Abstract
Glutamate signaling in the brain is one of the most studied targets in the alcohol research field. Here, we report the current understanding of how the excitatory neurotransmitter glutamate, its receptors, and its transporters are involved in low, episodic, and heavy alcohol use. Specific animal behavior protocols can be used to assess these different drinking levels, including two-bottle choice, operant self-administration, drinking in the dark, the alcohol deprivation effect, intermittent access to alcohol, and chronic intermittent ethanol vapor inhalation. Importantly, these methods are not limited to a specific category, since they can be interchanged to assess different states in the development from low to heavy drinking. We encourage a circuit-based perspective beyond the classic mesolimbic-centric view, as multiple structures are dynamically engaged during the transition from positive- to negative-related reinforcement to drive alcohol drinking. During this shift from lower-level alcohol drinking to heavy alcohol use, there appears to be a shift from metabotropic glutamate receptor-dependent behaviors to N-methyl-D-aspartate receptor-related processes. Despite high efficacy of the glutamate-related pharmaceutical acamprosate in animal models of drinking, it is ineffective as treatment in the clinic. Therefore, research needs to focus on other promising glutamatergic compounds to reduce heavy drinking or mediate withdrawal symptoms or both.
Keywords: glutamate, alcohol, addiction, two-bottle choice, self-administration, drinking in the dark, intermittent access to alcohol, chronic intermittent ethanol vapor
Introduction
Glutamate, the most prevalent excitatory neurotransmitter in the central nervous system, has long been associated with the excitotoxicity of alcohol withdrawal. Repeated episodes of alcohol withdrawal can generate aberrant behaviors such as hypermotility and increased seizures, which are classically thought to be related to an excitable state caused by increased glutamate action in the brain 1– 4. These hyperglutamatergic periods of alcohol deprivation between heavy drinking events may be kindled across time, in a process like electrophysiological kindling 5. Since this hypothesis is generally well accepted in the field, many have explored glutamatergic targets for new alcohol use disorder medications 6. However, since an acute injection of ethanol also increases glutamate in the nucleus accumbens (NAc) 7, a site heavily associated with both reward and stress, it suggests that there is a continuum of engagement through the transition from low to heavy drinking regulated by glutamate signaling. We focus on circuits that become recruited among subcortical structures beyond the classic mesolimbic-centric perspective.
There are distinct pharmacological classes of glutamate receptors, including ionotropic (iGluR) and metabotropic (mGluR) glutamate receptors and glutamate transporters that have been linked to a wide variety of alcohol-related phenotypes. In brief, iGluRs encompass α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors with 1–4 subunits (GluA1–4), N-methyl-D-aspartate (NMDA) receptors with two obligatory GluN1 subunits and combinations of GluN2(A–D) assemblies, and kainite receptors (GluK1–5). GluN receptors are more sensitive to alcohol than GluA and GluK 8– 10. Also, allosteric modulation of the GluN2B binding site can produce changes in alcohol-related behaviors. In contrast to the ligand-gated cation-selective ion channel iGluRs, mGluRs are G-protein-coupled and form three distinct classes: group I (mGluR1 and mGluR5), group II (mGluR2 and mGluR3), and group III (mGluR4, mGluR6, mGluR7, and mGluR8). Glutamate clearance in the synapse can be controlled by reuptake through transporters like excitatory amino acid transporters (EAATs) and adenosine transporters (equilibrative nucleoside transporter, or ENT) into glia and vesicular glutamate transporters into neurons. This review synthesizes the extant behavioral pharmacological findings for the role of glutamate, its receptors, and its circuitry throughout the brain in several stages of the transition to alcohol use disorder. In light of clinical literature, three general phases within alcohol use disorders are discussed: low-level drinking, binge drinking, and heavy drinking with withdrawal. We highlight specific animal behavior protocols in these three categories, but importantly these methods can be applied among all phases in the development of alcohol dependence.
Low-level drinking
Ethanol consumption that causes less than 0.08 g/dL (or 80 mg/dL) blood alcohol concentrations (BACs), less than 17 mM in the brain, is considered a low dose. Typical low-alcohol doses would be equivalent to a social drinker with BACs in the range of 0.015–0.025 g/dL (15–25 mg/dL). However, a BAC of 0.04 g/dL is classified as driving under the influence (DUI) for commercial drivers or previous DUI offenders ( http://www.dmv.org/). With rodents that can readily metabolize alcohol, higher gram per kilogram (g/kg) alcohol concentrations may lead to BACs under 80 mg/dL.
Acute sub-intoxication doses of alcohol ingestion in humans can cause reduced strength of evoked field potentials in the prefrontal cortex (PFC), suggesting reduced excitability and functional connections 11. This is concordant with a 0.375 g/kg ethanol injection inhibiting PFC firing rate by approximately 20% versus baseline in anesthetized rats 12. In general, there is a paucity of clinical data for low-level alcohol consumption and glutamate activity because low-level drinkers are compared with heavy drinkers instead of abstinent people in clinical research.
Two-bottle choice
Two-bottle choice (2BC) involves offering the option to drink either a diluted ethanol-containing solution (concentrations range from 3 to 30%) or water for a fixed amount of time ( Table 1). 2BC allows for the measurement of both voluntary consumption and ethanol preference over water and can be used as a single protocol or be combined with others to generate the desired level of drinking. In other words, first-day BACs may indicate low-level drinking, but weeks of 2BC could produce intoxicating BACs. This section focuses on 2BC studies that assess baseline ethanol preference, but daily limited-access studies that generate more binge-like drinking are discussed in the next section.
Table 1. Descriptions of alcohol-related protocols.
| Method | Details | Ethanol g/kg achieved | Key references |
|---|---|---|---|
| Two-bottle choice (2BC) | 3–20% ethanol given in one bottle with a
secondary bottle of water usually for 24 hours |
≤10 g/kg per 24 hours (mice) | McClearn and Rodgers
183; Belknap,
Crabbe, and Young 184 |
| Operant self-administration | 9–15% ethanol (some add 2% sucrose)
reinforcements are self-administered with cue for 30–60 minutes |
≤90 mg/dL BAC (rats), ≤200 mg/dL
BAC (mice); ≤1.5 g/kg per 30 minutes (rats), ≤3 g/kg per 1 hour (mice) |
Elmer, Meisch, and George
185;
Melendez et al. 186; Faccidomo et al. 57 |
| Cue-induced or stress-
induced reinstatement |
10–15% ethanol reinforcements are self-
administered with cue, then extinction after cue is no longer paired with delivery of ethanol, finally reinstatement of ethanol-seeking behavior (lever pressing) occurs after ethanol-related cue or stressor is given |
≤90 mg/dL BAC (rats), ≤200 mg/dL
BAC (mice); ≤1.5 g/kg per 30 minutes (rats), ≤3 g/kg per 1 hour (mice) |
Lê
et al.
187; Chaudhri
et al.
188;
Shaham et al. 46 |
| Alcohol discrimination | Sucrose or food pellet reinforcement given upon
pressing the correct response lever after ethanol or vehicle injection; test sessions involve injecting a novel drug and measuring lever selection |
0.5–2 g/kg injection,
intraperitoneally or per os |
Grant 64; Kostowski and Bienkowski 65 |
| Drinking in the dark | 3 hours into the dark photoperiod, one bottle of
20% ethanol is given for 2–4 hours (mice) |
≤3 g/kg per 2 hours; ≤7 g/kg per
4 hours (mice) |
Rhodes
et al.
90; Thiele, Crabbe,
Boehm 91 |
| Scheduled high alcohol
consumption |
Water restriction for all but 90 minutes–10 hours,
and every 3rd/4th day 5, 7, or 10% ethanol is substituted for 10–30 minutes followed by water |
≤2 g/kg per 30 minutes;
≤100 mg/dL BAC (mice) |
Finn et al. 104; Tanchuk et al. 44 |
| Multiple scheduled access | Four 1-hour access periods to 15% and 30%
ethanol separated by 2 hours starting 1 hour into dark cycle, 5 consecutive days/week |
≤2 g/kg per 1 hour; ≤130 mg/dL
BAC (rats) |
Murphy
et al.
106; Bell
et al.
107; McBride
et al. 109 |
| Alcohol deprivation effect | 5–20% ethanol given every day for 6–8 weeks
with a 3- to 6-day deprivation then resumption of drinking |
Additional 2 g/kg per 24 hours over
baseline (rats); additional 4 g/kg per 24 hours over baseline (mice) |
Sinclair
et al.
189; Spanagel
et al.
112;
Melendez et al. 190 |
| Intermittent access to
alcohol |
Every other day, 2BC of 20% ethanol and water is
given for 24 hours, repeated for 4+ weeks |
≤250 mg/dL BAC (rats), ≤200 mg/dL
BAC (mice); ≤10 g/kg per 24 hours (rats); ≤25 g/kg per 24 hours (mice) |
Wise
131; Simms
et al.
132; Hwa
et al.
135;
Carnicella, Ron, and Barak 133 |
| Chronic intermittent ethanol
vapor |
14-hour ethanol vapor and 10-hour air (rats)
or 16-hour/8-hour (mice), repeated for 4+ weeks |
150–250 mg/dL BAC during exposure;
post-exposure, 6 g/kg per 24 hours (rats); ≤4 g/kg per 2 hours (mice) |
Goldstein
et al.
191; O’Dell
et al.
192;
Lopez and Becker 193 |
Listed are popular animal protocols for alcohol drinking and the amount of alcohol given to the animal. These methods produce relevant blood alcohol concentrations (BACs) in rodents and are not restricted to a low, episodic, or heavy drinking category. Protocols can be repeated to generate the intended level of drinking or be combined for more exploration of the drinking behavior.
The studies are unequivocal that NMDA and AMPA regulate 2BC drinking, and both competitive and non-competitive GluN antagonists reduce 2BC intake 13– 15. For example, NMDA and AMPA infused into the lateral hypothalamus can both increase 2BC consumption 14. However, GluN antagonists and glycine B site blockade can importantly reduce motor coordination to achieve these effects 13– 16. Similarly, GluN2A knockout mice show alcohol-induced impairments in motor coordination from wild-types (WTs) but do not show differences in consumption 17. Other glutamate-related knockout lines also do not differ in 2BC drinking compared with WTs (for example, AMPA GluR1, GluN1 glycine, and mGluR5) 18– 21. Pharmacological manipulations of mGluRs, specifically mGluR5 antagonists and mGluR7 agonists, are effective at reducing 2BC intake in rats 22– 24. In complementary experiments, blockade with mGluR7 antagonist MMPIP or shRNA in the NAc can increase low-dose alcohol intake and preference 24, 25. Since Homer2 knockout mice drink less than WTs in 2BC 26, it suggests that downstream signaling molecules are also important beyond glutamate receptor binding and clearance. It is worth mentioning that the US Food and Drug Administration (FDA)-approved medication for alcohol dependence, acamprosate, for which the glutamatergic mechanism of action is controversial, reduces 2BC drinking in rats 27. There is a glaring gap in the literature for which glutamatergic circuits in the brain may govern low-dose ethanol drinking. We need this critical information for insight into higher-dose plasticity.
Another important variable on the outcome of 2BC drinking and potential neuroadaptations is strain. Classic comparisons contrast between drinking behavior of C57BL/6J mice and DBA/2J mice, yet many inbred strains have been assessed for 2BC 28. Although specific sucrose-fading procedures can be used to induce ethanol drinking in DBA/2J mice (for example, 29) or bypassing ethanol taste altogether (for example, 30), this mouse strain drinks much less than C57BL/6J mice. 2BC preference may be related to strain differences in the effect of glutamate and NMDA on the brain in vitro 31, 32 and differences in gene expression in response to acute ethanol 33. Also, alcohol-preferring (P) rats, genetically selected for high alcohol drinking, have a loss of the mGluR2 receptors that may contribute to escalated alcohol intake 34. Comparing high-drinking and low-drinking strains caused from trait selection or from inbred lines would increase the understanding of how glutamate-related genes influence drinking behavior.
Operant self-administration of alcohol
Operant self-administration is a powerful method for mice, rats, and monkeys to assess ethanol reinforcement. Via these methods, rodents will typically self-administer amounts ranging from 0.5 to 2 g/kg depending on factors such as session length, reinforcement schedule, and alcohol concentration by pressing a lever, spinning a wheel, or poking the nose into a receptacle ( Table 1). Uncompetitive GluN antagonists ketamine and memantine reduce operant responding for ethanol with mechanistic target of rapamycin signaling, likely regulating the anti-alcohol effects of ketamine 35. Both mGluR5 and mGluR1 blockade and mGluR7-positive allosteric modulation decrease alcohol self-administration in rats and mice 36– 40, particularly in the NAc 41, 42. As in 2BC studies, some have seen ethanol-induced sedation and hypnosis with mGluR5 antagonist MPEP and mGluR2/3 antagonist LY341495 43 and non-specific reductions in sucrose self-administration 39, 44. This may be due in part to mGluR5 influencing D1 receptors in seeking behavior 45.
Self-administration training techniques are also useful to investigate cue-induced reinstatement, or seeking behavior, following extinction of the alcohol-paired cues ( Table 1). In operant self-administration protocols, cue-induced reinstatement or stress-induced reinstatement of alcohol seeking after a period of extinction training is also interpreted to be a form of relapse 46 ( Table 1). We discuss the literature here instead of the relapse section, as no alcohol is consumed during reinstatement tests. There have been mixed reports for the ability of competitive GluN antagonists to affect reinstatement 47, 48. For mGluRs, it is not surprising that mGluR5 antagonism and mGluR2/3 agonism reduce cue-induced reinstatement, alcohol seeking in Pavlovian spontaneous recovery, and enhanced sensitivity to the attenuation of conditioned reinstatement 49– 52, but there are varying reports for whether these compounds affect baseline self-administration. Gass et al. 53 found evidence for increased glutamate transmission from the basolateral amygdala (BLA) to NAc core during cue-induced reinstatement of alcohol seeking. Glutamate transmission and transport may be mediated through adenosine ENT1 54 since N-acetylcysteine and ceftriaxone, which alter glial reuptake and release of glutamate, also alter alcohol self-administration 55. Downstream signaling molecules such as PKCε, ERK, and CaMKII/AMPA in the PFC and amygdala have been well established in alcohol self-administration and cue-induced reinstatement 56– 59. Specifically, amygdalar CaMKII/AMPA activation promotes self-administration and drinking 60– 62, whereas inhibition of CaMKII in the PFC increases the positive reinforcing effects of alcohol 63. Others have explored the activation of mGluR2 amygdala to hippocampus pathway in cue-induced alcohol seeking, where mGluR-mediated synaptic depression is impaired in the hippocampus 34. It seems that subregions of the amygdala and also the PFC are recruited during this low-level drinking.
Alcohol-discriminative stimulus effects
Alcohol discrimination tasks are useful to assess the neurobiological mechanisms underlying the discriminative stimulus effects (for example, interoceptive effects) of low and high alcohol doses ( Table 1). However, it is important to note that these tasks do not involve alcohol drinking but rather experimenter-administered alcohol. We have known for decades that the discriminative stimulus properties of ethanol are mediated by GluNs and GABAA 64– 66. Specifically, lower alcohol doses (for example, 0.5–1 g/kg) engage GABA receptor systems whereas higher doses (>2 g/kg) involve NMDA receptor systems. Rats and cynomolgus monkeys can discriminate alcohol from glutamate release inhibitors and NMDA ligands, showing that they have partial alcohol-like effects 67, 68. This is different from the discrimination of acamprosate, where acamprosate fails to substitute for an alcohol cue, suggesting that it is not a substitution drug 69. Besheer et al. have shown that alcohol discrimination is co-regulated by mGluR5 in the NAc and the mGluR2/3 in the amygdala 70– 73 and that inhibition of MEK/ERK(1/2) in the amygdala, but not NAc, potentiates the effects of a low alcohol dose 74. Recent work with stress hormone corticosterone links both mGluR5 and mGluR2/3 in the sensitivity to alcohol 75, 76, suggesting a role for neuropeptide modulation of glutamatergic circuits. Furthermore, in addition to the NAc, a functional role for the medial PFC (mPFC) in modulating sensitivity to low alcohol doses has been shown 66, 77. An interesting contribution from the Holmes lab shows that GluN2B in corticostriatal circuits governs choice learning and choice shifting 78. Although this learning is not in the presence of alcohol, they show a dissociation between OFC GluN2B in choice shifting and dorsal striatum GluN2B in choice learning. These findings suggest it is possible that learning about alcohol through discrimination tasks recruits distinct populations of both iGluR and mGluR in subcortical sites, although more research is required to confirm how this contrasts from habitual learning in the striatum.
Overall, there is ample evidence demonstrating PFC plasticity in alcohol-seeking behavior and low-dose alcohol drinking at a stage engaging positive reinforcement and the euphoric effects of the drug. Although 2BC studies have tested several facets of the glutamate system using knockout mice, there is a gap of knowledge in iGluRs in alcohol self-administration studies. This may be confounded by the fact that competitive GluRN antagonists mimic the interoceptive properties of alcohol. More recent studies have implicated GluRA in the rostromedial tegmental nucleus in alcohol seeking 79. Another behavioral outcome of low-dose acute, self-administered alcohol (1 g/kg) is an increase in inter-male aggression in a subset of mice 80. Memantine, neramexane, and mGluR5 antagonist MTEP interacted with alcohol to further increase alcohol-heightened aggression in mice, whereas mGluR2/3 agonist LY379268 did not 81. CRF type-1 receptors regulate serotonin function from the dorsal raphe nuclei (DRN)- mPFC to alter alcohol-heightened aggression 82, so glutamate may influence the mPFC for the expression of low-dose alcohol-related behavior.
Episodic drinking through binges and relapse
Binge drinking, defined as BACs greater than 0.08 g/dL or 80 mg/dL within 2 hours, is common among most strata of US adults and leads to an increased susceptibility for developing chronic alcoholism 83, 84. This section focuses on hazardous, episodic, binge drinking. However, epidemiological reports have found that there are almost as many binge-drinking episodes among moderate drinkers as among heavy drinkers in the US 83, so binge and relapse behavior represents the hazardous transition between moderate and heavy drinking. We focus on changes in glutamate plasticity to inform us on dramatic neurobiological events across species.
Binge alcohol drinkers have increased glutamate-to-creatine ratios and lower GABA concentrations in the anterior cingulate cortex (ACC) than do low alcohol drinkers 85, 86 presumably with glutamatergic perturbations. Repeated 2–3.4 g/kg alcohol injections increase accumbal and hippocampal glutamate compared with water-injected animals 1, 87, 88. This confirms a study in which young adults with depression had a positive correlation between the level of alcohol use and glutamate in the hippocampus 89.
Drinking in the dark
The prototypical procedure in mice to induce binge-like drinking is giving one bottle of alcohol, offered 3 hours into the active dark photoperiod for 2–4 hours, termed drinking in the dark (DID) ( Table 1) 90, 91. C57BL/6J mice typically drink 2–5 g/kg in a session. Even two alcohol “binges” in adolescent rats are sufficient to abolish long-term synaptic depression in hippocampal slices and to evoke cognitive deficits via a short-lasting, repeated blockade of GluN, inducing a change in the receptor subunit composition 92. An earlier DID study showed that both acamprosate and MPEP decreased DID intake without affecting sugar or water drinking 93. Others have gone on to show that mGluR5 signaling affects PKCε in the NAc or central amgydala (CeA) to regulate DID 94, 95. Specifically, repeated DID for 30 days elevates CeA levels of glutamate-associated proteins of Homer2a/b, mGluR1a, GluN2B, and PLCε 24 hours after withdrawal from binge drinking 95. Intra-CeA and intra-NAc mGluR1 negative allosteric modulator JNJ-16259685 also reduces DID intake 96, 97. More recent studies have isolated downstream factors after DID such as mGluRs affecting AMPA receptor trafficking proteins like eukaryotic elongation factor 2 or decreased amygdalar CaMKIIαT286 phosphorylation 98, 99. Importantly, this effect was isolated to the amygdala but not NAc or dorsal striatum. This may be related to the lack of difference in frequency and amplitude of spontaneous excitatory post-synaptic current (sEPSC) in dorsolateral striatum and dorsomedial striatum medium spiny neurons between 6 weeks’ DID and water-drinking mice 100. Also, moving away from the classic mesolimbic pathway, others have identified a novel ventral tegmental area (VTA)–bed nucleus of the stria terminalis (BNST) CRF circuit in DID 101. CRF-R1 antagonists can reduce DID through intact CRF-R2 signaling, and inhibiting VTA-projecting BNST CRF neurons reduces DID 101. Repeated 2 g/kg alcohol injections result in enhanced GluN-mediated LTP in VTA dopamine neurons 102, so it is likely that this VTA-BNST glutamate pathway is altered during binge drinking in DID in a similar fashion.
Beyond DID, there are other daily limited-access procedures that lead to binge drinking in rodents. Permutations of DID exist, such as 2-hour daily access for 14 days in C57BL/6J mice, to study other facets of binge-like drinking, such as tolerance 103. The scheduled high alcohol consumption (SHAC) protocol involves water restriction for all but 90 minutes of water access, and every fourth day alcohol replaces water for 10–30 minutes 104. Systemic administration of mGluR5 antagonist MPEP decreases SHAC intake but also sucrose self-administration 44. Further studies have found a role for mGluR5-Homer2-PI3K signaling in the NAc in SHAC intake 105, which can be replicated in the DID protocol 94. Another limited-access protocol is multiple scheduled access (MSA), in which P rats are offered four 1-hour 2BC sessions separated by 2 hours across the dark cycle 5 days per week 106. Changes in gene expression in the NAc and amygdala after weeks of MSA drinking in P rats have been extensively studied 107– 109, so what is needed is targeting how glutamate interacts between the sites through mGluRs and iGluRs 110. MSA can lead to a transient increase in alcohol drinking after a weekend of deprivation 107, an alcohol deprivation effect (ADE), so it incorporates episodic drinking in both limited-access binge drinking and relapse-like drinking.
Alcohol deprivation effect
Relapse is also episodic in nature, both in the clinic and modeled with animals. Relapse, a hallmark of alcohol use disorders, is the resumption of drinking following a prolonged period of abstinence. With animals, experimenters can model relapse through the expression of the ADE. In this method, alcohol-drinking animals are deprived of alcohol for a period of time (for example, days to weeks), and then following this deprivation period, an escalation in alcohol drinking is observed following re-exposure to alcohol ( Table 1). Intra-PFC glutamate and acamprosate separately reduce the ADE 111, 112. However, many other glutamatergic compounds—GluN/glycine receptor antagonist L-701,324, GluN2B selective antagonist ifenprodil, GluN channel blocker neramexane, GluA/GluK antagonist CNQX, and Na + channel blocker lamotrigine—attenuate the ADE similar to alcohol seeking during cue-induced reinstatement 47, 48. To the best of our knowledge, there are no reports for the involvement of iGluR or mGluR circuitry in the ADE, but we hypothesize that it would be similar to plastic changes in DID or operant self-administration circuitry.
It appears that episodic drinking, the amorphous transition between low-dose and high-dose intake, engages both reward-related and stress-related glutamate brain processes. A single DID protocol is mGluR5 antagonist-responsive, whereas repeated DID for a month alters changes in downstream glutamate proteins. Multiple glutamatergic compounds reduce the ADE and cue-induced reinstatement, so perhaps these protocols in combination with others would be more apt for screening medications for the clinic.
Heavy drinking and withdrawal
Heavy drinking is defined as consuming five or more drinks on the same occasion on each of five or more days in the past month 113. People who exhibit heavy drinking may or may not fall into the category of mild, moderate, or severe alcohol use disorder on the basis of the accompanying psychological symptoms 114. As mentioned earlier, heavy drinking can be different across species. Most clinical literature focuses on alcoholics, whereas rodent studies do not have the commodity of an overarching term. For example, heavy drinking in outbred rats can be 6 g/kg per day, whereas in mice it may be 15 g/kg per day. The subsequent analysis considers heavy drinking and withdrawal for the particular species.
Tsai et al. 115 originally reported that alcohol-dependent patients have increased glutamate and glycine in the cerebrospinal fluid during withdrawal, with accompanying reduced GABA concentrations. With proton magnetic resonance spectroscopy, increased glutamate levels have been associated with more years spent drinking, loss-of-control alcohol use, and craving during detoxification in heavy drinkers or non-treatment-seeking alcoholics 116– 118. This glutamate dysfunction is localized to the NAc and the ACC with a positive correlation between craving and glutamate and glutamine in these regions 118, 119. GluN compounds like ketamine, memantine, and d-cycloserine mimic the subjective effects of alcohol in recovering alcoholics 120– 122. However, it is unfortunate that clinical trials with memantine or FDA-approved acamprosate did not prevent relapse compared with placebo in alcohol-dependent patients in large-scale double-blind experiments 123– 127. In a massive genetics study, Schumann et al. 128 reported that genetic variations in GluN2A have the greatest relevance for human alcohol dependence among 10 glutamatergic probe genes, yet increased GluN2B expression and GluN2C in the ACC and dorsolateral PFC during withdrawal can indicate likelihood of alcohol craving and risk for relapse 129, 130. It appears that the ACC is a distinct site for glutamate plasticity in heavy drinking.
Intermittent access to alcohol
Cycles of binging and withdrawal occur in the transition to developing an alcohol use disorder. We can model voluntary alcohol drinking in between periods of abstinence, or alcohol deprivation, with 24-hour intermittent access to 2BC alcohol 131– 133. Weeks of intermittent alcohol access can lead to drinking despite adverse consequences 134 and signs of withdrawal such as handling-induced convulsions and decreased social interactions 135, 136. Giving access to alcohol for a 24-hour period may cause variability in when animals choose to drink, so researchers can also measure fluid consumption during the initial 2-, 4-, and/or 6-hour access within the 24-hour period. With this, front-loading behavior may be observed accompanied by high BACs after 2-hour access ( Table 1) 135. Additionally, smaller segments within 24-hour access allow drug manipulations to be assessed 137, 138.
Acamprosate reduces intermittent alcohol drinking in rats but not continuous-access alcohol drinking 132. Confirming clinical reports, outbred mice drinking on intermittent access to alcohol for 8 weeks show increased extracellular glutamate in the mPFC during withdrawal compared with 1 week of drinking and compared with water drinkers 136. Early reports with intermittent-access drinking in rats, drinking 7 g/kg per day, have enhanced post-synaptic GluA function in VTA neurons in the absence of any change in pre-synaptic glutamate release 139. Similarly, glutamatergic and GABAergic synaptic transmission are altered in the striatum of non-human primates with extended access for 3 years 140. Six months of continuous access and intermittent access to alcohol consumption in P rats produce selective increases in group 1 mGluR/Homer2/GluN2 expression within the NAc core and CeA 141, 142. Intermittent alcohol can produce short-term increases in Homer/glutamate receptor expression within both the NAc core and the CeA, which may increase the aversion of early alcohol withdrawal and consequently augment the negative reinforcing properties of alcohol. Modulators of the glutamate transporters reduce heavy drinking on a continuous-access schedule (15% and 30% ethanol) in P rats and the increased extracellular glutamate compared with water drinkers 143. These P rats also have enhanced expression of glutamate transporters EAAT2/GLT1 and xCT in the NAc and PFC, suggesting a role for targeting glutamate uptake in heavy drinking 144. Long-term intermittent alcohol recruits GABA and CRF neurons in the mPFC during withdrawal and disconnects the PFC–CeA pathway, suggesting that dysregulation of mPFC interneurons may be an early index of glutamate/GABA neuroadaptation in alcohol dependence 145. Impaired executive control over motivated behavior accompanies negative reinforcement during withdrawal. Seif et al. 146 show that cortical activation of NAc hyperpolarization-active GluN mediates aversion-resistant intermittent alcohol intake. Both the mPFC to NAc core and insula to NAc core mediate both quinine- and footshock-resistant alcohol drinking on an intermittent-access schedule. It appears that corticolimbic sites are integral to glutamate plasticity caused by chronic intermittent drinking.
Withdrawal from chronic intermittent ethanol vapor and other forced alcohol methods
There are several other protocols that forcibly induce a post-dependent state in animals, such as repeated high-dose alcohol injections, alcohol liquid diet, and chronic intermittent ethanol (CIE) vapor exposure ( Table 1). Studies on brain glutamate during alcohol withdrawal have been most extensively explored using these methods, since they surpass the aversive taste of alcohol drinking solutions to induce heavy BACs. However, it is important to note that CIE is used to render rodents ethanol-dependent to subsequently increase voluntary ethanol intake, not only to maintain high BACs 147– 149. Microdialysis studies have shown increased glutamate in the striatum 150, NAc 87, 151– 153, and hippocampus 1, 154 during withdrawal in alcohol-exposed rats and mice. These results are similar to 5 g/kg alcohol gavage injections for 2–4 weeks causing increased glutamate in striatum, hippocampus, and substantia nigra 8–12 hours after the last ingestion 155. To counteract excitotoxicity, acamprosate and GluN antagonists have been used to decrease alcohol drinking and to alleviate symptoms of alcohol withdrawal, including increased glutamate tone and convulsive events 112, 151, 156– 158. It is important to note that pharmacologically increasing glutamate transmission in the NAc with TBOA, a glutamate reuptake inhibitor, can increase drinking in both non-dependent and CIE-dependent mice 153. Alternatively, decreasing glutamate transmission in the NAc by activating group II mGluRs reduces drinking, although the effect was stronger in dependent mice. These results comparing glutamate in non-dependent and dependent animals have similar directionality with different magnitudes, so there may be separate but overlapping actions in the NAc for treating drinking versus withdrawal symptoms with glutamatergic compounds.
In accordance with clinical studies, the PFC is a large target of glutamate plasticity in alcohol dependence. CIE results in increased GluN-mediated activity in the mPFC and increased GluN1 and GluN2B subunit expression 159, 160. Mice that show “compulsive-like” behaviors after CIE exhibit increased NMDA currents in the orbitofrontal cortex compared with air-exposed controls 160. Rescue of infralimbic PFC mGluR2 deficit restores control over alcohol-seeking behavior 161. It appears that mGluR2 and mGluR5 can target symptoms of withdrawal (but see 162). Acamprosate improved attention set-shifting of alcohol-exposed animals but did not alter the concurrent changes in synaptic transmission or membrane excitability of mPFC neurons, indicating that the changes are not the pharmacological targets of acamprosate in the recovery of mPFC functions 163. Abulseoud et al. 164 showed that attenuation of alcohol withdrawal by ceftriaxone induced upregulation of glutamate transporter EAAT2. Reduction of EAAT2 likely contributes to a hyperglutamatergic state in the ENT1 knockout mice 54, 165, 166. Some have suggested that increasing glutamate uptake through transporters has a potential therapeutic role in the treatment of alcohol dependence 167 (but see 168). Aberrations in PFC function entangle reduced executive control and poor decision making in alcoholics 169.
The extended amygdala—composed of the BNST, BLA, and CeA—is particularly vulnerable to glutamate plasticity caused by CIE treatment. Chronic alcohol exposure produces neuroadaptations in glutamatergic transmission in the CeA 170, 171, and GluN2B-containing GluNs are most sensitive to CIE 170, 172, 173. CIE, but not continuous vapor exposure, increases BNST GluN-mediated EPSCs, not from altered glutamate release but from an increase in GluN2-containing GluN 174, suggesting that repeated cycles of exposure and withdrawal are necessary for these adaptations to occur. CIE enhances long-term potentiation formation in the BNST in GluN2B knockout mice through extrasynaptic GluN 175. Stress-induced alterations in anxiety-like behavior were absent following bilateral infusion of GluK1 agonist ATPA into the BLA, which augmented BLA GABAergic neurotransmission, and stress increased the amplitude of sEPSC and miniature inhibitory post-synaptic current 176. A regulatory stress neuropeptide could be nociceptin, since nociceptin application decreases glutamate transmission and blocks alcohol-induced effects in the CeA of naïve and CIE rats, but nociceptin antagonist revealed tonic inhibitory activity of nociceptin on evoked CeA glutamatergic transmission only in alcohol-dependent rats 177. Changes in the extended amygdala indicate a transition from positive reinforcement to negative reinforcement as stress neuropeptides like nociceptin, CRF, and dynorphin are more engaged 178.
Together, chronic forced or voluntary access to alcohol affects glutamate in multiple subcortical sites like the PFC and extended amygdala, and this agrees with the clinical literature. In addition to these sites, many others have examined the hippocampus as a crux of CIE-induced glutamatergic changes. Group I mGluRs and GluN2B-containing GluNs in CA1 and cortex impair LTD, reduce spine density, and disrupt learning 179, 180 (but see 138). This may be related to the enhanced stress systems recruited during repeated exposure to and withdrawal from alcohol. In line with this hypothesis, corticohippocampal GluN2B is engaged during repeated swim stress 181. This circuitry is also recruited in other addictive disorders. Glutamate homeostasis is a mediator of long-term drug-seeking behavior, especially through disruptions of the cysteine/glutamate exchanger and EAAT2/GLT1 182. Alterations in glutamate transmission after chronic alcohol exposure and withdrawal are evident, but some effects are also likely to be unique to withdrawal alone. Future research can tease apart these dynamic distinctions or suggest that they are interconnected.
Discussion
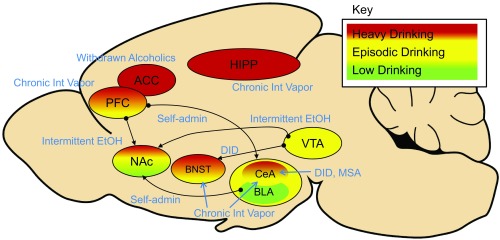
Across all phases of alcohol drinking, glutamate is a critical regulator of subcortical plasticity in the brain. We have mapped some relevant regions of interest according to their involvement in low, moderate, or heavy drinking ( Figure 1), but more work can be done to study how these sites work on a circuit level. Downstream signaling factors like CaMKII are important in the PFC and amygdala in operant self-administration. Binge drinking in the DID protocol also affects mGluR5 in the CeA and CRF in the BNST in connection with mesolimbic targets. Glutamate in the ACC and PFC is heavily disrupted in alcoholics, which is supported by preclinical research using intermittent access to alcohol or CIE. Electrophysiological studies also reveal a role for GluN2 in the extended amygdala in alcohol withdrawal, related to negative affect. Furthermore, glutamate transmission in circuits stemming from the NAc represents an overlap in circuitry from light to episodic to heavy drinking in a limited-access model. The roles of glutamate transporters and the interaction with glia are better understood at both ends of the drinking spectrum (2BC and CIE), but more can be learned through intermediate protocols that reveal the transition to heavy drinking. Overall, there appears to be a shift from mGluR-dependent behaviors to GluN-related processes transitioning from lower-level alcohol drinking to heavy alcohol drinking. The efficacy of acamprosate in animal models of drinking is high, in sharp contrast to its ineffective treatment in the clinic. Therefore, research needs to focus on other promising glutamatergic compounds to reduce heavy drinking or mediate withdrawal symptoms or both.
Figure 1. A sagittal representation of subcortical structures and their circuitry related to different stages during the transition from low-level drinking to heavy alcohol use.
Regions of interest in red indicate involvement in heavy drinking, yellow in episodic drinking, and green in lower-level drinking. Known connections start with the black circle and finish with the black arrowhead. Animal drinking protocols are depicted in blue italics. ACC, anterior cingulate cortex; BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CeA, central amygdala; HIPP, hippocampus; NAc, nucleus accumbens; PFC, prefrontal cortex; VTA, ventral tegmental area.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
William Griffin, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA
William McBride, Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN, USA
Funding Statement
This work was funded in part by National Institutes of Health grants R01AA025582 (to TLK and JB), R01AA019454 and U01AA017668 (to TLK), and R01AA019682 (to JB) and the NARSAD Independent Award (to TLK) and T32AA007573 (to LSH).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Chefer V, Meis J, Wang G, et al. : Repeated exposure to moderate doses of ethanol augments hippocampal glutamate neurotransmission by increasing release. Addict Biol. 2011;16(2):229–37. 10.1111/j.1369-1600.2010.00272.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rossetti ZL, Carboni S, Fadda F: Glutamate-induced increase of extracellular glutamate through N-methyl-D-aspartate receptors in ethanol withdrawal. Neuroscience. 1999;93(3):1135–40. 10.1016/S0306-4522(99)00250-X [DOI] [PubMed] [Google Scholar]
- 3. Poldrugo F, Snead OC, 3rd: Electroencephalographic and behavioral correlates in rats during repeated ethanol withdrawal syndromes. Psychopharmacology (Berl). 1984;83(2):140–6. 10.1007/BF00429722 [DOI] [PubMed] [Google Scholar]
- 4. Becker HC, Hale RL: Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal "kindling". Alcohol Clin Exp Res. 1993;17(1):94–8. 10.1111/j.1530-0277.1993.tb00731.x [DOI] [PubMed] [Google Scholar]
- 5. Ballenger JC, Post RM: Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133(1):1–14. 10.1192/bjp.133.1.1 [DOI] [PubMed] [Google Scholar]
- 6. Holmes A, Spanagel R, Krystal JH: Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl). 2013;229(3):539–54. 10.1007/s00213-013-3226-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Selim M, Bradberry CW: Effect of ethanol on extracellular 5-HT and glutamate in the nucleus accumbens and prefrontal cortex: comparison between the Lewis and Fischer 344 rat strains. Brain Res. 1996;716(1–2):157–64. 10.1016/0006-8993(95)01385-7 [DOI] [PubMed] [Google Scholar]
- 8. Allgaier C: Ethanol sensitivity of NMDA receptors. Neurochem Int. 2002;41(6):377–82. 10.1016/S0197-0186(02)00046-3 [DOI] [PubMed] [Google Scholar]
- 9. Lovinger DM, White G, Weight FF: Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243(4899):1721–4. 10.1126/science.2467382 [DOI] [PubMed] [Google Scholar]
- 10. Mirshahi T, Woodward JJ: Ethanol sensitivity of heteromeric NMDA receptors: effects of subunit assembly, glycine and NMDAR1 Mg 2+-insensitive mutants. Neuropharmacology. 1995;34(3):347–55. 10.1016/0028-3908(94)00155-L [DOI] [PubMed] [Google Scholar]
- 11. Kähkönen S, Wilenius J, Nikulin VV, et al. : Alcohol reduces prefrontal cortical excitability in humans: a combined TMS and EEG study. Neuropsychopharmacology. 2003;28(4):747–54. 10.1038/sj.npp.1300099 [DOI] [PubMed] [Google Scholar]
- 12. Tu Y, Kroener S, Abernathy K, et al. : Ethanol inhibits persistent activity in prefrontal cortical neurons. J Neurosci. 2007;27(17):4765–75. 10.1523/JNEUROSCI.5378-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McMillen BA, Joyner PW, Parmar CA, et al. : Effects of NMDA glutamate receptor antagonist drugs on the volitional consumption of ethanol by a genetic drinking rat. Brain Res Bull. 2004;64(3):279–84. 10.1016/j.brainresbull.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 14. Chen YW, Barson JR, Chen A, et al. : Glutamatergic input to the lateral hypothalamus stimulates ethanol intake: role of orexin and melanin-concentrating hormone. Alcohol Clin Exp Res. 2013;37(1):123–31. 10.1111/j.1530-0277.2012.01854.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lockridge A, Romero G, Harrington J, et al. : Timing-dependent reduction in ethanol sedation and drinking preference by NMDA receptor co-agonist d-serine. Alcohol. 2012;46(4):389–400. 10.1016/j.alcohol.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 16. Debrouse L, Hurd B, Kiselycznyk C, et al. : Probing the modulation of acute ethanol intoxication by pharmacological manipulation of the NMDAR glycine co-agonist site. Alcohol Clin Exp Res. 2013;37(2):223–33. 10.1111/j.1530-0277.2012.01922.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boyce-Rustay JM, Holmes A: Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit. Psychopharmacology (Berl). 2006;187(4):455–66. 10.1007/s00213-006-0448-6 [DOI] [PubMed] [Google Scholar]
- 18. Cowen MS, Schroff KC, Gass P, et al. : Neurobehavioral effects of alcohol in AMPA receptor subunit (GluR1) deficient mice. Neuropharmacology. 2003;45(3):325–33. 10.1016/S0028-3908(03)00174-6 [DOI] [PubMed] [Google Scholar]
- 19. Kiefer F, Jahn H, Koester A, et al. : Involvement of NMDA receptors in alcohol-mediated behavior: mice with reduced affinity of the NMDA R1 glycine binding site display an attenuated sensitivity to ethanol. Biol Psychiatry. 2003;53(4):345–51. 10.1016/S0006-3223(02)01486-5 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Blednov YA, Harris RA: Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: relationship to acamprosate actions. Int J Neuropsychopharmacol. 2008;11(6):775–93. 10.1017/S1461145708008584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bird MK, Kirchhoff J, Djouma E, et al. : Metabotropic glutamate 5 receptors regulate sensitivity to ethanol in mice. Int J Neuropsychopharmacol. 2008;11(6):765–74. 10.1017/S1461145708008572 [DOI] [PubMed] [Google Scholar]
- 22. McMillen BA, Crawford MS, Kulers CM, et al. : Effects of a metabotropic, mglu5, glutamate receptor antagonist on ethanol consumption by genetic drinking rats. Alcohol Alcohol. 2005;40(6):494–7. 10.1093/alcalc/agh200 [DOI] [PubMed] [Google Scholar]
- 23. Cowen MS, Djouma E, Lawrence AJ: The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther. 2005;315(2):590–600. 10.1124/jpet.105.090449 [DOI] [PubMed] [Google Scholar]
- 24. Bahi A, Fizia K, Dietz M, et al. : Pharmacological modulation of mGluR7 with AMN082 and MMPIP exerts specific influences on alcohol consumption and preference in rats. Addict Biol. 2012;17(2):235–47. 10.1111/j.1369-1600.2010.00310.x [DOI] [PubMed] [Google Scholar]
- 25. Bahi A: Viral-mediated knockdown of mGluR7 in the nucleus accumbens mediates excessive alcohol drinking and increased ethanol-elicited conditioned place preference in rats. Neuropsychopharmacology. 2013;38(11):2109–19. 10.1038/npp.2012.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Szumlinski KK, Lominac KD, Oleson EB, et al. : Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005;25(30):7054–61. 10.1523/JNEUROSCI.1529-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boismare F, Daoust M, Moore N, et al. : A homotaurine derivative reduces the voluntary intake of ethanol by rats: are cerebral GABA receptors involved? Pharmacol Biochem Behav. 1984;21(5):787–9. 10.1016/s0091-3057(84)80020-9 [DOI] [PubMed] [Google Scholar]
- 28. Yoneyama N, Crabbe JC, Ford MM, et al. : Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42(3):149–60. 10.1016/j.alcohol.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCool BA, Chappell AM: Persistent enhancement of ethanol drinking following a monosodium glutamate-substitution procedure in C57BL6/J and DBA/2J mice. Alcohol. 2014;48(1):55–61. 10.1016/j.alcohol.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fidler TL, Dion AM, Powers MS, et al. : Intragastric self-infusion of ethanol in high- and low-drinking mouse genotypes after passive ethanol exposure. Genes Brain Behav. 2011;10(3):264–75. 10.1111/j.1601-183X.2010.00664.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Z, Chow SY: Effects of glutamate, N-methyl- D-aspartate, high potassium, and hypoxia on unit discharges in CA1 area of hippocampal slices of DBA and C57 mice. Epilepsia. 1995;36(2):196–206. 10.1111/j.1528-1157.1995.tb00980.x [DOI] [PubMed] [Google Scholar]
- 32. Wanat MJ, Sparta DR, Hopf FW, et al. : Strain specific synaptic modifications on ventral tegmental area dopamine neurons after ethanol exposure. Biol Psychiatry. 2009;65(8):646–53. 10.1016/j.biopsych.2008.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kerns RT, Ravindranathan A, Hassan S, et al. : Ethanol-responsive brain region expression networks: implications for behavioral responses to acute ethanol in DBA/2J versus C57BL/6J mice. J Neurosci. 2005;25(9):2255–66. 10.1523/JNEUROSCI.4372-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou Z, Karlsson C, Liang T, et al. : Loss of metabotropic glutamate receptor 2 escalates alcohol consumption. Proc Natl Acad Sci U S A. 2013;110(42):16963–8. 10.1073/pnas.1309839110 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Sabino V, Narayan AR, Zeric T, et al. : mTOR activation is required for the anti-alcohol effect of ketamine, but not memantine, in alcohol-preferring rats. Behav Brain Res. 2013;247:9–16. 10.1016/j.bbr.2013.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hodge CW, Miles MF, Sharko AC, et al. : The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl). 2006;183(4):429–38. 10.1007/s00213-005-0217-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schroeder JP, Overstreet DH, Hodge CW: The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl). 2005;179(1):262–70. 10.1007/s00213-005-2175-9 [DOI] [PubMed] [Google Scholar]
- 38. Cowen MS, Krstew E, Lawrence AJ: Assessing appetitive and consummatory phases of ethanol self-administration in C57BL/6J mice under operant conditions: regulation by mGlu5 receptor antagonism. Psychopharmacology (Berl). 2007;190(1):21–9. 10.1007/s00213-006-0583-0 [DOI] [PubMed] [Google Scholar]
- 39. Salling MC, Faccidomo S, Hodge CW: Nonselective suppression of operant ethanol and sucrose self-administration by the mGluR7 positive allosteric modulator AMN082. Pharmacol Biochem Behav. 2008;91(1):14–20. 10.1016/j.pbb.2008.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adams CL, Cowen MS, Short JL, et al. : Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol-seeking in rats. Int J Neuropsychopharmacol. 2008;11(2):229–41. 10.1017/S1461145707007845 [DOI] [PubMed] [Google Scholar]
- 41. Besheer J, Faccidomo S, Grondin JJ, et al. : Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32(2):209–21. 10.1111/j.1530-0277.2007.00570.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Besheer J, Grondin JJ, Cannady R, et al. : Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry. 2010;67(9):812–22. 10.1016/j.biopsych.2009.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sharko AC, Hodge CW: Differential modulation of ethanol-induced sedation and hypnosis by metabotropic glutamate receptor antagonists in C57BL/6J mice. Alcohol Clin Exp Res. 2008;32(1):67–76. 10.1111/j.1530-0277.2007.00554.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tanchuck MA, Yoneyama N, Ford MM, et al. : Assessment of GABA-B, metabotropic glutamate, and opioid receptor involvement in an animal model of binge drinking. Alcohol. 2011;45(1):33–44. 10.1016/j.alcohol.2010.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parkitna JR, Sikora M, Gołda S, et al. : Novelty-seeking behaviors and the escalation of alcohol drinking after abstinence in mice are controlled by metabotropic glutamate receptor 5 on neurons expressing dopamine d1 receptors. Biol Psychiatry. 2013;73(3):263–70. 10.1016/j.biopsych.2012.07.019 [DOI] [PubMed] [Google Scholar]
- 46. Shaham Y, Shalev U, Lu L, et al. : The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl). 2003;168(1–2):3–20. 10.1007/s00213-002-1224-x [DOI] [PubMed] [Google Scholar]
- 47. Bäckström P, Hyytiä P: Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28(4):558–65. 10.1097/01.ALC.0000122101.13164.21 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Vengeliene V, Bachteler D, Danysz W, et al. : The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology. 2005;48(6):822–9. 10.1016/j.neuropharm.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 49. Bäckström P, Bachteler D, Koch S, et al. : mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29(5):921–8. 10.1038/sj.npp.1300381 [DOI] [PubMed] [Google Scholar]
- 50. Bäckström P, Hyytiä P: Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528(1–3):110–8. 10.1016/j.ejphar.2005.10.051 [DOI] [PubMed] [Google Scholar]
- 51. Rodd ZA, McKinzie DL, Bell RL, et al. : The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res. 2006;171(2):207–15. 10.1016/j.bbr.2006.03.032 [DOI] [PubMed] [Google Scholar]
- 52. Kufahl PR, Martin-Fardon R, Weiss F: Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR 2/3 agonist LY379268 and increased functional activity of mGluR 2/3 in rats with a history of ethanol dependence. Neuropsychopharmacology. 2011;36(13):2762–73. 10.1038/npp.2011.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gass JT, Sinclair CM, Cleva RM, et al. : Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addict Biol. 2011;16(2):215–28. 10.1111/j.1369-1600.2010.00262.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen J, Nam HW, Lee MR, et al. : Altered glutamatergic neurotransmission in the striatum regulates ethanol sensitivity and intake in mice lacking ENT1. Behav Brain Res. 2010;208(2):636–42. 10.1016/j.bbr.2010.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weiland A, Garcia S, Knackstedt LA: Ceftriaxone and cefazolin attenuate the cue-primed reinstatement of alcohol-seeking. Front Pharmacol. 2015;6:44. 10.3389/fphar.2015.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Olive MF, McGeehan AJ, Kinder JR, et al. : The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol. 2005;67(2):349–55. 10.1124/mol.104.003319 [DOI] [PubMed] [Google Scholar]
- 57. Faccidomo S, Besheer J, Stanford PC, et al. : Increased operant responding for ethanol in male C57BL/6J mice: specific regulation by the ERK 1/2, but not JNK, MAP kinase pathway. Psychopharmacology (Berl). 2009;204(1):135–47. 10.1007/s00213-008-1444-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Faccidomo S, Salling MC, Galunas C, et al. : Operant ethanol self-administration increases extracellular-signal regulated protein kinase (ERK) phosphorylation in reward-related brain regions: selective regulation of positive reinforcement in the prefrontal cortex of C57BL/6J mice. Psychopharmacology (Berl). 2015;232(18):3417–30. 10.1007/s00213-015-3993-z [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Schroeder JP, Spanos M, Stevenson JR, et al. : Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK 1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55(4):546–54. 10.1016/j.neuropharm.2008.06.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cannady R, Fisher KR, Durant B, et al. : Enhanced AMPA receptor activity increases operant alcohol self-administration and cue-induced reinstatement. Addict Biol. 2013;18(1):54–65. 10.1111/adb.12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cannady R, Fisher KR, Graham C, et al. : Potentiation of amygdala AMPA receptor activity selectively promotes escalated alcohol self-administration in a CaMKII-dependent manner. Addict Biol. 2016. 10.1111/adb.12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Salling MC, Faccidomo SP, Li C, et al. : Moderate Alcohol Drinking and the Amygdala Proteome: Identification and Validation of Calcium/Calmodulin Dependent Kinase II and AMPA Receptor Activity as Novel Molecular Mechanisms of the Positive Reinforcing Effects of Alcohol. Biol Psychiatry. 2016;79(6):430–42. 10.1016/j.biopsych.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Faccidomo S, Reid GT, Agoglia AE, et al. : CaMKII inhibition in the prefrontal cortex specifically increases the positive reinforcing effects of sweetened alcohol in C57BL/6J mice. Behav Brain Res. 2016;298(Pt B):286–90. 10.1016/j.bbr.2015.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grant KA: Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav. 1999;64(2):261–7. 10.1016/S0091-3057(99)00075-1 [DOI] [PubMed] [Google Scholar]
- 65. Kostowski W, Bieńkowski P: Discriminative stimulus effects of ethanol: neuropharmacological characterization. Alcohol. 1999;17(1):63–80. 10.1016/S0741-8329(98)00035-4 [DOI] [PubMed] [Google Scholar]
- 66. Hodge CW, Cox AA: The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology (Berl). 1998;139(1–2):95–107. 10.1007/s002130050694 [DOI] [PubMed] [Google Scholar]
- 67. Hundt W, Danysz W, Hölter SM, et al. : Ethanol and N-methyl-D-aspartate receptor complex interactions: a detailed drug discrimination study in the rat. Psychopharmacology (Berl). 1998;135(1):44–51. 10.1007/s002130050484 [DOI] [PubMed] [Google Scholar]
- 68. Vivian JA, Waters CA, Szeliga KT, et al. : Characterization of the discriminative stimulus effects of N-methyl- D-aspartate ligands under different ethanol training conditions in the cynomolgus monkey ( Macaca fascicularis). Psychopharmacology (Berl). 2002;162(3):273–81. 10.1007/s00213-002-1086-2 [DOI] [PubMed] [Google Scholar]
- 69. Spanagel R, Zieglgänsberger W, Hundt W: Acamprosate and alcohol: III. Effects on alcohol discrimination in the rat. Eur J Pharmacol. 1996;305(1–3):51–6. 10.1016/0014-2999(96)00176-8 [DOI] [PubMed] [Google Scholar]
- 70. Besheer J, Cox AA, Hodge CW: Coregulation of ethanol discrimination by the nucleus accumbens and amygdala. Alcohol Clin Exp Res. 2003;27(3):450–6. 10.1097/01.ALC.0000057036.64169.C1 [DOI] [PubMed] [Google Scholar]
- 71. Besheer J, Stevenson RA, Hodge CW: mGlu 5 receptors are involved in the discriminative stimulus effects of self-administered ethanol in rats. Eur J Pharmacol. 2006;551(1–3):71–5. 10.1016/j.ejphar.2006.08.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Besheer J, Grondin JJ, Salling MC, et al. : Interoceptive effects of alcohol require mGlu5 receptor activity in the nucleus accumbens. J Neurosci. 2009;29(30):9582–91. 10.1523/JNEUROSCI.2366-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cannady R, Grondin JJ, Fisher KR, et al. : Activation of group II metabotropic glutamate receptors inhibits the discriminative stimulus effects of alcohol via selective activity within the amygdala. Neuropsychopharmacology. 2011;36(11):2328–38. 10.1038/npp.2011.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Besheer J, Fisher KR, Cannady R, et al. : Intra-amygdala inhibition of ERK 1/2 potentiates the discriminative stimulus effects of alcohol. Behav Brain Res. 2012;228(2):398–405. 10.1016/j.bbr.2011.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Besheer J, Fisher KR, Jaramillo AA, et al. : Stress hormone exposure reduces mGluR5 expression in the nucleus accumbens: functional implications for interoceptive sensitivity to alcohol. Neuropsychopharmacology. 2014;39(10):2376–86. 10.1038/npp.2014.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jaramillo AA, Randall PA, Frisbee S, et al. : Activation of mGluR2/3 following stress hormone exposure restores sensitivity to alcohol in rats. Alcohol. 2015;49(6):525–32. 10.1016/j.alcohol.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jaramillo AA, Randall PA, Frisbee S, et al. : Modulation of sensitivity to alcohol by cortical and thalamic brain regions. Eur J Neurosci. 2016;44(8):2569–80. 10.1111/ejn.13374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Brigman JL, Daut RA, Wright T, et al. : GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nat Neurosci. 2013;16(8):1101–10. 10.1038/nn.3457 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Fu R, Zuo W, Gregor D, et al. : Pharmacological Manipulation of the Rostromedial Tegmental Nucleus Changes Voluntary and Operant Ethanol Self-Administration in Rats. Alcohol Clin Exp Res. 2016;40(3):572–82. 10.1111/acer.12974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Miczek KA, Barros HM, Sakoda L, et al. : Alcohol and heightened aggression in individual mice. Alcohol Clin Exp Res. 1998;22(8):1698–705. 10.1111/j.1530-0277.1998.tb03968.x [DOI] [PubMed] [Google Scholar]
- 81. Newman EL, Chu A, Bahamon B, et al. : NMDA receptor antagonism: escalation of aggressive behavior in alcohol-drinking mice. Psychopharmacology (Berl). 2012;224(1):167–77. 10.1007/s00213-012-2734-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Quadros IM, Hwa LS, Shimamoto A, et al. : Prevention of alcohol-heightened aggression by CRF-R1 antagonists in mice: critical role for DRN-PFC serotonin pathway. Neuropsychopharmacology. 2014;39(12):2874–83. 10.1038/npp.2014.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Naimi TS, Brewer RD, Mokdad A, et al. : Binge drinking among US adults. JAMA. 2003;289(1):70–5. 10.1001/jama.289.1.70 [DOI] [PubMed] [Google Scholar]
- 84. Bates ME, Labouvie EW: Adolescent risk factors and the prediction of persistent alcohol and drug use into adulthood. Alcohol Clin Exp Res. 1997;21(5):944–50. 10.1111/j.1530-0277.1997.tb03863.x [DOI] [PubMed] [Google Scholar]
- 85. Lee E, Jang DP, Kim JJ, et al. : Alteration of brain metabolites in young alcoholics without structural changes. Neuroreport. 2007;18(14):1511–4. 10.1097/WNR.0b013e3282ef7625 [DOI] [PubMed] [Google Scholar]
- 86. Silveri MM, Cohen-Gilbert J, Crowley DJ, et al. : Altered anterior cingulate neurochemistry in emerging adult binge drinkers with a history of alcohol-induced blackouts. Alcohol Clin Exp Res. 2014;38(4):969–79. 10.1111/acer.12346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kapasova Z, Szumlinski KK: Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32(4):617–31. 10.1111/j.1530-0277.2008.00620.x [DOI] [PubMed] [Google Scholar]
- 88. Ward RJ, Colivicchi MA, Allen R, et al. : Neuro-inflammation induced in the hippocampus of 'binge drinking' rats may be mediated by elevated extracellular glutamate content. J Neurochem. 2009;111(5):1119–28. 10.1111/j.1471-4159.2009.06389.x [DOI] [PubMed] [Google Scholar]
- 89. Hermens DF, Chitty KM, Lee RS, et al. : Hippocampal glutamate is increased and associated with risky drinking in young adults with major depression. J Affect Disord. 2015;186:95–8. 10.1016/j.jad.2015.07.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 90. Rhodes JS, Best K, Belknap JK, et al. : Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84(1):53–63. 10.1016/j.physbeh.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 91. Thiele TE, Crabbe JC, Boehm SL, 2nd: "Drinking in the Dark" (DID): a simple mouse model of binge-like alcohol intake. Curr Protoc Neurosci. 2014;68:9.49.1–12. 10.1002/0471142301.ns0949s68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Silvestre de Ferron B, Bennouar KE, Kervern M, et al. : Two Binges of Ethanol a Day Keep the Memory Away in Adolescent Rats: Key Role for GLUN2B Subunit. Int J Neuropsychopharmacol. 2015;19(1): pii: pyv087. 10.1093/ijnp/pyv087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gupta T, Syed YM, Revis AA, et al. : Acute effects of acamprosate and MPEP on ethanol Drinking-in-the-Dark in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32(11):1992–8. 10.1111/j.1530-0277.2008.00787.x [DOI] [PubMed] [Google Scholar]
- 94. Cozzoli DK, Courson J, Caruana AL, et al. : Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol Clin Exp Res. 2012;36(9):1623–33. 10.1111/j.1530-0277.2012.01776.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cozzoli DK, Courson J, Rostock C, et al. : Protein Kinase C Epsilon Activity in the Nucleus Accumbens and Central Nucleus of the Amygdala Mediates Binge Alcohol Consumption. Biol Psychiatry. 2016;79(6):443–51. 10.1016/j.biopsych.2015.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 96. Cozzoli DK, Courson J, Wroten MG, et al. : Binge alcohol drinking by mice requires intact group1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology. 2014;39(2):435–44. 10.1038/npp.2013.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lum EN, Campbell RR, Rostock C, et al. : mGluR1 within the nucleus accumbens regulates alcohol intake in mice under limited-access conditions. Neuropharmacology. 2014;79:679–87. 10.1016/j.neuropharm.2014.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Meyers JL, Salling MC, Almli LM, et al. : Frequency of alcohol consumption in humans; the role of metabotropic glutamate receptors and downstream signaling pathways. Transl Psychiatry. 2015;5(6):e586. 10.1038/tp.2015.70 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 99. Agoglia AE, Holstein SE, Reid G, et al. : CaMKIIalpha-GluA1 Activity Underlies Vulnerability to Adolescent Binge Alcohol Drinking. Alcohol Clin Exp Res. 2015;39(9):1680–90. 10.1111/acer.12819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wilcox MV, Cuzon Carlson VC, Sherazee N, et al. : Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology. 2014;39(3):579–94. 10.1038/npp.2013.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rinker JA, Marshall SA, Mazzone CM, et al. : Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake. Biol Psychiatry. 2016; pii: S0006-3223(16)30006-3. 10.1016/j.biopsych.2016.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bernier BE, Whitaker LR, Morikawa H: Previous ethanol experience enhances synaptic plasticity of NMDA receptors in the ventral tegmental area. J Neurosci. 2011;31(14):5205–12. 10.1523/JNEUROSCI.5282-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 103. Linsenbardt DN, Moore EM, Griffin KD, et al. : Tolerance to ethanol's ataxic effects and alterations in ethanol-induced locomotion following repeated binge-like ethanol intake using the DID model. Alcohol Clin Exp Res. 2011;35(7):1246–55. 10.1111/j.1530-0277.2011.01459.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Finn DA, Belknap JK, Cronise K, et al. : A procedure to produce high alcohol intake in mice. Psychopharmacology (Berl). 2005;178(4):471–80. 10.1007/s00213-004-2039-8 [DOI] [PubMed] [Google Scholar]
- 105. Cozzoli DK, Goulding SP, Zhang PW, et al. : Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci. 2009;29(27):8655–68. 10.1523/JNEUROSCI.5900-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Murphy JM, Gatto GJ, Waller MB, et al. : Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol. 1986;3(5):331–6. 10.1016/0741-8329(86)90010-8 [DOI] [PubMed] [Google Scholar]
- 107. Bell RL, Kimpel MW, Rodd ZA, et al. : Protein expression changes in the nucleus accumbens and amygdala of inbred alcohol-preferring rats given either continuous or scheduled access to ethanol. Alcohol. 2006;40(1):3–17. 10.1016/j.alcohol.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 108. Bell RL, Kimpel MW, McClintick JN, et al. : Gene expression changes in the nucleus accumbens of alcohol-preferring rats following chronic ethanol consumption. Pharmacol Biochem Behav. 2009;94(1):131–47. 10.1016/j.pbb.2009.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. McBride WJ, Kimpel MW, Schultz JA, et al. : Changes in gene expression in regions of the extended amygdala of alcohol-preferring rats after binge-like alcohol drinking. Alcohol. 2010;44(2):171–83. 10.1016/j.alcohol.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bell RL, Hauser SR, McClintick J, et al. : Ethanol-Associated Changes in Glutamate Reward Neurocircuitry: A Minireview of Clinical and Preclinical Genetic Findings. Prog Mol Biol Transl Sci. 2016;137:41–85. 10.1016/bs.pmbts.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Salimov RM, Salimova NB: The alcohol-deprivation effect in hybrid mice. Drug Alcohol Depend. 1993;32(2):187–91. 10.1016/0376-8716(93)80012-4 [DOI] [PubMed] [Google Scholar]
- 112. Spanagel R, Hölter SM, Allingham K, et al. : Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol. 1996;305(1–3):39–44. 10.1016/0014-2999(96)00174-4 [DOI] [PubMed] [Google Scholar]
- 113. (SAMHSA), S. A. and M. H. S. A: 2014 National Survey on Drug Use and Health (NSDUH). 2014. Reference Source [Google Scholar]
- 114. Association A.P: Diagnostic and Statistical Manual of Mental Disorders. 2013. Reference Source [Google Scholar]
- 115. Tsai GE, Ragan P, Chang R, et al. : Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal. Am J Psychiatry. 1998;155(6):726–32. [DOI] [PubMed] [Google Scholar]
- 116. Yeo RA, Thoma RJ, Gasparovic C, et al. : Neurometabolite concentration and clinical features of chronic alcohol use: a proton magnetic resonance spectroscopy study. Psychiatry Res. 2013;211(2):141–7. 10.1016/j.pscychresns.2012.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ende G, Hermann D, Demirakca T, et al. : Loss of control of alcohol use and severity of alcohol dependence in non-treatment-seeking heavy drinkers are related to lower glutamate in frontal white matter. Alcohol Clin Exp Res. 2013;37(10):1643–9. 10.1111/acer.12149 [DOI] [PubMed] [Google Scholar]
- 118. Bauer J, Pedersen A, Scherbaum N, et al. : Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology. 2013;38(8):1401–8. 10.1038/npp.2013.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mon A, Durazzo TC, Meyerhoff DJ: Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend. 2012;125(1–2):27–36. 10.1016/j.drugalcdep.2012.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Krystal JH, Petrakis IL, Webb E, et al. : Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Arch Gen Psychiatry. 1998;55(4):354–60. 10.1001/archpsyc.55.4.354 [DOI] [PubMed] [Google Scholar]
- 121. Krystal JH, Petrakis IL, Limoncelli D, et al. : Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacology. 2003;28(11):2020–8. 10.1038/sj.npp.1300252 [DOI] [PubMed] [Google Scholar]
- 122. Krystal JH, Petrakis IL, Limoncelli D, et al. : Characterization of the interactive effects of glycine and D-cycloserine in men: further evidence for enhanced NMDA receptor function associated with human alcohol dependence. Neuropsychopharmacology. 2011;36(3):701–10. 10.1038/npp.2010.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Evans SM, Levin FR, Brooks DJ, et al. : A pilot double-blind treatment trial of memantine for alcohol dependence. Alcohol Clin Exp Res. 2007;31(5):775–82. 10.1111/j.1530-0277.2007.00360.x [DOI] [PubMed] [Google Scholar]
- 124. Anton RF, O'Malley SS, Ciraulo DA, et al. : Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–17. 10.1001/jama.295.17.2003 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 125. Mann K, Lemenager T, Hoffmann S, et al. : Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addict Biol. 2013;18(6):937–46. 10.1111/adb.12012 [DOI] [PubMed] [Google Scholar]
- 126. Bisaga A, Evans SM: Acute effects of memantine in combination with alcohol in moderate drinkers. Psychopharmacology (Berl). 2004;172(1):16–24. 10.1007/s00213-003-1617-5 [DOI] [PubMed] [Google Scholar]
- 127. Krupitsky EM, Neznanova O, Masalov D, et al. : Effect of memantine on cue-induced alcohol craving in recovering alcohol-dependent patients. Am J Psychiatry. 2007;164(3):519–23. 10.1176/ajp.2007.164.3.519 [DOI] [PubMed] [Google Scholar]
- 128. Schumann G, Johann M, Frank J, et al. : Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch Gen Psychiatry. 2008;65(7):826–38. 10.1001/archpsyc.65.7.826 [DOI] [PubMed] [Google Scholar]
- 129. Biermann T, Reulbach U, Lenz B, et al. : N-methyl-D-aspartate 2b receptor subtype (NR2B) promoter methylation in patients during alcohol withdrawal. J Neural Transm (Vienna). 2009;116(5):615–22. 10.1007/s00702-009-0212-2 [DOI] [PubMed] [Google Scholar]
- 130. Bach P, Kirsch M, Hoffmann S, et al. : The effects of single nucleotide polymorphisms in glutamatergic neurotransmission genes on neural response to alcohol cues and craving. Addict Biol. 2015;20(6):1022–32. 10.1111/adb.12291 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 131. Wise RA: Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29(3):203–10. 10.1007/BF00414034 [DOI] [PubMed] [Google Scholar]
- 132. Simms JA, Steensland P, Medina B, et al. : Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32(10):1816–23. 10.1111/j.1530-0277.2008.00753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Carnicella S, Ron D, Barak S: Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48(3):243–52. 10.1016/j.alcohol.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hopf FW, Chang SJ, Sparta DR, et al. : Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res. 2010;34(9):1565–73. 10.1111/j.1530-0277.2010.01241.x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 135. Hwa LS, Chu A, Levinson SA, et al. : Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35(11):1938–47. 10.1111/j.1530-0277.2011.01545.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hwa LS, Nathanson AJ, Shimamoto A, et al. : Aggression and increased glutamate in the mPFC during withdrawal from intermittent alcohol in outbred mice. Psychopharmacology (Berl). 2015;232(16):2889–902. 10.1007/s00213-015-3925-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hwa LS, Debold JF, Miczek KA: Alcohol in excess: CRF 1 receptors in the rat and mouse VTA and DRN. Psychopharmacology (Berl). 2013;225(2):313–27. 10.1007/s00213-012-2820-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. McGuier NS, Padula AE, Lopez MF, et al. : Withdrawal from chronic intermittent alcohol exposure increases dendritic spine density in the lateral orbitofrontal cortex of mice. Alcohol. 2015;49(1):21–7. 10.1016/j.alcohol.2014.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Stuber GD, Hopf FW, Hahn J, et al. : Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol Clin Exp Res. 2008;32(10):1714–20. 10.1111/j.1530-0277.2008.00749.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Cuzon Carlson VC, Seabold GK, Helms CM, et al. : Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology. 2011;36(12):2513–28. 10.1038/npp.2011.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Obara I, Bell RL, Goulding SP, et al. : Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res. 2009;33(11):1924–34. 10.1111/j.1530-0277.2009.01030.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Szumlinski KK, Ary AW, Lominac KD, et al. : Accumbens Homer2 overexpression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology. 2008;33(6):1365–78. 10.1038/sj.npp.1301473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Das SC, Yamamoto BK, Hristov AM, et al. : Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. 10.1016/j.neuropharm.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Rao PS, Bell RL, Engleman EA, et al. : Targeting glutamate uptake to treat alcohol use disorders. Front Neurosci. 2015;9:144. 10.3389/fnins.2015.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. George O, Sanders C, Freiling J, et al. : Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A. 2012;109(44):18156–61. 10.1073/pnas.1116523109 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 146. Seif T, Chang SJ, Simms JA, et al. : Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16(8):1094–100. 10.1038/nn.3445 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 147. Griffin WC, 3rd, Lopez MF, Becker HC: Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res. 2009;33(11):1893–900. 10.1111/j.1530-0277.2009.01027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Griffin WC, 3rd: Alcohol dependence and free-choice drinking in mice. Alcohol. 2014;48(3):287–93. 10.1016/j.alcohol.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Gilpin NW, Smith AD, Cole M, et al. : Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol Clin Exp Res. 2009;33(12):2113–23. 10.1111/j.1530-0277.2009.01051.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Rossetti ZL, Carboni S: Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283(1–3):177–83. 10.1016/0014-2999(95)00344-K [DOI] [PubMed] [Google Scholar]
- 151. Dahchour A, De Witte P, Bolo N, et al. : Central effects of acamprosate: part 1. Acamprosate blocks the glutamate increase in the nucleus accumbens microdialysate in ethanol withdrawn rats. Psychiatry Res. 1998;82(2):107–14. 10.1016/S0925-4927(98)00016-X [DOI] [PubMed] [Google Scholar]
- 152. Melendez RI, Hicks MP, Cagle SS, et al. : Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29(3):326–33. 10.1097/01.ALC.0000156086.65665.4D [DOI] [PubMed] [Google Scholar]
- 153. Griffin WC, 3rd, Haun HL, Hazelbaker CL, et al. : Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39(3):707–17. 10.1038/npp.2013.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Dahchour A, De Witte P: Effect of repeated ethanol withdrawal on glutamate microdialysate in the hippocampus. Alcohol Clin Exp Res. 1999;23(10):1698–703. 10.1111/j.1530-0277.1999.tb04063.x [DOI] [PubMed] [Google Scholar]
- 155. Claus D, Kim JS, Kornhuber ME, et al. : [Effect of ethanol on the neurotransmitters glutamate and GABA]. Arch Psychiatr Nervenkr (1970). 1982;232(2):183–9. 10.1007/BF00343699 [DOI] [PubMed] [Google Scholar]
- 156. Erden BF, Ozdemirci S, Yildiran G, et al. : Dextromethorphan attenuates ethanol withdrawal syndrome in rats. Pharmacol Biochem Behav. 1999;62(3):537–41. 10.1016/S0091-3057(98)00175-0 [DOI] [PubMed] [Google Scholar]
- 157. Grant KA, Valverius P, Hudspith M, et al. : Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176(3):289–96. 10.1016/0014-2999(90)90022-X [DOI] [PubMed] [Google Scholar]
- 158. Stepanyan TD, Farook JM, Kowalski A, et al. : Alcohol withdrawal-induced hippocampal neurotoxicity in vitro and seizures in vivo are both reduced by memantine. Alcohol Clin Exp Res. 2008;32(12):2128–35. 10.1111/j.1530-0277.2008.00801.x [DOI] [PubMed] [Google Scholar]
- 159. Kroener S, Mulholland PJ, New NN, et al. : Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7(5):e37541. 10.1371/journal.pone.0037541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Radke AK, Jury NJ, Kocharian A, et al. : Chronic EtOH effects on putative measures of compulsive behavior in mice. Addict Biol. 2017;22(2):423–434. 10.1111/adb.12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Meinhardt MW, Hansson AC, Perreau-Lenz S, et al. : Rescue of infralimbic mGluR 2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci. 2013;33(7):2794–806. 10.1523/JNEUROSCI.4062-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Olive MF, Becker HC: Effects of the mGluR2/3 agonist LY379268 and the mGluR5 antagonist MPEP on handling-induced convulsions during ethanol withdrawal in mice. Alcohol. 2008;42(3):191–7. 10.1016/j.alcohol.2008.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hu W, Morris B, Carrasco A, et al. : Effects of acamprosate on attentional set-shifting and cellular function in the prefrontal cortex of chronic alcohol-exposed mice. Alcohol Clin Exp Res. 2015;39(6):953–61. 10.1111/acer.12722 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 164. Abulseoud OA, Camsari UM, Ruby CL, et al. : Attenuation of ethanol withdrawal by ceftriaxone-induced upregulation of glutamate transporter EAAT2. Neuropsychopharmacology. 2014;39(7):1674–84. 10.1038/npp.2014.14 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 165. Lee MR, Hinton DJ, Wu J, et al. : Acamprosate reduces ethanol drinking behaviors and alters the metabolite profile in mice lacking ENT1. Neurosci Lett. 2011;490(2):90–5. 10.1016/j.neulet.2010.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Nam HW, Lee MR, Zhu Y, et al. : Type 1 equilibrative nucleoside transporter regulates ethanol drinking through accumbal N-methyl-D-aspartate receptor signaling. Biol Psychiatry. 2011;69(11):1043–51. 10.1016/j.biopsych.2011.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Sari Y: Potential therapeutic role of glutamate transporter 1 for the treatment of alcohol dependence. OA Alcohol. 2013;1(1):6. 10.13172/2053-0285-1-1-574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Griffin WC, Ramachandra VS, Knackstedt LA, et al. : Repeated cycles of chronic intermittent ethanol exposure increases basal glutamate in the nucleus accumbens of mice without affecting glutamate transport. Front Pharmacol. 2015;6:27. 10.3389/fphar.2015.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. George O, Koob GF: Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2010;35(2):232–47. 10.1016/j.neubiorev.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Roberto M, Schweitzer P, Madamba SG, et al. : Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004;24(7):1594–603. 10.1523/JNEUROSCI.5077-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Freeman K, Staehle MM, Vadigepalli R, et al. : Coordinated dynamic gene expression changes in the central nucleus of the amygdala during alcohol withdrawal. Alcohol Clin Exp Res. 2013;37(Suppl 1):E88–100. 10.1111/j.1530-0277.2012.01910.x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 172. Floyd DW, Jung KY, McCool BA: Chronic ethanol ingestion facilitates N-methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J Pharmacol Exp Ther. 2003;307(3):1020–9. 10.1124/jpet.103.057505 [DOI] [PubMed] [Google Scholar]
- 173. Carpenter-Hyland EP, Woodward JJ, Chandler LJ: Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24(36):7859–68. 10.1523/JNEUROSCI.1902-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kash TL, Baucum AJ, 2nd, Conrad KL, et al. : Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2009;34(11):2420–9. 10.1038/npp.2009.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Wills TA, Klug JR, Silberman Y, et al. : GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis. Proc Natl Acad Sci U S A. 2012;109(5):E278–87. 10.1073/pnas.1113820109 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 176. Masneuf S, Lowery-Gionta E, Colacicco G, et al. : Glutamatergic mechanisms associated with stress-induced amygdala excitability and anxiety-related behavior. Neuropharmacology. 2014;85:190–7. 10.1016/j.neuropharm.2014.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Kallupi M, Varodayan FP, Oleata CS, et al. : Nociceptin/orphanin FQ decreases glutamate transmission and blocks ethanol-induced effects in the central amygdala of naive and ethanol-dependent rats. Neuropsychopharmacology. 2014;39(5):1081–92. 10.1038/npp.2013.308 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 178. Koob GF: A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. 10.1016/j.neuron.2008.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Reynolds AR, Berry JN, Sharrett-Field L, et al. : Ethanol withdrawal is required to produce persisting N-methyl-D-aspartate receptor-dependent hippocampal cytotoxicity during chronic intermittent ethanol exposure. Alcohol. 2015;49(3):219–27. 10.1016/j.alcohol.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Brigman JL, Wright T, Talani G, et al. : Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci. 2010;30(13):4590–600. 10.1523/JNEUROSCI.0640-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Kiselycznyk C, Svenningsson P, Delpire E, et al. : Genetic, pharmacological and lesion analyses reveal a selective role for corticohippocampal GLUN2B in a novel repeated swim stress paradigm. Neuroscience. 2011;193:259–68. 10.1016/j.neuroscience.2011.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Reissner KJ, Kalivas PW: Using glutamate homeostasis as a target for treating addictive disorders. Behav Pharmacol. 2010;21(5–6):514–22. 10.1097/FBP.0b013e32833d41b2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. McClearn GE, Rodgers DA: Genetic factors in alcohol preference of laboratory mice. J Comp Physiol Psychol. 1961;54:116–9. 10.1037/h0042530 [DOI] [Google Scholar]
- 184. Belknap JK, Crabbe JC, Young ER: Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl). 1993;112(4):503–10. 10.1007/BF02244901 [DOI] [PubMed] [Google Scholar]
- 185. Elmer GI, Meisch RA, George FR: Mouse strain differences in operant self-administration of ethanol. Behav Genet. 1987;17(5):439–51. 10.1007/BF01073111 [DOI] [PubMed] [Google Scholar]
- 186. Melendez RI, Rodd-Henricks ZA, Engleman EA, et al. : Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res. 2002;26(3):318–25. 10.1111/j.1530-0277.2002.tb02540.x [DOI] [PubMed] [Google Scholar]
- 187. Lê AD, Quan B, Juzytch W, et al. : Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl). 1998;135(2):169–74. 10.1007/s002130050498 [DOI] [PubMed] [Google Scholar]
- 188. Chaudhri N, Sahuque LL, Schairer WW, et al. : Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35(3):783–91. 10.1038/npp.2009.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Sinclair JD, Walker S, Jordan W: Behavioral and physiological changes associated with various durations of alcohol deprivation in rats. Q J Stud Alcohol. 1973;34(3):744–57. [PubMed] [Google Scholar]
- 190. Melendez RI, Middaugh LD, Kalivas PW: Development of an alcohol deprivation and escalation effect in C57BL/6J mice. Alcohol Clin Exp Res. 2006;30(12):2017–25. 10.1111/j.1530-0277.2006.00248.x [DOI] [PubMed] [Google Scholar]
- 191. Goldstein DB: Relationship of alcohol dose to intensity of withdrawal signs in mice. J Pharmacol Exp Ther. 1972;180(2):203–15. [PubMed] [Google Scholar]
- 192. O'Dell LE, Roberts AJ, Smith RT, et al. : Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28(11):1676–82. 10.1097/01.ALC.0000145781.11923.4E [DOI] [PubMed] [Google Scholar]
- 193. Lopez MF, Becker HC: Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl). 2005;181(4):688–96. 10.1007/s00213-005-0026-3 [DOI] [PubMed] [Google Scholar]