, and stress/strain tunable structural color. Adv Mater. 2010;22:5110–4. doi: 10.1002/adma.201002509. [DOI] [PubMed] [Google Scholar]
- 333.Sun J, Zhao X, Illeperuma WRK, Chaudhuri O, Oh KH, Mooney DJ, Vlassak JJ, Suo Z. Highly stretchable and tough hydrogels. Nature. 2012;489:133–6. doi: 10.1038/nature11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Bae YH, Okano T, Hsu R, Kim SW. Thermo-sensitive polymers as on-off switches for drug release. Die Makromolekulare Chemie, Rapid Communications. 1987;8:481–5. [Google Scholar]
- 335.Matsuo ES, Tanaka T. Kinetics of discontinuous volume--phase transition of gels. J Chem Phys. 1988;89:1695–703. [Google Scholar]
- 336.Zhang X, Yang Y, Chung T. Effect of Mixed Solvents on Characteristics of Poly(N-isopropylacrylamide) Gels. Langmuir. 2002;18:2538–42. [Google Scholar]
- 337.Kamenjicki M, Lednev IK, Mikhonin A, Kesavamoorthy R, Asher SA. Photochemically controlled photonic crystals. Adv Funct Mater. 2003;13:774–80. [Google Scholar]
- 338.Reese CE, Mikhonin AV, Kamenjicki M, Tikhonov A, Asher SA. Nanogel Nanosecond Photonic Crystal Optical Switching. J Am Chem Soc. 2004;126:1493–6. doi: 10.1021/ja037118a. [DOI] [PubMed] [Google Scholar]
- 339.Tokarev I, Minko S. Stimuli-responsive hydrogel thin films. Soft Matter. 2009;5:511–24. [Google Scholar]
- 340.Kuckling D. Responsive hydrogel layers-from synthesis to applications. Colloid Polym Sci. 2009;287:881–91. [Google Scholar]
- 341.Stuart MAC, Huck WTS, Genzer J, Muller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S. Emerging applications of stimuli-responsive polymer materials. Nat Mater. 2010;9:101–13. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- 342.Sharp JS, Jones RAL. Micro-buckling as a Route Towards Surface Patterning. Adv Mater. 2002;14:799–802. [Google Scholar]
- 343.Cheng S, Zhang J, Zhuo R. Macroporous poly(N-isopropylacrylamide) hydrogels with fast response rates and improved protein release properties. J Biomed Mater Res A. 2003;67A:96–103. doi: 10.1002/jbm.a.10062. [DOI] [PubMed] [Google Scholar]
- 344.Wu XS, Hoffman AS, Yager P. Synthesis and characterization of thermally reversible macroporous poly(N-isopropylacrylamide) hydrogels. Journal of Polymer Science, Part A: Polymer Chemistry. 1992;30:2121–9. [Google Scholar]
- 345.Zhang J, Cheng S, Huang S, Zhuo R. Temperature-Sensitive Poly(N-isopropylacrylamide) Hydrogels with Macroporous Structure and Fast Response Rate. Macromolecular Rapid Communications. 2003;24:447–51. [Google Scholar]
- 346.Zhang X, Yang Y, Chung T, Ma K. Preparation and Characterization of Fast Response Macroporous Poly(N-isopropylacrylamide) Hydrogels. Langmuir. 2001;17:6094–9. [Google Scholar]
- 347.Caykara T, Kucuktepe S, Turan E. Swelling characteristics of thermo- sensitive poly[(2-diethylaminoethyl methacrylate)-co-(N,N-dimethylacrylamide)] porous hydrogels. Polym Int. 2007;56:532–7. [Google Scholar]
- 348.Zhao B, Moore JS. Fast pH- and Ionic Strength-Responsive Hydrogels in Microchannels. Langmuir. 2001;17:4758–63. [Google Scholar]
- 349.Zhao Q, Sun J, Zhou Q. Synthesis of macroporous poly(N-isopropylacrylamide) hydrogel with ultrarapid swelling-deswelling properties. J Appl Polym Sci. 2007;104:4080–7. [Google Scholar]
- 350.Yan Q, Hoffman AS. Synthesis of macroporous hydrogels with rapid swelling and deswelling properties for delivery of macromolecules. Polymer. 1995;36:887–9. [Google Scholar]
- 351.Xue W, Hamley IW, Huglin MB. Rapid swelling and deswelling of thermoreversible hydrophobically modified poly(N-isopropylacrylamide) hydrogels prepared by freezing polymerisation. Polymer. 2002;43:5181–6. [Google Scholar]
- 352.Zhang X, Zhuo R. Preparation of fast responsive, temperature-sensitive poly(N-isopropylacrylamide) hydrogel. Macromolecular Chemistry and Physics. 1999;200:2602–5. [Google Scholar]
- 353.Serizawa T, Wakita K, Akashi M. Rapid Deswelling of Porous Poly(N-isopropylacrylamide) Hydrogels Prepared by Incorporation of Silica Particles. Macromolecules. 2002;35:10–2. [Google Scholar]
- 354.Yoshida R, Uchida K, Kaneko Y, Sakai K, Kikuchi A, Sakurai Y, Okano T. Comb-type grafted hydrogels with rapid deswelling response to temperature changes. Nature. 1995;374:240–2. [Google Scholar]
- 355.Annaka M, Tanaka C, Nakahira T, Sugiyama M, Aoyagi T, Okano T. Fluorescence Study on the Swelling Behavior of Comb-Type Grafted Poly(N-isopropylacrylamide) Hydrogels. Macromolecules. 2002;35:8173–9. [Google Scholar]
- 356.Kamata H, Chung U, Shibayama M, Sakai T. Anomalous volume phase transition in a polymer gel with alternative hydrophilic–amphiphilic sequence. Soft Matter. 2012;8:6876. [Google Scholar]
- 357.Kaneko Y, Nakamura S, Sakai K, Aoyagi T, Kikuchi A, Sakurai Y, Okano T. Rapid Deswelling Response of Poly(N-isopropylacrylamide) Hydrogels by the Formation of Water Release Channels Using Poly(ethylene oxide) Graft Chains. Macromolecules. 1998;31:6099–105. [Google Scholar]
- 358.Zhang J, Cheng S, Zhuo R. Poly(vinyl alcohol)/poly(N-isopropylacrylamide) semi-interpenetrating polymer network hydrogels with rapid response to temperature changes. Colloid & Polymer Science. 2003;281:580–3. [Google Scholar]
- 359.Flory PJ. Theory of Elastic Mechanisms in Fibrous Proteins. J Am Chem Soc. 1956;78:5222–35. [Google Scholar]
- 360.Huck WTS. Responsive polymers for nanoscale actuation. Materials Today. 2008;11:24–32. [Google Scholar]
- 361.Byun M, Santangelo CD, Hayward RC. Swelling-driven rolling and anisotropic expansion of striped gel sheets. Soft Matter. 2013;9:8264. [Google Scholar]
- 362.Wu J, Lin Y, Sun J. Anisotropic volume change of poly(N-isopropylacrylamide)-based hydrogels with an aligned dual-network microstructure. Journal of Materials Chemistry. 2012;22:17449. [Google Scholar]
- 363.Homma M, Seida Y, Nakano Y. Evaluation of optimum condition for designing high-performance electro-driven polymer hydrogel systems. J Appl Polym Sci. 2000;75:111. [Google Scholar]
- 364.Zrinyi M, Szabo D, Barsi L. Magnetic Field Sensitive Polymeric Actuators. J Intell Mater Syst Struct. 1998;9:667–71. [Google Scholar]
- 365.Zrínyi M, Barsi L, Büki A. Deformation of ferrogels induced by nonuniform magnetic fields. J Chem Phys. 1996;104:8750–6. [Google Scholar]
- 366.Zrinyi M, Barsi L, Büki A. Ferrogel: a new magneto-controlled elastic medium. Polym Gels Networks. 1997;5:415–27. [Google Scholar]
- 367.Bednarek S. Magnetoelastic properties of a ferroelast within an organo-silicon polymer matrix. J Magn Magn Mater. 1997;166:91–6. [Google Scholar]
- 368.Varga Z, Filipcsei G, Zrínyi M. Smart composites with controlled anisotropy. Polymer. 2005;46:7779–87. [Google Scholar]
- 369.Kim YS, Liu M, Ishida Y, Ebina Y, Osada M, Sasaki T, Hikima T, Takata M, Aida T. Thermoresponsive actuation enabled by permittivity switching in an electrostatically anisotropic hydrogel. 2015:1–6. doi: 10.1038/nmat4363. [DOI] [PubMed] [Google Scholar]
- 370.Yue YF, Haque MA, Kurokawa T, Nakajima T, Gong JP. Lamellar Hydrogels with High Toughness and Ternary Tunable Photonic Stop-Band. Adv Mater. 2013;25:3106–10. doi: 10.1002/adma.201300775. [DOI] [PubMed] [Google Scholar]
- 371.Tadao S, Takashi N. Preparation and Application of High-Performance Superabsorbent Polymers. In: Buchholz FL, Peppas NA, editors. Superabsorbent Polymers. Washington: American Chemical Society; 1994. pp. 112–27. [Google Scholar]
- 372.Dzenis Y. Spinning Continuous Fibers for Nanotechnology. Science. 2004;304:1917–9. doi: 10.1126/science.1099074. [DOI] [PubMed] [Google Scholar]
- 373.Burger C, Hsiao BS, Chu B. Nanofibrous materials and their applications. Annual Review of Materials Research. 2006;36:333–68. [Google Scholar]
- 374.Jahan KI, Goponenko A, Dzenis YA. Electrospun nanofibrous materials as stimuli-responsive polymerized hydrogels. PolyChar 23 Symposia; 2015; p. 68. [Google Scholar]
- 375.Papkov D, Zou Y, Andalib MN, Goponenko A, Cheng SZD, Dzenis YA. Simultaneously strong and tough ultrafine continuous nanofibers. ACS Nano. 2013;7:3324–31. doi: 10.1021/nn400028p. [DOI] [PubMed] [Google Scholar]
- 376.Beese AM, Papkov D, Li S, Dzenis YA, Espinosa HD. In situ transmission electron microscope tensile testing reveals structure-property relationships in carbon nanofibers. Carbon. 2013;60:246–53. [Google Scholar]
- 377.Liu Y, Dzenis Y. Explicit 3D finite element model of continuous nanofiber networks. Submitted 2016. [Google Scholar]
- 378.Jin Xing, Hsieh You-Lo. Anisotropic Dimensional Swelling of Membranes of Ultrafine Hydrogel Fibers. Macromol Chem Phys. 2005;206:1745–51. [Google Scholar]
- 379.Acharya M, Arumugam GK, Heiden PA. Dual Electric Field Induced Alignment of Electrospun Nanofibers. Macromolecular Materials and Engineering. 2008;293:666–74. [Google Scholar]
- 380.Wang B, Li B, Xiong J, Li CY. Hierarchically Ordered Polymer Nanofibers via Electrospinning and Controlled Polymer Crystallization. Macromolecules. 2008;41:9516–9521. [Google Scholar]
- 381.Dong H, Wang D, Sun G, Hinestroza JP. Assembly of Metal Nanoparticles on Electrospun Nylon 6 Nanofibers by Control of Interfacial Hydrogen-Bonding Interactions. Chemistry of Materials. 2008;20:6627–6632. [Google Scholar]
- 382.Chen S, Sun G. High Sensitivity Ammonia Sensor Using a Hierarchical Polyaniline/Poly(ethylene-co-glycidyl methacrylate) Nanofibrous Composite Membrane. ACS Applied Materials & Interfaces. 2013;5:6473–6477. doi: 10.1021/am402217s. [DOI] [PubMed] [Google Scholar]
- 383.Huang C, Soenen SJ, Rejman J, Lucas B, Braeckmans K, Demeester J, De Smedt SC. Stimuli-responsive electrospun fibers and their applications. Chem Soc Rev. 2011;40:2417–34. doi: 10.1039/c0cs00181c. [DOI] [PubMed] [Google Scholar]
- 384.Wan F, Tang Z, He W, Chu B. A chemistry/physics pathway with nanofibrous scaffolds for gene delivery. Phys Chem Chem Phys. 2010;12:12379–89. doi: 10.1039/c002515a. [DOI] [PubMed] [Google Scholar]
- 385.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–98. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Ji W, Sun Y, Yang F, van den Beucken JJ, Fan M, Chen Z, Jansen JA. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm Res. 2011;28:1259–72. doi: 10.1007/s11095-010-0320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197–211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 388.Cao S, Hu B, Liu H. Synthesis of pH-responsive crosslinked poly[styrene-co-(maleic sodium anhydride)] and cellulose composite hydrogel nanofibers by electrospinning. Polym Int. 2009;58:545–51. [Google Scholar]
- 389.Chen H, Hsieh Y. Ultrafine hydrogel fibers with dual temperature- and pH-responsive swelling behaviors. Journal of Polymer Science Part A: Polymer Chemistry. 2004;42:6331–9. [Google Scholar]
- 390.Singamaneni S, McConney ME, Tsukruk VV. Swelling-induced folding in confined nanoscale responsive polymer gels. ACS Nano. 2010;4:2327–37. doi: 10.1021/nn901886y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.