Abstract
High altitude flight in rarefied, extremely cold and hypoxic air is a very challenging activity. Only a few species of birds can achieve it. Hitherto, the structure of the lungs of such birds has not been studied. This is because of the rarity of such species and the challenges of preparing well-fixed lung tissue. Here, it was posited that in addition to the now proven physiological adaptations, high altitude flying birds will also have acquired pulmonary structural adaptations that enable them to obtain the large amounts of oxygen (O2) needed for flight at high elevation, an environment where O2 levels are very low. The Andean goose (Chloephaga melanoptera) normally resides at altitudes above 3000 meters and flies to elevations as high as 6000 meters where O2 becomes limiting. In this study, its lung was morphologically- and morphometrically investigated. It was found that structurally the lungs are exceptionally specialized for gas exchange. Atypically, the infundibulae are well-vascularized. The mass-specific volume of the lung (42.8 cm3.kg-1), the mass-specific respiratory surface area of the blood-gas (tissue) barrier (96.5 cm2.g-1) and the mass-specific volume of the pulmonary capillary blood (7.44 cm3.kg-1) were some of the highest values so far reported in birds. The pulmonary structural specializations have generated a mass-specific total (overall) pulmonary morphometric diffusing capacity of the lung for oxygen (DLo2) of 0.119 mlO2.sec-1.mbar-1.kg-1, a value that is among some of the highest ones in birds that have been studied. The adaptations of the lung of the Andean goose possibly produce the high O2 conductance needed to live and fly at high altitude.
Introduction
Biologists have long strived to understand how differences in the functional designs of the vertebrate respiratory systems correlate with metabolism, lifestyle and environment inhabited [1–4]. The adaptations that support avian flight at extreme altitude have been particularly perplexing [5–12]. Energetically, powered (active) flight is the most costly mode of locomotion [13–16]. It exacts various physiological and morphological adaptations [17]. Birds that fly at extreme altitude, the so-called ‘super birds’ [18], operate under very low ambient temperature and dry, rarefied and hypoxic air [19, 20]. To avoid the challenges presented by flight at high elevation, lowland resident birds that cannot tolerate extreme hypoxia [5] cover long distances to avoid flying over high obstacles [21].
The respiratory physiology of birds that fly at high altitude differs profoundly from that of low level flying ones [6]. For example, in the bar-headed goose (Anser indicus) [11, 12, 19], the Andean goose (Chloephaga melanoptera) [22, 23], the Tibetan chicken (Gallus gallus) [24] and the Ruppell’s griffon vulture (Gyps rueppellii) [25], mutations of hemoglobin have generated molecular configurations that increase O2 affinity [23–28]. Bar-headed geese in particular have evolved increased capacity of transporting O2 along the path from the atmosphere to the mitochondria [19]. Recently, it was shown that Andean geese, lifelong residents of the Andes, have evolved a different strategy of matching O2 supply to demand in hypoxic environments: while bar-headed geese match O2 supply and demand during rest in low O2 environments with large increases in ventilation [6, 28], the Andean geese exhibit very modest ventilatory response and instead greatly increase the amount of O2 extracted from each breath [29]. The latter suggests that Andean geese possess pulmonary structural adaptations that increase the capacity for O2 acquisition.
While the unique functional design of the avian respiratory system [30–39] undoubtedly increases O2 uptake efficiency, details on the structure of the lungs of the elite high-altitude flying birds are totally lacking. This is because of rarity of such species and the challenges posed in obtaining adequately fixed lung tissues. Of the known 63 species and 15 orders of volant and non-volant bird species on which pulmonary morphometric data exist [33, 36, 37, 39], none of them is an extreme high-altitude flyer. Here, we examined the lungs of the Andean goose, a large bird that lives at altitude between 3,000 and 6,000 meters of the Andes [40] to determine possible morphological specializations that may support its lifestyle.
The abbreviations used in the text are defined in Table 1 and the morphometric values are given as mean ±SD.
Table 1. Definition of abbreviations.
| AC | Air capillary |
| BC | Blood capillary |
| BGB | Blood-gas (tissue) barrier |
| Deo2 | Morphometric diffusing capacity of the erythrocyte |
| DLo2 | Total (overall) morphometric pulmonary diffusing capacity of the lung |
| Dmo2 | Membrane diffusing capacity of the total barrier, i.e., the blood-gas (tissue) barrier and the plasma layer |
| LP | Lung parenchyma |
| PL | Parabronchial lumen |
| S(t) | Surface area of the blood-gas (tissue) barrier |
| S(t).V(LP)-1 | Surface area of the blood-gas (tissue) barrier (S(t)) per unit volume of the lung parenchyma (V(LP)), i.e., the surface density of the respiratory surface area |
| VL | Volume of the lung |
| V(LP) | Volume of the lung parenchyma |
| V(PCB) | Volume of the pulmonary capillary blood |
| V(PCB).S(t)-1 | Volume of the pulmonary capillary blood (V(PCB)) per unit surface area of the blood-gas (tissue) barrier |
| τhb | Harmonic mean thickness of the total barrier, i.e., the distance between the respiratory surface and the surface of the erythrocyte cell membrane (the air-hemoglobin pathway) |
| τht | Harmonic mean thickness of the blood-gas (tissue) barrier |
Materials and methods
Ethical clearance, location and method of capture of the birds
Lungs were obtained from three wild Andean geese (Chloephaga melanoptera) captured and raised in San Pedro de Casta, Perú at an altitude of 3180 meters. Animals were collected under permit # 36087–2012 from the Gestión Florestral y de Fauna Sylvestre, Ministerio de Agricultura, Republica del Perú. They were two years old when sampled. All procedures were conducted according to guidelines approved by the Animal Care Committee at the University of British Columbia (A16-0019) in accordance with the Canadian Council on Animal Care.
Fixation of the lungs
The birds were weighed and then killed by intravenous injection of propofol into the tibiotarsal vein (>20 mg/kg). With the body in a supine position, the lungs and the air sacs were fixed by intratracheal instillation with 2.3% glutaraldehyde buffered with sodium cacodylate (osmolarity 350 mOsm and pH 7.4) at a pressure head of 3000 Pa (1 cm H2O = 1 mbar = 102 Pa). The top of the funnel, which was constantly topped up, was held 30 cm above the sternum of the supine bird. When it stopped flowing by gravity, the body wall was repeatedly gently squeezed to expel the air from the lung and the air sacs to achieve better penetration of the fixative. When the fixative finally stopped flowing, the trachea was ligated and the fixative left in the respiratory system for ~4 hours. Thereafter, the lungs were removed from their costovertebral attachments and immersed in fixative.
Determination of the lung volume (VL)
In the laboratory, the extrapulmonary primary bronchus was trimmed close to the hilum and the adhering fat and connective tissue elements removed before the VL was determined by the weight displacement method of Scherle [41].
Sampling of the lung and microscopic and morphometric analyses of the lung
Details on lung sampling, tissue processing for microscopy and morphometric analyses are given in Maina [42]. They are succinctly outlined below.
Sampling and analysis of the main structural components of the lung at the light microscopic level of magnification
The left lung of one of the birds was cut into five slices along the costal sulci and the slices in turn cut into halves just dorsal to the primary bronchus. The ten half-slices were processed and embedded in paraffin wax with the cranial face directed anteriorly. Sections were cut at 8 μm thickness and the first technically adequate slice stained with hematoxylin and eosin. The volume densities of the lung parenchyma (LP), the lumina of the parabronchi (tertiary bronchi) and the secondary bronchi, the blood vessels larger than blood capillaries (BCs) and the primary bronchus were determined field-by-field by point-counting using a 100-point Zeiss integrating graticule at a final magnification of x100. The absolute volumes of the structural parameters were calculated from the VL, the reference space.
Sampling and analysis of the structural components of the LP at the transmission electron microscopic level of magnification
The right lungs of the three birds were cut into five slices along the costovertebral sulci and the slices then cut into halves just dorsal to the primary bronchus. From the cranial surfaces of each of the ten half slices, 1 mm thick slices were cut and laid out flat. A transparent acetate paper with a quadratic lattice grid on which squares were numbered was then dropped onto the surface of the slice to avoid bias. Six random numbers that fell within the range of those on the part of the grid that lay on the slice were generated from a free computer software (https://www.random.org/integers) and small pieces of lung tissue taken from the areas (squares) where the numbers were located. When a random number fell on a large blood vessel or an airway (components of the lung that were analyzed at light microscopic level), an extra random number was generated until six pieces of tissue were sampled from the LP. The pieces of tissue were cut to a size of ~1 mm3 and processed for transmission electron microscopy. From the group of blocks processed from the pieces of tissue from each half slice of the lung (normally more than six in number), one block was picked at random and semithin sections cut and stained with toluidine blue. The block face was trimmed to an appropriate shape and size by removing the non-parenchymatous structures. Ultrathin sections were cut, mounted on 200-square wire-mesh coated copper grids and stained with lead citrate and counter-stained with uranyl acetate. For determination of the volume densities and the surface areas of the components of the LP, eight electron micrographs were taken from a predetermined corner (top right) of the grid (to avoid bias) from each section, at a primary magnification of x4,400. The same number of electron micrographs (80) was taken from the same areas of the grid squares at a higher primary magnification of x13,000 for the determination of the harmonic mean thickness of the blood-gas (tissue) barrier (τht) and the harmonic mean thickness of the total barrier, i.e., the distance between the respiratory surface and the erythrocyte cell membrane (τhb). The images were enlarged by a factor of x2.5 and a quadratic lattice grid superimposed on top. The volume densities of the components of the LP, namely the BCs, the air capillaries (ACs) and the structural tissue of the LP were determined by point-counting, the surface areas by intersection counting and the τhb and the τht by intercept length measurement using a logarithmic scale [43, 44]. The absolute volumes of components of the LP were calculated as the product of their volume densities and the volume of the LP, the reference space.
Morphometric modeling of the lung of the Andean goose for conductance of oxygen
After determining the relevant morphometric parameters, the membrane diffusing capacity (DMo2), the morphometric diffusing capacity of the erythrocyte (Deo2) and the total morphometric pulmonary diffusing capacity of the lung for oxygen (DLo2) were determined by applying the appropriate O2 permeation coefficient (Kto2) and the O2 uptake coefficient of the whole blood (Θo2) [43, 44]. The revised model of Weibel et al. [45] was used to determine the DMo2 and the DLo2.
Results
Morphological findings
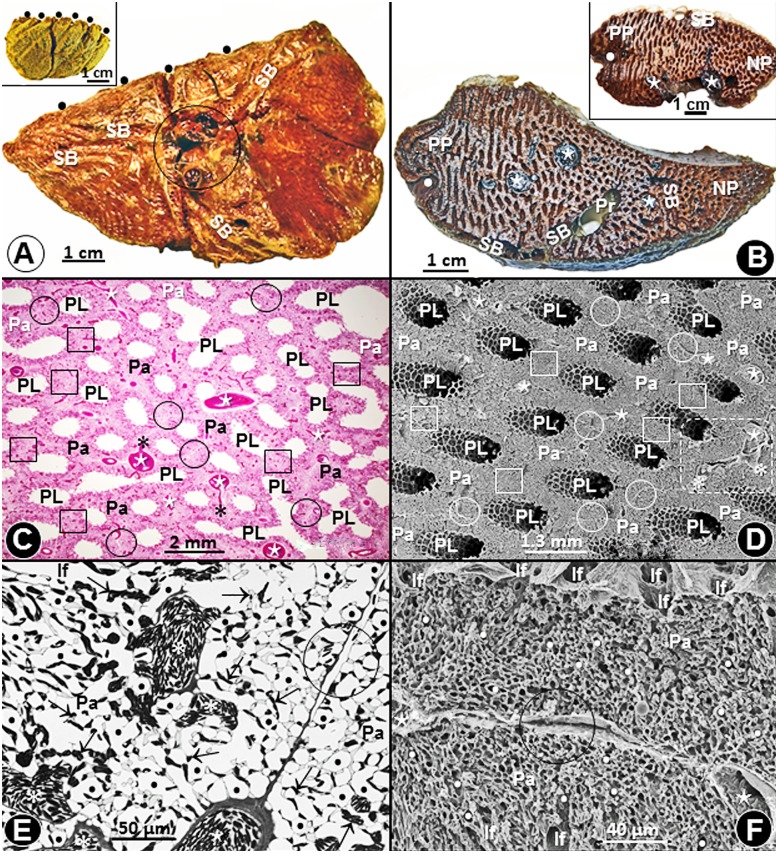
The cone-shaped lungs (Fig 1A) of the Andean goose were firmly attached to the ribs and the vertebrae across six costovertebral sulci (Fig 1A and 1B). In some parts of the lung, interparabronchial septa were present while in others they were lacking (Fig 1C–1F). Interparabronchial blood vessels gave rise to relatively smaller intraparabronchial ones (Figs 1C–1E, 2A and 2B), the atria were conspicuous (Figs 1C, 1D and 2A–2D) and the infundibulae were intensely vascularized (Fig 2C–2F). This is an uncharacteristic feature of the lungs of birds that have so far been studied where the surface of the infundibulae is nonvascular (Fig 3A) with BCs being located in the LP. Probably to optimize respiratory surface area, occasionally, single BCs surround ACs (Fig 3B). Fig 3C shows an extravasated erythrocyte that measures ~13 μm in diameter lying next to an AC and one of the cells lodged into an AC (Insert). While most of the BCs connected by means of epithelial-epithelial cell retinaculae that also separated the ACs (Fig 3D and 3E), some BCs attached directly (Fig 3D and 3E). The blood-gas (tissue) barrier (BGB) comprised an epithelial cell, an endothelial cell and a common basement membrane (Fig 3F) and was rather uniform in thickness (Fig 3D–3F).
Fig 1. Lung, parabronchi and interparabronchial septa of the lung of the Andean goose (Chloephaga melanoptera).
(A) Medial- and lateral (insert) views of the cone-shaped lung. •, costovertebral sulci; SB, secondary bronchi; circle, hilum. (B) Transverse slices of the lung showing large blood vessels (⋆), secondary bronchi (SB), a primary bronchus (Pr), paleopulmonic parabronchi (PP), neopulmonic parabronchi (NP) and a costovertebral sulcus (•). (C, D) Histological- (C) and scanning electron (D) micrographs showing parabronchial lumina (PL) that are surrounded by parenchyma (Pa). ⋆, interparabronchial blood vessels; *, intraparabronchial blood vessels; circle (O), areas where interparabronchial septa exist; square (□), areas where interparabronchial septa are missing. The area bounded by the dashed outline in D is enlarged on 2B. (E, F) Toluidine blue stained- (E) and scanning electron (F) micrographs showing interparabronchial septa (circle, O). Pa, parenchyma; ⋆, interparabronchial blood vessels; *, intraparabronchial blood vessels; •, air capillaries; ↑, blood capillaries; If, infundibulae.
Fig 2. Parabronchi, interparabronchial septa and infundibulae of the lung of the Andean goose (Chloephaga melanoptera).
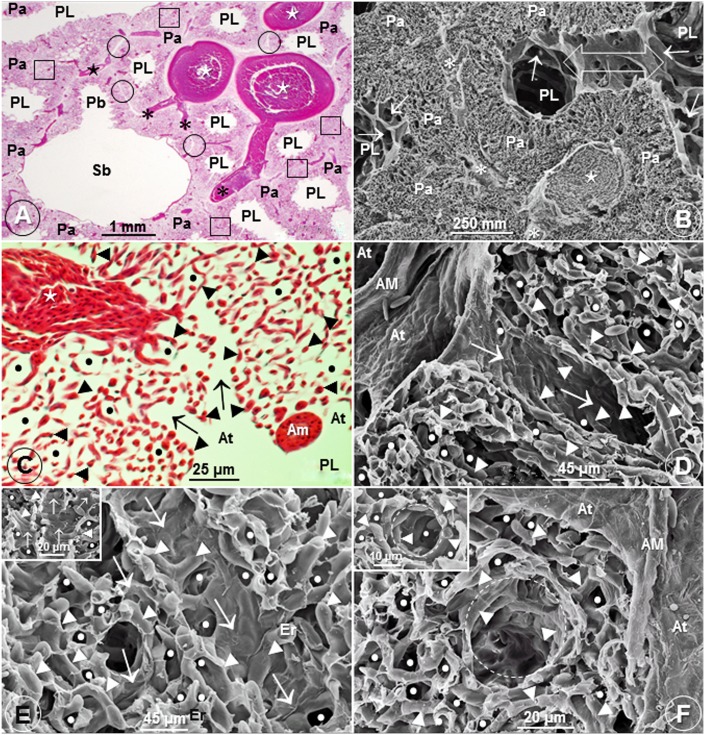
(A, B) Histological- (A) and scanning electron (B) micrographs of the lung showing a secondary bronchus (Sb) giving rise to a parabroncus (Pb) (A) and an interparabronchial artery (⋆) giving rise to intraparabronchial arteries (*) (B). PL, parabronchial lumina; Pa, parenchyma; circle (O), areas where interparabronchial septa exist; square (□), areas where interparabronchial septa are missing; arrows, atria; open double sided arrow (B), area where adjacent parabronchi anastomose. The area shown in figure B is an enlargement of the dashed enclosed area in Fig 1D. (C-F) Histological- (C) and scanning electron (Figs D-F) micrographs showing the intense vascularization of the infundibulae (↑) (C-E) and dashed circles (F). PL (C), parabronchial lumen; ⋆ (C), intraparabronchial blood vessel; AM (D, F), atrial muscle; At (D, F), atria; ►, blood capillaries; •, air capillaries; Er (E), erythrocytes.
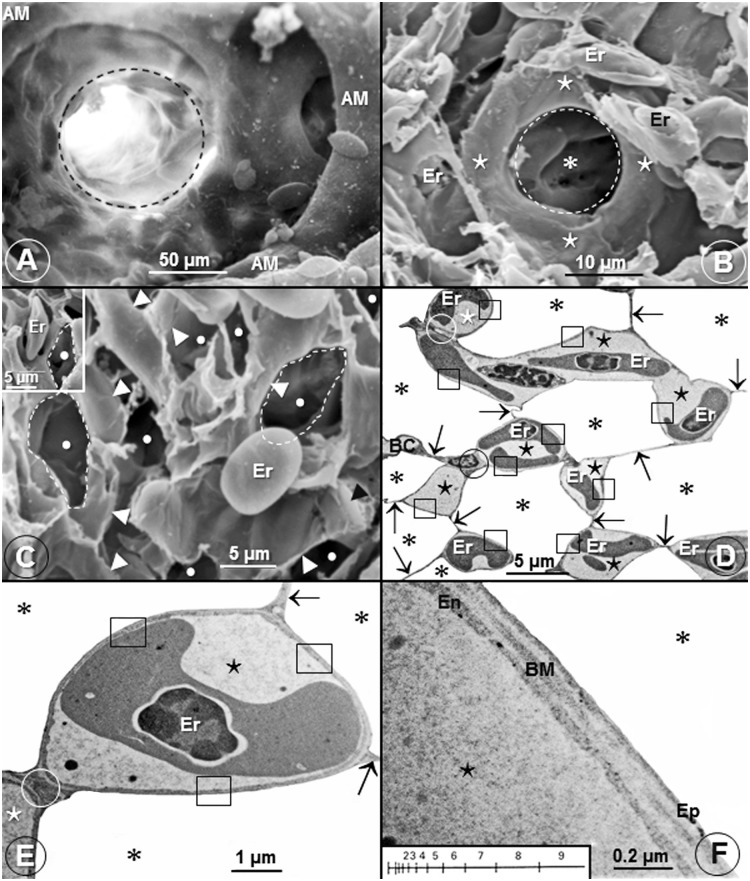
Fig 3. Infundibulae, air capillaries, blood capillaries and the blood-gas (tissue) barrier of the lung of the domestic fowl (Gallus gallus variant domesticus) and the Andean goose (Chloephaga melanoptera).
(A) A scanning electron micrograph showing an avascular infundibulum (dashed circle) of the lung of the domestic fowl. AM, atrial muscle. (B) Scanning electron micrograph of the lung of the Andean goose (Chloephaga melanoptera) showing an air capillary (* encircled by a dashed circle) which is surrounded by a single blood capillary (⋆). Er, erythrocytes. (C) Scanning electron micrograph showing lung parenchyma with extravasated erythrocytes (Er) that correspond in size (diameter) with those of the air capillaries (•). Some of the air capillaries are outlined with dashes lines. •, air capillaries; ►, blood capillaries. In the insert, an erythrocyte (Er) has slotted into an air capillary. (D, E) Low- (D) and high (E) magnification transmission electron micrographs showing air capillaries (*) and blood capillaries (⋆). In E, a blood capillary (⋆) is almost completely surrounded by air in the air capillaries (*). ↑, epithelial-epithelial cells connections; □, blood-gas (tissue) barrier; Er, erythrocytes; circle (O), blood capillary-blood capillary connection. (F) High magnification transmission electron micrograph showing the structure of the blood-gas (tissue) barrier. It [blood-gas (tissue) barrier] consists of an epithelial cell (Ep), an endothelial cell (En) and a basement membrane (BM). ⋆, blood capillary; *, air capillary. The insert shows a logarithmic scale that was used to determine the harmonic mean thickness of the blood-gas (tissue) barrier and that of the total barrier.
Morphometric findings
The mean body mass and VL of the Andean geese were respectively 2,636±144 g and 112±5.23 cm3 (S1 Table). The volume densities of the main structural components of the lung were: lung parenchyma (LP) = 61.7±7.4%; parabronchi and secondary bronchi = 25.8±3.7%; blood vessels larger than BCs = 9.5±1.4% and; primary bronchus = 3.0±5.2% (S2 Table). Concerning the structural components of the LP, the volume densities of the ACs, the BCs and the structural tissue were respectively 63.4±3.0, 28.2±2.9 and 8.4±0.13% (S3 Table). The surface area of the BGB was 2.52±0.025 m2 (S4 Table) and the τht and the τhb (S5 Table), were respectively 0.222±0.01 μm and 0.474±0.08 μm and the Deo2, the DMo2 and the DLo2 (S6 Table) were respectively 0.221±0.02, 3.87±0.89 and 0.312±0.03 mlO2.sec-1.mbar-1.
Comparison of some pulmonary morphometric parameters of the lung of the Andean goose with those of some species of birds is shown on Table 2 (S7 Table) and with those of the population of birds on which data are available (S8 Table) on Figs 4–9.
Table 2. Comparison of some pulmonary morphometric parameters of the Andean goose (Chloephaga melanoptera) with those of other species of birds.
BM, body mass; τht, harmonic mean thickness of the blood-gas (tissue) barrier; VL.BM-1, volume of the lung per unit body mass; V(PCB).BM-1, volume of the pulmonary capillary blood per unit body mass; V(PCB).S(t)-1, volume of the pulmonary capillary blood per unit surface area of the blood-gas tissue barrier; S(t).BM-1, surface area of the blood-gas (tissue) barrier per unit body mass; S(t).V(LP)-1, surface area of the blood-gas (tissue) barrier per unit volume of the lung parenchyma; DLo2.kg-1, total (overall) morphometric pulmonary diffusing capacity of the lung per unit body mass.
| Common English name/ Latin name | BM (kg) | τht (μm) | V(L).BM-1 (cm3.kg-1) | V(PCB).BM-1 cm3.kg-1 | V (PCB).S(t)-1 (cm3.m-2) | S(t).BM-1 (cm2.g-1) | S(t).V(LP)-1 (mm2.mm-3) | DLo2.kg-1 (mlO2.sec-1.mbar-1.kg-1)Θ |
|---|---|---|---|---|---|---|---|---|
| Andean Goosea (Chloephaga melanoptera) | 2.64 | 0.222 | 42.8 | 7.44 | 0.8 | 96.5 | 330 | 0.119 |
| Violet-Eared Hummingbirdb (Colibri coruscans) | 0.007 | 0.099 | 42.9 | 7.00 | 1.0 | 87.1 | 389 | -β |
| African Rock Martinc (Hirundo fuligula) | 0.014 | 0.090 | 24.1 | 5.46 | 0.7 | 86.5 | 353 | 5.284 |
| House Sparrowc (Passer domesticus) | 0.026 | 0.096 | 29.8 | 6.31 | 0.9 | 63.0 | 389 | 3.819 |
| Budgerigard (Mellopsitacus undulates) | 0.040 | 0.117 | 28.3 | 4.39 | 1.1 | 42.6 | 317 | 0.072 |
| Rock Doved (Columba livia) | 0.220 | 0.161 | 34.3 | 5.01 | 1.3 | 39.8 | 254 | 0.122 |
| Spectacled Guillemote (Larus argentatus) | 0.740 | 0.153 | 27.8 | 4.34 | 1.5 | 22.1 | 236 | 0.045 |
| Muscovy Duckf (Cairina moschata) | 1.630 | 0.199 | 30.0 | 4.31 | 1.5 | 30.0 | 200 | 0.079 |
| Domestic Fowlg (Gallus domesticus) | 2.140 | 0.318 | 12.6 | 1.63 | 1.6 | 8.7 | 172 | 0.020 |
| Graylag Goosee (Anser anser) | 3.840 | 0.118 | 30 | 3.25 | 1.4 | 23.1 | 253 | 0.059 |
| Humboldt Penguinh (Spheniscus humboldti) | 4.500 | 0.530 | 30.4 | 8.02 | 4.4 | 18.1 | 116 | 0.067 |
| Emui (Dromaius novaehollandiae) | 30.00 | 0.232 | 36.7 | 5.55 | 1.7 | 5.4 | 82 | 0.017 |
| Ostrichj (Struthio camelus) | 45.00 | 0.560 | 38.1 | 5.46 | 2.1 | 30.1 | 98.3 | 0.086 |
Sources of data:
aThis study;
bDubach [57];
cMaina [54];
fVidyadaran et al. [52];
gAbdalla et al. [49];
hMaina and King [61];
iMaina and King [55];
jMaina and Nathaniel [56].
Θ: In the lung of the Andean goose on which the revised model [45] was used, the value of the DLo2 was 31.6% greater than that determined by the older model [44]. This factor (31.6%) was used to adjust the values for the other species of birds (shown on the table) that had been calculated using the older model [43].
β: The total morphometric pulmonary diffusing capacity of the violet eared hummingbird was not reported by Dubach [57] nor were sufficient data given to allow the value to be calculated.
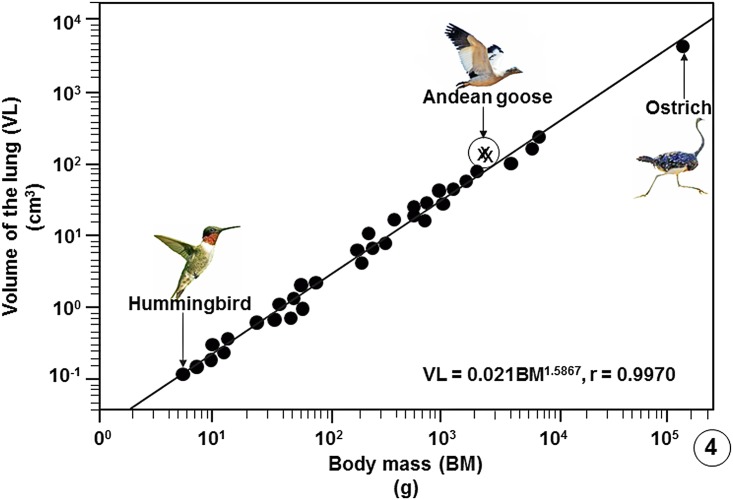
Fig 4. Regression line plotted on logarithmic x-y axes showing the correlation between the Volumes of the Lungs (VL) of birds on which data are available against Body Masses (BM).
The values of the three specimens of the Andean goose (Chloephaga melanoptera) that were investigated in this study lie above the common regression line of the bird population. The data on which the regression line was plotted are summarized in publications [33], [37], [39] and [54–56] and are given in data supporting this paper (S8 Table).
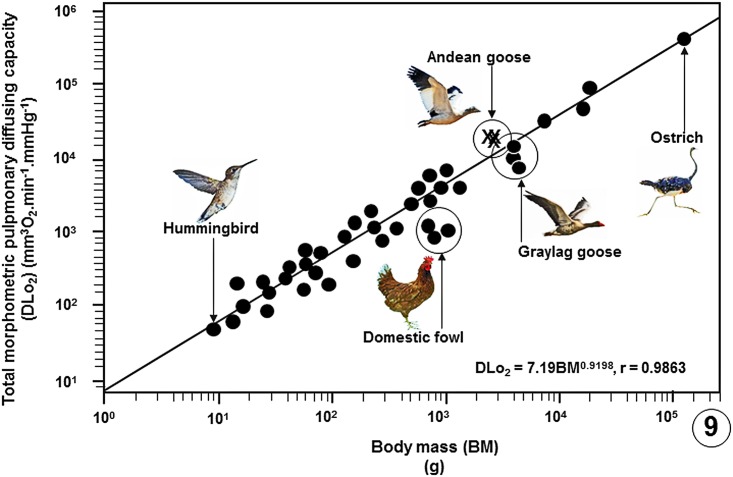
Fig 9. Regression line plotted on logarithmic x-y axes showing the correlation between the total morphometric pulmonary diffusing capacities of the lungs for oxygen (DLo2) of the birds that have been studied against Body Masses (BM).
The values of the three specimens of the Andean goose (Chloephaga melanoptera) that were investigated in this study lie above the common regression line of the bird population. The data on which the regression line was plotted are summarized in publications [33], [37], [39] and [54–56] and given in data supporting this paper (S8 Table).
Discussion
This study reports that certain morphological- and morphometric specializations occur in the lung of the Andean goose (Chloephaga melanoptera). Such features should increase the gas exchange efficiency of the lung and may explain the high pulmonary O2 extraction rates that were recently reported in this species by Lague et al. [28]. The Andean goose is one of the largest birds in the Western Hemisphere that lives at an altitude of above 3000 m in valleys and plateaus of the Andes from Peru to southern Chile. The species is non-migratory and may breed at elevations as high as 5,500 meters but rarely ever descends below 2,000 m [40].
Morphological findings
Compared with the lungs of the other species of birds that have so far been studied where the infundibulae are avascular [33, 36] (Fig 3A), in the lung of the Andean goose, the structural units are intensely vascularized (Fig 3C–3F). To an undermined extent, this feature should substantially increase the respiratory surface area and the volume of the pulmonary capillary blood (V(pcb)). Location of infundibular BCs next to the parabronchial lumen (PL) (Fig 2C–2F) results in the following features that increase respiratory efficiency: (a) the infundibular dead-space air is reduced or possibly eliminated, (b) the infundibular BCs are exposed to inspired air with the highest possible partial pressure of O2 and (c) the BCs are not affected by the process of ‘O2 screening’ [46, 47].
The significance of the sparse distribution of the interparabronchial septa in the lung of the Andean goose (Figs 1C, 1D and 2A) is unclear. Scheid [48] contended that since no air pressure differences exist between the parabronchi in the avian lung, little if any air moves between them. If the assertion is correct, the sparse distribution of the interparabronchial septa may be of no functional consequence in the lung of the Andean goose. The septa may be a phylogenetic relic carried over during the evolution of the lung. Interparabronchial septa are well-developed in the lungs of the galliform birds [49–53] while they are totally lacking in those of the passerine ones [53, 54].
Morphometric findings
In the avian lung, the mean volume density of the LP is 49% [33, 36, 39, 52–54]. The values range from 18 to 78% respectively in the lungs of the emu (Dromaius novaehollandiae) [55] to that of the ostrich (Struthio camelus) [56]. For the Andean goose, the mean value of 62% lies on the upper end of the range in birds. The highest mass-specific respiratory surface areas in birds of 87.1 and 86.5 cm2.g-1 have respectively been reported in the lungs of a 7.3 g violet-eared hummingbird (Colibri coruscans) [57] and a 13.7 g African rock martin (Hirundo fuligula) [33, 36, 37, 39, 58]. The value of 96.5 cm2.g-1 for the lung of the Andean goose is the highest so far reported in a bird (Table 2). The very high mass specific respiratory surface area of 800 cm2g-1 that was reported for an unnamed species of hummingbird by Stanislaus [59] should be treated with caution since it is not explained how the value was determined.
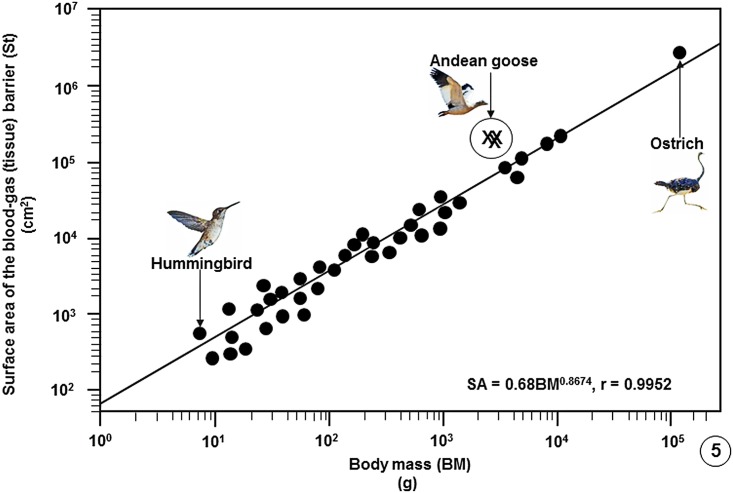
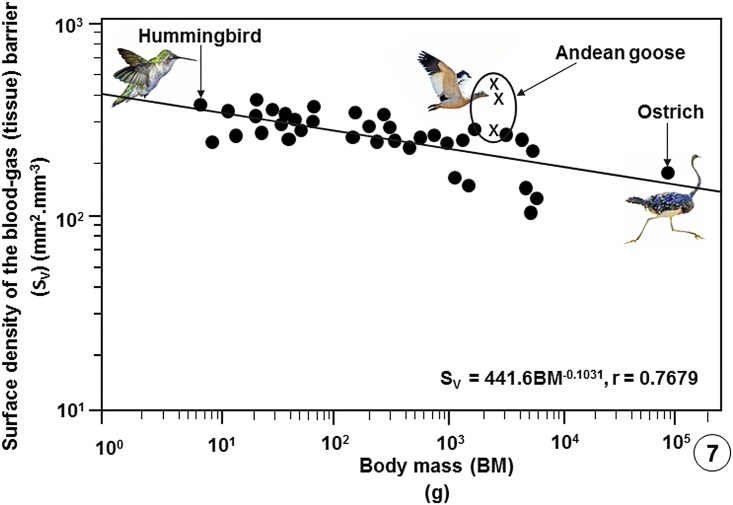
In vertebrate lungs, respiratory surface area is increased by subdivision of the LP [33, 36, 39] and/or gross enlargement of the lung [60]. Stereologically, surface area (S) per unit volume (V) is designated surface density (SV) [43]. The SV of the BGB, i.e., the surface area of the BGB per unit volume of the lung parenchyma (S(t).V(LP)-1), scales inversely with body mass [33, 36, 39]. S(t).V(LP)-1 indicates the intensity of the subdivision (compartmentalization) of the LP and therefore the relative sizes (diameters) of the terminal respiratory units in a lung. In the avian lung, the respiratory units (the ACs) range in diameter from 3 μm in the smaller species [54] to 20 μm in that of the ostrich [56]. In this study, while the diameters of the ACs were not directly determined, their sizes corresponded to those of the erythrocytes of ~13 μm (Fig 3C). The large S(t).V(LP)-1 of 330±70.6 mm2.mm-3 that exceeds the values of most birds (Table 2) shows that the very small ACs are generated by intense subdivision of the LP. In birds that have so far been investigated, a S(t).V(LP)-1 of 82 mm2.mm-3 was reported in the lung of the emu [55] and the highest ones of 389 mm2.mm-3 in those of the violet-eared hummingbird [57] and the house sparrow (Passer domesticus) [33, 36, 39]. The extreme subdivision of the LP of the avian lung explains the unexpected outcome that while the avian lung is 27% smaller compared to that of a mammal of equivalent body mass and the volume density of the LP is about one-half that of a mammalian lung [1, 33, 36, 39], the respiratory surface in a bird lung is ~15% greater.
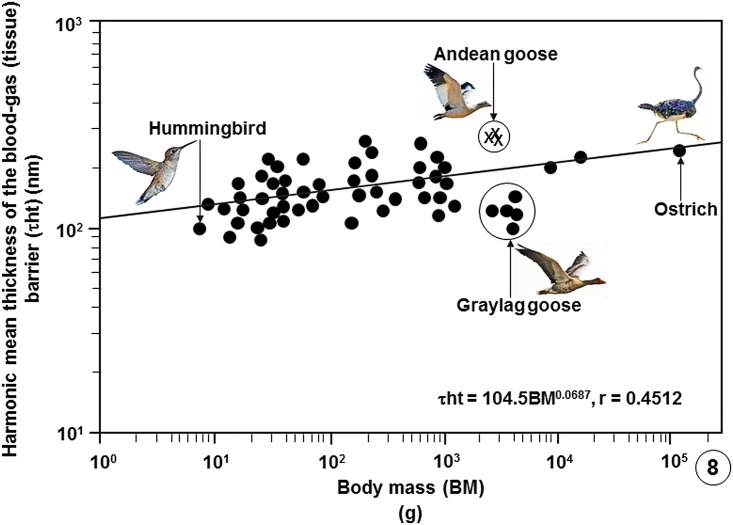
In birds, the thinnest BGBs (τht) have been reported in the lungs of the violet-eared hummingbird (0.099 μm) [57], the house sparrow (0.096 μm) and the African rock martin (0.090 μm) [33, 36, 39]. The thickest BGBs occur in the ostrich (0.56 μm) [56] and the Humboldt penguin (Spheniscus humboldti) (0.53 μm) [61] lungs. For the Andean goose, the mean τht of 0.222 μm was not relatively remarkably thin (Table 2; Fig 8). A τht of 0.118 μm was reported in the lung of a 3.84 kg body mass low altitude flying greylag goose (Anser anser) [33, 36, 37, 58]. For a bird that lives and flies in a cold and hypoxic environment where heart rate and cardiac output may need to be constantly high, the thicker BGB in the lung of the Andean goose may help avert structural failure of the BGB, a feature that has been reported to occur in the avian lungs [62, 63]. The particularly thick BGB of the lung of the Humboldt penguin [61] and the presence of plentiful connective tissue elements, especially of collagen in the BGB [64], was attributed to the capacity of the lung tolerating high hydrodynamic pressures during dives.
Fig 8. Regression line plotted on logarithmic x-y axes showing the correlation between the harmonic mean thicknesses of the blood-gas (tissue) barriers (τht) of the lungs of birds that have been studied against Body Masses (BM).
The values of the three specimens of the Andean goose (Chloephaga melanoptera) investigated in this study lie above the common regression line of the bird population. Showing that the tissue barrier of the Andean goose is not particularly thin, the tissue barrier of the relatively larger, low altitude dwelling greylag goose (Anser anser) is relatively much thinner. The data on which the regression line was plotted are summarized in publications [33], [37], [39] and [54–56] and given in data supporting this paper (S8 Table).
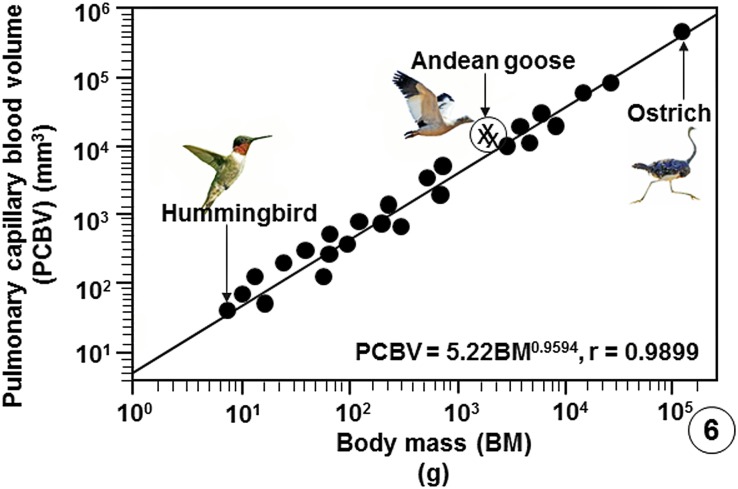
Gas exchangers, including the lungs, are characteristically intensely vascularized [65–69]. The greater the degree of vascularization the larger the volume of blood in the BCs [33, 36, 37, 39, 54]. In the African rock martin, 29% of the volume of the lung consists of blood, with 79% of it located in the BCs [33, 36, 37, 39, 54]. For the Andean goose, the volume of blood comprised 30% of the VL, with 65% of it in the BCs. Among the data available on the bird lungs, the mass-specific V(PCB) of the lung of the Andean goose (7.44 cm3.kg-1) is only surpassed by that of the diving Humboldti penguin (8.02 cm3.kg-1) [60] (Table 2). The ratio of the V(PCB) to the total respiratory surface (SA), i.e., V(PCB).S(t)-1, the so-called capillary loading [70], indicates the degree of exposure of pulmonary capillary blood to air [65, 70], with low values indicating high gas exchange efficiency. In the avian lungs that have been investigated, V(PCB).S(t)-1 ranges from 0.7 cm3.m-2 in the African rock martin [33, 36, 37, 39, 54] to 4.4 cm3.m-2 in the Humboldt penguin [55]. For the lung of the Andean goose, the particularly low value of 0.8 cm3.m-2 points out to a specialization for gas exchange.
Among the data available on the avian lungs, the mass-specific DMo2 of the lung of the Andean goose of 1.479 mlO2.sec-1.mbar-1.kg is only surpassed by that of the violet-eared hummingbird [57] while the mass-specific DLo2 (0.119 mlO2.sec-1.mbar-1.kg) is only surpassed by that of the lung of the African rock martin [33, 36, 37, 39, 54] (Table 2). Because the DLo2 integrates the diffusing capacities, i.e., the conductances, of the structural components that form the air-hemoglobin pathway [42–45], structurally, the parameter it is the most comprehensive estimator of the gas exchange capacity of a lung. The large mass-specific DLo2 of the lung of the Andean goose is consistent with and undoubtedly contributes to the high pulmonary O2 extraction rates reported for this species by Lague et al. [28].
Compared with data on lungs of species of bird that have so far been investigated, the Andean goose has relatively larger lungs (Fig 4), larger surface area of the BGB (Fig 5), larger V(PCB) (Fig 6), greater surface density of the BGB (Fig 7) and the BGB is moderately thin (Fig 8). These specializations have generated relatively larger DLo2 of the lung of the Andean goose (Fig 9).
Fig 5. Regression line plotted on logarithmic x-y axes showing the correlation between the surface areas of the blood-gas (tissue) barriers (St) of the lungs of birds on which data are available against Body Masses (BM).
The values of the three specimens of the Andean goose (Chloephaga melanoptera) that were investigated in this study lie above the regression line of the bird population. The data on which the regression line was plotted are summarized in publications [33], [37], [39] and [54–56] and are given in data supporting this paper (S8 Table).
Fig 6. Regression line plotted on logarithmic x-y axes showing the correlation between the Pulmonary Capillary Blood Volume (PCBV) of the lungs of birds that have been studied against Body Masses (BM).
The values of the three specimens of the Andean goose (Chloephaga melanoptera) that were investigated in this study lie above the regression line of the bird population. The data on which the regression line was plotted are summarized in publications [33], [37], [39] and [54–56] and are given in data supporting this paper (S8 Table).
Fig 7. Regression line plotted on logarithmic x-y axes showing the correlation between the surface densities of the blood-gas (tissue) barriers per unit volume of the lung parenchyma (SV) of the lungs of the birds that have been studied against Body Masses (BM).
The values of the three specimens of the Andean goose (Chloephaga melanoptera) investigated in this study lie above the common regression line of the bird population. The data on which the regression line was plotted are summarized in publications [33], [37], [39] and [54–56] and given in data supporting this paper (S8 Table).
Comment on the modeling of the oxygen diffusing capacity of the avian lung and future research directions
Here, the revised model of Weibel et al. [45] was used to determine the DMo2 and the DLo2 of the lung of the Andean goose. To compare the value of the DLo2 of the lung of the Andean goose with those of species of birds that had been calculated using the older model of Weibel [44], the values were adjusted by a factor of 31.6%. This was the ratio of the value of the DLo2 of the Andean goose calculated by the revised model of Weibel et al. [45] to that calculated by the older model of Weibel [44]. Because the current study is the only one where the two models have been applied and compared on the lung of a species of bird, the adjustment factor should be considered tentative. Taking into account the large structural and functional differences between the avian (parabronchial) lung and the mammalian (bronchioalveolar) one, it was considered judicious to adjust the avian DLo2 values that had been determined with the older model of Weibel [44] with an avian lung derived adjustment factor rather than by applying the adjustment factors that were derived from the mammalian lung in Weibel et al. [45]. The application of the two models on the avian lung should be assessed on lungs of a larger number of species birds.
Following this study, it will be of great interest to find out whether the pulmonary specializations that have been found in the lung of the Andean goose characterize the lungs of all high altitude flying birds or different adaptive strategies exist in the lungs of the other birds that fly to high elevations.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Garnet Martens, Bradford Ross, and Derrick Horne of the UBC Bio-Imaging Facility for assistance with preparation of the tissues; Quinton Dos Santos of the Spectrum Unit of the University of Johannesburg helped with light microscopic photography; Daniel K. Maina assisted with the processing of the digital images and; E. Bautista and L. Alza particularly assisted with the acquisition of the Andean geese.
Data Availability
The supporting data are all contained within the Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Gehr P, Mwangi DK, Ammann A, Maloiy GMO, Taylor CR, Weibel ER. Design of the mammalian respiratory system: V. Scaling morphometric diffusing capacity to body mass: wild and domestic animals. Respir Physiol. 1981; 44:61–86. [DOI] [PubMed] [Google Scholar]
- 2.Suarez RK, Darveau CA, Childress JJ. Metabolic scaling: a many-spleandoured thing. Comp Biochem Physiol B, Biochem Mol Biol. 2004; 139:531–541. 10.1016/j.cbpc.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 3.White CR, Kearney MR. Metabolic scaling in animals: methods, empirical results and theoretical explanations. Comp Physiol. 2014; 4:231–256. [DOI] [PubMed] [Google Scholar]
- 4.Gillooly JF, Gomez JP, Mavrodiev EV, Rong Y, McLamore ES. Body mass scaling of passive oxygen diffusion in endotherms and ectotherms. Proc Natl Acad Sci, USA. 2016; 113:5340–5345. 10.1073/pnas.1519617113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black CP, Tenney SM. Oxygen transport during progressive hypoxia in high altitude and sea level water-fowl. Respir Physiol. 1980; 39:217–239. [DOI] [PubMed] [Google Scholar]
- 6.Scott GR, Hawkes LA, Frappell PB, Butler PJ, Bishop CM, Milsom WK. How bar- headed geese fly over the Himalayas. Physiology. 2015; 30:107–115. 10.1152/physiol.00050.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fedde MR, Orr JA, Shams H, Scheid P. Cardiopulmonary function in exercising bar-headed geese during normoxia and hypoxia. Respir Physiol. 1989; 77:239–262. [DOI] [PubMed] [Google Scholar]
- 8.Butler PJ. High fliers: the physiology of bar-headed geese. Comp Biochem Physiol A Mol Integr Physiol. 2010; 156:325–329. 10.1016/j.cbpa.2010.01.016 [DOI] [PubMed] [Google Scholar]
- 9.Altshuler DL, Dudley R. The physiology and biomechanics of avian flight at high altitude. Integr Comp Biol. 2006; 46:4–8. [DOI] [PubMed] [Google Scholar]
- 10.Köppen U, Yakovlev A, Barth R, Kaatz M, Berthold P. Seasonal migrations of four individual bar-headed geese Anser indicus from Kyrgyzstan followed by satellite telemetry. J Ornithol. 2010; 151:703–712. [Google Scholar]
- 11.Hawkes LA, Balachandran S, Batbayar N, Butler PJ, Frappell PB, Milsom WK et al. The trans-Himalayan flights of bar-headed geese (Anser indicus). Proc Natl Acad Sci, USA. 2011; 108:9516–9519. 10.1073/pnas.1017295108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bishop CM, Spivey RJ, Hawkes LA, Batbayar N, Chua B, Frappell PB et al. The roller coaster flight strategy of bar-headed geese conserves energy during Himalayan migrations. Science. 2015; 347:250–254. 10.1126/science.1258732 [DOI] [PubMed] [Google Scholar]
- 13.Tucker VA. Energetics of natural avian flight In: Paynter RA, editor. Avian energetics. Cambridge (MA): Nuttal Ornithological Club; 1974. pp. 298–333. [Google Scholar]
- 14.Wells DJ. Muscle performance in hovering hummingbirds. J Exp Biol. 1993; 178:39–57. [Google Scholar]
- 15.Nudds RL, Bryant DM. The energy cost of short flights in birds. J Exp Biol. 2000; 203: 1561–1582. [DOI] [PubMed] [Google Scholar]
- 16.Tobalske BW, Hedrik TL, Dial KP, Biewener AA. Comparative power curves in bird flight. Nature. 2003; 421:363–366. 10.1038/nature01284 [DOI] [PubMed] [Google Scholar]
- 17.Maina JN. What it takes to fly: the novel respiratory structural and functional adaptations in birds and bats. J Exp Biol. 2000; 203:3045–3064. [DOI] [PubMed] [Google Scholar]
- 18.Whiteman L. The high life. http://audubonmagazine.org/birds/birds0011.html. 2000.
- 19.Scott GR. Elevated performance: the unique physiology of birds that fly at high altitudes. J Exp Biol. 2011; 214:2455–2462. 10.1242/jeb.052548 [DOI] [PubMed] [Google Scholar]
- 20.Altshuler DL, Dudley R. The physiology and biomechanics of avian flight at high altitude. Integr Comp Biol. 2006; 46:4–8. [DOI] [PubMed] [Google Scholar]
- 21.Irwin DE, Irwin JH. Siberian migratory divides: the role of seasonal migration in speciation In: Marra GR, editor. Birds of two worlds: the ecology and evolution of migration. Baltimore (MD): Johns Hopkins University Press; 2005. pp. 27–40. [Google Scholar]
- 22.McCracken KG, Barger CP, Sorenson MD. Phylogenetic and structural analysis of the HbA (αA/βA) and HbD (αD/βA) hemoglobin genes in two high-altitude waterfowl from the Himalayas and the Andes: bar-headed goose (Anser indicus) and Andean goose (Chloephaga melanoptera). Mol Phylogenet Evol. 2010; 56:649–658. 10.1016/j.ympev.2010.04.034 [DOI] [PubMed] [Google Scholar]
- 23.Hiebl I, Braunitzer G, Schneeganss D. The primary structures of the major and minor hemoglobin-components of adult Andean goose (Chloephaga melanoptera, Anatidae): the mutation Leu-Serin in position 55 of the beta-chains. Biol Chem Hoppe-Seyler. 1987; 368:1559–1569. [DOI] [PubMed] [Google Scholar]
- 24.Gou X, Li N, Lian L, Yan D, Zhang H, Wei Z, Wu C. Hypoxic adaptations of hemoglobin in Tibetan chick embryo: high oxygen-affinity mutation and selective expression. Comp Biochem Physiol. 2007; 147B:147–155. [DOI] [PubMed] [Google Scholar]
- 25.Weber RE, Hiebl I, Braunitzer G. High altitude and hemoglobin function in the vultures, Gyps rueppellii and Aegypius monachus. Biol Chem Hoppe Seyler. 1988; 369:233–240. [PubMed] [Google Scholar]
- 26.Jessen TH, Weber RE, Fermi G, Tame J, Braunitzer G. Adaptation of bird hemoglobins to high altitudes: demonstration of molecular mechanism by protein engineering. Proc Natl Acad Sci, USA. 1991; 88:6519–6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Hua ZQ, Tame JRH, Lu GY, Zhang RJ, Gu XC. The crystal structure of a high oxygen affinity species of hemoglobin (bar-headed goose hemoglobin in the oxy form). J Mol Biol. 1996; 255:484–493. 10.1006/jmbi.1996.0040 [DOI] [PubMed] [Google Scholar]
- 28.Scott GR, Milsom WK. Control of breathing and adaptation to high altitude in the bar-headed goose. Am J Physiol Regul Integr Comp Physiol. 2007; 293:R379–R391. 10.1152/ajpregu.00161.2007 [DOI] [PubMed] [Google Scholar]
- 29.Laguë SL, Chua B, Alza L, Scott GR, Frappell PB, Zhong Y et al. High-altitude champions: divergent strategies in birds for obtaining oxygen in extreme hypoxia. J. Exp. Biol. 2017: In press. [Google Scholar]
- 30.Duncker HR. The lung-air sac system of birds. A contribution to the functional anatomy of the respiratory apparatus. Ergeb Anat Entwicklung. 1971; 45:1–171. [PubMed] [Google Scholar]
- 31.Scheid P. Avian respiratory system and gas exchange In: Sutton JR, Coates G, Remmers JE, editors. Hypoxia: the adaptations. Burlington (Ontario): BC Decker Inc; 1990. pp 4–7. [Google Scholar]
- 32.Fedde MR. The structure and gas flow pattern in the avian lung. Poult Sci. 1980; 59:2642–2653. [DOI] [PubMed] [Google Scholar]
- 33.Maina JN. The design of the lung-air sac system of birds: development, structure, and function. Heidelberg: Springer-Verlag; 2005. [Google Scholar]
- 34.King AS. Structural and functional aspects of the avian lung and its air sacs. Intern Rev Gen Exp Zool. 1966; 2:171–267. [Google Scholar]
- 35.McLelland J. Anatomy of the lungs and air sacs In: King AS, McLelland J, editors. Form and function in birds, Vol. 4. London: Academic Press; 1989. pp. 221–279. [Google Scholar]
- 36.Maina JN. The morphometry of the avian lung In: King AS, McLelland J, editors. Form and function in birds, Vol.4. London: Academic Press; 1989. pp. 307–368. [Google Scholar]
- 37.Maina JN, West JB. Thin and strong! The bioengineering dilemma in the structural and functional design of the blood-gas barrier. Physiol Rev. 2005; 85:811–844. 10.1152/physrev.00022.2004 [DOI] [PubMed] [Google Scholar]
- 38.Maina JN. Pivotal debates and controversies on the structure and function of the avian respiratory system: setting the record straight. Biol Rev. 2016; in press. [DOI] [PubMed] [Google Scholar]
- 39.Maina JN, King AS, Settle G. An allometric study of the pulmonary morphometric parameters in birds, with mammalian comparison. Phil Trans R Soc Lond. 1989; 326B:1–57. [DOI] [PubMed] [Google Scholar]
- 40.Fjeldså J, Krabbe N. Birds of the High Andes: a manual to the birds of the temperate zone of the Andes and Patagonia, South America. Copenhagen (Denmark): Zoological Museum of the University of Copenhagen; 1990. [Google Scholar]
- 41.Scherle WF. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970; 26:57–60. [PubMed] [Google Scholar]
- 42.Maina JN. Some recent advances of the study and understanding of the functional design of the avian lung: morphological and morphometric perspectives. Biol Rev. 2002; 77:97–152. [DOI] [PubMed] [Google Scholar]
- 43.Weibel ER. Morphometry: stereological theory and practical methods In: Gill J, editor. Models of lung disease: microscopy and structural methods. New York: Marcel Dekker; 1990; pp. 199–251. [Google Scholar]
- 44.Weibel ER. Morphometric estimation of pulmonary diffusion capacity. I. Model and method. Respir Physiol. 1970/71; 11:54–75. [DOI] [PubMed] [Google Scholar]
- 45.Weibel ER, Federspiel WJ, Fryder-Doffey F, Hsia CW, König M, Stalder-Navarro L et al. Morphometric model for pulmonary diffusing capacity I. Membrane diffusing capacity. Respir Physiol. 1993; 93:125–149. [DOI] [PubMed] [Google Scholar]
- 46.Sapoval B, Filoche M, Weibel ER. Smaller is better—but not too small: a physical scale for the design of the mammalian pulmonary acinus. Proc Natl Acad Sci, USA. 2016; 99:10411–10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsia CCW, Hyde DM, Weibel ER. Lung structure and the intrinsic challenges of gas exchange. Comp Physiol. 2016; 6:827–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheid P. Mechanisms of gas exchange in bird lungs. Rev Physiol Biochem Pharmacol. 1979; 86:137–186. [DOI] [PubMed] [Google Scholar]
- 49.Abdalla MA, Maina JN, King AS, King DZ, Henry J. Morphometrics of the avian lung. 1. The domestic fowl, Gallus domesticus. Respir Physiol. 1982; 47:267–278. [DOI] [PubMed] [Google Scholar]
- 50.Abdalla MA, Maina JN. Quantitative analysis of the exchange tissue of the avian lung (Galliformes). J Anat, Lond. 1981; 134:677–680. [Google Scholar]
- 51.Maina JN. A morphometric comparison of the lungs of two species of birds of different exercise capacities. J Anat, Lond. 1982; 134:604–605. [Google Scholar]
- 52.Vidyadaran MK, King AS, Kassim H. Deficient anatomical capacity for oxygen uptake of the developing lung of the female domestic fowl when compared with red-jungle fowl. Schweischrift Arch Tiere. 1987; 129:225–237. [PubMed] [Google Scholar]
- 53.Maina JN, Abdalla MA, King AS. Light microscopic morphometry of the lungs of 19 avian species. Acta Anat. 1982; 112:264–270. [DOI] [PubMed] [Google Scholar]
- 54.Maina JN. Morphometrics of the avian lung. 3. The structural design of the passerine lung. Respir Physiol. 1984; 55:291–309. [DOI] [PubMed] [Google Scholar]
- 55.Maina JN, King AS. The lung of the emu, Dromaius novaehollandiae: a microscopic and morphometric study. J Anat, Lond. 1989; 163:67–74. [PMC free article] [PubMed] [Google Scholar]
- 56.Maina JN, Nathaniel C. A qualitative and quantitative study of the lung of an ostrich, Struthio camelus. J Exp Biol. 2001; 204:2313–2330. [DOI] [PubMed] [Google Scholar]
- 57.Dubach M. Quantitative analysis of the respiratory system of the house sparrow, budgerigar, and violet-eared hummingbird. Respir Physiol. 1981; 46:43–60. [DOI] [PubMed] [Google Scholar]
- 58.Maina JN. Morphometrics of the avian lung. 4. The structural design of the charadriiform lung. Respir Physiol. 1987; 68:99–119. [DOI] [PubMed] [Google Scholar]
- 59.Stanislaus M. Untersuchungen an der Kolibrilunge. Zeits Morphol Tiere. 1937; 33:261–289. [Google Scholar]
- 60.Maina JN. The lungs of the volant vertebrates—birds and bats: how are they relatively structurally optimized for this elite mode of locomotion? In: Weibel ER, Taylor CR, Bolis L, editors. Diversity in biological design: symmorphosis—fact or fancy? New York: Cambridge University Press; 1997; pp. 177–185. [Google Scholar]
- 61.Maina JN, King AS. A morphometric study of the lung of a humboldti penguin (Spheniscus humboldti). Zentrabl Vet Med Ser C, Anat Histol Embryol. 1987; 16:293–297. [PubMed] [Google Scholar]
- 62.Maina JN, Jimoh SA. Structural failures of the blood-gas barrier and the epithelial-epithelial cell connections in the different vascular regions of the lung of the domestic fowl, Gallus gallus variant domesticus, at rest and during exercise. BioOpen. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maina JN, Sikiru AJ. Study of stress induced failure of the blood-gas barrier and the epithelial-epithelial cells connections of the lung of the domestic fowl, Gallus gallus variant domesticus after vascular perfusion. Biomed Engineer Comput Biol. 2013; 5:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Welsch U, Aschauer B. Ultrastructural observations on the lung of the emperor penguin (Apternodytes forsteri). Cell Tissue Res. 1986; 243:137–144. [Google Scholar]
- 65.Maina JN. Morphological and morphometric properties of the blood-gas barrier: comparative perspectives In: Makanya AN, editor. The vertebrate blood-gas barrier in health and disease: structure, development and remodeling. Heidelberg: Springer; 2015; pp. 15–38. [Google Scholar]
- 66.Maina JN. Bioengineering aspects in the design of gas exchangers: comparative evolutionary, morphological, functional, and molecular perspectives. Heidelberg: Springer; 2011. [Google Scholar]
- 67.Maina JN. Functional morphology of the vertebrate respiratory organs. Lebanon (NH): Oxford and IBH Publishing Company; 2002. [Google Scholar]
- 68.Maina JN. Fundamental structural aspects in the bioengineering of the gas exchangers: comparative perspectives. Heidelberg: Springer Verlag; 2002. [DOI] [PubMed] [Google Scholar]
- 69.Maina JN. Critical appraisal of some factors pertinent to the functional designs of the gas exchangers. Cell Tissue Res. 2017; 367:747–767. 10.1007/s00441-016-2549-9 [DOI] [PubMed] [Google Scholar]
- 70.Maina JN. Comparative respiratory morphology and morphometry: the functional design of the respiratory systems In: Gilles R, editor. Advances in comparative and environmental physiology. Berlin: Springer; 1994; pp. 111–232. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The supporting data are all contained within the Supporting Information files.