Abstract
Background
The burden of osteoporosis in the Asia-Pacific region is not well characterized. The Medication Use Patterns, Treatment Satisfaction, and Inadequate Control of Osteoporosis Study in the Asia-Pacific Region (MUSIC OS-AP) was designed to better understand the association of gastrointestinal events with patient-reported outcomes in postmenopausal women of this region.
Methods
MUSIC OS-AP is a prospective, multinational, observational cohort study of postmenopausal women ≥ 50 years of age diagnosed with osteoporosis. The study was conducted in five Asia-Pacific countries: Australia, New Zealand, Taiwan, Korea, and India. MUSIC OS-AP has three components: a physician questionnaire, a retrospective chart review, and a prospective cohort study. The physician questionnaire investigated the role of gastrointestinal events in physicians' pharmacologic management of osteoporosis. The retrospective chart review, also completed by physicians, recorded rate of osteoporosis treatment and the types of osteoporosis medications prescribed to osteoporosis patients. The prospective cohort study investigated the associations between gastrointestinal events and patient-reported outcomes among patients taking oral medications for osteoporosis as well as reasons for non-treatment in patients who remained untreated.
The prospective cohort study enrolled two groups of patients: untreated, and treated with oral osteoporosis medications. Untreated patients completed only the baseline surveys, providing information on gastrointestinal event rates, quality of life, health care resource use, and reasons for non-treatment. Treated patients, who were either new to osteoporosis medication or continuing an ongoing medication course, completed surveys at baseline and 3, 6, and 12 months post-baseline. The evaluations recorded patient characteristics, gastrointestinal events, health-related and osteoporosis-specific quality of life, health care resource use, medication adherence, and satisfaction with treatment.
Results
Physicians at 59 sites completed the physician questionnaire, and data for 300 patients from 26 sites were abstracted for the retrospective chart review. Enrollment and baseline data collection for the prospective cohort study were conducted between July 2013 and August 2014 for 301 untreated and 3287 treated patients, of whom 1416 were new users and 1871 were experienced users of oral osteoporosis medications.
Conclusions
The results of MUSIC OS-AP will highlight the association of gastrointestinal events with patient-reported outcomes among postmenopausal women with osteoporosis and elucidate physicians' management of gastrointestinal events among this patient population in the Asia-Pacific region.
Abbreviations: ADEOS, Adherence Evaluation of Osteoporosis treatment; BMD, bone mineral density; EQ-5D-3L, European Quality of Life-5 Dimensions; GI, gastrointestinal; MUSIC-OS, Medication Use Patterns, Treatment Satisfaction, and Inadequate Control of Osteoporosis Study; OPAQ-SV, Osteoporosis Assessment Questionnaire; OPSAT-Q, Osteoporosis Patient Satisfaction Questionnaire
Keywords: Osteoporosis, Postmenopausal, Research design, Gastrointestinal diseases, Patient satisfaction, Quality of life, Medication adherence
Highlights
-
•
The burden of osteoporosis in the Asia-Pacific region is not well characterized.
-
•
MUSIC OS-AP is a prospective, multinational cohort study of osteoporotic women.
-
•
Study subjects are from Australia, New Zealand, Taiwan, Korea, and India.
-
•
The study will assess the association of GI events with patient-reported outcomes.
1. Introduction
The prevalence of osteoporosis in older women (i.e., aged ≥ 50) living in Asia-Pacific countries is comparable to that in Europe and North America (Wade et al., 2014), with values ranging from 23% in Australia (Henry et al., 2011) to 38% in Korea (Park et al., 2014) and 50% or higher in India (Meeta et al., 2013). The World Health Organization found that, in the year 2000, approximately 45% of osteoporotic fractures occurring worldwide were in Southeast Asian and Western Pacific countries, including Australia, New Zealand, China, and Korea (Johnell & Kanis, 2006).
Although osteoporosis is common in the Asia-Pacific region, information on the pharmacologic treatment of osteoporosis, e.g., treatment rates, treatment satisfaction, and the effects of treatment on health care resource use and quality of life, is scarce. In Australia, the self-reported rate of pharmacologic treatment of osteoporosis in a population-based sample aged ≥ 50 years was 54.1% (Gill et al., 2012), and in Korea, the rate of administration of osteoporosis drugs to postmenopausal women with physician-diagnosed osteoporosis was 42.1% (Lee et al., 2014). Bisphosphonates were the most commonly prescribed pharmacotherapy in both studies (Gill et al., 2012, Lee et al., 2014), and alendronate was the most commonly used bisphosphonate in the Australian study (Gill et al., 2012). Beyond this, little is known about the management of osteoporosis in Asia-Pacific countries or about osteoporosis patients' experience with pharmacologic treatment. In particular, the gastrointestinal (GI) events that are so common among users of osteoporosis therapy in US (Tosteson et al., 2003) and European (Ringe and Moller, 2009, Payer et al., 2009) populations are virtually uncharacterized in patients in the Asia-Pacific region.
The Medication Use Patterns, Treatment Satisfaction, and Inadequate Control of Osteoporosis Study in the Asia-Pacific Region (MUSIC OS-AP) was designed to address this information gap by querying physicians' approaches to treating patients with GI events, determining rates of pharmacologic treatment and reasons for non-treatment, and assessing the association of GI events with treatment patterns (i.e., adherence, persistence, discontinuation, switching) and patient-reported outcomes (i.e., treatment satisfaction, health care resource use, and quality of life) in osteoporosis patients in the Asia-Pacific region.
The primary objectives of MUSIC OS-AP were to describe: (i) the frequency of GI events among postmenopausal women receiving pharmacologic treatment for osteoporosis; (ii) the association between GI events and adherence to, discontinuation of, and switching between osteoporosis medications; and (iii) the association between GI events and health-related quality of life, treatment satisfaction, and health care resource utilization. Secondary objectives were: (i) to describe the physician's approach to the management of osteoporosis patients with GI events; (ii) to estimate the rates of pharmacologic treatment and non-treatment of osteoporosis; and (iii) to determine the factors associated with the decision of whether or not to treat osteoporosis with pharmacotherapy in clinical practice.
2. Materials and methods
2.1. Study design
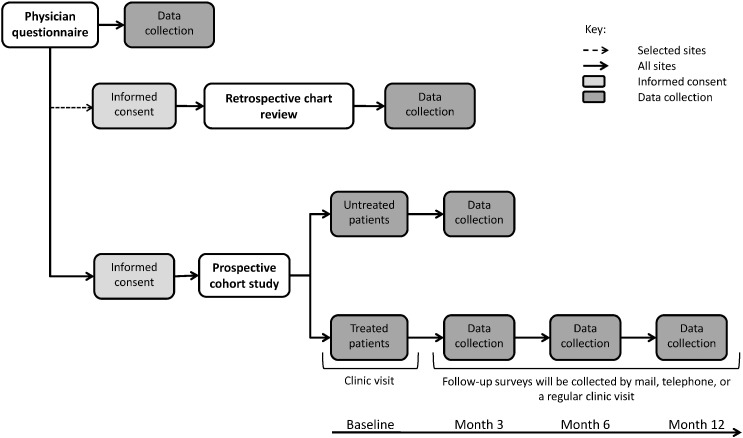
The design of MUSIC OS-AP is shown in Fig. 1. Details of the design of MUSIC OS are given in the publication describing the closely related MUSIC OS-EU study, which was carried out in Canada and Europe (Modi et al., 2015). Like the EU study, MUSIC OS-AP included three components: a physician questionnaire, a retrospective chart review, and a prospective cohort study. Sites were selected for participation in the study based on their responses to the Site Assessment Questionnaire and in such a way that their distribution was representative of real world clinical practice for osteoporosis in each country. Factors such as the investigator's experience in conducting clinical research, interest in participation, and ability to dedicate time and resources to the study were considered. Site investigators completed the physician questionnaire and retrospective review of patients' charts. All sites completed ethics reviews according to their local ethics board requirements. Patients fulfilling the selection criteria were allocated to either the treated or untreated patient group of the prospective cohort study. Baseline assessments were conducted for all patients at the enrollment visit, while follow-up questionnaires were completed only by treated patients at 3, 6, and/or 12 months after enrollment (Fig. 1).
Fig. 1.
Design of MUSIC OS-AP.
2.2. Physician questionnaire
The physician questionnaire collected information regarding the physician's standard practices regarding the diagnosis and treatment of patients with osteoporosis and his or her perspective on current osteoporosis treatment approaches and medication adherence. Treatment approaches were examined specifically with regards to the management of patients with GI events by asking how often physicians witnessed GI events (e.g., heartburn, upset stomach, nausea, or pain) and how often GI sensitivity impacted their decision to prescribe osteoporosis treatment and their choice of medication. Physicians were asked about their treatment strategies (e.g., prescribe a gastroprotective agent, recommend a drug holiday, or switch to another medication) with patients who either had pre-existing GI problems or developed GI problems while taking osteoporosis therapy.
2.3. Retrospective chart review
In the retrospective chart review, the questions were limited to whether the patient was receiving pharmacologic therapy—oral or injected, calcium and/or vitamin D, or none—for their osteoporosis. Physicians from 26 randomly selected sites answered the questions on behalf of their most recent patients.
2.4. Prospective cohort study
The prospective cohort component of this study enrolled both untreated and treated patients. Eligibility and exclusion criteria were the same as in the MUSIC OS-EU study (Modi et al., 2015). Briefly, patients were eligible for enrollment in the prospective cohort study if they were postmenopausal women, at least 50 years of age, had osteoporosis in their physician's judgment (with or without a BMD test), and provided informed consent. Patients were excluded if they had been diagnosed with Parkinson's disease or any other neuromuscular disease or Paget's disease; were currently treated with any injected medication for osteoporosis; had been switched between oral pharmacologic osteoporosis medications within the past 3 months; or were currently or formerly (past 90 days) enrolled in a clinical trial.
The untreated study arm comprised a maximum of 300 participants. Simultaneously, a maximum of 3300 participants with osteoporosis who were receiving oral pharmacologic agents were targeted for enrollment in the treated group. Pharmacologic treatments included bisphosphonates (e.g., alendronate, risedronate, and ibandronate), calcitonin, strontium ranelate, and selective estrogen-receptor modulators (raloxifene and bazedoxifene). Calcium and/or vitamin D and estrogen and/or hormone replacement therapy were considered supplemental to pharmacologic therapy. Treated participants were further classified as new users or experienced users, defined respectively as patients who had been receiving oral pharmacologic therapy for less than 3 months and patients receiving the same oral pharmacologic therapy for at least 3 months prior to enrollment.
Untreated patients completed only the baseline survey (Table 1). Demographics, risk factors for osteoporosis, fracture history, medical history, osteoporosis medications, bone mineral density (BMD) test results (T-scores), and vitamin D/calcium use were documented, as were reasons for non-treatment and GI events in the prior 6 months. In addition, health care resource utilization in the prior 3 months, and quality of life over the prior 2 weeks, were assessed.
Table 1.
Schedule of assessments.
| Baseline (office visit) |
Follow-up assessmentsa |
|||
|---|---|---|---|---|
| 3 months | 6 months | 12 months | ||
| Physician questionnaireb | √ | |||
| Retrospective chart reviewb | √ | |||
| Informed consent and eligibility | √ | |||
| Demographics and risk factors for osteoporosis | √ | |||
| Osteoporosis disease assessment | √ | |||
| Medical history | √ | |||
| Bone mineral density test/T-scorec | √ | √ | √ | √ |
| Participant treatment concernsd | √ | √ | √ | √ |
| Medications (osteoporosis, GI, vitamin D and calcium)e | √ | √ | √ | √ |
| Gastrointestinal events | √ | √ | √ | √ |
| Healthcare resource utilization questionnaire | √ | √ | √ | √ |
| ADEOS adherence scalea | √ | √ | √ | √ |
| Treatment satisfaction questionnaire for medications (OPSAT-Q)a | √ | √ | √ | |
| Health related quality of life (EQ-5D-3L) | √ | √ | √ | |
| Health related quality of life (OPAQ-SV) | √ | √ | √ | |
| New falls and fractures | √ | √ | √ | |
| Adverse eventsc | √ | √ | √ | |
ADEOS, Adherence Evaluation of Osteoporosis treatment; EQ-5D-3L, European Quality of Life-5 Dimensions; GI, gastrointestinal; OPAQ-SV, Osteoporosis Assessment Questionnaire; OPSAT-Q, Osteoporosis Patient Satisfaction Questionnaire.
Not collected for untreated participants. Study completion/premature discontinuation form was collected for all treated participants. If participant discontinued prior to 12 month follow-up visit, the reason for discontinuation, including death and death date (if applicable), was recorded.
The physician questionnaire and retrospective chart review were completed prior to the commencement of the prospective component of the study. Physician questionnaire was completed by the Principal Investigator at all sites. The retrospective chart review was completed by a randomly selected subset of sites.
Collected by the physician during any clinical office visit, if applicable.
Collected for untreated and treated participants at baseline and for treated patients thereafter.
At baseline, all applicable medications were recorded, while during follow-up only those medications that had been changed or discontinued were recorded.
Treated study participants completed the baseline survey and were then followed for 12 months. Data collected at baseline only were: patient demographics, risk factors for osteoporosis, fracture history, medical history, osteoporosis medications, BMD test results (T-scores), and vitamin D/calcium use. Patient-reported outcomes collected at baseline and all follow-up visits included GI events, health care resource use, and medication adherence. Health-related quality of life, treatment satisfaction, and adverse events were assessed in treated patients at follow-up visits according to the schedule shown in Table 1.
GI events were defined as the following clinical symptoms: heartburn/acid reflux, upset stomach/indigestion, nausea/vomiting, pain behind the breastbone, pain on swallowing or food sticking, stomach pain above or below the navel, diarrhea or constipation, and bloating. General health-related and osteoporosis-specific quality of life were measured by the European Quality of Life-5 Dimensions (EQ-5D-3 L) questionnaire (Oemar & Oppe, n.d.) and the Osteoporosis Assessment Questionnaire (OPAQ-SV) (Randell et al., 1998), respectively. Patient-reported health care resource use was categorized as hospitalizations, fractures, surgeries, or visits to a general practitioner, specialist, or emergency department in the preceding 3–6 months. Medication adherence was measured by the Adherence Evaluation of Osteoporosis treatment (ADEOS) questionnaire (Breuil et al., 2012), and treatment satisfaction by the Osteoporosis Patient Satisfaction Questionnaire (OPSAT-Q) (Flood et al., 2006). Untreated participants did not complete the ADEOS or OPSAT-Q questionnaires. Changes in osteoporosis medications (new medications and medication discontinuations), occurrences of new falls, fractures, and adverse events were also recorded at the follow-up visits.
All questionnaires were made available in the local language of the participating clinics, as follows: in Australia and New Zealand, English; in Korea, Korean; in Taiwan, traditional Chinese; and in India, Bengali, Gujarati, Hindi, Kannada, Marathi, Tamil, Telugu, or English (as needed). The translations of the self-administered and telephone versions of the EQ-5D-3L questionnaire were supplied by Euroqol and were certified as linguistically validated. However, the face-to-face questionnaire was not available in all languages needed for this study, so it was translated (forward and back) by a Euroqol preferred translation vendor following their standard guidelines. Similarly, validated translations of the OPAQ-SV, ADEOS, and OPSAT-Q questionnaires were not available for all languages included in this study. These questionnaires were translated (forward and back) into the languages listed above and certified by a qualified translation vendor. The translations were not linguistically validated due to timeline and budget constraints, but the authors/license holders of the questionnaires approved the use of the translations for the MUSIC OS-AP study.
2.5. Data collection and analysis
Patient recruitment and baseline data collection occurred between July 2013 and August 2014. Study staff enrolled patients at routine office visits, obtained informed consent, and administered the baseline evaluation. Treated patients were provided with the month 3 follow-up questionnaire at the time of the baseline visit. Months 6 and 12 questionnaires were mailed to treated patients prior to their respective follow-up dates, and site personnel called participants to remind them when their questionnaires were due. Data were collected at each study site and entered into a secure, internet-based Electronic Data Capture system (ClinStream™).
The anticipated analyses are descriptive and no a priori hypothesis is proposed. Future publications will present analyses of baseline data from the physician questionnaire and reasons for non-treatment among untreated patients. Publications on the prospective component of the study will describe outcomes such as GI events, health care resource use, medication adherence, health-related quality of life, treatment satisfaction, and adverse events. These patient-reported outcomes will be assessed over time (baseline, 6 months, and 12 months) and compared across patients with and without GI events. Least squares mean differences between patients with and without GI events will be adjusted for common covariates such as age, history of GI events, new/experienced user status, body mass index, highest level of education, age at menopause, predominant treatment, hours of physical exercise per week, number of previous fractures, number of previous falls, parental hip fracture, current smoking, glucocorticoid use, co-morbidities, alcohol consumption ≥ 3 units per day, and country of residence. Assuming an attrition rate of 15%–20%, the proposed sample size of 3300 treated participants was calculated to permit a final evaluable population of 2700 subjects. This was expected to be sufficient for the descriptive and exploratory analyses anticipated and to permit comparisons between patients with and without GI events..
3. Results
3.1. Physician questionnaire and retrospective chart review
Physicians from 59 sites agreed to participate in the study: 20 from Australia/New Zealand, 15 from India, 15 from Korea, and 9 from Taiwan (Table 2). The study site sample comprised 16 primary care clinics and 43 specialty centers (Table 2). Physician specialties included rheumatology, endocrinology, bone mineralogy, geriatrics, orthopedics, and osteology. Physicians at all sites completed the physician questionnaire. Data for 300 patients from 26 sites were abstracted for the retrospective chart review (Table 2).
Table 2.
Study site distribution.
| Australia/New Zealand | India | Korea | Taiwan | Total | |
|---|---|---|---|---|---|
| Total | 20 | 15 | 15 | 9 | 59 |
| Specialty center | 9 | 14 | 13 | 7 | 43 |
| Primary care clinic | 11 | 1 | 2 | 2 | 16 |
| Participated in retrospective chart review | 9 | 1 | 9 | 7 | 26 |
3.2. Prospective cohort study
All 59 sites participated in the prospective cohort study. A total of 3588 patients were enrolled in this component of the study.
3.2.1. Characteristics of untreated patients
The characteristics of the 301 untreated patients are presented in Table 3. Untreated patients were, on average, 63.0 years of age. The majority of untreated patients (56%) were West Asian, with the rest of the study population comprising East Asians (32%) and Caucasians (12%).This group had a mean (SD) FRAX score of 5.8 (5.4), and 18% of them reported having prior osteoporotic fractures.
Table 3.
Characteristics of patients enrolled in the prospective cohort component of MUSIC OS-APa.
| Untreated patients (N = 301) | Treated patients |
|||
|---|---|---|---|---|
| New users (N = 1416) | Experienced users (N = 1871) | All patients (N = 3287) | ||
| Age, mean (SD) years | 63.0 (9.3) | 62.9 (9.5) | 67.3 (9.0) | 65.4 (9.5) |
| Age at menopause, mean (SD) yearsb | 48.5 (4.8) | 47.2 (5.6) | 48.4 (5.1) | 47.9 (5.4) |
| Race, n (%)c | ||||
| Caucasian | 36 (12%) | 80 (6%) | 374 (20%) | 454 (14%) |
| East Asian | 97 (32%) | 372 (26%) | 1107 (59%) | 1479 (45%) |
| West Asians | 167 (56%) | 963 (68%) | 389 (21%) | 1352 (41%) |
| Other | 0 (0%) | 1 (0%) | 0 (0%) | 1 (0%) |
| Height, mean (SD) cm | 154.7 (6.4) | 153.6 (6.9) | 154.5 (6.6) | 154.1 (6.7) |
| Weight, mean (SD) kg | 62.6 (12.5) | 60.9 (11.8) | 57.8 (10.9) | 59.1 (11.4) |
| Highest level of education, n (%) | ||||
| High school or less | 203 (68%) | 1067 (75%) | 1333 (71%) | 2400 (73%) |
| Non-university certificate or diploma | 16 (5%) | 47 (3%) | 92 (5%) | 139 (4%) |
| University degree or higher | 73 (24%) | 240 (17%) | 347 (19%) | 587 (18%) |
| Prefer not to answer | 8 (3%) | 62 (4%) | 98 (5%) | 160 (5%) |
| Physical exercise, mean (SD) hours/week | 4.0 (4.1) | 3.1 (4.7) | 4.6 (5.3) | 4.0 (5.1) |
| Hypothyroidism, n (%) | 67 (22%) | 204 (14%) | 230 (12%) | 434 (13%) |
| Mean FRAX score (SD)d | 5.8 (5.4) | 10.6 (11.1) | 15.5 (13.7) | 12.8 (12.5) |
| OP risk factors, n (%) | ||||
| Alcohol use (≥ 3 units per day) | 2 (1%) | 5 (0%) | 10 (1%) | 15 (0%) |
| Current smoking | 4 (1%) | 14 (1%) | 21 (1%) | 35 (1%) |
| Glucocorticoid use | 10 (3%) | 36 (3%) | 78 (4%) | 114 (3%) |
| Parental hip fracture | 18 (6%) | 42 (3%) | 140 (7%) | 182 (6%) |
| Prior OP fractures | 53 (18%) | 225 (16%) | 482 (26%) | 707 (22%) |
| Rheumatoid arthritis | 27 (9%) | 103 (7%) | 167 (9%) | 270 (8%) |
| Secondary osteoporosis | 18 (6%) | 36 (3%) | 127 (7%) | 163 (5%) |
cm, centimeter; kg, kilogram; OP, osteoporosis; SD, standard deviation.
Two patients, one untreated and one experienced user, were excluded from this table because of missing data on GI events.
One experienced user and one new user had missing data.
Caucasian was defined as European, Mediterranean, Middle Eastern, or North African Descent. East Asian was defined as Chinese, Korean, Japanese, or Taiwanese. West Asian was defined as Indian or Pakistani. Other included only those identifying as New Zealand Maori.
FRAX scores were recorded for 21, 89, 72, and 161 patients in the respective columns.
3.2.2. Characteristics of treated patients
The 3287 treated patients included 1416 new users and 1871 experienced users (Table 3). Treated patients were, on average, 65.4 years of age. The largest ethnic group in this study arm was East Asians (45%), followed by West Asians (41%) and Caucasians (14%). Treated patients had a mean (SD) FRAX score of 12.8 (12.5), and 22% of them had a prior osteoporotic fracture.
4. Discussion
The MUSIC OS-AP study will address several areas in which information about osteoporosis treatment in the Asia-Pacific region is lacking. One of these areas is the physician's perspective of the management of osteoporosis and the factors, including GI events, associated with the decision to treat osteoporosis. Complementary to this will be the patient's perspective of pharmacologic management of osteoporosis—specifically the reasons for non-treatment and satisfaction with the selection of treatment. A single study from Korea reported that composite scores on the OPSAT-Q were higher (better) in monthly versus weekly bisphosphonate users, in non-smokers versus current or past smokers, and in non-users versus users of acid-related medications (Oh et al., 2012). MUSIC OS-AP will provide data from Korea and other Asia-Pacific countries for comparison with these results, as well as information on how patient satisfaction changes over time and with the occurrence of GI events. This will be the first-recorded data on these topics for most of the countries involved.
Another area lacking data, and one of the primary emphases of MUSIC OS-AP, is the role of GI events in the treatment of osteoporosis. Two studies from Asia-Pacific countries have shown that treatment of osteoporosis with bisphosphonates resulted in esophageal injury. In a nationwide case–control study in Taiwan, use of alendronate increased the risk of upper GI bleeding (hazard ratio 1.32) and lower GI bleeding (hazard ratio 1.84) in both men and women (Peng et al., 2014). In an endoscopy study of healthy postmenopausal Korean women, 10 out of 16 experienced mucosal damage after taking alendronate daily for two weeks (Mok et al., 2013). The association of osteoporosis treatment with mild GI events is less clear (Tseng et al., 2006), perhaps because GI problems are common among adults in Asia-Pacific countries (Chang et al., 2012, Guarner, n.d, Ghoshal et al., 2011), regardless of whether they are being treated for osteoporosis. There is also little information from Asia-Pacific countries about the association of GI events with health care resource use and quality of life of osteoporosis patients. MUSIC OS-AP will address these gaps by determining the rate of GI events in treated and untreated patients and assessing the association of GI events with treatment rates, treatment patterns, and other patient-reported outcomes in osteoporotic postmenopausal women.
Distinctive features of MUSIC OS-AP are its use of a physician questionnaire to assess factors predictive of osteoporosis treatment, the inclusion of an untreated group to assess patient-reported reasons for and clinical correlates of non-treatment, and its focus on the link between GI events and patient-reported outcomes. MUSIC OS-AP will acquire data on several rarely addressed patient-reported outcomes, including health care resource use, satisfaction with treatment, and quality of life. Measurement of GI events by patient report may provide a more relevant depiction of the rate of GI events among osteoporosis patients than can be gleaned solely from chart reviews or claims database analyses. Patient-reported measures, however, may be subject to a reporting bias and to variations in patient compliance with study procedures. For example, at-home completion of the questionnaires may not occur exactly during the time frame specified in the study.
One limitation of the study design is that patients were not required to report the severity of the GI events; in fact, GI events typically considered severe (e.g., those involving bleeding or perforation) were not included in the patient questionnaire. Another limitation is that the retrospective chart review will reflect the percentage of patients prescribed medication, not necessarily the percentage of patients who fill and subsequently take their medications. Finally, although the size of MUSIC OS-AP will allow it to detect the true rate of GI events and the impact of GI events on persistence with treatment, the inclusion of women from fundamentally different ethnic groups and cultures may limit the applicability of the combined results. For this reason, country-specific analyses will be a useful feature of subsequent publications.
In conclusion, MUSIC OS-AP will highlight the association of GI events with the management of osteoporosis and with patient-reported outcomes in developing and developed countries of the Asia-Pacific region. MUSIC OS-AP will enable determination of the rates of a broad range of GI events among both treated and untreated patients, and provide a clearer understanding of the association of GI events with persistence with treatment, quality of life, treatment satisfaction, and osteoporosis- and GI-related health care utilization.
Disclosures
A. Modi and SS are employees of Merck & Co., Inc. and own stock in the company. PRE has received research funding from Merck & Co., Inc., Amgen, Novartis, GlaxoSmithKline, and Eli Lilly and honoraria from Merck & Co., Inc., Amgen, ViiV Healthcare. XY is an Associate Director of the Center for Observational and Real-World Evidence at Merck & Co., Inc. MSL, YKM, and A. Mithal have no conflicts of interest to report.
The study was funded by Merck & Co., Inc. Other than through the employer relationship disclosed, Merck & Co., Inc. did not have a role in the study design, data collection, interpretation of the data, in writing of the manuscript, and in the decision to submit the manuscript for publication.
Author contributions
Conception and design of the study: A. Modi, PRE, MSL, YKM, A. Mithal, and SS.
Analysis and/or interpretation of the data: A. Modi, XY, and SS.
Drafting and revision of the manuscript: A. Modi, PRE, MSL, YKM, A. Mithal, XY, and SS.
Approval of the final version of the manuscript: A. Modi, PRE, MSL, YKM, A. Mithal, XY, and SS.
Acknowledgments
The authors thank the following MUSIC OS-AP site investigators: in Taiwan, Ning-Sheng Lai, Yang-Hwei Tsuang, Horng-Chaung Hsu, James Cheng-Chung Wei, Shao-Keh Hsu, Hongsen Chiang, Ting-Kuo Chang, Kang Lu, and Chung-Hwan Chen; In India, Harvinder Singh Chhabra, Rajesh Malhotra, Arun Bhanot, Lalit Duggal, Jyotsna Oak, Vaishali Desh Mukh, Parag Sancheti, Bharat Dave, Vikash Kapor, An Roy, Thomas Paul, Mala Dharmalingam, Rakesh Rajput, Abhay Nene, and Harshad Shah; in Australia and New Zealand, Graeme Jones, Gustavo Duque, Nigel Gilchrist, Ego Seeman, Jeremy Allen, Lyn March, Fred Delooze, John Wark, Robert Will, Michael Campbell, and Charles Inderjeeth; in South Korea, Moo II Kang, Hyun Koo Yoon, Yoon-Sok Chung, Dongwon Byun, In Ju Kim, Deog-Yoon Kim, Dong Jin Chung, Sung-hee Choi, Seong Bin Hong, Yumie Rhee, Jung hee Kim, Kyong-Chol Kim, Dong-Hyeok Shin, Seon-Hwa Lee, and Choon-Hee Chung.
The authors also thank Anna Kaufman, MPH, and Melissa Stauffer, PhD, in collaboration with SCRIBCO, for medical writing assistance.
References
- Wade S.W., Strader C., Fitzpatrick L.A., Anthony M.S., O'Malley C.D. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch. Osteoporos. 2014;9:182. doi: 10.1007/s11657-014-0182-3. [DOI] [PubMed] [Google Scholar]
- Henry M.J., Pasco J.A., Nicholson G.C., Kotowicz M.A. Prevalence of osteoporosis in Australian men and women: Geelong osteoporosis study. Med J Aust. 2011;195:321–322. doi: 10.5694/mja11.10571. [DOI] [PubMed] [Google Scholar]
- Park E.J., Joo I.W., Jang M.J., Kim Y.T., Oh K., Oh H.J. Prevalence of osteoporosis in the Korean population based on Korea National Health And Nutrition Examination Survey (KNHANES), 2008–2011. Yonsei Med. J. 2014;55:1049–1057. doi: 10.3349/ymj.2014.55.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeta H.C.V., Marwah R., Sahay R., Kalra S., Babhulkar S. Clinical practice guidelines on postmenopausal osteoporosis: an executive summary and recommendations. J Midlife Health. 2013;4:107–126. doi: 10.4103/0976-7800.115293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnell O., Kanis J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- Gill T.K., Taylor A.W., Hill C.L., Phillips P.J. Osteoporosis in the community: sensitivity of self-reported estimates and medication use of those diagnosed with the condition. Bone Joint Res. 2012;1:93–98. doi: 10.1302/2046-3758.15.2000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Lee Y.H., Moon S.H., Lee Y.S. Influence of insurance benefit criteria on the administration rate of osteoporosis drugs in postmenopausal females. Clin Orthop Surg. 2014;6:56–61. doi: 10.4055/cios.2014.6.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson A.N., Grove M.R., Hammond C.S., Moncur M.M., Ray G.T., Hebert G.M. Early discontinuation of treatment for osteoporosis. Am J Med. 2003;115:209–216. doi: 10.1016/s0002-9343(03)00362-0. [DOI] [PubMed] [Google Scholar]
- Ringe J.D., Moller G. Differences in persistence, safety and efficacy of generic and original branded once weekly bisphosphonates in patients with postmenopausal osteoporosis: 1-year results of a retrospective patient chart review analysis. Rheumatol. Int. 2009;30:213–221. doi: 10.1007/s00296-009-0940-5. [DOI] [PubMed] [Google Scholar]
- Payer J., Cierny D., Killinger Z., Sulkova I., Behuliak M., Celec P. Preferences of patients with post-menopausal osteoporosis treated with bisphosphonates—the viva ii study. J Int Med Res. 2009;37:1225–1229. doi: 10.1177/147323000903700430. [DOI] [PubMed] [Google Scholar]
- Modi A., Sen S., Adachi J.D., Adami S., Cortet B., Cooper A.L. Rationale and design of MUSIC OS-EU: an international observational study of the treatment of postmenopausal women for osteoporosis in Europe and Canada. Clin. Exp. Rheumatol. 2015;33:537–544. [PubMed] [Google Scholar]
- Oemar M, Oppe M. EQ-5D-3L User Guide: Basic information on how to use the EQ-5D-3L instrument. http://www.euroqol.org/about-eq-5d/publications/user-guide.html. Accessed December 6, 2014.
- Randell A.G., Bhalerao N., Nguyen T.V., Sambrook P.N., Eisman J.A., Silverman S.L. Quality of life in osteoporosis: reliability, consistency, and validity of the osteoporosis assessment questionnaire. J Rheumatol. 1998;25:1171–1179. [PubMed] [Google Scholar]
- Breuil V., Cortet B., Cotte F.E., Arnould B., Dias-Barbosa C., Gaudin A.F. Validation of the adherence evaluation of osteoporosis treatment (ADEOS) questionnaire for osteoporotic post-menopausal women. Osteoporos. Int. 2012;23:445–455. doi: 10.1007/s00198-011-1555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood E.M., Beusterien K.M., Green H., Shikiar R., Baran R.W., Amonkar M.M. Psychometric evaluation of the osteoporosis patient treatment satisfaction questionnaire (OPSAT-Q), a novel measure to assess satisfaction with bisphosphonate treatment in postmenopausal women. Health Qual Life Outcomes. 2006;4:42. doi: 10.1186/1477-7525-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh K.W., Kim D.Y., Lee Y.S., Kang M.I. Osteoporosis patient treatment satisfaction questionnaire in postmenopausal women intermittently treated with oral bisphosphonates: the bravo study. J. Bone Miner. Metab. 2012;30:359–366. doi: 10.1007/s00774-011-0326-0. [DOI] [PubMed] [Google Scholar]
- Peng Y.L., Hu H.Y., Luo J.C., Hou M.C., Lin H.C., Lee F.Y. Alendronate, a bisphosphonate, increased upper and lower gastrointestinal bleeding: risk factor analysis from a nationwide population-based study. Osteoporos. Int. 2014;25:1617–1623. doi: 10.1007/s00198-014-2647-z. [DOI] [PubMed] [Google Scholar]
- Mok J.O., Jung C.H., Kim C.H., Ryu C.B., Kim Y.J., Kim S.J. Endoscopic comparison of alendronate alone and the enteric-coated alendronate with calcitriol combination in postmenopausal Korean females. Korean J Intern Med. 2013;28:694–700. doi: 10.3904/kjim.2013.28.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng L.N., Sheu W.H., Ho E.S., Lan H.H., Hu C.C., Kao C.H. Effects of alendronate combined with hormone replacement therapy on osteoporotic postmenopausal Chinese women. Metabolism. 2006;55:741–747. doi: 10.1016/j.metabol.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Chang F.Y., Chen P.H., Wu T.C., Pan W.H., Chang H.Y., Wu S.J. Prevalence of functional gastrointestinal disorders in Taiwan: questionnaire-based survey for adults based on the Rome III criteria. Asia Pac. J. Clin. Nutr. 2012;21:594–600. [PubMed] [Google Scholar]
- Guarner F, Lázaro, Gascón, Royo, Eximan, Herrero. Map of digestive disorders & diseases. http://www.worldgastroenterology.org/assets/downloads/pdf/wdhd/2008/events/map_of_digestive_disorders_2008.pdf. Accessed February 25, 2015.
- Ghoshal U.C., Singh R., Chang F.Y., Hou X., Wong B.C., Kachintorn U. Epidemiology of uninvestigated and functional dyspepsia in Asia: facts and fiction. J Neurogastroenterol Motil. 2011;17:235–244. doi: 10.5056/jnm.2011.17.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]