Abstract
The aim of this work was to study the effects of cyclosporine (CsA), tacrolimus (FK-506), and rapamycin (RAPA) on bone mass, femoral microstructure, femoral biomechanical properties, and bone remodeling in healthy adult male rats.
Forty-eight 5-month-old male Wistar rats were used. CsA (2 mg/kg/day), FK-506 (3 mg/kg/day), RAPA (1.25 mg/kg/day), or water (0.5 ml/rat/day, control group) were administered orally for 3 months.
After sacrifice, mean values of immunosuppressants in blood were: CsA (670.4 ng/ml), FK-506 (19.2 ng/ml), and RAPA (4.8 ng/ml). Levels of biochemical parameters were normal in all groups. Femoral BMD was decreased in FK-506 and RAPA groups and lumbar BMD in FK-506 group. Trabecular volume fraction (BV/TV) decreased only in FK-506 group. RAPA and CsA affected femoral cortical structure, but FK-506 did not. FK-506 produced an increase in bone remodeling, and CsA a decrease. FK-506 group showed a decrease in biomechanical parameters relative to all groups. RAPA group showed a decrease in ultimate stress vs control group, and CsA group presented an increase in biomechanical parameters versus control group.
We found that administration of both RAPA and FK-506 as monotherapy for healthy rats produced osteopenia. CsA treatment only produces slight damages in the cortical zone of the femur.
Keywords: Immunosuppressants, Cyclosporine, Tacrolimus, Rapamycin, Osteopenia, Bone quality
1. Introduction
With the use of new and more powerful immunosuppressive drugs, survival rates in patients receiving solid-organ transplantation have improved significantly due to effective control of acute rejection episodes. However, a substantial number of works describe the development of osteoporosis among patients who have received transplantation of organs such as the kidney, heart, or liver (Monegal et al., 2001, Al-Gabri et al., 2005, Maalouf and Shane, 2005, Kulak et al., 2012, Wang et al., 2013, Monegal et al., 2013). These patients have increased risk of vertebral and nonvertebral fracture (Ramsey-Goldman et al., 1999). The rate of bone loss increases during the first year after kidney or heart transplantation but experiences a stabilization at the lumbar level. However, in parts of the skeleton where cortical bone is predominant, like the hip, an accelerated rhythm of bone loss is maintained over the years following transplantation (Delmas, 2001). Osteoporosis and increased risk of fracture have a negative impact on the quality of life of patients. In view of the morbidity and mortality associated with hip and vertebral fractures, such a pattern will influence the quality of life of these patients.
The many factors contributing to the pathogenesis of osteoporosis after transplantation include the underlying disease (e.g., primary biliary cirrhosis), concurrent disorders, hypogonadism, postoperative malnutrition and immobilization, and treatment with glucorticoids. The negative effects of glucocorticoids on bone are known (Delmas, 2001). In recent years, however, the accepted dose of glucocorticoids after transplantation has been significantly reduced, and the action of immunosuppressants themselves on bone cannot be overlooked as a potential negative factor. Cyclosporin (CsA), FK-506 (tacrolimus), or rapamycin (RAPA) are immunosuppressants used in many transplantation patients. In experimental models, CsA has been shown to induce bone loss through an increase in bone resorption (Tsuruoka et al., 2005). Indeed, a correlation has been observed between CsA serum concentrations and increased bone remodeling in patients with heart (Thiébaud et al., 1996) or liver (Giannini et al., 2000) transplantation. While it is currently accepted that FK-506 presents minor secondary effects when compared to CsA, there are also works that show that this immunosuppressant increases bone remodeling and brings about a significant decrease in bone mineral density (BMD) in human trabecular bone (Stempfle et al., 1998) and in experimental models (Kirino et al., 2004). RAPA, on the other hand, is a more novel treatment. According to several reports, its effects on bone are lower than those of other immunosuppressants (Romero et al., 1995, Campistol et al., 2005). In spite of the importance of the problem, there are few works in the literature that study the causes of bone loss in patients who have undergone solid-organ transplantation.
The importance of this question led us to study the actions of 3 commonly used immunosuppressants on bone quality: CsA, FK-506, and RAPA. Due to the difficulty of performing this study in humans as a result of heterogeneity in age, transplanted organ, sex, and initial patient status, we decided to use male adult rats as a homogenous experimental model. Rats have been previously used by many investigators to study bone loss and the positive and negative effects of various drugs on loss of bone (Zhu, 2010).
The aim of this work was: 1) to establish an experimental model of administration of immunosuppressants to healthy male rats in which the level of blood immunosuppressants was similar to the levels clinically accepted for human patients treated with these drugs; and 2) to use this experimental model to comparatively study the effects produced by CsA, FK-506, and RAPA on BMD, trabecular and cortical bone microstructure, biomechanical properties, and bone remodeling through the levels of biochemical markers of bone turnover.
2. Materials and methods
2.1. Animals
Forty-eight five-month-old male Wistar rats weighing 405 ± 32 g (mean ± SD) were used. The animals were kept under constant living conditions (22 °C, 12 h per day of light–dark cycles), and food (standard laboratory chow) and water were available ad libitum.
The animals were randomized to 4 different groups: SHAM (n = 12), in which each rat received 300 μl/day of distilled water by oral gavage; CsA (n = 12), in which each rat received 2 mg/kg/day of CsA by oral gavage; RAPA, in which each rat received 1.25 mg/kg/day of RAPA by oral gavage; FK-506, in which each rat received 3 mg/kg/day of FK-506 by oral gavage. Treatment was maintained for 3 months.
2.2. Immunosuppressant preparation
Doses of immunosuppressants were prepared according to a medium weight of the experimental rats of 400 g. Cyclosporine A (Sandimmun, 100mg, Novartis) was diluted in distilled water (150 µl of CsA in 5475 µl of distilled water). 300 µl of this dilution was administered daily by oral gavage to each rat (2 mg/kg/day). FK-506 (Tacrolimus, Prograf 5mg, capsules). Each day, the content of 4 capsules (20 mg) was diluted in 2 ml of ethanol to solubilize the product and 3 ml of distilled water were added. 300 µl of this dilution was administered daily by oral gavage to each rat (3 mg/kg/ day). Rapamicine (Rapamune, 1 mg/ml, oral solution, Wyeth) was administered directly to rats from pharmaceutical preparation, 500 µl/rat by oral gavage daily (1.25 mg/kg/day). Each rat of the SHAM group received daily 300 µl of distilled water by oral gavage.
The day following the last treatment, the experimental animals were weighed and sacrificed by exsanguination while under ether anesthesia. Blood samples were obtained by cardiac puncture (2 ml with EDTA for determination of immunosuppressant levels in total blood) and 7 ml without any additive for serum determinations. Total blood was immediately used to determine immunosuppressant levels, and serum aliquots were frozen at − 80 °C until determination of biochemical markers of bone turnover or other biochemical determinations. Once the blood was collected, the animals were frozen at − 20 °C until determination of BMD in previously thawed animals. Prior to BMD analyses, the left femurs were excised and cleaned of adjacent tissue. The right femur was also excised and cleaned for computerized microtomographic analysis (μCT) and biomechanical testing. Lumbar spine BMD was determined in situ. Repeated freeze–thaw cycles have been shown to have no influence on the mechanical properties of bone (Borchers et al., 1995). All the experiments with animals were carried out according to Spanish law regarding the use, protection, and care of experimental animals (Royal Decree 53/2013).
2.3. Blood immunosuppressants
2.3.1. CsA
The automated Dimension® CSA method uses an immunoassay technique in which free and CsA-bound antibody-enzyme species are separated using magnetic particles. CsA was measured in whole blood samples (EDTA tubes) that were previously mixed and lysed. Sensitivity of the methods was 25 ng/ml and inter- and intra-assay variation coefficients were < 11% and < 15.9%, respectively.
2.3.2. FK-506
FK-506 levels were measured in an IMx analyzer (Abbott, Germany), which performs microparticle enzyme analysis (MEIA). FK-506 was measured in whole blood samples (EDTA tubes) previously extracted using a precipitation reagent. The sensitivity of the method was 4.1 ng/ml and inter- and intra-assay variation coefficients were < 4% and < 10%, respectively.
2.3.3. RAPA
RAPA levels were measured in an IMx analyzer (Abbott, Germany). This autoanalyzer also performs MEIA. RAPA was measured in whole blood samples (EDTA tubes) previously extracted using a precipitation reagent. The sensitivity of the method was 1 ng/ml and inter- and intra-assay variation coefficients were < 7% and < 9%, respectively.
2.4. BMD
BMD was determined in situ in the lumbar spine (L2, L3, and L4) and in the whole left femur by dual energy X-ray densitometry (DEXA) using a HOLOGIC QDR-1000 TM (S/N 277) (Hologic, Inc., Waltham, MA, USA) with small-animal software (Gala Paniagua et al., 1998). Intra-assay and inter-assay variation coefficients were < 0.53% and < 1.2%, respectively. The femur scans were analyzed for BMD of the whole femur. The scans of the L2, L3, and L4 vertebrae were analyzed for BMD of the whole 3 vertebrae and the results were expressed as the mean of the values obtained.
2.5. Trabecular and cortical microarchitecture analysis of femur by microCT
The distal region of the right femur was analyzed by μCT (Skyscan N.V., Aartselaar, Belgium) imaged with an X-ray tube voltage of 100 kV and a current of 100 μA, and with a 1.0 mm aluminum filter. The trabecular and cortical bone femur region were studied. For more details, see reference De La Piedra et al. (2011).
2.6. Biomechanical testing
The femora were subjected to mechanical testing with a Microtest EM1/10/FR/m testing machine (Microtest, S.A., Madrid, Spain) and 3-point bending strength was measured. The extrinsic biomechanical properties of bone include ultimate force, stiffness, and work to failure. The intrinsic biomechanical properties include ultimate stress, apparent young modulus, and toughness. For more details, see reference De La Piedra et al. (2011).
2.7. Biochemical markers of bone turnover
Serum bone Gla protein (Osteocalcin) (BGP) was determined by ELISA for the specific quantitative determination of rat osteocalcin levels (Rat-MID Osteocalcin, IDS, UK). The sensitivity of this assay was 50 ng/ml. The intra- and inter-assay coefficients of variation of the method were < 5.0% and < 6.6%, respectively.
Serum aminoterminal propeptide of collagen I (PINP) was assayed by a specific serum ELISA for rat PINP (Rat/Mouse PINP EIA, IDS, UK). Assay sensitivity was 0.7 ng/ml. The intra- and inter-assay variation coefficients of the method were < 5.0% and < 8.2%, respectively.
Serum 5b isoenzyme of tartrate-resistant acid phosphatase (TRAP) was measured by an ELISA specific for rat TRAP (RatTRAP Assay, IDS, UK). The sensitivity of the assay was 0.1 U/L. Intra- and inter-assay variation coefficients of the method were < 5.0% and < 5.5%, respectively.
Serum C-telopeptide of type I collagen (CTX) was measured by an ELISA specific for rat CTX (RatLaps ELISA, IDS, UK). The sensitivity of the assay was 2.0 ng/ml. Intra- and inter-assay variation coefficients of the method were < 5.6% and < 10.5%, respectively.
2.8. 25(OH) vitamin D analysis
The 25(OH) vitamin D was determined in rat serum by ELISA (25-hydroxyvitamin D EIA, IDS, UK). The sensitivity of the assay was 2 ng/ml. Intra- and inter-assay variation coefficients of the method were < 5.6% and < 6.4%, respectively.
2.9. PTH analysis
Serum PTH was determined in rat serum by ELISA (Rat Bioactive Intact PTH ELISA kit, Immutopics, USA). This technique is a third-generation PTH assay in which only (1–84) molecule (intact PTH) are measured. The sensitivity of the assay was 3 pg/ml. Intra- and inter-assay variation coefficients of the method were < 3.9% and < 8%, respectively.
2.10. Other biochemical determinations
Calcium, creatinine, gamma-glutamyl-transpeptidase (gamma-GTP), and alkaline phosphatase (AP) in rat serum were determined in a VITROS 5.1 autoanalyzer of the integrated system VITROS 5600 (Ortho Clinical Diagnostics). Sensitivity and intra- and inter-assay variation coefficients of the methods were, respectively: calcium 1.4 mg/dl, < 1.5% and < 1.9%; creatinine 0.05 mg/dl, < 1% and < 2.5%; gamma-GTP 10 UI/L, < 1.2% and < 1.6%; and AP 20 IU/L, < 1.5% and < 1.9%.
2.11. Statistical analyses
The results of the experiments were expressed as the mean ± SD of the different parameters. A non-parametric method, the Mann–Whitney test (Medcalc Software Program, Belgium), was used to compare the different treatment groups. P-values < 0.05 were accepted as denoting a significant difference.
3. Results
3.1. Experimental model: blood levels of immunosuppressants
Administration of immunosuppressants to rats over 3 months at a dose that is similar to the dose/kg ratio used in humans produced undetectable blood levels of FK-506 and RAPA, though also producing acceptable levels of CsA (Table 1). We administered increasing amounts of FK-506 and RAPA until we reached acceptable blood levels of immunosuppressants (data not shown). Definitive doses of immunosuppressants are shown in the lower part of Table 1: 2 mg/kg/day of CsA, 3 mg/kg/day of FK-506, and 1.25 mg/kg/day of RAPA, and this was the experimental model used in the present work.
Table 1.
Blood levels of immunosuppressants.
| Time after administration | Immunosuppressant dose |
||
|---|---|---|---|
| CsA 2 mg/kg/day |
FK-506 0.10 mg/kg/day |
RAPA 0.05 mg/kg/day |
|
| 2 h | 550 ± 47 ng/ml | Undetectable | Undetectable |
| 24 h | 200 ± 23 ng/ml | Undetectable | Undetectable |
| Time after administration | Immunosuppressant dose |
||
| CsA 2 mg/kg/day |
FK-506 3 mg/kg/day |
RAPA 1.25 mg/kg/day |
|
| 2 h | 670 ± 50 ng/ml | 19 ± 2 ng/ml | 10 ± 1.5 ng/ml |
| 24 h | 153 ± 20 ng/ml | 4 ± 0.6 ng/ml | 4 ± 1.4 ng/ml |
Blood levels of immunosuppressants after the daily administration of the indicated doses of cyclosporine (CsA), tacrolimus (FK-506) and rapamycin (RAPA) during 3 months to five-month-old male Wistar rats (n = 3 rats/group).
3.2. Bone remodeling markers
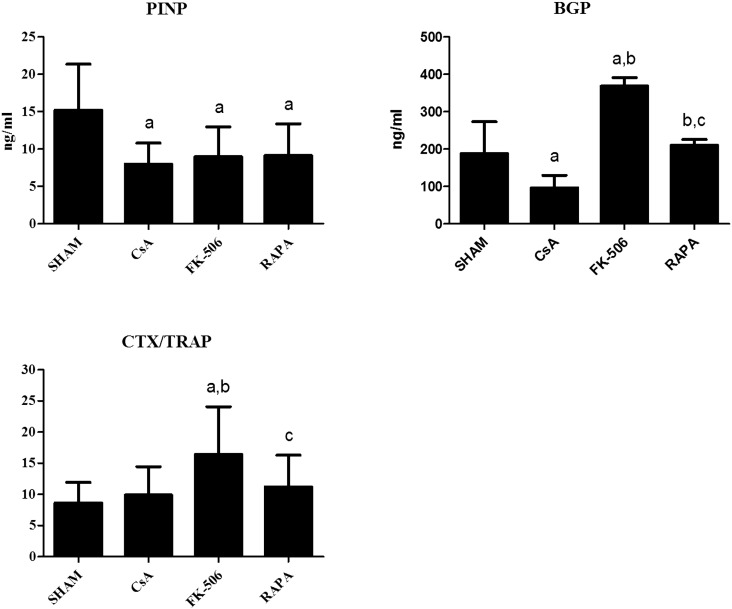
Fig. 1 shows the serum levels of the biochemical markers of bone formation PINP and BGP and the biochemical index of bone resorption CTX/TRAP following administration of CsA, FK-506, and RAPA to the rats over 3 months according to the doses indicated in the Materials and methods section above. Levels of PINP decreased significantly in the 3 groups of treated animals with respect to the untreated group, and no significant differences were detected between the treatment groups. BGP levels decreased significantly in the group treated with CsA with respect to the control group and increased significantly in the group treated with FK-506. The group treated with RAPA did not show significant differences with respect to the untreated group. Levels of CTX/TRAP were significantly increased with respect to untreated rats only in the FK-506 treated group, with no differences found in the CsA and RAPA groups.
Fig. 1.
Biochemical markers of bone turnover in five-month-old male Wistar rats treated with cyclosporine (CsA, 2 mg/kg/day), FK-506 (0.1 mg/kg/da), and rapamycin (RAPA, 0.05 mg/kg/day) over three months. Statistical significance: Mann–Whitney test; a: significant vs. control; b: significant vs. CsA; c: significant vs. FK-506. PINP: CsA vs. control p < 0.01; FK-506 vs. control p < 0.05; RAPA vs. control p < 0.05. BGP: CsA vs. control p < 0.05, FK-506 vs. control p < 0.001, FK-506 vs. CsA p < 0.001, RAPA vs. CsA p < 0.01, RAPA vs. FK-506 p < 0.001. CTX/TRAP: FK-506 vs. control p < 0.01, FK-506 vs. CsA p < 0.01, RAPA vs. FK-506 p < 0.05.
3.3. Bone mineral density
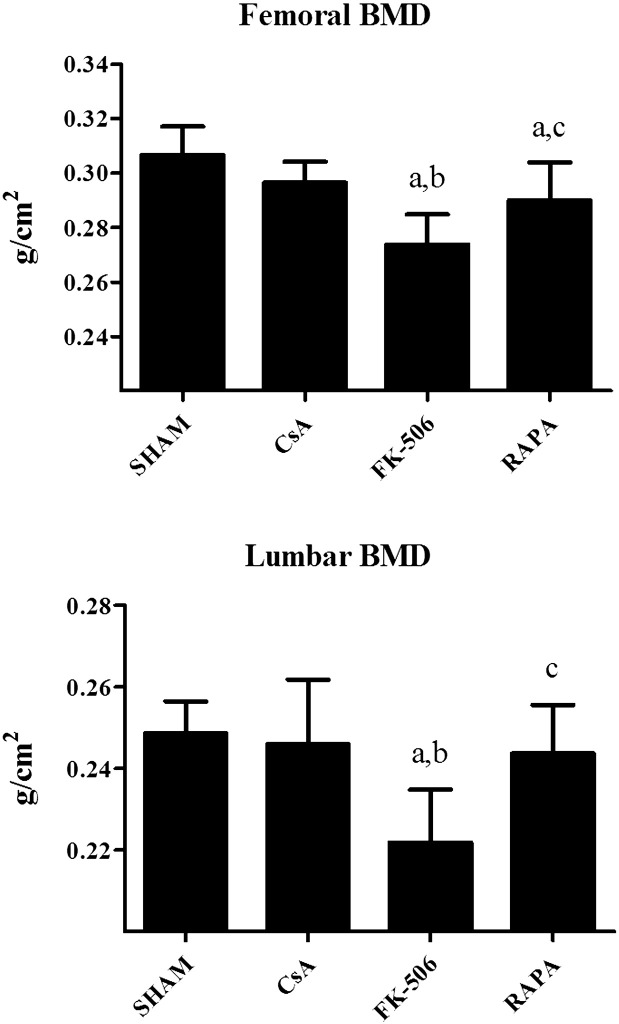
Fig. 2 shows femoral (F) and lumbar (L) BMD in the rats treated with immunosuppressants. FK-506 and RAPA produced a significant decrease in FBMD with respect to the control group, and the decrease in the FK-506 group was higher than in the RAPA group. CsA treatment did not produce any significant change in FBMD. LBMD decreased significantly only in the group treated with FK-506.
Fig. 2.
Femoral and lumbar BMD in five-month-old male Wistar rats treated with cyclosporine (CsA, 2 mg/kg/day), FK-506 (0.1 mg/kg/day) and rapamycin (RAPA, 0.05 mg/kg/day) during three months. Statistical significance: Mann–Whitney test; a: significant vs, control; b: significant vs. CsA; c: significant vs. FK-506. Femoral BMD: FK-506 vs. control p < 0.001, FK-506 vs CsA p < 0.001, RAPA vs. control p < 0.05, RAPA vs. FK-506 p < 0.01. Lumbar BMD: FK-506 vs. control p < 0.001, FK-506 vs. CsA p < 0.001, RAPA vs. FK-506 p < 0.01.
3.4. Bone microstructure
3.4.1. Femoral trabecular microstructure
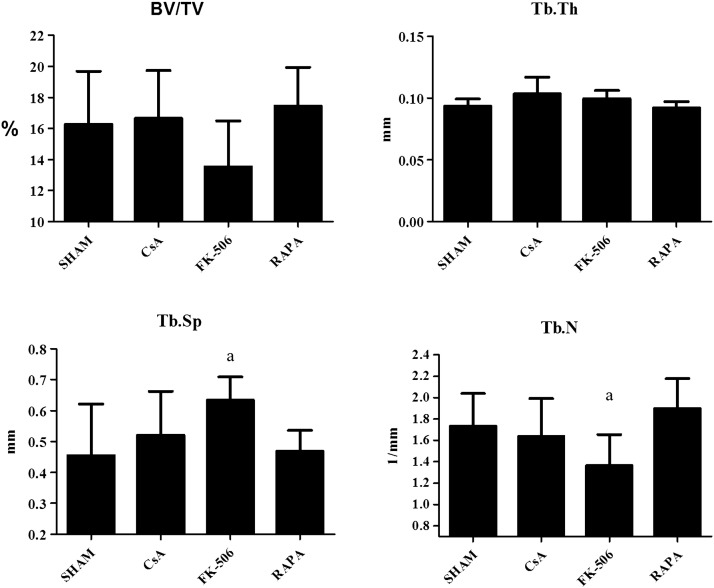
Fig. 3 shows the percentage of volume of bone tissue with respect to total bone volume (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N) in the femur of the different groups of rats treated for 3 months with CsA, FK-506, and RAPA. Only the group treated with FK-506 experienced a significant decrease in BV/TV and Tb.N and an increase in Tb.Sp with respect to the control group. Neither CsA nor RAPA produced changes in femoral trabecular microstructure.
Fig. 3.
Percent of bone volume with respect to total bone volume (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and number of trabeculae (Tb.N) in five-month-old male Wistar rats treated with cyclosporine (CsA, 2 mg/kg/day), FK-506 (0.1 mg/kg/da), and rapamycin (RAPA, 0.05 mg/kg/day) over three months. Statistical significance: Mann–Whitney test; a: significant vs, control; BV/TV: control vs FK-506: p < 0.05 (2). Tb.Sp: control vs FK-506: p < 0.01. Tb.N: control vs FK-506: p < 0.01.
3.4.2. Femoral cortical microstructure
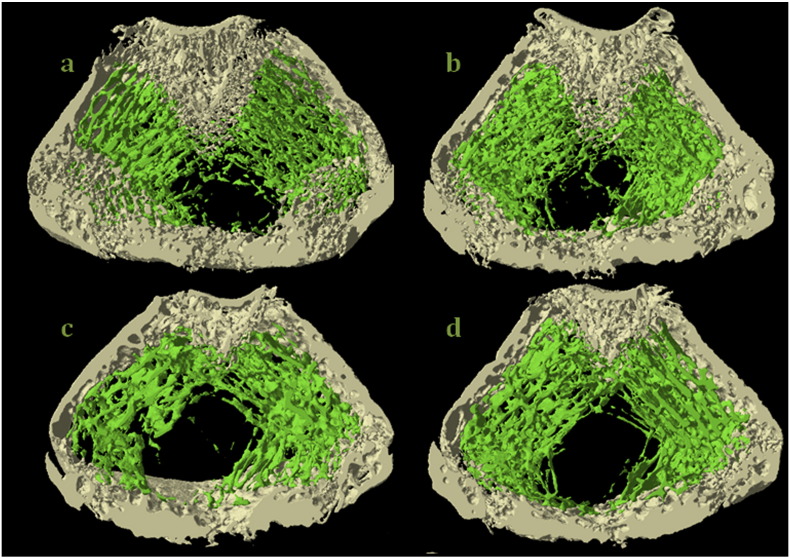
Table 2 shows the microstructural parameters of the cortical bone of rat femora treated with CsA, FK-506, and RAPA. A detrimental effect of RAPA was found in the structure of the femoral cortical bone, with decreases in cortical area (− 9.75%, p < 0.05) and rotational inertia moment (− 39.3%, p < 0.002). CsA also affected femoral cortical structure, decreasing cortical area (− 7.1%, p < 0.05), and rotational IM (− 14.5%, p < 0.05). FK-506 did not affect femoral cortical structure. Fig. 4 shows 4 representative images of the 3D microtomographic sections, both of trabecular and cortical zones of the femur of the rats of the 4 studied groups. A decrease in the trabecular zone was observed in the section that corresponds to FK-506 treated rats, as well as a slight decrease in the cortical zone in the group treated with RAPA.
Table 2.
Microstructural parameters of the cortical bone of rat femora treated with CsA, FK-506, and RAPA.
| SHAM | CsA | FK-506 | RAPA | |
|---|---|---|---|---|
| Cortical area, mm2 | 6.81 ± 0.41 | 6.33 ± 0.23a | 6.33 ± 0.55 | 6.10 ± 0.50a |
| Rotational inertia moment, mm4 | 35.3 ± 3.3 | 30.2 ± 3.9a | 30.7 ± 6.3 | 28.4 ± 3.8a |
| Cortical thickness, mm | 0.290 ± 0.041 | 0.313 ± 0.023 | 0.325 ± 0.030 | 0.300 ± 0.037 |
Microstructural parameters of the cortical of femur of five-month-old male Wistar rats treated with cyclosporine (CsA, 2 mg/kg/day), FK-506 (0.1 mg/kg/da) and rapamycin (RAPA, 0.05 mg/kg/day) during three months. Statistical significance: Mann–Whitney test; a vs. SHAM. In all the cases the statistical significance was p < 0.05 with the exception of rotational inertia moment RAPA vs SHAM p < 0.01.
Fig. 4.
Representative images of the 3D microstructure sections, both of trabecular and cortical zones of the femur of five-month-old male Wistar rats treated with cyclosporine (CsA, 2 mg/kg/day), FK-506 (0.1 mg/kg/da) and rapamycin (RAPA, 0.05 mg/kg/day) during three months. a: Control, b: cyclosporine, c: tacrolimus and d: rapamycin.
3.5. Biochemical parameters
Treatment with CsA, FK-506, or RAPA did not produce any alteration in the levels of serum calcium, creatinine, gamma-GTP, AP, 25(OH) vitamin D, or bio-PTH compared to the untreated group.
3.6. Biomechanical properties of the femur
Table 3 shows the results of the bending test carried out in the femur of the rats. The group of rats treated with FK-506 showed a significant decrease in ultimate load, ultimate displacement, and work to failure with respect to all the studied groups and a decrease in ultimate strain and toughness versus the rats treated with CsA and RAPA, with no changes observed in ultimate stress. The RAPA group showed a decrease in ultimate stress with respect to the control group, and the CsA group presented an increase in ultimate displacement, work to failure, and ultimate strain versus the control group.
Table 3.
Results of the bending test carried out in the femur of the rats treated with CsA, FK-506 and RAPA.
| SHAM | CsA | FK-506 | RAPA | ||
|---|---|---|---|---|---|
| Extrinsic properties (structural) | |||||
| Ultimate load, N | Fult | 186 ± 14 | 178 ± 10 | 158 ± 15a,b,c | 172 ± 16 |
| Ultimate displacement, mm | δult | 0.69 ± 0.14 | 0.79 ± 0.07a | 0.61 ± 0.14b,c | 0.77 ± 0.06 |
| Work to failure, mJ | U | 75 ± 20 | 90 ± 17a | 61 ± 15a,b,c | 83 ± 13 |
| Intrinsic properties (material) | |||||
| Ultimate stress, MPa | σult | 164 ± 19 | 156 ± 25 | 155 ± 12 | 150 ± 10a |
| Ultimate strain | Ɛult | 0.06 ± 0.01 | 0.08 ± 0.01a | 0.05 ± 0.01b,c | 0.07 ± 0.01 |
| Toughness, MPa | u | 6.3 ± 1.6 | 7.5 ± 1.5 | 5.4 ± 1.2b,c | 6.8 ± 0.9 |
Results of the bending test applied to the femur of five-month-old male Wistar rats treated with cyclosporine (CsA, 2 mg/kg/day), FK-506 (0.1 mg/kg/da) and rapamycin (RAPA, 0.05 mg/kg/day) during three months. Statistical significance: Mann–Whitney test; p < 0.05; a vs. SHAM, b vs. cyclosporine, c vs. RAPA.
3.7. Biochemical parameters
Table 4 shows the levels of serum calcium, creatinine, gamma-GTP, AP, 25(OH) vitamin D, and bio-PTH in the 4 groups of rats studied. Treatment with CsA, FK-506, or RAPA did not produce any alteration in the levels of these parameters with respect to the untreated group.
Table 4.
Biochemical parameters in the serum rats treated with CsA, FK-506 and RAPA.
| Creatinine mg/dl |
Calcium mg/dl |
GGTP IU/l |
ALP IU/l |
25(OH)vitD ng/ml |
PTH pg/ml |
|
|---|---|---|---|---|---|---|
| SHAM | 0.610 ± 0.054 | 9.567 ± 0.242 | 1.167 ± 0.408 | 144.83 ± 23.28 | 26.0 ± 4.3 | 60.3 ± 36.7 |
| CsA | 0.63 ± 0.073 | 9.300 ± 0.253 | 1.000 ± 0.000 | 145.33 ± 26.77 | 29.3 ± 7.2 | 66.9 ± 26.8 |
| FK-506 | 0.652 ± 0.050 | 9.767 ± 0.294 | 1.000 ± 0.000 | 160.33 ± 54.55 | 22.2 ± 3.8 | 88.2 ± 57.2 |
| RAPA | 0.608 ± 0.031 | 9.533 ± 0.197 | 1.000 ± 0.000 | 159.67 ± 25.60 | 27.3 ± 4.4 | 63.8 ± 34.1 |
Biochemical parameters in the serum of five-month-old male Wistar rats treated with cyclosporine (CsA, 2 mg/kg/day), FK-506 (0.1 mg/kg/day), and rapamycin (RAPA, 0.05 mg/kg/day) over three months. Statistical significance: Mann–Whitney test. No significant statistical differences between groups were observed.
4. Discussion
One of the main problems when studying the action of immunosuppressants in humans is the lack of homogeneity of the population in terms of age, transplanted organ, and patient status; homogeneity is required to perform definitive statistical studies. The use of an experimental model eliminates these difficulties. For this reason, the first objective of our work was to design an experimental model with conditions as similar as possible to those of patients. Thus, we tried to have similar serum levels of CsA, FK-506, and RAPA to those admitted as valid in patients treated with these drugs. Doing this ensured that the effects of immunosuppressants on bone cells of animals would be similar to the effect produced by immunosuppressants on human bone cells. We started administering the same concentrations of immunosuppressants per kg of body weight that usually were administered to humans; however, with the exception of CsA, we observed undetectable levels of immunosuppressants in blood. By increasing the dose of administered immunosuppressants, we reached levels of CsA (2 mg/kg/), FK-506 (3 mg/kg), and RAPA (1.25 mg/kg) that, when administered daily, produced the desired levels of immunosuppressants in the blood of the rats.
Another important problem in the study of the effects of immunosuppressants in humans is the fact that patients usually take several drugs simultaneously, therefore making it difficult to isolate the effects of each one. For this reason, we have administered only one immunosuppressant to each group of rats so as to study their effects separately.
It is important to note that many experimental works on the effects of immunosuppressants on rat bone do not measure serum levels of immunosuppressants, and in many cases the administered dose is very high.
When we analyzed the changes in bone remodeling markers after the treatment with immunosuppressants, only FK-506 produced a significant increase in BGP (marker of bone formation and bone remodeling) and the rate CTX/TRAP5b, showing a significant increase in bone remodeling after the administration of this immunosuppressant. The fact that an increase of PINP was not observed does not rule out a transient increase in this marker and a return to normal levels after 3 months of treatment. So, high levels of PINP were described during the 2 first months after ovariectomy in rats, and returned to basal levels after 8 weeks (Rissanen et al., 2008). Treatment with CsA or RAPA did not seem to produce a state of high remodeling unless it was transient, and we found that a decrease in bone remodeling was produced by CsA if we take into account values of PINP and BGP.
It is difficult to make a comparison between our results and those of other authors, because in many cases they use different doses of immunosuppressants, different ages of rats, and different times of exposure to the drugs.
With CsA doses of 1 or 5 mg/kg 3 times/week for 4 months (our dose is intermediate 2 mg/kg/day), Erben et al. (2003) found an increase in BGP in 9-month-old male Fisher rats, which contrasts with our results, since we did not detect changes in this bone marker. In the same work, this author found a transient increase in the resorption bone marker urinary deoxypyridinoline (Dpyr) with CsA treatment, although the levels for this marker seemed to return to normality after 4 months of treatment, which mirrors our findings about CTX/TRAP5b. Our finding of a general increase of bone remodeling after the treatment with FK-506 agrees with that of Kirino et al. (2004), who found a significant increase of BGP and Dpyr in 8-week-old Sprague–Dawley rats that were treated for 5 weeks with 1 mg/kg/day of FK-506 administered intraperitoneally. According to these authors, this dose would be equivalent to 3.2 mg/kg orally, which is the dose we employed in the present work. Spolidorio et al. (2007) found an increase in the resorption marker FATR following administration of CsA (a finding we did not reach with the CTX/TRAP marker) in rats treated with 10 mg/kg/day of CsA (a dose 5 times higher than ours). Another important point is that these authors used 50 g rats, many of which were younger than ours, a fact which could have caused the different effects of immunosuppressants on bone remodeling. However, with these same animals, these authors did not find changes in the levels of FATR after the administration of FK-506 (1 mg/kg/day versus 3 mg/kg/day in our work).
With respect to BMD, our results show that CsA did not affect levels of lumbar or femoral BMD. Although a slight decrease in cortical area and IM (cortical microstructure) was observed, the trabecular microstructure was normal and femoral microstructure was not affected. In the case of RAPA administration, lumbar BMD was not affected, but femoral BMD was decreased. This is due to the fact that trabecular microstructure was not affected, although there was a larger decrease than in the case of CsA administration in the cortical microstructure (cortical area and IM). However, FK-506 administration produced a serious alteration in trabecular microstructure (less BV/TV and Tb.Th) and a greater Tb.Sp. This fact produces a significant decrease in femoral and lumbar BMD although cortical microstructure is not affected.
There is a great controversy in the literature about the effects produced by CsA on bone mass. Erben et al. (2003) found deleterious and transient effects on bone mass when administering 15 mg/kg/day of CsA to male rats and also observed positive effects on female rats. Cvetkovic et al. (1994), who administered 15 mg/kg/day of CsA to male Sprague–Dawley rats over 28 days, found a decrease in trabecular volume. Romero et al. (1995) administered 15 mg/kg/day of CsA to Sprague–Dawley rats that were 10 weeks old and found a 66% decrease in trabecular area. Similar results were found by Kawana et al. (1996) and Katz et al. (1994). The difference between the results of these authors and ours could be due to their elevated dose of CsA compared with ours (15 mg/kg/day vs. 2 mg/kg/day). However, Abdelhadi et al. (2002), who administered our same dose to growing rats, did not find variations in the trabecular volume of tibial metaphysis, but they did find a decrease when administering 30 mg/kg/day. Nassar et al. (2013) observed an imbalance of the alveolar bone homeostasia in a model of experimental periodontitis after the administration of 10 mg/kg of CsA over 30 days. All the studies that show undesirable effects on the bone of rats by CsA use high doses of CsA that could be considered toxic. In our study, blood levels in the rats were similar to those of treated patients and in conditions in which bone damage is undetectable. In an interesting work, Jäger et al. (2012) demonstrated that CsA blood levels of < 200 ng/ml did not induce high turnover osteopenia in aged rats, while higher levels induced bone loss. This controversy on the effects of CsA on bone has also been observed in human studies. Works carried out in heart-transplanted patients (Thiébaud et al., 1996, Shane et al., 1993) show a deleterious effect of CsA on bone mass, while other studies with monotherapy of CsA in kidney transplant do not show bone-mass loss.
More works agree with our results reviewing the effects produced administering FK-506 to rats. Kirino et al. (2004) administered 1 mg/kg/day of FK-506 intraperitoneally for 5 weeks and, like in our work, recorded a decrease in trabecular volume. Fukunaga et al. (2004), who administered intramuscularly 1 mg/kg/day of FK-506 to rats, also observed a decrease in the femoral trabecular area. An interesting finding in the work of these authors is that they did not find any difference in the cortical bone of the rats treated with FK-506; this result is in agreement with ours. Cvetkovic et al. (1994) also found a decrease in trabecular volume in rats administered 5 mg/kg/day of FK-506. A decrease of 56% in trabecular area was also observed by Romero et al. (1995) when the same dose of FK-506 was administered. Administering the same dose as in our work (3 mg/kg/day of FK-506) to growing rats, Abdelhadi et al. (2002) found a decrease in tibial metaphysis and the same results were observed with 1 mg/kg/day administered over 5 weeks in Sprague–Dawley rats. Kosugi et al. (2013), using a morphometric analysis, found that FK-506 produced osteoporosis in the femur and mandibular condyle in Sprague–Dawley rats treated for 5 weeks. However, results of other authors contrast with ours. Inoue et al. (2000), who administered 10 mg/kg/day of CsA or 1 mg/kg/day of FK-506 to male rats over 10 weeks, found a decrease in femoral BMD with both treatments, although this decrease was more pronounced with CsA. The difference from our results could be due to the high dose administered. Folwarczna et al. (2009) administering a low dose of FK-506 (0.3 mg/kg/day) to adult male rats, found that this dose increased bone formation. Results that differed from ours were found by Spolidorio et al. (2007). These authors administered 10 mg/kg/day of CsA subcutaneously to one group of rats and 1 mg/kg/day of FK-506 to another group. After this, the group treated with CsA was treated with FK-506. Surprisingly, FK-506 reverted bone loss, increasing bone volume.
There are fewer works about RAPA. One of the factors that may have influence its scant clinical use and the low number of experimental works are the difficultly to determine RAPA levels by HPLC until 2006. From this date, an automatic commercial method similar to that of FK-506 was developed, thereby making it possible to use this compound. The results of Joffe et al. (1993) agree with ours. A dose of 2.5 mg/kg/day of RAPA for 14 days did not affect trabecular volume. Similarly, Romero et al. (1995) and Joffe et al. (1993) demonstrated that RAPA administration did not produce changes in trabecular volume.
All the parameters analyzed about bone quality lead to a higher or lower bone resistance that really is the more important parameter, responsible of the possible fracture. As we can observe in our results on the biomechanical properties of the femur in rats treated with immunosuppressants, treatment with FK-506 produced the most negative effects, especially in the extrinsic properties (ultimate load and work to failure). Regarding intrinsic properties, although there is no significant difference between the group treated with this immunosuppressant and the control group, their values are significantly lower than those of groups treated with CsA and RAPA. Surprisingly, CsA treatment produced a variation in extrinsic parameters (ultimate displacement and work to failure) and in the intrinsic (ultimate strain) in the sense that the bone of rats treated with CsA is even more resistant than the bone of the control group.
As for the general biochemical parameters of rats, we did not find significant differences in the levels of serum calcium, gamma-GTP, creatinine, or total AP. This fact indicates that immunosuppressants at these doses did not produce renal or hepatic damage. We did not find any change in PTH levels after 3 months of treatment. Other authors, such as Kirino et al. (2004), found a transient increase in PTH levels in rats treated with FK-506 after 3 weeks of treatment, later returning to normal levels. Cvetkovic et al. (1994) did not find changes in calcium or PTH after CsA treatment administered to rats, but they found changes after 28 days of FK-506 treatment. In works with patients, some authors demonstrated that immunosuppressants produced bone loss through a secondary hyperparathyroidism, although other authors did not observe the same (Rubin and Bilezikian, 2002). We did not observe any alteration in the levels of 25(OH) vitamin D after the administration of the 3 immunosuppressants.
In conclusion, in our in vivo experiments we found that both RAPA and FK-506 administered as monotherapy to normal rats produce osteopenia. RAPA only affects cortical bone structure, while losses in bone mass due to FK-506 are deeper and affect cortical and trabecular zones. CsA treatment only produces slight damages in the cortical zone of the femur, and bones of animals treated with this drug are even more resistant to flexion than those of control rats. Findings on the biomechanical parameters of resistance to flexion in this work correlated with findings in BMD and bone microstructural parameters in the rats treated with CsA, FK-506, and RAPA. On the other hand, the increase in bone remodeling found after the administration of FK-506 could explain the decrease found in bone mass, while with CsA a decrease in bone remodeling is observed.
Disclosures
All authors state that they have no conflicts of interest.
Acknowledgments
This study was funded by a grant of Fondo de Investigación Sanitaria FIS, Spain.
Authors' roles: Study design: MR, MDC, and CP. Study conduct: CP. Data collection: MR, MM, and MMF. Data analysis: MR, MM, MMF, JRC, and DG. Data interpretation: MR, MDC, JRC, and CP. Drafting manuscript: MR, MMF, and CP. Revision of manuscript content: MR, MMF, and CP. Approving final version of manuscript: MR, MM, MMF, MDC, JRC, DG, and CP. CP takes responsibility for the integrity of the data analysis.
References
- Abdelhadi M., Ericzon B.-G., Hultenby K., Sjöden G., Reinholt F.P., Nordenström J. Structural skeletal impairment induced by immunosuppressive therapy in rats: cyclosporine A vs tacrolimus. Transpl. Int. 2002;15:180–187. doi: 10.1007/s00147-002-0413-1. [DOI] [PubMed] [Google Scholar]
- Al-Gabri S., Zadrazil J., Krejcí K., Horák P., Bachleda P. Changes in bone mineral density and selected metabolic parameters over 24 months following renal transplantation. Transplant. Proc. 2005;37:1014–1019. doi: 10.1016/j.transproceed.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Borchers R.E., Gibson L.J., Burchardt H., Hayes W.C. Effects of selected thermal variables on the mechanical properties of trabecular bone. Biomaterials. 1995;16:545–551. doi: 10.1016/0142-9612(95)91128-l. [DOI] [PubMed] [Google Scholar]
- Campistol J.M., Holt D.W., Epstein S., Gioud-Paquet M., Rutault K., Burke J.T. Bone metabolism in renal transplant patients treated with cyclosporine or sirolimus. Transpl. Int. 2005;18:1028–1035. doi: 10.1111/j.1432-2277.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- Cvetkovic M., Mann G.N., Romero D.F., Liang X.G., Ma Y., Jee W.S. The deleterious effects of long-term cyclosporine A, cyclosporine G, and FK506 on bone mineral metabolism in vivo. Transplantation. 1994;57:1231–1237. doi: 10.1097/00007890-199404270-00016. [DOI] [PubMed] [Google Scholar]
- De La Piedra C., Quiroga I., Montero M., Dapia S., Caeiro J.R., Rubert M. Daily or monthly ibandronate prevents or restores deteriorations of bone mass, architecture, biomechanical properties and markers of bone turnover in androgen-deficient aged rats. Aging Male. 2011;14:220–230. doi: 10.3109/13685538.2010.518176. [DOI] [PubMed] [Google Scholar]
- Delmas P.D. Osteoporosis in patients with organ transplants: a neglected problem. Lancet. 2001;357:325–326. doi: 10.1016/s0140-6736(00)03632-1. [DOI] [PubMed] [Google Scholar]
- Erben R.G., Brunner K.S., Breig B., Eberle J., Goldberg M., Hofbauer L.C. Skeletal effects of cyclosporin A are gender related in rats. Endocrinology. 2003;144:40–49. doi: 10.1210/en.2002-220513. [DOI] [PubMed] [Google Scholar]
- Folwarczna J., Kaczmarczyk-Sedlak I., Pytlik M., Nowińska B., Cegieła U., Sliwiński L. Effect of low-dose tacrolimus coadministered with raloxifene on the skeletal system in male rats. Acta Pol. Pharm. 2009;66:207–212. [PubMed] [Google Scholar]
- Fukunaga J., Yamaai T., Yamachika E., Ishiwari Y., Tsujigiwa H., Sawaki K. Expression of osteoclast differentiation factor and osteoclastogenesis inhibitory factor in rat osteoporosis induced by immunosuppressant FK506. Bone. 2004;34:425–431. doi: 10.1016/j.bone.2003.05.003. [DOI] [PubMed] [Google Scholar]
- Gala Paniagua J., Díaz-Curiel M., de la Piedra Gordo C., Castilla Reparaz C., Torralbo García M. Bone mass assessment in rats by dual energy X-ray absorptiometry. Br. J. Radiol. 1998;71:754–758. doi: 10.1259/bjr.71.847.9771386. [DOI] [PubMed] [Google Scholar]
- Giannini S., Nobile M., Ciuffreda M., Iemmolo R.M., Dalle Carbonare L., Minicuci N. Long-term persistence of low bone density in orthotopic liver transplantation. Osteoporos. Int. 2000;11:417–424. doi: 10.1007/s001980070109. [DOI] [PubMed] [Google Scholar]
- Inoue T., Kawamura I., Matsuo M., Aketa M., Mabuchi M., Seki J. Lesser reduction in bone mineral density by the immunosuppressant, FK506, compared with cyclosporine in rats. Transplantation. 2000;70:774–779. doi: 10.1097/00007890-200009150-00011. [DOI] [PubMed] [Google Scholar]
- Jäger W., Xu H., Wlcek K., Schüler C., Rubel F., Erben R.G. Gender- and dose-related effects of cyclosporin A on hepatic and bone metabolism. Bone. 2012;50:140–148. doi: 10.1016/j.bone.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Joffe I., Katz I., Sehgal S., Bex F., Kharode Y., Tamasi J. Lack of change of cancellous bone volume with short-term use of the new immunosuppressant rapamycin in rats. Calcif. Tissue Int. 1993;53:45–52. doi: 10.1007/BF01352014. [DOI] [PubMed] [Google Scholar]
- Katz I., Li M., Joffe I., Stein B., Jacobs T., Liang X.G. Influence of age on cyclosporin A-induced alterations in bone mineral metabolism in the rat in vivo. J. Bone Miner. Res. 1994;9:59–67. doi: 10.1002/jbmr.5650090109. [DOI] [PubMed] [Google Scholar]
- Kawana K., Takahashi M., Kushida K., Hoshino H., Sakata S., Inoue T. The effect of cyclosporin A administration on bone metabolism in the rat evaluated by biochemical markers. J. Endocrinol. Invest. 1996;19:499–504. doi: 10.1007/BF03349007. [DOI] [PubMed] [Google Scholar]
- Kirino S., Fukunaga J., Ikegami S., Tsuboi H., Kimata M., Nakata N. Regulation of bone metabolism in immunosuppressant (FK506)-treated rats. J. Bone Miner. Metab. 2004;22:554–560. doi: 10.1007/s00774-004-0523-1. [DOI] [PubMed] [Google Scholar]
- Kosugi K., Yonezu H., Kawashima S., Honda K., Arai Y., Shibahara T. A longitudinal study of the effect of experimental osteoporosis on bone trabecular structure in the rat mandibular condyle. Cranio. 2013;31:140–150. doi: 10.1179/crn.2013.022. [DOI] [PubMed] [Google Scholar]
- Kulak C.A.M., Cochenski Borba V.Z., Kulak J., Ribeiro Custódio M. Osteoporosis after solid organ transplantation. Minerva Endocrinol. 2012;37:221–231. [PubMed] [Google Scholar]
- Maalouf N.M., Shane E. Osteoporosis after solid organ transplantation. J. Clin. Endocrinol. Metab. 2005;90:2456–2465. doi: 10.1210/jc.2004-1978. [DOI] [PubMed] [Google Scholar]
- Monegal A., Navasa M., Guañabens N., Peris P., Pons F., Martinez de Osaba M.J. Bone disease after liver transplantation: a long-term prospective study of bone mass changes, hormonal status and histomorphometric characteristics. Osteoporos. Int. 2001;12:484–492. doi: 10.1007/s001980170094. [DOI] [PubMed] [Google Scholar]
- Monegal A., Navasa M., Peris P., Colmenero J., Cuervo A., Muxí A. Bone disease in patients awaiting liver transplantation. Has the situation improved in the last two decades? Calcif. Tissue Int. 2013;93:571–576. doi: 10.1007/s00223-013-9797-4. [DOI] [PubMed] [Google Scholar]
- Nassar P.O., Felipetti F.A., Nassar C.A., Spolidorio L.C. Evaluation of effect of cyclosporine A on the bone tissue with induced periodontal disease to ligature in rats. Transplant. Proc. 2013;45:778–782. doi: 10.1016/j.transproceed.2012.02.029. [DOI] [PubMed] [Google Scholar]
- Ramsey-Goldman R., Dunn J.E., Dunlop D.D., Stuart F.P., Abecassis M.M., Kaufman D.B. Increased risk of fracture in patients receiving solid organ transplants. J. Bone Miner. Res. 1999;14:456–463. doi: 10.1359/jbmr.1999.14.3.456. [DOI] [PubMed] [Google Scholar]
- Rissanen J.P., Suominen M.I., Peng Z., Morko J., Rasi S., Risteli J. Short-term changes in serum PINP predict long-term changes in trabecular bone in the rat ovariectomy model. Calcif. Tissue Int. 2008;82:155–161. doi: 10.1007/s00223-007-9101-6. [DOI] [PubMed] [Google Scholar]
- Romero D.F., Buchinsky F.J., Rucinski B., Cvetkovic M., Bryer H.P., Liang X.G. Rapamycin: a bone sparing immunosuppressant? J. Bone Miner. Res. 1995;10:760–768. doi: 10.1002/jbmr.5650100513. [DOI] [PubMed] [Google Scholar]
- Rubin M.R., Bilezikian J.P. Clinical review 151: the role of parathyroid hormone in the pathogenesis of glucocorticoid-induced osteoporosis: a re-examination of the evidence. J. Clin. Endocrinol. Metab. 2002;87:4033–4041. doi: 10.1210/jc.2002-012101. [DOI] [PubMed] [Google Scholar]
- Shane E., Rivas M.C., Silverberg S.J., Kim T.S., Staron R.B., Bilezikian J.P. Osteoporosis after cardiac transplantation. Am. J. Med. 1993;94:257–264. doi: 10.1016/0002-9343(93)90057-v. [DOI] [PubMed] [Google Scholar]
- Spolidorio L.C., Nassar P.O., Nassar C.A., Spolidorio D.M.P., Muscará M.N. Conversion of immunosuppressive monotherapy from cyclosporin a to tacrolimus reverses bone loss in rats. Calcif. Tissue Int. 2007;81:114–123. doi: 10.1007/s00223-007-9040-2. [DOI] [PubMed] [Google Scholar]
- Stempfle H.U., Werner C., Echtler S., Assum T., Meiser B., Angermann C.E. Rapid trabecular bone loss after cardiac transplantation using FK506 (tacrolimus)-based immunosuppression. Transplant. Proc. 1998;30:1132–1133. doi: 10.1016/s0041-1345(98)00181-x. [DOI] [PubMed] [Google Scholar]
- Thiébaud D., Krieg M.A., Gillard-Berguer D., Jacquet A.F., Goy J.J., Burckhardt P. Cyclosporine induces high bone turnover and may contribute to bone loss after heart transplantation. Eur. J. Clin. Invest. 1996;26:549–555. doi: 10.1046/j.1365-2362.1996.00170.x. [DOI] [PubMed] [Google Scholar]
- Tsuruoka S., Schwartz G.J., Ioka T., Yamamoto H., Ando H., Fujimura A. Citrate reverses cyclosporin A-induced metabolic acidosis and bone resorption in rats. Am. J. Nephrol. 2005;25:233–239. doi: 10.1159/000085969. [DOI] [PubMed] [Google Scholar]
- Wang T.K.M., O'Sullivan S., Gamble G.D., Ruygrok P.N. Bone density in heart or lung transplant recipients—a longitudinal study. Transplant. Proc. 2013;45:2357–2365. doi: 10.1016/j.transproceed.2012.09.117. [DOI] [PubMed] [Google Scholar]
- Zhu Ke H. Animal models for osteoporosis research. Bone. 2010;47:S349–S350. [Google Scholar]