Abstract
One of the most common human autoimmune diseases, alopecia areata (AA), is characterized by sudden, often persisting and psychologically devastating hair loss. Animal models have helped greatly to elucidate critical cellular and molecular immune pathways in AA. The two most prominent ones are inbred C3H/HeJ mice which develop an AA-like hair phenotype spontaneously or after experimental induction, and healthy human scalp skin xenotransplanted onto SCID mice, in which a phenocopy of human AA is induced by injecting IL-2-stimulated PBMCs enriched for CD56+/NKG2D+ cells intradermally. The current review critically examines the pros and cons of the available AA animal models and how they have shaped our understanding of AA pathobiology, and the development of new therapeutic strategies.
AA is thought to arise when the hair follicle’s (HF) natural immune privilege (IP) collapses, inducing ectopic MHC class I expression in the HF epithelium and autoantigen presentation to autoreactive CD8+ T cells. In common with other autoimmune diseases, upregulation of IFN-γ and IL-15 is critically implicated in AA pathogenesis, as are NKG2D and its ligands, MICA, and ULBP3.
The C3H/HeJ mouse model was used to identify key immune cell and molecular principles in murine AA, and proof-of-principle that Janus kinase (JAK) inhibitors are suitable agents for AA management in vivo, since both IFN-γ and IL-15 signal via the JAK pathway. Instead, the humanized mouse model of AA has been used to demonstrate the previously hypothesized key role of CD8+ T cells and NKG2D+ cells in AA pathogenesis and to discover human-specific pharmacologic targets like the potassium channel Kv1.3, and to show that the PDE4 inhibitor, apremilast, inhibits AA development in human skin. As such, AA provides a model disease, in which to contemplate general challenges, opportunities, and limitations one faces when selecting appropriate animal models in preclinical research for human autoimmune diseases.
Keywords: Alopecia areata, Autoimmunity, C3H, HeJ mouse model, Alopecia areata humanized mouse model, Hair follicle, T lymphocytes, NKG2D, Immune privilege, Janus kinase(JAK)-1, Jak3
1. Introduction
When selecting appropriate animal models to understand the pathogenesis and treatment prospects for human autoimmune diseases, one faces a number of challenges, research opportunities, and limitations. These can be exemplarily explored when contemplating the most common inflammatory hair loss disorder, alopecia areata (AA), whose clinical and histological features are so distinctive that they usually do not pose a major diagnostic challenge (Fig. 1) [1–3].
Fig. 1.
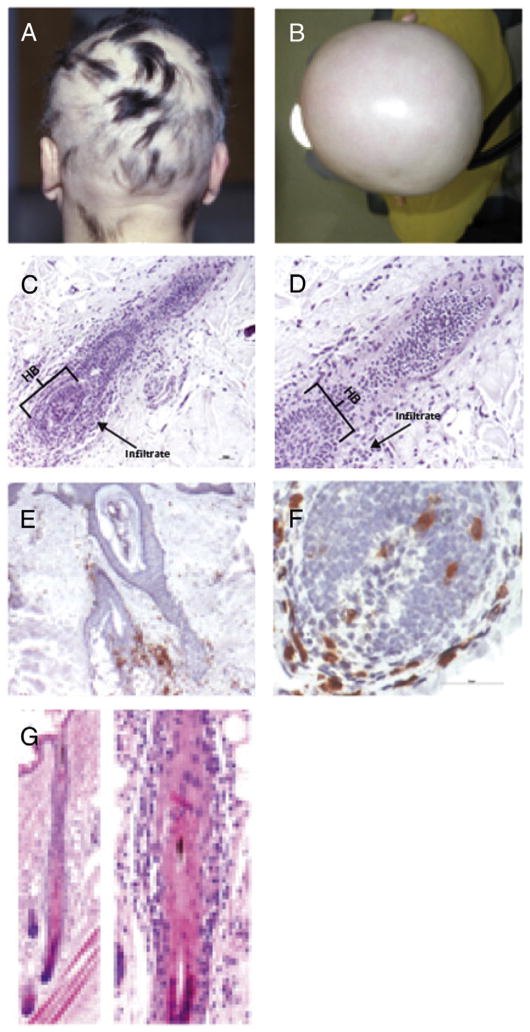
Clinical images of AA patients and classical histology of lesional AA HFs in C3H/HeJ and in the humanized mice (A). Characteristic hair phenotype of patient with severe AA. (B) Total hair loss of the scalp named alopecia totals. (C) Histologic feature demonstrates dystrophic hair follicles with dense perifollicular and intrafollicular lymphocytic infiltrate around the lower part of hair follicle and the HB in human skin graft injected with PBMCs enriched for NKG2D+ and CD56+ cells. (D) An absence of the infiltrate around the mid hair follicle. (E) Low magnification of imunohistochemical staining shows dense perifollicular CD8+ cells infiltrates along the lower part of hair follicle in human skin graft injected with IL-2 enriched PBMCs. (F) High magnification demonstrating hair bulb with intra-and perifollicular CD8+ cells. (G) Histologic features of graft-induced alopecia areata in C3H/HeJ mice. A late anagen hair with intra- and perifollicular mononuclear cell infiltrates along the whole vertical section of the hair follicle, not only around the hair bulb of the hair follicle, as is observed in human alopecia areata. Photographs courtesy of XXX (Panel C); XXXXX (Panel G).
AA is also one of the most common of the human autoimmune diseases [1,4,5], with an estimated prevalence in the USA of 20.2 per 100,000 individuals and a calculated lifetime risk of approximately 1.7% [6], i.e. an autoimmune disease incidence rivaled only by type 1 diabetes mellitus and rheumatoid arthritis. It is interesting to note that these most prevalent cases of disease-causing anti-self-reactivity all represent examples of antigen-specific, T cell-mediated, and strictly organ-specific autoimmunity [4,7]. Recently, a retrospective analysis of the most currently available, continuous 20-year period (1990–2009) suggested that, like other autoimmune diseases, the incidence of AA may be steadily increasing [8].
In addition to the fact that no cause-directed therapy of AA is available to date, and that AA management in daily clinical practice remains unsatisfactory [1,9,10], it is even more urgent to develop more effective treatment strategies that target key events in AA pathobiology. To achieve this goal, certain animal models, discussed below, have proven to be very instructive [11].
2. AA disease immunopathogenesis
While many factors have been implicated in the pathogenesis of AA, it is now clear that the immune system is the major player, with T cells and a collapse of the physiological immune privilege (IP) of the HF [12] playing critical roles [1,5,10]. The normal hair follicle (HF) represents a site of relative IP, because defined regions of its epithelium (bulge, bulb) do not express MHC class I and class II molecules, and because a number of immunoinhibitory cytokines and neuropeptides create an immunoinhibitory milieu [12–18]. Even the few intraepithelial Langerhans cells found within the HF epithelium below its stem cell region, the bulge, are immunologically functionally impaired, as they fail to express MHC class II [19]. That collapse of this HF IP is a condition sine qua non for AA to occur [20] is now widely accepted [1,4,5,10].
However, it had been unclear why immunologically privileged, MHC class I-negative anagen HFs are not attacked by NK cells, which detect and lyse cells that fail to express MHC class I [21–23]. Instead, only very few cells that express CD56 or NKG2D (two markers expressed by human NK cells, NKT cells, and some CD8+ T cells) are found around normal human scalp HFs [18,24]. Instead, in AA, the number of perifollicular NKG2D+ cells and the HF expression of activating NKG2D ligands are massively up-regulated [18,25] (see Supplementary text 1). Therefore, maintenance of the physiological IP of the HF also entails as yet incompletely understood mechanisms that suppress undesired NK cell activation; this mechanism appears to be defective in AA [18,25].
A large body of experimental and clinical evidence supports the “IP collapse” model of AA pathogenesis, and the key role played by autoreactive effector CD8+ T cells [20], whose selective transfer or deletion suffices to induce or block the disease in suitable animal models [1,5,12,13] (Fig. 2). While AA, is, thus, clearly a T cell-dependent, antigen- and organ-specific autoimmune disease that selectively attacks growing (=anagen) HFs, nails, and (rarely) retina pigment epithelium [1,26], it is not restricted to humans and occurs in many different mammalian species, including mice and rats [27,28]. Furthermore, it is evident that AA is associated with the incidence of other autoimmune diseases, with which AA shares some genetic predisposing features such as MHC/HLA haplotype associations [29–33].
Fig. 2. The pathogenesis of AA.
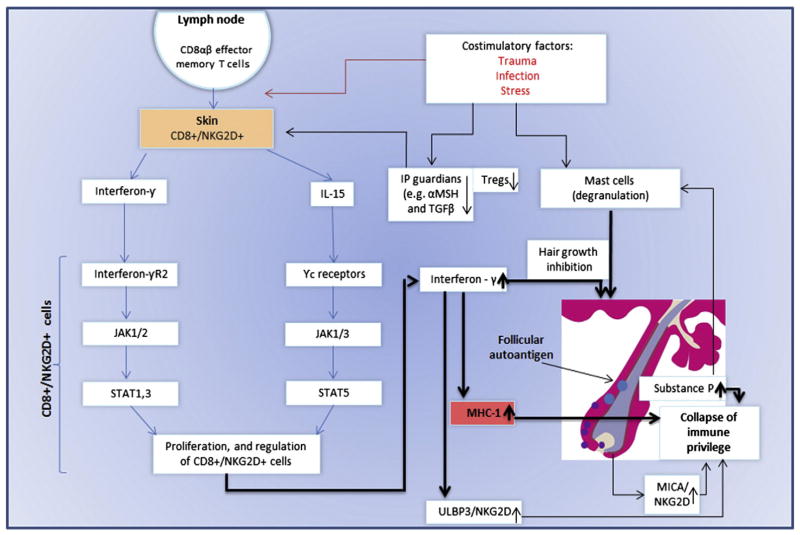
CD8αβ + effector memory t as well as NK cells expressing NKG2D produce a large amount of IFN-γ in regional lymph nodes in of affected AA C3H/HeJ mouse model [68]. These cells were also detected in human lesional areas of AA [18,68]. Affymetrix microarray analysis demonstrated INF response gene and IL-15 (74). IL-15R is expressed on cd8 + cells surrounding the human AA [68], and serve as a growth factor to effectors’ CD8 + and NK cells [74,131]. The common gamma chain (γc) (expressed by il-15R) signals through Janus kinase (JAK) 1 and 3, whereas INF-γ through JAK 1 and 2. JAKs activation leads to the phosporylation and activation of STATS which will translocate to the nucleus. This will lead to proliferation and activation of preexisting autoreacive CD8+ T cells [68]. These cells produce large amount of INF-γ which will induce MHC class I in hair follicle leading to the IP collapse and the ability of the CD8+ cells and NK cells, to attack the hair follicle and induce AA. This process may occur in AA patients with genetic background that may also be predisposed to environmental triggers such as trauma, infection and stress (with the release of substance P). Furthermore, in both mice and man, a decrease number of T regulatory cells (Tregs) were observed [132]. T-regs are known to be a crucial player in the preventing autoimmune phenomena. Recently, an interaction of perifollicular mast cells and CD8+ cells was observed. This interaction may impact IP maintenance, or, under inflammatory condition, IP collapsed.
The bulk of the currently available evidence suggests that the following elements must come together to initiate the visible clinical hair loss phenotype [1,4,5].
First and foremost, HFs must enter the active growth phase of the hair cycle (anagen) along with a collapse of the anagen HF’s physiological state of IP. This IP collapse, which can most effectively be induced by IFN-γ or substance P [17,34,35], presumably leads to changes in the quality and quantity of the expressed self-antigen repertoire [36–38], rendering HFs, which now ectopically express MHC class I-presented autoantigens, vulnerable as potential targets of anti-self immune reactivity [1,12](see Supplementary text 2).
Next, a perifollicular T cell infiltrate, with CD8+ T cells often seen to be infiltrating the anagen hair bulb epithelium. This has encouraged the hypothesis that these represent the functionally most important population of antigen-specific autoreactive T cells [39].
In parallel with the accumulation of this infiltrate, there is a dramatic increase in inflammatory mediators, which include, besides IFN-γ, other chemokines, cytokines, endogenous TLR ligands, and alterations in selected intracutaneous mediators such as retinoids [5,40,41]. These abnormalities are thought to further contribute to IP collapse and to the HF damage (dystrophy) caused by autoreactive CD8+ T cells recognizing and attacking cells that express follicular autoantigens, and IFN-γ, which in itself induces HF dystrophy and induces premature entry of the HF into catagen [42].
Inflammation-induced HF dystrophy and premature catagen induction, together, underlie the clinical hair loss phenotype of AA, as the dystrophic HF epithelium loses the ability to sufficiently anchor the hair shaft, which is subsequently shed and lost, causing alopecia, while premature catagen entry of anagen HFs terminates their production of a pigmented hair shaft [1].
Even in the most severe cases, AA is thought to be reversible, since the HF’s epithelial stem cells are usually not attacked [1]. Thus, AA frequently remits spontaneously, presumably as a result of reestablishment of HF IP [1]. One major challenge of future AA therapy should be, then, to accelerate and promote this process (for detailed review, see [1,4,5,13,43,44]) (Fig. 2).
Besides CD8+ and/or NKG2D+ cells, the role of additional immunocytes the immunopathology of AA has recently come under scrutiny, namely, that of regulatory T cells [45,46] and of perifollicular mast cells interacting with CD8+ T cells [47] (Fig. 2: AA pathobiology)(see Supplementary texts 3). Moreover, autoreactive T cells may arise in the rare situation of lack of the autoimmune regulator (AIRE), a transcriptional regulator expressed in medullary thymic epithelial cells, that enables the elimination of autoreactive T cells through negative selection [48], as in the recessive autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy (APECED) [49]. However, even though an AIRE risk haplotype may constitute an important component in an individual’s relative risk to develop AA [49], the role of AIRE in the pathobiology of classical AA remains unclear.
3. Murine models of AA
To further elucidate the pathogenesis of human AA, namely, how IP collapse is induced, and the relative importance of CD8+ versus other NKG2D+ cells, Tregs and other potential players in this process, appropriate animal models have proven indispensable [11]. Luckily, several animal models exist, and the “assay menu” from which one can pick is attractive (see below and Tables 1 and 2; unfortunately, the interesting Dundee experimental balding rat model of AA [50,51] is now unavailable and therefore not further discussed).
Table 1.
The presence of AA criteria in the various animal models.
| Animal mode | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Criteria | DEBR rat [50, 51] | Classical C3H/HeJ Mice [58, 59] | C3H/HeJ with skin graft-induced hair loss [52] | Transfer of lymph node cells of AA C3H/HeJ cultured with non-specific stimulator, to naïve mice [64] | Clonal CD8+ TCR transgenic 1MOG244.1 T Lymphocyte-Mediated hair loss [125] | Human AA scalp skin/SCID mice injected with scalp T cells [96] | Normal human scalp skin/SCID* mice injected with IL-2 enriched PBMCs [102] |
| Clinical phenotype of AA is characterized by sudden hair loss with temporarily hair regrowth. | [51,52] + | Regrowth <3% [58] | Regrowth <3% [58] | + | + | + | + |
| Spontaneously arising chain of pathogenesis events leading to AA | + | + | − | − | − | − | − |
| Histological feature of mononuclear infiltrate around and within the hair bulb. | [126] + | + [84] | [84] + | + | + | + | + |
| Abnormal expression of both major histocompatibility complex (MHC) I and II along the lower part of the hair follicles. | + [126] | [127] + | [128] + | + | NA | + | + |
| Lesional alopecia areata hair follicles are characterized by peri- and intrafollicular-CD8+ T cells. | + [126] | + [58] | + [58] | + | + | + | + |
| An excessive natural killer (NK) cell activities, as well as overexpression of NK-activating receptors (NKG2D) and NKG2D-activating ligands are observed in lesional areas of AA patients AA patients (MICA and ULBP3). | NA | NA | NA | NA | NA | NA | + |
| The hair loss pattern is categorize to focal and diffuse pattern | + [27] | + [27] | + [27] | + | + | − | − |
| The inflammation is specific for anagen hairs and causes anagen arrest. | + [55,126] | + [58] | + [58] | + | + | + | + |
| Retained the number of the follicular structures with decreased number of the terminal follicles and increased number of telogen structure | + [129] | + | + [84] | + | NA | + | + |
NA – Not analyzed.
The “Dundee experimental balding rat (DEBR)” was the first reported animal model of AA, but it is not used anymore [50,51]. Still, the DEBR model was the first to demonstrate that AA is actually a T cell-mediated disease, since depletion of CD8+ cells induced complete hair regrowth, and depletion of CD4+ cells partially restored hair growth in AA-affected rats [50,51].
Table 2.
Major characteristic features of the alopecia areata animal models.
| With autologous scalp T cells Typical properties |
DEBR rat [50,51] | Classical C3H/HeJ Mice [58,59] | C3H/HeJ with skin graft-induced hair loss [52,62] | Transfer of lymph node cells of AA C3H/HeJ cultured with non-specific stimulator, to naïve mice [64] | Clonal CD8+ TCR transgenic 1MOG244.1 T lymphocyte-mediated hair loss [125] | Human AA scalp skin/SCID mice injected with scalp T cells [96] | Normal Human scalp skin/SCID* mice injected with IL-2 enriched PBMCs [102] |
|---|---|---|---|---|---|---|---|
| Skin source | Rat | Mouse | Mouse | Mouse | C57BL/6 J mice | Human | Human |
| Spontaneous hair growth | Positive | Positive | Positive | Positive | No data | Positive | Positive |
| Grooming induced hair loss | Negative | Positive | Positive | Positive | No data | Negative | Negative |
| Location of the infiltrates and immuno-phenotype** | Around and within the hair bulb CD8 > CD4 | Above the hair bulb CD8 < CD4 | Above the hair bulb CD8 < CD4 | Above the hair bulb CD8 < CD4 | Above the hair bulb. In severe cases-lack of follicles | Around and within the hair bulb as in human AACD8 | Around and within the hair bulb as in human AA. CD8 > CD4 |
| Applicability of model outside strain originally reported | Negative-AA susceptibility genes are either lost or recessive upon out-breeding | Rare-AA susceptibility genes are either lost or recessive upon out-breeding to most strains develop spontaneous AA | Not tested due to cross-strain transplant incompatibility | Not tested due to cross-strain transplant incompatibility | Not tested due to cross-strain transplant incompatibility | Positive-Compatible with immune-compromised recipient mouse strains | Positive-Compatible with immune-compromised recipient mouse strains |
| Disadvantages | Low frequency and late onset. | Low frequency and late onset | Not typical histologic feature of human AA. | Not typical histologic feature of human AA. 3. Mice environment | 1. Complicated model. 2. Not typical histologic feature of human AA. | 1. Many biopsies from each patient are required, 2. Complicated model | Reliance on primary human donor tissue allows no role for model propagation via mouse inter-breeding |
| Potential for pre-clinical drug screening | Not realistic | Not realistic | With limited value due to the murine–specific disease pathology., i.e., not responding to agents such as KV1.3 | Establishing and expanding the model is possible; however, limited value due to murine –specific disease pathology, i.e., not responding to agents such as KV1.3. | Establishing and expanding the model is possible; however, limited value due to murine –specific disease pathology, i.e., not responding to agents such as KV1.3. | Not realistic | Can be used widely due to the ease of creating the model, and its reliance on healthy human donor tissue and human disease pathology. |
C.B-17/IcrHsd-PrkdcSCIDLystbg-J mouse, the autosomal recessive beige mutation backcrossed/intercrossed to the C.B-17/Icr-Prkdcscid mouse line results in diminished NK cell activity [130].
Histological feature of human AA is characterized by cellular infiltrates around and within hair follicles; CD4+ cells dominants over CD8 + cells.
3.1. C3H/HeJ inbred mouse model of AA
The C3H/HeJ model [11,52,53] has long dominated basic AA in vivo research (Tables 1 and 2). This mouse model has additionally produced many novel results with important implications for human AA by accessing the powerful tools of mouse genetics, including engineered transgenesis and knockout approaches, and cross-breeding for QTL analysis [54–56]. It has been observed that transfer of CD8+ cells alone suffices to induce localized AA-like hair loss, thus confirming the IP collapse theory of AA [20], whereas the additional transfer of CD4+/CD25− cells promotes systemic AA. In contrast, transfer of CD4+/CD25+ (Treg) cells blocks disease onset in the C3H/HeJ mouse model [57], underscoring the important regulatory function of CD4+ T cells, at least in the pathobiology of murine AA-like hair loss in this mouse strain.
Up to 20% of aging C3H/HeJ mice spontaneously develop an AA-like hair loss phenotype, yet with major variations in the incidence and timing of hair loss [11,56,58–62]. However, the occurrence of the hair loss phenotype can be accelerated by transferring full-thickness lesional skin grafts from affected aging mice to young, 10-week-old female recipients [52,63]. Most recently, the McElwee group has further refined this model by showing that the clinical AA phenotyp can be induced in recipient C3H/HeJ naive mice via adoptive transfer of lymph node cells from AA+ donors, after culturing the former in the presence of IL-7 and IL-15 (Table 1). This procedure enabled the generation of a large number of AA-affected mice within several weeks, without the need for lesional skin transplantation [64].
The C3H/HeJ model has been instrumental in confirming a crucial role for IFN-γ in AA pathogenesis [65], an integral part of the IP collapse theory of AA [1,12,17]: Interferon-gamma-deficient mice are resistant to the development of AA [54], while the injection of neutralizing IFN-γ antibody can induce hair regrowth in alopecic mice [66].
The C3H/HeJ AA mouse model has also provided insight into the role that can be played by psychoemotional stressors in AA pathogenesis, a concept long suspected by clinicians due to repeated testimonies from AA patients claiming stress-triggering or stress-aggravation of their hair loss [65]. For example, it was shown that AA can be potentiated by inducing perifollicular neurogenic inflammation and by inducing HF IP collapse via injection of substance P [35], a key stress-related neuropeptide that had been shown to induce IP collapse in human HFs ex vivo [34]. Substance P is released from the peripheral termini of sensory nerve fibers in the skin and acts as a promoter of neurogenic skin inflammation which inhibits hair growth and induces premature catagen [34,35,67].
Global transcriptional profiling analyses of human and C3H/HeJ AA lesions consistently identified upregulation of numerous IFN-regulated genes and of immunostimulatory cytokines such as IL-2 and IL-15 [68]. Upregulation of IL-15 and its receptor subunit IL-15Rα in AA hair follicles [68,69] were also observed (Fig. 2). Like IFN-γ, IL-15 signals via a pathway that involves Janus kinase (JAK)-1, Jak3, and signal transducer; in addition, IL-15 signaling utilizes the activator of transcription, STAT-5 [70,71]. IL-15 increases both innate and antigen-specific immune responses and – like IFN-γ – has been implicated in the pathogenesis of several other immune diseases [69,72]. Therefore, both IFN-γ and IL-15 are promising therapeutic targets [69,73,74], whose identification has been facilitated by the C3H/HeJ mouse model of AA (see Supplementary text 4). Among pleiotropic functions, IL-15 acts as a pro-inflammatory cytokine and stimulates self-reactive memory T cells. Indeed, anti-IL-15Rβ antibodies prevented the induction of AA in C3H/HeJ mice [68].
Accordingly, an impressive preventive and therapeutic effect on AA was observed in C3H/HeJ mice following therapy with JAK inhibitors (Ruxolitinib and Tofacitinib) [68]. This is currently being followed by trials in human patients, where some have been successfully treated with oral Ruxolitinib, an inhibitor of both JAK1 and JAK2 [68,75,76]. This approach is further encouraged by a most recent report that pharmacological inhibition of JAK–STAT signaling can promote the growth of normal murine and human HFs [77]. Therefore, it will be most interesting to learn whether this treatment strategy generates reproducible and long-lasting hair regrowth results in larger cohorts of AA patients, while avoiding unwanted systemic immune suppression.
Although the C3H/HeJ model is important in the field of AA, it has some limitations, mostly centered around potential differences between inbred-mouse and human-specific genetics. First, the C3H/HeJ strain that has been used for almost all preclinical AA disease modeling carries a homozygous null mutant allele of the Tlr4 gene [78], while functionally equivalent Tlr4 mutations are not associated with human AA (as opposed to TLR1 poymorphisms [79]). However, even C3H/HeN mice, which are wild-type at the Tlr4 locus, are susceptible to an AA-like disease [56], suggesting that this peculiar TLR4 defect is not of major pathobiological relevance to the development of AA-like lesions in C3H/HeJ mice.
Second, in terms of AA pathobiology, MICA, whose potentially important role in AA development we have covered above [18], is a distantly MHC-related and MHC-linked highly polymorphic gene [80] that is strikingly absent in mice, while it is present in most mammals [81–83]. In view of the increasing recognition of NKG2D ligands like MICA in AA pathobiology [18,25,68] this constitutes a significant difference from human AA that must be kept in mind when interpreting data derived with the C3H/HeJ AA model.
Third, another problem with this AA mouse model is that its immunopathology differs substantially from that of the human disease in that CD8+ and CD4+ cells are observed essentially along the entire HF axis, including and sometimes even only around the bulge [63,84] (Table 2). Instead, the characteristic “swarm of bees” perifollicular infiltrate in lesional human AA skin typically occurs only around and inside the hair bulb [47,62,85,86]. Instead, peri- and intra-bulge infiltrates typically characterize irreversible cicatrical alopecias, where HF stem cells are destroyed [87,88], while even long-standing human AA lesions can remit spontaneously year after the onset of hair loss [87].
Fourth, other potential pharmacologic targets for future AA therapy are human-specific, such as that described below for human effector-memory-T cell-specific voltage-gated potassium channels, Kv1.3 [89]. In fact, K+ channel expression by murine T cells is quite dissimilar from that of human T cells. Hence, inbred mice cannot serve as a valid model for evaluating therapeutic effects of selected immunoinhibitory agents of interest in AA, such as Kv1.3 blockers [90].
Fifth, CTLA4, plays a crucial role in maintaining a balanced immune response, and has attracted great interest for its potential involvement in the pathogenesis of AA and other autoimmune diseases [60,61,25,91–93]. However, while the CTLA4 genomic region has several polymorphic SNP markers associated with human AA [92,93], similar observations have not been found in C3H/HeJ. In human disease, allelic susceptibility differences appear to be outside the amino acid coding region for the mature transmembrane receptor, although one missense SNP occurs in the signal peptide, and another SNP occurs in the 3′ untranslated region of the mRNA [94,95]. Thus, one key feature that links CTLA4 to AA pathobiology, namely, CTLA4 polymorphisms, is not adequately represented in the C3H/HeJ AA mouse model.
Sixth and last, it remains entirely unclear how predictive for the human condition the C3H/HeJ model is for identifying candidate (auto-) antigens, since disease-driving (auto-) antigenic peptides that are loaded onto MHC class I molecules are unlikely to be identical between the species and should not be expected to be identifiable in mice.
3.2. The humanized AA mouse model
When the first humanized AA mouse model was described almost 20 years ago, i.e. the transplantation of lesional human AA skin onto SCID mice [96], it contributed an important advance in the field, since it permitted one, for the first time, to study human AA in a preclinical in vivo setting [13]. Moreover, this model conclusively documented the key role of CD8+ T cells and anagen HF-derived autoantigens, and the importance of CD4+ T cell help in the development of alopecia in scalp skin transplants from AA patients, and provided the first functional evidence for the IP collapse theory of AA [97,98].
However, the initial humanized mouse model, had major practical limitations that precludes its widespread use, since it required the availability of substantial amounts of diseased human scalp skin from AA patients, including the autologous, intracutaneous T cell populations that resided in the donor lesional skin [13,96]. This made the model so difficult to handle that its use remained restricted to the lab from whence it originated.
Therefore, a more practical “humanized” mouse model of AA with a wider range of applications was sought. First, split-thickness transplants of healthy human corporeal skin were grafted onto beige SCID mice, which lack T and B lymphocytes, and possess extremely low NK activity [99–101]. After engraftment and healing, grafts underwent intracutaneous injection of IL-2-cultured human PBMCs, where the culture period with high-dose IL-2 produced a cell population enriched for expression of NK cell markers (CD56, NKG2D).
Given that high numbers and activities of CD56+ or NKG2D+ cells are associated with AA [18], it was hypothesized that AA lesions may also be inducible in healthy, full-thickness human scalp skin transplants upon injecting IL-2-activated PBMCs enriched for NKG2D+ and/or CD56+ cells. This was indeed the case, since a clinical and immunohistological phenocopy of human AA hair loss lesions could be induced (Fig. 1), irrespective of whether the injected, activated, and pre-selected PBMC populations from healthy donors without a prior history of AA were autologous or allogeneic to the scalp skin transplant [89,102,103].
Intriguingly, this represents one of the very few animal models in which a previously healthy human organ and its appendages (here, scalp skin and terminal anagen scalp HFs) can be experimentally transformed into a tissue that develops a phenocopy of a major human autoimmune disease [102] (See Table 1).
This humanized AA model can easily be applied to testing new candidate therapeutic agents for human AA and has already resulted in identification of two promising new candidate agents for future AA management, i.e. apremilast [103], and a potassium voltage-gated channel 3 (KV1.3) blocker [89]. Orally administered apremilast is a small molecule inhibitor of phosphodiesterase 4 (PDE4) with potent anti-inflammatory activities. The agent has been approved by the FDA for treatment of psoriatic arthritis and moderate-to-severe psoriasis [104,105] and is currently being considered for clinical trials for AA. The KV1.3 blocker is known to suppress the production of pro-inflammatory cytokine like IFN-γ and IL-2 and T cell proliferation in human effector memory T cells [106,107]. Neither of these agents appears to cause systemic immune suppression, and both reportedly have a favorable toxicity profile in vivo [108,109]. Thus, this new humanized mouse model of AA is well-suited to identify novel candidate agents for the treatment of human AA.
By comparison, this modified version of the humanized AA mouse model [89,102,103], which employs scalp skin and PBMCs from healthy donors, rather than human AA skin xenotransplants [96], does not adequately model the genetic and other AA risk factors that may contribute to initiation of the human disease [25,33].
Instead, besides its usefulness as a screening system for new candidate AA therapeutics, this model permits one to dissect at least some minimal elements that suffice to induce a phenocopy of the human disease. Moreover, it has provided the first functional evidence that excessive activities of IL-2-stimulated human NKG2D+ cells, including massive IFN-g secretion by these cells, suffices to induce AA lesions in previously healthy human skin—and this notably in the apparent absence of genetic or other AA risk factors [102].
This questions whether genetic risk factors are as critical to the induction of AA as is often proclaimed [25,33,110,111], and also whether all forms of human AA require the presence of preexisting autoreactive CD8+ T cells that recognize an MHC class I-presented autoantigen [12,20]. Therefore, it has already been proposed that AA may best be viewed as a stereotypic HF response pattern to a spectrum of diverse inflammation-related damaging events, that all share the induction of IP collapse and HF dystrophy in the anagen hair bulb, resulting in the AA hair phenotype [5].
Yet, the predecessor of the new model had utilized lesional skin from human AA patients to generate a humanized AA mouse model [96]. Therefore, this older assay variant may be recruited to dissect the relative functional contribution of patient genetics to the pathobiology of AA initiation [5,112,113,114] (Supplementary text 5).
One initial critical question regarding this new AA model was whether the inflammatory infiltrate generated is HF-specific, a signature feature of AA. This is the case, since IFN-γ + cells and CD8+/NKG2D+ disease-inducing T cells are observed primarily around and within the damaged HFs in injected human skin xenotransplants, while other skin compartments (e.g. the central and distal HF as well as the epidermis in the human skin transplants) do not show prominent inflammatory cell infiltrates—just as in spontaneously occurring human AA. Therefore, the new humanized mouse system seems to effectively model the immune effector activity that causes human lesions.
Another critical question was whether the new model actually represented a GvHD response that drove an AA-like disease phenotype. This can be excluded [1] since a) autologous versus allogeneic tissue and PBMCs produced identical hair loss results [102], b) all classical histopathological, and immunohistological signs of GvHD are missing even when allogeneic PBMCs are used to induce AA lesions in human skin xenotransplants [102], and c) since the typical response of human HFs undergoing GvHD is cictricial alopecia, not the non-scarring, reversible alopecia of AA [88,115].
Since large numbers of high-dose IL-2-stimulated PBMCs are required to induce the AA phenotype in the humanized mouse model, one may also ask whether the relatively large number of cells injected into the xenografts does not simply cause severe tissue stress and toxicity resulting in alopecia, thus somehow non-specifically inducing AA. However, this can be ruled out, since injection of the same number of various types of control cells into grafts fails to induce AA lesions [102].
The key role of NKG2D+ cells (incl. NK and CD8+ T cells) is increasingly recognized not only in AA pathobiology [18,25,68,25] (Supplementary text 2), but also in type I diabetes mellitus, multiple sclerosis, rheumatoid arthritis, and Crohn’s disease [116–120].
Therefore, it is conceivable that the new humanized AA mouse model may also be employed to dissect pathways and immune response patterns shared between these organ-specific autoimmune diseases. Moreover, the model can be utilized to characterize inherited or acquired imbalances between disease-protective and disease-promoting NKG2D+ cell populations [121].
4. Conclusions and perspectives
As immune-therapy of human autoimmune diseases has made enormous progress over the past years – at a speed not anticipated a decade ago – the previously neglected mini-organ affected so psychologically disturbingly by AA may yet turn out to be one of the most responsive organ systems for therapeutic immune-intervention [122–124].
Lessons learned from preclinical AA research on how to prevent the experimental induction of hair loss lesions and how to stimulate hair re-growth in established disease promise to be relevant for the management of related, medically more important, but less easily accessible and experimentally tractable, organ-specific autoimmune diseases –namely to those autoimmune diseases characterized by IP collapse and a key pathogenic role of IFN-γ, IL-15, and/or NKG2D+ cells, such as type I diabetes, multiple sclerosis, and rheumatoid arthritis.
In order to probe the efficacy of new immune-intervention strategies in AA, the field can now choose from two mutually complementary mouse models, the C3H/HeJ model and human skin xenotransplants on SCID mice, each of which comes along with well-defined limitations, pros, and cons that one needs to keep in mind when using them experimentally and when interpreting the data generated with these models. Yet, both have already identified encouraging, specific, and clinically promising new therapeutic strategies that are directly relevant to AA patients. A more widespread use of these intriguing preclinical models in autoimmune disease research will surely foster this trend and will advance the field well beyond immunologically induced hair loss.
Supplementary Material
Take-home messages.
Alopecia Areata is classical T cell mediated autoimmune disorder which can shade light on many other autoimmune conditions.
Several animals models using human skin grafts transplanted into nude mice or specific inbreed mice can serve as an excellent tool to further elucidate the pathogenesis of AA.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.autrev.2016.03.008.
References
- 1.Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366:1515–25. doi: 10.1056/NEJMra1103442. [DOI] [PubMed] [Google Scholar]
- 2.D’Ovidio R. Alopecia areata: news on diagnosis, pathogenesis and treatment. G Ital Dermatol Venereol. 2014;149:25–45. [PubMed] [Google Scholar]
- 3.Hordinsky MK. Overview of alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S13–5. doi: 10.1038/jidsymp.2013.4. [DOI] [PubMed] [Google Scholar]
- 4.Islam N, Leung PS, Huntley AC, et al. The autoimmune basis of alopecia areata: a comprehensive review. Autoimmun Rev. 2015;14:81–9. doi: 10.1016/j.autrev.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 5.McElwee KJ, Gilhar A, Tobin DJ, et al. What causes alopecia areata? Exp Dermatol. 2013;22:609–26. doi: 10.1111/exd.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update: part I. clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:77–188. doi: 10.1016/j.jaad.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 7.Tsai S, Santamaria P. MHC class II polymorphisms, autoreactive T-cells, autoimmunity. Front Immunol. 2013;10:321. doi: 10.3389/fimmu.2013.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirzoyev SA, Schrum AG, Davis MD, et al. Lifetime incidence risk of alopecia areata estimated at 2. 1% by Rochester Epidemiology Project, 1990–2009. J Invest Dermatol. 2014;134:1141–2. doi: 10.1038/jid.2013.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro J. Current treatment of alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S42–4. doi: 10.1038/jidsymp.2013.14. [DOI] [PubMed] [Google Scholar]
- 10.Guo H, Cheng Y, Shapiro J, et al. The role of lymphocytes in the development and treatment of alopecia areata. Expert Rev Clin Immunol. 2015;11:1335–51. doi: 10.1586/1744666X.2015.1085306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundberg JP, McElwee K, Brehm MA, et al. Animal models for alopecia areata: what and where? J Investig Dermatol Symp Proc. 2015;17:23–6. doi: 10.1038/jidsymp.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paus R, Nickoloff BJ, Ito T. A ‘hairy’ privilege. Trends Immunol. 2005;26:32–40. doi: 10.1016/j.it.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Gilhar A, Paus R, Kalish RS. Lymphocytes, neuropeptides, and genes involved in alopecia areata. J Clin Invest. 2007;117:2019–27. doi: 10.1172/JCI31942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Marr AK, Breitkopf T, et al. Hair follicle mesenchyme-associated PD-L1 regulates T-cell activation induced apoptosis: a potential mechanism of immune privilege. J Invest Dermatol. 2014;134:736–45. doi: 10.1038/jid.2013.368. [DOI] [PubMed] [Google Scholar]
- 15.Breitkopf T, Lo BK, Leung G, et al. Somatostatin expression in human hair follicles and its potential role in immune privilege. J Invest Dermatol. 2013;133:1722–30. doi: 10.1038/jid.2013.53. [DOI] [PubMed] [Google Scholar]
- 16.Kang H, Wu WY, Lo BK, et al. Hair follicles from alopecia areata patients exhibit alterations in immune privilege-associated gene expression in advance of hair loss. J Invest Dermatol. 2010;130:2677–80. doi: 10.1038/jid.2010.180. [DOI] [PubMed] [Google Scholar]
- 17.Ito T, Ito N, Bettermann A, et al. Collapse and restoration of MHC class-I-dependent immune privilege: exploiting the human hair follicle as a model. Am J Pathol. 2004;164:623–34. doi: 10.1016/S0002-9440(10)63151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito T, Ito N, Saatoff M, et al. Maintenance of hair follicle immune privilege Is linked to prevention of NK Cell attack. J Invest Dermatol. 2008;128:1196–206. doi: 10.1038/sj.jid.5701183. [DOI] [PubMed] [Google Scholar]
- 19.Gilhar A. Collapse of immune privilege in alopecia areata: coincidental or substantial? J Invest Dermatol. 2010;130:2535–7. doi: 10.1038/jid.2010.260. [DOI] [PubMed] [Google Scholar]
- 20.Paus R, Slominski A, Czarnetzki BM. Is alopecia areata an autoimmune-response against melanogenesis-related proteins, exposed by abnormal MHC class I expression in the anagen hair bulb? Yale J Biol Med. 1993;66:541–54. [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Song Y, Bakker AB, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–2. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 23.Janeway CA, Travers P, Walport M, et al. Immunobiology. New York: Garland Science Press; 2005. pp. 37–102. [Google Scholar]
- 24.Christoph T, Müller-Röver S, Audring H, et al. The human hair follicle immune system: cellular composition and immune privilege. Br J Dermatol. 2000;142:862–73. doi: 10.1046/j.1365-2133.2000.03464.x. [DOI] [PubMed] [Google Scholar]
- 25.Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113–7. doi: 10.1038/nature09114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandhi D, Singal A, Gupta, et al. Ocular alterations in patients of alopecia areata. J Dermatol. 2009;36:262–8. doi: 10.1111/j.1346-8138.2009.00636.x. [DOI] [PubMed] [Google Scholar]
- 27.McElwee KJ, McElwee KJ, Boggess D, et al. Comparison of alopecia areata in human and nonhuman mammalian species. Pathobiology. 1998;66:90–107. doi: 10.1159/000028002. [DOI] [PubMed] [Google Scholar]
- 28.Sundberg JP, Oliver RF, McElwee KJ, et al. Alopecia areata in humans and other mammalian species. J Invest Dermatol. 1995;104(5 Suppl):32S–3S. doi: 10.1038/jid.1995.51. [DOI] [PubMed] [Google Scholar]
- 29.Barahmani N, de Andrade AM, Slusser JP, et al. Major histocompatibility complex class I chain-related gene A polymorphisms and extended haplotypes are associated with familial alopecia areata. J Invest Dermatol. 2006;126:74–8. doi: 10.1038/sj.jid.5700009. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Mir A, Zlotogorski A, Gordon D, et al. Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. Am J Hum Genet. 2007;80:316–28. doi: 10.1086/511442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyakhovitsky A, Shemer A, Amichai B. Increased prevalence of thyroid disorders in patients with new onset alopecia areata. Australas J Dermatol. 2015;56:103–6. doi: 10.1111/ajd.12178. [DOI] [PubMed] [Google Scholar]
- 32.Noso S, Park C, Babaya N, et al. Organ specificity in autoimmune diseases: thyroid and islet autoimmunity in alopecia areata. J Clin Endocrinol Metab. 2015;100:1976–83. doi: 10.1210/jc.2014-3985. [DOI] [PubMed] [Google Scholar]
- 33.Betz RC, Petukhova L, Ripke S, et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat Commun. 2015;22:6–5966. doi: 10.1038/ncomms6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters EM, Liotiri S, Bodó E, et al. Probing the effects of stress mediators on the human hair follicle: substance P holds central position. Am J Pathol. 2007;171:1872–86. doi: 10.2353/ajpath.2007.061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siebenhaar F, Sharov AA, Peters EM, et al. Substance P as an immunomodulatory neuropeptide in a mouse model for autoimmune hair loss (alopecia areata) J Invest Dermatol. 2007;127:1489–97. doi: 10.1038/sj.jid.5700704. [DOI] [PubMed] [Google Scholar]
- 36.Pinto S, Michel C, Schmidt-Glenewinkel H, et al. Overlapping gene coexpression patterns in human medullary thymic epithelial cells generate self-antigen diversity. Proc Natl Acad Sci U S A. 2013;10:E3497–505. doi: 10.1073/pnas.1308311110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein L, Hinterberger M, Wirnsberger G, et al. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–344. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- 38.Nakayama M, McDaniel K, Fitzgerald-Miller L, et al. Regulatory vs. inflammatory cytokine T-cell responses to mutated insulin peptides in healthy and type 1 diabetic subjects. Proc Natl Acad Sci U S A. 2005;112:4429–34. doi: 10.1073/pnas.1502967112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertolini M, Uchida Y, Paus R. Toward the clonotype analysis of alopecia areata-specific, intralesional human CD8+ T lymphocytes. J Investig Dermatol Symp Proc. 2015;17:9–12. doi: 10.1038/jidsymp.2015.31. [DOI] [PubMed] [Google Scholar]
- 40.Duncan FJ, Silva KA, Johnson CJ, et al. Endogenous retinoids in the pathogenesis of alopecia areata. J Invest Dermatol. 2013;133:334–43. doi: 10.1038/jid.2012.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito T, Tokura Y. The role of cytokines and chemokines in the T-cell-mediated auto-immune process in alopecia areata. Exp Dermatol. 2014;23:787–91. doi: 10.1111/exd.12489. [DOI] [PubMed] [Google Scholar]
- 42.Ito T, Ito N, Saathoff M, et al. Interferon-gamma is a potent inducer of catagen-like changes in cultured human anagen hair follicles. Br J Dermatol. 2005;152:623–31. doi: 10.1111/j.1365-2133.2005.06453.x. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Yan B, Wang H, et al. Hair regrowth in alopecia areata patients following stem cell educator therapy. BMC Med. 2015;20:13–87. doi: 10.1186/s12916-015-0331-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lattouf C, Jimenez JJ, Tosti A, et al. Treatment of alopecia areata with simvastatin/ezetimibe. J Am Acad Dermatol. 2015;72:359–61. doi: 10.1016/j.jaad.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Han YM, Sheng YY, Xu F, et al. Imbalance of T-helper 17 and regulatory T cells in patients with alopecia areata. J Dermatol. 2015;42:981–8. doi: 10.1111/1346-8138.12978. [DOI] [PubMed] [Google Scholar]
- 46.Shin BS, Furuhashi T, Nakamura M, et al. Impaired inhibitory function of circulating CD4 + CD25+ regulatory T cells in alopecia areata. J Dermatol Sci. 2013;70:141–3. doi: 10.1016/j.jdermsci.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Bertolini M, Zilio F, Rossi A, et al. Abnormal interactions between perifollicular mast cells and CD8+ T-cells may contribute to the pathogenesis of alopecia areata. PLoS One. 2014;9:e94260. doi: 10.1371/journal.pone.0094260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kisand K, Peterson P, Laan M. Lymphopenia-induced proliferation in aire-deficient mice helps to explain their autoimmunity and differences from human patients. Front Immunol. 2014;5:51. doi: 10.3389/fimmu.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wengraf DA, McDonagh AJ, Lovewell TR, et al. Genetic analysis of autoimmune regulator haplotypes in alopecia areata. Tissue Antigens. 2008;71:206–12. doi: 10.1111/j.1399-0039.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- 50.McElwee KJ, Spiers EM, Oliver RF. Partial restoration of hair growth in the DEBR model for alopecia areata after in vivo depletion of CD4+ T cells. Br J Dermatol. 1999;140:432–7. doi: 10.1046/j.1365-2133.1999.02705.x. [DOI] [PubMed] [Google Scholar]
- 51.McElwee KJ, Spiers EM, Oliver RF. In vivo depletion of CD8+ T cells restores hair growth in the DEBR model for alopecia areata. Br J Dermatol. 1996;135:211–7. [PubMed] [Google Scholar]
- 52.McElwee KJ, Boggess D, King LE, Jr, et al. Experimental induction of alopecia areata-like hair loss in C3H/HeJ mice using full-thickness skin grafts. J Invest Dermatol. 1998;111:797–803. doi: 10.1046/j.1523-1747.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- 53.McElwee KJ, Hoffmann R, Freyschmidt-Paul P, et al. Resistance to alopecia areata in C3H/HeJ mice is associated with increased expression of regulatory cytokines and a failure to recruit CD4+ and CD8+ cells. J Invest Dermatol. 2002;119:1426–33. doi: 10.1046/j.1523-1747.2002.19620.x. [DOI] [PubMed] [Google Scholar]
- 54.Freyschmidt-Paul P, McElwee KJ, Hoffmann R, et al. Interferon-gamma-deficient mice are resistant to the development of alopecia areata. Br J Dermatol. 2006;155:515–21. doi: 10.1111/j.1365-2133.2006.07377.x. [DOI] [PubMed] [Google Scholar]
- 55.McElwee KJ, Hoffmann R. Alopecia areata – animal models. Clin Exp Dermatol. 2002;27:410–7. doi: 10.1046/j.1365-2230.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- 56.Sundberg JP, Berndt A, Silva KA, et al. Alopecia areata: updates from the mouse perspective. J Investig Dermatol Symp Proc. 2013;16:S23–4. doi: 10.1038/jidsymp.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McElwee KJ, Freyschmidt-Paul P, Hoffmann R, et al. Transfer of CD8(+) cells induces localized hair loss whereas CD4(+)/CD25(−) cells promote systemic alopecia areata and CD4(+)/CD25(+) cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol. 2005;124:947–57. doi: 10.1111/j.0022-202X.2005.23692.x. [DOI] [PubMed] [Google Scholar]
- 58.Sundberg JP, Cordy WR, King LE., Jr Alopecia areata in aging C3H/HeJ mice. J Invest Dermatol. 1994;102:847–56. doi: 10.1111/1523-1747.ep12382416. [DOI] [PubMed] [Google Scholar]
- 59.Sundberg JP, Boggess D, Montagutelli X, et al. C3H/HeJ mouse model for alopecia areata. J Invest Dermatol. 1995;104(5 Suppl):16S–7S. doi: 10.1038/jid.1995.38. [DOI] [PubMed] [Google Scholar]
- 60.Sundberg JP, Boggess D, Silva KA, et al. Major locus on mouse chromosome 17 and minor locus on chromosome 9 are linked with alopecia areata in C3H/HeJ mice. J Invest Dermatol. 2003;120:771–5. doi: 10.1046/j.1523-1747.2003.12135.x. [DOI] [PubMed] [Google Scholar]
- 61.Sundberg JP, Silva KA, Li R, et al. Adult-onset alopecia areata is a complex polygenic trait in the C3H/HeJ mouse model. J Invest Dermatol. 2004;123:294–7. doi: 10.1111/j.0022-202X.2004.23222.x. [DOI] [PubMed] [Google Scholar]
- 62.Sun J, Silva KA, McElwee KJ, et al. The C3H/HeJ mouse and DEBR rat models for alopecia areata: review of preclinical drug screening approaches and results. Exp Dermatol. 2008;17:793–805. doi: 10.1111/j.1600-0625.2008.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silva KA, Sundberg JP. Surgical methods for full-thickness skin grafts to induce alopecia areata in C3H/HeJ mice. Comp Med. 2013;63:392–7. [PMC free article] [PubMed] [Google Scholar]
- 64.Wang EH, Khosravi-Maharlooei M, Jalili RB, et al. Transfer of alopecia areata to C3H/HeJ mice using cultured lymph node-derived cells. J Invest Dermatol. 2015;135:2530–2. doi: 10.1038/jid.2015.176. [DOI] [PubMed] [Google Scholar]
- 65.Freyschmidt-Paul P, Zoller M, McElwee KJ, et al. The functional relevance of the type 1 cytokines IFN-gamma and IL-2 in alopecia areata of C3H/HeJ mice. J Investig Dermatol Symp Proc. 2005;10:282–3. doi: 10.1111/j.0022-202X.2005.10130_5.x. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura M, Jo J, Tabata Y, et al. Controlled delivery of T-box21 small interfering RNA ameliorates autoimmune alopecia (alopecia areata) in a C3H/HeJ mouse model. Am J Pathol. 2008;172:650–8. doi: 10.2353/ajpath.2008.061249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paus R, Arck P. Neuroendocrine perspectives in alopecia areata: does stress play a role? J Invest Dermatol. 2009;129:1324–6. doi: 10.1038/jid.2009.111. [DOI] [PubMed] [Google Scholar]
- 68.Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043–9. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fuentes-Duculan J, Gulati N, Bonifacio KM, Kunjravia N, Zheng X, Suárez-Fariñas M, Shemer A, Guttman-Yassky E, Krueger JG. Biomarkers of alopecia areata disease activity and response to corticosteroid treatment. Exp Dermatol. 2015 doi: 10.1111/exd.12918. http://dx.doi.org/10.1111/exd.12918 [Epub ahead of print] [DOI] [PubMed]
- 70.Cooley ID, Read KA, Oestreich KJ. Trans-presentation of IL-15 modulates STAT5 activation and bcl-6 expression in TH1 cells. Sci Rep. 2015;5:15722. doi: 10.1038/srep15722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marçais A, Cherfils-Vicini J, Viant C, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol. 2014;15:749–57. doi: 10.1038/ni.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jabri B, Abadie V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat Rev Immunol. 2015;15:771–83. doi: 10.1038/nri3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castela E, Le Duff F, Butori C, et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol. 2014;150:748–51. doi: 10.1001/jamadermatol.2014.504. [DOI] [PubMed] [Google Scholar]
- 74.Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waldmann TA. The biology of IL-15: implications for cancer therapy and the treatment of autoimmune disorders. J Investig Dermatol Symp Proc. 2013;16:S28–30. doi: 10.1038/jidsymp.2013.8. [DOI] [PubMed] [Google Scholar]
- 76.Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EBioMed. 2015;2:351–5. doi: 10.1016/j.ebiom.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harel S, Higgins CA, Cerise JE. Pharmacologic inhibition of JAK–STAT signaling promotes hair growth. Sci Adv. 2015;1:e1500973. doi: 10.1126/sciadv.1500973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamath AB, Behar SM. Toll-like receptor 4-defective C3H/HeJ mice are not more susceptible than other C3H substrains to infection with Mycobacterium tuberculosis. Infect Immun. 2003;71:4112–8. doi: 10.1128/IAI.71.7.4112-4118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seok H, Suh DW, Jo B, et al. Association between TLR1 polymorphisms and alopecia areata. Autoimmunity. 2014;47:372–7. doi: 10.3109/08916934.2014.910769. [DOI] [PubMed] [Google Scholar]
- 80.Moran D, Morishima S, Malkki M, et al. Identification of the MICA*070 allele by sequencing and phasing. Hum Immunol. 2013;74:557–61. doi: 10.1016/j.humimm.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ters KT, von Herrath MG, Homann D. Autoimmunity and autoimmune diseases. In: Paul W, editor. Fundamental Immunology. 7. Chap. 44 Philadelphia, PA, USA: Kluwer/Lippincott, Williams & Wilkins; 2013. pp. 1069–112. [Google Scholar]
- 82.Groh V, Bahram S, Bauer S, et al. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci U S A. 1996;93:12445–50. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steinle A, Groh V, Spies T. Diversification, expression, and gamma delta T cell recognition of evolutionarily distant members of the MIC family of major histocompatibility complex class I-related molecules. Proc Natl Acad Sci U S A. 1998;95:12510–5. doi: 10.1073/pnas.95.21.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McElwee KJ, Silva K, Boggess D, et al. Alopecia areata in C3H/HeJ mice involves leukocyte-mediated root sheath disruption in advance of overt hair loss. Vet Pathol. 2003;40:643–50. doi: 10.1354/vp.40-6-643. [DOI] [PubMed] [Google Scholar]
- 85.Weedon D. Weedon’s Skin Pathology, 2-Volume set: expert consult - online and print, 3e. 3. 2009. [Google Scholar]
- 86.Khoury EL, Price VH, Greenspan JS. HLA-DR expression by hair follicle keratinocytes in alopecia areata: evidence that it is secondary to the lymphoid infiltration. J Invest Dermatol. 1988;90:193–200. doi: 10.1111/1523-1747.ep12462213. [DOI] [PubMed] [Google Scholar]
- 87.Harries MJ, Paus R. The pathogenesis of primary cicatricial alopecias. Am J Pathol. 2010;177:2152–62. doi: 10.2353/ajpath.2010.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ito T. Hair follicle is a target of stress hormone and autoimmune reactions. J Dermatol Sci. 2010;60:67–73. doi: 10.1016/j.jdermsci.2010.09.006. http://dx.doi.org/10.1016/j.jdermsci.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 89.Gilhar A, Keren A, Shemer A, et al. Blocking potassium channels (Kv1.3): a new treatment option for alopecia areata? J Invest Dermatol. 2013;133:2088–91. doi: 10.1038/jid.2013.141. [DOI] [PubMed] [Google Scholar]
- 90.Beeton C, Wulff H, Standifer NE, et al. Kv1. 3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci U S A. 2006;103:17414–9. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sundberg JP, McElwee KJ, Carroll JM, et al. Hypothesis testing: CTLA4 co-stimulatory pathways critical in the pathogenesis of human and mouse alopecia areata. J Invest Dermatol. 2011;131:2323–4. doi: 10.1038/jid.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.John KK, Brockschmidt FF, Redler S, et al. Genetic variants in CTLA4 are strongly associated with alopecia areata. J Invest Dermatol. 2011;131:1169–72. doi: 10.1038/jid.2010.427. [DOI] [PubMed] [Google Scholar]
- 93.Megiorni F, Mora B, Maxia C, et al. Cytotoxic T-lymphocyte antigen 4 (CTLA4) +49AG and CT60 gene polymorphisms in alopecia areata: a case–control association study in the Italian population. Arch Dermatol Res. 2013;305:665–70. doi: 10.1007/s00403-013-1348-3. [DOI] [PubMed] [Google Scholar]
- 94.de Jong VM, Zaldumbide A, van der Slik AR, et al. Variation in the CTLA4 3′UTR has phenotypic consequences for autoreactive T cells and associates with genetic risk for type 1 diabetes. Genes Immun. 2016;17:75–8. doi: 10.1038/gene.2015.51. [DOI] [PubMed] [Google Scholar]
- 95.Ban Y, Tozaki T, Taniyama M, et al. Association of a CTLA-4 3′ untranslated region (CT60) single nucleotide polymorphism with autoimmune thyroid disease in the Japanese population. Autoimmunity. 2005;38:151–3. doi: 10.1080/08916930500050319. [DOI] [PubMed] [Google Scholar]
- 96.Gilhar A, Ullmann Y, Berkutzki T, et al. Autoimmune hair loss (alopecia areata) transferred by T lymphocytes to human scalp explants on SCID mice. J Clin Invest. 1998;101:62–7. doi: 10.1172/JCI551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gilhar A, Landau M, Assy B, et al. Mediation of alopecia areata by cooperation between CD4+ and CD8+ T lymphocytes: transfer to human scalp explants on prkdc(scid) mice. Arch Dermatol. 2002;138:916–22. doi: 10.1001/archderm.138.7.916. [DOI] [PubMed] [Google Scholar]
- 98.Gilhar A, Landau M, Assy B, et al. Transfer of alopecia areata in the human scalp graft/prkdc(scid) (SCID) mouse system is characterized by a TH1 response. Clin Immunol. 2003;106:181–7. doi: 10.1016/s1521-6616(02)00042-6. [DOI] [PubMed] [Google Scholar]
- 99.Teicher BA, editor. Tumor models in cancer research. New York: Springer Science + Business Media, LLC; 2011. [Google Scholar]
- 100.Thomsen M, Galvani S, Canivet C, et al. Reconstitution of immunodeficient SCID/beige mice with human cells: applications in preclinical studies. Toxicol. 2008;246:18–23. doi: 10.1016/j.tox.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 101.Mosier DE, Stell KL, Gulizia RJ, et al. Homozygous scid/scid; beige/beige mice have low levels of spontaneous or neonatal T cell-induced B cell generation. J Exp Med. 1993;177:191–4. doi: 10.1084/jem.177.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gilhar A, Keren A, Shemer A, et al. Autoimmune disease induction in a healthy human organ: a humanized mouse model of alopecia areata. J Invest Dermatol. 2013;133:844–7. doi: 10.1038/jid.2012.365. [DOI] [PubMed] [Google Scholar]
- 103.Keren A, Shemer A, Ullmann Y, Paus R, et al. The PDE4 inhibitor, apremilast, suppresses experimentally induced alopecia areata in human skin in vivo. J Dermatol Sci. 2015;77:74–6. doi: 10.1016/j.jdermsci.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 104.Poole RM, Ballantyne AD. Apremilast: first global approval. Drugs. 2014;74:825–37. doi: 10.1007/s40265-014-0218-4. [DOI] [PubMed] [Google Scholar]
- 105.Chiricozzi A, Caposiena D, Garofalo V, et al. A new therapeutic for the treatment of moderate to severe plaque psoriasis: apremilast. Expert Rev Clin Immunol. 2015 doi: 10.1586/1744666X.2016.1134319. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 106.Azam P, Sankaranarayanan A, Homerick D, et al. Targeting effector memory T cells with the small molecule Kv1. 3 blocker PAP-1 suppresses allergic contact dermatitis. J Invest Dermatol. 2007;127:1419–29. doi: 10.1038/sj.jid.5700717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ueyama A, Imura K, Kasai-Yamamoto E, et al. Kv1. 3 blockers ameliorate allergic contact dermatitis by preferentially suppressing effector memory T cells in a rat model. Clin Exp Dermatol. 2013;38:897–903. doi: 10.1111/ced.12097. [DOI] [PubMed] [Google Scholar]
- 108.Busa S, Kavanaugh A. Drug safety evaluation of apremilast for treating psoriatic arthritis. Expert Opin Drug Saf. 2015;14:979–85. doi: 10.1517/14740338.2015.1031743. [DOI] [PubMed] [Google Scholar]
- 109.Pereira LE, Villinger F, Wulff H, et al. Pharmacokinetics, toxicity, and functional studies of the selective Kv1. 3 channel blocker 5-(4-phenoxybutoxy)psoralen in rhesus macaques. Exp Biol Med (Maywood) 2007;232:1338–54. doi: 10.3181/0705-RM-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramot Y, Zlotogorski A. Molecular genetics of alopecias. Curr Probl Dermatol. 2015;47:87–96. doi: 10.1159/000369408. [DOI] [PubMed] [Google Scholar]
- 111.Redler S, Angisch M, Heilmann S, et al. Immunochip-based analysis: high-density genotyping of immune-related loci sheds further light on the autoimmune genetic architecture of alopecia areata. J Invest Dermatol. 2015;135:919–291. doi: 10.1038/jid.2014.459. [DOI] [PubMed] [Google Scholar]
- 112.Garzorz N, Alsisi M, Todorova A, et al. Dissecting susceptibility from exogenous triggers: the model of alopecia areata and associated inflammatory skin diseases. J Eur Acad Dermatol Venereol. 2015;29:2429–3245. doi: 10.1111/jdv.13325. [DOI] [PubMed] [Google Scholar]
- 113.Kim SK, Chung JH, Park HJ, et al. Polymorphisms in the promoter regions of the CXCL1 and CXCL2 genes contribute to increased risk of alopecia areata in the Korean population. Genet Mol Res. 2015;14:9667–74. doi: 10.4238/2015.August.14.29. [DOI] [PubMed] [Google Scholar]
- 114.Biran R, Zlotogorski A, Ramot Y. The genetics of alopecia areata: new approaches, new findings, new treatments. J Dermatol Sci. 2015;78:11–20. doi: 10.1016/j.jdermsci.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 115.Harries MJ, Trueb RM, Tosti A, et al. How not to get scar(r)ed: pointers to the correct diagnosis in patients with suspected primary cicatricial alopecia. Br J Dermatol. 2009;160:482–501. doi: 10.1111/j.1365-2133.2008.09008.x. [DOI] [PubMed] [Google Scholar]
- 116.Van Belle TL, Ling E, Haase C, et al. NKG2D blockade facilitates diabetes prevention by antigen-specific Tregs in a virus-induced model of diabetes. J Autoimmun. 2013;40:66–73. doi: 10.1016/j.jaut.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 117.Guerra N, Pestal K, Juarez T, et al. A selective role of NKG2D in inflammatory and autoimmune diseases. Clin Immunol. 2013;149:432–9. doi: 10.1016/j.clim.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ruck T, Bittner S, Gross CC, et al. CD4 + NKG2D+ T cells exhibit enhanced migratory and encephalitogenic properties in neuroinflammation. PLoS One. 2013;8:e81455. doi: 10.1371/journal.pone.0081455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Andersson AK, Sumariwalla PF, McCann FE, et al. Blockade of NKG2D ameliorates disease in mice with collagen-induced arthritis: a potential pathogenic role in chronic inflammatory arthritis. Arthritis Rheum. 2011;63:2617–29. doi: 10.1002/art.30460. 2011. [DOI] [PubMed] [Google Scholar]
- 120.Pariente B, Mocan I, Camus M, et al. Activation of the receptor NKG2D leads to production of Th17 cytokines in CD4+ T cells of patients with Crohn’s disease. Gastro-enterology. 2011;141:217–26. doi: 10.1053/j.gastro.2011.03.061. [DOI] [PubMed] [Google Scholar]
- 121.Kaufman G, d’Ovidio R, Kaldawy A, et al. An unexpected twist in alopecia areata pathogenesis: are NK cells protective and CD49b + T cells pathogenic? Exp Dermatol. 2010;19:e347–9. doi: 10.1111/j.1600-0625.2010.01106.x. [DOI] [PubMed] [Google Scholar]
- 122.Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–81. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Divito SJ, Kupper TS. Inhibiting Janus kinases to treat alopecia areata. Nat Med. 2014;20:989–90. doi: 10.1038/nm.3685. [DOI] [PubMed] [Google Scholar]
- 124.Bray N. Autoimmune disease: getting to the root of hair loss in alopecia. Nat Rev Drug Discov. 2014;13:724–5. doi: 10.1038/nrd4443. [DOI] [PubMed] [Google Scholar]
- 125.Alli R, Nguyen P, Boyd K, et al. A mouse model of clonal CD8+ T lymphocyte-mediated alopecia areata progressing to alopecia universalis. J Immunol. 2012;188:477–86. doi: 10.4049/jimmunol.1100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang JG, Oliver RF. Immunohistological study of the development of the cellular infiltrate in the pelage follicles of the DEBR model for alopecia areata. Br J Dermatol. 1994;130:405–14. doi: 10.1111/j.1365-2133.1994.tb03371.x. [DOI] [PubMed] [Google Scholar]
- 127.Freyschmidt-Paul P, Sundberg JP, Happle R, et al. Successful treatment of alopecia areata-like hair loss with the contact sensitizer squaric acid dibutylester (SADBE) in C3H/HeJ mice. J Invest Dermatol. 1999;113:61–8. doi: 10.1046/j.1523-1747.1999.00640.x. [DOI] [PubMed] [Google Scholar]
- 128.Freyschmidt-Paul P, Seiter S, Zöller M, et al. Treatment with an anti-CD44v10-specific antibody inhibits the onset of alopecia areata in C3H/HeJ mice. J Invest Dermatol. 2000;115:653–7. doi: 10.1046/j.1523-1747.2000.00113.x. [DOI] [PubMed] [Google Scholar]
- 129.McElwee KJ, Freyschmidt-Paul P, Sundberg JP, et al. The pathogenesis of alopecia areata in rodent models. J Investig Dermatol Symp Proc. 2003;8:6–11. doi: 10.1046/j.1523-1747.2003.12164.x. [DOI] [PubMed] [Google Scholar]
- 130.Subramanian L, Blumenfeld H, Tohn R, et al. NKT cells stimulated by long fatty acyl chain sulfatides significantly reduce the incidence of type 1 diabetes in nonobese diabetic mice [corrected] PLoS One. 2012;7:e37771. doi: 10.1371/journal.pone.0037771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 132.Zöller AM, McElwee KJ, Engel P, et al. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol. 2002;118:983–92. doi: 10.1046/j.1523-1747.2002.01745.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.