Abstract
We examined the individual and combined effects of teriparatide and anti-RANKL (receptor activator of nuclear factor κB ligand) monoclonal antibody in ovariectomized mice. Three-month-old female C57BL/6 mice were ovariectomized (OVX) or sham operated. Four weeks after OVX, they were assigned to 3 different groups to receive anti-RANKL monoclonal antibody (Ab) alone (5 mg/kg single injection at 4 weeks after OVX, Ab group), teriparatide alone (80 μg/kg daily injection for 4 weeks from 4 weeks after OVX, PTH group), or mAb plus teriparatide (Ab + PTH group). Mice were sacrificed 8 weeks after OVX. Bone mineral density (BMD) was measured at the femur and lumbar spine. Hind limbs were subjected to histological and histomorphometric analysis. Serum osteocalcin and CTX-I levels were measured to investigate the bone turnover. Compared with Ab group, Ab + PTH group showed a significant increase in BMD at distal femur and femoral shaft. Cortical bone volume was significantly increased in PTH and Ab + PTH groups compared with Ab group. Bone turnover in Ab + PTH group was suppressed to the same degree as in Ab group. The number of TRAP-positive multinucleated cells was markedly reduced in Ab and Ab + PTH groups. These results suggest that combined treatment of teriparatide with anti-RANKL antibody has additive effects on BMD in OVX mice compared with individual treatment.
Keywords: RANKL, Parathyroid hormone, Ovariectomy, Osteoporosis, Combination therapy, Mouse
Highlights
-
•
The effects of anti-RANKL antibody and teriparitide in ovariectomized mice was studied.
-
•
Anti-RANKL antibody and teriparatide increased bone mass at the distinct regions.
-
•
Combination of anti-RANKL antibody and teriparatide had additive effects.
-
•
Teriparitide, but not anti-RANKL antibody, increased periosteal bone formation.
1. Introduction
Skeletal homeostasis is maintained in a strictly regulated balance between bone resorption and bone formation (Baron, 1989, Boyle et al., 2003, Harada and Rodan, 2003). Osteoclasts are multinucleated giant cells of hematopoietic origin and are responsible for both physiologic and pathologic bone resorption (Roodman, 1999, Roodman, 2004, Tanaka, 2007, Takayanagi et al., 2000). Receptor activator of nuclear factor κB ligand (RANKL) is a member of the tumor necrosis factor (TNF) superfamily cytokine and plays an essential role in the differentiation, function, and survival of osteoclasts (Lacey et al., 1998, Yasuda et al., 1998). The essential role of RANKL in osteoclastogenesis and bone resorption has been established by the findings that the targeted disruption of RANKL or its receptor RANK induced osteopetrosis in mice due to an impaired osteoclast differentiation (Dougall et al., 1999, Kong et al., 1999), while knockout of osteoprotegerin (OPG), a physiologic inhibitor of RANKL, induced severe osteoporosis caused by increased bone resorption by osteoclasts (Mizuno et al., 1998). RANKL–RANK pathways are also involved in pathologic bone loss such as postmenopausal osteoporosis, rheumatoid arthritis, and metastatic bone diseases (Takayanagi et al., 2000, Roodman, 2004). Therefore, regulating the RANKL–RANK pathway is one of the best therapeutic approaches to conquer these pathological conditions (Tanaka et al., 2005).
Denosumab is a fully human monoclonal antibody that specifically binds to human RANKL and strongly inhibits its interaction with RANK (Bekker et al., 2004, McClung et al., 2006, Lewiecki et al., 2007). Subcutaneous administration of 60 mg denosumab every 6 months resulted in a rapid reduction of bone turnover and a significant increase in bone mineral density (BMD) in postmenopausal women with low bone mass (McClung et al., 2006). In addition, denosumab significantly reduced vertebral, nonvertebral, and hip fracture risks in women with postmenopausal osteoporosis compared with placebo in the pivotal 3 year FREEDOM trial (Cummings et al., 2009).
On the other hand, intermittent injection of teriparatide (recombinant human PTH [1–34], PTH), is the only bone anabolic agent currently available for the treatment of osteoporosis. The precise mechanisms how PTH increases bone formation in vivo still remains elusive, but previous studies have shown that osteoclasts may be required for the anabolic effect of PTH (Black et al., 2003, Finkelstein et al., 2003). There has been a controversy regarding the combination therapy of teriparatide and anti-resorptive agents such as bisphosphonates. Previous clinical studies have indicated that amino bisphosphonate treatment does not augment the anabolic effect of PTH (Finkelstein et al., 2003, Finkelstein et al., 2006, Tsai et al., 2013). However, recent clinical study demonstrated the additive effects of denosumab on PTH-induced BMD increase (Tsai et al., 2013) although previous animal studies of combination of RANKL inhibitors and PTH have not shown consistent results (Furuya et al., 2011, Samadfam et al., 2007). In this study, we examined the effect of combined anti-RANKL monoclonal antibody and PTH in ovariectomized mice to uncover the mechanism of action of the combination therapy.
2. Materials and methods
2.1. Reagents and animals
Anti-murine monoclonal RANKL antibody (OYC1, hereinafter referred to Ab, Orient Yeast Co., Tokyo, Japan) was obtained as previously reported (Furuya et al., 2011). Teriparatide was provided from Asahikasei Pharmaceutical Co. Ltd. (Tokyo, Japan). Twelve-week-old virgin female C57BL/6 N mice were purchased from Sankyo Labo Service Co. (Tokyo, Japan). All mice were housed under specific pathogen-free conditions and exposed to a 12-h light–12-h dark cycle and treated with humane care under the approval of the Animal Care and Use Committee of the University of Tokyo.
2.2. Treatment protocols
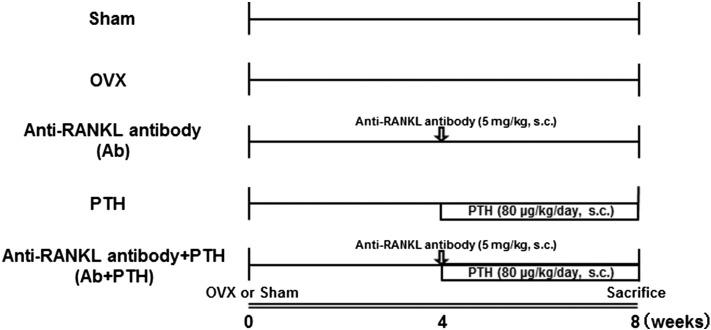
The study design is shown schematically in Fig. 1. Mice were assigned to five different groups. Four groups were ovariectomized (OVX) and one group was sham operated (Sham). Four weeks after the surgeries, mice in the OVX groups were either untreated (OVX group) or treated with Ab (single injection of 5 mg/kg) (Ab group), teriparatide (80 μg/kg/day for 4 weeks) (PTH group), or antibody plus PTH (Ab + PTH group). All mice were sacrificed 8 weeks after the operation. Sera was obtained using capillary blood collection tube with serum separator (Becton, Dickinson and Company, Sparks, MD), and concentrations of C-telopeptide (CTx) (RatLaps ELISA; Nordic Bioscience, Herlev, Denmark) and osteocalcin (mouse osteocalcin EIA, Biomedical Technologies Inc., Stoughton, MA) were measured.
Fig. 1.
Experimental protocol.
Mice were assigned to five different groups (n = 5–6). Four groups were ovariectomized (OVX), and one group was sham operated and remained untreated for 4 weeks after the operation. Four weeks after surgery, treatment with anti-RANKL mAb (5 mg/kg s.c., once at 4 weeks after OVX), PTH (80 μg/kg/day s.c., daily injection for 4 weeks), or Ab plus PTH was started in the OVX groups. All mice were sacrificed 8 weeks after operation.
2.3. Radiological analysis
Micro CT scanning of the distal femur was performed using a ScanXmate-L090 Scanner (Comscantechno Co., Ltd., Yokohama, Japan). Three-dimensional microstructural image data were reconstructed and structural indices were calculated using TRI/3D-BON software (RATOC Systems, Osaka, Japan). BMD in the femur and lumbar spine was measured using a bone mineral analyzer (PIXImus Densitometer; GE Medical Systems, Waukesha, WI).
2.4. Histological and histomorphometric analysis
For histological analysis, fixed and undecalcified femurs were embedded in glycol methacrylate, and 3 μm sections in the distal femur were longitudinally cut and stained with toluidine blue (TB) or tartrate-resistant acid phosphatase (TRAP). Histomorphometric measurements were made at × 400 magnification in the secondary spongiosa area of distal femur. For double labeling, mice were injected subcutaneously with 20 mg/kg body weight of tetracycline hydrochloride on day 4 and 16 mg/kg body weight of calcein on day 1 before sacrifice.
2.5. Statistical analysis
Each series of experiments was repeated at least three times. The results are expressed as the mean ± SD. Statistical analyses were performed using a two-tailed unpaired Student's t test or ANOVA analysis.
3. Results
3.1. Effects of anti-RANKL antibody and PTH on OVX mice
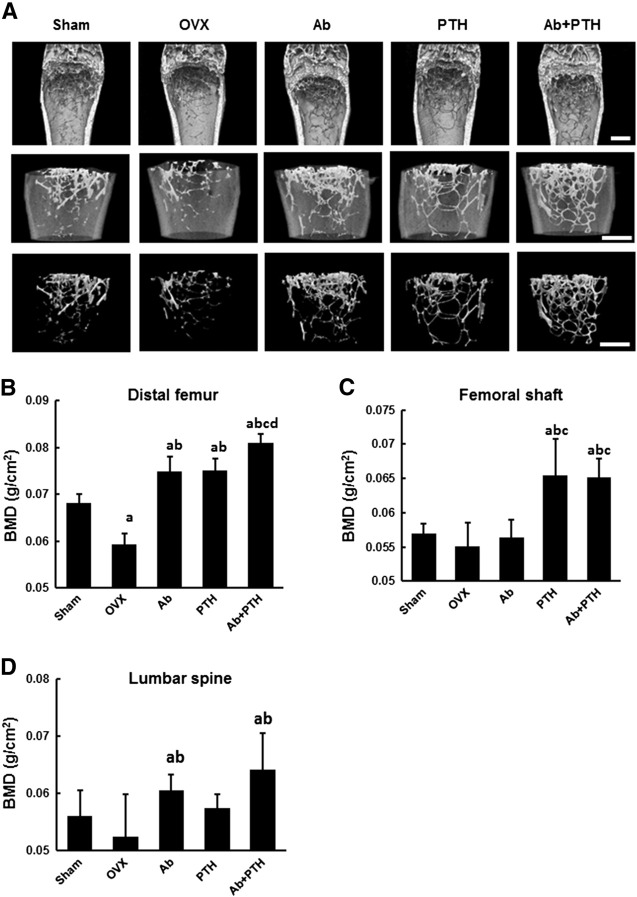
In the distal femur, Ab and PTH groups showed a significant increase in BMD compared to OVX group and Ab + PTH group exhibited a greater increase than Ab and PTH groups (Fig. 2B). The additive effect of Ab and PTH was confirmed by micro CT analysis (Fig. 2A). In contrast, PTH and Ab + PTH treatment exhibited a significant increase in BMD in the femoral shaft, which was not observed in Ab group (Fig. 2C). In the lumbar spine, Ab and Ab + PTH groups showed a significant increase in BMD (Fig. 2D).
Fig. 2.
Effect of anti-RANKL monoclonal antibody on the anabolic effect of PTH.
(A) Representative micro CT of the distal femur of the sham, OVX, Ab, PTH, and Ab + PTH groups at 8 weeks after surgery. Scale bars: 800 μm. (B) BMD of the distal femur at 8 weeks after surgery, i.e., after 4 weeks of treatment with anti-RANKL mAb, PTH, or Ab + PTH. (a) p < 0.01 vs. Sham, (b) p < 0.01 vs. OVX, (c) p < 0.01 vs. Ab, (d) p < 0.01 vs. PTH. (C) BMD of the femoral shaft at 8 weeks after surgery, i.e., after 4 weeks of treatment with anti-RANKL mAb, PTH, or Ab + PTH. (a) p < 0.01 vs. Sham, (b) p < 0.01 vs. OVX, (c) p < 0.01 vs. Ab. (D) BMD of the lumbar spine at 8 weeks after surgery, i.e., after 4 weeks of treatment with anti-RANKL mAb, PTH, or Ab + PTH. (a) p < 0.05 vs. Sham, (b) p < 0.05 vs. OVX. Data are shown as the mean ± SD (n = 5–6).
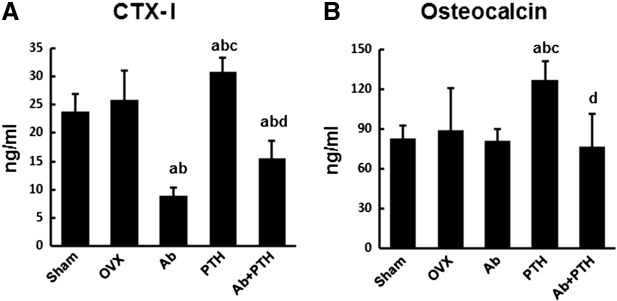
We then analyzed the serum bone turnover markers, CTX-I and osteocalcin, 8 weeks after ovariectomy. Serum CTX-I levels were significantly suppressed in Ab group and increased in PTH group compared to OVX group. CTX-I levels were also suppressed in Ab + PTH group, and no significant difference was observed between Ab and Ab + PTH groups (Fig. 3A). Osteocalcin levels were significantly higher in PTH groups than OVX group, but no significant change was observed in Ab and Ab + PTH groups (Fig. 3B). These results indicate that the bone turnover is maintained at low levels by anti-RANKL antibody treatment even after PTH treatment.
Fig. 3.
Serum concentration of bone metabolic markers.
(A) Serum CTX-I. (B) Serum osteocalcin. (a) p < 0.01 vs. Sham, (b) p < 0.01 vs. OVX, (c) p < 0.01 vs. Ab, (d) p < 0.01 vs. PTH. Data are mean ± SD (n = 5–6).
3.2. Histology and histomorphometry
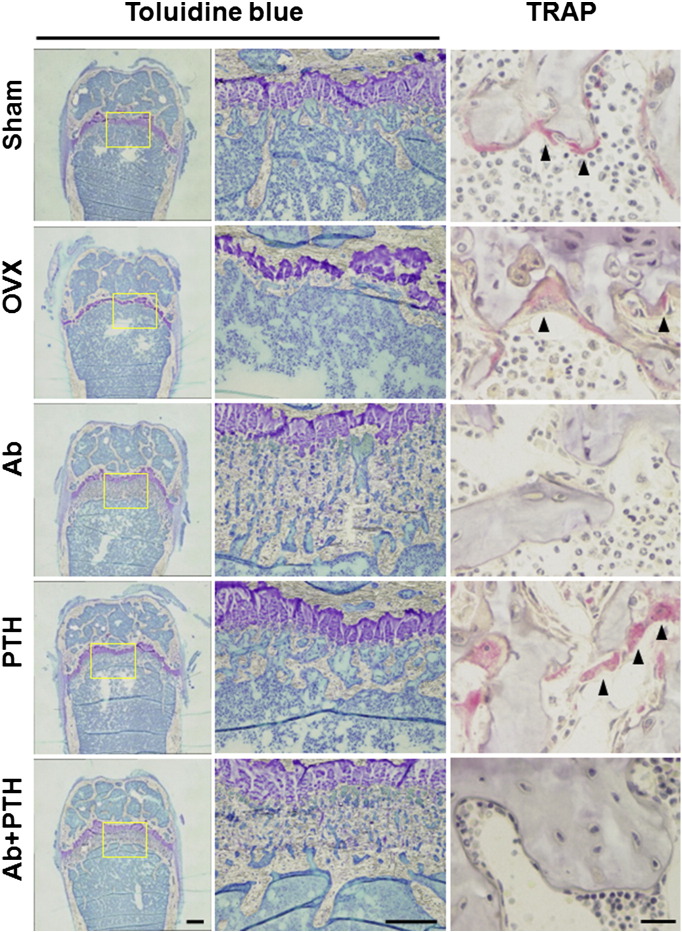
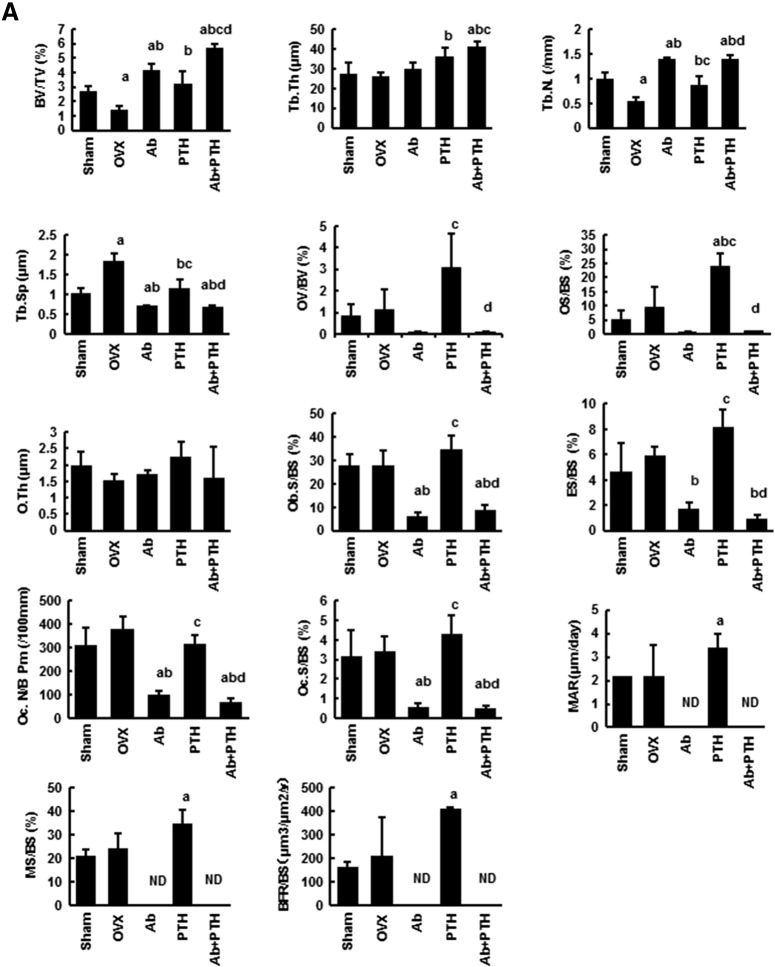
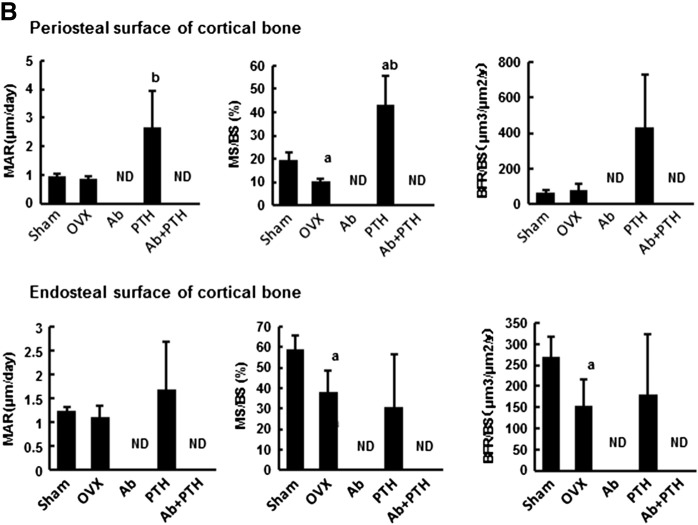
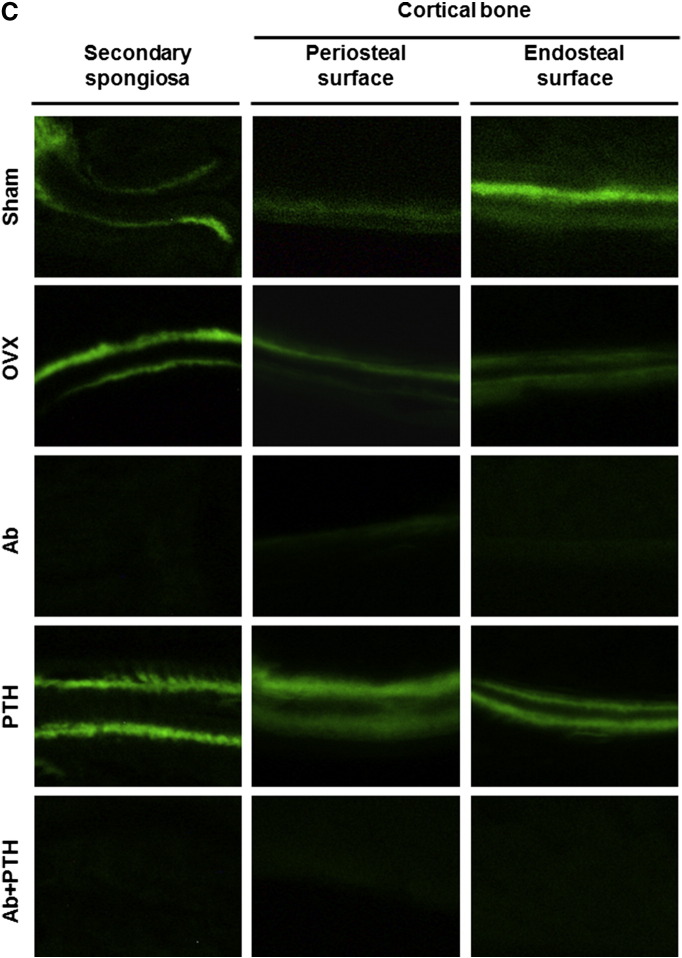
As shown in Fig. 4 by toluidine blue staining of the distal femur, Ab treatment and PTH treatment increased BMD at the distinct regions. Ab treatment mainly increased BMD in the primary spongiosa region, while PTH increased that in the secondary spongiosa. Ab + PTH treatment increased BMD in both primary and secondary spongiosa regions (Fig. 4). TRAP staining of the distal femur showed a marked decrease in osteoclasts in Ab and Ab + PTH groups and increase in PTH group (Fig. 4). Histomorphometric analysis in the trabecular bone in the distal femur demonstrated a significant increase in BV/TV in Ab and PTH groups, and the combination of Ab and PTH exhibited an additive effect. Ab treatment markedly decreased osteoblast parameters such as OV/BV, OS/BS, Ob.S/BS, MAR, MS/BS, and BFR/BS as well as osteoclast parameters such as ES/BS, OcN/BS, and Oc.S/BS, and these parameters remained low even in the combination of Ab with PTH (Fig. 5A). In the cortical bone region (femoral shaft), marked increase in MAR and MS/BS was observed by PTH treatment in the periosteal surface, which was strongly suppressed by combination treatment with Ab. In contrast, in the endosteal surface, significant reduction in MAR, MS/BS, and BFR/BS was observed in Ab-treated mice, which was not recovered by treatment with PTH (Fig. 5B). Representative data of double labeling are provided in Fig. 5C.
Fig. 4.
Distal femur stained with toluidine blue or TRAP.
Decalcified sections of distal femur in sham, OVX, Ab, PTH, or Ab + PTH group stained with toluidine blue are shown at low magnification (left panel) and higher magnification (middle panel). Bars: 100 μm (left) and 50 μm (middle). Decalcified sections of distal femur in sham, OVX, Ab, PTH, or Ab + PTH group stained with TRAP (right panel). Bar: 20 μm.
Fig. 5.
Bone histomorphometry of the secondary spongiosa in distal femur and femoral shaft.
(A) Histomorphometry of distal femur. Histomorphometric parameters: the bone volume/tissue volume (BV/TV, %), trabecular bone thickness (Tb.Th, μm), trabecular number (Tb. N.,/mm), trabecular bone separation (Tb.S., μm), osteoid volume/bone volume (OV/BV, %), osteoid surface/bone surface (OS/BS, %), and osteoid thickness (O.Th, μm). Osteoblast surface/bone surface (Ob.S/BS, %), eroded surface/bone surface (ES/BS, %), osteoclast number/bone perimeter (Oc N./B. Pm,/100 mm), osteoclast surface/bone surface (Oc.S/BS, %), mineral apposition rate (MAR, μm/day), mineralizing surface per bone surface (MS/BS, %), and bone formation rate/bone surface (BFR/BS, mm3/mm2/year). ND: not detected. (a) p < 0.05 vs. Sham, (b) p < 0.05 vs. OVX, (c) p < 0.05 vs. Ab, (d) p < 0.05 vs. PTH. Data are mean ± SD (n = 3–4). (B) Histomorphometry of femoral shaft. ND: not detected. (a) p < 0.05 vs. Sham, (b) p < 0.05 vs. OVX, (c) p < 0.05 vs. Ab, (d) p < 0.05 vs. PTH. Data are mean ± SD (n = 3–4). (C) Representative pictures of double labeling with tetracycline hydrochloride and calcein.
4. Discussion
The usefulness of combination therapy of anti-resorptives with anabolic agents in osteoporosis patients is still controversial. Previous studies have demonstrated that combination with bisphosphonates such as alendronic acid and zoledronic acid does not exert additive effects on PTH-induced increase in BMD in the clinical setting (Black et al., 2003, Finkelstein et al., 2003, Cosman et al., 2011, Li et al., 2012). This is at least partly because suppression of bone resorption inhibits bone resorption-dependent release of coupling factors from bone matrix. However, several animal studies showed that PTH exhibits anabolic effects even in the presence of anti-resorptives. Pierroz et al. (2010) demonstrated that treatment of RANK −/− mice, which completely lack osteoclasts, with high dose PTH still increased serum osteocalcin and trabecular BMD. In addition, recent clinical study showed that denosumab and PTH had additive effects on BMD (Tsai et al., 2013).
We previously reported that a single subcutaneous injection of anti-RANKL antibody OYC1 markedly augmented BMD in normal mice. We here analyzed the effect of combination treatment of intermittent PTH and anti-RANKL antibody in ovariectomized mice (Furuya et al., 2011). Consistent with the recent clinical results of combined PTH and denosumab treatment, we found that Ab had additive effects on PTH in BMD increase in the distal femur. In contrast, Ab did not appear to increase BMD of femoral shaft, or have additive effects on PTH, indicating that the additive effects of Ab on PTH were only observed in the trabecular bone. Interestingly, histological analysis exhibited that these two reagents increased BMD at distinct regions. Ab mainly increased BMD at the primary spongiosa region, while PTH did so at the secondary spongiosa region. The combination of Ab and PTH increased both primary and secondary spongiosa regions. In addition, at the cortical regions, PTH increased bone formation at the periosteal surface but not at the endosteal surface, which was completely suppressed by Ab. PTH did not increase bone formation or bone resorption at the endosteal surface, while Ab treatment suppressed both bone formation and bone resorption. These results suggest that these two reagents have distinct properties in the function of increasing BMD, in particular, their target regions. Pierroz et al. (2010) showed that combined treatment of PTH with denosumab in human RANKL transgenic mice did not have additive effects on BMD as compared to denosumab alone. They discussed that this may be because too much suppression of bone resorption impairs the anabolic effects of PTH. Although the reason for the discrepancy between their results and our data is not clear, we speculate that the bone dynamics in human RANKL transgenic mice may be somehow different from that in OVX mice.
We found that Ab and PTH increased BMD at the distinct regions of the distal femur, primary spongiosa, and secondary spongiosa, and the combination of Ab and PTH increased BMD in both of these regions. In addition, PTH, but not Ab, increased periosteal bone formation, and Ab treatment suppressed PTH-induced periosteal bone formation. Consistent with this observation, there were no additive effect of Ab on PTH-induced BMD increase in the femoral shaft. There are limitations in the present study. In particular, this is a mouse study, and it may not be directly applied to humans. In addition, molecular basis of the site-specific effect of Ab and PTH is not clear. Future human study will reveal the effect of the combination therapy on the skeletal tissues in a further detail.
Edited by: Peter Ebeling
References
- Baron R. Molecular mechanisms of bone resorption by the osteoclast. Anat. Rec. 1989;224:317–324. doi: 10.1002/ar.1092240220. [DOI] [PubMed] [Google Scholar]
- Bekker P.J., Holloway D.L., Rasmussen A.S., Murphy R., Martin S.W., Leese P.T., Holmes G.B., Dunstan C.R., DePaoli A.M. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J. Bone Miner. Res. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- Black D.M., Greenspan S.L., Ensrud K.E., Palermo L., McGowan J.A., Lang T.F., Garnero P., Bouxsein M.L., Bilezikian J.P., Rosen C.J. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N. Engl. J. Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Cosman F., Eriksen E.F., Recknor C., Miller P.D., Guanabens N., Kasperk C., Papanastasiou P., Readie A., Rao H., Gasser J.A., Bucci-Rechtweg C., Boonen S. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1–34)] in postmenopausal osteoporosis. J. Bone Miner. Res. 2011;26:503–511. doi: 10.1002/jbmr.238. [DOI] [PubMed] [Google Scholar]
- Cummings S.R., San Martin J., McClung M.R., Siris E.S., Eastell R., Reid I.R., Delmas P., Zoog H.B., Austin M., Wang A., Kutilek S., Adami S., Zanchetta J., Libanati C., Siddhanti S., Christiansen C. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- Dougall W.C., Glaccum M., Charrier K., Rohrbach K., Brasel K., De Smedt T., Daro E., Smith J., Tometsko M.E., Maliszewski C.R., Armstrong A., Shen V., Bain S., Cosman D., Anderson D., Morrissey P.J., Peschon J.J., Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein J.S., Hayes A., Hunzelman J.L., Wyland J.J., Lee H., Neer R.M. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N. Engl. J. Med. 2003;349:1216–1226. doi: 10.1056/NEJMoa035725. [DOI] [PubMed] [Google Scholar]
- Finkelstein J.S., Leder B.Z., Burnett S.M., Wyland J.J., Lee H., de la Paz A.V., Gibson K., Neer R.M. Effects of teriparatide, alendronate, or both on bone turnover in osteoporotic men. J. Clin. Endocrinol. Metab. 2006;91:2882–2887. doi: 10.1210/jc.2006-0190. [DOI] [PubMed] [Google Scholar]
- Furuya Y., Mori K., Ninomiya T., Tomimori Y., Tanaka S., Takahashi N., Udagawa N., Uchida K., Yasuda H. Increased bone mass in mice after single injection of anti-receptor activator of nuclear factor-kappaB ligand-neutralizing antibody: evidence for bone anabolic effect of parathyroid hormone in mice with few osteoclasts. J. Biol. Chem. 2011;286:37023–37031. doi: 10.1074/jbc.M111.246280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S., Rodan G.A. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- Kong Y.Y., Yoshida H., Sarosi I., Tan H.L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A.J., Van G., Itie A., Khoo W., Wakeham A., Dunstan C.R., Lacey D.L., Mak T.W., Boyle W.J., Penninger J.M. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Lacey D.L., Timms E., Tan H.L., Kelley M.J., Dunstan C.R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S., Hsu H., Sullivan J., Hawkins N., Davy E., Capparelli C., Eli A., Qian Y.X., Kaufman S., Sarosi I., Shalhoub V., Senaldi G., Guo J., Delaney J., Boyle W.J. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Lewiecki E.M., Miller P.D., McClung M.R., Cohen S.B., Bolognese M.A., Liu Y., Wang A., Siddhanti S., Fitzpatrick L.A. Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J. Bone Miner. Res. 2007;22:1832–1841. doi: 10.1359/jbmr.070809. [DOI] [PubMed] [Google Scholar]
- Li Y.F., Zhou C.C., Li J.H., Luo E., Zhu S.S., Feng G., Hu J. The effects of combined human parathyroid hormone (1–34) and zoledronic acid treatment on fracture healing in osteoporotic rats. Osteoporos. Int. 2012;23:1463–1474. doi: 10.1007/s00198-011-1751-6. [DOI] [PubMed] [Google Scholar]
- McClung M.R., Lewiecki E.M., Cohen S.B., Bolognese M.A., Woodson G.C., Moffett A.H., Peacock M., Miller P.D., Lederman S.N., Chesnut C.H., Lain D., Kivitz A.J., Holloway D.L., Zhang C., Peterson M.C., Bekker P.J. Denosumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2006;354:821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- Mizuno A., Amizuka N., Irie K., Murakami A., Fujise N., Kanno T., Sato Y., Nakagawa N., Yasuda H., Mochizuki S., Gomibuchi T., Yano K., Shima N., Washida N., Tsuda E., Morinaga T., Higashio K., Ozawa H. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem. Biophys. Res. Commun. 1998;247:610–615. doi: 10.1006/bbrc.1998.8697. [DOI] [PubMed] [Google Scholar]
- Pierroz D.D., Bonnet N., Baldock P.A., Ominsky M.S., Stolina M., Kostenuik P.J., Ferrari S.L. Are osteoclasts needed for the bone anabolic response to parathyroid hormone? A study of intermittent parathyroid hormone with denosumab or alendronate in knock-in mice expressing humanized RANKL. J. Biol. Chem. 2010;285:28164–28173. doi: 10.1074/jbc.M110.101964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodman G.D. Cell biology of the osteoclast. Exp. Hematol. 1999;27:1229–1241. doi: 10.1016/s0301-472x(99)00061-2. [DOI] [PubMed] [Google Scholar]
- Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- Samadfam R., Xia Q., Goltzman D. Pretreatment with anticatabolic agents blunts but does not eliminate the skeletal anabolic response to parathyroid hormone in oophorectomized mice. Endocrinology. 2007;148:2778–2787. doi: 10.1210/en.2006-1475. [DOI] [PubMed] [Google Scholar]
- Takayanagi H., Iizuka H., Juji T., Nakagawa T., Yamamoto A., Miyazaki T., Koshihara Y., Oda H., Nakamura K., Tanaka S. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43:259–269. doi: 10.1002/1529-0131(200002)43:2<259::AID-ANR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Tanaka S. Signaling axis in osteoclast biology and therapeutic targeting in the RANKL/RANK/OPG system. Am. J. Nephrol. 2007;27:466–478. doi: 10.1159/000106484. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Nakamura K., Takahasi N., Suda T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL–RANK signaling system. Immunol. Rev. 2005;208:30–49. doi: 10.1111/j.0105-2896.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- Tsai J.N., Uihlein A.V., Lee H., Kumbhani R., Siwila-Sackman E., McKay E.A., Burnett-Bowie S.A., Neer R.M., Leder B.Z. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013;382:50–56. doi: 10.1016/S0140-6736(13)60856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., Tomoyasu A., Yano K., Goto M., Murakami A., Tsuda E., Morinaga T., Higashio K., Udagawa N., Takahashi N., Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]