Abstract
Substance use disorders (SUDs) can be viewed as a pathology of neuroadaptation. The pharmacological overstimulation of neural mechanisms of reward, motivated learning and memory leads to drug-seeking behavior. A critical characteristic of SUDs is the appearance of craving, the motivated desire and urge to use, which is a main focus of current pharmacological and behavioral therapies. Recent proof-of-concept studies have tested the effects of non-invasive brain stimulation on craving. Although its mechanisms of action are not fully understood, this approach shows interesting potential in tuning down craving and possibly consumption of diverse substances. This article reviews available results on the use of repetitive transcranial magnetic stimulation (rTMS) and transcranial electrical stimulation (tES) in SUDs, specifically tobacco, alcohol and psychostimulant use disorders. We discuss several important factors that need to be addressed in future works to improve clinical assessment and effects of non-invasive brain stimulation in SUDs. Factors discussed include brain stimulation devices and parameters, study designs, brain states and subjects’ characteristics.
Introduction
Substance use disorders (SUDs) have been extensively studied in animal and human models in the last decades and growing lines of investigation suggest that SUDs are the end product of a neuroadaptative pathology. The pharmacological usurpation of neural mechanisms of reward, motivated learning and memory can lead to drug-seeking behavior and craving (Feil and Zangen, 2010; Hyman, 2007; Kalivas and O’Brien, 2007; Koob and Volkow, 2009). Despite extensive research, current treatments including pharmacological and behavioral therapies have limited efficacy in reducing craving and promoting complete abstinence of substance use (Anton et al., 2006) and SUDs still have a costly impact on healthcare worldwide. There is thus a need to explore novel approaches to treat SUDs.
Non-invasive brain stimulation (NIBS) techniques, namely repetitive transcranial magnetic stimulation (rTMS) and transcranial electrical stimulation (tES) are being investigated as new approaches to reduce craving and treat SUDs. rTMS uses the principle of electromagnetic induction to modulate cortical excitability. Repeated sessions of rTMS can induce long-lasting and durable neurophysiological changes in targeted brain structures and modulate behaviours associated with cortical functioning (e.g., (Wagner et al., 2004). The neurophysiological effects of rTMS are dependent on several parameters, including the frequency of stimulation, the number of pulses delivered per train of stimulation, the intertrain interval, the length of the stimulation period and the targeted cortical area. tES uses direct or alternative electrical current, applied to the scalp traveling from a positive (cathode) to a negative (anode) electrode. Under the anode, cortical neurons are thought to be facilitated whereas under the cathode, cortical neurons are believed to be suppressed (Nitsche et al., 2003). rTMS is approved by the FDA for the treatment of refractory major depression (George et al., 1997; 1995; O’Reardon et al., 2007; Pascual-Leone et al., 1996). rTMS and tES have also been studied with success in the reduction of auditory hallucinations and positive symptoms in schizophrenia, neuropathic pain and anxiety disorders (Kuo et al., 2013; Wassermann and Lisanby, 2001).
Both, rTMS and tES have shown some promise in the treatment of SUDs. Although preliminary, emerging results provide encouraging data (Feil and Zangen, 2010; Jansen et al., 2013; Wing et al., 2012). The goal of this review is to present the current state of understanding of craving and SUDs and summarize the findings on the use of NIBS in SUDs. We focus here on craving because it is now a DSM 5 criterion for SUD. Craving is also considered a predictive factor of relapse following a quit attempt because of abnormal cue-reactivity in SUD patients (Baker et al., 2012; Goudriaan et al., 2010; Paulus et al., 2005; Hone-Blanchet et al., 2014) but this concept is still disputed (Perkins, 2012). For instance, in tobacco use disorder (TUD), it is suggested that therapies for smoking cessation should tune down cue-induced craving (Ferguson and Shiffman, 2009). Therefore, new alternative therapeutic modalities, aiming at controlling craving and promoting long-term abstinence, are needed.
Craving is a complex concept, encompassing neurobiological and psychosocial variables. It is defined as the desire for the previously experienced effects of a psychoactive drug, motivated by internal and external cues (Hyman, 2007). Repeated and intense firing of dopamine neurons provides the pleasurable sensations associated with substance intake. Although psychoactive substances act through an extremely wide range of active compounds with specific neuropharmacological mechanisms of action (Stahl, 2005), an essential pharmacological endpoint remains dopamine release through the mesocorticolimbic pathways. Intoxication is thus associated with diverse effects, dependent on the substance’s properties, and repetition of intake and binges allow the replication of this dopamine-firing pattern. More importantly, this repeated pattern associates incentive salience to external stimuli and promotes drug-related goal-directed actions and motivational behavior. Dopamine signaling from the ventral tegmental area to the ventral striatum (e.g., nucleus accumbens) and prefrontal cortices initiates drug-seeking behaviour, but recruitment of the central nucleus of the amygdala, ventral pallidum and dorsal striatum eventually reinforces compulsive drug-seeking. During withdrawal, the shift from constant drug-related reward to abstinence is primarily characterized with reduced DA neurons firing. This recruits the extended amygdala (including the central nucleus of the amygdala and the shell part of the nucleus accumbens) that mediates limbic and motor influxes, resulting in the appearance of a broad range of withdrawal symptoms (Koob & Volkow, 2009). Moreover, this metabolic stress results in elevated corticotropin-releasing factor in the central amygdala, thus facilitating the impact of drug cues and stressors on the possibility of relapse. Stress is a key part in the maintenance of SUDs, as it is thought to facilitate the reinstatement of drug-seeking behavior through the sustained action of corticotropin-releasing factor in the amygdala. Corticostriatal glutamatergic pathways from the prefrontal cortices to the nucleus accumbens mediate drug-induced reinstatement. Furthermore, the recruitment of the basolateral nucleus of the amygdala and hippocampus is also important in the attribution and valuation of drug-cues, thus being extremely important in drug-related cue-reinstatement (Koob & Volkow, 2009).
It has been shown in multiple works that craving can be induced by environmental cues alone, independently from the state of abstinence (Franklin et al., 2007). Successful abstinence is consistently associated with the capacity to resist craving. Therefore, reduction of craving and/or ability to resist craving, may thus represent critical objectives and major therapeutical outcomes across SUDs (see Figure 1). A balance between reflective (i.e decision-making, executive) and reflexive (i.e reward-biased, impulsive) systems is thought to regulate drug-related behavior, and more generally, reward-associated behavior and response to craving (Bechara et al., 2005). The reflective system exerts top-down control on the impulsive system, thus regulating emotions and affective states. However, decision-making is a complex process that requires integration of information and can thus be influenced by the reflexive system. Chronic drug consumption would facilitate a hyperactivity and hypersensitivity of the reflexive system, overcoming the reflective system. This neurocognitive model is strongly based on neuroanatomical organization of the reward system, with a limbic drive circuit comprising projections from the medial prefrontal cortex to the nucleus accumbens (the reflexive system), and an executive control circuit comprising projections from the DLPFC to the dorsal part of the striatum (the reflective system) (Hanlon et al., 2015).
Figure 1.
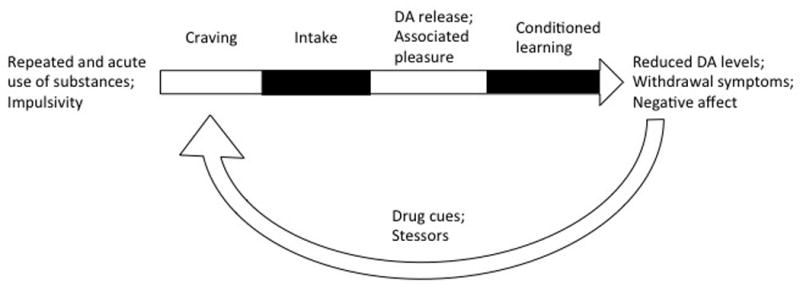
Illustration of the development and reinstatement of craving in SUDs.
There are currently various experimental measurements of craving. Craving can be induced by transient abstinence and by presentation of salient sensorial cues. Measure of craving presently relies on the use of standardized questionnaires. Although physiological measurements (i.e.: skin conductance, heartbeat rate, serum cortisol) are also used in some studies, they are not reliable and objective correlates of craving, hence making craving an important but difficult outcome measure.
The following sections review results focusing on the changes relative to craving and substance use induced by NIBS in the context of TUD, alcohol use disorder (AUD) and psychostimulant use disorder. Of note, studies detailed below do not use the same definition of TUD, AUD, and psychostimulant use disorders and many were completed before the DSM 5 edition. We thus use the authors’ own terminology in the description of subjects and substance use. Finally, we propose possible mechanisms underlying the beneficial effects of NIBS in reducing craving and critical methodological factors to consider for future works.
Noninvasive brain stimulation in tobacco use disorder
Cigarette smoking is still the leading cause of premature death and illness in many countries. Currently available pharmacotherapeutical alternatives, such as bupropion and varenicline, decrease nicotine craving by respectively stimulating the dopamine and GABA pathways but reported side effects are important. Nicotine replacement therapy (nicotine gums, patches and inhalators) replaces nicotine inhaled during cigarette smoking. Although these methods may help transiently decrease craving for cigarettes, smoking cessation rates appear to not exceed 35% despite use of these treatments (Benowitz, 2009).
Neural substrates of tobacco use disorders
Nicotine inhaled from cigarette smoke is carried from the lungs to the brain where it selectively binds to nicotinic cholinergic receptors (nAChRs). Direct stimulation of these receptors activates the liberation of dopamine in the mesocorticolimbic pathways, among other neurotransmitters. Acute and repeated chronic effects of nicotine result in the activation of the prefrontal cortex, visual areas and thalamus. Nicotine intake increases dopamine concentration in the ventral tegmental area (VTA), nucleus accumbens (NA) and striatum, which is thought to provide the pleasurable and arousing effects of cigarette smoking. The stimulation of dopamine pathways also allows the liberation of GABA and glutamate, neurotransmitters that respectively have inhibiting and facilitating effects on dopamine transmission. Chronic use of nicotine tones down the inhibitory action of GABA release but maintains the release of glutamate, which facilitates the release of dopamine and enhances the reinforcing effects of nicotine. Other hypotheses of the development of nicotine SUD point at the reduction of the activity of monoamine oxidase A and B (MAO-A and MAO-B), both enzymes involved in the catalysis of catecholamines (Schwartz and Benowitz, 2010). Importantly, nicotine also stimulates the release of acetylcholine, serotonin, norepinephrine and endorphins, triggering a global neurophysiological response which may induce alterations in neuronal activity and excitability (Markou, 2008). Single and paired-pulse TMS paradigms as applied for example by Lang et al. (2007) have demonstrated that chronic nicotine intake may increase cortical inhibition, with smaller amplitude of motor evoked potentials and increased afferent inhibition in smokers compared to healthy controls.
There is also rich neuroimaging literature showing the activation of a complex network which includes the dorsolateral prefrontal cortex (DLPFC), orbitofrontal cortex (OFC), and the striatum during craving-inducing exposure to smoking cues (Brody, 2006; Goldstein and Volkow, 2002; McBride et al., 2006; Wilson et al., 2004). Importantly, the DLPFC is a cortical relay in the mesocortical dopamine pathway, stimulated during craving and substance use and thus a key brain structure in the development and maintenance of TUD. The DLPFC has thus been the main brain region targeted with NIBS.
Repetitive transcranial magnetic stimulation in tobacco use disorders
Studies have investigated the effect of single or repeated sessions of rTMS on craving for cigarettes and level of consumption of cigarettes in smokers. The first studies were conducted to evaluate the effect of a single rTMS session over the left DLPFC on craving. Johann et al. (2003) found that smokers rated craving for cigarettes lower on a visual analog scale (VAS) after a session of active high frequency rTMS (20 Hz; 90% of resting motor threshold (RMT), compared to sham rTMS (Johann et al., 2003). However, in a double-blind crossover study with 2 active (20 Hz; 90% RMT) and 2 sham sessions, Eichhammer et al. (2003) evaluated craving with a similar VAS and found no differences in craving between sham and active conditions. Nonetheless, they did find that subjects smoked less cigarettes after active rTMS as compared to sham (Eichhammer et al., 2003). Following those two pioneering studies, Amiaz et al. (2009) assessed the effects of rTMS on cue-induced craving and urinary cotinine. In this parallel study, 48 treatment-seeking smokers were individually assigned to one of the following categories; rTMS (10 Hz; 100% RMT) with presentation of smoking cues, rTMS with presentation of neutral cues, sham rTMS with presentation of smoking cues, and sham rTMS with presentation of neutral cues. rTMS sessions were administered during 10 consecutive weekdays. Craving was assessed before, immediately after and 6 months after the end of the rTMS arm. Urinary cotinine levels were measured before stimulation on days 1, 5 and 10. Smokers who received active rTMS (with neutral or smoking cues) reported a decrease in craving and a decrease in cigarette consumption and urinary cotinine levels, but these effects were not significant after the follow-up period of six months (Amiaz et al., 2009). Rose et al. (2011) tested the effect of combining bilateral rTMS with a smoking-cue condition. The smoking-cue condition consisted of presentation of smoking-related pictures and holding a cigarette and a lighter. There was also a neutral cue condition, which consisted of presentation of neutral pictures and holding a pencil and rubber eraser. They delivered low frequency (1 Hz; 90% RMT) and high frequency bilateral rTMS (10 Hz; 90% RMT) over the superior frontal gyrus (SFG). All participants received both types of stimulation and an active control condition with stimulation (1 Hz; 90% RMT) to the motor cortex. High frequency rTMS to the SFG induced craving during the smoking cue condition, whereas low frequency rTMS had no significant effect. In an additional condition, they combined stimulation with controlled inhalation of actual cigarette smoke, by means of a controlled cigarette puff volume apparatus. Prior to stimulation, subjects inhaled a cigarette with the apparatus to determine the volume of smoke normally inhaled with each puff. During stimulation, subjects inhaled this volume of cigarette smoke. In this condition, active high frequency rTMS decreased craving ratings (Rose et al., 2011). Taken together, these results provide interesting insight on the role of the SFG in provoking opposite behavioural impact following stimulation: increasing or suppressing craving. More recently, Li et al. (2013) used a crossover paradigm in which subjects received sham and active rTMS (10Hz, 100% RMT) over the left DLPFC, with a one-week interval separating the two rTMS sessions. Subjects were presented neutral and cigarette-smoking visual cues, before and after stimulation, and completed the Questionnaire of Smoking Urges. Active as compared to sham rTMS reduced craving ratings (Li et al., 2013a). When comparing these results with the ones from Johann (2003) and Eichhammer (2003), the authors propose that these immediate effects may partly be ascribable to the increased number of pulses during the stimulation period (1000 pulses per session for Johann et al. and Eichhammer et al.; 3000 pulses per session for Li et al.). Interestingly, greater reduction in smoking cravings were correlated with greater FTND scores and number of cigarettes smoked per day at baseline. The authors thus suggest that rTMS may be of particular clinical importance in difficult-to-treat smokers. Pripfl et al. (2013) investigated the neural mechanisms underlying nicotine-craving reduction. They used high frequency rTMS (10Hz; 90%MT) applied to the left DLPFC in smokers who were required to remain abstinent for 6 hours before stimulation. They also recorded EEG delta brain waves before and after stimulation, which are presumably associated to activity in the dopamine pathways and thought to be a neurobiological correlate of craving. They found that both craving ratings and EEG delta power were reduced after active stimulation as compared to sham (Pripfl et al., 2013). These results suggest that rTMS may impact the dopamine reward pathways and may be one of its primary mechanisms of action in the reduction of craving. Finally, Dinur-Klein et al. (2014) investigated the preclinical efficacy of 13 sessions of either 10Hz rTMS, 1Hz rTMS or sham stimulation on craving and cigarette smoking (measured with urinary cotinine levels) in 115 treatment-seeking smokers. The have used an H-shape coil, which allowed to target both the lateral PFC and insula (Zangen et al., 2005). Craving levels were measured with the Short Tobacco Craving Questionnaire and visual smoking cues were presented to the subjects before each rTMS sessions. High frequency rTMS significantly reduced both craving and cigarette smoking compared to low frequency rTMS (1 Hz) and sham rTMS, reaching an abstinence rate of 33% at a 6-month follow-up (Dinur-Klein et al., 2014). The authors suggest that stimulation of reward sensitive areas in deeper layers of the cortex, such as the insula, may be more efficient to decrease cravings and achieve abstinence.
In summary, over a total of eight experiments of rTMS in TUD, five have found a decrease in tobacco-related craving, two have reported no changes, and one observed an increase in craving. In regards to stimulation parameters, all five studies reporting decreases in craving used high frequency rTMS, three targeted the left DLPFC, one targeted the midline SFG and one targeted the lateral PFC. On the two studies reporting no changes, one delivered 1 Hz rTMS and one 20 Hz rTMS. The only study reporting craving increase used low frequency rTMS. In regards to subjects’ characteristics, two studies among the five that reported a decrease in craving required subjects to be abstinent before stimulation and two did not require abstinence. One study reporting craving decrease tested treatment-seekers. In terms of experimental paradigm, three of the five studies used a cue-provoked paradigm (see Table 1).
Table 1.
Summary of studies assessing craving with TMS and tES in TUD.
| Study | Subjects (N) | Design | NIBS parameters | Targeted regions | Abstinence Level | Measures | Main results |
|---|---|---|---|---|---|---|---|
| Johann et al., 2003 | Smokers (11) | Sham-controlled, Crossover | rTMS 1 session 20Hz 90% RMT 1000 pulses |
L DLPFC | 12-hr abstinence | “Desire to smoke” scored on VAS | Craving: decreased (active vs. sham) |
| Eichhammer et al., 2003 | Smokers (14) | Double-blind, Sham-controlled. Crossover | rTMS 2 sessions 20Hz 90% RMT 1000 pulses |
L DLPFC | 12-hr abstinence | “Desire to smoke” scored on VAS | Craving: no change (active vs. sham) |
| Number of smoked cigarettes during ad libitum 6-hr period following procedures | Intake: decreased (active vs. sham) | ||||||
| Amiaz et al., 2009 | Treatment-seeking smokers (48) | Double-blind, Sham-controlled, Parallel | rTMS 10 sessions 10Hz 100% RMT 1000 pulses |
L DLPFC | No abstinence | sTCQ scored on VAS before and after neutral and smoking cues | Cue-provoked craving : decreased in smoking cue condition (active vs sham) after the intervention, but not at the 6-month follow up |
| Urinary cotinine level Subjective self-report of cigarette intake |
Intake: decreased number of smoked cigarette in neutral and smoking cue conditions (active vs. sham) after the intervention, but not at the 6-month follow up. Decreased concentration of urinary cotinine in real stimulation groups (not measured at follow-up). | ||||||
| Rose et al., 2011 | Smokers (15) | Single-blind, Crossover | rTMS 1 session |
No abstinence | Shiffman-Jarvik Questionnaire before and after smoking and neutral cues and cigarette manipulation |
Cue-provoked craving : increased with presentation of smoking cues (10Hz vs. 1Hz over SFG). Decreased with presentation of neutral cues (10Hz vs. 1Hz over SFG; 1Hz over SFG vs. 1Hz over M1). |
|
| 1Hz 90% RMT 450 pulses |
SFG | ||||||
| 10Hz 90% RMT 4500 pulses |
SFG | ||||||
| Li et al., 2013 | Non-treatment seeking smokers (16) | Double-blind, Sham-controlled, Crossover | rTMS 2 sessions 10Hz 100% RMT 3000 pulses |
L DLPFC | 2-hr abstinence | Questionnaire of Smoking Urges before and after cue-proved paradigm | Cue-provoked paradigm: active compared to sham rTMS reduced cravings. |
| Pripfl et al., 2013 | Smokers (14) | Single-blind, Sham-controlled, Crossover | rTMS 1 session 10 Hz 90% MT 1200 pulses |
L DLPFC Neuronavigated |
6-hr abstinence | Craving rating on the item “How strong is your desire to smoke?” before and after smoking cues | Cue-provoked craving : cravings were reduced (active vs sham). |
| Hayashi et al., 2013 | Smokers (10) | Single-blind, Sham-controlled, Crossover | rTMS 4 sessions 1 Hz |
L DLPFC | 4-hr abstinent or no abstinence | “I’m craving a cigarette right now” scored on a VAS | Craving: no change. |
| Dinur-Klein et al., 2014 | Smokers (115) | Double-blind, Sham-controlled, Parallel | rTMS 13 sessions |
No abstinence | Standard craving questionnaire before and after smoking cues | Craving: reduction in HF groups vs sham and LF; greater reduction in group presented with smoking cues (HF vs. LF). | |
| High frequency | Lateral PFC/insula | ||||||
| Low-frequency | Lateral PFC/insula | Urinary cotinine | Intake: reduction of cigarette consumption (urinary cotinine) in HF groups vs sham and LF. | ||||
| Fregni et al., 2008 | Smokers (24) | Double-blind, Sham-controlled Crossover | tES 1 session 2mA 20min |
Anodal L DLPFC coupled with cathodal R DLPFC | No abstinence | 5-items craving scored on VAS*.before andafter smoking and manipulation of cigarettes. | Cue-provoked craving: decreased (active using either configurations vs. sham) |
| Anodal R DLPFC coupled with cathodal L DLPFC | |||||||
| Boggio et al., 2009 | Smokers (27) | Double-blind, Sham-controlled Parallel | tES 5 sessions 2mA 10min |
Anodal L DLPFCcoupled with cathodal R DLPFC | No abstinence | 5-items craving scored on VAS 1* before and after smoking cues and manipulation | Cue-induced craving: decreased (active vs. sham) |
| Cigarette intake | Intake: decreased in the number of cigarettes smoked in a dose-dependant effect (active vs. sham) | ||||||
| Xu et al., 2013 | Smokers (24) | Single-blind design Sham-controlled Crossover | tES 1 session 2 mA 20min |
Anodal L DLPFC coupled with cathodal R Supra-orbital | Overnight abstinence; minimum of 10-hr | Urge to Smoke Scale before and after smoking cues and manipulation | Cue-provoked craving: no changes (active vs sham). |
| Fecteau et al., 2013 | Treatment-seeking smokers (12) | Double-blind, Sham-controlled Crossover | tES 5 sessions 2 mA 30min |
Anodal R DLPFC coupled with cathodal L DLPFC | No abstinence | Questionnaire of Smoking Urges scored on VAS before and after smoking cues and manipulation | Craving: decrease in Desire to smoke subscale; no changes in other subscales (active vs. sham) |
| Number of smoked cigarettes | Intake: decreased in the number of cigarettes smoked after the 5-day session and up to 4 days after the end of the tDCS regimen | ||||||
| Meng et al., 2014 | Smokers (30) | Double-blind, Sham-controlled Parallel | tES 1 session 1 mA 20min |
Anodal over both occipital lobes coupled with cathodal over both sides of FPT | No abstinence | Cigarette intake | Intake: bilateral stimulation reduced cigarette intake |
| Anodal over the L FPT couped with cathodal over R FPT |
1 “I have a desire for a cigarette right now”, 2 “If it were possible, I would smoke now”, 3 “All I want right now is a cigarette”, 4 “I have an urge for a cigarette, 5 “ I crave a cigarette right now”
Transcranial electric stimulation in tobacco use disorders
tES applied over the DLPFC may also induce changes in craving and cigarette consumption in TUD. A first study by Fregni et al. (2008) sought to determine if tES could modulate cigarette craving in smokers. This study employed a randomized, double-blind, sham-controlled crossover design. Craving were elicited with the presentation of a smoking-related video, and rated before and after the video cue on a VAS. A single session of anodal stimulation of either the right or left DLPFC coupled with cathodal stimulation of the contralateral DLPFC (20min; 2 mA) decreased craving when compared to sham stimulation (Fregni et al., 2008). A subsequent study by Boggio et al. (2009) sought to determine if repeated sessions of anodal stimulation of left DLPFC coupled with cathodal stimulation of the right DLPFC (20min; 2 mA) might induce a longer-lasting decrease in the number of smoked cigarettes and craving. Active stimulation decreased the number of smoked cigarettes compared to sham tES throughout the 5-day intervention. Interestingly, the effect was reported as dose-dependent, as the effect of tES seemed to gain magnitude depending on the number of sessions. Moreover, active tES diminished craving ratings compared to sham tES (Boggio et al., 2009). We recently investigated the effect of tES (30min, 2 mA, anodal over the left DLPFC coupled with cathodal over the right DLPFC) on the number of smoked cigarettes in smokers who wished to quit (Fecteau et al., 2014). Subjects received two five-day regimens of active and sham tES. The number of smoked cigarettes was collected throughout the experiment. We found that active as compared to sham tES reduced the number of smoked cigarettes and the effect lasted up to four days after the end of the stimulation session. Subjects also performed two versions the Ultimatum Game task, the original task with monetary reward and a modified version of the task with cigarettes as reward. The Ultimatum Game assesses cognitive processes involving decision-making. Subjects can either accept or reject an offer made by a proposer. The offer consists of splitting a reward in unequal parts, to the advantage of the subject or not. If the subject accepts the offer, the reward is split as proposed, but if not, no one receives the reward (e.g., Sanfey, 2003). Active tES induced a more conservative approach in the Ultimatum Game as smokers refused more offers of cigarettes. This study thus suggests that tES applied over the DLPFC may modulate reward-sensitive processes. Of interest, Pripfl et al. (2013) have shown that anodal stimulation (with 3 electrodes) of the right DLPFC coupled with cathodal of the left DLPFC (15min; 0.45 mA) improved controllability of impulsivity in smokers. There were no effects with cathodal stimulation of the right DLFPC coupled with anodal tES of the left DLPFC and sham stimulation. Although this work did not investigate craving, it suggests differential effects of tES in smokers and healthy subjects on a cognitive process, impulsivity, known to be impaired in some smokers. Xu et al. (2013) used anodal tES (20min; 2 mA) over the left DLPFC, with the cathode over the right supraorbital area, in smokers to assess changes in craving and negative affects with the Profile of Mood States questionnaire. Active tES reduced tension, anxiety and depression indices, but did not change craving (Xu et al., 2013). Finally, Meng et al. (2014) have studied the effects of a single session of tES on the number of cigarettes smoked on the following day and attention bias to smoking-related cues using an eye-tracking system. There were 3 stimulation conditions (20 min, 1 mA): cathodal stimulation over the frontal-parietal-temporal area (FPT) of both hemispheres with anodal stimulation over both occipital cortices; cathodal stimulation over the right FPT and anodal stimulation over the right occipital cortex; and sham tES. Main findings (FPT) of both hemispheres with anodal stimulation over the right FPT and anodal stimulation over the were a decrease of cigarette consumption in subjects who received bilateral cathodal tES over the FPT and an attentional shift from smoking-related to neutral cues as compared to the other groups (Meng et al., 2014). In summary, these results provide interesting support for the clinical potential of tES in TUD (see Table 1).
Over a total of four studies assessing the use of tES on tobacco-related craving, all delivering 2 mA, two have reported a decrease in craving, one reported a decrease in craving in one subscale over a total of four, and one study found no changes in craving (see Table 1). The three studies reporting a decrease in craving targeted both DLPFCs whereas the study reporting no changes applied anodal stimulation to the left DLPFC and cathodal over the right supraorbital area. In regards to subjects, three of the studies reporting decreases in craving did not require subjects to be abstinent, whereas subjects were abstinent for a minimum of ten hours in the other study. Only one study recruited treatment-seekers and all four studies used a cue-provoked paradigm.
Noninvasive brain stimulation in alcohol use disorder
Alcohol is the most widely used psychoactive substance and AUD is a frequent condition worldwide. AUD is a severe condition, preoccupying social issue and a complex phenomenon as various concomitant psychiatric conditions, such as mood disorders, are encountered among alcoholic patients (Vengeliene et al., 2009).
Neural substrates of alcohol use disorders
Ethanol is the main psychoactive ingredient in alcohol intake and acts as a CNS depressant. As a nonselective pharmacological agent, ethanol easily penetrates the brain blood barrier and acts primarily by disrupting receptors and ion channels, affecting a broad range of neurotransmitter systems within the human brain. Its poor selectivity makes it difficult to identify which of its effects are ascribable to a particular neural system or receptor interaction. Following heavy consumption and intoxication, alcohol inhibits L-type Ca2+ channels and facilitates the opening of G-protein activated K+ channels, resulting in the inhibition of NMDA receptors activation. This reduces excitation and allows the enhancement of GABAA receptor function, increasing inhibition. The resulting concentration of ethanol in blood and affinity of the subunit of the receptor or channel interacting with ethanol are thought to be the main parameters depicting alcohol’s psychoactive effect (Vengeliene et al., 2009). Alcohol’s action on intrinsic membrane properties can lead to various molecular mechanisms involving neurotransmitters and second messengers, including serotoninergic (5-HT3) receptors present on GABAergic interneurons. Potentiation of 5-HT3Rs by alcohol consumption enhances the inhibitory action of GABA. Moreover, activation of 5-HT3 and nAChRs facilitates the release of dopamine and leads to the modulation of glutamate, GABA, acetylcholine and norepinephrine transmissions (Vengeliene et al., 2009). Therefore, the pleasurable and reinforcing properties of ethanol depend on the indirect stimulation of the mesolimbic pathway, mediated by the modulation of glutamatergic systems and disinhibition of dopamine neurons. Additionally, results also suggest that ethanol may directly excite dopamine neurons in the VTA and thus stimulate the release of dopamine (Sulzer, 2011). It is also thought that the decrease in K+ currents and activation of inward currents by acetaldehyde, a metabolite of ethanol, may be partly responsible for dopamine neurons firing (Sulzer, 2011). However, it is important to note that even though dopamine remains a key player in alcohol reinforcement, opiates and cannabinoids are also involved in the initiation and maintenance of AUD. In humans, approved pharmacotherapies for AUD comprise disulfiram (an acetaldehyde dehydrogenase inhibitor), naltrexone (an opioid-receptor antagonist) and acamprosate (acts on normalizing NMDA hyperexcitability during withdrawal)(Lev-Ran et al., 2012). The aforementioned compounds are relatively effective in diminishing alcohol craving but also induce serious side effects.
Repetitive transcranial magnetic stimulation in alcohol use disorders
In one of the first rTMS studies in AUD, Mishra et al. (2011) administered ten daily sessions of 10 Hz rTMS (110% RMT) over the right DLPFC to 30 AUD patients and compared the effects to that of sham stimulation in 15 patients. They evaluated craving for alcohol with the Alcohol Craving Questionnaire after each rTMS session. They also evaluated craving one month after the final rTMS session. Patients had to remain sober during ten consecutive days before the rTMS session. Active compared to sham rTMS reduced craving scores after the ten days of stimulation. The authors propose that rTMS over the DLPFC may facilitate the stimulation of dopamine pathways through the meso-fronto-limbic connections. They also suggest a possible transhemispheric suppression of the left DLPFC may have resulted from the stimulation of the right DLPFC. Although these hypotheses remain to be tested in future work, this study highlights the potential efficacy of high-frequency rTMS in diminishing craving intensity in AUD (Mishra et al., 2011). Höppner et al. (2011) have also assessed the effect of high frequency rTMS in 19 female AUD patients, stimulating the left DLPFC in ten consecutive session with 20 Hz rTMS (90% RMT). They assessed craving for alcohol with the Obsessive Compulsive Drinking Scale (OCDS) and depressive symptoms with two different scales (Hamilton Scale and Beck’s Inventory). No significant differences were found in craving and mood when comparing active and sham rTMS groups (Höppner et al., 2011). Herremans et al. (2012) focused on the effect of a single session of high frequency rTMS (20Hz; 110% RMT) over the right DLPFC on alcohol craving. The study regrouped 36 AUD patients detoxified for 12 days and evaluated alcohol craving with the OCDS before and after the single rTMS session. The authors report no significant differences in alcohol craving when comparing active to sham rTMS groups. It is worth noting that the rTMS was delivered at home and that these patients had stopped taking medication (benzodiazepine) before the experiment (Herremans et al., 2012). Following this study, the same research group (Herremans et al., 2013) assessed the effect of a single session of rTMS (20Hz; 110% RMT) over the right DLPFC in AUD patients detoxified for 14 days. They measured alcohol craving with the OCDS and found no effect of active or sham rTMS on craving, similarly to their first study. Finally, a single case report from De Ridder et al. (2011) delivered 15 sessions of 1Hz rTMS over the medial frontal cortex to decrease alcohol craving in a severe AUD patient. The patient gained beneficial effects over withdrawal symptoms for three months post-treatment. The patient relapsed after this three-month period and was treated again with five rTMS daily session and relapsed again three weeks post-treatment (De Ridder et al., 2011). Although this case report includes a single subject, it highlights the transient effects obtainable with repeated sessions of rTMS.
In sum, two studies (Mishra et al. 2011; De Ridder et al. 2011) have shown a significant decrease in alcohol craving following active stimulation and three studies have reported negative findings (Herremans et al, 2012, 2013; Höppner et al., 2011). Diverse stimulation parameters and different types of patients may have caused the important discrepancies between results in the aforementioned studies. In regards to stimulation parameters, one study used 10Hz over the right DLPFC and the other 1 Hz over the dorsal ACC. The three unsuccessful studies all used 20 Hz, one over the left DLPFC and two over the right DLPFC. All studies required their subjects to be abstinent, as most of them were treatment-seeking in-clinic patients. One of the two studies reporting a decrease in craving used a cue-provoked paradigm and the other did not. The three studies reporting no changes in craving did not use a cue-provoked paradigm to assess the effect of rTMS on craving. In regards to craving assessments, one study used the ACQ-NOW with 5 factors and one used a single item VAS. The other studies used the OCDS (see Table 2).
Table 2.
Summary of studies assessing craving with TMS and tES in AUD.
| Author, year | Subjects (N) | Experimental Design | NIBS parameters | Targeted regions | Abstinence Level | Craving and intake measures | Main results |
|---|---|---|---|---|---|---|---|
| Mishra et al., 2011 | Alcoholics (45) | Single blind, Sham-controlled, Parallel | rTMS 10 sessions 10 Hz 110% RMT 1000 pulses |
R DLPFC | Abstinent, in-clinic | ACQ-NOW 5 factors and GCI 2 factors |
Craving: Reduced immediately after the stimulation period and after 1 month (active vs. sham). |
| Höppner et al., 2011 | Alcoholics (19) | Single-blind, Sham-controlled, Parallel | rTMS 10 sessions 20Hz 90% RMT 1000 pulses |
L DLPFC | Abstinent, in-clinic | OCDS | Craving: No changes (active vs. sham) |
| De Ridder et al., 2011 | Detoxified Alcoholics (1) | Case report | rTMS 15 sessions 1 Hz 600 pulses |
Dorsal ACC | Abstinent | Craving VAS before and after drinking cues Intake: Blood alcohol volume assessed with a breathalizer |
Cue-provoked craving: Reduced for three months after termination of the stimulation protocol (3 weeks). Intake: Reduced during the stimulation protocol Withdrawal symptoms: reduced. |
| Herremans et al., 2012 | Detoxified alcoholics (36) | Single-blind, Sham-controlled, Parallel | rTMS 1 sessions 20 Hz 110% RMT 1560 pulses |
R DLPFC | Abstinent, in-clinic | 14 items OCDS | Craving: No changes (active vs. sham) |
| Herremans et al., 2013 | Detoxified alcoholics (29) | Single blind, Sham-controlled, Crossover | rTMS 20 Hz 110% RMT 1560 pulses |
R DLPFC | Abstinent, in-clinic | 14 items OCDS | Craving: No changes (active vs. sham) |
| Boggio et al., 2008 | Detoxified alcoholics (13) | Double-blind, Sham-controlled Crossover | tES 1 session 2 mA 20 min |
Anodal L DLPFC coupled with cathodal R DLPFC; Anodal R DLPFC coupled with cathodal L DLPFC |
Abstinent | 16 items AUQ before and after drinking and neutral cues | Cue-provoked craving: Reduced (active (both configurations) vs. sham). |
| Nakamura-Palacios et al., 2012 | Alcoholics (49) | Single-blind, Sham-controlled, Parallel | tES 1 session 1 mA 10 min |
Anodal L DLPFC, Cathodal R supradeltoid area | 7 days abstinence | 5 items OCDS before and after drinking and neutral cues |
Craving: no changes (active vs sham) |
| da Silva et al., 2013 | Male, Lesch’s type IV alcoholics (13) | Single-blind, Sham-controlled, Parallel | tES 5 weekly sessions during 5 consecutive weeks 2 mA 20 min |
Anodal L DLPFC coupled with cathodal R supraorbital area | Abstinent in-clinic patients |
5 items OCDS before and after drinking cues |
Cue-provoked craving: active and sham tDCS decreased cravings |
| den Uyl et al., 2015 | Hazardous drinkers (41) | Double-blind, Sham-controlled, Parallel | tES 1 session 1 mA 10 min |
Anodal L DLPFC coupled with cathodal R supraorbital area; Anodal L IFG coupled with cathodal R supraorbital area |
No abstinence | 14 items AAAQ before and after tDCS | Craving: Reduced after active tDCS with anode over L DLPFC and cathode over R supraorbital area |
Transcranial electric stimulation in alcohol use disorders
Boggio et al, (2008) have investigated the effect tES on alcohol craving, with 13 abstinent AUD patients who were administered bilateral tES over the right and left DLPFCs. In this cue-elicited paradigm, patients were presented alcohol-related visual cues to induce craving before stimulation. Both active tES electrode montages (anodal to the left with cathodal to the right; cathodal to the left with anodal to the right; both conditions at 2mA for 20 min) reduced craving compared to sham stimulation (Boggio et al., 2008). More recently, Nakamura-Palacios et al. (2012) used 1mA tES during 10 min in 7-days abstinent alcoholics to decrease craving (Anodal left DLPFC; cathodal right supradeltoid area) (Nakamura-Palacios et al., 2012). They found no significant changes in craving on the OCDS. da Silva et al. (2013) studied the effect of five weekly tES sessions (anodal stimulation of the left DLPFC coupled with cathodal stimulation of the right supradeltoid area, 2mA for 20min) in a clinical population of alcoholics. They focused on the effects on craving, event-related potentials following presentation of alcohol-related visual cues, and depression and anxiety symptoms. Active tES and sham stimulation decreased craving and depressive symptoms. This decrease in both measurements was larger and more significant in the active tES group. Active compared to sham tES also triggered DLPFC activity during the presentation of alcohol-related cues. There was no significant effect on anxiety symptoms. Furthermore, 2 out of 6 patients in the active group and 6 out of 7 in the sham group remained abstinent during the five-week trial, the others relapsed (da Silva et al., 2013). Finally, den Uyl and colleagues (2015) studied the effect of tDCS over alcohol craving in hazardous drinkers from a student population. They compared two electrode montages; one with the anode over the left DLPFC and the cathode over the right supraorbital area, and one with the anode over the left IFG and the right supraorbital area. They measured craving with the Alcohol Approach and Avoidance Questionnaire (AAAQ) before and after tDCS but did not include a cue-provoked paradigm. Active tDCS with the anode over the left DLPFC reduced craving compared to sham (den Uyl et al., 2015).
Over a total of four studies, three have reported a decrease in craving, with two studies using 2mA and one 1mA. One study used a bilateral montage with the anode and cathode over both DLPFCs, and two delivered anodal stimulation of the left DLPFC and cathodal over the right supradeltoid area. The fourth study which did not report changes in craving delivered 1 mA tDCS with anodal stimulation of the left DLPFC and cathodal of the right supraorbital area. In regards to the patients, they were all abstinent across the studies, except for the den Uyl study, which included hazardous drinkers among a student population. In regards to craving measurements, between the three successful studies, one used the AUQ, one the OCDS and one the AAAQ. All studies used a cue-provoked paradigm, except for the den Uyl study which only measured baseline craving before and after tDCS. The study from Nakamura-Palacios and colleagues (2012), which reported no changes, used the OCDS to assess alcohol craving but did not use a cue-provoked paradigm (see Table 2).
Noninvasive brain stimulation in psychostimulant use disorders
Cocaine is a powerful psychostimulant of the CNS and thus directly stimulates the release of dopamine in the mesocorticolimbic pathways. It is a highly addictive substance, with an estimate 1.7 million patients in the US (NIDA, 2012). Cocaine use disorder is strongly characterized by relapse and recidivism (Dackis and O’Brien, 2001; Stahl, 2005). Methamphetamine (METH), a methylated derivative of amphetamines, is similarly addictive and stimulating to the dopamine circuits. Its popularity has reached epidemic proportions throughout the world, with an estimated 25 million users worldwide (Cadet and Krasnova, 2009). Its pharmacological action is of longer duration and chronic intake can result in severe and long-lasting neuropsychiatric adverse effects, due to its neurotoxic effects on the dopamine and serotonin terminals (Marshall and O’Dell, 2012; Völlm et al., 2004).
Neural substrates of psychostimulant use disorders
Cocaine acts as an indirect agonist at dopamine receptors and as an inhibitor of monoamine transporters (such as DAT, the dopamine transporter). Therefore, its double action stimulates the release of dopamine and blocks the reuptake mechanism of dopamine neurons (Stahl, 2005), causing an accumulation of dopamine in the synaptic cleft. This causes dopamine concentration to reach a nonphysiological level and drastically enhances dopamine transmission. Behavioral effects on cocaine users are mainly mediated by these mechanisms although cocaine also has effects on norepinephrinergic and serotoninergic neurons. METH also stimulates release of dopamine and, partially, serotonin, and additionally transiently reverses monoamine transporters (DAT, SERT) thus increasing extracellular concentration of such transmitters (Völlm et al. 2004).
Repeated and chronic psychostimulants intake eventually facilitates the down-regulation of D2 receptors concentration in the prefrontal cortex, leading to an increase in D1 receptors signaling, thought to increase glutamate transmission and facilitate glutamate release in cortico-limbic projections. Eventually, this leads to the augmentation of dendritic spines, insertion of AMPA receptors and the increase in AMPA receptor sensitivity of the postsynaptic density of GABA medium spiny neurons of the NA, which will affect the metabotropic glutamate receptors (mGluRs) (Kalivas, 2007a; Kalivas et al., 2006). In fact, partial inhibition of glutamate release in the DLPFC and partial blockade of mGluRs are pharmacological experimental avenues to suppress cocaine use disorder, as demonstrated in studies in which blocking AMPA receptors on the NA inhibited relapse (Cornish and Kalivas, 2000; Kalivas, 2007b; Kalivas et al., 2005). Targeting GABA transmission is another interesting avenue to regulate psychostimulant craving. GABA levels in the ventral pallidum (VP) have been reported lower than normal in patients with cocaine abuse and decrease in GABA concentrations in this structure has been associated with cocaine-seeking behaviors and development of cocaine sensitization (Kalivas, 2007b). Increase of glutamate release near limbic structures during cocaine administration is thought to modulate GABA transmission and GABA projections from the NA to the VP, among other structures. Interestingly, outputs from the NA to the VP are also peptidergic, and chronic psychostimulant intake can induce long-term changes in opioid peptides regulation, namely on β-endorphin in the NA and dynorphin in striatal neurons. All opioid receptors from the basal ganglia are probably involved in the rewarding effect of cocaine administration, although μ- and δ -opioid receptors are most cited (Trigo et al., 2010).
As for TUD and AUD, the prefrontal areas are of crucial importance in the development and maintenance of psychostimulants use disorder. For instance, reduced metabolism of the OFC observed in cocaine and METH users is thought to modulate dopamine transmission and facilitate reward sensitivity (Volkow, 2002; Volkow et al., 2011). Studies using NIBS in these populations have thus focused on the DLPFC as a brain target in an effort to decrease craving by modulating activity in the prefrontal region and its connected network.
Repetitive transcranial magnetic stimulation in psychostimulant use disorders
Two studies have sought to test the clinical potential of rTMS in the reduction of cocaine craving. In the first one, Camprodon et al. (2007) have administered two sessions of rTMS (10 Hz, 90 % RMT) in randomized order over the right and left DLPFC of each patient. The six patients were in-clinic and seeking treatment for cocaine use disorder. They completed craving VAS before, after and 4 hours after each of the two rTMS session, which were scheduled at a week interval. Cocaine craving were decreased after rTMS applied to the right, but not the left hemisphere (Camprodon et al., 2007). Although these preliminary results are interesting, the authors suggest that a bigger sample size is needed to confirm the effect and the laterality segregation observed in the main effect. In a second study, Politi et al. (2008) investigated the effect of 10 daily sessions of 15 Hz rTMS over the left DLPFC (100% RMT) in 36 detoxification patients diagnosed with cocaine use disorder. Craving reports and psychosomatic symptoms of abstinence were assessed at each of the ten days (Politi et al., 2008). The results showed a gradual and significant reduction of craving following the course of the rTMS protocol, with the most significant changes occurring at the seventh session according to authors. Finally, Li et al. (2013) have used a single session of 1 Hz rTMS targeting the left DLFPC to decrease METH craving in non-treatment seeking METH users. They found out that active compared to sham rTMS induced craving (Li et al., 2013b). The authors propose that inhibitory action of low frequency rTMS over the prefrontal cortex may allow increased activity of the craving-related subcortical regions Two of the four experiments described demonstrate that rTMS has potential in the reduction of psychostimulant craving, with several subsequent sessions providing a stronger effect (see Table 3). However, it remains evident that more thorough research has to be conducted, taking into account the type of patient and the optimal stimulation parameters, namely the stimulation target as discussed in (Fecteau et al., 2010).
Table 3.
Summary of studies assessing craving with TMS and tES in psychostimulant use disorder.
| Author, year | Subjects (N) | Experimental Design | NIBS parameters | Targeted regions | Abstinence Level | Craving measures | Main results |
|---|---|---|---|---|---|---|---|
| Camprodon et al., 2007 | Detoxified cocaine users (6) | Crossover | rTMS 1 session 10 Hz 90% RMTT 2000 pulses |
L DLPFC R DLPFC |
Abstinent | 15 items VAS | Craving: Reduced cocaine craving when comparing ratings before and after rTMS to the R DLPFC. No effect on cocaine cravings when comparing ratings before and after rTMS to the L DLPFC. |
| Politi et al., 2008 | Detoxified cocaine users (36) | – | rTMS 10 sessions 15Hz 100% RMT 600 pulses |
L DLPFC | Abstinent | Clinical evaluation of psychopathologic symptoms of craving | Craving: Reduced cocaine craving gradually along the course of stimulation protocol. |
| Li et al., 2013 | Non-treatment seeking METH-dependent users (10) | Single-blind, Sham-controlled, Crossover, Control group | rTMS 1 session 15 min 1 Hz 100% MT 900 pulses |
L DLPFC | Abstinent | 1-item VAS with 0 being “not craving at all” and 10 being “the most craving I’ve ever had”, Before and after drug cues |
Cue-provoked craving: active to sham rTMS compared induced cravings in METH-users. |
| Shahbabaie et al., 2014 | METH-dependent patients (30) | Double-blind, Sham-controlled, Crossover | tES 2 mA 20 min |
Anodal R DLPFC coupled with cathodal L supraorbital area | 1-week abstinent | 1-itemVAS on subjective craving | Cue-provoked craving: active tDCS decreased cravings at rest but increased cravings when administered during exposure to drug cues. |
| Conti et al., 2014 | Crack-cocaine users (13) | Single-blind, Sham-controlled, Parallel | tES 5 sessions 20 min 2 mA |
Anodal R DLPFC coupled with cathodal L DLPFC | Abstinent (minimum of 31days) | Brief Cocaine Craving Questionnaire 7 items Before and after drug cues |
Cue-provoked craving: no change |
The two studies reporting a decrease in craving used high frequency rTMS; one targeted the left DLPFC and the other the right DLPFC. The study reporting no change used high frequency rTMS over the left DLPFC and the study reporting an increase in craving delivered 1Hz rTMS over the left DLPFC. In regards to subjects, they were abstinent in all studies. In regards to outcome measures, the two conditions showing a decrease in craving and the one showing no change did not use a cue-provoked paradigm, whereas the study demonstrating an increase in craving used a cue-provoked paradigm (see Table 3).
Transcranial electric stimulation in psychostimulant use disorders
One study has assessed the effect of tES on cocaine-related cue reactivity (Conti et al., 2014). In this work, they measured event-related potentials cue-reactivity in abstinent crack-cocaine users. They measured craving before and after the first tES session, and after the fifth and final tES sessions. They found that a single session of anodal tES (2 mA, 20min) over the right DLPFC coupled with cathodal tES over the left DLPFC did not decrease craving compared to sham tES (see Table 3). Moreover, there was no effect after 5 sessions of active tES compared to sham. There was no significant effect on cocaine intake at 3-months follow-up, but found that 5 subjects in the active tES group and one for the sham group had remained abstinent (Conti and Nakamura-Palacios, 2014).
One study has investigated the effect of tES in abstinent male METH-dependent subjects (Shahbabaie et al., 2014). This was a randomized, sham-controlled, double-blind, cross-over design and subjects received one active and one sham stimulation session, with the anode over the right DLPFC and the cathode over the left supraorbital area at 2 mA 20 min with a wash-out period of 72 hours. Craving levels were measured before, during and after each tES session on a 1-item VAS, and were asked to rate their METH craving spontaneously. There was an effect of time (pre, during, post) and stimulation (sham, active) but interaction did not reach significance.
Over two studies using tES for psychostimulant craving, one demonstrated a decrease in craving and the other did not. They used the same stimulation parameters (20min, 2mA). The one reporting a decrease in craving applied anodal to the right DLPFC and cathodal to the left supraorbital area (Shahbabaie et al., 2014) whereas the one that did not applied anodal stimulation over the right DLPFC and cathodal over the left DLPFC (Conti et al., 2014). In regards to patients, the study reporting a decrease in craving used 1-week abstinent subjects whereas the other study required a minimum of 31-days of abstinence. Both studies used a cue-provoked paradigm (see Table 3).
General discussion
Studies presented in this review provide insight for the use of NIBS in reducing craving and consumption of addictive substances. A recent meta-analysis has explored the effects of rTMS and tES on craving reduction for diverse substances and provided statistical evidence that these techniques can decrease craving levels for food, nicotine, alcohol and marijuana (Jansen et al., 2013). They found an effect size of 0.476, which encourages future investigations. Of interest, there was no significant difference between rTMS and tES in decreasing craving. Moreover, the magnitude of the effects was not different across substances. The authors also tested whether targeting the left or right DLPFC with NIBS induced greater benefits on craving. Although there was no significant statistical difference, greater craving suppression was reported when targeting the right hemisphere.
Although the results described in this review mainly come from experimental studies, it is worth comparing NIBS with traditional pharmacology and behavioral interventions. SUDs have a high prevalence worldwide, available therapies that are clinically efficient are relatively rare. Most available pharmacotherapies rely either on pharmacological blockade of sensitive receptors or on pharmacological substitution of the substance’s active component. In TUD, nicotine replacement therapy and bupropion or varenicline are the most used alternatives for smoking cessation. In AUD, main medications use to limit alcohol withdrawal symptoms include anticonvulsants, antipsychotics and benzodiazepines with limited success (Amato et al., 2011). In psychostimulant use disorder, most common treatments include the use of indirect dopamine agonists that seem to increase psychostimulant abstinence (Pérez-Mañà et al., 2011). In opioid use disorder, methadone maintenance therapy seems an effective replacement therapy that decreases heroin use, but its primary impact is to retain patients in treatment and does not reduce the high mortality rate of patients (Mattick et al., 2009). In sum, current available pharmacotherapies for SUDs are effective for some patients, but still do not meet overall clinical needs (Castells et al., 2010). Behavioral interventions are helpful to achieve clinical success but do not seem to comprise all facets of substance use disorders (Knapp et al., 2008). Overall, meta-analytical researches suggest that, across substances, combination of pharmacological treatment and behavioural intervention may increase clinical success and ameliorate clinical attendance and patient retention (Amato et al., 2011; Knapp et al., 2008; Stead et al., 2012).
As currently understood, NIBS mechanisms of action in craving would suggest a similarity with pharmacological replacement therapy; if indeed tES and rTMS can provoke dopamine release. Similarly to the conclusions reached in the aforementioned studies, we believe that combination with behavioural intervention would be paramount in the development of successful clinical therapy comprising NIBS. rTMS applied to the DLPFC (George et al., 1996; Pascual-Leone et al., 1996b; O’Reardon et al., 2007) and tES applied over the DLPFC also appears to induce beneficial effects in reucing depressive symptoms. Mechanisms associated with these approaches are still uncovered. It is possible that these NIBS protocols targeting the DLPFC and known to elevate mood, may in turn have a beneficial impact in SUD symptoms, such as decreasing craving and consumption. More studies are needed to assess such potential therapeutic mechanisms of action of rTMS and tES in SUDs. It has also been reported that rTMS (Baeken et al., 2009) and tES (Antal et al., 2014) applied over the DLPFC decreased stress by potentially modulating the HPA axis. Such effects might also contribute to positive effects of NIBS observed in various pathologies including major depression and SUDs. It is well described that stress plays a major role in major depression, through hyper reactivity to stressors, and SUDs, through reinstatement of craving. It has been suggests that some beneficial effects of NIBS in such psychopathologies (major depression and SUDs) may share a common pathway (Brunelin & Fecteau, 2014). There is still however a crucial need for the development of a biophysical model of rTMS and tES to decipher their potentially numerous mechanisms of action of clinical effects.
We have discussed several theoretical and technical issues related to the potential use of NIBS in SUDs. It is also worth addressing the potential side effects of such devices. Most common side effects of rTMS are headaches, neck pain and transient hearing changes (Rossi et al., 2009). For tES, side effects reported in the general literature seem similar that the ones reported in studies including patients with SUDs stated in this article. They include headaches, mild tingling, dizziness and increased skin sensibility at the location of electrodes. However, no studies reported disruption of the protocol or resignation of subjects due to side effects. In the context of eventually using NIBS in the treatment of SUDs, it is also important to think of possible side effects. If stimulation of the prefrontal cortex does entail an excitatory effect over cortical and subcortical structures, as suggested by several authors, and promotes the indirect stimulation of the dopamine pathways (Cho & Strafella, 2009), one may propose that NIBS would develop “addictive properties” if administered chronically. Similarly to pharmacological replacement therapy (e.g., nicotine replacement therapy) or cross-sensitization studies (i.e., when a substance is replaced with another substance), NIBS could technically create a shift in SUDs. We propose that this “side effect” would in fact be beneficial as it would, given ideal NIBS parameters and continuous professional counseling, reduce cravings through stimulation of the dopamine pathways, limit the advent of withdrawal symptoms and gradually deplete neuropsychological vulnerability to environmental substance cues and stress associated with substance craving. However, this hypothesis relies on a simplistic explanation of NIBS mechanisms of action; actual mechanisms of action may be far more complex and likely recruit cerebral structures other than the prefrontal cortex and striatum, thus recruiting supplementary neurotransmitter systems other than glutamate and dopamine.
Although experimental results are promising, the mechanisms of action of NIBS underlying its potential in reducing craving and substance use have yet to be characterized and several questions remain unanswered. Here we delimitate four general categories of factors that can mediate the effects rTMS or tES on craving: 1) stimulation parameters, 2) subjects’ brain state, 3), experimental measures of craving and 4) the sample characteristics.
1) The effects of stimulation parameters on craving
Stimulation parameters vary across reported studies, and this has to be taken into account for several reasons. First, mechanisms underlying the effects of rTMS and tES remain elusive, even in the healthy resting brain. The possibility for these two techniques of not sharing the same neurophysiological effects cannot be put aside, especially across SUDs. As mentioned in the introduction, rTMS and tES likely have different neurophysiological effects. Specificity of future studies and combination with neuroimaging modalities (e.g., Hone-Blanchet et al., 2015; Bestmann & Feredoes, 2013) will likely help elucidate these differences.
In addition to this difference between the rTMS and tES devices, the choice of the brain target, the nature of stimulation (presumably known to be excitatory or inhibitory), duration and intensity, all critical aspects in conducting a clinically relevant stimulation protocol, vary highly across studies in SUDs.
Most studies have targeted the DLPFC with rTMS or tES to diminish craving and substance intake in SUDs. Although a complex network is involved in craving in SUDs, the DLPFC may be one of the only cortical locations targetable by NIBS to impact the dopamine pathways and the insula (Garavan, 2010; Kalivas et al., 2005; Naqvi and Bechara, 2009). Indeed, the DLPFC receives direct input from the dopamine mesocortical pathway, originating from the VTA, and projects to the basal ganglia, hippocampus and thalamus (Goldman-Rakic, 1996). It has been reported that rTMS to the DLPFC can enhance dopamine transmission in the striatum (Pogarell et al., 2007), ACC and OFC (Ko and Strafella, 2011); and tES can modulate GABA and glutamate transmission, as shown by the stimulation of the primary motor cortex (Nitsche and Liebetanz, 2004; Stagg et al., 2009). Thus, several authors have proposed that the reduction in craving following stimulation may be mediated by the frontolimbic connections of the DLPFC with structures of the basal ganglia. Moreover, connections from the OFC to the striatum and amygdala, regulating motivational behavior and reward, may also be involved in the observed effect. The DLPFC is also a crucial relay in decision-making processes, and is thought to be essential in the behavioral regulation of craving throughout the prefrontal-striatal pathways (Kober and Mende-Siedlecki, 2010). In brief, among a complex network that has been associated with SUDs, the DLPFC seems to be the best candidate to be non-invasively targeted with NIBS to reduce craving across SUDs. We propose that excitatory stimulation of the DLPFC may act on GABA and glutamate transmission and thus indirectly facilitate dopamine release in the mesocortical pathway, thus transiently reducing substance craving (see Figure 2). The DLPFC has been largely targeted in the studies mentioned in this article. Although this region remains of critical interest, because it is easily targetable from the scalp and relevant in SUDs, other regions may be tested in future work. The insula for instance is becoming a target of primary importance in SUDs. Located inside the lateral sulcus, it receives inputs from the thalamus, parietal, occipital, temporal and frontal cortices. Moreover, it has reciprocal connections with the amygdala and nucleus accumbens. It is activated during drug craving and this activation is correlated with ratings of craving. Pharmacological inactivation or lesions to the insula lead to a disruption of substance craving in animal (Contreras et al., 2007) and humans (Navqi et al., 2007), thus suggesting a paramount role in the maintenance of SUDs. The study by Dinur-Klein and colleagues (2014) used the TMS H-coil, designed to presumably reach subcortical structures such as the insula, with promising results. Similarly, a preliminary study describing TMS administration to decrease chronic pain targeted the right posterior-superior insula in healthy controls, showing changes in cold perception (Ciampi de Andrade et al., 2012). Another target of interest in neuropsychiatric applications of NIBS is the cingulate cortex. Neuroimaging studies have shown that the dorsal part of the ACC is implicated in drug-related cue-reactivity and deficits in inhibitory control among patients with SUDs (Hong et al., 2009; Janes et al., 2013; Yücel et al., 2007). Targeting the ACC, or another region of the prefrontal matric, could thus act on specific aspects of craving. However, the available biophysical models of TMS and tES strongly suggest that targeting one prefrontal region may very well entail neurophysiological effects on surrounding regions. Of interest, a study from Cho and Strafella (2009) has shown that rTMS of the left DLPFC induces dopamine release in the ispilateral ACC and orbitofrontal cortex. This advocates for a better understanding of the mechanisms of action TMS and tES to determine what is best: stimulation of a target for effects over this target or stimulation of a target for effects over neighboring regions.
Figure 2.
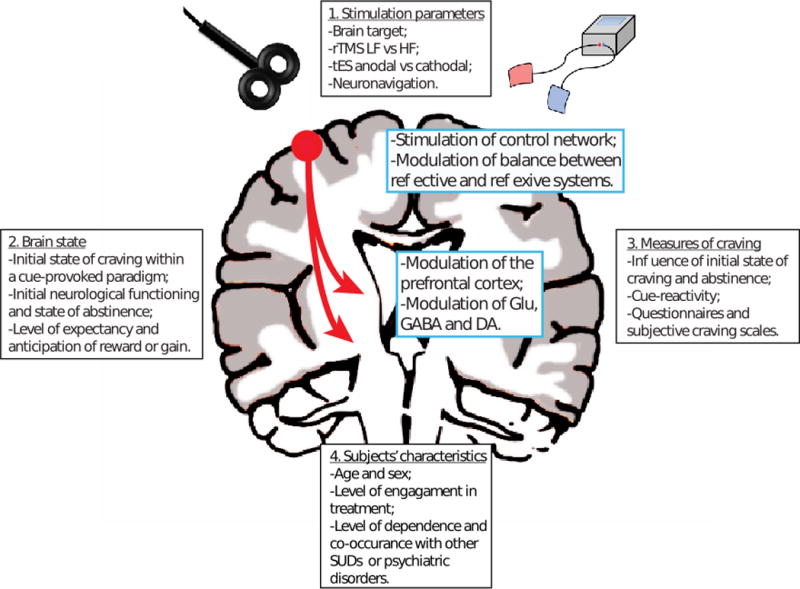
Summary of the four general categories of factors that can mediate the effects rTMS or tES on craving.
Changes ascribable to neurostimulation are in need of further characterization in humans but animal models will also contribute at deciphering the effects of NIBS that reduce craving. In this regard, Pedron et al. have demonstrated for the first time that anodal tES might be a valid and practical therapeutic alternative in reducing craving. Two daily 20 min sessions of tES for five consecutive days significantly increased working memory and decreased depression-related and addiction-related behaviours (Pedron et al., 2013). Although more results are needed, this consists in important preclinical evidence for the use of NIBS in SUD research. Other animal works have shown that rTMS can enhance dopamine transmission in the mesolimbic pathway (Keck et al., 2002) and tES may increase extracellular dopamine levels in the basal ganglia (Tanaka et al., 2013). It remains important to remember that although SUDs all have the dopamine pathways as a common denominator, several other neurotransmitter systems are involved in this pathology of neuroadaptation.
Precise localization of the brain target is another critical factor in safe and accurate delivery of brain stimulation. When targeting the DLPFC with rTMS, studies have largely used anatomical information provided by neurological atlases and mapping of Brodman areas, thus localizing the DLPFC as the midpoint of a line drawn from two points taken anteriorly and laterally 5cm from the vertex. Other studies have also located the DLPFC by moving 6cm anteriorly from the primary motor cortex. TMS-compatible neuronavigation methods offer more reliability, as they allow registering a tridimensional brain target with the TMS coil to ensure pulses are given at the same spot (Rusjan et al., 2010; Hone-Blanchet et al., 2015). Moreover, such neuronavigation system can also use previously acquired MR anatomical information of a given SUD patient and thus offer more precision and importantly, take into account inter-individual variability (Fox et al., 2012). Only one rTMS study reported in this review has used neuronavigation (Pripfl et al., 2013), whereas the rest have used manual methods for the localization of the DLPFC or did not report the method of brain targeting. Studies have shown that neuronavigation compared to manual localization of brain targets can increase the effect of brain stimulation and may help provide greater clinical benefits (Bashir et al., 2013). When delivering tES, all reviewed studies have used the 10–20 EEG International System to locate the DLPFC (electrodes positioned over F3 and F4).
Studies have targeted the DLPFC with low or high frequencies rTMS or anodal and/or cathodal tES to suppress craving. These stimulation parameters are known to induce different direct and distal effects in the brain as shown by neuroimaging studies in the healthy resting brain. These effects have been demonstrated by fMRI, MR spectroscopy and with electrophysiology. For instance, low frequency rTMS over the DLPFC has been shown to enhance BOLD activation in the contralateral and ipsilateral prefrontal regions (Nahas et al., 2001). Of interest, in a comparative study in healthy subjects, Eldaief et al. (2011) have shown that low frequency and high frequency rTMS of the left posterior inferior parietal lobule have differential effect on the default mode network, with increased connectivity in the low frequency condition.
Different effects are also observed with tES. Anodal tES applied over the prefrontal cortex can reduce blood oxygenation level-dependent (BOLD) signals in the areas under the electrodes and in the relevant network related to the cognitive processes tested (Holland et al., 2011; Meinzer et al., 2012; Saiote et al., 2013). Cathodal tES over M1 has been demonstrated to reduce glutamate release, and anodal tES to reduce GABA concentration in the primary motor cortex region as measured by MR spectroscopy (Stagg et al., 2009).
Anodal tES over the DLPFC can also increase resting state functional connectivity strength between the area under electrodes and regions involved in the default mode network in healthy subjects (Keeser et al., 2011; Park et al., 2013). This is critical, especially for patients with SUDs as they show impaired regulation of such networks (Ma et al., 2011). As proposed by (Hanlon et al., 2012), one important question remaining is Should we target the primary site of craving (i.e., pushing down the hot spot) or should we target neighboring regions (i.e., pulling the activity away from the hotspot)? Then, in regards to NIBS, the main question is Which stimulation parameters should we use, excitatory and/or inhibitory, to reduce craving? This brings us to the importance of brain state to suppress craving with rTMS or tES.
2) The importance of brain state to reduce craving with NIBS
Brain state likely varies throughout an experiment; before, during and after stimulation, which is of crucial importance in the issue of diminishing substance craving with NIBS. Craving has been extensively studied using cue-elicited tasks and fMRI. Cue-elicited paradigms have shown changes in BOLD signals in a complex network, including the DLPFC, OFC, striatum, thalamus, VTA and amygdala (Brody, 2006; Goldstein and Volkow, 2002; McBride et al., 2006; Wilson et al., 2004). In regards to activations observed in the DLPFC, it is not clear yet whether the right and/or the left DLPFC are critical to craving. Studies have shown that the DLPFC is differentially activated depending on treatment status and expectancy, abstinence and withdrawal symptoms, and explicit regulation in tobacco and cocaine use disorder. The DLPFC may be thus implicated in differential ways across SUDs (Jasinska et al., 2014).
Of further importance, not only experimentally-induced craving leads to a differential pattern of brain activity, but even the addicted brain at rest displays differential pattern of brain activity compared to a healthy resting brain. For instance, more severe dependence to nicotine as assessed by the Fagerstrom Test for Nicotine Dependence questionnaire is associated with weaker resting state functional connectivity between the striatum and dorsal anterior cingulated. It is even suggested that the abnormal resting state functional connectivity in cingulate-striatal fibers may serve as an in vivo marker for TUD (Hong et al., 2009; Sutherland et al., 2012). Hence, a crucial question is Should we apply NIBS in a craving brain or in a resting brain? So far, subjects’ brain state has not been controlled and varies importantly in the studies using NIBS to reduce craving reviewed here. Some studies (e.g., Fregni et al, 2008) have collected craving ratings using a cue-elicited paradigm immediately before tES. In this study, tES was applied in smokers craving for cigarettes, although this “elicited brain state” was not part of the planned design. Other studies have presented SUD-related pictures and neutral pictures during delivery of NIBS (e.g. Rose et al. 2013). These behavioral paradigms, even when presenting solely neutral cues, have also likely induced a specific brain pattern rather than mimicking the resting brain, such as activating regions involved in attentional processing. Other factors can also play a role in brain state in the context of SUD. For instance, level of expectancy (e.g., if the subject can consume right after the experiment or only after a few hours after the experiment) elicited different brain activations and needs to be controlled in future studies (McBride et al., 2006). This brings us to the third factor, which is the outcome assessment, including the impact of brain state during craving assessment.
3) Experimental measures of craving
Studies using NIBS to suppress craving have collected craving ratings in different brain states. For instance, some studies asked their subjects to be abstinent for a number of hours before the experiment, other experimentally induced craving by presenting substances-related cues. It can be discussed that abstinence-induced and cue-induced craving may ultimately represent the same neurological phenomena, but works have demonstrated that in smokers, craving can be induced with cues a few minutes after nicotine smoking (Franklin et al., 2007), suggesting that cue-reactivity may work independently from pharmacological stimulation and abstinence state. Additionally, it is often proposed that cue-induced craving paradigms may be a more ecologically valid approach, as recuperating SUDs patients are sensitive to environmental drug-related cues (Ferguson and Shiffman, 2009; Hone-Blanchet et al., 2014).
Craving assessment design in itself is also important in determining the effect of NIBS in SUDs. The multiple questionnaires used to assess craving in NIBS studies vary in quality, based on the number of items and subscales and the level of standardization. For instance, some studies asked subjects to rate their craving level by asking a single question (e.g., How much do you desire to smoke?). This is a methodological choice that allows the measure of craving at the exact moment (if for instance tested multiple times during a 10-min tES session), which would be difficult if using a longer, more detailed questionnaire. However, one question does not fully capture the different aspects of craving, and from a methodological standpoint likely limits the evaluation of the whole construct of craving and statistical power. We, for instance, reported significant changes in craving only for one specific subscale among the four subscales (Desire to smoke, Anticipation of positive outcome, Relief from negative affect, and Intention to smoke) within the standardized Questionnaire of Smoking Urges from Tiffany and Drobes (1991)(Fecteau et al., 2014). It is thus possible that NIBS only modulates some specific aspects of craving. Another methodological aspect that is essential to consider when discussing studies using NIBS to reduce craving is how ratings of craving were reported. Studies have used either VAS or categorical craving scores. As reported by several authors, categorical scores may not capture small changes in craving that VAS may capture.
Finally, other methodological aspects important for any NIBS studies are also relevant when addressing SUDs. For instance, control of sham condition and blinding levels are critical in an effort to assess the clinical relevance of NIBS in SUDs and several studies have not reported how these were done. Avoidance of carry over effects is also essential in this regard within repeated sessions paradigms, to determine the effect of the tested stimulation design itself. In line with this, in crossover paradigms, craving assessments need to be assessed before each arm to provide a secure baseline for experimental measurements.
4) Tested subjects who may benefit from NIBS?
Finally, patients’ characteristics are important to consider when assessing the effects of NIBS on craving in SUDs. For example, age and sex can influence craving (Potenza et al., 2012; Robbins et al., 1998) and the effects of NIBS (Freitas et al., 2013; Meinzer et al., 2012).
Some factors specific to SUDs are also important. Neuroimaging studies reported that patients’ level of engagement towards treatment and anticipation of benefits may impact results, supporting the importance of identifying patients as treatment-seeking or not. For instance, fMRI studies reported differential patterns of activity, especially involving the DLPFC, in regards to whether subjects were treatment-seekers or not (Wilson et al., 2004). Hence, this differential pattern of brain activity related to expectancy status is likely to impact the effects of NIBS. The level of dependence is also important, as neuroimaging studies reported differential patterns of activity accordingly (Hong et al., 2009), which is also likely interfering with the effects of NIBS.
In the reviewed studies, most patients with AUD also had TUD. In some other studies, patients displayed depressive and anxiety symptoms. Co-occurrence of mood disorders and SUDs (Pettinati et al., 2013) is frequent and is of particular importance, as targeting the DLPFC with NIBS is also known to modulate mood in healthy subjects (e.g. Pascual-Leone et al. 1996; George et al. 1996) and reducing depressive symptoms in patients with depression (e.g., O’Reardon et al., 2007) and in SUD patients (Camprodon et al, 2007). We thus cannot rule out whether NIBS alleviate mood in patients with SUD and whether these effects may in turn partially suppress craving. Subjective level of stress may also influence and/or induce craving, and this relationship is well documented (Potenza et al., 2012; Sinha, 2011; Sinha et al., 2006).
In sum, we propose that identifying and inducing the ideal brain state to obtain the greatest craving reduction with NIBS should be a priority in the amelioration of such alternatives. In the same perspective, combination of NIBS with medication for smoking cessation also has to be considered. Although several clinical studies have used such combination in pain (Fregni et al., 2006) and schizophrenia (Brunelin et al., 2012), no results are readily available in SUDs. This poses the challenges of identifying the possible deleterious interference of NIBS with medication, and determining the optimal dosage of neurostimulation concurrently with medication.
Highlights.
We have reviewed studies using rTMS and tES to reduce craving in SUDs
Both brain stimulation techniques can induce significant changes in craving
Craving reduction did not reach clinical significance in all studies
Methodological discrepancies may explain the different conclusions
Parameters need to be addressed in future works to promote therapeutic potential
Acknowledgments
Funding:
This work was supported by a scholarship from Centre Interdisciplinaire de Recherche en Réadaptation et Intégration Sociale and Fonds de la Recherche du Quebec, Sante to AHB, grants from the Sidney R. Baer Jr. Foundation, the National Institutes of Health (R01HD069776, R01NS073601, R21 MH099196, R21 NS082870, R21 NS085491, R21 HD07616, UL1 RR025758), and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH 8KL2TR000168-05) to APL, and grants from the National Science and Engineering Research Council and Canada Research Chair to SF. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health or the Sidney R. Baer Jr. Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the alcohol withdrawal syndrome. Cochrane Database Syst Rev. 2011;6:CD008537. doi: 10.1002/14651858.CD008537.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction. 2009;104:653–660. doi: 10.1111/j.1360-0443.2008.02448.x. [DOI] [PubMed] [Google Scholar]
- Antal A, Fischer T, Saiote C. Transcranial electrical stimulation modifies the neuronal response to psychosocial stress exposure. Hum Brain Mapp. 2014;35:3750–3759. doi: 10.1002/hbm.22434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift RM, Weiss RD, Williams LD, Zweben A. Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Baker TB, Breslau N, Covey L, Shiffman S. DSM criteria for tobacco use disorder and tobacco withdrawal: a critique and proposed revisions for DSM-5*. Addiction. 2012;107:263–275. doi: 10.1111/j.1360-0443.2011.03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeken C, De Raedt R, Leyman L. The impact of one HF-rTMS session on mood and salivary cortisol in treatment of resistant unipolar melancholic depressed patients. J Affect Disord. 2009;113:100–108. doi: 10.1016/j.jad.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Bashir S, Perez JM, Horvath JC, Pascual-Leone A. Differentiation of motor cortical representation of hand muscles by navigated mapping of optimal TMS current directions in healthy subjects. J Clin Neurophysiol. 2013;30:390–395. doi: 10.1097/WNP.0b013e31829dda6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive approach. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of Nicotine: Addiction, Smoking-Induced Disease, and Therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Feredoes E. Combined neurostimulation and neuroimaging in cognitive neuroscience: past, present and future. Ann NY Acad Sci. 2013;1296:11–13. doi: 10.1111/nyas.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio P, Liguori P, Sultani N, Rezende L. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neuroscience. 2009;463:82–86. doi: 10.1016/j.neulet.2009.07.041. [DOI] [PubMed] [Google Scholar]
- Boggio P, Sultani N, Fecteau S, Merabet L. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: A double-blind, sham-controlled study. Drug Alcohol Depen. 2008;92:55–60. doi: 10.1016/j.drugalcdep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Brody AL. Functional brain imaging of tobacco use and dependence. J Psychiatr Res. 2006;40:404–418. doi: 10.1016/j.jpsychires.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelin J, Mondino M, Gassab L, Haesebaert F, Gaha L, Suaud-Chagny MF, Saoud M, Mechri A, Poulet E. Examining transcranial direct-Current Stimulation (tdCS) as a treatment for Hallucinations in Schizophrenia. Am J Psychiatry. 2012;169:719–724. doi: 10.1176/appi.ajp.2012.11071091. [DOI] [PubMed] [Google Scholar]
- Brunelin J, Fecteau S. Can the effects of noninvasive brain stimulation alleviating neuropsychiatric symptoms result from a common beneficial regulation of the hypothalamic-pituitary-adrenal axis? Brain Stim. 2015;8:173–176. doi: 10.1016/j.brs.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN. International Review of Neurobiology. 1st. Elsevier Inc; 2009. Chapter 5 – Molecular Bases of Methamphetamine-Induced Neurodegeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camprodon JA, Martínez-Raga J, Alonso-Alonso M, Shih MC, Pascual-Leone A. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depen. 2007;86:91–94. doi: 10.1016/j.drugalcdep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Castells X, Casas M, Pérez-Maña C, Roncero C, Vidal X, Capellà D. Efficacy of psychostimulant drugs for cocaine dependence. Cochrane Database Syst Rev. 2010;2:CD007380. doi: 10.1002/14651858.CD007380.pub3. [DOI] [PubMed] [Google Scholar]
- Ciampi de Andrade D, Galhardoni R, Pinto LF, Lancelotti R, Rosi J, Jr, Marcolin MA, Teixeira MJ. Into the Island: A new technique of non-invasive cortical stimulation of the insula. Clin Neurophysiol. 2012;42:363–368. doi: 10.1016/j.neucli.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulated dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. Plos One. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti CL, Nakamura-Palacios EM. Bilateral Transcranial Direct Current Stimulation Over Dorsolateral Prefrontal Cortex Changes the Drug-cued Reactivity in the Anterior Cingulate Cortex of Crack-cocaine Addicts. Brain Stimul. 2014;7:130–132. doi: 10.1016/j.brs.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva MC, Conti CL, Klauss J, Alves LG, do Nascimento Cavalcante HM, Fregni F, Nitsche MA, Nakamura-Palacios EM. Behavioral effect sof transcranial Direct Current Stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J Physiol Paris. 2013;107:493–502. doi: 10.1016/j.jphysparis.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O’Brien C. Cocaine dependence: a disease of the brain’s reward centers. J Subst Abuse Treat. 2001:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- De Ridder D, Vanneste S, Kovacs S, Sunaert S, Dom G. Transient alcohol craving suppression by rTMS of dorsal anterior cingulate: An fMRI and LORETA EEG study. Neurosci Lett. 2011;496:5–10. doi: 10.1016/j.neulet.2011.03.074. [DOI] [PubMed] [Google Scholar]
- Dinur-Klein L, Dannon P, Hadar A, Rosenberg O, Roth Y, Kotler M, Zangen A. Smoking cessation induced by the deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol Psychiatry. 2014;76:742–749. doi: 10.1016/j.biopsych.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Eichhammer P, Johann M, Kharraz A, Binder H, Pittrow D, Wodarz N, Hajak G. High-Frequency Repetitive Transcranial Magnetic Stimulation Decreases Cigarette Smoking. J Clin Psych. 2003;64:951–953. doi: 10.4088/jcp.v64n0815. [DOI] [PubMed] [Google Scholar]
- Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci USA. 2011;108:21229–21234. doi: 10.1073/pnas.1113103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Agosta S, Hone-Blanchet A, Fregni F, Boggio P, Ciraulo D, Pascual-Leone A. Drug and Alcohol Dependence. Drug Alcohol Depen. 2014;140:78–84. doi: 10.1016/j.drugalcdep.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Fregni F, Boggio PS, Camprodon JA, Pascual-Leone A. Neuromodulation of Decision-Making in the Addictive Brain. Subst Abuse. 2010;45:1766–1786. doi: 10.3109/10826084.2010.482434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil J, Zangen A. Brain stimulation in the study and treatment of addiction. Neurosci Biobehav R. 2010;34:559–574. doi: 10.1016/j.neubiorev.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR. Limbic Activation to Cigarette Smoking Cues Independent of Nicotine Withdrawal: A Perfusion fMRI Study. Neuropsychopharmacol. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Lima MC, Ferreira MJL, Wagner T, Rigonatti SP, Castro AW, Souza DR, Riberto M, Freedman SD, Nitsche MA, Pascual-Leone A. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122:197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Fregni F, Liguori P, Fecteau S, Nitsche M. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J Clin Psych. 2008;69:32–40. doi: 10.4088/jcp.v69n0105. [DOI] [PubMed] [Google Scholar]
- Freitas C, Farzan F, Pascual-Leone A. Assessing brain plasticity across the lifespan with transcranial magnetic stimulation: why, how, and what is the ultimate goal? Front Neurosci. 2013;7:42. doi: 10.3389/fnins.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H. Insula and drug cravings. Brain Struct Funct. 2010;214:593–601. doi: 10.1007/s00429-010-0259-8. [DOI] [PubMed] [Google Scholar]
- George MS, Wassermann EM, Kimbrell TA, Little JT, Williams WE, Danielson AL, Greenberg BD, Hallett M, Post RM. Mood Improvement Following Daily Left Prefrontal Repetitive Transcranial Magnetic Stimulation in Patients With Depression: A Placebo-Controlled Crossover Trial. Am J Psychiatry. 1997;154:1752–1756. doi: 10.1176/ajp.154.12.1752. [DOI] [PubMed] [Google Scholar]
- George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, Hallett M, Post RM. Daily repetitive transcranila magnetic stimulation (rTMS) improves mood in depression. NeuroReport. 1995;6:1853–1857. doi: 10.1097/00001756-199510020-00008. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional. Philos Trans R Soc Lond, B, Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug Addiction and Its Underlying Neurobiological Basis: Neuroimaging Evidence for the Involvement of the Frontal Cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, De Ruiter MB, van den Brink W, Oosterlaan J, Veltman DJ. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: an fMRI study. Addict Biol. 2010;15:491–503. doi: 10.1111/j.1369-1600.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Jones EM, Li X, Hartwell KJ, Brady KT, George MS. Individual variability in the locus of prefrontal craving for nicotine: implications for brain stimulation studies and treatments. Drug Alcohol Depen. 2012;125:239–243. doi: 10.1016/j.drugalcdep.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Austelle CW, DeVries W, Mithoefer O, Badran BW, George MS. what goes up can come down: novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain Res. 2015 doi: 10.1016/j.brainres.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans SC, Baeken C, Vanderbruggen N, Vanderhasselt MA, Zeeuws D, Santermans L, De Raedt R. No influence of one right-sided prefrontal HF-rTMS session on alcohol craving in recently detoxified alcohol-dependent patients: Results of a naturalistic study. Drug Alcohol Depen. 2012;120:209–213. doi: 10.1016/j.drugalcdep.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Herremans SC, Vanderhasselt MA, De Raedt R, Baeken C. Reduced Intra-individual Reaction Time Variability During a Go-NoGo Task in Detoxified Alcohol-Dependent Patients After One Right-Sided Dorsolateral Prefrontal HF-rTMS Session. Alcohol Alcoholism. 2013:1–6. doi: 10.1093/alcalc/agt054. [DOI] [PubMed] [Google Scholar]
- Holland R, Leff AP, Josephs O, Galea JM, Desikan M, Price CJ, Rothwell JC, Crinion J. Speech Facilitation by Left Inferior Frontal Cortex Stimulation. Curr Biol. 2011;21:1403–1407. doi: 10.1016/j.cub.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone-Blanchet A, Wensing T, Fecteau S. The use of virtual reality in craving assessment and cue-exposure therapy in substance use disorders. Front Hum Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone-Blanchet A, Salas ER, Celnik P, Kallo A, Schar M, Puts NAJ, Harris AD, Barker PB, Fecteau S, Early CJ, Allen RP, Edden RA. Co-registration of magnetic resonance spectroscopy and transcranial magnetic stimulation. J Neurosci Meth. 2015;242:52–57. doi: 10.1016/j.jneumeth.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GV, Stein EA. Association of Nicotine Addiction and Nicotine’s Actions With Separate Cingulate Cortex Functional Circuits. Arch Gen Psych. 2009;66:431–442. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höppner J, Broese T, Wendler L, Berger C, Thome J. Repetitive transcranial magnetic stimulation (rTMS) for treatment of alcohol dependence. World J Biol Psych. 2011:57–62. doi: 10.3109/15622975.2011.598383. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: A Disease of Learning and Memory. Focus. 2007;5:220. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Jansen JM, Daams JG, Koeter MWJ, Veltman DJ, van den Brink W, Goudriaan AE. Effects of non-invasive neurostimulation on craving: A meta-analysis. Neurosci Biobehav R 37. 2013;10:2472–2480. doi: 10.1016/j.neubiorev.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Janes AC, Jensen JE, Farmer SL, Frederick B, Pizzagalli DA, Lukas SE. GABA levels in the dorsal anterior cingulate cortex associated with difficulty ignoring smoking-related cues in tobacco-dependent volunteers. Neuropsychopharmacol. 2013;38:1113–1120. doi: 10.1038/npp.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johann M, Wiegand R, Kharraz A, Bobbe G, Sommer G, Hajak G, Wodarz N, Eichhammer P. Repetitiv Transcranial Magnetic Stimulation in Nicotine Dependence. Psychiatr Prax. 2003;30:129–131. doi: 10.1055/s-2003-39733. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Cocaine and amphetamine-like psychostimulants: neurocircuitry and glutamate neuroplasticity. Dialogues Clin Neurosci. 2007a;9:389–397. doi: 10.31887/DCNS.2007.9.4/pkalivas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Neurobiology of Cocaine Addiction: Implications for New Pharmacotherapy. Am J Addict. 2007b;16:71–78. doi: 10.1080/10550490601184142. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug Addiction as a Pathology of Staged Neuroplasticity. Neuropsychopharmacol. 2007;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Peters J, Knackstedt L. Animal Models and Brain Circuits in Drug Addiction. Molecular Interventions. 2006;6:339–344. doi: 10.1124/mi.6.6.7. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Keck ME, Welt T, Müller MB, Erhardt A, Ohl F, Toschi N, Holsboer F, Sillaber I. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacol. 2002;43:101–109. doi: 10.1016/s0028-3908(02)00069-2. [DOI] [PubMed] [Google Scholar]
- Keeser D, Padberg F, Reisinger E, Pogarell O, Kirsch V, Palm U, Karch S, Moller HJ, Nitsche MA, Mulert C. Prefrontal direct current stimulation modulates resting EEG and event-related potentials in healthy subjects: A standardized low resolution tomography (sLORETA) study. NeuroImage. 2011;55:644–657. doi: 10.1016/j.neuroimage.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Knapp WP, Soares B, FArrell M, Silva de Lima M. Psychosocial interventions for cocaine and psychostimulant amphetamines related disorders. Cochrane Database Syst Rev. 2007;3:CD003023. doi: 10.1002/14651858.CD003023.pub2. [DOI] [PubMed] [Google Scholar]
- Ko JH, Strafella AP. Dopaminergic Neurotransmission in the Human Brain: New Lessons from Perturbation and Imaging. Neuroscientist. 2011 doi: 10.1177/1073858411401413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P. Prefrontal–striatal pathway underlies cognitive regulation of craving. PNAS. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacol. 2009;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MF, Paulus W, Nitsche MA. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage. 2013;85:948–960. doi: 10.1016/j.neuroimage.2013.05.117. [DOI] [PubMed] [Google Scholar]
- Lang N, Hasan A, Sueske E, Paulus W, Nitsche MA. Cortical Hypoexcitability in Chronic Smokers? A Transcranial Magnetic Stimulation Study. Neuropsychopharmacol. 2007;33:2517–2523. doi: 10.1038/sj.npp.1301645. [DOI] [PubMed] [Google Scholar]
- Lev-Ran S, Balchand K, Lefebvre L, Araki KF, Le Foll B. Pharmacotherapy of alcohol use disorders and concurrent psychiatric disorders: a review. Can J Psychiatry. 2012;57:342–349. doi: 10.1177/070674371205700603. [DOI] [PubMed] [Google Scholar]
- Li X, Malcolm RJ, Huebner K, Hanlon CA, Taylor JJ, Brady KT, George MS, See RE. Drug and Alcohol Dependence. Drug Alcohol Depen. 2013a;133:641–646. doi: 10.1016/j.drugalcdep.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Malcolm RJ, Huebner K, Hanlon CA, Taylor JJ, Brady KT, George MS, See RE. Low frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex transiently increases cue-induced craving for methamphetamine: A preliminary study. Drug Alcohol Depen. 2013b;133:641–646. doi: 10.1016/j.drugalcdep.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Fu XM, Li N, Wang CX, Zhang H, Qian RB, Xu HS, Hu X, Zhang DR. Abnormal Brain Default-Mode Network Functional Connectivity in Drug Addicts. PLoS ONE. 2011;6:16560. doi: 10.1371/journal.pone.0016560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A. Neurobiology of nicotine dependence. Phil Trans R Soc B: Biol Sci. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JF, O’Dell SJ. Methamphetamine influences on brainand behavior: unsafe at any speed? TINS. 2012;35:536–545. doi: 10.1016/j.tins.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. doi: 10.1002/14651858.CD002209. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of Expectancy and Abstinence on the Neural Response to Smoking Cues in Cigarette Smokers: an fMRI Study. Neuropsychopharmacol. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Antonenko D, Lindenberg R, Hetzer S, Ulm L, Avirame K, Flaisch T, Floel A. Electrical Brain Stimulation Improves Cognitive Performance by Modulating Functional Connectivity and Task-Specific Activation. J Neurosci. 2012;32:1859–1866. doi: 10.1523/JNEUROSCI.4812-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Liu C, Yu C, Ma Y. Journal of Psychiatric Research J Psychiatr Res. 2014:1–7. doi: 10.1016/j.jpsychires.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Mishra BR, Nizamie SH, Das B, Praharaj SK. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addict. 2011;105:49–55. doi: 10.1111/j.1360-0443.2009.02777.x. [DOI] [PubMed] [Google Scholar]
- Nahas Z, Lomarev M, Roberts DR, Shastri A, Lorberbaum JP, Teneback C, McConnell K, Vincent DJ, Li X, George MS, Bohning DE. Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity-dependent bilateral effects as measured by interleaved BOLD fMRI. Biol Psychiatry. 2001;50:712–720. doi: 10.1016/s0006-3223(01)01199-4. [DOI] [PubMed] [Google Scholar]
- Nakamura-Palacios EM, de Almeida Benevides MC, da Penha Zago-Gomes M, de Oliveira RWD, de Vasconcellos VF, de Castro LNP, da Silva MC, Ramos PA, Fregni F. Auditory event-related potentials (P3) and cognitive changes induced by frontal direct current stimulation in alcoholics according to Lesch alcoholism typology. Int J Neuropsychopharmacol. 2012;15:601–616. doi: 10.1017/S1461145711001040. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. TINS. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche M, Liebetanz D. GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur J Neurosci. 2004;19:2720–2726. doi: 10.1111/j.0953-816X.2004.03398.x. [DOI] [PubMed] [Google Scholar]
- Nitsche M, Nitsche M, Klein C, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003;114:600–604. doi: 10.1016/s1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackeim HA. Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biol Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Park CH, Chang WH, Park JY, Shin YI, Kim ST, Kim YH. Neurosci Lett. 2013;539:7–10. doi: 10.1016/j.neulet.2013.01.047. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Rubio B, Pallardò F, Català MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. The Lancet. 1996;348:233–238. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural Activation Patterns of Methamphetamine-Dependent Subjects During Decision Making Predict Relapse. Arch gen psych. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Pedron S, Monnin J, Haffen E, Sechter D, Van Waes V. Repeated Transcranial Direct Current Stimulation Prevents Abnormal Behaviors Associated with Abstinence from Chronic Nicotine Consumption. Neuropsychopharmacol. 2014;39:981–988. doi: 10.1038/npp.2013.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Maña C, Castells X, Vidal X, Casas M, Capellà D. Efficacy of indirect dopamine agonists for psychostimulant dependence: A systematic review and meta-analysis of randomized controlled trials. J Subs Abuse Treatment. 2011;40:109–122. doi: 10.1016/j.jsat.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Subjective Reactivity to Smoking Cues as a Predictor of Quitting Success. Nicotine Tob Res. 2012;14:383–387. doi: 10.1093/ntr/ntr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, O’Brien CP, Dundon WD. Current status of co-occurring mood and substance use disorders: a new therapeutic target. Am J Psychiatry. 2013;170:23–30. doi: 10.1176/appi.ajp.2012.12010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogarell O, Koch W, Pöpperl G, Tatsch K, Jakob F, Mulert C, Grossheinrich N, Rupprecht R, Möller HJ, Hegerl U, Padberg F. Acute prefrontal rTMS increases striatal dopamine to a similar degree as d-amphetamine. Psychiar Res-Neuroim. 2007;156:251–255. doi: 10.1016/j.pscychresns.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Politi E, Fauci E, Santoro A, Smeraldi E. Daily Sessions of Transcranial Magnetic Stimulation to the Left Prefrontal Cortex Gradually Reduce Cocaine Craving. Am J Addict. 2008;17:345–346. doi: 10.1080/10550490802139283. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Hong KIA, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural Correlates of Stress-Induced and Cue-Induced Drug Craving: Influences of Sex and Cocaine Dependence. Am J Psychiatry. 2012;169 doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pripfl J, Neumann R, Köhler U, Lamm C. Effects of transcranial direct current stimulation on risky decision making are mediated by ‘hot’ and ‘cold’ decisions, personality, and hemisphere. Eur J Neurosci. 2013;38:3778–3785. doi: 10.1111/ejn.12375. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depen. 1998;53:223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Rose JE, McClernon FJ, Froeliger B, Behm FM, Preud’homme X, Krystal AD. Repetitive Transcranial Magnetic Stimulation of the Superior Frontal Gyrus Modulates Craving for Cigarettes. Biol Psychiatry. 2011;70:794–799. doi: 10.1016/j.biopsych.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Rusjan PM, Barr MS, Farzan F, Arenovich T, Maller JJ, Fitzgerald PB, Daskalakis ZJ. Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum Brain Mapp. 2010;31:1643–1652. doi: 10.1002/hbm.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiote C, Turi Z, Paulus W, Antal A. Combining functional magnetic resonance imaging with transcranial electrical stimulation. Fr Human Neurosci. 2013;7:1–7. doi: 10.3389/fnhum.2013.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey AG. The Neural Basis of Economic Decision-Making in the Ultimatum Game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Benowitz NL. Nicotine Addiction. N Engl J Med. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbabaie A, Golesorkhi M, Zamanian B, Ebrahimpoor M, Keshvari F, Nejati V, Fregni F, Ekhtiari H. State dependent effect of transcranial direct current stimulation (tDCS) on methamphetamine craving. Int J Neuropsychopharmacol. 2014:1–8. doi: 10.1017/S1461145714000686. [DOI] [PubMed] [Google Scholar]
- Sinha R. Effects of Adrenal Sensitivity, Stress- and Cue-Induced Craving, and Anxiety on Subsequent Alcohol Relapse and Treatment Outcomes. Arch gen psych. 2011;68:942. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-Induced Cocaine Craving and Hypothalamic-Pituitary-Adrenal Responses Are Predictive of Cocaine Relapse Outcomes. Arch gen psych. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O’Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H. Polarity-Sensitive Modulation of Cortical Neurotransmitters by Transcranial Stimulation. J Neurosci. 2009;29:5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM. Essential Psychopharmacology. Second. Cambridge University Press; 2005. [Google Scholar]
- Stead LF, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2012;10:CD008286. doi: 10.1002/14651858.CD008286.pub2. [DOI] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. NeuroImage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Takano Y, Tanaka S, Hironaka N, Kobayashi K, Hanakawa T, Watanabe K, Honda M. Transcranial direct-current stimulation increases extracellular dopamine levels in the rat striatum. Front Syst Neurosci. 2013;7:1–8. doi: 10.3389/fnsys.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo JM, Martin-García E, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: a common substrate in drug addiction. Drug and alcohol dependence. 2010;108:183–194. doi: 10.1016/j.drugalcdep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2009;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. Role of Dopamine, the Frontal Cortex and Memory Circuits in Drug Addiction: Insight from Imaging Studies. Neurobiol Learn Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. PNAS. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völlm BA, de Araujo IE, Cowen PJ, Rolls ET, Kringelbach ML, Smith KA, Jezzard P, Heal RJ, Matthews PM. Methamphetamine Activates Reward Circuitry in Drug Naïve Human Subjects. Neuropsychopharmacol. 2004;29:1715–1722. doi: 10.1038/sj.npp.1300481. [DOI] [PubMed] [Google Scholar]
- Wagner T, Gangitano M, Romero R, Théoret H, Kobayashi M, Anschel D, Ives J, Cuffin N, Schomer D, Pascual-Leone A. Intracranial measurement of current densities induced by transcranial magnetic stimulation in the human brain. Neurosci Lett. 2004;354:91–94. doi: 10.1016/s0304-3940(03)00861-9. [DOI] [PubMed] [Google Scholar]
- Wassermann E, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin Neurophysiol. 2001;112:1367–1377. doi: 10.1016/s1388-2457(01)00585-5. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing VC, Barr MS, Wass CE, Lipsman N, Lozano AM, Daskalakis ZJ, George TP. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 2012;6:221–230. doi: 10.1016/j.brs.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Xu J, Fregni F, Brody AL, Rahman AS. Transcranial direct current stimulation reduces negative affect but not cigarette craving in overnight abstinent smokers. Front Psychiatry. 2013;4:112. doi: 10.3389/fpsyt.2013.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Lubman DI, Harrison BJ, Fornito A, Allen NB, Wellard RM, Roffel K, Clarke K, Forman SD, Pantelis C. A combined spectroscopic and functional MRI investigation of the dorsal anterior cingulate region in opiate addiction. Mol Psychiatry. 2007;12:691–702. doi: 10.1038/sj.mp.4001955. [DOI] [PubMed] [Google Scholar]


