Abstract
In this review on respiratory assistance, we aim to discuss the following recent advances: the optimization and customization of mechanical ventilation, the use of high-frequency oscillatory ventilation, and the role of noninvasive ventilation. The prevention of ventilator-induced lung injury and diaphragmatic dysfunction is now a key aspect in the management of mechanical ventilation, since these complications may lead to higher mortality and prolonged length of stay in intensive care units. Different physiological measurements, such as esophageal pressure, electrical activity of the diaphragm, and volumetric capnography, may be useful objective tools to help guide ventilator assistance. Companies that design medical devices including ventilators and respiratory monitoring platforms play a key role in knowledge application. The creation of a ventilation consortium that includes companies, clinicians, researchers, and stakeholders could be a solution to promote much-needed device development and knowledge implementation.
Keywords: ventilator-induced lung injury, diaphragmatic dysfunction, mechanical ventilation
Introduction
Respiratory failure is the leading cause of admission to pediatric intensive care units (PICUs) 1– 3. Mechanical ventilation (MV) is a lifesaving therapy, allowing the support of patients with respiratory failure with the objectives of improving gas exchange and decreasing work of breathing. MV consists of a pressurized volume of gas delivered by either an invasive (tracheal tube or tracheostomy) or a non-invasive interface. MV is particularly challenging in children because of the heterogeneity of this population in terms of age, weight, and pathophysiology.
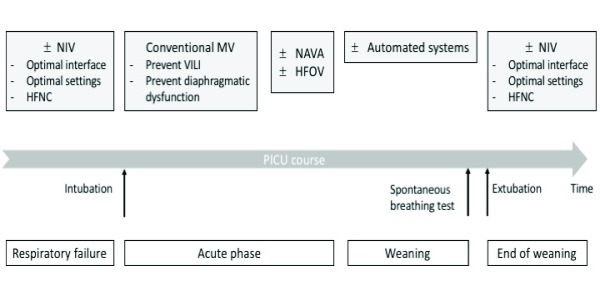
In this brief review, we aim to discuss the current clinical challenges in pediatric ventilatory assistance outside of the neonatal patient population. We will focus this discussion on recent advances regarding 1) optimization and individualization of patient–ventilator interactions during MV, 2) application of high-frequency oscillatory ventilation (HFOV), and 3) the role of noninvasive ventilation (NIV) ( Table 1 and Figure 1).
Figure 1. Schematic representation of recent advances in mechanical ventilation of critically ill children.
HFNC, high-flow nasal cannula; HFOV, high-frequency oscillatory ventilation; MV, mechanical ventilation; NAVA, neurally adjusted ventilatory assist; NIV, noninvasive ventilation; VILI, ventilator-induced lung injury.
Table 1. Key messages suggested by the recent advances in pediatric ventilator assistance.
|
Optimization/
individualization of MV |
To limit ventilator-induced lung injury using transpulmonary pressure and volumetric
capnography monitoring |
| To limit diaphragmatic dysfunction by monitoring electrical activity of the diaphragm | |
| To better identify the timing of extubation with spontaneous breathing trials using
CPAP mode or T-Tube |
|
| Modes of MV | To consider NAVA to improve patient–ventilator interaction |
| To still consider high-frequency oscillatory ventilation in the most severe pediatric
ARDS not adequately supported with optimally set conventional ventilation |
|
| NIV | To consider NIV as a first-line support in many pathologies |
| To consider high-flow nasal cannula to improve comfort and tolerance of NIV | |
| To select the optimal interface according to the patient among all that are available
nowadays |
ARDS, acute respiratory distress syndrome; CPAP, continuous positive airway pressure; MV, mechanical ventilation; NAVA, neurally adjusted ventilatory assist; NIV, noninvasive ventilation.
Advances in optimization and customization of mechanical ventilation in children
Advances in the management of mechanical ventilation to limit ventilator-induced lung injury: transpulmonary pressure and capnography
The use of a global lung-protective ventilatory strategy, referring to low tidal volume and high levels of positive end-expiratory pressure (PEEP), in order to prevent ventilator-induced lung injury (VILI) improved survival in patients with acute respiratory distress syndrome (ARDS) 4– 8. In daily practice, the only way to assess the respiratory mechanics and the effects of MV on the lung itself are the ventilatory pressure, flow, and volume measured by the ventilator. However, relying on only these parameters during MV (plateau pressure level and tidal volume prescribed) may be misleading and may provide inaccurate assessment of the risk of VILI, since such variables do not accurately describe lung dynamics. Indeed, these recording variables reflect the respiratory system as a whole and do not take into account important pathophysiological features (e.g. chest wall compliance, intrinsic inspiratory/expiratory respiratory effort, heterogeneity of lung disease, etc.). Currently, new challenges are to optimize and customize MV by individualized monitoring at the bedside in order to avoid barotrauma, volotrauma, atelectrauma, and biotrauma 9. To do so, transpulmonary pressure and capnography monitoring are helpful.
The driving pressure is a key variable for clinicians to optimize protective volume and inspiratory pressure in order to avoid lung stress and strain 5, 10. The driving pressure is the ratio of the tidal volume to the static respiratory system compliance (ΔP=V T/C RS); it is also equivalent to the plateau pressure minus the PEEP (ΔP = Pplat – PEEP). A recent study by Amato et al. on adult ARDS reported that among different ventilation variables, driving pressure was most strongly and independently associated with survival. Indeed, a decrease in driving pressure concomitant to a reduction in tidal volume or an increase in PEEP were associated with increased survival, while differences in tidal volume were not associated with different survival rates when the driving pressure was constant 5. In ARDS, the proportion of lung available for ventilation is markedly decreased; therefore, the driving pressure (and consequently tidal volume) should be adapted to this reality rather than using only predicted body weight 11. However, it is important to note that this approach should be adapted depending on the patient’s condition. In particular, the impact of a given driving pressure might not be similar in patients with low chest wall compliance 12. It is the reason why there is an increasing interest in the monitoring of transpulmonary pressure to guide ventilatory assistance adjustments.
The transpulmonary pressure, defined by the difference between the airway pressure and pleural pressure, should be considered as the lung-distending pressure. This pressure measurement is closely correlated with lung strain and risk of VILI 13. Esophageal pressure is a good surrogate for pleural pressure and its measurement is valuable to the assessment of lung strain in mechanically ventilated patients. Despite controversies regarding the interpretation of absolute values of esophageal pressure, a recent paper reviewed the usefulness of this tool in ventilation management 14. Measurement of esophageal pressure is the only way to distinguish the effect of pressure on the lung and the chest wall. When a given amount of pressure is delivered, it is of great importance in some situations to better know which percentage is distending the lung (potentially harmful to the lungs) and which amount is distending the chest wall. As an example in ARDS, at the end of expiration, transpulmonary pressure can be negative (when pleural pressure exceeds end-expiratory airway pressure) and induces collapse of the alveoli. This may expose these parts of the lungs to being repeatedly reopened and recollapsed at each breath. To protect the lung from VILI, one would like to find a balance between protecting aerated units from over-distension and recruiting unstable units, thereby reducing tissue damage associated with their cyclic recruitment/derecruitment. The titration of PEEP based on esophageal pressure measurement 15– 17 has been proposed in patients with ARDS. Talmor et al. showed that oxygenation and lung compliance were significantly improved in patients managed by a ventilatory strategy including esophageal pressure measurement 12. This recent interest in transplumonary pressure has contributed to the development of such monitoring in several ventilators (Avea ventilator-CareFusion® and G5-Hamilton Medical®, for example). An example of clinical information given by esophageal pressure monitoring is given in Figure 2. Unfortunately, such ventilators are not available in all units while no dedicated monitor is able to provide this measurement. We believe that the use of transpulmonary pressure has to be developed and, nowadays, we include this monitoring in our clinical practice in the management of difficult-to-ventilate children with low lung and chest wall (and/or low abdominal) compliances. However, more research in this field is needed to validate the best strategy to quantify esophageal pressure in children and to confirm its utility in ventilation titration. In particular, the impact of mediastinum weight is taken into account by some authors in adult studies, but it has not been examined in pediatric patients. Beyond the estimation of absolute pleural pressure, we also use esophageal pressure monitoring to assess the work of breathing in invasive ventilation and NIV (see below) 18– 20.
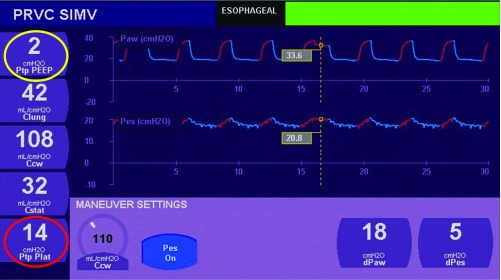
Figure 2. Esophageal and transpulmonary pressures measured in an 8-year-old morbidly obese patient with the Avea ventilator ® from CareFusion.
(1) Transpulmonary pressure (TPP = plateau pressure – esophageal pressure: to calculate TPP, esophageal pressure is used as a surrogate of intrapleural pressure) is 14 cmH 2O at the end of insufflation (red circle). This means that despite a positive plateau pressure of 34 cmH 2O in this obese child, lung parenchyma does not experience much distension (14 cmH 2O) at the end of insufflation. (2) Positive end expiration pressure (PEEP) set on the ventilator is 2 cmH 2O above esophageal pressure (yellow circle). This means that there is minimal risk of lung collapse at the end of expiration. Figure adapted from RM DiBlasi database with permission.
Volumetric capnography (Vcap) is also a novel tool which allows the measurement of physiological and alveolar dead space at the bedside 21– 23. In this technique, expired CO 2 is plotted against the tidal volume for each breath. Vcap analysis gives a global index of ventilation/perfusion (V/Q) mismatch, containing shunt and indices of lung efficiency (physiological and alveolar dead space). Vcap can help to set PEEP to obtain the lowest physiological and alveolar dead space, the lowest arterial to end-tidal CO 2 gradient (PaCO 2–ETCO 2 gradient), and the optimal alveolar plateau slope (SIII) that reflect V/Q heterogeneity 24– 27. We believe that Vcap will help clinicians to set PEEP routinely in the near future. We already use it to better predict PaCO 2 in mechanically ventilated children 23, and CO 2 measurement obtained by Vcap is already included in a closed loop system dedicated to MV management 28.
The role of diaphragmatic function in the management of mechanical ventilation
Increasing evidence suggests that MV is associated with diaphragmatic dysfunction and atrophy, also known as ventilator-induced diaphragmatic dysfunction 29– 31. To limit such consequences on the diaphragm, specific efforts should be addressed to reduce the duration of MV and to optimize ventilator settings. Improving individualized MV at bedside to limit diaphragmatic weakness is a great challenge but is essential to successfully wean patients from MV and decrease poor outcomes 30, 32, 33.
Monitoring of the electrical activity of the diaphragm (EAdi) provides new information to clinicians in order to assess diaphragm function and the impact of ventilation on the diaphragm muscle that can lead to rapidly progressive diaphragmatic weakness 30, 32. EAdi has been shown to reflect the patient ventilatory drive, and it is well correlated with work of breathing based on short-term physiological studies 34, 35. EAdi permits the detection of periods of blunted drive secondary to overassistance 36, which likely favor the risk of diaphragm dysfunction. It therefore may be used as a tool to adjust ventilatory support 37, to detect tonic activity of the diaphragm (which reflects the effort of the patient to increase the lung volume) 38, and to assess patient–ventilator asynchrony 39. When combined with pressure or volume delivered, EAdi measurements permit the assessment of diaphragm neuroventilatory (V T/EAdi) or neuromechanical (ΔP/EAdi) efficiency 40. In the only pediatric study on this topic to date, Wolf et al. observed that the ability to generate a higher diaphragmatic activity for the same tidal volume in pressure support ventilation (PSV) was a predictor of successful extubation 41.
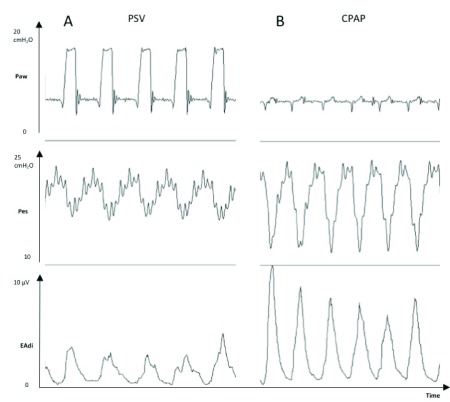
As shown in Figure 3, both EAdi and esophageal pressure can provide similar clinical information regarding the patient’s work of breathing. Although these tools are correlated in most clinical situations, they can differ in patients with diaphragmatic dysfunction. It is therefore important to emphasize that EAdi represents respiratory drive and not diaphragmatic contractility.
Figure 3.
Tracings of a mechanically ventilated patient showing the increases in esophageal pressure swings (Pes, cmH 2O) and electrical activity of the diaphragm (EAdi, μV) from a period with pressure support ventilation (PSV) +8 cmH 2O and positive end-expiratory pressure +5 cmH 2O (Panel A) to a period with continuous positive airway pressure (CPAP) +5 cmH 2O only (Panel B).
Paw, mean airway opening pressure.
This technology requires a specific nasogastric catheter equipped with distal electrodes (NAVA catheter, Maquet Critical Care, Solna, Sweden) connected to a dedicated Servo-I ventilator (Maquet Critical Care, Solna, Sweden). The main clinical application of EAdi monitoring is the neurally adjusted ventilatory assist mode (NAVA), a mode of ventilation which uses the EAdi to trigger and cycle-off breathing efforts. The NAVA level and the EAdi determine the amount of ventilator assistance. NAVA has many advantages compared to conventional MV, including improved patient–ventilator synchrony 39, 42– 45, the potential for a reduction in barotrauma (secondary to a decline of inspiratory pressure and tidal volume) 23, 39, 42, 44, 46, a possible decrease in atelectrauma 47, and, finally, improved diaphragmatic efficiency 40. Moreover, NAVA improves unloading of the respiratory muscles and prevents the risk of over-assistance through downregulation of EAdi induced by increased assistance 37. A recent randomized trial was conducted in children to test the clinical impact of NAVA 48. The feasibility of NAVA in clinical practice was confirmed, and NAVA was associated with lower FiO 2 requirements and lower inspiratory pressures. A trend for shorter duration of ventilation was observed, but it did not reach statistical significance. Nowadays, we use the NAVA mode routinely, in particular in difficult-to-wean children, in children who have undergone cardiac surgery, or any case in which the promotion of assisted ventilation and avoidance of diaphragm rest is important. EAdi is also routinely used to detect diaphragm contractility recovery in children with neuromuscular disease (e.g. botulism, Guillain-Barré syndrome, and cervical trauma).
Advances in weaning from mechanical ventilation
Owing to MV’s potential complications, such as VILI 49 and severe diaphragmatic atrophy 30, 32, it is imperative that it be discontinued as soon as the patient is capable of sustaining spontaneous breathing. On the other hand, premature extubation may also be problematic, as higher mortality rates have been reported in patients with extubation failure 2, 50. Consequently, when and how to perform MV weaning are key questions in critically ill patients. The identification of extubation readiness is usually based on clinical judgement, according to the respiratory, neurological, and hemodynamic status. However, this practice remains greatly subjective, while the timing of extubation is challenging. Therefore, efficient processes to safely reduce and remove ventilator support are necessary.
Clinical and research efforts have focused on early identification of weaning readiness. Some authors suggest the use of written protocols to assist clinicians in the management of weaning MV, but their usage in clinical practice remains limited for several reasons 51: (1) providing and following protocols are time consuming, resulting in fluctuation in protocol implementation and compliance; (2) clinical instructions may not be explicit enough, resulting in variable interpretations of the protocol; and (3) protocols are generally specific to one organization, leading to a certain heterogeneity in clinical practice.
The development of the closed-loop system (CLS) (computerized protocol implementing its recommendations without caregiver intervention) has resolved some of these issues 52. While optimizing ventilatory support on a continuous basis according to the patient’s respiratory condition, CLS offers consistent orders that constrain interpretation variations among caregivers, potentially resulting in a more efficient application of protocols. The use of CLS leads to a quicker adjustment of ventilator settings assessed by a reduction of time between the assessment of patient status and medical order, and medical order and clinical execution 53.
Two CLSs are commercialized for respiratory weaning: SmartCare/PS ® (Dräger Medical, Lubeck, Germany) and IntelliVent ® (Hamilton Medical, Bonaduz, Switzerland). These systems automatically reduce the level of support when the patient’s respiratory rate, tidal volume, and end tidal CO 2 (EtPCO 2) are within acceptable ranges. In adults, these systems reduced the weaning time without increasing adverse events 54. Currently, only two trials, one for each of these two technologies, have been conducted in children, and their findings regarding safety and duration of weaning process are encouraging 28, 53. A significant limitation of these systems remains the minimal weight/age required (15 kg for Smartcare/PS ® and 7 kg with Intellivent ®) and they cannot be used in case of significant leaks around the endotracheal tube. We believe that these automated systems will improve the management of MV and therefore the outcome of patients, allowing the customization of ventilator support according to each child’s condition. However, companies and researchers should now focus their efforts on algorithms adapted to our pediatric population.
During the weaning process, identifying whether or not patients will be able to breathe spontaneously after extubation is a significant challenge. The recent consensus conference on pediatric ARDS (PALICC) has addressed this question and recommended that spontaneous breathing trials (SBTs) or extubation readiness tests should be performed 55.
Determining inclusion criteria for SBT initiation has been a difficult challenge because of the broad patient population, different modes of ventilation, and lack of consensus for acceptable SBT parameters. Another limitation is appropriate timing for starting SBT. For these reasons, some patients who qualify for SBTs may not be recognized, which may result in a prolonged ventilation course. Some institutions are now using electronic data pooled from ventilators and electronic medical records to develop explicit software rules and algorithms (decision support) to help identify patients who may be ready for SBT. Assuming a patient has met certain parameters for SBT criteria (EtCO 2, SpO 2, tidal volume, respiratory rate, inspiratory pressure, etc.), the electronic medical record can provide visual cues to help remind clinicians that their patient is ready for SBT.
In adults undergoing SBT, the use of an inspiratory pressure of 5 to 8 cmH 2O is recommended 56. In children, very few data exist regarding the optimal method to conduct a SBT. Interestingly, a physiologic study conducted by Khemani et al., comparing a SBT with a continuous positive airway pressure (CPAP) of 5 cmH 2O versus pressure support of 10 cmH 2O, concluded that pressure support significantly underestimates the potential for post extubation breathing efforts 57. According to this recent study, we recommend performing a SBT in CPAP mode or with a T-tube. However, it should be noted that respiratory efforts observed during CPAP trial will be reflective of the efforts observed after extubation but will be larger than during SBT with PSV. Therefore, it is not surprising to observe increased efforts during CPAP, which should not lead to delay in extubation unless they appear to be objectively poorly tolerated.
During weaning, esophageal pressure measurement can be a useful tool to assess the work of breathing. A robust parameter which can be derived from esophageal pressure and transdiaphragmatic pressure, i.e. the difference between esophageal pressure and gastric pressure, is the pressure-time-product. This parameter was used as a tool to assess work of breathing and optimize ventilation support in children with different diseases 18, 20. Jubran et al. showed that esophageal pressure trend during a SBT provided an accurate prediction of weaning outcome 58. Over the course of a SBT, esophageal pressure-time-product remained unchanged in successfully weaned patients. In contrast, weaning failure patients developed marked and progressive increase in esophageal pressure-time-product (up to 4-fold above the normal value) as a result of an increase in the mechanical load of the respiratory muscles 58.
Advances in high-frequency oscillatory ventilation
HFOV has been commonly used for decades in neonatal, pediatric, and adult populations 58. Clinical trials have demonstrated that HFOV is associated with an oxygenation improvement in patients with acute lung injury or ARDS 59– 61. However, the clinical use of HFOV in this population has decreased. Recent studies demonstrated an association between early use of HFOV and worse outcome in terms of mortality in adult 62 and pediatric populations 63, 64. However, several biases have been highlighted in the two pediatric studies regarding the methodology 65– 67. As suggested by Rettig et al., the mortality in patients with ARDS supported by HFOV may be linked to the disease category itself rather than the use of HFOV 68. Given these limitations and with regard to our clinical experience, we consider, as supported by the PALICC, HFOV to still be a rescue therapy in some children with severe ARDS.
Advances in noninvasive ventilation
NIV is defined as the delivery of MV without an endotracheal tube or tracheostomy. NIV comprises both CPAP and bilevel positive airway pressure (BiPAP) ventilation. NIV is increasingly used in PICUs 69, 70. In the last decade, the potential indications for NIV in critically ill patients have grown considerably, and the performance of this mode of support has greatly improved. In children developing ARDS, NIV can be considered as a first line of treatment in milder disease 55. Despite the lack of clear guidelines, this mode of support definitely has its place in the treatment of a wide range of pathologies in children, including pneumonia, upper airway obstruction, post-extubation respiratory failure, acute chest syndrome, and asthma 70.
The use of NIV has recently evolved because of the emergence of high-flow nasal cannula (HFNC). This modality is now available from a number of manufacturers and has been widely adopted in pediatric practice. Different mechanisms have been hypothesized to account for the clinical benefits, including washout of the nasopharyngeal dead space, reduction of work of breathing, decrease in airway resistance, and improvement of pulmonary compliance 71, 72. HFNC has been able to provide a mean pharyngeal pressure of 4 cmH 2O when used at a flow of 2 L/kg/minute 73, but this effect is variable. In clinical use, HFNC allows improvement of comfort and tolerance to NIV and reduction of air leak, gastric distension, and skin injuries, especially in younger children. The literature is still poor to identify the specific population that would benefit from this technology 18, 74. The role of HFNC outside the PICU still needs to be investigated, and we currently restrict HFNC use in the PICU. More evidence is expected from several ongoing randomized controlled trials (TRAMONTANE study, NCT02457013; Hi-Flo study, NCT01498094; HHFNC study, NCT01662544). We believe that, within a few years, the role of HFNC will be better defined and potentially widened.
The optimal interface for NIV in children has recently been discussed as a key aspect in respiratory management 75. A large variety of devices recently emerged, including nasal, oronasal, and total face masks and helmet. Because mask-fit pressure is spread over a larger surface beyond the nose area, total face masks appear to be more comfortable than oronasal masks 76. This device was shown to be as efficient as oronasal mask in terms of breathing pattern, gas exchange, and outcome in adults 77. The helmet is also increasingly used 70 and should be considered as a feasible alternative for NIV in children, as suggested by the results of a recent randomized controlled trial comparing the use of a helmet and a face mask in children 78. As for total face masks, preliminary data are pointing towards the helmet as an interface to increase comfort and decrease skin injury and air leaks 79.
Finally, to improve NIV success, the achievement of an adequate patient–ventilator synchrony is crucial 19. Although the performance of ventilators has improved within the last few years, patient–ventilator asynchrony in NIV remains a significant issue. As with invasive ventilation, tools to improve patient–ventilator synchrony during NIV have been recently investigated. EAdi monitoring and noninvasive NAVA are feasible and well tolerated in PICU patients with patient–ventilator synchrony improvement 80, 81. Monitoring esogastric pressure offers another way to improve patient–ventilator interaction during NIV. In infants 82 and children 19, esophageal pressure measurement has been shown to be a valuable tool to assess patient–ventilator interaction and to optimize ventilatory settings ( Figure 1).
Conclusion
There have been major advances in the management of mechanically ventilating children over the last 3 years. The implementation of this new knowledge in usual practice is a challenge, as advances occur not only in the respiratory field but also in many fields that pediatric intensivists must digest. In such a situation, companies that design medical devices including ventilators and respiratory monitoring platforms play a key role in the application of knowledge. The creation of a ventilation consortium that includes companies, caregivers, researchers, and stakeholders could be a solution to promote knowledge implementation.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Robert DiBlasi, Department of Respiratory Therapy, Seattle Children's Hospital, Seattle, Washington, USA
Thomas Keens, Pediatric Pulmonology, Keck School of Medicine, Children's Hospital Los Angeles, University of Southern California, California, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Hammer J: Acute respiratory failure in children. Paediatr Respir Rev. 2013;14(2):64–9. 10.1016/j.prrv.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 2. Farias JA, Fernández A, Monteverde E, et al. : Mechanical ventilation in pediatric intensive care units during the season for acute lower respiratory infection: a multicenter study. Pediatr Crit Care Med. 2012;13(2):158–64. 10.1097/PCC.0b013e3182257b82 [DOI] [PubMed] [Google Scholar]
- 3. Khemani RG, Smith LS, Zimmerman JJ, et al. : Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5 Suppl 1):S23–40. 10.1097/PCC.0000000000000432 [DOI] [PubMed] [Google Scholar]
- 4. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–8. 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Amato MB, Meade MO, Slutsky AS, et al. : Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–55. 10.1056/NEJMsa1410639 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. ARDS Definition Task Force, . Ranieri VM, Rubenfeld GD, et al. : Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Villar J, Kacmarek RM, Pérez-Méndez L, et al. : A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006;34(5):1311–8. 10.1097/01.CCM.0000215598.84885.01 [DOI] [PubMed] [Google Scholar]
- 8. Briel M, Meade M, Mercat A, et al. : Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865–73. 10.1001/jama.2010.218 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Slutsky AS, Ranieri VM: Ventilator-induced lung injury. N Engl J Med. 2014;370(22):980. 10.1056/NEJMc1400293 [DOI] [PubMed] [Google Scholar]
- 10. Chiumello D, Carlesso E, Brioni M, et al. : Airway driving pressure and lung stress in ARDS patients. Crit Care. 2016;20:276. 10.1186/s13054-016-1446-7 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Rimensberger PC, Cheifetz IM: Ventilatory support in children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5 suppl 1):S51–60. 10.1097/PCC.0000000000000433 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Talmor D, Sarge T, Malhotra A, et al. : Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359(20):2095–104. 10.1056/NEJMoa0708638 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Chiumello D, Carlesso E, Cadringher P, et al. : Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med. 2008;178(4):346–55. 10.1164/rccm.200710-1589OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Akoumianaki E, Maggiore SM, Valenza F, et al. : The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189(5):520–31. 10.1164/rccm.201312-2193CI [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Pintado M, de Pablo R, Trascasa M, et al. : Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care. 2013;58(9):1416–23. 10.4187/respcare.02068 [DOI] [PubMed] [Google Scholar]
- 16. Rodriguez PO, Bonelli I, Setten M, et al. : Transpulmonary pressure and gas exchange during decremental PEEP titration in pulmonary ARDS patients. Respir Care. 2013;58(5):754–63. [DOI] [PubMed] [Google Scholar]
- 17. Soroksky A, Esquinas A: Goal-directed mechanical ventilation: are we aiming at the right goals? A proposal for an alternative approach aiming at optimal lung compliance, guided by esophageal pressure in acute respiratory failure. Crit Care Res Pract. 2012;2012: 597932. 10.1155/2012/597932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Essouri S, Durand P, Chevret L, et al. : Optimal level of nasal continuous positive airway pressure in severe viral bronchiolitis. Intensive Care Med. 2011;37(12):2002–7. 10.1007/s00134-011-2372-4 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Fauroux B, Nicot F, Essouri S, et al. : Setting of noninvasive pressure support in young patients with cystic fibrosis. Eur Respir J. 2004;24(4):624–30. 10.1183/09031936.04.0000137603 [DOI] [PubMed] [Google Scholar]
- 20. Khirani S, Ramirez A, Aloui S, et al. : Continuous positive airway pressure titration in infants with severe upper airway obstruction or bronchopulmonary dysplasia. Crit Care. 2013;17(4):R167. 10.1186/cc12846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verscheure S, Massion PB, Verschuren F, et al. : Volumetric capnography: lessons from the past and current clinical applications. Crit Care. 2016;20(1):184. 10.1186/s13054-016-1377-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Suarez-Sipmann F, Bohm SH, Tusman G: Volumetric capnography: the time has come. Curr Opin Crit Care. 2014;20(3):333–9. 10.1097/MCC.0000000000000095 [DOI] [PubMed] [Google Scholar]
- 23. Baudin F, Bourgoin P, Brossier D, et al. : Noninvasive Estimation of Arterial CO2 From End-Tidal CO2 in Mechanically Ventilated Children: The GRAeDIENT Pilot Study. Pediatr Crit Care Med. 2016;17(12):1117–23. 10.1097/PCC.0000000000000935 [DOI] [PubMed] [Google Scholar]
- 24. Suter PM, Fairley B, Isenberg MD: Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med. 1975;292(6):284–9. 10.1056/NEJM197502062920604 [DOI] [PubMed] [Google Scholar]
- 25. Tusman G, Suarez-Sipmann F, Böhm SH, et al. : Monitoring dead space during recruitment and PEEP titration in an experimental model. Intensive Care Med. 2006;32(11):1863–71. 10.1007/s00134-006-0371-7 [DOI] [PubMed] [Google Scholar]
- 26. Tusman G, Suarez-Sipmann F, Bohm SH, et al. : Capnography reflects ventilation/perfusion distribution in a model of acute lung injury. Acta Anaesthesiol Scand. 2011;55(5):597–606. 10.1111/j.1399-6576.2011.02404.x [DOI] [PubMed] [Google Scholar]
- 27. Kallet RH: Measuring dead-space in acute lung injury. Minerva Anestesiol. 2012;78(11):1297–305. [PubMed] [Google Scholar]
- 28. Jouvet P, Eddington A, Payen V, et al. : A pilot prospective study on closed loop controlled ventilation and oxygenation in ventilated children during the weaning phase. Crit Care. 2012;16(3):R85. 10.1186/cc11343 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Vassilakopoulos T, Petrof BJ: Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;169(3):336–41. 10.1164/rccm.200304-489CP [DOI] [PubMed] [Google Scholar]
- 30. Jaber S, Petrof BJ, Jung B, et al. : Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183:364–71. 10.1164/rccm.201004-0670OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Petrof BJ, Hussain SN: Ventilator-induced diaphragmatic dysfunction: what have we learned? Curr Opin Crit Care. 2016;22(1):67–72. 10.1097/MCC.0000000000000272 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Levine S, Nguyen T, Taylor N, et al. : Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358(13):1327–35. 10.1056/NEJMoa070447 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Hudson MB, Smuder AJ, Nelson WB, et al. : Both high level pressure support ventilation and controlled mechanical ventilation induce diaphragm dysfunction and atrophy. Crit Care Med. 2012;40(4):1254–60. 10.1097/CCM.0b013e31823c8cc9 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Bellani G, Mauri T, Coppadoro A, et al. : Estimation of patient's inspiratory effort from the electrical activity of the diaphragm. Crit Care Med. 2013;41(6):1483–91. 10.1097/CCM.0b013e31827caba0 [DOI] [PubMed] [Google Scholar]
- 35. Ducharme-Crevier L, Du Pont-Thibodeau G, Emeriaud G: Interest of monitoring diaphragmatic electrical activity in the pediatric intensive care unit. Crit Care Res Pract. 2013;2013:384210. 10.1155/2013/384210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Emeriaud G, Larouche A, Ducharme-Crevier L, et al. : Evolution of inspiratory diaphragm activity in children over the course of the PICU stay. Intensive Care Med. 2014;40(11):1718–26. 10.1007/s00134-014-3431-4 [DOI] [PubMed] [Google Scholar]
- 37. Colombo D, Cammarota G, Bergamaschi V, et al. : Physiologic response to varying levels of pressure support and neurally adjusted ventilatory assist in patients with acute respiratory failure. Intensive Care Med. 2008;34(11):2010–8. 10.1007/s00134-008-1208-3 [DOI] [PubMed] [Google Scholar]
- 38. Emeriaud G, Beck J, Tucci M, et al. : Diaphragm electrical activity during expiration in mechanically ventilated infants. Pediatr Res. 2006;59(5):705–10. 10.1203/01.pdr.0000214986.82862.57 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Bordessoule A, Emeriaud G, Morneau S, et al. : Neurally adjusted ventilatory assist improves patient-ventilator interaction in infants as compared with conventional ventilation. Pediatr Res. 2012;72(2):194–202. 10.1038/pr.2012.64 [DOI] [PubMed] [Google Scholar]
- 40. Di Mussi R, Spadaro S, Mirabella L, et al. : Impact of prolonged assisted ventilation on diaphragmatic efficiency: NAVA versus PSV. Crit Care. 2016;20:1. 10.1186/s13054-015-1178-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Wolf GK, Walsh BK, Green ML, et al. : Electrical activity of the diaphragm during extubation readiness testing in critically ill children. Pediatr Crit Care Med. 2011;12(6):e220–4. 10.1097/PCC.0b013e3181fe28fc [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. de La Oliva P, Schuffelmann C, Gomez-Zamora A, et al. : Asynchrony, neural drive, ventilatory variability and COMFORT: NAVA versus pressure support in pediatric patients. A non-randomized cross-over trial. Intensive Care Med. 2012;38(5):838–46. 10.1007/s00134-012-2535-y [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Clement KC, Thurman TL, Holt SJ, et al. : Neurally triggered breaths reduce trigger delay and improve ventilator response times in ventilated infants with bronchiolitis. Intensive Care Med. 2011;37(11):1826–32. 10.1007/s00134-011-2352-8 [DOI] [PubMed] [Google Scholar]
- 44. Alander M, Peltoniemi O, Pokka T, et al. : Comparison of pressure-, flow-, and NAVA-triggering in pediatric and neonatal ventilatory care. Pediatr Pulmonol. 2012;47(1):76–83. 10.1002/ppul.21519 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Vignaux L, Grazioli S, Piquilloud L, et al. : Optimizing patient-ventilator synchrony during invasive ventilator assist in children and infants remains a difficult task*. Pediatr Crit Care Med. 2013;14(7):e316–25. 10.1097/PCC.0b013e31828a8606 [DOI] [PubMed] [Google Scholar]
- 46. Breatnach C, Conlon NP, Stack M, et al. : A prospective crossover comparison of neurally adjusted ventilatory assist and pressure-support ventilation in a pediatric and neonatal intensive care unit population. Pediatr Crit Care Med. 2010;11(1):7–11. 10.1097/PCC.0b013e3181b0630f [DOI] [PubMed] [Google Scholar]
- 47. Blankman P, Hasan D, van Mourik MS, et al. : Ventilation distribution measured with EIT at varying levels of pressure support and Neurally Adjusted Ventilatory Assist in patients with ALI. Intensive Care Med. 2013;39(6):1057–62. 10.1007/s00134-013-2898-8 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Kallio M, Peltoniemi O, Anttila E, et al. : Neurally adjusted ventilatory assist (NAVA) in pediatric intensive care--a randomized controlled trial. Pediatr Pulmonol. 2015;50(1):55–62. 10.1002/ppul.22995 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Kneyber MC, Zhang H, Slutsky AS: Ventilator-induced lung injury. Similarity and differences between children and adults. Am J Respir Crit Care Med. 2014;190(3):258–65. 10.1164/rccm.201401-0168CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kurachek SC, Newth CJ, Quasney MW, et al. : Extubation failure in pediatric intensive care: a multiple-center study of risk factors and outcomes. Crit Care Med. 2003;31(11):2657–64. 10.1097/01.CCM.0000094228.90557.85 [DOI] [PubMed] [Google Scholar]
- 51. Randolph AG, Wypij D, Venkataraman ST, et al. : Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA. 2002;288(20):2561–8. 10.1001/jama.288.20.2561 [DOI] [PubMed] [Google Scholar]
- 52. Wysocki M, Jouvet P, Jaber S: Closed loop mechanical ventilation. J Clin Monit Comput. 2014;28(1):49–56. 10.1007/s10877-013-9465-2 [DOI] [PubMed] [Google Scholar]
- 53. Jouvet PA, Payen V, Gauvin F, et al. : Weaning children from mechanical ventilation with a computer-driven protocol: a pilot trial. Intensive Care Med. 2013;39(5):919–25. 10.1007/s00134-013-2837-8 [DOI] [PubMed] [Google Scholar]
- 54. Lellouche F, Mancebo J, Jolliet P, et al. : A multicenter randomized trial of computer-driven protocolized weaning from mechanical ventilation. Am J Respir Crit Care Med. 2006;174(8):894–900. 10.1164/rccm.200511-1780OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pediatric Acute Lung Injury Consensus Conference Group: Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5):428–39. 10.1097/PCC.0000000000000350 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Ouellette DR, Patel S, Girard TD, et al. : Liberation From Mechanical Ventilation in Critically Ill Adults: An Official American College of Chest Physicians/American Thoracic Society Clinical Practice Guideline: Inspiratory Pressure Augmentation During Spontaneous Breathing Trials, Protocols Minimizing Sedation, and Noninvasive Ventilation Immediately After Extubation. Chest. 2017;151(1):166–80. 10.1016/j.chest.2016.10.036 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Khemani RG, Hotz J, Morzov R, et al. : Pediatric extubation readiness tests should not use pressure support. Intensive Care Med. 2016;42(8):1214–22. 10.1007/s00134-016-4387-3 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Jubran A, Tobin MJ: Pathophysiologic basis of acute respiratory distress in patients who fail a trial of weaning from mechanical ventilation. Am J Respir Crit Care Med. 1997;155(3):906–15. 10.1164/ajrccm.155.3.9117025 [DOI] [PubMed] [Google Scholar]
- 59. Arnold JH, Anas NG, Luckett P, et al. : High-frequency oscillatory ventilation in pediatric respiratory failure: a multicenter experience. Crit Care Med. 2000;28(12):3913–9. 10.1097/00003246-200012000-00031 [DOI] [PubMed] [Google Scholar]
- 60. Sud S, Sud M, Friedrich JO, et al. : High frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): systematic review and meta-analysis. BMJ. 2010;340:c2327. 10.1136/bmj.c2327 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Arnold JH, Hanson JH, Toro-Figuero LO, et al. : Prospective, randomized comparison of high-frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. Crit Care Med. 1994;22(10):1530–9. 10.1097/00003246-199422100-00006 [DOI] [PubMed] [Google Scholar]
- 62. Ferguson ND, Cook DJ, Guyatt GH, et al. : High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368(9):795–805. 10.1056/NEJMoa1215554 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Gupta P, Green JW, Tang X, et al. : Comparison of high-frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. JAMA Pediatr. 2014;168(3):243–9. 10.1001/jamapediatrics.2013.4463 [DOI] [PubMed] [Google Scholar]
- 64. Bateman ST, Borasino S, Asaro LA, et al. : Early High-Frequency Oscillatory Ventilation in Pediatric Acute Respiratory Failure. A Propensity Score Analysis. Am J Respir Crit Care Med. 2016;193(5):495–503. 10.1164/rccm.201507-1381OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kneyber MC, Markhorst DG: Do We Really Know How to Use High-Frequency Oscillatory Ventilation in Critically Ill Children? Am J Respir Crit Care Med. 2016;193(9):1067–8. 10.1164/rccm.201512-2418LE [DOI] [PubMed] [Google Scholar]
- 66. Kneyber MC, van Heerde M, Markhorst DG: It is too early to declare early or late rescue high-frequency oscillatory ventilation dead. JAMA Pediatr. 2014;168(9):861. 10.1001/jamapediatrics.2014.961 [DOI] [PubMed] [Google Scholar]
- 67. Samransamruajkit R: It Is Too Early to Say No Place for High-Frequency Oscillatory Ventilation in Children with Respiratory Failure. Am J Respir Crit Care Med. 2016;194(4):521–2. 10.1164/rccm.201603-0530LE [DOI] [PubMed] [Google Scholar]
- 68. Rettig JS, Smallwood CD, Walsh BK, et al. : High-Frequency Oscillatory Ventilation in Pediatric Acute Lung Injury: A Multicenter International Experience. Crit Care Med. 2015;43(12):2660–7. 10.1097/CCM.0000000000001278 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Essouri S, Chevret L, Durand P, et al. : Noninvasive positive pressure ventilation: five years of experience in a pediatric intensive care unit. Pediatr Crit Care Med. 2006;7(4):329–34. 10.1097/01.PCC.0000225089.21176.0B [DOI] [PubMed] [Google Scholar]
- 70. Wolfler A, Calderini E, Iannella E, et al. : Evolution of Noninvasive Mechanical Ventilation Use: A Cohort Study Among Italian PICUs. Pediatr Crit Care Med. 2015;16(5):418–27. 10.1097/PCC.0000000000000387 [DOI] [PubMed] [Google Scholar]
- 71. Pham TM, O'Malley L, Mayfield S, et al. : The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr Pulmonol. 2015;50(7):713–20. 10.1002/ppul.23060 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Frizzola M, Miller TL, Rodriguez ME, et al. : High-flow nasal cannula: impact on oxygenation and ventilation in an acute lung injury model. Pediatr Pulmonol. 2011;46(1):67–74. 10.1002/ppul.21326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Milesi C, Baleine J, Matecki S, et al. : Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? A physiologic study. Intensive Care Med. 2013;39(6):1088–94. 10.1007/s00134-013-2879-y [DOI] [PubMed] [Google Scholar]
- 74. Wraight TI, Ganu SS: High-flow nasal cannula use in a paediatric intensive care unit over 3 years. Crit Care Resusc. 2015;17(3):197–201. [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Mortamet G, Amaddeo A, Essouri S, et al. : Interfaces for noninvasive ventilation in the acute setting in children. Paediatr Respir Rev. 2016; pii: S1526-0542(16)30117-8. 10.1016/j.prrv.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 76. Chacur FH, Vilella Felipe LM, Fernandes CG, et al. : The total face mask is more comfortable than the oronasal mask in noninvasive ventilation but is not associated with improved outcome. Respiration. 2011;82(5):426–30. 10.1159/000324441 [DOI] [PubMed] [Google Scholar]
- 77. Ozsancak A, Sidhom SS, Liesching TN, et al. : Evaluation of the total face mask for noninvasive ventilation to treat acute respiratory failure. Chest. 2011;139(5):1034–41. 10.1378/chest.10-1905 [DOI] [PubMed] [Google Scholar]
- 78. Chidini G, Calderini E, Cesana BM, et al. : Noninvasive continuous positive airway pressure in acute respiratory failure: helmet versus facial mask. Pediatrics. 2010;126(2):e330–6. 10.1542/peds.2009-3357 [DOI] [PubMed] [Google Scholar]
- 79. Codazzi D, Nacoti M, Passoni M, et al. : Continuous positive airway pressure with modified helmet for treatment of hypoxemic acute respiratory failure in infants and a preschool population: a feasibility study. Pediatr Crit Care Med. 2006;7(5):455–60. 10.1097/01.PCC.0000235246.68050.3A [DOI] [PubMed] [Google Scholar]
- 80. Ducharme-Crevier L, Beck J, Essouri S, et al. : Neurally adjusted ventilatory assist (NAVA) allows patient-ventilator synchrony during pediatric noninvasive ventilation: a crossover physiological study. Crit Care. 2015;19:44. 10.1186/s13054-015-0770-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Thille AW, Cabello B, Galia F, et al. : Reduction of patient-ventilator asynchrony by reducing tidal volume during pressure-support ventilation. Intensive Care Med. 2008;34(8):1477–86. 10.1007/s00134-008-1121-9 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Houtekie L, Moerman D, Bourleau A, et al. : Feasibility Study on Neurally Adjusted Ventilatory Assist in Noninvasive Ventilation After Cardiac Surgery in Infants. Respir Care. 2015;60(7):1007–14. 10.4187/respcare.03624 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation