Abstract
Serotonin (5-HT) stimulated the release of arachidonic acid in hippocampal neurons cocultured with glial cells but not in glial cultures alone. Similar results were observed for the 5-HT-stimulated release of inositol phosphates. These results suggest a neural but not glial origin of both responses. Pharmacological studies suggested that release of arachidonic acid and inositol phosphates was mediated by a type 2 5-HT (5-HT2) receptor. 5-HT-stimulated release of arachidonic acid was also detected in cortical neurons, which contain high levels of 5-HT2 receptors, but not striatum, spinal cord, or cerebellar granule cells, which have very low levels or are devoid of 5-HT2 receptors. The phorbol ester phorbol 12-myristate 13-acetate augmented the 5-HT-stimulated release of arachidonic acid but inhibited the 5-HT-stimulated release of inositol phosphates. 5-HT-stimulated release of arachidonic acid, but not inositol phosphates, was dependent on extracellular calcium. 5-HT stimulated the release of [3H]lysophosphatidylcholine from [3H]choline-labeled cells with no increase in the release of [3H]choline or phospho[3H]choline. These data suggest that 5-HT stimulated the release of arachidonic acid in hippocampal neurons through the activation of phospholipase A2, independent of the activation of phospholipase C.
Full text
PDF
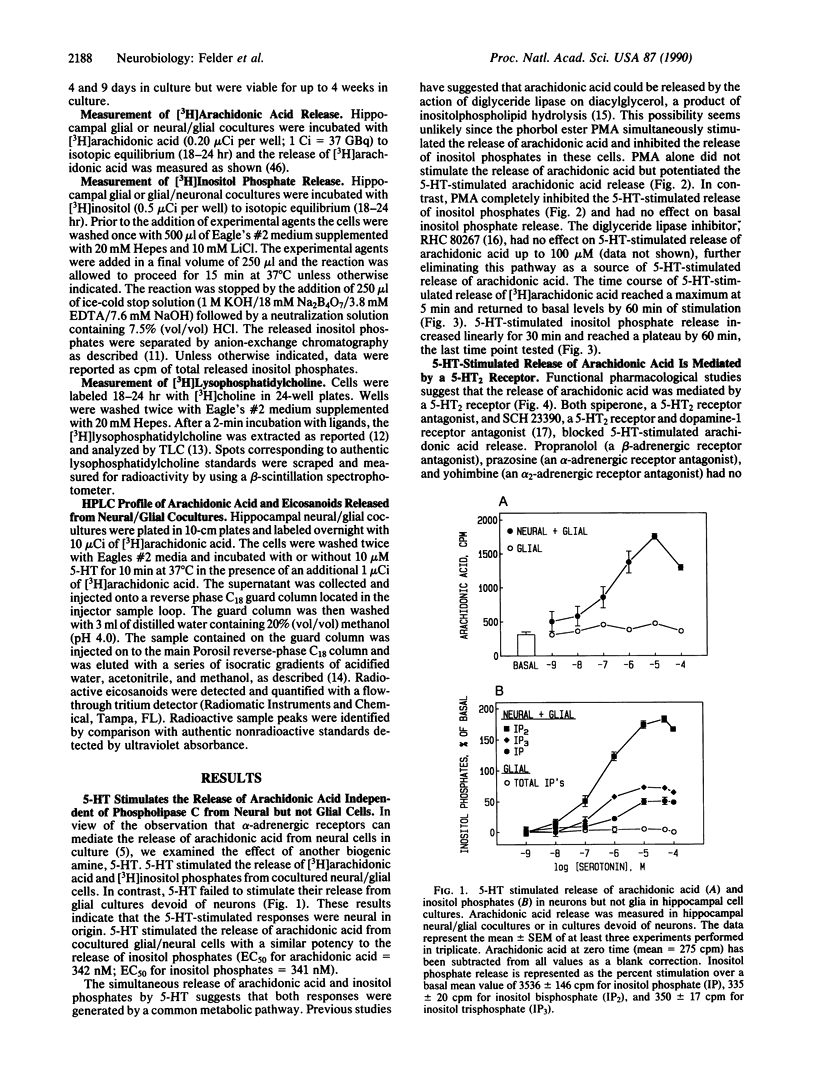
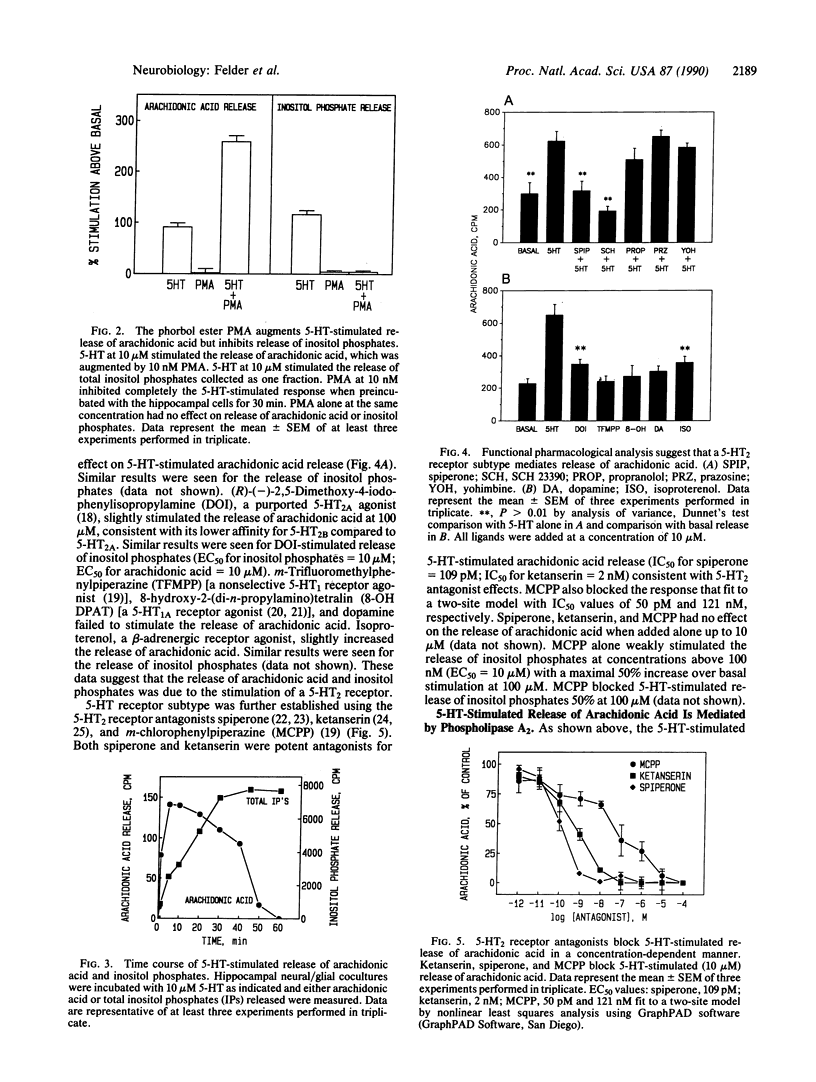
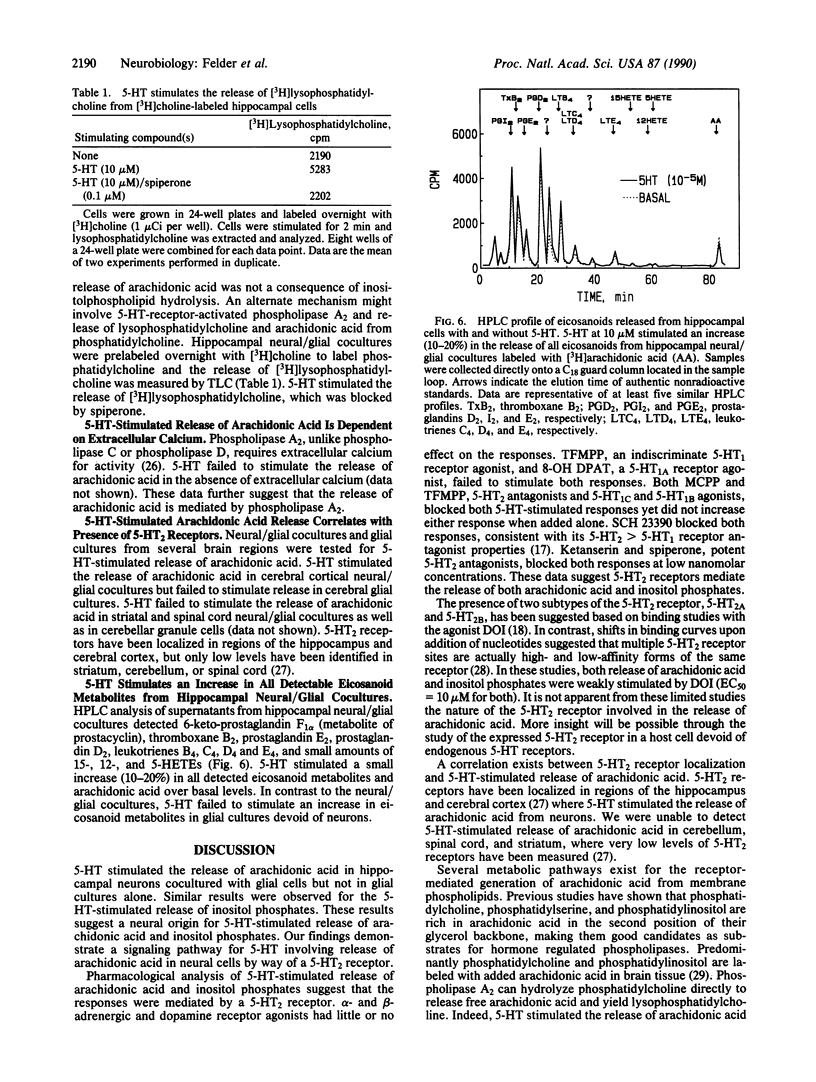

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidsson L. E., Hacksell U., Nilsson J. L., Hjorth S., Carlsson A., Lindberg P., Sanchez D., Wikstrom H. 8-Hydroxy-2-(di-n-propylamino)tetralin, a new centrally acting 5-hydroxytryptamine receptor agonist. J Med Chem. 1981 Aug;24(8):921–923. doi: 10.1021/jm00140a002. [DOI] [PubMed] [Google Scholar]
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerve K. S., Daae L. N., Bremer J. The selective loss of lysophospholipids in some commonly used lipid-extraction procedures. Anal Biochem. 1974 Mar;58(1):238–245. doi: 10.1016/0003-2697(74)90463-1. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6374–6378. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M. G protein regulation of phospholipase A2. Mol Neurobiol. 1989 Fall;3(3):155–171. doi: 10.1007/BF02935629. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin B. R., Brann M. R., Buckley N. J., Ma A. L., Bonner T. I., Axelrod J. Stimulation of arachidonic acid release and inhibition of mitogenesis by cloned genes for muscarinic receptor subtypes stably expressed in A9 L cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8698–8702. doi: 10.1073/pnas.85.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn P. J., Sanders-Bush E. Relative efficacies of piperazines at the phosphoinositide hydrolysis-linked serotonergic (5-HT-2 and 5-HT-1c) receptors. J Pharmacol Exp Ther. 1987 Aug;242(2):552–557. [PubMed] [Google Scholar]
- Coughlin S. R., Moskowitz M. A., Antoniades H. N., Levine L. Serotonin receptor-mediated stimulation of bovine smooth muscle cell prostacyclin synthesis and its modulation by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7134–7138. doi: 10.1073/pnas.78.11.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGeorge J. J., Noronha J. G., Bell J., Robinson P., Rapoport S. I. Intravenous injection of [1-14C]arachidonate to examine regional brain lipid metabolism in unanesthetized rats. J Neurosci Res. 1989 Nov;24(3):413–423. doi: 10.1002/jnr.490240311. [DOI] [PubMed] [Google Scholar]
- Dumuis A., Sebben M., Haynes L., Pin J. P., Bockaert J. NMDA receptors activate the arachidonic acid cascade system in striatal neurons. Nature. 1988 Nov 3;336(6194):68–70. doi: 10.1038/336068a0. [DOI] [PubMed] [Google Scholar]
- Gammon C. M., Allen A. C., Morell P. Bradykinin stimulates phosphoinositide hydrolysis and mobilization of arachidonic acid in dorsal root ganglion neurons. J Neurochem. 1989 Jul;53(1):95–101. doi: 10.1111/j.1471-4159.1989.tb07299.x. [DOI] [PubMed] [Google Scholar]
- Hicks P. E., Schoemaker H., Langer S. Z. 5HT-receptor antagonist properties of SCH 23390 in vascular smooth muscle and brain. Eur J Pharmacol. 1984 Oct 15;105(3-4):339–342. doi: 10.1016/0014-2999(84)90628-9. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Clapham D. E. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989 Jun 9;244(4909):1174–1176. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- Korte K., Casey M. L. Phospholipid and neutral lipid separation by one-dimensional thin-layer chromatography. J Chromatogr. 1982 Oct 8;232(1):47–53. doi: 10.1016/s0378-4347(00)86006-5. [DOI] [PubMed] [Google Scholar]
- Lazarewicz J. W., Wroblewski J. T., Palmer M. E., Costa E. Activation of N-methyl-D-aspartate-sensitive glutamate receptors stimulates arachidonic acid release in primary cultures of cerebellar granule cells. Neuropharmacology. 1988 Jul;27(7):765–769. doi: 10.1016/0028-3908(88)90088-3. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Gommeren W., Van Gompel P., Wynants J., Janssen P. F., Laduron P. M. Receptor-binding properties in vitro and in vivo of ritanserin: A very potent and long acting serotonin-S2 antagonist. Mol Pharmacol. 1985 Jun;27(6):600–611. [PubMed] [Google Scholar]
- Leysen J. E., Niemegeers C. J., Van Nueten J. M., Laduron P. M. [3H]Ketanserin (R 41 468), a selective 3H-ligand for serotonin2 receptor binding sites. Binding properties, brain distribution, and functional role. Mol Pharmacol. 1982 Mar;21(2):301–314. [PubMed] [Google Scholar]
- Llinás R., McGuinness T. L., Leonard C. S., Sugimori M., Greengard P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc Natl Acad Sci U S A. 1985 May;82(9):3035–3039. doi: 10.1073/pnas.82.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon R. A., Davis K. H., Titeler M. 3H-DOB (4-bromo-2,5-dimethoxyphenylisopropylamine) labels a guanyl nucleotide-sensitive state of cortical 5-HT2 receptors. Mol Pharmacol. 1987 Feb;31(2):194–199. [PubMed] [Google Scholar]
- McKenna D. J., Peroutka S. J. Differentiation of 5-hydroxytryptamine2 receptor subtypes using 125I-R-(-)2,5-dimethoxy-4-iodo-phenylisopropylamine and 3H-ketanserin. J Neurosci. 1989 Oct;9(10):3482–3490. doi: 10.1523/JNEUROSCI.09-10-03482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemiss D. N., Fozard J. R. 8-Hydroxy-2-(di-n-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur J Pharmacol. 1983 May 20;90(1):151–153. doi: 10.1016/0014-2999(83)90230-3. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Ordway R. W., Walsh J. V., Jr, Singer J. J. Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science. 1989 Jun 9;244(4909):1176–1179. doi: 10.1126/science.2471269. [DOI] [PubMed] [Google Scholar]
- Pazos A., Cortés R., Palacios J. M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 1985 Nov 4;346(2):231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- Peters S. P., Schulman E. S., Liu M. C., Hayes E. C., Lichtenstein L. M. Separation of major prostaglandins, leukotrienes, and monoHETEs by high performance liquid chromatography. J Immunol Methods. 1983 Nov 25;64(3):335–343. doi: 10.1016/0022-1759(83)90441-6. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Shapiro E., Zipkin R., Schwartz J. H., Feinmark S. J. Formation and action of 8-hydroxy-11,12-epoxy-5,9,14-icosatrienoic acid in Aplysia: a possible second messenger in neurons. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1721–1725. doi: 10.1073/pnas.86.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D., Volterra A., Dale N., Siegelbaum S. A., Kandel E. R., Schwartz J. H., Belardetti F. Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of Aplysia sensory cells. Nature. 1987 Jul 2;328(6125):38–43. doi: 10.1038/328038a0. [DOI] [PubMed] [Google Scholar]
- Schaefer A., Komlós M., Seregi A. Effects of biogenic amines and psychotropic drugs on endogenous prostaglandin biosynthesis in the rat brain homogenates. Biochem Pharmacol. 1978 Jan 15;27(2):213–218. doi: 10.1016/0006-2952(78)90303-9. [DOI] [PubMed] [Google Scholar]
- Schmidt A. W., Peroutka S. J. 5-Hydroxytryptamine receptor "families". FASEB J. 1989 Sep;3(11):2242–2249. doi: 10.1096/fasebj.3.11.2673898. [DOI] [PubMed] [Google Scholar]
- Segal M. Rat hippocampal neurons in culture: responses to electrical and chemical stimuli. J Neurophysiol. 1983 Dec;50(6):1249–1264. doi: 10.1152/jn.1983.50.6.1249. [DOI] [PubMed] [Google Scholar]
- Sutherland C. A., Amin D. Relative activities of rat and dog platelet phospholipase A2 and diglyceride lipase. Selective inhibition of diglyceride lipase by RHC 80267. J Biol Chem. 1982 Dec 10;257(23):14006–14010. [PubMed] [Google Scholar]
- Taki T., Kanfer J. N. Partial purification and properties of a rat brain phospholipase. J Biol Chem. 1979 Oct 10;254(19):9761–9765. [PubMed] [Google Scholar]
- Wolf B. A., Turk J., Sherman W. R., McDaniel M. L. Intracellular Ca2+ mobilization by arachidonic acid. Comparison with myo-inositol 1,4,5-trisphosphate in isolated pancreatic islets. J Biol Chem. 1986 Mar 15;261(8):3501–3511. [PubMed] [Google Scholar]
- Wolf R. A., Gross R. W. Identification of neutral active phospholipase C which hydrolyzes choline glycerophospholipids and plasmalogen selective phospholipase A2 in canine myocardium. J Biol Chem. 1985 Jun 25;260(12):7295–7303. [PubMed] [Google Scholar]
- Wolfe L. S. Eicosanoids: prostaglandins, thromboxanes, leukotrienes, and other derivatives of carbon-20 unsaturated fatty acids. J Neurochem. 1982 Jan;38(1):1–14. doi: 10.1111/j.1471-4159.1982.tb10847.x. [DOI] [PubMed] [Google Scholar]


